Electrochemical and Optical Properties of D-A-A-A-D Azomethine Triad and Its NIR-Active Polymer
Abstract
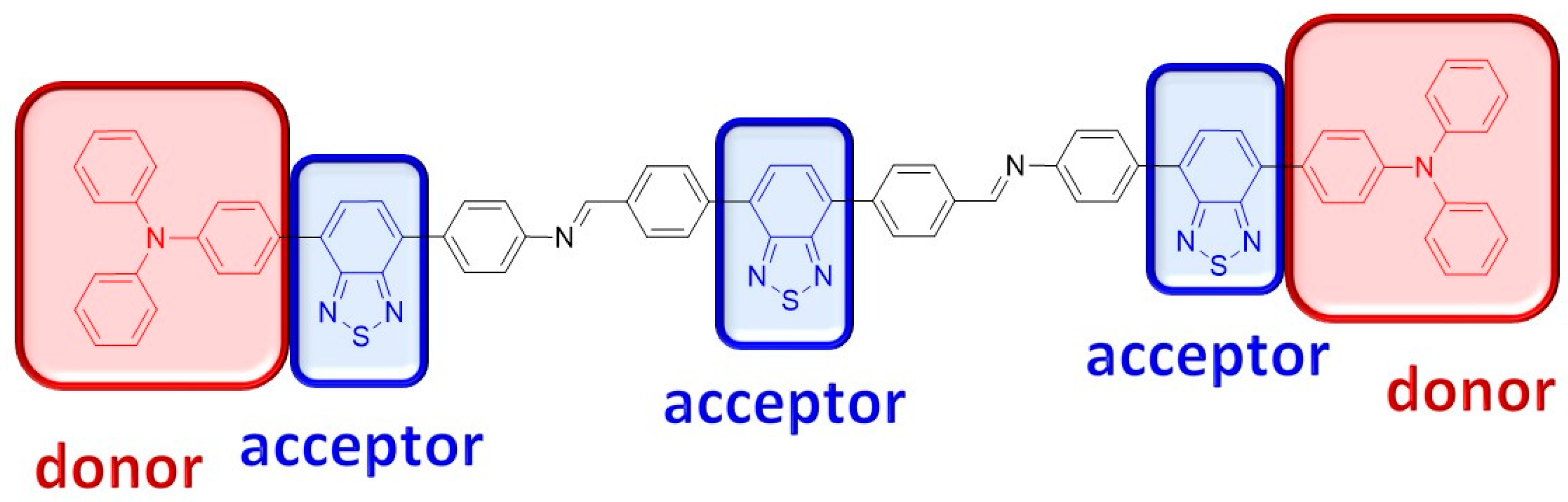
:1. Introduction
2. Results and Discussion
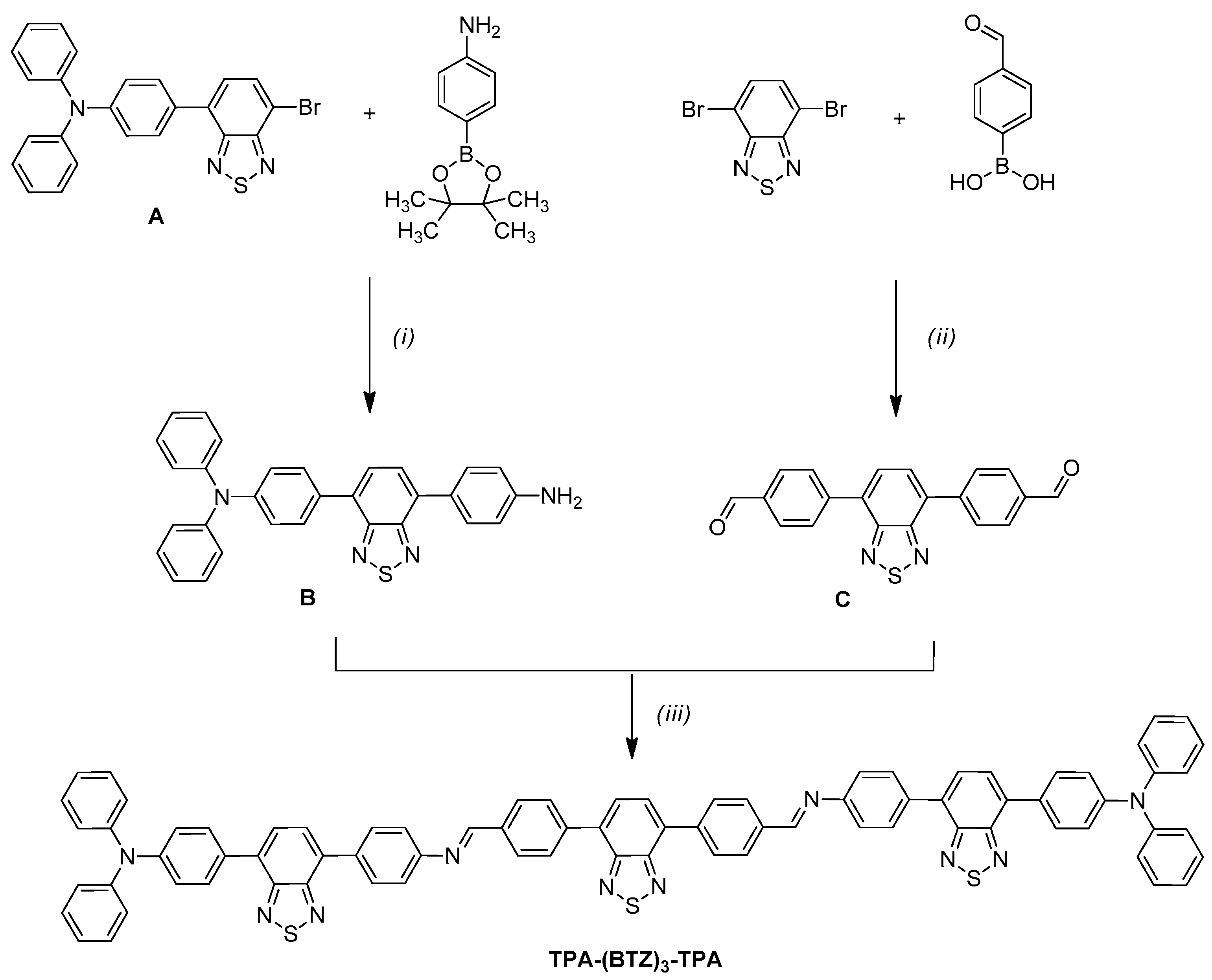
2.1. Synthesis of the Dye
2.2. Optical Properties
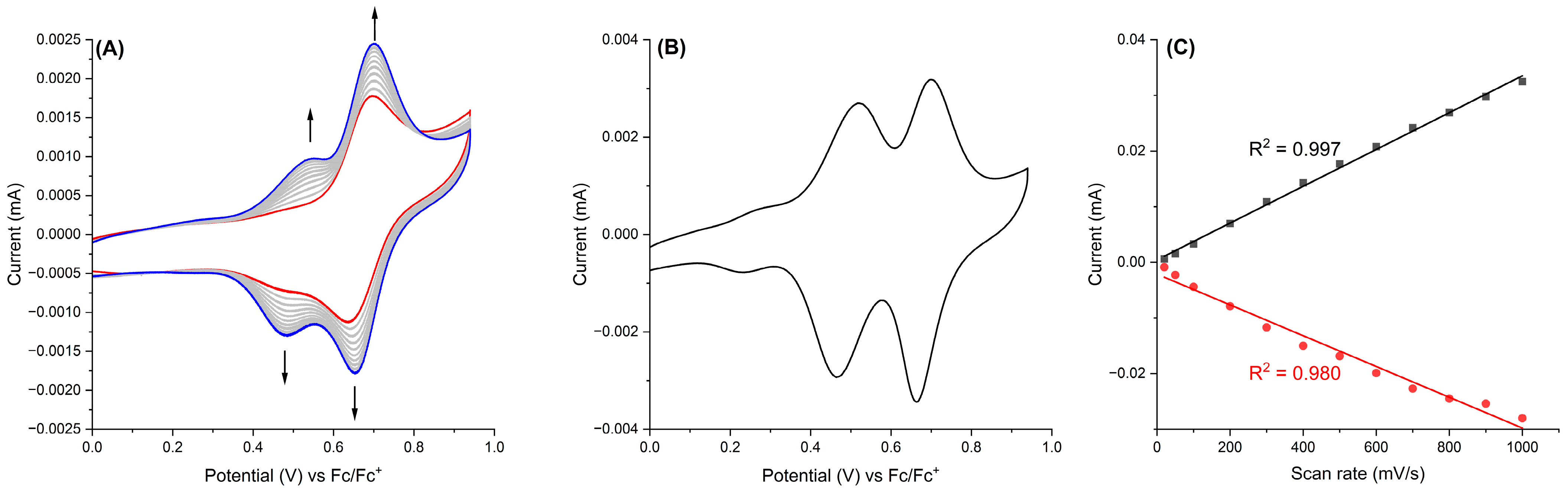
2.3. Electropolymerization and Characterization
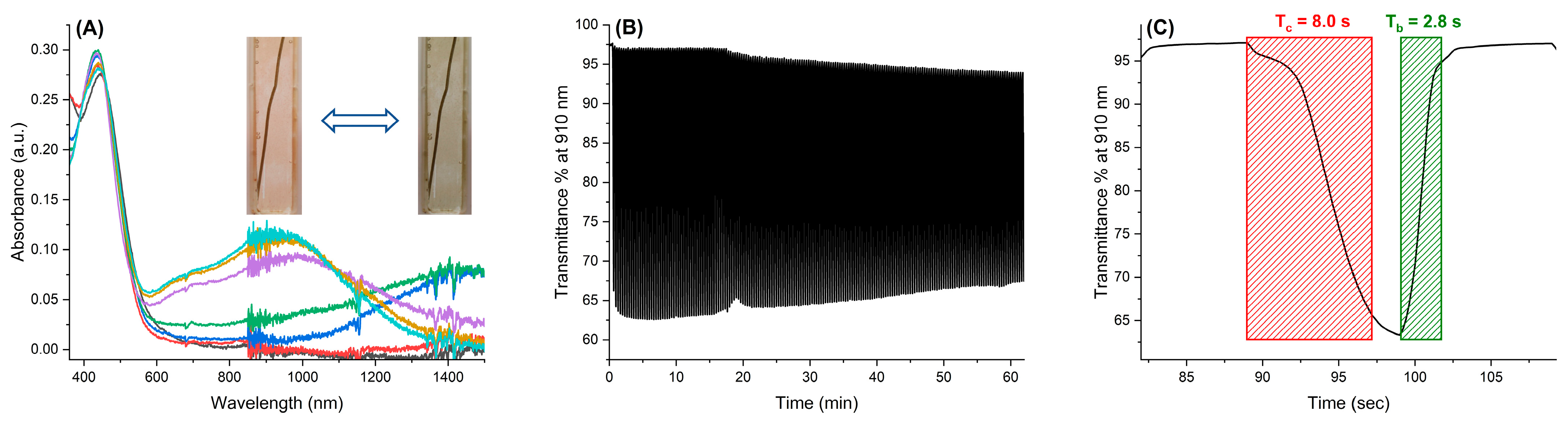
2.4. Spectroelectrochemical Properties
3. Materials and Methods
- 4-(4-Bromo-2,1,3-benzothiadiazol-7-yl)-N,N-diphenylaniline A:
- 4-(4-Phenylamino-2,1,3-benzothiadiazol-7-yl)-N,N-diphenylaniline B:
- 4-[4-(4-formylphenyl)-2,1,3-benzothiadiazol-7-yl]benzaldehyde C:
- Azomethine triad TPA-(BTZ)3-TPA:
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bolduc, A.; Mallet, C.; Skene, W.G. Survey of recent advances of in the field of π-conjugated heterocyclic azomethines as materials with tuneable properties. Sci. China Chem. 2013, 56, 3–23. [Google Scholar] [CrossRef]
- Yang, C.J.; Jenekhe, S.A. Conjugated aromatic poly(azomethines). 1. Characterization of structure, electronic spectra, and processing of thin films from soluble complexes. Chem. Mater. 1991, 3, 878–887. [Google Scholar] [CrossRef]
- Bolduc, A.; Al Ouahabi, A.; Mallet, C.; Skene, W.G. Insight into the Isoelectronic Character of Azomethines and Vinylenes Using Representative Models: A Spectroscopic and Electrochemical Study. J. Org. Chem. 2013, 78, 9258–9269. [Google Scholar] [CrossRef]
- Charland-Martin, A.; Collier, G.S. Understanding Degradation Dynamics of Azomethine-containing Conjugated Polymers. Macromolecules 2024, 57, 6146–6155. [Google Scholar] [CrossRef]
- Filiatrault, H.L.; Muras, K.; Wałęsa-Chorab, M.; Skene, W.G. On-Substrate Preparation of a Poly(triphenylamino azomethine) for Electrochromic Devices. Polymers 2024, 16, 2440. [Google Scholar] [CrossRef] [PubMed]
- Wałęsa-Chorab, M.; Skene, W.G. Leveraging reversible bonds for property modification of electrochromes and their immobilization by dual modes: Thermal and electrochemical polymerization. Prog. Org. Coatings 2024, 187, 108113. [Google Scholar] [CrossRef]
- Lerond, M.; Bélanger, D.; Skene, W.G. Surface immobilized azomethine for multiple component exchange. Soft Matter 2017, 13, 6639–6646. [Google Scholar] [CrossRef]
- Wałȩsa-Chorab, M.; Skene, W.G. Engaging the reversible bonds of an immobilized styreno-thiophene film. Cryst. Growth Des. 2020, 20, 5688–5697. [Google Scholar] [CrossRef]
- Abbasi, A.; Rezvani, Z.; Nejati, K. Synthesis and properties of new liquid crystalline compounds containing an alkoxyphenylazo group. Dyes Pigment. 2006, 70, 71–75. [Google Scholar] [CrossRef]
- Ng, S.C.; Chan, H.S.O.; Wong, P.M.L.; Tan, K.L.; Tan, B.T.G. Novel heteroarylene polyazomethines: Their syntheses and characterizations. Polymer 1998, 39, 4963–4968. [Google Scholar] [CrossRef]
- Fujii, S.; Minami, S.; Urayama, K.; Suenaga, Y.; Naito, H.; Miyashita, O.; Imoto, H.; Naka, K. Beads-on-String-Shaped Poly(azomethine) Applicable for Solution Processing of Bilayer Devices Using a Same Solvent. ACS Macro Lett. 2018, 7, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.C.; Shim, S.C. Synthesis and properties of a novel polyazomethine, the polymer with high photoconductivity and second-order optical nonlinearity. Synth. Met. 2000, 114, 91–95. [Google Scholar] [CrossRef]
- Napierała, S.; Kubicki, M.; Wałęsa-Chorab, M. Toward Electrochromic Metallopolymers: Synthesis and Properties of Polyazomethines Based on Complexes of Transition-Metal Ions. Inorg. Chem. 2021, 60, 14011–14021. [Google Scholar] [CrossRef] [PubMed]
- Sicard, L.; Navarathne, D.; Skalski, T.; Skene, W.G. On-Substrate Preparation of an Electroactive Conjugated Polyazomethine from Solution-Processable Monomers and its Application in Electrochromic Devices. Adv. Funct. Mater. 2013, 23, 3549–3559. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Skene, W.G. Investigation of an electroactive immobilized azomethine for potential electrochromic use. Sol. Energy Mater. Sol. Cells 2019, 200, 109977. [Google Scholar] [CrossRef]
- Leliège, A.; Barik, S.; Skene, W.G. Photopatternable Electrochromic Materials from Oxetane Precursors. ACS Appl. Mater. Interfaces 2014, 6, 6920–6929. [Google Scholar] [CrossRef]
- Sonker, E.; Tiwari, R.; Kumar, K.; Krishnamoorthi, S. Electrical properties of new polyazomethines. SN Appl. Sci. 2020, 2, 1123. [Google Scholar] [CrossRef]
- Garbay, G.; Giraud, L.; Gali, S.M.; Hadziioannou, G.; Grau, E.; Grelier, S.; Cloutet, E.; Cramail, H.; Brochon, C. Divanillin-Based Polyazomethines: Toward Biobased and Metal-Free π-Conjugated Polymers. ACS Omega 2020, 5, 5176–5181. [Google Scholar] [CrossRef]
- Li, G.; Yu, K.; Noordijk, J.; Meeusen-Wierts, M.H.M.; Gebben, B.; oude Lohuis, P.A.M.; Schotman, A.H.M.; Bernaerts, K.V. Hydrothermal polymerization towards fully biobased polyazomethines. Chem. Commun. 2020, 56, 9194–9197. [Google Scholar] [CrossRef]
- Wałȩsa-Chorab, M.; Skene, W.G. On-substrate polymerization-a versatile approach for preparing conjugated polymers suitable as electrochromes and for metal ion sensing. RSC Adv. 2014, 4, 19053–19060. [Google Scholar] [CrossRef]
- Leung, A.C.W.; MacLachlan, M.J. Schiff Base Complexes in Macromolecules. J. Inorg. Organomet. Polym. Mater. 2007, 17, 57–89. [Google Scholar] [CrossRef]
- Ketcham, K.A.; Swearingen, J.K.; Castiñeiras, A.; Garcia, I.; Bermejo, E.; West, D.X. Iron(III), cobalt(II,III), copper(II) and zinc(II) complexes of 2-pyridineformamide 3-piperidylthiosemicarbazone. Polyhedron 2001, 20, 3265–3273. [Google Scholar] [CrossRef]
- Pająk, A.K.; Kotowicz, S.; Gnida, P.; Małecki, J.G.; Ciemięga, A.; Łuczak, A.; Jung, J.; Schab-Balcerzak, E. Synthesis and Characterization of New Conjugated Azomethines End-Capped with Amino-thiophene-3,4-dicarboxylic Acid Diethyl Ester. Int. J. Mol. Sci. 2022, 23, 8160. [Google Scholar] [CrossRef]
- Iwan, A.; Palewicz, M.; Chuchmała, A.; Gorecki, L.; Sikora, A.; Mazurek, B.; Pasciak, G. Opto(electrical) properties of new aromatic polyazomethines with fluorene moieties in the main chain for polymeric photovoltaic devices. Synth. Met. 2012, 162, 143–153. [Google Scholar] [CrossRef]
- Hindson, J.C.; Ulgut, B.; Friend, R.H.; Greenham, N.C.; Norder, B.; Kotlewski, A.; Dingemans, T.J. All-aromatic liquid crystal triphenylamine-based poly(azomethine)s as hole transport materials for opto-electronic applications. J. Mater. Chem. 2010, 20, 937–944. [Google Scholar] [CrossRef]
- Petrus, M.L.; Bouwer, R.K.M.; Lafont, U.; Athanasopoulos, S.; Greenham, N.C.; Dingemans, T.J. Small-molecule azomethines: Organic photovoltaics via Schiff base condensation chemistry. J. Mater. Chem. A 2014, 2, 9474–9477. [Google Scholar] [CrossRef]
- Dufresne, S.; Skene, W.G. Optoelectronic property tailoring of conjugated heterocyclic azomethines—The effect of pyrrole, thiophene and furans. J. Phys. Org. Chem. 2012, 25, 211–221. [Google Scholar] [CrossRef]
- Schab-Balcerzak, E.; Iwan, A.; Krompiec, M.; Siwy, M.; Tapa, D.; Sikora, A.; Palewicz, M. New thermotropic azomethine–naphthalene diimides for optoelectronic applications. Synth. Met. 2010, 160, 2208–2218. [Google Scholar] [CrossRef]
- Amin, M.F.; Gnida, P.; Kotowicz, S.; Małecki, J.G.; Siwy, M.; Nitschke, P.; Schab-Balcerzak, E. Spectroscopic and Physicochemical Investigations of Azomethines with Triphenylamine Core towards Optoelectronics. Materials 2022, 15, 7197. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-K.; Lin, Y.-C.; Ho, J.-C.; Yang, W.-C.; Chen, W.-C. Biomass-Derived Degradable Poly(azomethine)s for Flexible Bistable Photonic Transistor Memories. ACS Sustain. Chem. Eng. 2022, 10, 5268–5277. [Google Scholar] [CrossRef]
- Pan, L.; Hu, B.; Zhu, X.; Chen, X.; Shang, J.; Tan, H.; Xue, W.; Zhu, Y.; Liu, G.; Li, R.-W. Role of oxadiazole moiety in different D–A polyazothines and related resistive switching properties. J. Mater. Chem. C 2013, 1, 4556. [Google Scholar] [CrossRef]
- Liu, C.-L.; Chen, W.-C. Conjugated Polymers for Memory Device Applications. In Electrical Memory Materials and Devices; The Royal Society of Chemistry: London, UK, 2015; pp. 233–255. [Google Scholar]
- Cai, G.; Wang, J.; Lee, P.S. Next-Generation Multifunctional Electrochromic Devices. Acc. Chem. Res. 2016, 49, 1469–1476. [Google Scholar] [CrossRef]
- Yıldırım, M.; Kaya, İ.; Aydın, A. Azomethine coupled fluorene–thiophene–pyrrole based copolymers: Electrochromic applications. React. Funct. Polym. 2013, 73, 1167–1174. [Google Scholar] [CrossRef]
- Gautier, Y.; Skene, W.G. Effect of azomethine structural modification of electrochromic performance. J. Mater. Chem. C 2024, 12, 3589–3603. [Google Scholar] [CrossRef]
- Koole, M.; Frisenda, R.; Petrus, M.L.; Perrin, M.L.; van der Zant, H.S.J.; Dingemans, T.J. Charge transport through conjugated azomethine-based single molecules for optoelectronic applications. Org. Electron. 2016, 34, 38–41. [Google Scholar] [CrossRef]
- Ma, X.; Niu, H.; Wen, H.; Wang, S.; Lian, Y.; Jiang, X.; Wang, C.; Bai, X.; Wang, W. Synthesis, electrochromic, halochromic and electro-optical properties of polyazomethines with a carbazole core and triarylamine units serving as functional groups. J. Mater. Chem. C 2015, 3, 3482–3493. [Google Scholar] [CrossRef]
- Farcas, A.; Jarroux, N.; Ghosh, I.; Guégan, P.; Nau, W.M.; Harabagiu, V. Polyrotaxanes of Pyrene–Triazole Conjugated Azomethine and α-Cyclodextrin with High Fluorescence Properties. Macromol. Chem. Phys. 2009, 210, 1440–1449. [Google Scholar] [CrossRef]
- Georgiev, A.; Yordanov, D.; Dimov, D.; Zhivkov, I.; Nazarova, D.; Weiter, M. Azomethine phthalimides fluorescent E→Z photoswitches. J. Photochem. Photobiol. A Chem. 2020, 393, 112443. [Google Scholar] [CrossRef]
- Bishop, S.; Tremblay, M.-H.; Gellé, A.; Skene, W.G. Understanding Color Tuning and Reversible Oxidation of Conjugated Azomethines. J. Phys. Chem. A 2019, 123, 2687–2693. [Google Scholar] [CrossRef]
- Marin, L.; van der Lee, A.; Shova, S.; Arvinte, A.; Barboiu, M. Molecular amorphous glasses toward large azomethine crystals with aggregation-induced emission. New J. Chem. 2015, 39, 6404–6420. [Google Scholar] [CrossRef]
- Krishnaveni, K.; Gurusamy, S.; Rajakumar, K.; Sathish, V.; Thanasekaran, P.; Mathavan, A. Aggregation induced emission (AIE), selective fluoride ion sensing and lysozyme interaction properties of Julolidinesulphonyl derived Schiff base. J. Photochem. Photobiol. A Chem. 2022, 427, 113822. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Tremblay, M.H.; Skene, W.G. Hydrogen-Bond and Supramolecular-Contact Mediated Fluorescence Enhancement of Electrochromic Azomethines. Chemistry 2016, 22, 11382–11393. [Google Scholar] [CrossRef] [PubMed]
- Gather, M.C.; Köhnen, A.; Meerholz, K. White Organic Light-Emitting Diodes. Adv. Mater. 2011, 23, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, S.; Callaghan, L.; Skene, W.G. Conjugated Fluorenes Prepared from Azomethines Connections-II: The Effect of Alternating Fluorenones and Fluorenes on the Spectroscopic and Electrochemical Properties. J. Phys. Chem. B 2009, 113, 15541–15549. [Google Scholar] [CrossRef] [PubMed]
- Orlova, N.; Nikolajeva, I.; Pučkins, A.; Belyakov, S.; Kirilova, E. Heterocyclic Schiff Bases of 3-Aminobenzanthrone and Their Reduced Analogues: Synthesis, Properties and Spectroscopy. Molecules 2021, 26, 2570. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, K.; Mishra, M.; Singh, V.P. 8(E)-4-[{2-(2,4-dinitrophenyl)hydrazono}benzene-1,3-diol] as a solvatochromic Schiff base and chromogenic signaling of water content by its deprotonated form in acetonitrile. RSC Adv. 2014, 4, 27556. [Google Scholar] [CrossRef]
- Takeda, Y. Modulating the Photophysical Properties of Twisted Donor–Acceptor–Donor π-Conjugated Molecules: Effect of Heteroatoms, Molecular Conformation, and Molecular Topology. Acc. Chem. Res. 2024, 57, 2219–2232. [Google Scholar] [CrossRef]
- Muras, K.; Kubicki, M.; Wałęsa-Chorab, M. Benzochalcodiazole-based donor-acceptor-donor non-symmetric small molecules as dual-functioning electrochromic and electrofluorochromic materials. Dyes Pigment. 2023, 212, 111098. [Google Scholar] [CrossRef]
- Deng, B.; Guo, F.; Duan, N.; Yang, S.; Tian, H.; Sun, B. A Solvatochromic Fluorescent Probe for Solvent Polarity Detection Using a Smartphone. ChemistrySelect 2022, 7, e202200766. [Google Scholar] [CrossRef]
- Nath, S.; Bhattacharya, B.; Sarkar, U.; Singh, T.S. Solvent Effects on the Photophysical Properties of a Donor–acceptor Based Schiff Base. J. Fluoresc. 2022, 32, 1321–1336. [Google Scholar] [CrossRef]
- Nath, S.; Bhattacharya, B.; Sarkar, U.; Singh, T.S. Photophysical investigation of a donor-acceptor based Schiff base in solvents of varying polarities. J. Mol. Struct. 2022, 1255, 132435. [Google Scholar] [CrossRef]
- Gautam, P.; Misra, R.; Koukaras, E.N.; Sharma, A.; Sharma, G.D. Donor–acceptor–acceptor–donor small molecules for solution processed bulk heterojunction solar cells. Org. Electron. 2015, 27, 72–83. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhu, Q.; Tang, T.; Shah, K.W.; Xu, J. Diversity of electron acceptor groups in donor–acceptor type electrochromic conjugated polymers. Sol. Energy Mater. Sol. Cells 2019, 197, 32–75. [Google Scholar] [CrossRef]
- Dou, L.; Liu, Y.; Hong, Z.; Li, G.; Yang, Y. Low-Bandgap Near-IR Conjugated Polymers/Molecules for Organic Electronics. Chem. Rev. 2015, 115, 12633–12665. [Google Scholar] [CrossRef]
- Pluczyk-Malek, S.; Honisz, D.; Akkuratov, A.; Troshin, P.; Lapkowski, M. Tuning the electrochemical and optical properties of donor-acceptor D-A2-A1-A2-D derivatives with central benzothiadiazole core by changing the A2 strength. Electrochim. Acta 2021, 368, 137540. [Google Scholar] [CrossRef]
- Kurowska, A.; Zassowski, P.; Kostyuchenko, A.S.; Zheleznova, T.Y.; Andryukhova, K.V.; Fisyuk, A.S.; Pron, A.; Domagala, W. Effect of donor to acceptor ratio on electrochemical and spectroscopic properties of oligoalkylthiophene 1,3,4-oxadiazole derivatives. Phys. Chem. Chem. Phys. 2017, 19, 30261–30276. [Google Scholar] [CrossRef]
- Chang, Z.-F.; Jing, L.-M.; Chen, B.; Zhang, M.; Cai, X.; Liu, J.-J.; Ye, Y.-C.; Lou, X.; Zhao, Z.; Liu, B.; et al. Rational design of asymmetric red fluorescent probes for live cell imaging with high AIE effects and large two-photon absorption cross sections using tunable terminal groups. Chem. Sci. 2016, 7, 4527–4536. [Google Scholar] [CrossRef]
- Barik, S.; Skene, W.G. Turning-on the Quenched Fluorescence of Azomethines through Structural Modifications. Eur. J. Org. Chem. 2013, 2013, 2563–2572. [Google Scholar] [CrossRef]
- Li, W.; Yuan, F.; Xu, N.; Mei, S.; Chen, Z.; Zhang, C. Triphenylamine-triazine polymer materials obtained by electrochemical polymerization: Electrochemistry stability, anions trapping behavior and electrochromic-supercapacitor application. Electrochim. Acta 2021, 384, 138344. [Google Scholar] [CrossRef]
- Nowacki, M.; Wałęsa-Chorab, M. Influence of temperature on electrochemical and electrochromic properties of naphthalenediimide-triphenylamine-based polymer. Prog. Org. Coat. 2023, 182, 107691. [Google Scholar] [CrossRef]
- Fu, W.; Chen, H.; Han, Y.; Wang, W.; Zhang, R.; Liu, J. Electropolymerization of D-A-D type monomers consisting of triphenylamine and substituted quinoxaline moieties for electrochromic devices. New J. Chem. 2021, 45, 19082–19087. [Google Scholar] [CrossRef]
- Fu, W.; Chen, H.; Yi, X.; Zhang, R.; Liu, J. Electrochemical polymerization of D-A-D type monomers consisting of triphenylamine and benzo[1,2-b:4,5-b′]dipyrazine units for multicolor electrochromism. Eur. Polym. J. 2022, 173, 111274. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Liao, W.K.; Liou, G.S. A comparative study of redox-active, ambipolar electrochromic triphenylamine-based polyimides prepared by electrochemical polymerization and conventional polycondensation methods. Polym. Chem. 2018, 9, 236–248. [Google Scholar] [CrossRef]
- Chandra Santra, D.; Mondal, S.; Malik, S. Design of triphenylamine appended anthracene derivatives: Electro-polymerization and their electro-chromic behaviour. RSC Adv. 2016, 6, 81597–81606. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Chen, Y.-Z. Electrosynthesis of redox-active and electrochromic polymer films from triphenylamine-cored star-shaped molecules end-capped with arylamine groups. Eur. Polym. J. 2018, 99, 422–436. [Google Scholar] [CrossRef]
- Santra, D.C.; Nad, S.; Malik, S. Electrochemical polymerization of triphenylamine end-capped dendron: Electrochromic and electrofluorochromic switching behaviors. J. Electroanal. Chem. 2018, 823, 203–212. [Google Scholar] [CrossRef]
- Guven, N.; Şener Cemaloğlu, Ö.; Camurlu, P. Fast Switching Triphenylamine-Based Electrochromic Polymers with Fluorene Core: Electrochemical Synthesis and Optoelectronic Properties. J. Electrochem. Soc. 2022, 169, 026511. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Lin, J.-W. Facile preparation of electrochromic poly(amine–imide) films from diimide compounds with terminal triphenylamino groups via electrochemical oxidative coupling reactions. Polym. Chem. 2014, 5, 6770–6778. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Tang, J.H.; He, Y.Q.; Shao, J.Y.; Gong, Z.L.; Zhong, Y.W. Multistate Redox Switching and Near-Infrared Electrochromism Based on a Star-Shaped Triruthenium Complex with a Triarylamine Core. Sci. Rep. 2016, 6, 35253. [Google Scholar] [CrossRef]
- Wang, C.; Wang, M.; Zhang, Y.; Zhao, J.; Fu, C. A new electrochromic copolymer which switched between neutral black and oxidized transmissive. RSC Adv. 2016, 6, 80002–80010. [Google Scholar] [CrossRef]
- Yao, C.J.; Yao, J.; Zhong, Y.W. Metallopolymeric films based on a biscyclometalated ruthenium complex bridged by 1,3,6,8-tetra(2-pyridyl)pyrene: Applications in near-infrared electrochromic windows. Inorg. Chem. 2012, 51, 6259–6263. [Google Scholar] [CrossRef] [PubMed]
- Wałęsa-Chorab, M.; Banasz, R.; Kubicki, M.; Patroniak, V. Dipyrromethane functionalized monomers as precursors of electrochromic polymers. Electrochim. Acta 2017, 258, 571–581. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, M.; Wei, J.; Zhang, Y.; Zhang, S.; Gao, Y.; Li, J.; Lu, P.; Yang, B.; Ma, Y. Stable p/n-Dopable Conducting Redox Polymers for High-Voltage Pseudocapacitor Electrode Materials: Structure-Performance Relationship and Detailed Investigation into Charge-Trapping Effect. Adv. Energy Mater. 2017, 7, 1701063. [Google Scholar] [CrossRef]
- Mangione, M.I.; Spanevello, R.A.; Rumbero, A.; Heredia, D.; Marzari, G.; Fernandez, L.; Otero, L.; Fungo, F. Electrogenerated Conductive Polymers from Triphenylamine End-Capped Dendrimers. Macromolecules 2013, 46, 4754–4763. [Google Scholar] [CrossRef]
- Yen, H.J.; Liou, G.S. Novel blue and red electrochromic poly(azomethine ether)s based on electroactive triphenylamine moieties. Org. Electron. 2010, 11, 299–310. [Google Scholar] [CrossRef]
- Liu, F.; Bai, J.; Yu, G.; Ma, F.; Hou, Y.; Niu, H. Synthesis, electrochromic properties and flash memory behaviors of novel D-A-D polyazomethines containing EDOT and thiophene units. Org. Electron. 2020, 77, 105538. [Google Scholar] [CrossRef]
- Liu, F.; Cong, Z.; Yu, G.; Niu, H.; Hou, Y.; Wang, C.; Wang, S. Novel D-A-D conjugated polymers based on tetraphenylethylene monomer for electrochromism. Opt. Mater. 2020, 100, 109658. [Google Scholar] [CrossRef]
- Kadish, K.M.; Anderson, J.E. Purification of solvents for electroanalysis: Benzonitile. Pure Appl. Chem. 1987, 59, 703–714. [Google Scholar] [CrossRef]
- Wang, L.; Xia, Q.; Hou, M.; Yan, C.; Xu, Y.; Qu, J.; Liu, R. A photostable cationic fluorophore for long-term bioimaging. J. Mater. Chem. B 2017, 5, 9183–9188. [Google Scholar] [CrossRef]
- Tang, Y.; Huang, H.; Peng, B.; Chang, Y.; Li, Y.; Zhong, C. A thiadiazole-based covalent triazine framework nanosheet for highly selective and sensitive primary aromatic amine detection among various amines. J. Mater. Chem. A 2020, 8, 16542–16550. [Google Scholar] [CrossRef]
- Deng, D.; Zou, Y.; Chen, Z.; Liu, S.; Yang, Y.; Pu, S. Finely regulated benzothiadiazole derivatives: Aggregation-induced emission (AIE), hypso- or bathochromic mechanofluorochromic behaviors, and multilevel information encryption applications. Dyes Pigment. 2023, 211, 111051. [Google Scholar] [CrossRef]
- Song, H.; Li, T.Y.; Pan, Y.; Han, X.; Guo, Y.; Shi, L.; Song, M.-P. Covalent organic nanocage with aggregation induced emission property and detection for Hg2+ as fluorescence sensors. Dyes Pigment. 2023, 219, 111584. [Google Scholar] [CrossRef]
- Niu, J.; Wang, Y.; Zou, X.; Tan, Y.; Jia, C.; Weng, X.; Deng, L. Infrared electrochromic materials, devices and applications. Appl. Mater. Today 2021, 24, 101073. [Google Scholar] [CrossRef]
- Jelle, B.P. Solar radiation glazing factors for window panes, glass structures and electrochromic windows in buildings—Measurement and calculation. Sol. Energy Mater. Sol. Cells 2013, 116, 291–323. [Google Scholar] [CrossRef]
- Gu, C.; Jia, A.-B.; Zhang, Y.-M.; Zhang, S.X.-A. Emerging Electrochromic Materials and Devices for Future Displays. Chem. Rev. 2022, 122, 14679–14721. [Google Scholar] [CrossRef]


| Solvent | λabs (nm) | λem (nm) | Stokes Shift (cm−1) | Quantum Yield (%) |
|---|---|---|---|---|
| Toluene | 443 | 577 | 5242 | 35 |
| Dioxane | 438 | 580 | 5590 | 37 |
| Tetrahydrofuran | 439 | 602 | 6168 | 32 |
| DCM | 435 | 618 | 6808 | 27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roszyk, M.; Wałęsa-Chorab, M. Electrochemical and Optical Properties of D-A-A-A-D Azomethine Triad and Its NIR-Active Polymer. Molecules 2024, 29, 4470. https://doi.org/10.3390/molecules29184470
Roszyk M, Wałęsa-Chorab M. Electrochemical and Optical Properties of D-A-A-A-D Azomethine Triad and Its NIR-Active Polymer. Molecules. 2024; 29(18):4470. https://doi.org/10.3390/molecules29184470
Chicago/Turabian StyleRoszyk, Mateusz, and Monika Wałęsa-Chorab. 2024. "Electrochemical and Optical Properties of D-A-A-A-D Azomethine Triad and Its NIR-Active Polymer" Molecules 29, no. 18: 4470. https://doi.org/10.3390/molecules29184470
APA StyleRoszyk, M., & Wałęsa-Chorab, M. (2024). Electrochemical and Optical Properties of D-A-A-A-D Azomethine Triad and Its NIR-Active Polymer. Molecules, 29(18), 4470. https://doi.org/10.3390/molecules29184470






