Mechanisms of Atomic Oxygen Erosion in Fluorinated Polyimides Investigated by Molecular Dynamics Simulations
Abstract
:1. Introduction
2. Results and Discussion
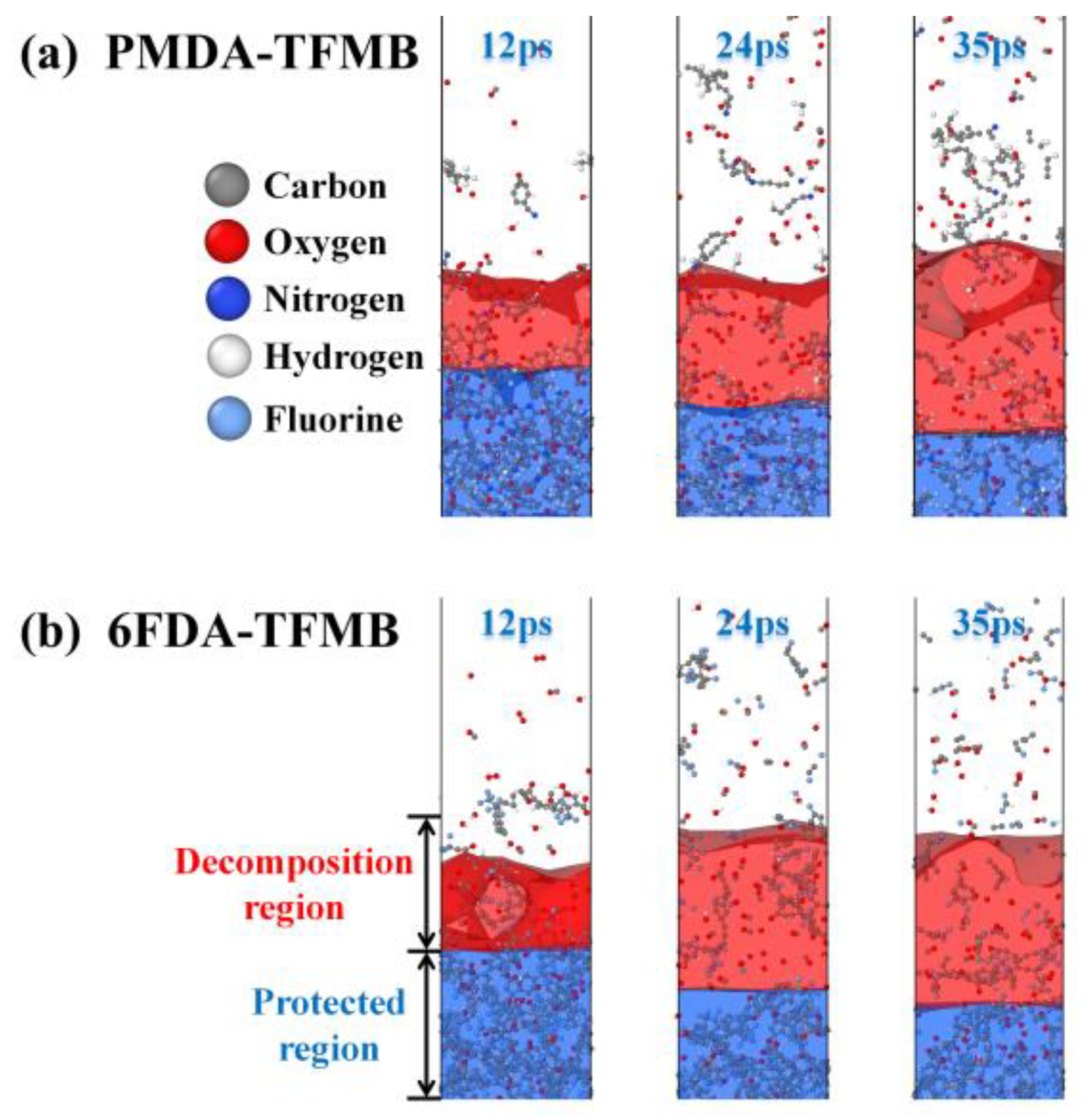
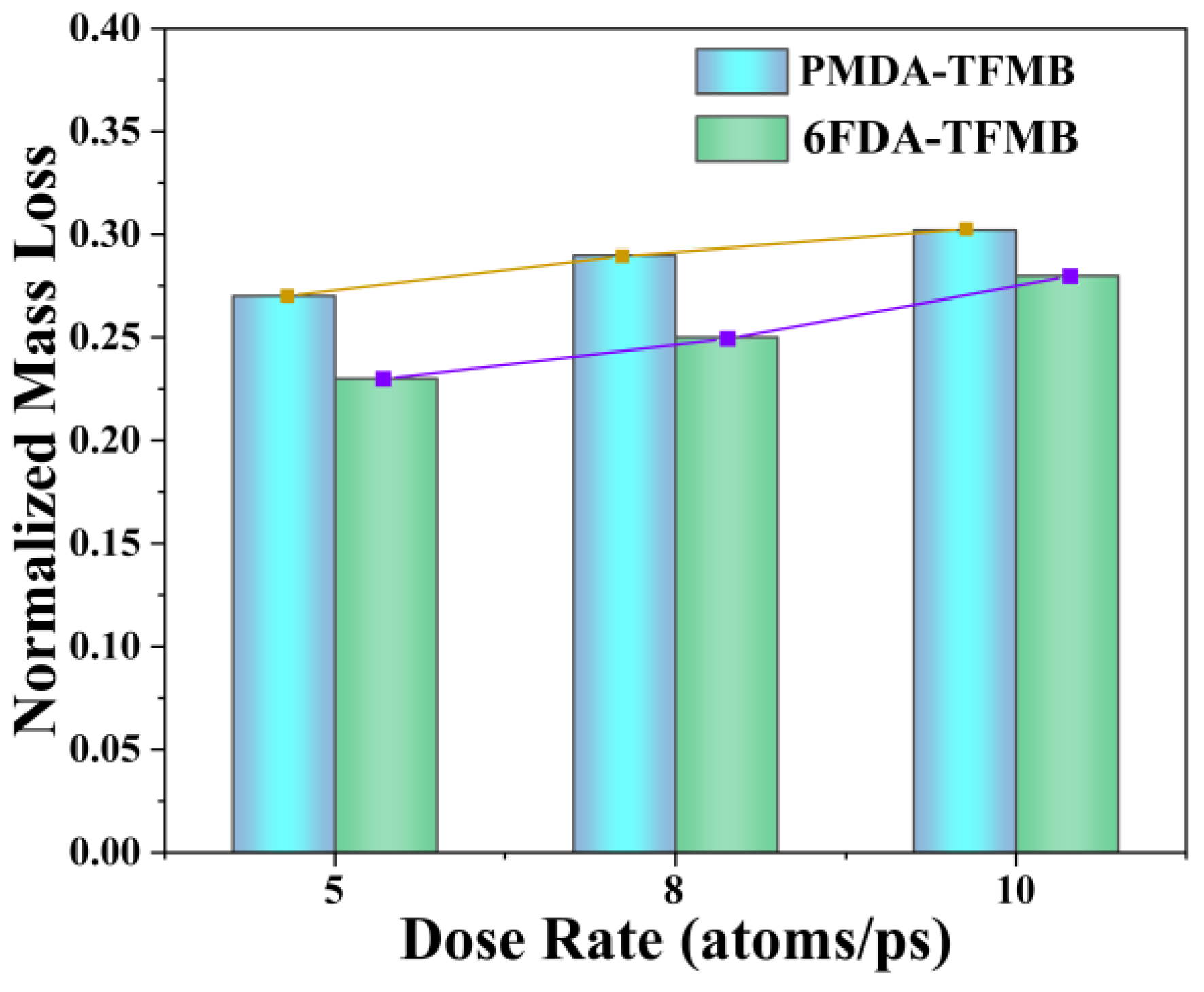
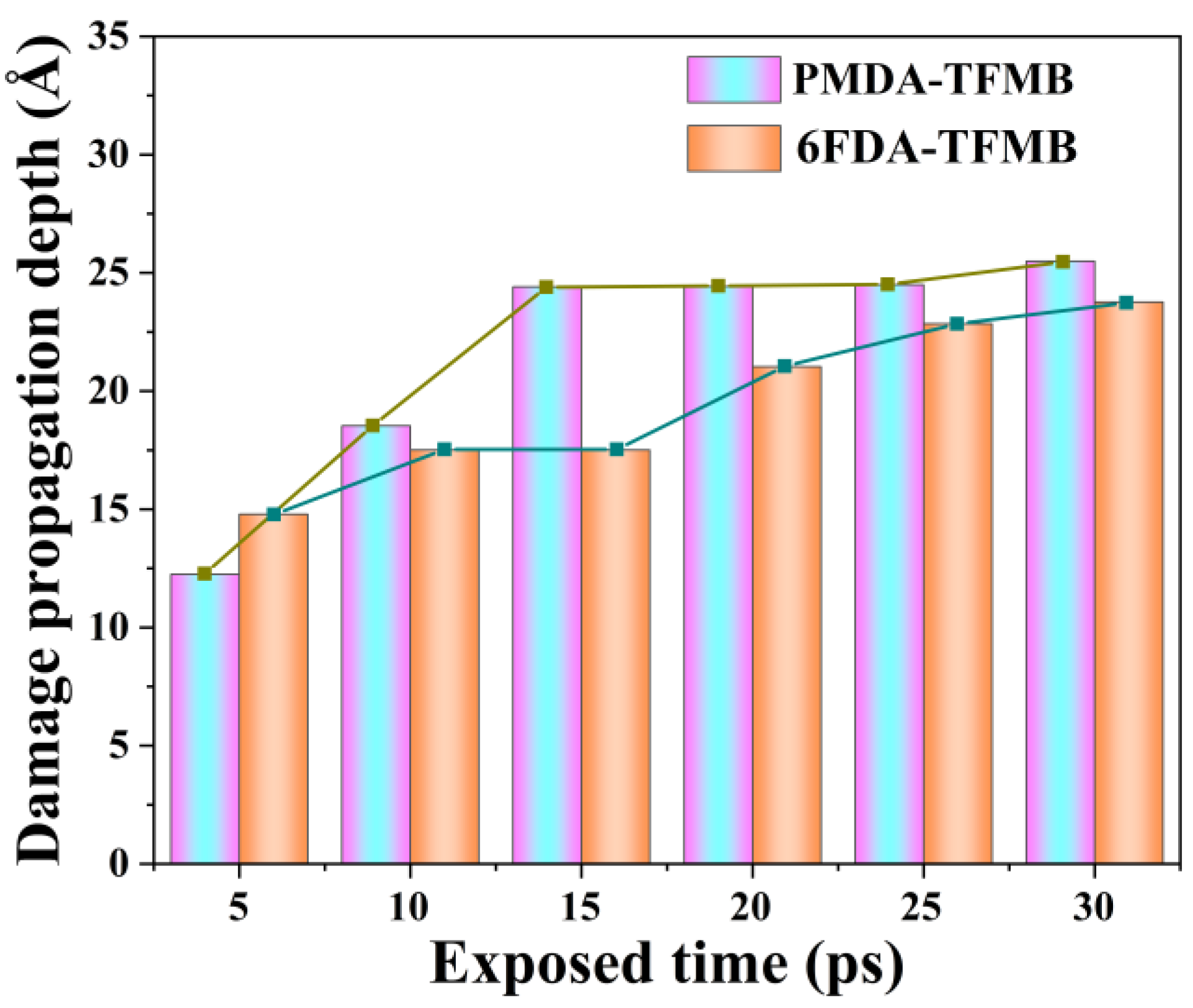
2.1. Erosion Kinetics of Fluorinated Polyimides
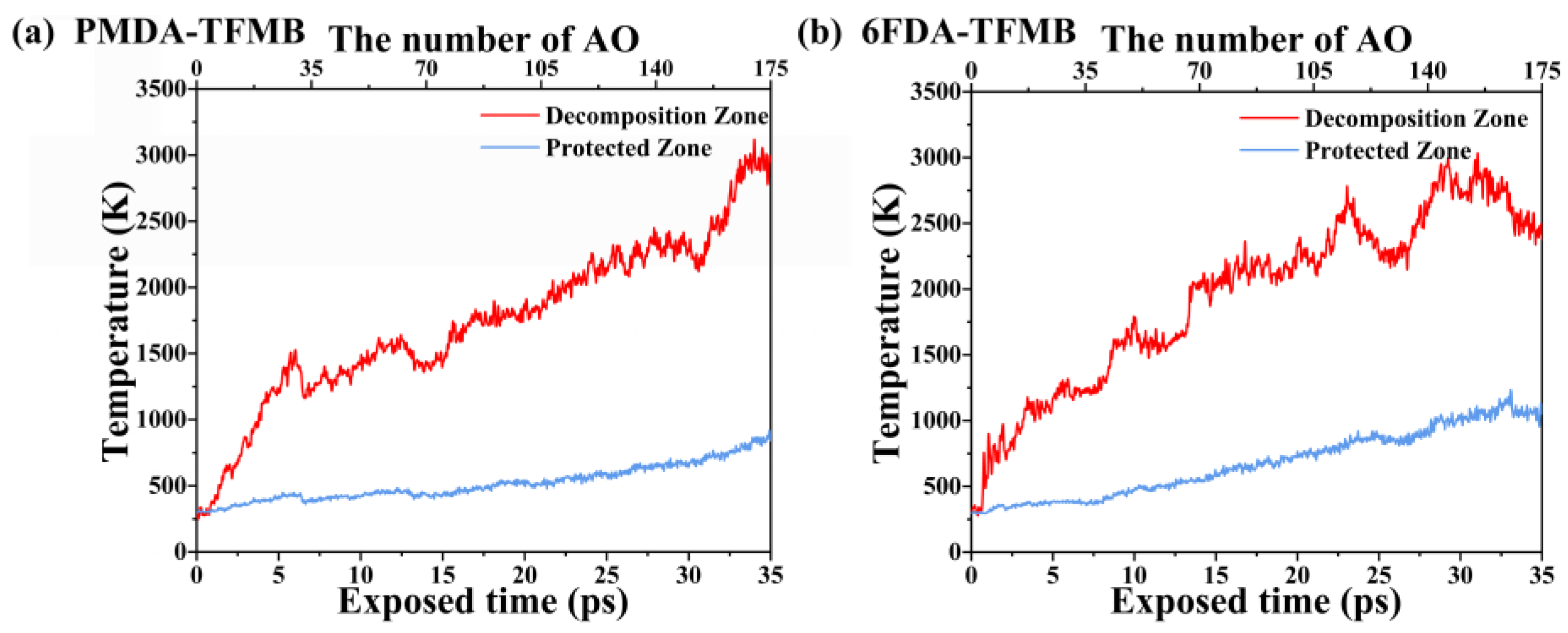
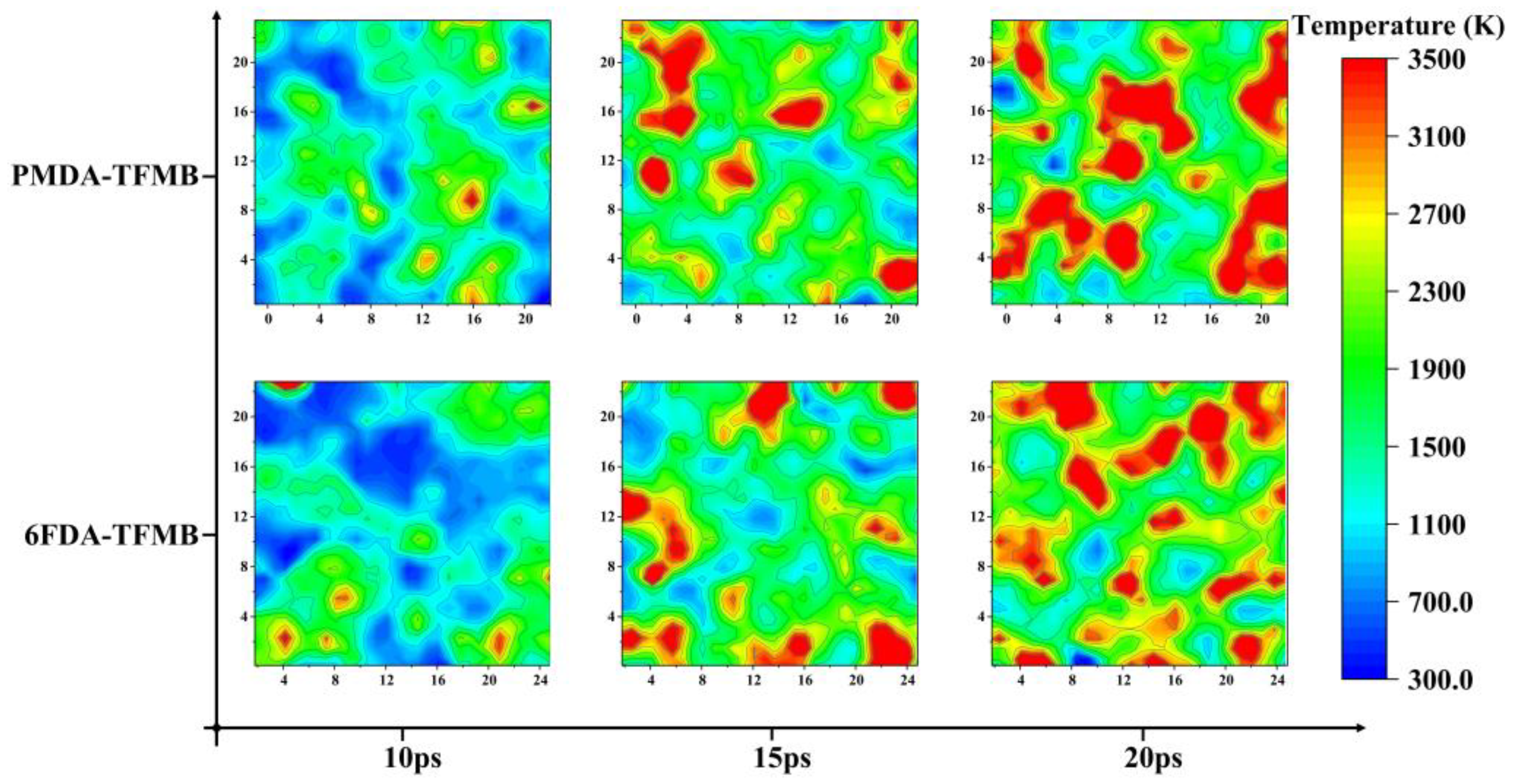
2.2. Temperature Evolution during AO Erosion
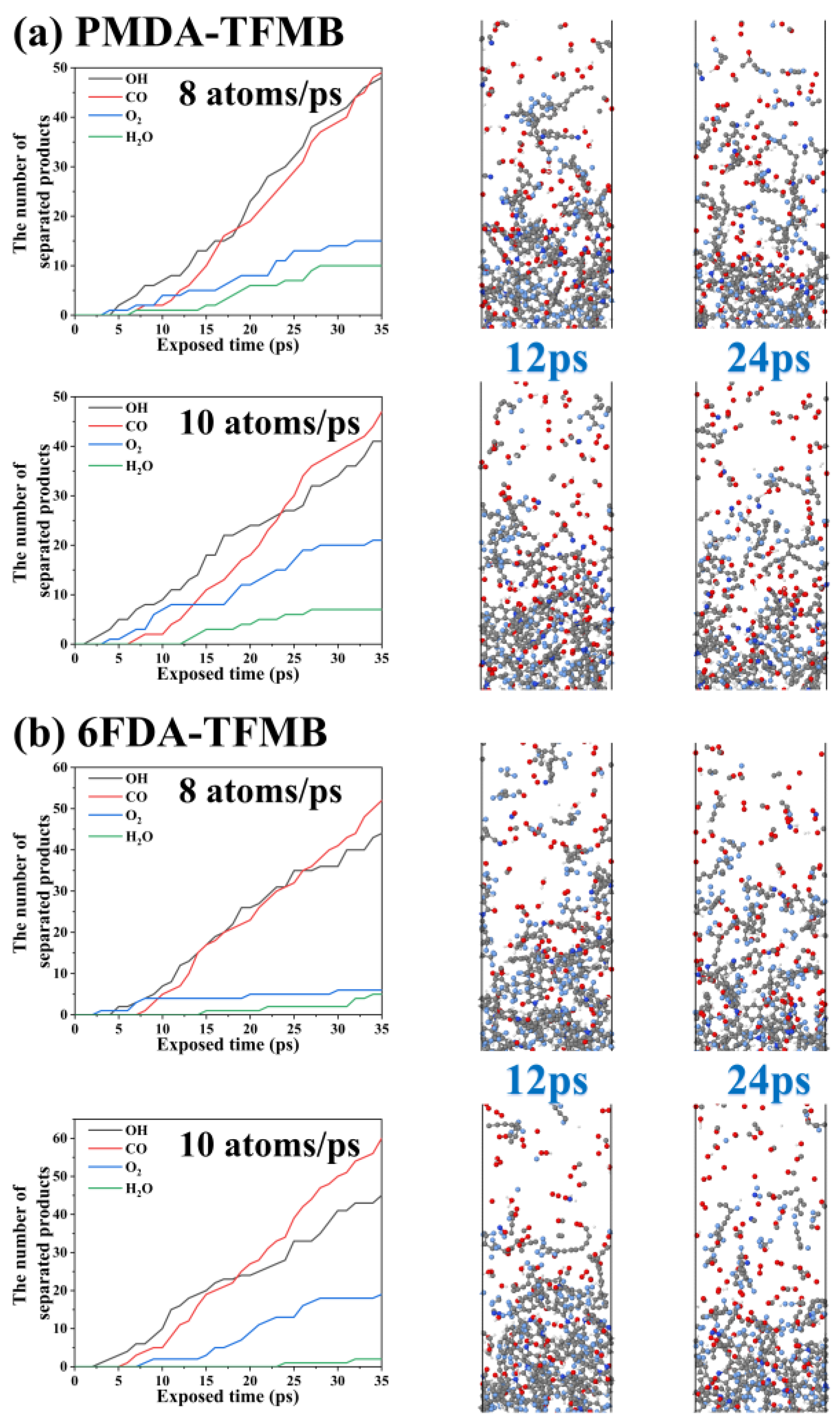
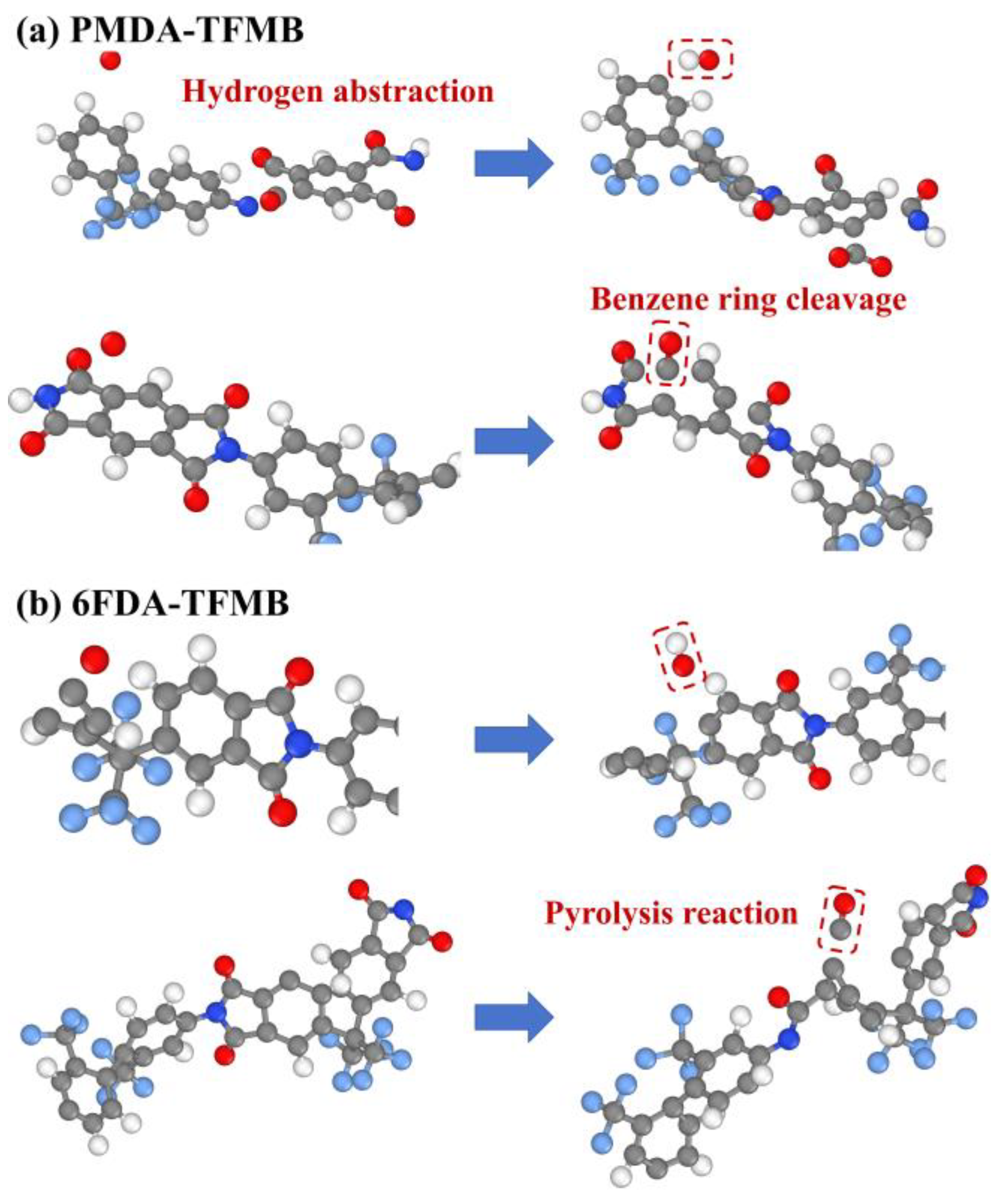
2.3. AO Erosion Product Analysis
3. Methods and Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in Aromatic Polyimide Films for Electronic Applications: Preparation, Structure and Properties. Polymers 2022, 6, 1269. [Google Scholar] [CrossRef] [PubMed]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, e1807738. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Liu, X.; Wang, R.; Lu, C.; Wen, X.; Tu, G. Research Progress and Application of Polyimide-Based Nanocomposites. Nanomaterials 2023, 4, 656. [Google Scholar] [CrossRef] [PubMed]
- Xi, S.; Wang, Y.; Zhang, X.; Cao, K.; Su, J.; Shen, J.; Wang, X. Fire-resistant Polyimide-Silica Aerogel Composite Aerogels with Low Shrinkage, Low Density and High Hydrophobicity for Aerospace Applications. Polym. Test. 2023, 129, 108259. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Ouyang, C.; Yi, S.; Liu, S. Porous Highly Fluorinated Polyimide/Polydopamine Nanocomposite Films with Simultaneously Enhanced Toughness, UV-Shielding and Photostability for Aerospace Applications. Polym. Test. 2023, 118, 107899. [Google Scholar] [CrossRef]
- Tao, L.; Yang, H.; Liu, J.; Fan, L.; Yang, S. Synthesis and Characterization of Highly Optical Transparent and Low Dielectric Constant Fluorinated Polyimides. Polymer 2009, 25, 6009–6018. [Google Scholar] [CrossRef]
- Xia, A.; Guo, H.; Qiu, X.; Ding, M.; Gao, L. Syntheses and Properties of Polyimides Derived from Diamines Containing 2,5-Disubstituted Pyridine Group. J. Appl. Polym. Sci. 2006, 2, 1844–1851. [Google Scholar] [CrossRef]
- Xiao, T.; Fan, X.; Fan, D.; Li, Q. High Thermal Conductivity and Low Absorptivity/Emissivity Properties of Transparent Fluorinated Polyimide Films. Polym. Bull. 2017, 11, 4561–4575. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Tseng, I.-H.; Huang, S.-L.; Hsieh, C.-W. Enhancement of Dimensional Stability and Optical Transparency of Colorless Organo-Soluble Polyimide by Incorporation of Silica and Cosolvent. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 48–56. [Google Scholar] [CrossRef]
- Ni, H.-J.; Liu, J.-G.; Wang, Z.-H.; Yang, S.-Y. A Review on Colorless and Optically Transparent Polyimide Films: Chemistry, Process and Engineering Applications. J. Ind. Eng. Chem. 2015, 28, 16–27. [Google Scholar] [CrossRef]
- Choi, C.H.; Sohn, B.H.; Chang, J.-H. Colorless and Transparent Polyimide Nanocomposites: Comparison of the Properties of Homo- and Co-polymers. J. Ind. Eng. Chem. 2013, 19, 1593–1599. [Google Scholar] [CrossRef]
- He, X.; Zhang, S.; Zhou, Y.; Zheng, F.; Lu, Q. The “Fluorine Impact” on Dielectric Constant of Polyimides: A Molecular Simulation Study. Polymer 2022, 254, 125073. [Google Scholar] [CrossRef]
- Zuo, H.-T.; Gan, F.; Dong, J.; Zhang, P.; Zhao, X.; Zhang, Q.-H. Highly Transparent and Colorless Polyimide Film with Low Dielectric Constant by Introducing Meta-Substituted Structure and Trifluoromethyl Groups. Chin. J. Polym. Sci. 2020, 39, 455–464. [Google Scholar] [CrossRef]
- Zhu, G.; Xing, J.; Mushtaq, N.; Fang, X.; Chen, G. Synthesis and Characterization of High Tg and Colorless Polyamide-Imide Films Derived from Amide-Containing Diamines with Different Trifluoromethyl Substitution. Polymer 2024, 302, 127033. [Google Scholar] [CrossRef]
- Shi, Y.; Li, J.; Wu, W.; Ni, J.; Wei, B.; Liu, C.; Huang, X. Preparation and Characterisation of Heat-Resistant Fluorinated Co-Polyimides and Their Gas Transport Characteristics. High Perform. Polym. 2024, 4, 230–241. [Google Scholar] [CrossRef]
- Barra, G.; Guadagno, L.; Raimondo, M.; Santonicola, M.G.; Toto, E.; Ciprioti, S.V. A Comprehensive Review on the Thermal Stability Assessment of Polymers and Composites for Aeronautics and Space Applications. Polymers 2023, 15, 3786. [Google Scholar] [CrossRef]
- Goverapet, S.; van Duin, A.C. Molecular-Dynamics-Based Study of the Collisions of Hyperthermal Atomic Oxygen with Graphene Using the ReaxFF Reactive Force Field. Chem. Eur. J. 2011, 115, 13269–13280. [Google Scholar]
- Rahnamoun, A.; van Duin, A. Reactive Molecular Dynamics Simulation on the Disintegration of Kapton, POSS Polyimide, Amorphous Silica, and Teflon During Atomic Oxygen Impact Using the ReaxFF Reactive Force-Field Method. Chem. Eur. J. 2014, 118, 2780–2787. [Google Scholar] [CrossRef]
- Suliga, A.; Jakubczyk, M.E.; Hamerton, I.; Viquerat, A. Analysis of Atomic Oxygen and Ultraviolet Exposure Effects on Cycloaliphatic Epoxy Resins Reinforced with Octa-Functional POSS. Acta Astronaut. 2018, 142, 103–111. [Google Scholar] [CrossRef]
- Clausi, M.; Santonicola, G.M.; Schirone, L.; Laurenzi, S. Analysis of Ultraviolet Exposure Effects on the Surface Properties of Epoxy/Graphene Nanocomposite Films on Mylar Substrate. Acta Astronaut. 2017, 134, 307–313. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Qian, Y.; Qi, H.; Li, J.; Sun, J. Mechanically Robust Atomic Oxygen-Resistant Coatings Capable of Autonomously Healing Damage in Low Earth Orbit Space Environment. Adv. Mater. 2018, 30, e1803854. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, Z.; Lee, C.; Wang, J. Unified Model for Low-Earth-Orbital Atomic-Oxygen and Atomic-Oxygen/Ultraviolet Induced Erosion of Polymeric Materials. Aerosp. Sci. Technol. 2016, 53, 194–206. [Google Scholar] [CrossRef]
- Qi, H.; Shi, Q.; Qian, Y.; Li, Y.; Xu, J.; Xu, C.; Xie, X. The Atomic Oxygen Erosion Resistance Effect and Mechanism of the Perhydropolysilazane-Derived SiOx Coating Used on Polymeric Materials in Space Environment. Polymers 2022, 2, 322. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, M.; Xu, C.; Luo, Y. Perhydropolysilazane Derived Silica Coating Protecting Kapton from Atomic Oxygen Attack. Thin Solid Films 2011, 3, 1063–1068. [Google Scholar] [CrossRef]
- Chen, S.; Wu, X.; Zhong, M.; Yan, D.; Huang, W. The Atomic Oxygen Resistant Study of a Transparent Polyimide Film Containing Phosphorus and Fluorine. Appl. Surf. Sci. 2023, 631, 157562. [Google Scholar]
- Shimamura, H.; Nakamura, T. Mechanical Properties Degradation of Polyimide Films Irradiated by Atomic Oxygen. Polym. Degrad. Stab. 2009, 9, 1389–1396. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, L.; Zou, L.; Wang, G.; Wang, Y. Analysis of Atomic Oxygen Erosion Resistance Mechanism of Polyimide Composite Materials Enhanced by Polyhydroxysilylazane. Appl. Surf. Sci. 2024, 657, 159814. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Zhang, C.; Yang, L.; Chu, L. Revealing the Mechanism on Steam Co-Gasification of Cellulose and Polyethylene: A Combined ReaxFF and DFT Study. Fuel 2023, 334, 126784. [Google Scholar] [CrossRef]
- Li, G.; Zheng, F.; Huang, Q.; Wang, J.; Niu, B.; Zhang, Y.; Long, D. Molecular Insight into Pyrolysis Processes via Reactive Force Field Molecular Dynamics: A State-of-the-Art Review. J. Anal. Appl. Pyrolysis 2022, 166, 105620. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, L.; Zou, L.; Ayubi, B.I.; Wang, Y. Mechanism Analysis and Potential Applications of Atomic Oxygen Erosion Protection for Kapton-Type Polyimide Based on Molecular Dynamics Simulations. Polymers 2024, 12, 1687. [Google Scholar] [CrossRef]
- Wei, D.; Zeng, F.; Cui, J. Damage and Degradation of Mechanical Properties of Polyimide-Based Materials under Atomic Oxygen Attack: A Molecular Dynamics Simulation Study. Comput. Mater. Sci. 2024, 243, 113110. [Google Scholar] [CrossRef]
- Zeng, F.; Peng, C.; Liu, Y.; Qu, J. Reactive Molecular Dynamics Simulations on the Disintegration of PVDF, FP-POSS, and Their Composite During Atomic Oxygen Impact. J. Phys. Chem. A 2015, 30, 8359–8368. [Google Scholar] [CrossRef]
- Jeon, I.; Lee, S.; Yang, S. Hyperthermal Erosion of Thermal Protection Nanocomposites Under Atomic Oxygen and N2 Bombardment. Int. J. Mech. Sci. 2023, 240, 107910. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, B. Atomic Oxygen Degradation of a Fluorine-Containing Colorless Polyimide. Polym. Degrad. Stab. 2023, 215, 110443. [Google Scholar] [CrossRef]
- Peng, W.-F.; Lei, H.-Y.; Zhang, X.-X.; Qiu, L.-H.; Huang, M.-J. Fluorine Substitution Effect on the Material Properties in Transparent Aromatic Polyimides. Chin. J. Polym. Sci. 2022, 7, 781–788. [Google Scholar] [CrossRef]
- Tong, H.; Hu, C.; Yang, S.; Ma, Y.; Guo, H.; Fan, L. Preparation of Fluorinated Polyimides with Bulky Structure and Their Gas Separation Performance Correlated with Microstructure. Polymer 2015, 69, 138–147. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Chen, W.; Xu, C.; Zhao, X.; Li, J.; Ren, Q. Synthesis and Properties of Fluorinated Polyimides with Multi-Bulky Pendant Groups. RSC Adv. 2017, 42, 26420–26427. [Google Scholar] [CrossRef]
- Pu, C.; Lin, D.; Xu, H.; Liu, F.; Gao, H.; Tian, G.; Wu, D. Clarifying the Effect of Chemical Structure on High-Temperature Resistance of Polyimides Based on DFT and ReaxFF Based Molecular Dynamic Simulation. Polymer 2022, 255, 125119. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Tersoff, J. Empirical Interatomic Potential for Silicon with Improved Elastic Properties. Phys. Rev. B 1988, 38, 9902–9905. [Google Scholar] [CrossRef]
- Brenner, D.W. Empirical Potential for Hydrocarbons for Use in Simulating the Chemical Vapor Deposition of Diamond Films. Phys. Rev. B 1990, 42, 9458–9471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G. Numerical Simulation on Atomic Oxygen Undercutting of Kapton Film in Low Earth Orbit. Acta Astronaut. 2010, 67, 388–395. [Google Scholar] [CrossRef]
- Banks, A.B.; Groh, D.K.K.; Miller, K.S. Low Earth Orbital Atomic Oxygen Interactions with Spacecraft Materials. MRS Proc. 2004, 851, NN8.1. [Google Scholar] [CrossRef]
- Rahnamoun, A.; van Duin, A. Reactive Molecular Dynamics Simulation on the Disintegration of Kapton and Silicon-Oxygen Compound in Kapton-POSS During Oxygen Bombardment. In Abstracts of Papers of the American Chemical Society; American Chemical Society: Washington, DC, USA, 2012. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhang, L.; Zou, L.; Ayubi, B.I.; Wang, Y. Mechanisms of Atomic Oxygen Erosion in Fluorinated Polyimides Investigated by Molecular Dynamics Simulations. Molecules 2024, 29, 4485. https://doi.org/10.3390/molecules29184485
Zhou S, Zhang L, Zou L, Ayubi BI, Wang Y. Mechanisms of Atomic Oxygen Erosion in Fluorinated Polyimides Investigated by Molecular Dynamics Simulations. Molecules. 2024; 29(18):4485. https://doi.org/10.3390/molecules29184485
Chicago/Turabian StyleZhou, Shengrui, Li Zhang, Liang Zou, Bilal Iqbal Ayubi, and Yiwei Wang. 2024. "Mechanisms of Atomic Oxygen Erosion in Fluorinated Polyimides Investigated by Molecular Dynamics Simulations" Molecules 29, no. 18: 4485. https://doi.org/10.3390/molecules29184485








