Biosorption Ability of Pharmaceutically Active Compounds by Anabaena sp. and Chroococcidiopsis thermalis
Abstract
1. Introduction
2. Results and Discussion
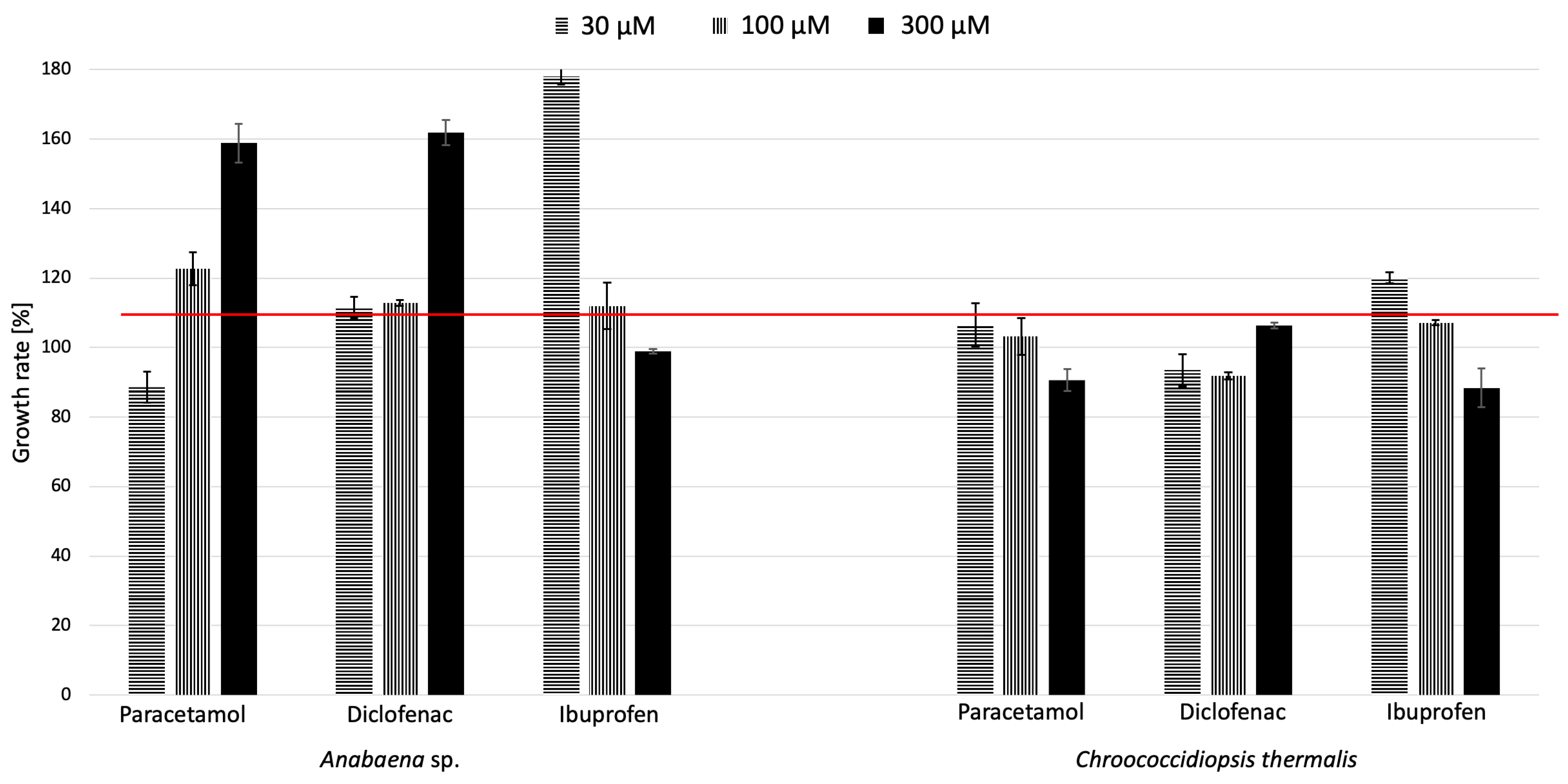
2.1. Effects of Paracetamol, Diclofenac, and Ibuprofen on the Growth of Cyanobacteria
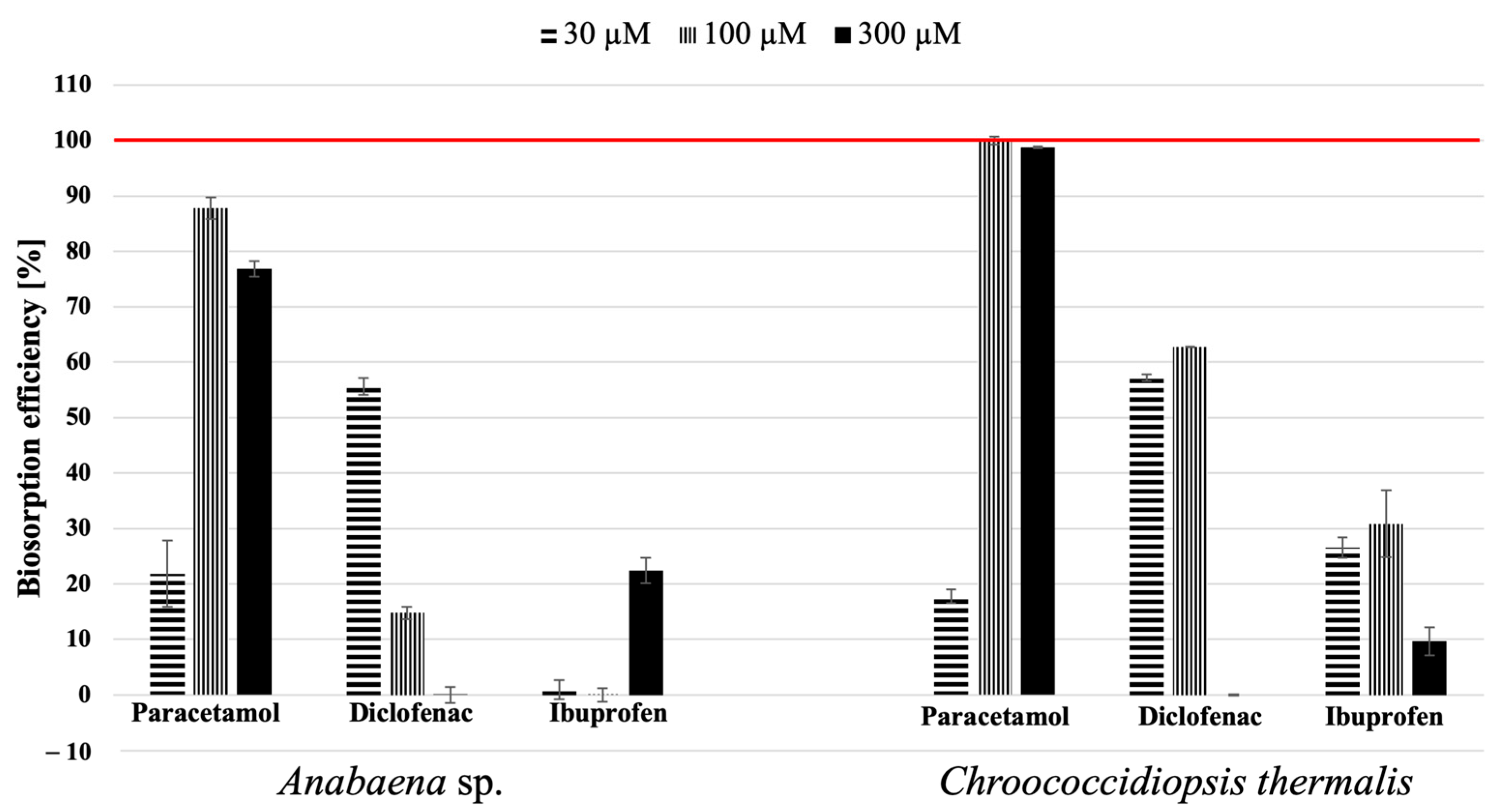
2.2. Biosorption Ability
2.3. Interaction of Tested Pharmaceuticals with the Cyanobacterial Cell Surface
2.4. Identification of Analgesics and Their Metabolites in a Post-Culture Medium Using the MS/MS Technique
3. Materials and Methods
3.1. Chemicals
3.2. Biosorption Experiments
- BioEPCM—efficiency of biosorption from the post-culture medium;
- BioECS—efficiency of biosorption on the cell surface;
- Se—peak area of the studied analytes in extracts of the post-culture medium;
- Sec—peak area of the studied analytes in the extracts from the cells’ surface;
- Sr—peak area of the studied analytes in the reference sample.
3.3. The Growth and Metabolic Response Studies
3.4. Statistical Analysis
3.5. LC-UV-MS/MS Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Non-Pharmaceutical Trade of OTC Drugs—Safety, Economy and Patient Expectations. 2023. Available online: https://pozaapteczny.pl/wp-content/uploads/2023/04/5944d3ce0a5455cd7c1b6a3ee53b3aa3bb323300.pdf (accessed on 30 March 2023). (In Polish).
- Barcellos, D.D.S.; Procopiuck, M.; Bollmann, H.A. Management of pharmaceutical micropollutants discharged in urban waters: 30 years of systematic review looking at opportunities for developing countries. Sci. Total Environ. 2022, 809, 151128. [Google Scholar] [CrossRef] [PubMed]
- Sellier, A.; Khaska, S.; Le Gal La Salle, C. Assessment of the occurrence of 455 pharmaceutical compounds in sludge according to their physical and chemical properties: A review. J. Hazard. Mater. 2022, 426, 128104. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, R.; Dzingelevičienė, R.; Bradauskaitė, A.; Lajevardipour, A.; Mlynska-Szultka, M.; Dzingelevičius, N.; Raugelė, S.; Razbadauskas, A.; Abbasi, S.; Rees, R.M.; et al. Pharmaceutical and Microplastic Pollution before and during the COVID-19 Pandemic in Surface Water, Wastewater, and Groundwater. Water 2022, 14, 3082. [Google Scholar] [CrossRef]
- Fabrega, J.; Carapeto, R. Regulatory review of the environmental risk assessment of veterinary medicinal products in the European Union, with particular focus on the centralised authorisation procedure. Environ. Sci. Eur. 2020, 32, 99. [Google Scholar] [CrossRef]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part II-Toxicity and Environmental Risk Assessment. Molecules 2020, 25, 1796. [Google Scholar] [CrossRef] [PubMed]
- Omotola, E.O.; Olatunji, O.S. Quantification of selected pharmaceutical compounds in water using liquid chromatography-electrospray ionisation mass spectrometry (LC-ESI-MS). Heliyon 2020, 6, e05787. [Google Scholar] [CrossRef]
- Gallardo-Altamirano, M.J.; Maza-Márquez, P.; Montemurro, N.; Rodelas, B.; Osorio, F.; Pozo, C. Linking microbial diversity and population dynamics to the removal efficiency of pharmaceutically active compounds (PhACs) in an anaerobic/anoxic/aerobic (A(2)O) system. Chemosphere 2019, 233, 828–842. [Google Scholar] [CrossRef]
- Kołecka, K.; Gajewska, M.; Caban, M. From the pills to environment—Prediction and tracking of non-steroidal anti-inflammatory drug concentrations in wastewater. Sci. Total Environ. 2022, 825, 153611. [Google Scholar] [CrossRef]
- Świacka, K.; Michnowska, A.; Maculewicz, J.; Caban, M.; Smolarz, K. Toxic effects of NSAIDs in non-target species: A review from the perspective of the aquatic environment. Environ. Pollut. 2020, 273, 115891. [Google Scholar] [CrossRef]
- Parolini, M. Toxicity of the Non-Steroidal Anti-Inflammatory Drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef]
- Fu, Q.; Fedrizzi, D.; Kosfeld, V.; Schlechtriem, C.; Ganz, V.; Derrer, S.; Rentsch, D.; Hollender, J. Biotransformation Changes Bioaccumulation and Toxicity of Diclofenac in Aquatic Organisms. Environ. Sci. Technol. 2020, 54, 4400–4408. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, B.L.; Ferreira, D.P.; Borges, S.F.; Ferreira, A.M.; Holanda, F.H.; Ucella-Filho, J.G.M.; Cruz, R.A.S.; Birolli, W.G.; Luque, R.; Ferreira, I.M. Diclofenac, ibuprofen, and paracetamol biodegradation: Overconsumed non-steroidal anti-inflammatories drugs at COVID-19 pandemic. Front. Microbiol. 2023, 14, 1207664. [Google Scholar] [CrossRef] [PubMed]
- Adnan, L.A.; Hadibarata, T.; Sathishkumar, P.; Mohd Yusoff, A.R. Biodegradation Pathway of Acid Red 27 by White-Rot Fungus Armillaria sp. F022 and Phytotoxicity Evaluation. CLEAN—Soil Air Water 2016, 44, 239–246. [Google Scholar] [CrossRef]
- Al Farraj, D.A.; Hadibarata, T.; Yuniarto, A.; Syafiuddin, A.; Surtikanti, H.K.; Elshikh, M.S.; Al Khulaifi, M.M.; Al-Kufaidy, R. Characterization of pyrene and chrysene degradation by halophilic Hortaea sp. B15. Bioprocess Biosyst. Eng. 2019, 42, 963–969. [Google Scholar] [CrossRef]
- Syafiuddin, A.; Boopathy, R. Role of anaerobic sludge digestion in handling antibiotic resistant bacteria and antibiotic resistance genes—A review. Bioresour. Technol. 2021, 330, 124970. [Google Scholar] [CrossRef]
- Del Álamo, A.C.; Pariente, M.I.; Molina, R.; Martínez, F. Advanced bio-oxidation of fungal mixed cultures immobilized on rotating biological contactors for the removal of pharmaceutical micropollutants in a real hospital wastewater. J. Hazard. Mater. 2022, 425, 128002. [Google Scholar] [CrossRef] [PubMed]
- Badia-Fabregat, M.; Lucas, D.; Pereira, M.A.; Alves, M.; Pennanen, T.; Fritze, H.; Rodríguez-Mozaz, S.; Barceló, D.; Vicent, T.; Caminal, G. Continuous fungal treatment of non-sterile veterinary hospital effluent: Pharmaceuticals removal and microbial community assessment. Appl. Microbiol. Biotechnol. 2016, 100, 2401–2415. [Google Scholar] [CrossRef]
- Balaji, S.; Kalaivani, T.; Rajasekaran, C. Biosorption of Zinc and Nickel and Its Effect on Growth of Different Spirulina Strains. CLEAN—Soil Air Water 2014, 42, 507–512. [Google Scholar] [CrossRef]
- Drzyzga, D.; Forlani, G.; Vermander, J.; Kafarski, P.; Lipok, J. Biodegradation of the aminopolyphosphonate DTPMP by the cyanobacterium Anabaena variabilis proceeds via a C-P lyase-independent pathway. Environ. Microbiol. 2017, 19, 1065–1076. [Google Scholar] [CrossRef]
- Morsy, F.M.; Hassan, S.H.A.; Koutb, M. Biosorption of Cd(II) and Zn(II) by Nostoc commune: Isotherm and Kinetics Studies. CLEAN—Soil Air Water 2011, 39, 680–687. [Google Scholar] [CrossRef]
- Vendruscolo, F.; da Rocha Ferreira, G.L.; Antoniosi Filho, N.R. Biosorption of hexavalent chromium by microorganisms. Int. Biodeterior. Biodegrad. 2017, 119, 87–95. [Google Scholar] [CrossRef]
- Jayashree, S.; Thangaraju, N.; Gnanadoss, J.J. Toxic effects of chromium on the aquatic cyanobacterium Oscillatoria sp and removal of chromium by biosorption. J. Exp. Sci. 2012, 3, 28–34. [Google Scholar]
- Cecal, A.; Humelnicu, D.; Rudic, V.; Cepoi, L.; Ganju, D.; Cojocari, A. Uptake of uranyl ions from uranium ores and sludges by means of Spirulina platensis, Porphyridium cruentum and Nostok linckia alga. Bioresour. Technol. 2012, 118, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Zinicovscaia, I.; Safonov, A.; Zelenina, D.; Ershova, Y.; Boldyrev, K. Evaluation of biosorption and bioaccumulation capacity of cyanobacteria Arthrospira (spirulina) platensis for radionuclides. Algal Res. 2020, 51, 102075. [Google Scholar] [CrossRef]
- Karatay, S.E.; Dönmez, G.; Aksu, Z. Effective biosorption of phenol by the thermophilic cyanobacterium Phormidium sp. Water Sci. Technol. 2017, 76, 3190–3194. [Google Scholar] [CrossRef]
- Tiwari, B.; Chakraborty, S.; Srivastava, A.K.; Mishra, A.K. Biodegradation and rapid removal of methyl parathion by the paddy field cyanobacterium Fischerella sp. Algal Res. 2017, 25, 285–296. [Google Scholar] [CrossRef]
- Mona, S.; Kumar, V.; Deepak, B.; Kaushik, A. Cyanobacteria: The Eco-Friendly Tool for the Treatment of Industrial Wastewaters. In Bioremediation of Industrial Waste for Environmental Safety: Biological Agents and Methods for Industrial Waste Management; Bharagava, R.N., Saxena, G., Eds.; Springer Singapore: Singapore, 2020; Volume 2, pp. 389–413. [Google Scholar]
- Fatima, S.; Asif, N.; Ahmad, R.; Fatma, T. Toxicity of NSAID drug (paracetamol) to nontarget organism-Nostoc muscorum. Environ. Sci. Pollut. Res. Int. 2020, 27, 35208–35216. [Google Scholar] [CrossRef]
- Kropidłowska, K.; Caban, M. Effect of salinity on the toxicity of diclofenac, ibuprofen and naproxen toward cyanobacterium Synechocystis salina. Chemosphere 2023, 338, 139521. [Google Scholar] [CrossRef]
- Pan, M.; Lyu, T.; Zhan, L.; Matamoros, V.; Angelidaki, I.; Cooper, M.; Pan, G. Mitigating antibiotic pollution using cyanobacteria: Removal efficiency, pathways and metabolism. Water Res. 2021, 190, 116735. [Google Scholar] [CrossRef]
- Pomati, F.; Netting, A.G.; Calamari, D.; Neilan, B.A. Effects of erythromycin, tetracycline and ibuprofen on the growth of Synechocystis sp. and Lemna minor. Aquat. Toxicol. 2004, 67, 387–396. [Google Scholar] [CrossRef]
- Vincent, W.F. Cyanobacteria. In Encyclopedia of Inland Waters, Likens, G.E., Ed.; Academic Press: Oxford, UK, 2009; pp. 226–232. [Google Scholar]
- Rezayian, M.; Niknam, V.; Ebrahimzadeh, H. Stress response in cyanobacteria. Iran J. Plant Physiol. 2019, 3, 2773–2787. [Google Scholar]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.H.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Massey, I.Y.; Al osman, M.; Yang, F. An overview on cyanobacterial blooms and toxins production: Their occurrence and influencing factors. Toxin Rev. 2022, 41, 326–346. [Google Scholar] [CrossRef]
- Watanabe, M.; Semchonok, D.A.; Webber-Birungi, M.T.; Ehira, S.; Kondo, K.; Narikawa, R.; Ohmori, M.; Boekema, E.J.; Ikeuchi, M. Attachment of phycobilisomes in an antenna-photosystem I supercomplex of cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 2512–2517. [Google Scholar] [CrossRef]
- Orosa, M.; Torres, E.; Fidalgo, P.; Abalde, J. Production and analysis of secondary carotenoids in green algae. J. Appl. Phycol. 2000, 12, 553–556. [Google Scholar] [CrossRef]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Functional properties of carotenoids originating from algae. J. Sci. Food Agric. 2013, 93, 5–11. [Google Scholar] [CrossRef]
- Fernández-Rojas, B.; Hernández-Juárez, J.; Pedraza-Chaverri, J. Nutraceutical properties of phycocyanin. J. Funct. Foods 2014, 11, 375–392. [Google Scholar] [CrossRef]
- Niemczyk, E.; Pogrzeba, J.; Adamczyk-Woźniak, A.; Lipok, J. Boronic Acids of Pharmaceutical Importance Affect the Growth and Photosynthetic Apparatus of Cyanobacteria in a Dose-Dependent Manner. Toxins 2020, 12, 793. [Google Scholar] [CrossRef]
- Khan, A.H.; Barros, R. Pharmaceuticals in Water: Risks to Aquatic Life and Remediation Strategies. Hydrobiology 2023, 2, 395–409. [Google Scholar] [CrossRef]
- Andersen, R.A. Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Sneha, G.R.; Annayya; Hembrom, B.B.; Varghese, E.; Yadav, R.K.; Abraham, G. Screening and selection of Anabaena spp. for desiccation tolerance through physiological parameters and multivariate analysis. J. Appl. Phycol. 2023, 35, 1273–1284. [Google Scholar] [CrossRef]
- Mandhata, C.P.; Bishoyi, A.K.; Sahoo, C.R.; Maharana, S.; Padhy, R.N. Insight to biotechnological utility of phycochemicals from cyanobacterium Anabaena sp.: An overview. Fitoterapia 2023, 169, 105594. [Google Scholar] [CrossRef] [PubMed]
- Fais, G.; Casula, M.; Sidorowicz, A.; Manca, A.; Margarita, V.; Fiori, P.L.; Pantaleo, A.; Caboni, P.; Cao, G.; Concas, A. Cultivation of Chroococcidiopsis thermalis Using Available In Situ Resources to Sustain Life on Mars. Life 2024, 14, 251. [Google Scholar] [CrossRef] [PubMed]
- Aguiló-Nicolau, P.; Galmés, J.; Fais, G.; Capó-Bauçà, S.; Cao, G.; Iñiguez, C. Singular adaptations in the carbon assimilation mechanism of the polyextremophile cyanobacterium Chroococcidiopsis thermalis. Photosynth. Res. 2023, 156, 231–245. [Google Scholar] [CrossRef]
- Nicolaisen, K.; Hahn, A.; Schleiff, E. The cell wall in heterocyst formation by Anabaena sp. PCC 7120. J. Basic Microbiol. 2009, 49, 5–24. [Google Scholar] [CrossRef] [PubMed]
- Hamida, R.S.; Ali, M.A.; Redhwan, A.; Bin-Meferij, M.M. Cyanobacteria—A Promising Platform in Green Nanotechnology: A Review on Nanoparticles Fabrication and Their Prospective Applications. Int. J. Nanomed. 2020, 15, 6033–6066. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, A.K.; Naraian, R. Cyanobacteria as biochemical energy source for the synthesis of inorganic nanoparticles, mechanism and potential applications: A review. 3 Biotech 2021, 11, 445. [Google Scholar] [CrossRef]
- Żyszka-Haberecht, B.; Poliwoda, A.; Lipok, J. Biocatalytic hydrogenation of the C=C bond in the enone unit of hydroxylated chalcones—Process arising from cyanobacterial adaptations. Appl. Microbiol. Biotechnol. 2018, 102, 7097–7111. [Google Scholar] [CrossRef]
- Drzyzga, D.; Lipok, J. Analytical insight into degradation processes of aminopolyphosphonates as potential factors that induce cyanobacterial blooms. Environ. Sci. Pollut. Res. 2017, 24, 24364–24375. [Google Scholar] [CrossRef]
- Nackiewicz, J.; Kołodziej, Ł.; Poliwoda, A.; Broda, M.A. Oxidation of diclofenac in the presence of iron(II) octacarboxyphthalocyanine. Chemosphere 2021, 265, 129145. [Google Scholar] [CrossRef]
- Nackiewicz, J.; Gąsowska-Bajger, B.; Kołodziej, Ł.; Poliwoda, A.; Pogoda-Mieszczak, K.; Skonieczna, M. Comparison of the degradation mechanisms of diclofenac in the presence of iron octacarboxyphthalocyanine and myeloperoxidase. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 287, 122113. [Google Scholar] [CrossRef]
- Lucas, F.W.; Mascaro, L.H.; Fill, T.P.; Rodrigues-Filho, E.; Franco-Junior, E.; Homem-de-Mello, P.; de Lima-Neto, P.; Correia, A.N. Diclofenac on boron-doped diamond electrode: From electroanalytical determination to prediction of the electrooxidation mechanism with HPLC-ESI/HRMS and computational simulations. Langmuir 2014, 30, 5645–5654. [Google Scholar] [CrossRef] [PubMed]
- Keen, O.S.; Thurman, E.M.; Ferrer, I.; Dotson, A.D.; Linden, K.G. Dimer formation during UV photolysis of diclofenac. Chemosphere 2013, 93, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Mackinney, B.G. Absorption of Light By Chlorophyll Solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Chamovitz, D.; Sandmann, G.; Hirschberg, J. Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate-limiting step in carotenoid biosynthesis. J. Biol. Chem. 1993, 268, 17348–17353. [Google Scholar] [CrossRef]
- Bennett, A.; Bogorad, L. Complementary chromatic adaptation in a filamentous blue-green alga. J. Cell Biol. 1973, 58, 419–435. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pogrzeba, J.; Poliwoda, A. Biosorption Ability of Pharmaceutically Active Compounds by Anabaena sp. and Chroococcidiopsis thermalis. Molecules 2024, 29, 4488. https://doi.org/10.3390/molecules29184488
Pogrzeba J, Poliwoda A. Biosorption Ability of Pharmaceutically Active Compounds by Anabaena sp. and Chroococcidiopsis thermalis. Molecules. 2024; 29(18):4488. https://doi.org/10.3390/molecules29184488
Chicago/Turabian StylePogrzeba, Jerzy, and Anna Poliwoda. 2024. "Biosorption Ability of Pharmaceutically Active Compounds by Anabaena sp. and Chroococcidiopsis thermalis" Molecules 29, no. 18: 4488. https://doi.org/10.3390/molecules29184488
APA StylePogrzeba, J., & Poliwoda, A. (2024). Biosorption Ability of Pharmaceutically Active Compounds by Anabaena sp. and Chroococcidiopsis thermalis. Molecules, 29(18), 4488. https://doi.org/10.3390/molecules29184488







