A Dy(III) Coordination Polymer Material as a Dual-Functional Fluorescent Sensor for the Selective Detection of Inorganic Pollutants
Abstract
1. Introduction
2. Results
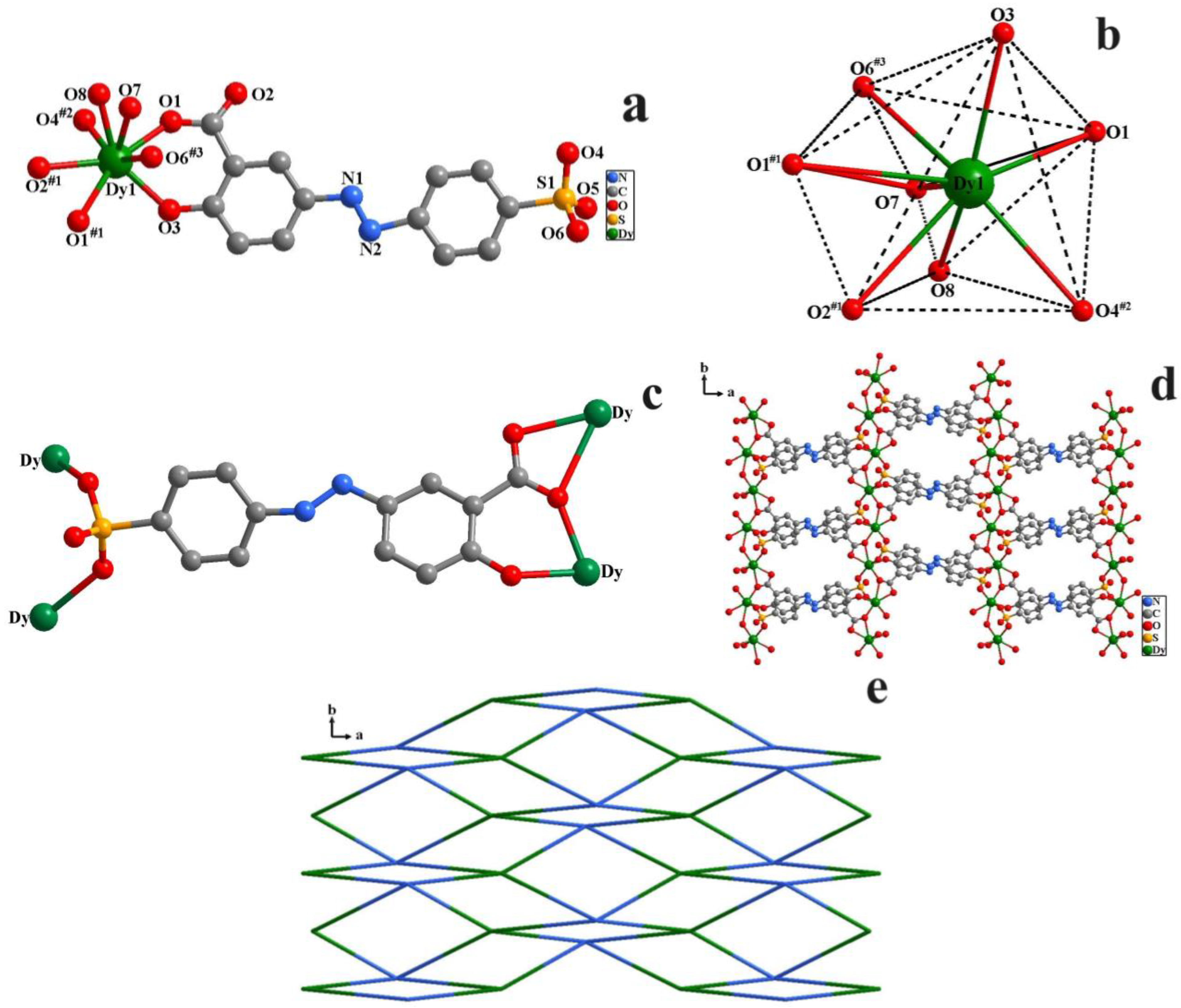
2.1. Structure Description of 1
2.2. Thermogravimetric Analysis
2.3. Powder X-ray Diffraction
2.4. Scanning Electron Microscopy
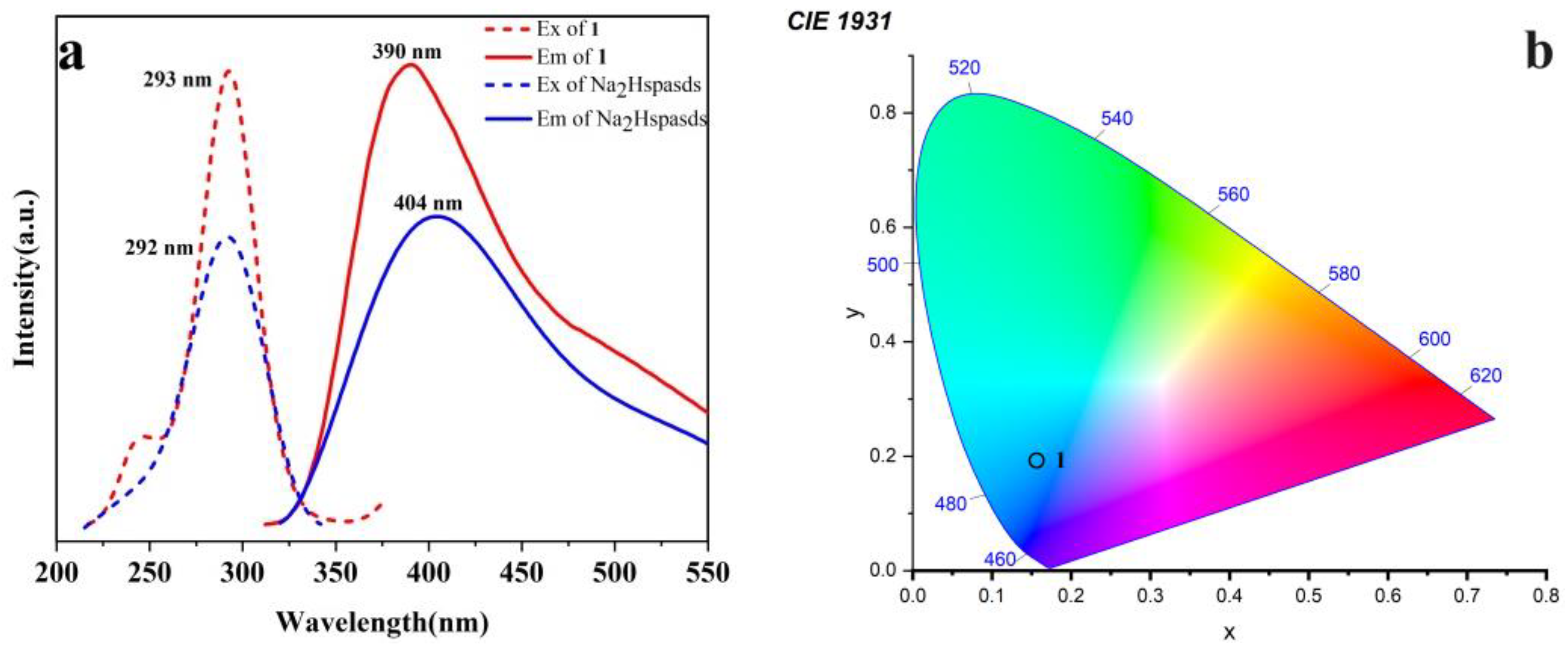
2.5. Photoluminescence Properties
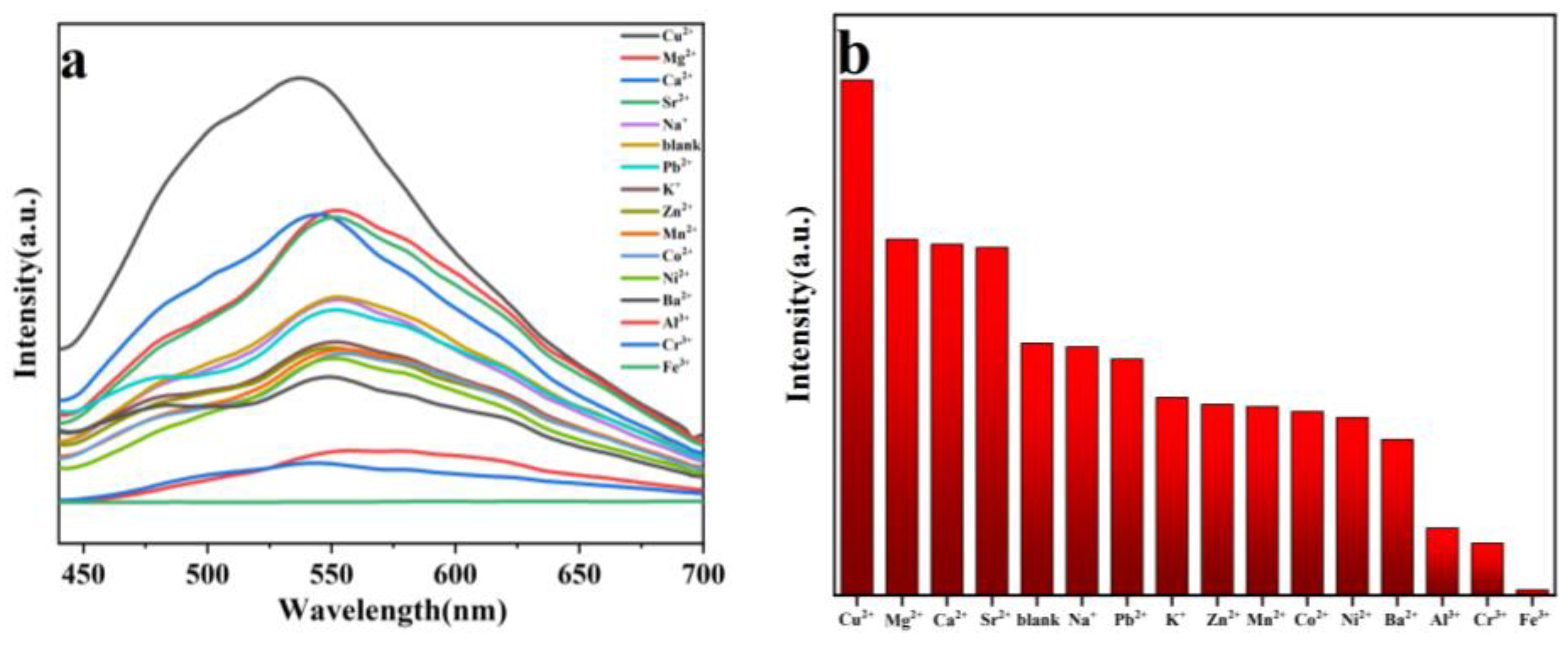
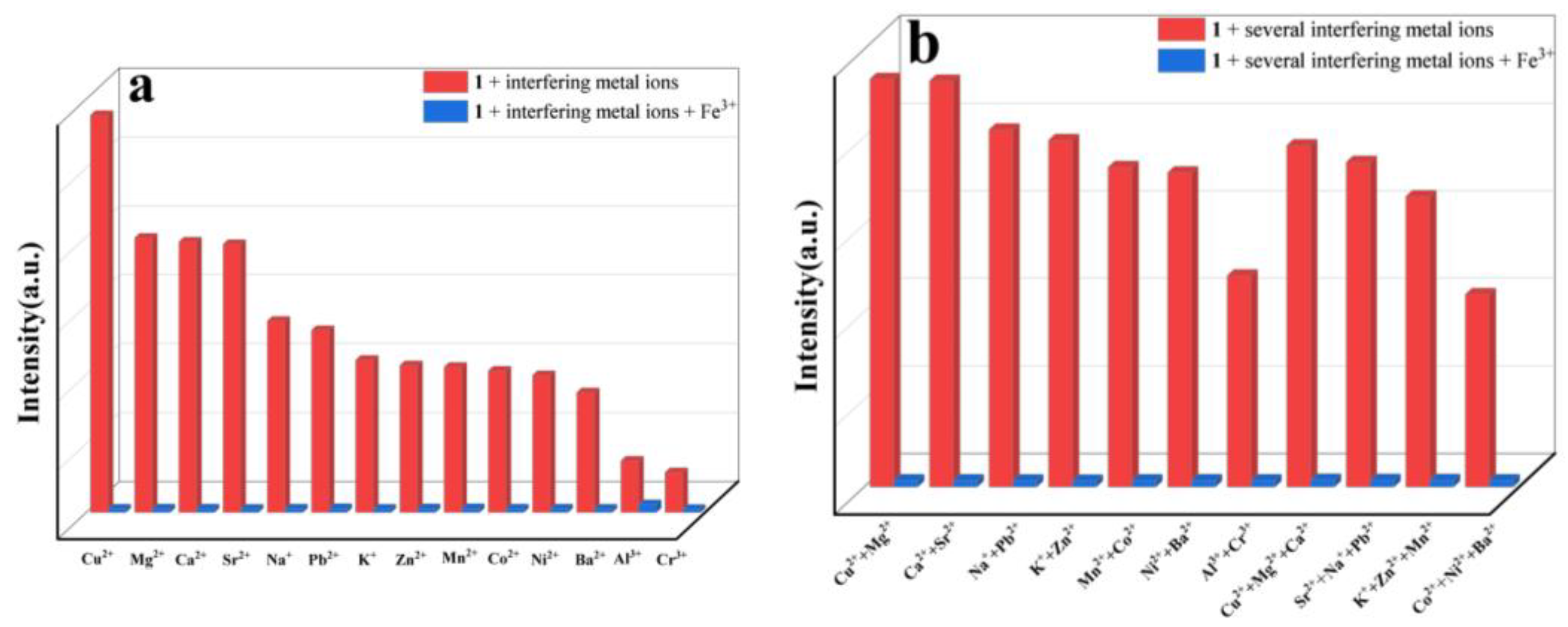
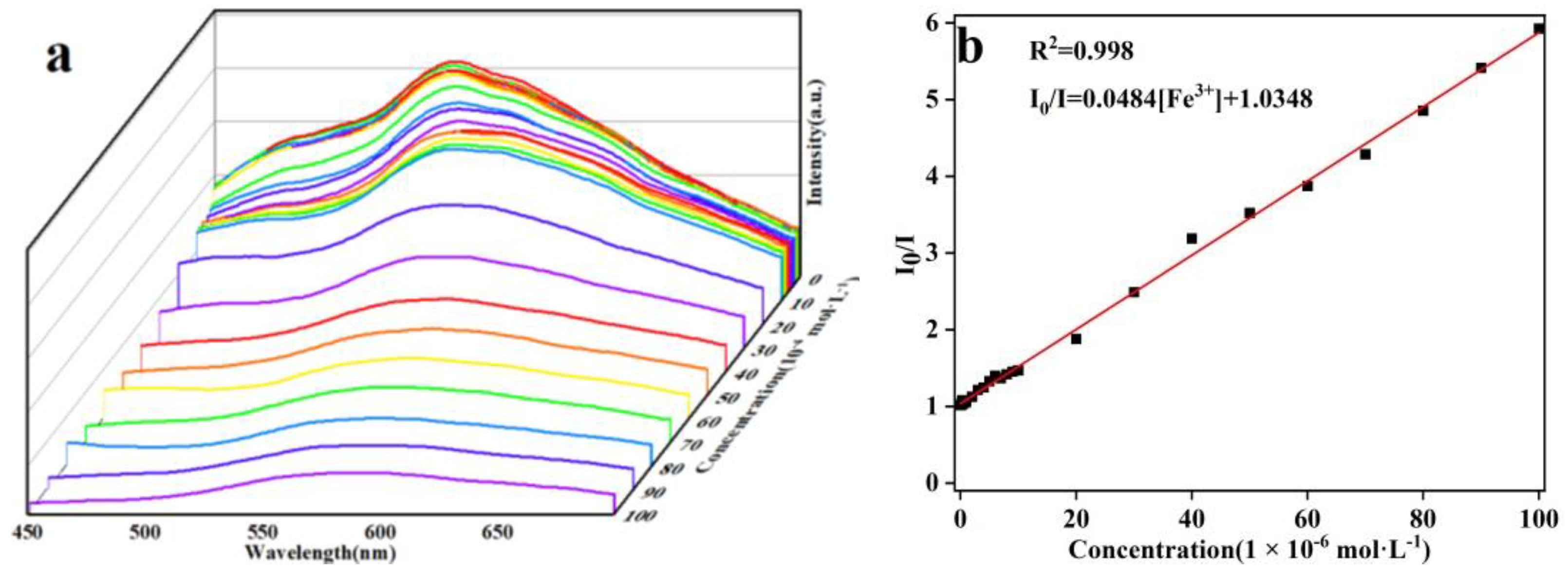
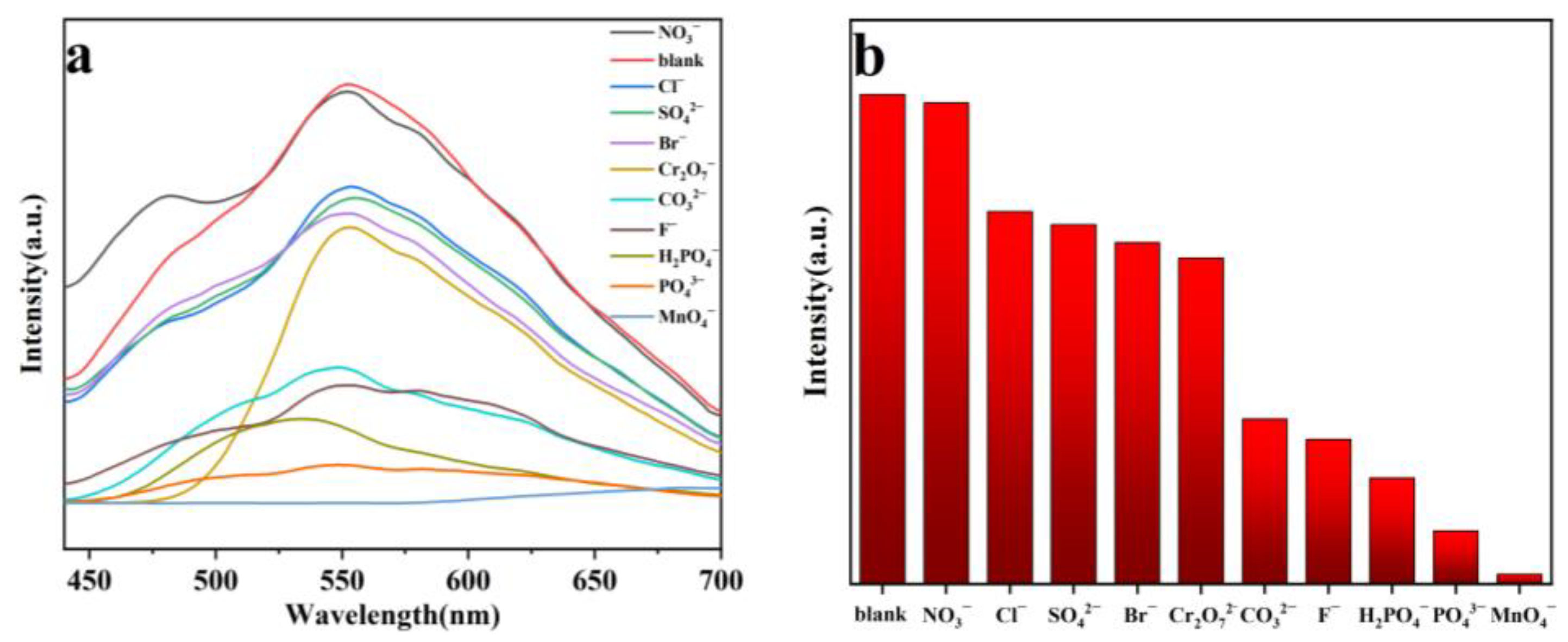
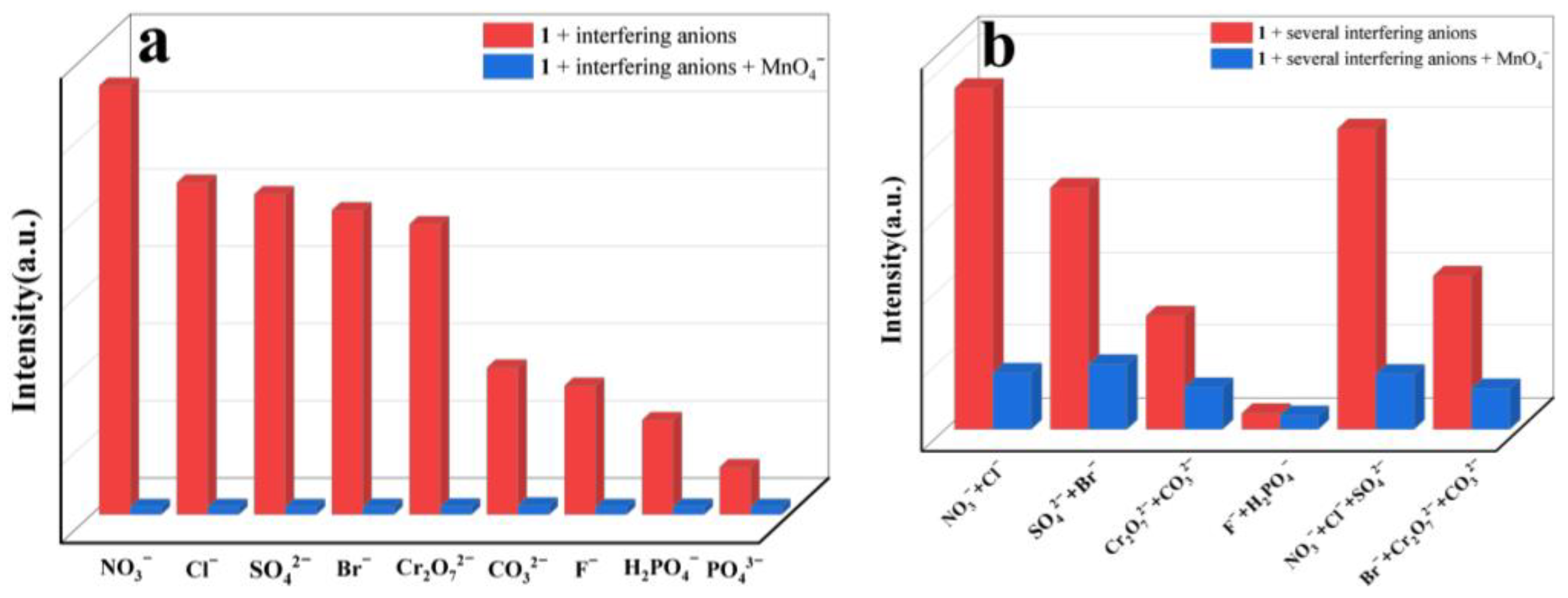
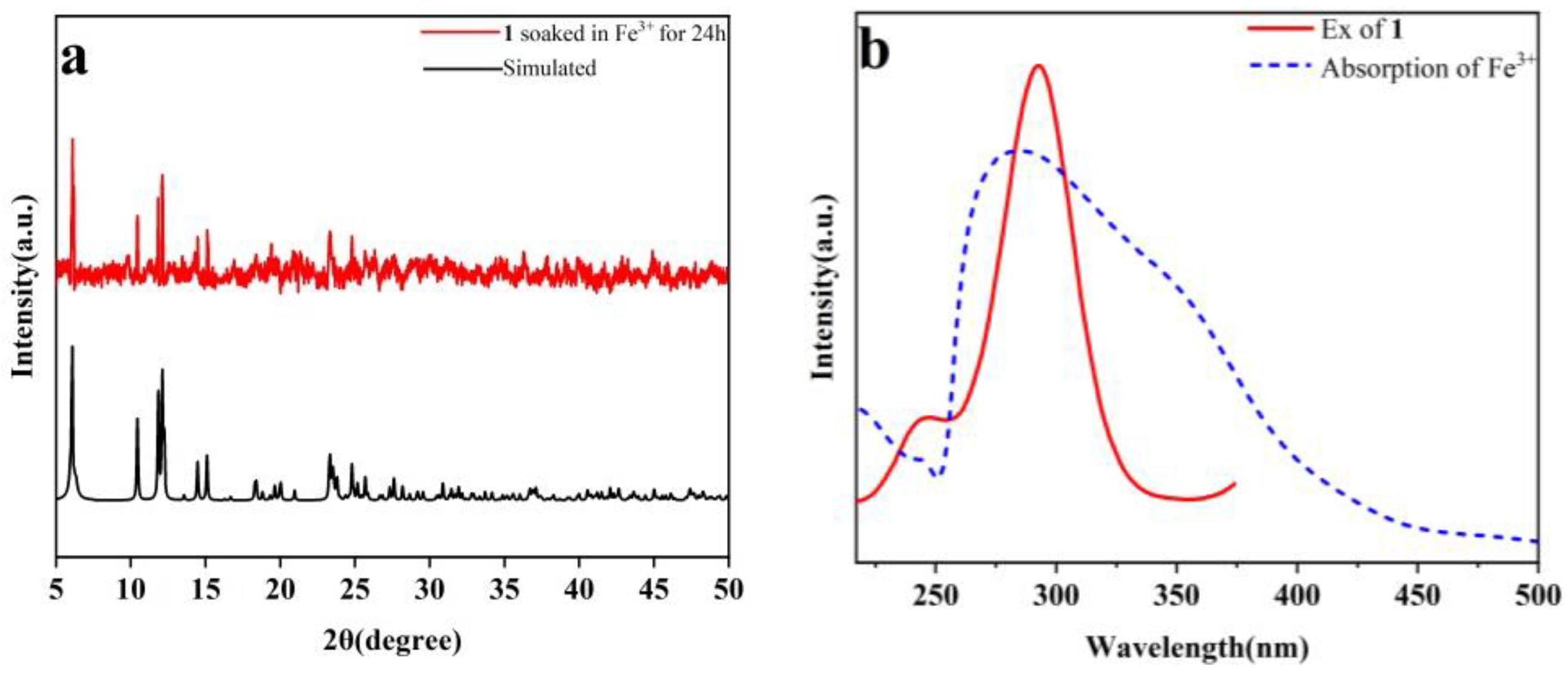
2.6. Selective Sensing of Fe3+
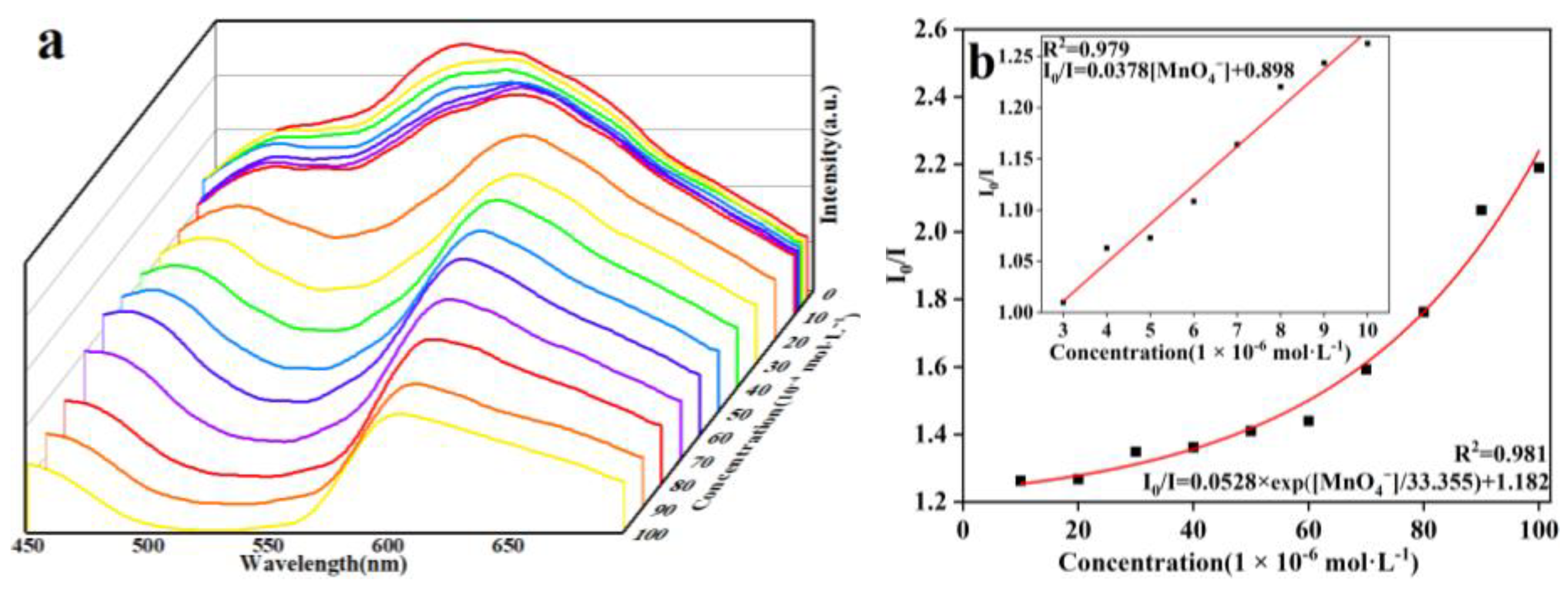
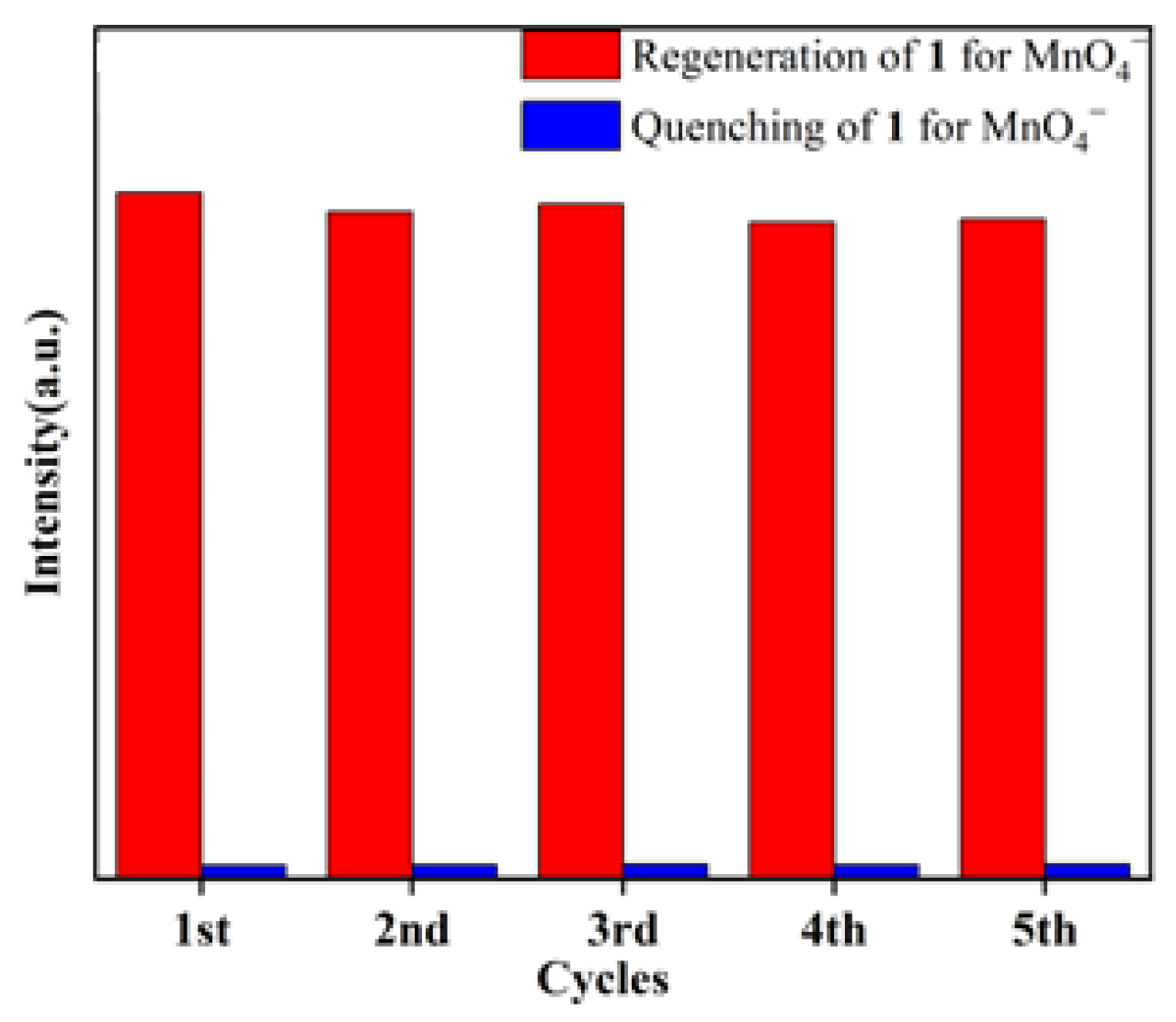
2.7. Selective Sensing of MnO4−
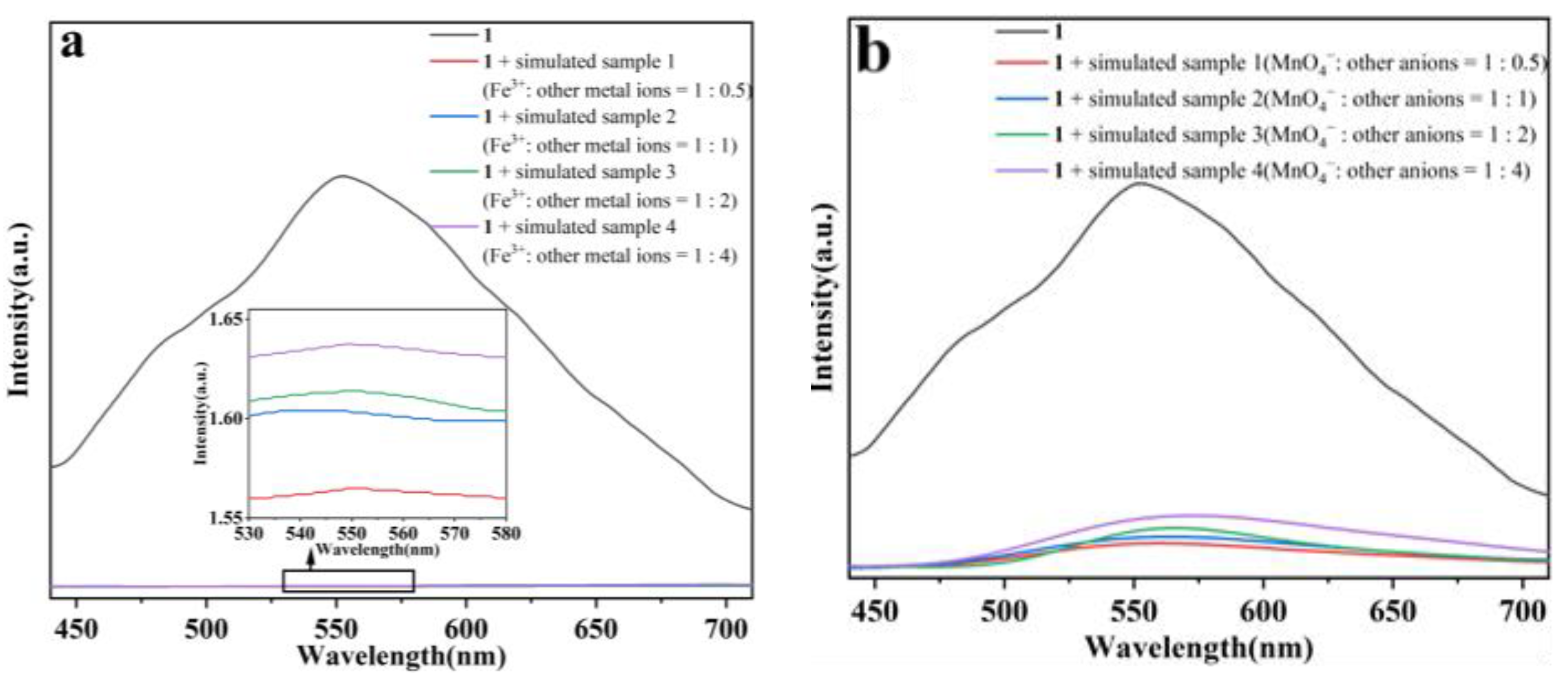
2.8. Practical Application
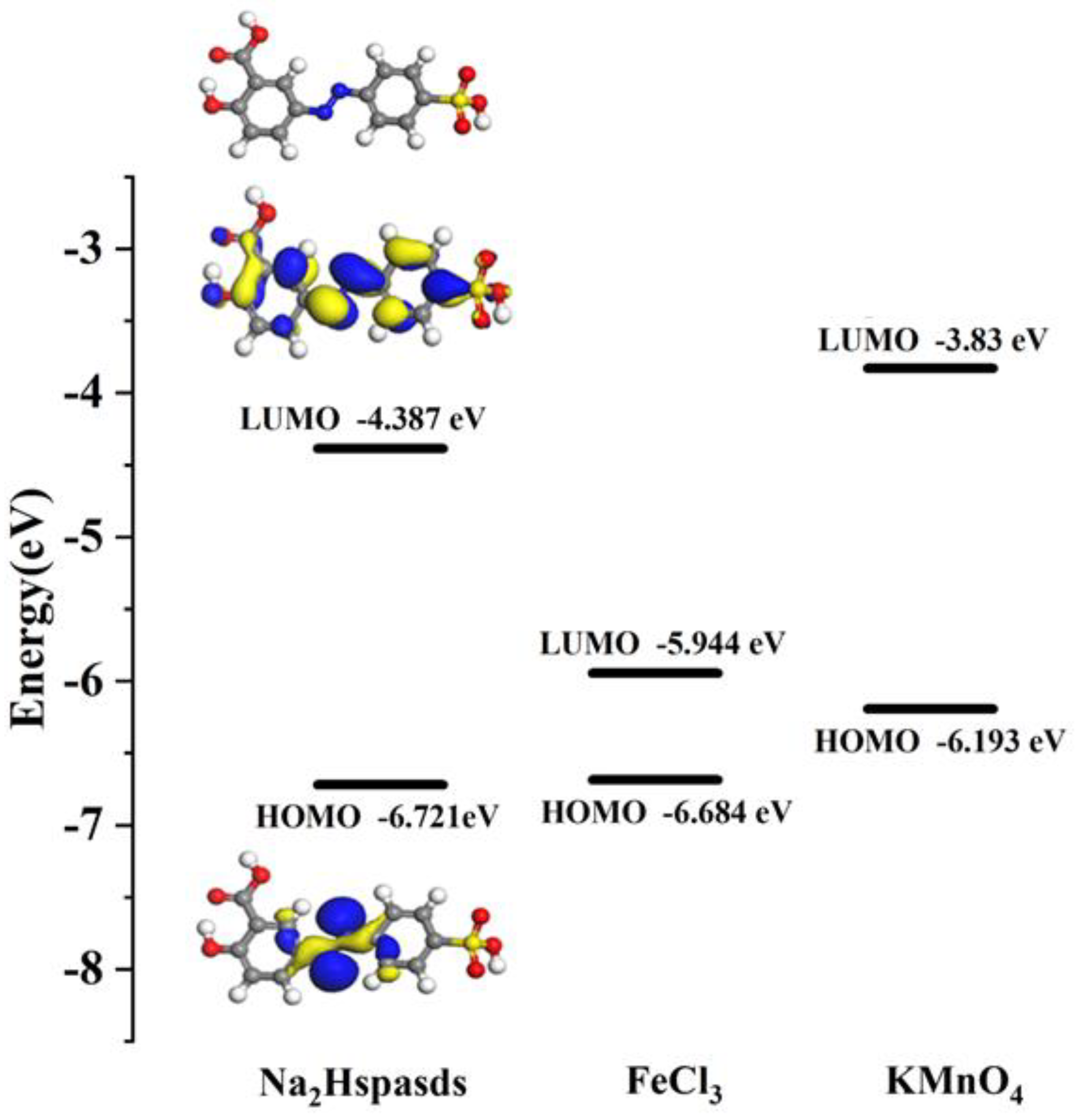
2.9. Quenching Mechanisms
3. Experimental Section
3.1. Materials and Methods
3.2. Synthesis of 1
3.3. X-ray Crystallography
3.4. Luminescence Sensing Experiments
3.5. Computational Approach
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Alexandrov, E.V.; Shevchenko, A.P.; Nekrasova, N.A.; Blatov, V.A. Topological methods for analysis and design of coordination polymers. Russ. Chem. Rev. 2022, 91, RCR5032. [Google Scholar] [CrossRef]
- Thoonen, S.; Hua, C. Chiral detection with coordination polymers. Chem.-Asian J. 2021, 16, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Chai, H.M.; Yan, J.L.; Zhang, G.Q.; Wang, J.X.; Ren, Y.X.; Gao, L.J. Five mesoporous lanthanide metal-organic frameworks: Syntheses, structures, and fluorescence sensing of Fe3+, Cr2O72−, and H2O2 and electrochemical sensing of trinitrophenol. Inorg. Chem. 2022, 61, 7286–7295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Didier, A. State of the art and prospects in metal-organic framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gao, Y.-P.; Lv, Y.-H.; Wang, L.; Han, Y.; Chang, F. A series of 2D and 3D coordination polymers containing multicore Mn-carboxyl chain and flexible polycarboxylate ligands: Synthesis, structure, and magnetic properties. J. Coord. Chem. 2021, 74, 1090–1105. [Google Scholar] [CrossRef]
- Akhtar, A.; Danish, M.; Asif, A.; Arshad, M.N.; Asiri, A.M. Docking assisted DNA-binding, biological screening, and nuclease activity of copper complexes derived from sulfonamides. J. Coord. Chem. 2021, 74, 2092–2110. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, J.; Tian, W. Terbium(III)-based coordination polymer with millimeter-size single crystals and high selectivity and sensitivity for folic acid. CrystEngComm 2023, 25, 945–952. [Google Scholar] [CrossRef]
- Du, Y.; Yang, H.R.; Liu, R.J.; Shao, C.Y.; Yang, L.R. Multi-responsive chemosensor for highly sensitive and selective detection of Fe3+, Cu2+, Cr2O72− and nitrobenzene based on luminescent lanthanide metal-organic framework. Dalton Trans. 2020, 49, 13003–13016. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhao, S.-N.; Zang, S.-Q.; Li, J. Functional metal-organic frameworks as effective sensors of gases and volatile compounds. Chem. Soc. Rev. 2020, 49, 6364–6401. [Google Scholar] [CrossRef]
- Guo, X.-Z.; Chen, S.-S.; Li, W.-D.; Han, S.-S.; Deng, F.; Qiao, R.; Zhao, Y. Series of cadmium(II) coordination polymers based on a versatile multi-N-donor tecton or mixed carboxylate ligands: Synthesis, structure, and selectively sensing property. ACS Omega 2019, 4, 11540–11553. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, S.; Guo, X.; Wang, Q.; Fu, L.; Mi, S.; Gao, P.; Su, Q.; Guo, H. Dual-function metal-organic framework as efficient turn-off sensor for water and unusual turn-on sensor for Ag+. Cryst. Growth Des. 2021, 21, 5108–5115. [Google Scholar] [CrossRef]
- Hou, Z.; Li, P.; Wang, H.-Y.; Li, Z.; Li, H. The construction of a lanthanide coordination polymer as ratiometric luminescent H2PO4− sensor. Dyes Pigments 2017, 147, 429–435. [Google Scholar] [CrossRef]
- Yang, M.-X.; Chen, L.-J.; Ye, Y.-Z.; Lin, X.-Y.; Lin, S. Four 3D Co(II) MOFs based on 2,4,6-tris(4-pyridyl)-1,3,5-triazine and polycarboxylic acid ligands and their derivatives as efficient electrocatalysts for oxygen reduction reaction. Dalton Trans. 2021, 50, 4904–4913. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Li, Q.-Y.; Li, J. Structures, fluorescence and magnetism of a series of coordination polymers driven by a tricarboxypyridine ligand. CrystEngComm 2022, 24, 6751–6761. [Google Scholar] [CrossRef]
- Li, R.-F.; Zhang, H.; Hong, M.-Z.; Shi, J.-G.; Liu, X.-F.; Feng, X. Two Co(II)/Ni(II) complexes based on nitrogenous heterocyclic ligands as high-performance electrocatalysts for the hydrogen evolution reaction. Dalton Trans. 2022, 51, 3970–3976. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Liu, J.; Han, B.; Li, G. Two imidazole multicarboxylate-based MOFs: Syntheses, structures and proton conductive properties. New J. Chem. 2021, 45, 16971–16977. [Google Scholar] [CrossRef]
- Wen, M.-Y.; Ren, L.; Cui, G.-H. Two Co(II) complexes containing pyridylbenzimidazole ligands as chemosensors for the sensing of levofloxacin, acetylacetone, and Ni2+ with high selectivity and sensitivity. CrystEngComm 2021, 23, 8563–8571. [Google Scholar] [CrossRef]
- Zhou, Y.; Hong, M.; Wu, X. Lanthanide-transition metal coordination polymers based on multiple N- and O-donor ligands. Chem. Commun. 2006, 135–143. [Google Scholar] [CrossRef]
- Sun, Y.-Q.; Cheng, Y.; Yin, X.-B. Dual-ligand lanthanide metal-organic framework for sensitive ratiometric fluorescence detection of hypochlorous acid. Anal. Chem. 2021, 93, 3559–3566. [Google Scholar] [CrossRef]
- Ji, G.; Liu, J.; Gao, X.; Sun, W.; Wang, J.; Zhao, S.; Liu, Z. A luminescent lanthanide MOF for selectively and ultra-high sensitively detecting Pb2+ ions in aqueous solution. J. Mater. Chem. A 2017, 5, 10200–10205. [Google Scholar] [CrossRef]
- Nisio, A.D.; Foresta, C. Water and soil pollution as determinant of water and food quality/contamination and its impact on male fertility. Reprod. Biol. Endocrinol. 2019, 17, 4. [Google Scholar] [CrossRef] [PubMed]
- Oloruntoba, A.; Omoniyi, A.O.; Shittu, Z.A.; Ajala, R.O.; Kolawole, S.A. Heavy metal contamination in soils, water, and food in nigeria from 2000–2019: A systematic review on methods, pollution level and policy implications. Water Air Soil Pollut. 2024, 235, 586. [Google Scholar] [CrossRef]
- Brugnara, C. Iron deficiency and erythropoiesis: New diagnostic approaches. Clin. Chem. 2003, 49, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Li, B.; You, Z.X.; Xing, Y.H.; Bai, F.Y.; Sun, L.-X.; Shi, Z. A smart sensing triazine hexacarboxylic metal-organic skeleton material: Synthesis, structure and multifunctional fluorescence detector. J. Mater. Chem. C 2021, 9, 3193–3203. [Google Scholar] [CrossRef]
- Ma, Y.-L.; Du, L.; Zhao, Q.-H. Synthesis, crystal structure, luminescent and magnetic properties of lanthanide coordination polymers based on a zwitterionic polycarboxylate ligand. Inorg. Chem. Commun. 2017, 77, 1–5. [Google Scholar] [CrossRef]
- An, X.; Wang, H.; Li, G. Structures and luminescent properties of two 2D coordination polymers containing Tb(III) or Dy(III) ions. J. Fluoresc. 2014, 24, 425–429. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied topological analysis of crystal structures with the program package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Yi, X.-G.; Chen, W.-T.; Huang, J.-G.; Zhang, D.-W.; Wang, Y.-F. Synthesis, structure, photoluminescent and semiconductor properties of, and theoretical calculations for, a novel zinc complex. J. Chem. Res. 2017, 41, 586–590. [Google Scholar] [CrossRef]
- Ge, Y.F.; Li, G.T.; Fu, D.X.; Liu, L.N.; Wu, B.L. Manganese(II) and zinc(II) coordination polymers based on 2-(5-bromo-pyridin-3-yl)-1H-imidazole-4,5-dicarboxylic acid: Synthesis, structure and properties. J. Coord. Chem. 2019, 72, 1820–1832. [Google Scholar] [CrossRef]
- Lin, C.; Wang, M.; Tang, J.; Zhu, Z.; Wu, P.; Hu, A.; Zhang, L.; Wang, J. A two-fold interpenetrated dual-emitting luminescent metal-organic framework as a ratiometric sensor for chromium(III). Inorg. Chem. 2021, 60, 16803–16809. [Google Scholar] [CrossRef] [PubMed]
- Dascălu, M.; Chibac-Scutaru, A.-L.; Roman, G. Detection of nitroaromatics by a Zn(II)-containing coordination polymer derived from a 1,2,3-triazole-based tricarboxylate ligand. J. Mol. Liq. 2023, 386, 122457. [Google Scholar] [CrossRef]
- Liu, L.-J.; Zhu, H.; Han, C.; Cui, G.-H.; Fu, L. Luminescent detecting of Fe3+ and Cr2O72− ions by three ternary 2D coordination polymers. Polyhedron 2021, 198, 115074. [Google Scholar] [CrossRef]
- Li, B.; Yan, Q.-Q.; Yong, G.-P. New porous coordination polymer reveals selective sensing of Fe3+, Cr2O72−, CrO42−, MnO4− and nitrobenzene, and stimuli responsive luminescent color conversions. J. Mater. Chem. C 2020, 8, 11786–11795. [Google Scholar] [CrossRef]
- Wang, X.; Xiao, H.; Zhang, M.; Lin, H.; Liu, G. Four luminescent metal-organic chain compounds based on semi-rigid N-donor ligands and 3-hydroxy-2-naphthoic acid for recognition of Fe3+ and Cr2O72− ions. Polyhedron 2020, 179, 114383. [Google Scholar] [CrossRef]
- Wang, J.; Chen, N.-N.; Qian, J.; Chen, X.-R.; Zhang, X.-Y.; Fan, L. Multi-responsive chemosensing and photocatalytic properties of three luminescent coordination polymers derived from a bifunctional 1,1′-di(4-carbonylphenyl)-2,2′-biimidazoline ligand. CrystEngComm 2020, 22, 6195–6206. [Google Scholar] [CrossRef]
- Zhang, X.-P.; Deng, X.-C.; Dong, G.-Y. A new water-stable cadmium(II) coordination polymer for luminescence sensing of chlortetracycline and Fe3+ ions in aqueous solution. J. Chem. Sci. 2024, 136, 21. [Google Scholar] [CrossRef]
- Liu, L.J.; Ran, Y.G.; Du, J.L.; Wang, Z.C.; Liu, M.; Mu, Y.J. A luminescent Cd(II) coordination polymer as a multi-responsive fluorescent sensor for Zn2+, Fe3+ and Cr2O72− in water with fluorescence enhancement or quenching. RSC Adv. 2021, 11, 11266. [Google Scholar] [CrossRef]
- Xu, T.-Y.; Nie, H.-J.; Li, J.-M.; Shi, Z.-F. Highly selective sensing of Fe3+/Hg2+ and proton conduction using two fluorescent Zn(II) coordination polymers. Dalton Trans. 2020, 49, 11129–11141. [Google Scholar] [CrossRef]
- Wang, X.; He, S.; Chen, J.; Wei, J.; Chen, C.; Shi, W.; Wu, D.; Fu, L.; Yang, T. A highly efficient lanthanide coordination polymer luminescent material for the multi-task detection of environmental pollutants. Dalton Trans. 2024, 53, 276–284. [Google Scholar] [CrossRef]
- Liu, D.; Dong, G.; Wang, X.; Nie, F.; Li, X. A luminescent eu coordination polymer with near-visible excitation for sensing and its homologues constructed from 1,4-benzenedicarboxylate and 1H-imidazo[4,5-f][1,10]-phenanthroline. CrystEngComm 2020, 22, 7877–7887. [Google Scholar] [CrossRef]
- Li, F.; Hong, Y.-S.; Zuo, K.-X.; Sun, Q.; Gao, E.-Q. Highly selective fluorescent probe for Hg2+ and MnO4− by the two-fold interpenetrating metal-organic framework with nitro functionalized linkers. J. Solid State Chem. 2019, 270, 509–515. [Google Scholar] [CrossRef]
- Zhao, X.-X.; Qin, Z.-B.; Li, Y.-H.; Cui, G.-H. Two luminescent cobalt(II) coordination polymers for selective sensing of MnO4− in water. Transit. Met. Chem. 2018, 43, 597–604. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Chen, F.-M.; Wen, Q.; Zhou, C.-C.; He, X.; Li, Y.; Liu, H.-F. Zn-based coordination polymers with tricarboxylic acid ligand: Fluorescence sensor toward Fe3+ and MnO4−. J. Mol. Struct. 2022, 1252, 132183. [Google Scholar] [CrossRef]
- Yan, Y.-T.; Cao, F.; Zhang, W.-Y.; Zhang, S.-S.; Zhang, F.; Wang, Y.-Y. Seven luminescent metal–organic frameworks constructed from 5-(triazol-1-yl)nicotinic acid: Luminescent sensors for CrVI and MnO4− ions in an aqueous medium. New J. Chem. 2018, 42, 9865–9875. [Google Scholar] [CrossRef]
- Ma, J.-X.; Xu, N.; Wang, Y.; Liu, G.-C.; Wang, X.-L. Three Zn(II) coordination polymers as dual-responsive luminescent probes for highly selective detection of Fe3+ cation and MnO4− anion. Z. Anorg. Allg. Chem. 2022, 648, e202100331. [Google Scholar] [CrossRef]
- Zhao, F.-H.; Feng, R.; Li, S.-Q.; Zhang, J.-J.; Yu, W.-C.; Li, Z.-L. Two water-stable Zn(II) coordination polymers as luminescent sensors for detection of uric acid in serum, MnO4−, and Cr(VI) oxo-anions in tap water. Cryst. Growth Des. 2024, 24, 5335–5348. [Google Scholar] [CrossRef]
- Xu, S.; Shi, J.-J.; Ding, B.; Liu, Z.-Y.; Wang, X.-G.; Zhao, X.-J.; Yang, E.-C. A heterometallic sodium(I)-europium(III)-organic layer exhibiting dual-responsive luminescent sensing for nitrofuran antibiotics, Cr2O72− and MnO4− anions. Dalton Trans. 2019, 48, 1823–1834. [Google Scholar] [CrossRef]
- Shi, J.-L.; Xu, P.; Wang, X.-G.; Ding, B.; Zhao, X.-J.; Yang, E.C. A dual-responsive luminescent terbium(III) chain for selective sensing of Fe3+ and MnO4− ions. Z. Anorg. Allg. Chem. 2018, 644, 1598–1606. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Li, X.; Yu, J.; Chi, W.; Wang, Z.; Zheng, H.; Sun, Z.-G.; Zhu, Y.; Jiao, C. A stable Mn(II) coordination polymer demonstrating proton conductivity and quantitative sensing of oxytetracycline in aquaculture. Dalton Trans. 2024, 53, 5034. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, Z.-Q.; Xu, Y.-L.; Chang, Z.; Bu, X.-H. Guest dependent structure and acetone sensing properties of a 2D Eu3+ coordination polymer. RSC Adv. 2017, 7, 2258. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Saleh, D.I.; Alhashmialameer, D.; Mahmoud, S.F.; Etaiw, S.E.H. Copper and silver nano-schiff base complexes: Efficient catalysts for triazole synthesis and antimicrobial assessment. J. Mol. Struct. 2024, 1316, 138910. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Y.; Li, Y.; Ma, Z.; Li, Q.; Wang, Q.; Xu, D.; Gao, J.; Gao, X.; Sun, H. Molecular level unveils anion exchange membrane fouling induced by natural organic matter via XDLVO and molecular simulation. Sci. Total Environ. 2024, 916, 170272. [Google Scholar] [CrossRef] [PubMed]
- Ryzhkov, M.V.; Enyashin, A.N.; Delley, B. Plutonium complexes in water: New approach to ab initio modeling. Radiochim. Acta 2021, 109, 327–342. [Google Scholar] [CrossRef]
- Ibrahim, S.M.; Al-Hossainy, A.F. Synthesis, Structural characterization, DFT, kinetics and mechanism of oxidation of bromothymol blue: Application to textile industrial wastewater treatment. Chem. Pap. 2021, 75, 297–309. [Google Scholar] [CrossRef]
- Bhattacharjee, C.R.; Goswami, P.; Mondal, P. Synthesis, reactivity, thermal, electrochemical and magnetic studies on iron(III) complexes of tetradentate Schiff base ligands. Inorg. Chim. Acta 2012, 387, 86–92. [Google Scholar] [CrossRef]



| 1 | |
|---|---|
| Chemical formula | C13H11N2O8SDy |
| Mr | 517.8 |
| Crystal system | Monoclinic |
| Space group | C2/m |
| Temperature (K) | 193 |
| a, b, c (Å) | 29.6268(18), 8.8632(6), 7.6514(5) |
| β (°) | 102.436(4) |
| V (Å3) | 1962.0(2) |
| Z | 4 |
| V (Å3) | 1962.0(2) |
| µ (mm−1) | 20.6 |
| Tmin, Tmax | 0.442, 0.751 |
| No. of measured, independent and | 1862, 1862, 1724 |
| observed [I > 2σ(I)] reflections | |
| Rint | 0.0903 |
| (sinθ/λ)max (Å−1) | 0.602 |
| R[F2 > 2σ(F2)], wR(F2), S | 0.085, 0.219, 1.12 |
| No. of reflections | 1862 |
| No. of parameters | 201 |
| No. of restraints | 188 |
| R1, ωR2 [I ≥ 2σ(I)] | 0.0848, 0.2074 |
| R1, ωR2 (all data) | 0.0966, 0.2185 |
| Δρmax, Δρmin (e·Å−3) | 2.79, −2.25 |
| Sensor | Ksv/M−1 | LOD/M | Reference |
|---|---|---|---|
| [Dy(spasds)(H2O)2]n | 4.84 × 104 | 9.30 × 10−7 | This work |
| [Co(L)(TBTA)(H2O)2·H2O]n | 1.42 × 104 | 1.61 × 10−6 | [33] |
| [Zn(L)2·2DMF]n | 1.92 × 104 | 2.34 × 10−5 | [34] |
| [Cd(bimbp)(3-HNA)2]n | 4.39 × 104 | 5.9 × 10−5 | [35] |
| [Zn(L)·H2O]n | 1.38 × 104 | 3.01 × 10−5 | [36] |
| [Cd(L)(nip)(H2O)·H2O]n | 5.53 × 103 | 7.16 × 10−6 | [37] |
| [Cd(btic)(phen)·0.5H2O]n | 4.959 × 104 | 1.524 × 10−4 | [38] |
| [Zn(Hssa)(1,4-bib)·H2O]n | 4.16 × 104 | 1.66 × 10−6 | [39] |
| [Eu(BDC)1.5(IP)(H2O)]·nH2O | 2.33 × 104 | 6.05 × 10−5 | [40] |
| [Tb2(ccpc)2(H2O)6]·1.5H2O | 1.022 × 104 | 1.71 × 10−6 | [41] |
| Sensor | Ksv/M−1 | LOD/M | Reference |
|---|---|---|---|
| [Dy(spasds)(H2O)2]n | 3.78 × 104 | 1.19 × 10−6 | This work |
| [Co(NPDC)(bpee)·DMF·2H2O]n | 4.26 × 103 | 1.5 × 10−6 | [42] |
| [Co2(L2)(1,4-chdc)2]n | 1.63 × 104 | 1.2 × 10−4 | [43] |
| [Zn(L)2·2DMF]n | 1.92 × 104 | 2.34 × 10−5 | [34] |
| [Zn(BTC)(Hdpa)·H2O]n | 2.46 × 104 | 8.86 × 10−6 | [44] |
| [Cd(L)2(H2O)2]n | 2.2 × 104 | 1.73 × 10−4 | [45] |
| [Zn(3-bpat)(5-mip)]n | 2.8 × 104 | 3.64 × 10−6 | [46] |
| [Zn2(OH)2(1,5-NDS)(Mbimb)]n | 7.3 × 103 | 4.33 × 10−6 | [47] |
| [Eu2Na(Hpddb)(pddb)2(CH3COO)2·2.5(DMA)]n | 2.84 × 103 | 5.99 × 10−6 | [48] |
| [Tb(H2O)(L)(TPA)·0.5H2O]n | 1.13 × 104 | 1.43 × 10−6 | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; An, B.; Li, S.; Chen, L.; Tao, L.; Fang, T.; Guan, L. A Dy(III) Coordination Polymer Material as a Dual-Functional Fluorescent Sensor for the Selective Detection of Inorganic Pollutants. Molecules 2024, 29, 4495. https://doi.org/10.3390/molecules29184495
Wang Y, An B, Li S, Chen L, Tao L, Fang T, Guan L. A Dy(III) Coordination Polymer Material as a Dual-Functional Fluorescent Sensor for the Selective Detection of Inorganic Pollutants. Molecules. 2024; 29(18):4495. https://doi.org/10.3390/molecules29184495
Chicago/Turabian StyleWang, Ying, Baigang An, Si Li, Lijiang Chen, Lin Tao, Timing Fang, and Lei Guan. 2024. "A Dy(III) Coordination Polymer Material as a Dual-Functional Fluorescent Sensor for the Selective Detection of Inorganic Pollutants" Molecules 29, no. 18: 4495. https://doi.org/10.3390/molecules29184495
APA StyleWang, Y., An, B., Li, S., Chen, L., Tao, L., Fang, T., & Guan, L. (2024). A Dy(III) Coordination Polymer Material as a Dual-Functional Fluorescent Sensor for the Selective Detection of Inorganic Pollutants. Molecules, 29(18), 4495. https://doi.org/10.3390/molecules29184495







