Dynamic Adsorption of Mn2+ from Acid Mine Drainage by Highly Active Immobilized Particles with Fe0/Fe2+ Enhanced SRB
Abstract
:1. Introduction
2. Results and Discussion
2.1. Dynamic Adsorption Performance of Fe0/2+-SRB for Mn2+
2.1.1. Breakthrough Curves of Fe0/2+-SRB for Mn2+ at Different Adsorption Layer Heights
2.1.2. Breakthrough Curves of Fe0/2+-SRB for Mn2+ at Different Inflow Velocity
2.1.3. Breakthrough Curves of Fe0/2+-SRB for Mn2+ at Different Initial Concentrations
2.2. Dynamic Adsorption Kinetics Models of Fe0/2+-SRB for Mn2+
2.3. Microscopic Characterization of Fe0/2+-SRB
2.3.1. X-ray Diffraction Analysis
2.3.2. Fourier Transform Infrared Spectroscopy Analysis
2.3.3. Scanning Electron Microscopy Analysis
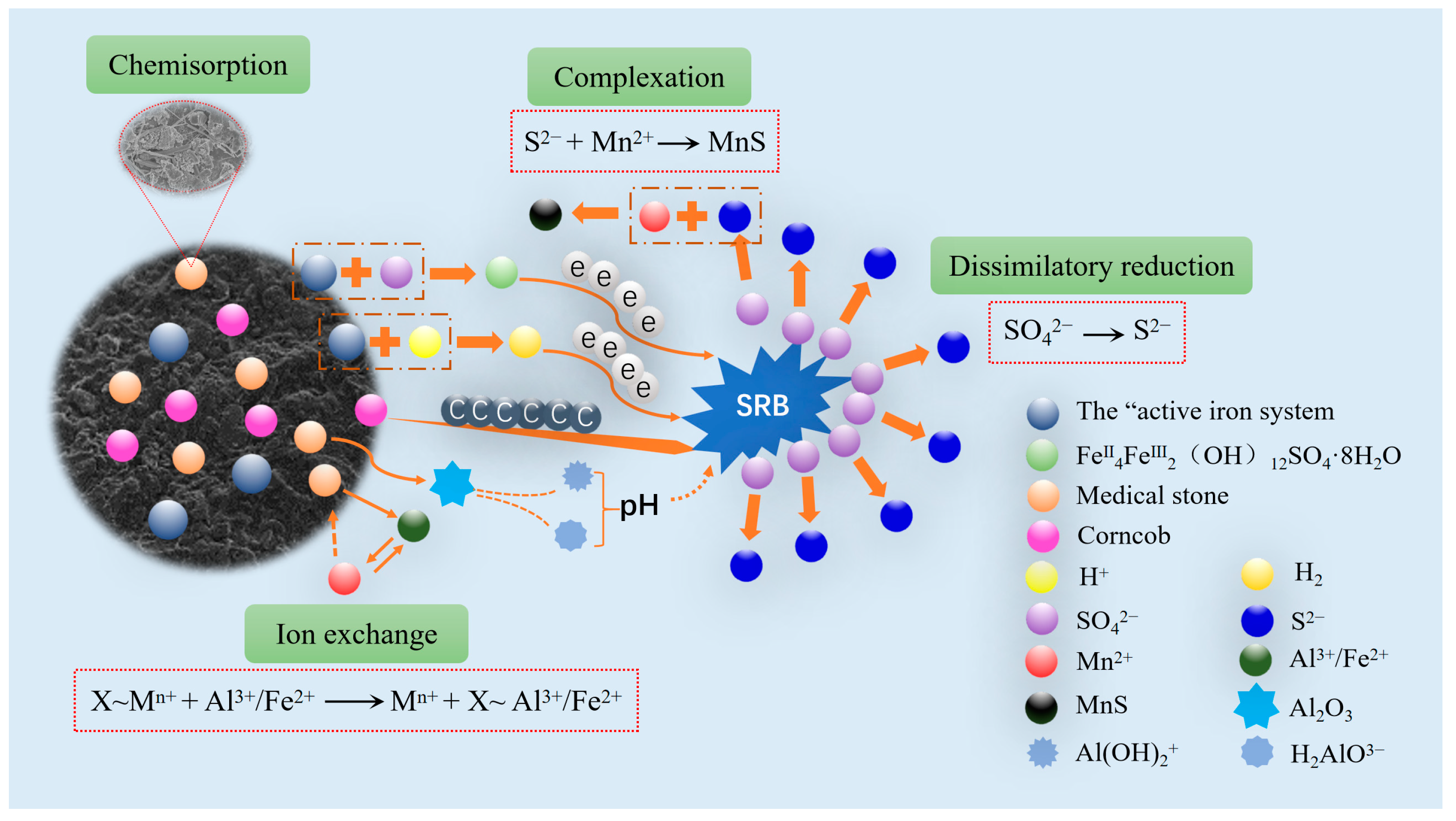
2.4. The Treatment Mechanism of AMD by Fe0/2+-SRB(3#)
2.5. Analysis of the Adaptability of Fe0/2+-SRB to AMD
2.5.1. Adaptability of Fe0/2+-SRB(3#) to Initial pH
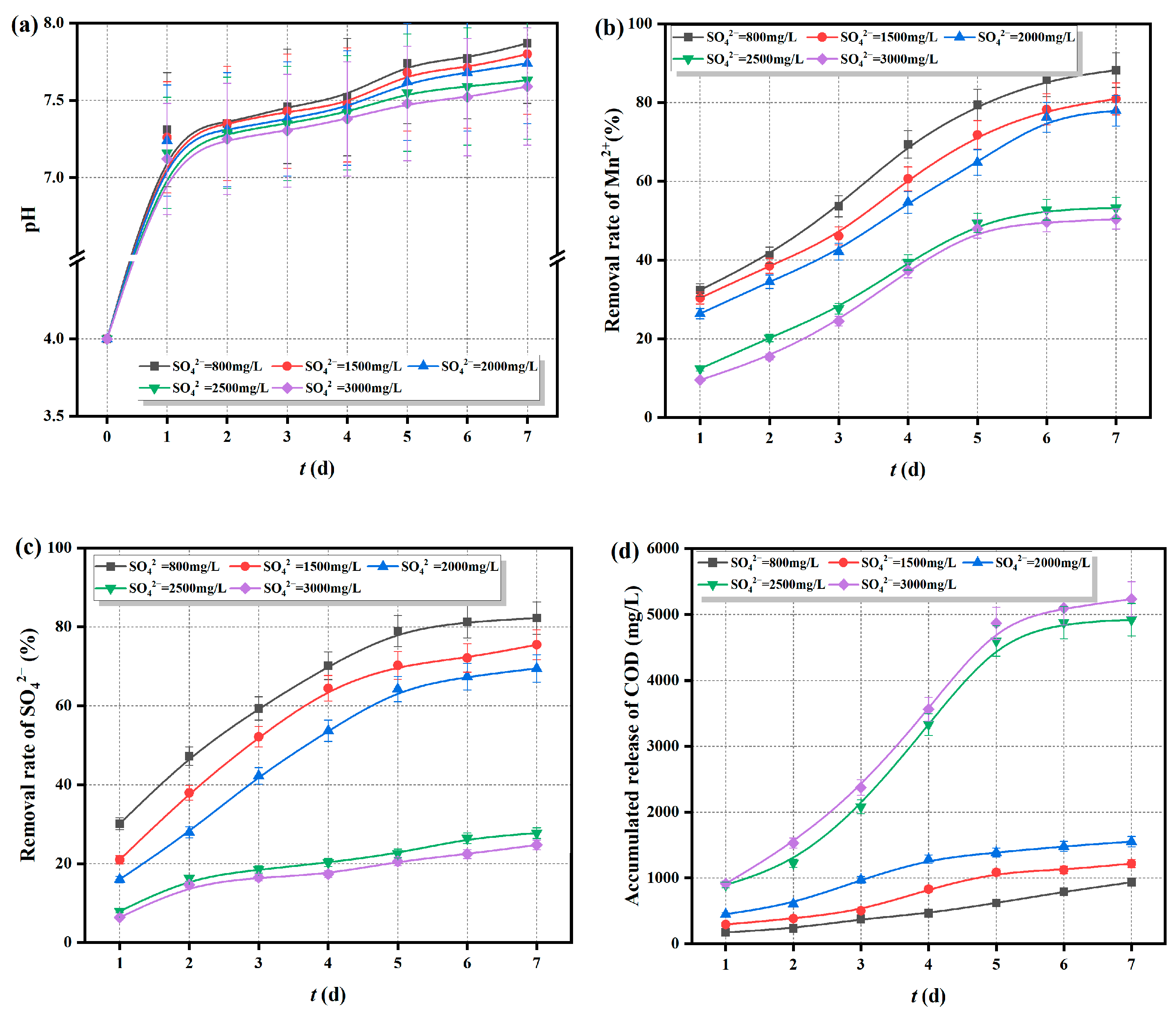
2.5.2. Adaptability of Fe0/2+-SRB(3#) to Initial Concentration of SO42−
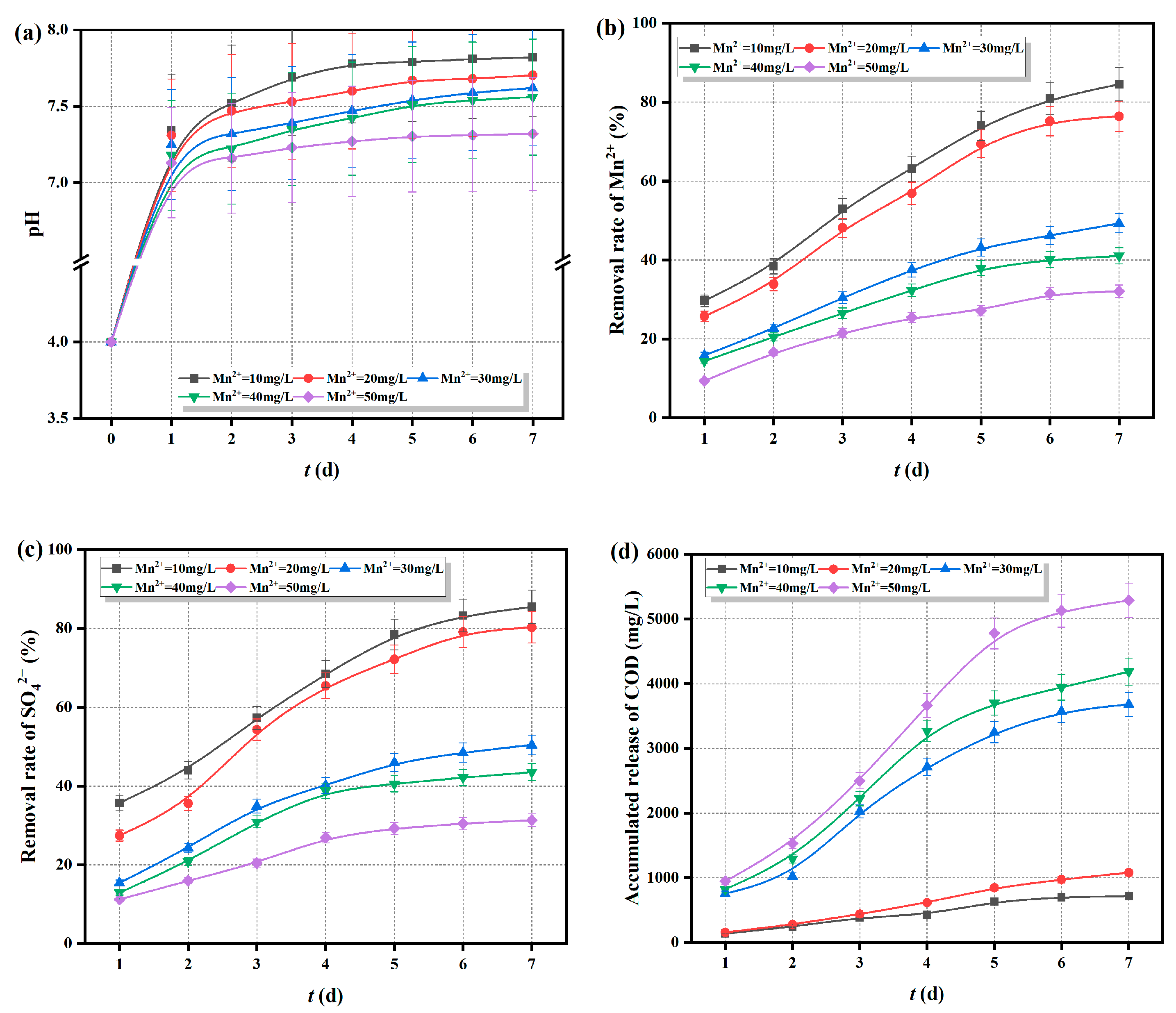
2.5.3. Adaptability of Fe0/2+-SRB(3#) to Initial Concentration of Mn2+
3. Materials and Methods
3.1. Materials and Simulated AMD
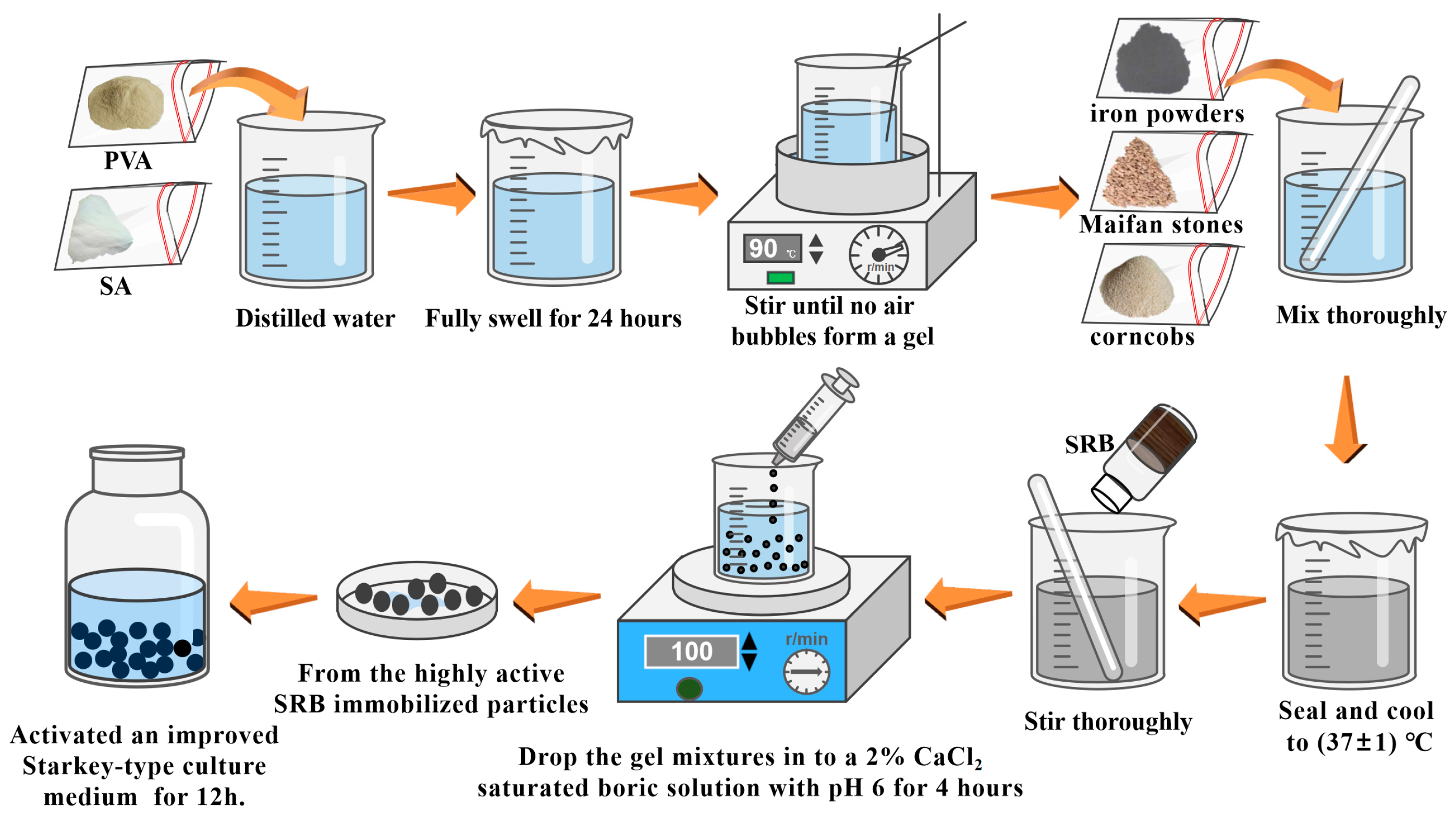
3.2. Preparation of Fe0/Fe2+-SRB Immobilized Particles
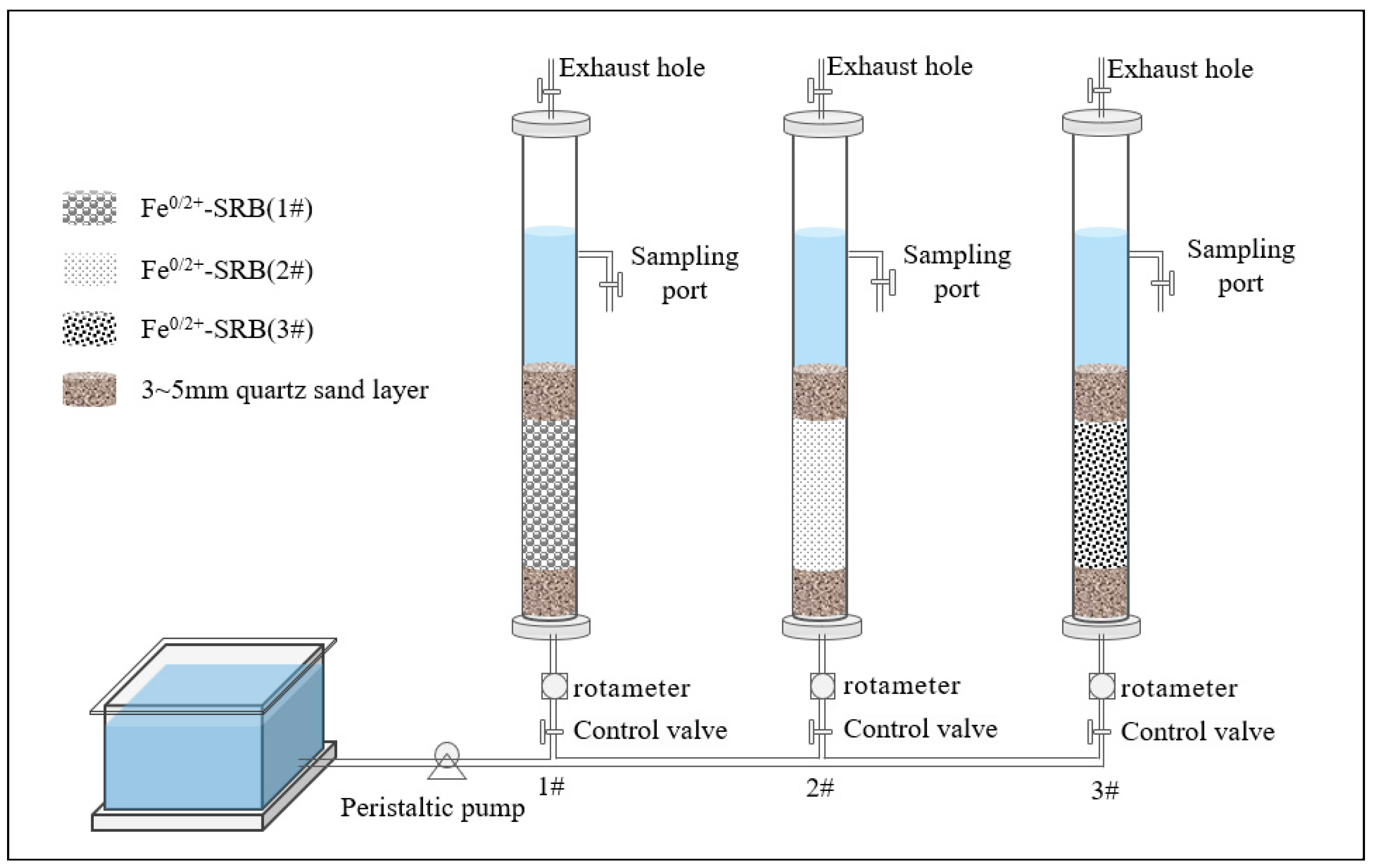
3.3. Dynamic Experiments
3.4. Breakthrough Curve of Mn2+ Adsorption by Fe0/2+-SRB
3.5. Dynamic Adsorption Kinetics Models of Mn2+ by Fe0/2+-SRB
3.6. Adaptability of Fe0/2+-SRB to AMD
3.7. Detection Items and Methods
4. Conclusions
- (1)
- When the adsorption layer height was 200 mm, the inflow rate was 5 × 10−5 m3/s, and the initial Mn2+ concentration was 10 mg/L, the dynamic adsorption capacity for Mn2+ in the constructed dynamic columns was maximized.
- (2)
- Compared with dynamic columns 1# and 2#, dynamic column 3# showed the best performance in treating AMD, indicating that the presence of the Fe0/Fe2+ composite system enhances the remediation capability of Fe0/2+-SRB. Additionally, the Thomas model accurately describes the adsorption kinetics of Mn2+ by Fe0/2+-SRB(3#).
- (3)
- In Fe0/2+-SRB(3#), there are interactions of chemical adsorption, ion exchange, dissimilation–reduction reaction, and surface complexation among the substrates. Mn2+ is primarily removed in the form of manganese sulfide (MnS), while Fe0/Fe2+ facilitates the dissimilatory reduction of SO42− by SRB, leading to the generation of S2−.
- (4)
- Fe0/2+-SRB(3#) can adapt to AMD with an initial pH greater than 4, SO42− concentration below 2500 mg/L, and Mn2+ concentration below 20 mg/L, making it an excellent adsorbent material for treating AMD.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sulonen, M.L.K.; Baeza, J.A.; Gabriel, D.; Guisasola, A. Optimisation of the operational parameters for a comprehensive bioelectrochemical treatment of acid mine drainage. J. Hazard. Mater. 2021, 409, 124944. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Routh, J.; Dario, M.; Sarkar, S.; Wei, L.Z.; Luo, D.G.; Liu, Y. Distribution and mobilization of heavy metals at an acid mine drainage affected region in South China, a post-remediation study. Sci. Total Environ. 2020, 724, 138122. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, K.; Banat, F.; Pham, Q.M.; Ho, S.H.; Ren, N.Q.; Show, P.L. Biological remediation of acid mine drainage: Review of past trends and current outlook. Environ. Sci. Ecotechnol. 2020, 2, 100024. [Google Scholar] [CrossRef] [PubMed]
- Acharya, B.S.; Kharel, G. Acid mine drainage from coal mining in the United States—An overview. J. Hydrol. 2020, 588, 125061. [Google Scholar] [CrossRef]
- Abdullah, N.; Yusof, N.; Lau, W.J.; Jaafar, J.; Ismail, A.F. Recent trends of heavy metal removal from water/wastewater by membrane technologies. J. Ind. Eng. Chem. 2019, 76, 17–38. [Google Scholar] [CrossRef]
- Park, J.; Kwon, E.; Chung, E.; Kim, H.; Battogtokh, B.; Woo, N.C. Environmental Sustainability of Open-Pit Coal Mining Practices at Baganuur, Mongolia. Sustain. Sci. 2020, 12, 248. [Google Scholar] [CrossRef]
- Xu, R.; Li, B.Q.; Xiao, E.Z.; Young, L.Y.; Sun, X.X.; Kong, T.L.; Dong, Y.R.; Wang, Q.; Yang, Z.H.; Chen, L.; et al. Uncovering microbial responses to sharp geochemical gradients in a terrace contaminated by acid mine drainage. Environ. Pollut. 2020, 261, 114226. [Google Scholar] [CrossRef]
- Kim, N.; Park, D. Biosorptive treatment of acid mine drainage: A review. Int. J. Environ. Sci. Technol. 2022, 19, 9115–9128. [Google Scholar] [CrossRef]
- Tong, L.; Fan, R.G.; Yang, S.C.; Li, C.L. Development and Status of the Treatment Technology for Acid Mine Drainage. Min. Metall. Explor. 2021, 38, 315–327. [Google Scholar] [CrossRef]
- Ogbughalu, O.T.; Gerson, A.R.; Qian, G.J.; Smart, R.S.C.; Schumann, R.C.; Kawashima, N.; Fan, R.; Li, J.; Short, M.D. Heterotrophic Microbial Stimulation through Biosolids Addition for Enhanced Acid Mine Drainage Control. Minerals 2017, 7, 105. [Google Scholar] [CrossRef]
- Rezaie, B.; Anderson, A. Sustainable resolutions for environmental threat of the acid mine drainage. Sci. Total Environ. 2020, 717, 137211. [Google Scholar] [CrossRef] [PubMed]
- Magowo, W.E.; Sheridan, C.; Rumbold, K. Global Co-occurrence of Acid Mine Drainage and Organic Rich Industrial and Domestic Effluent: Biological sulfate reduction as a co-treatment-option. J. Water Process Eng. 2020, 38, 101650. [Google Scholar] [CrossRef]
- Ayangbenro, A.S.; Olanrewaju, O.S.; Babalola, O.O. Sulfate-Reducing Bacteria as an Effective Tool for Sustainable Acid Mine Bioremediation. Front. Microbiol. 2018, 9, 1986. [Google Scholar] [CrossRef] [PubMed]
- Chai, G.D.; Wang, D.Q.; Zhang, Y.T.; Wang, H.; Li, J.K.; Jing, X.S.; Meng, H.Y.; Wang, Z.; Guo, Y.; Jiang, C.B.; et al. Effects of organic substrates on sulfate-reducing microcosms treating acid mine drainage: Performance dynamics and microbial community comparison. J. Environ. Manag. 2023, 330, 117148. [Google Scholar] [CrossRef] [PubMed]
- Neculita, C.M.; Zagury, G.J.; Bussière, B. Passive treatment of acid mine drainage in bioreactors using sulfate-reducing bacteria: Critical review and research needs. J. Environ. Qual. 2007, 36, 1–16. [Google Scholar] [CrossRef]
- Zhang, M.J.; Wang, J.L.; Liu, X.Y.; Lv, Y.; Yu, B.B.; Gao, B.W.; Yan, X. Mine tailings remediation via sulfate-reducing bacteria and iron-reducing bacteria: Heavy metals immobilization and biological sealing. Chem. Ecol. 2022, 38, 706–719. [Google Scholar] [CrossRef]
- Jiang, Y.T.; Yang, F.; Dai, M.; Ali, I.; Shen, X.; Hou, X.T.; Alhewairini, S.S.; Peng, C.S.; Naz, I. Application of microbial immobilization technology for remediation of Cr (VI) contamination: A review. Chemosphere 2022, 286, 131721. [Google Scholar] [CrossRef]
- Zhang, M.L.; Wang, H.X.; Han, X.M. Preparation of metal-resistant immobilized sulfate reducing bacteria beads for acid mine drainage treatment. Chemosphere 2016, 154, 215–223. [Google Scholar] [CrossRef]
- Zhang, M.L.; Wang, H.X. Preparation of immobilized sulfate reducing bacteria (SRB) granules for effective bioremediation of acid mine drainage and bacterial community analysis. Miner. Eng. 2016, 92, 63–71. [Google Scholar] [CrossRef]
- An, W.B.; Hu, X.C.; Chen, H.; Wang, Q.Q.; Zheng, Y.L.; Wang, J.H.; Di, J.Z. Experimental study on the treatment of AMD by SRB immobilized particles containing “active iron” system. PLoS ONE 2023, 18, 0295616. [Google Scholar] [CrossRef]
- Ruehl, M.D.; Hiibel, S.R. Evaluation of organic carbon and microbial inoculum for bioremediation of acid mine drainage. Miner. Eng. 2020, 157, 106554. [Google Scholar] [CrossRef]
- Patel, H. Fixed-bed column adsorption study: A comprehensive review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Liu, J.; Yi, Z.J.; Ou, Z.L.; Yang, T.H. Removal of Cr(VI) and methyl orange by activated carbon fiber supported nanoscale zero-valent iron in a continuous fixed bed column. Water Sci. Technol. 2020, 82, 732–746. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Gupta, S.; Mondal, P. Modeling of continuous adsorption of greywater pollutants onto sawdust activated carbon bed integrated with sand column. J. Environ. 2022, 10, 107155. [Google Scholar] [CrossRef]
- Arshadi, M.; Abdolmaleki, M.K.; Eskandarloo, H.; Azizi, M.; Abbaspourrad, A. Synthesis of Highly Monodispersed, Stable, and Spherical NZVI of 20-30 nm on Filter Paper for the Removal of Phosphate from Wastewater: Batch and Column Study. ACS Sustain. Chem. Eng. 2018, 6, 11662–11676. [Google Scholar] [CrossRef]
- Golie, W.M.; Upadhyayula, S. Continuous fixed-bed column study for the removal of nitrate from water using chitosan/alumina composite. J. Water Process Eng. 2016, 12, 58–65. [Google Scholar] [CrossRef]
- Ansari, A.; Nadres, E.T.; Do, M.; Rodrigues, D.F. Investigation of the removal and recovery of nitrate by an amine-enriched composite under different fixed-bed column conditions. Process Saf. Environ. 2021, 150, 365–372. [Google Scholar] [CrossRef]
- Manjunath, S.V.; Kumar, M. Simultaneous removal of antibiotic and nutrients via Prosopis juliflora activated carbon column: Performance evaluation, effect of operational parameters and breakthrough modeling. Chemosphere 2021, 262, 127820. [Google Scholar] [CrossRef] [PubMed]
- Aksu, Z.; Gonen, F. Biosorption of phenol by immobilized activated sludge in a continuous packed bed: Prediction of breakthrough curves. Process Biochem. 2004, 39, 599–613. [Google Scholar] [CrossRef]
- Lv, N.; Li, X.F. Phosphorus removal from wastewater using Ca-modified attapulgite: Fixed-bed column performance and breakthrough curves analysis. J. Environ. Manag. 2023, 328, 116905. [Google Scholar] [CrossRef]
- Chowdhury, Z.Z.; Zain, S.M.; Rashid, A.K. Breakth rough curve analysis for column dynamics sorption of Mn(II) ions from wastewater by using mangostana garcinia peel-based granular activated carbon. J. Chem. 2013, 2013, 959761. [Google Scholar] [CrossRef]
- Fallah, N.; Taghizadeh, M. Continuous fixed-bed adsorption of Mo(VI) from aqueous solutions by Mo(VI)-IIP: Breakthrough curves analysis and mathematical modeling. J. Environ. 2020, 8, 104079. [Google Scholar] [CrossRef]
- Guo, X.Y.; Hu, Z.Y.; Fu, S.O.; Dong, Y.R.; Jiang, G.L.; Li, Y. Experimental study of the remediation of acid mine drainage by Maifan stones combined with SRB. PLoS ONE 2022, 17, e0261823. [Google Scholar]
- Wang, J.; Song, F.X.; Zhang, B.Q.; Cui, W.W.; Tang, L.; Wang, S.W.; Gao, H.T. Experimental design for spectrometric determination of adsorption performance of modified Maifanshi on recovered Cr3+ wastewater. Exp. Technol. Manag. 2023, 40, 197–202. (In Chinese) [Google Scholar] [CrossRef]
- Yang, H.; Luo, B.H.; Zhang, Y.; Zhou, B.X.; Ahmed, S.M.; Liu, H.M.; Liu, X.W.; He, Y.; Xia, S.B. Study of Humic Acid Adsorption Character on Natural Maifan Stone: Characterization, Kinetics, Adsorption Isotherm, and Thermodynamics. ACS Omega 2020, 5, 7683–7692. [Google Scholar] [CrossRef]
- Falayi, T.; Ntuli, F. Removal of heavy metals and neutralisation of acid mine drainage with un-activated attapulgite. J. Ind. Eng. Chem. 2014, 20, 1285–1292. [Google Scholar] [CrossRef]
- Dong, Y.R.; Di, J.Z.; Wang, M.X.; Ren, Y.D. Experimental study on the treatment of acid mine drainage by modified corncob fixed SRB sludge particles. RSC Adv. 2019, 9, 19016–19030. [Google Scholar] [CrossRef]
- GB5749-2006; Standards for Drinking Water Quality. Standardization Administration of China: Beijing, China, 2006.
- Di, J.; Li, T.; Zhao, W. Treatment acid mine drainage by sulfate reducing bacteria using different biomass carbon sources. J. China Coal Soc. 2019, 44, 1915–1922. (In Chinese) [Google Scholar] [CrossRef]
- Aranda-García, E.; Cristiani-Urbina, E. Effect of pH on hexavalent and total chromium removal from aqueous solutions by avocado shell using batch and continuous systems. Environ. Sci. Pollut. R. 2019, 26, 3157–3173. [Google Scholar] [CrossRef]
- Hanbali, M.; Holail, H.; Hammud, H. Remediation of lead by pretreated red algae: Adsorption isotherm, kinetic, column modeling and simulation studies. Green Chem. Lett. Rev. 2014, 7, 342–358. [Google Scholar] [CrossRef]
- Claude, B.J.; Onyango, M.S. Continuous fixed-bed column adsorption of nickel (II) using recyclable three-dimensional cellulose nanocrystals-hydrogel: Bed depth service time, Thomas, Adams-Bohart, and Yoon-Nelson modelling. Can. J. Chem. Eng. 2024, 102, 2160–2171. [Google Scholar] [CrossRef]
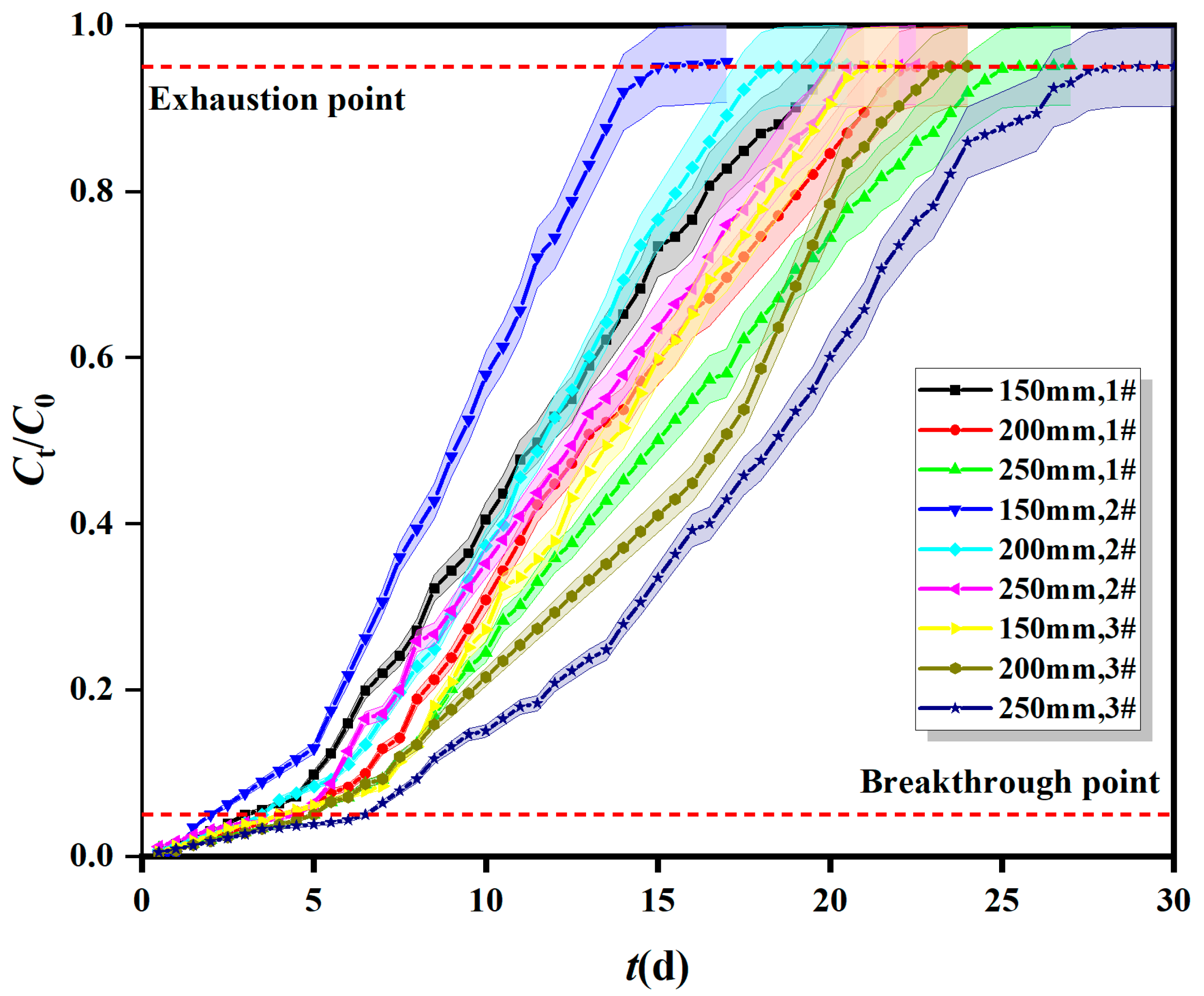
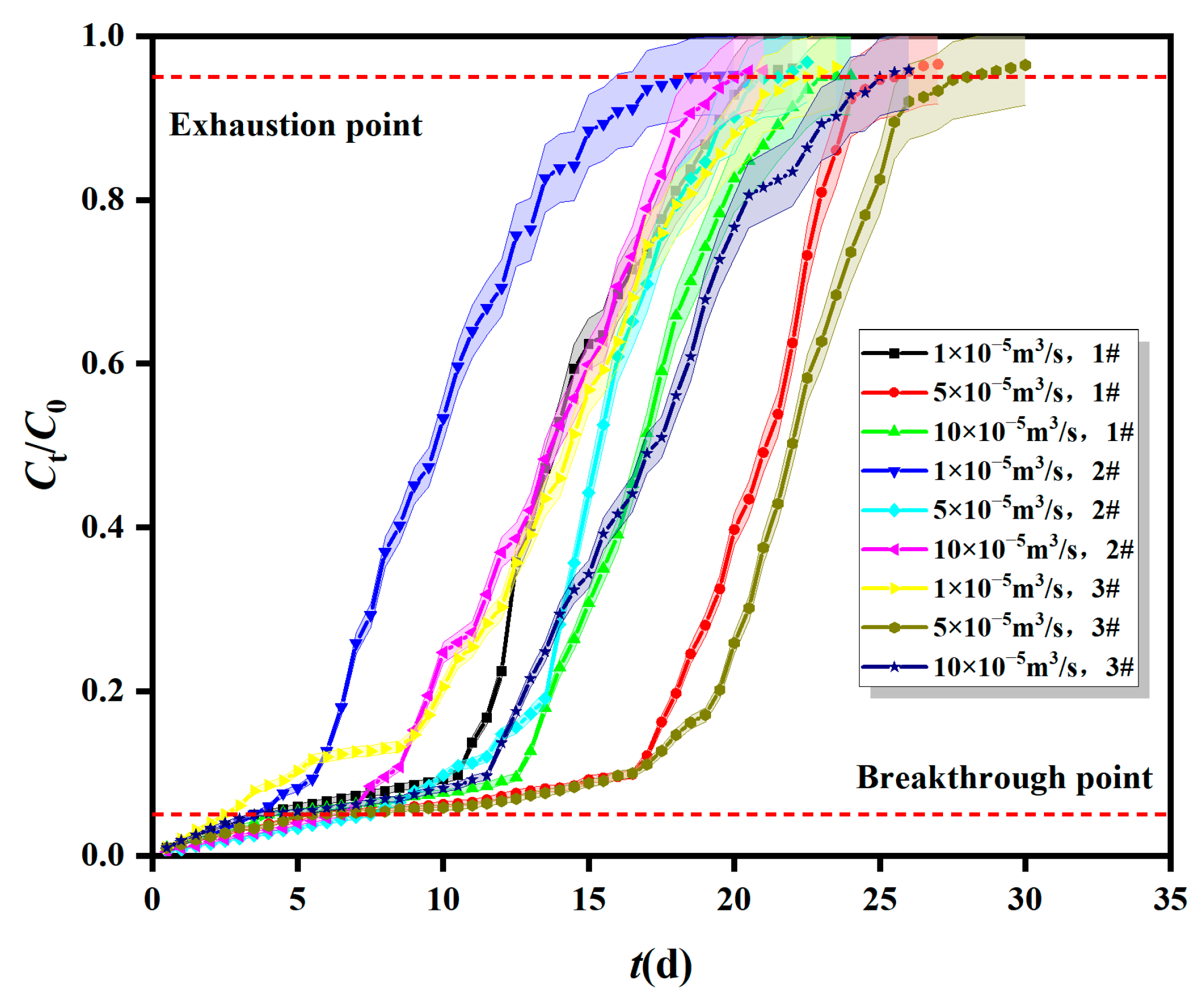
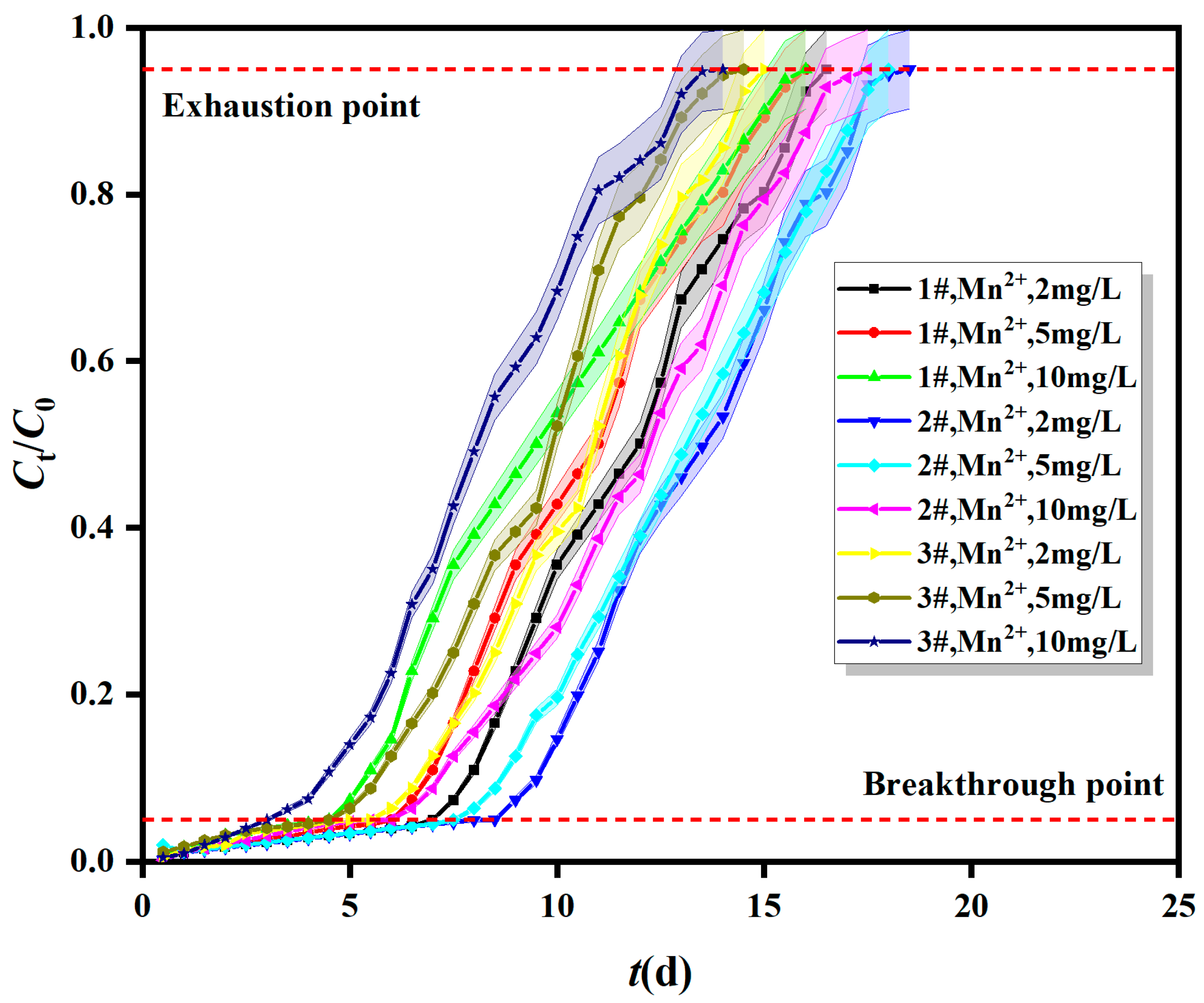


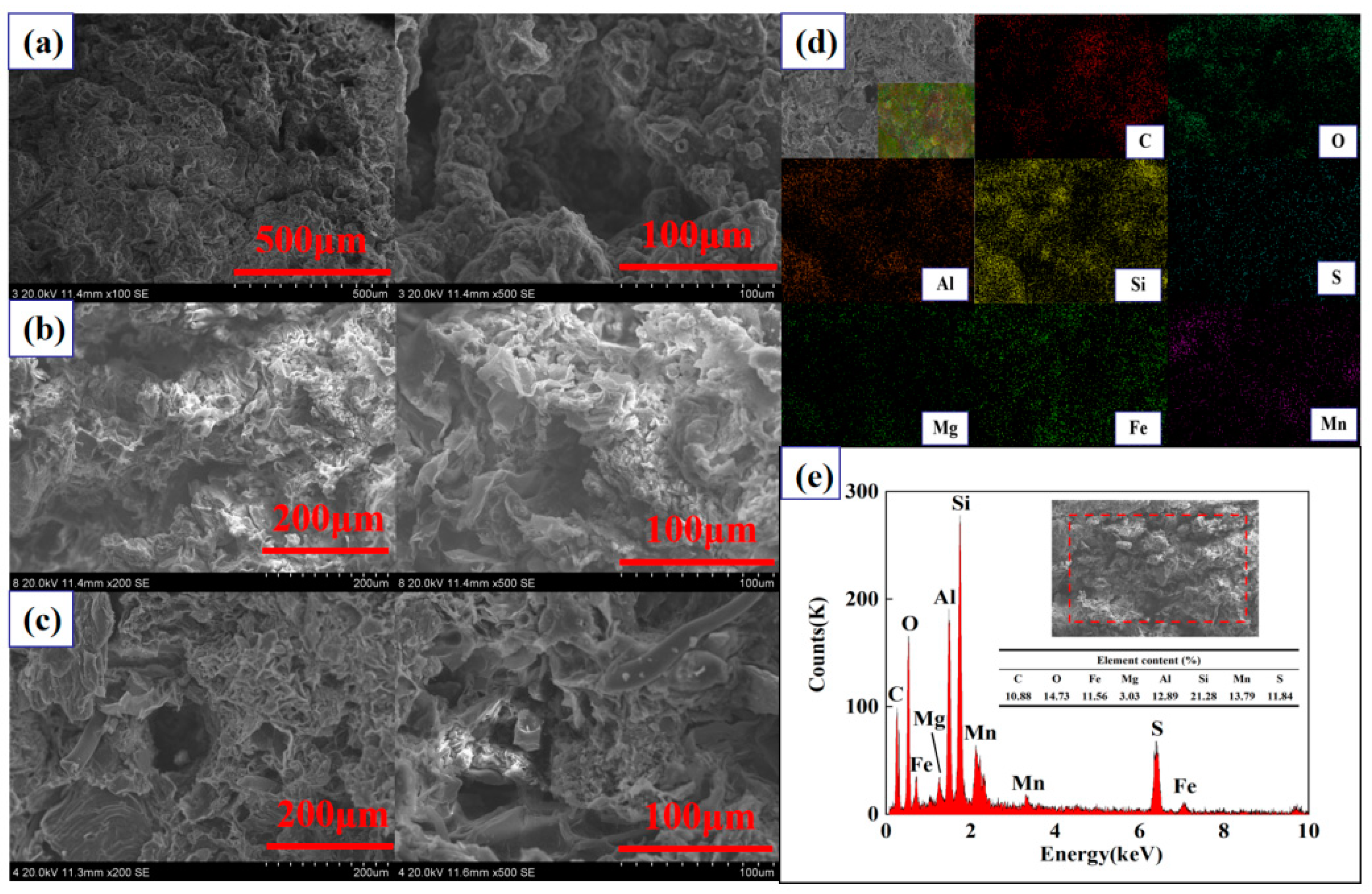

| Column | Different Adsorption Layer Heights | Different Inflow Velocity | Different Initial Concentrations | ||||||
|---|---|---|---|---|---|---|---|---|---|
| H (mm) | Qtotal (mg) | Qe (mg·g−1) | Q (m3·s−1) | Qtotal (mg) | Qe (mg·g−1) | C0 (mg·L−1) | Qtotal (mg) | Qe (mg·g−1) | |
| 1# | 150 | 1592.324 ± 79.616 | 5.3077 ± 0.2654 | 1 × 10−5 | 2015.303 ± 100.765 | 4.2879 ± 0.2144 | 2 | 258.265 ± 12.931 | 0.5495 ± 0.0275 |
| 200 | 1974.233 ± 78.712 | 4.2005 ± 0.2100 | 5 × 10−5 | 3686.187 ± 184.309 | 7.8430 ± 0.3922 | 5 | 561.209 ± 28.060 | 1.1941 ± 0.0597 | |
| 250 | 2387.693 ± 119.385 | 3.9143 ± 0.1957 | 10 × 10−5 | 2634.256 ± 131.713 | 5.6048 ± 0.2802 | 10 | 1002.388 ± 50.119 | 2.1327 ± 0.1066 | |
| 2# | 150 | 1086.530 ± 54.327 | 3.6218 ± 0.1181 | 1 × 10−5 | 1233.536 ± 61.677 | 2.6245 ± 0.1312 | 2 | 219.335 ± 10.967 | 0.4667 ± 0.0233 |
| 200 | 1523.578 ± 76.179 | 3.2417 ± 0.1621 | 5 × 10−5 | 2238.492 ± 111.925 | 4.7627 ± 0.2381 | 5 | 463.763 ± 23.188 | 0.9867 ± 0.0493 | |
| 250 | 1787.183 ± 89.359 | 2.9298 ± 0.1465 | 10 × 10−5 | 1883.551 ± 94.178 | 4.0076 ± 0.2004 | 10 | 755.581 ± 37.779 | 1.6076 ± 0.0804 | |
| 3# | 150 | 1958.153 ± 79.908 | 6.5272 ± 0.3264 | 1 × 10−5 | 2017.284 ± 100.864 | 4.2921 ± 0.2146 | 2 | 325.597 ± 16.280 | 0.6928 ± 0.0346 |
| 200 | 2452.581 ± 122.629 | 5.2183 ± 0.2609 | 5 × 10−5 | 4120.802 ± 206.040 | 8.7677 ± 0.4384 | 5 | 785.711 ± 39.286 | 1.6717 ± 0.0836 | |
| 250 | 3068.945 ± 153.447 | 5.0311 ± 0.2516 | 10 × 10−5 | 2719.803 ± 135.990 | 5.7868 ± 0.2893 | 10 | 1365.539 ± 68.277 | 2.8863 ± 0.1443 | |
| Fitting Parameters | Bohart–Adams Model | Thomas Model | ||||||
|---|---|---|---|---|---|---|---|---|
| H (mm) | Q (m3·s−1) | C0 (mg·L−1) | kAB (L·mg−1·min−1) | N0 (mg·L−1) | R2AB | kTh (L·mg−1·min−1) | q0 (mg·g−1) | R2Th |
| 150 | 1 × 10−5 | 10 | 8.341 × 10−6 | 4.086 | 0.914 | 3.314 × 10−6 | 6.554 | 0.989 |
| 200 | 1 × 10−5 | 10 | 7.254 × 10−6 | 3.947 | 0.923 | 3.105 × 10−6 | 5.252 | 0.978 |
| 250 | 1 × 10−5 | 10 | 5.247 × 10−6 | 3.214 | 0.955 | 2.494 × 10−6 | 5.055 | 0.991 |
| 200 | 1 × 10−5 | 10 | 5.264 × 10−6 | 3.524 | 0.825 | 2.483 × 10−6 | 4.320 | 0.985 |
| 200 | 5 × 10−5 | 10 | 6.584 × 10−6 | 8.259 | 0.893 | 3.096 × 10−6 | 9.054 | 0.994 |
| 200 | 10 × 10−5 | 10 | 5.748 × 10−6 | 6.058 | 0.875 | 2.785 × 10−6 | 5.783 | 0.986 |
| 200 | 1 × 10−5 | 2 | 8.547 × 10−6 | 0.351 | 0.904 | 19.844 × 10−6 | 0.702 | 0.995 |
| 200 | 1 × 10−5 | 5 | 7.458 × 10−6 | 1.458 | 0.897 | 6.623 × 10−6 | 1.672 | 0.994 |
| 200 | 1 × 10−5 | 10 | 6.251 × 10−6 | 2.225 | 0.910 | 4.963 × 10−6 | 2.875 | 0.988 |
| Producing Area | Composition and Content of Maifan Stone (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Na2O | Fe2O3 | CaO | K2O | MgO | TiO2 | MnO | P2O5 | Reference | |
| Shandong Province, Linyi | 67.90 | 15.75 | 5.45 | 2.82 | 2.51 | 1.59 | 0.66 | 0.32 | 0.06 | 0.056 | [33] |
| Shandong Province, Mengyin | 66.97 | 16.00 | 5.52 | 2.80 | 1.66 | 2.70 | 1.40 | — | — | — | [34] |
| Sample | Matrix Content (g) | Pore Structure Characteristics | |||||
|---|---|---|---|---|---|---|---|
| SRB | Iron Powder | Corncobs | Maifan Stones | Average Pore Volume (cm3·g−1) | Average Pore Diameter (nm) | Specific Surface Area (m2·g−1) | |
| Fe0/2+-SRB(1#) | 3.0 | 0.0 | 0.5 | 0.3 | 0.0153 | 7.8325 | 11.0725 |
| Fe0/2+-SRB(2#) | 0.0 | 0.6 | 0.0 | 0.3 | 0.0069 | 5.7564 | 9.5360 |
| Fe0/2+-SRB(3#) | 3.0 | 0.6 | 0.5 | 0.3 | 0.0325 | 10.3327 | 15.3420 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Wang, L.; An, W.; Wang, Q. Dynamic Adsorption of Mn2+ from Acid Mine Drainage by Highly Active Immobilized Particles with Fe0/Fe2+ Enhanced SRB. Molecules 2024, 29, 4497. https://doi.org/10.3390/molecules29184497
Chen H, Wang L, An W, Wang Q. Dynamic Adsorption of Mn2+ from Acid Mine Drainage by Highly Active Immobilized Particles with Fe0/Fe2+ Enhanced SRB. Molecules. 2024; 29(18):4497. https://doi.org/10.3390/molecules29184497
Chicago/Turabian StyleChen, He, Laigui Wang, Wenbo An, and Qiqi Wang. 2024. "Dynamic Adsorption of Mn2+ from Acid Mine Drainage by Highly Active Immobilized Particles with Fe0/Fe2+ Enhanced SRB" Molecules 29, no. 18: 4497. https://doi.org/10.3390/molecules29184497
APA StyleChen, H., Wang, L., An, W., & Wang, Q. (2024). Dynamic Adsorption of Mn2+ from Acid Mine Drainage by Highly Active Immobilized Particles with Fe0/Fe2+ Enhanced SRB. Molecules, 29(18), 4497. https://doi.org/10.3390/molecules29184497






