Unveiling the Influences of In Situ Carbon Content on the Structure and Electrochemical Properties of MoS2/C Composites
Abstract
:1. Introduction
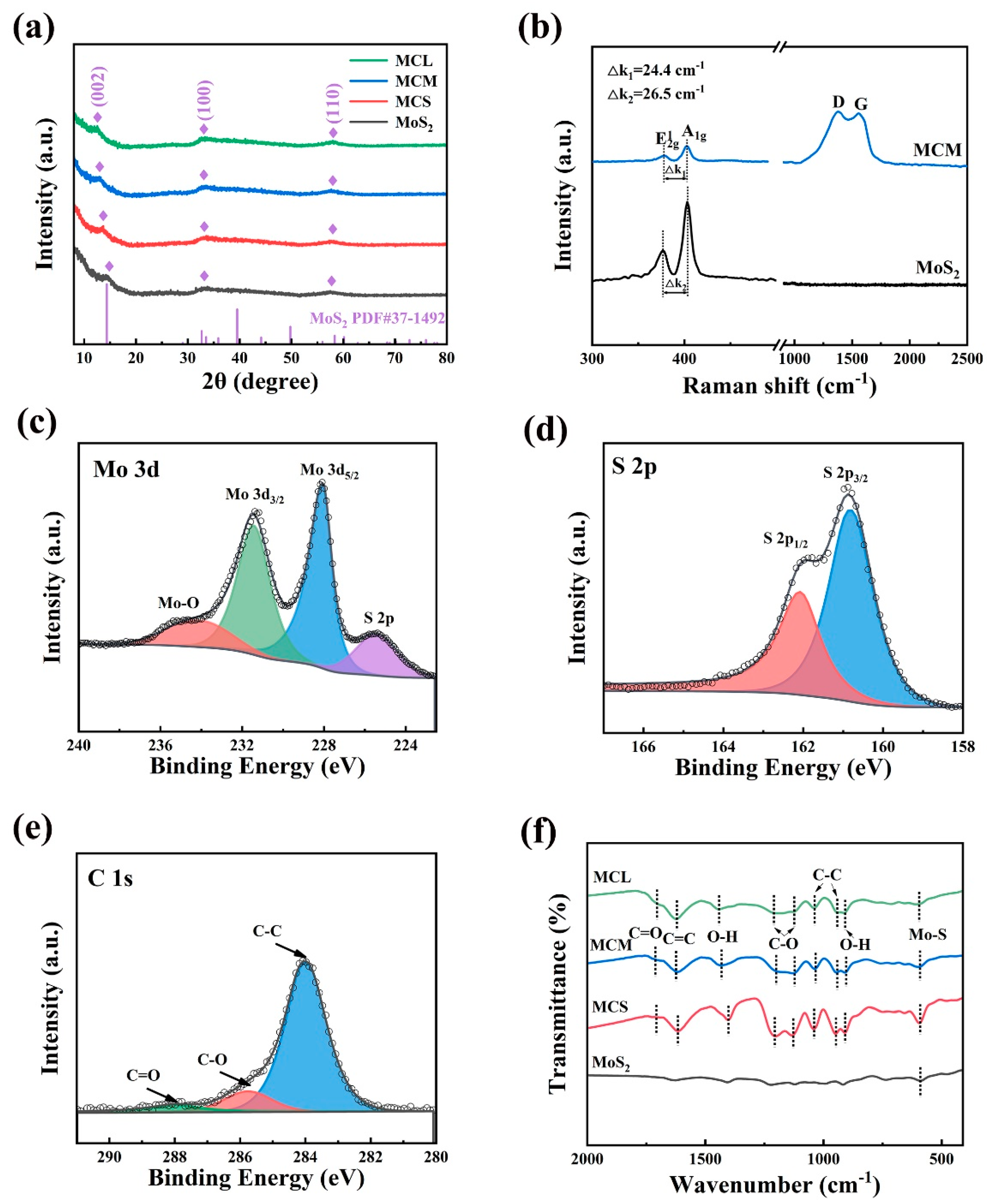
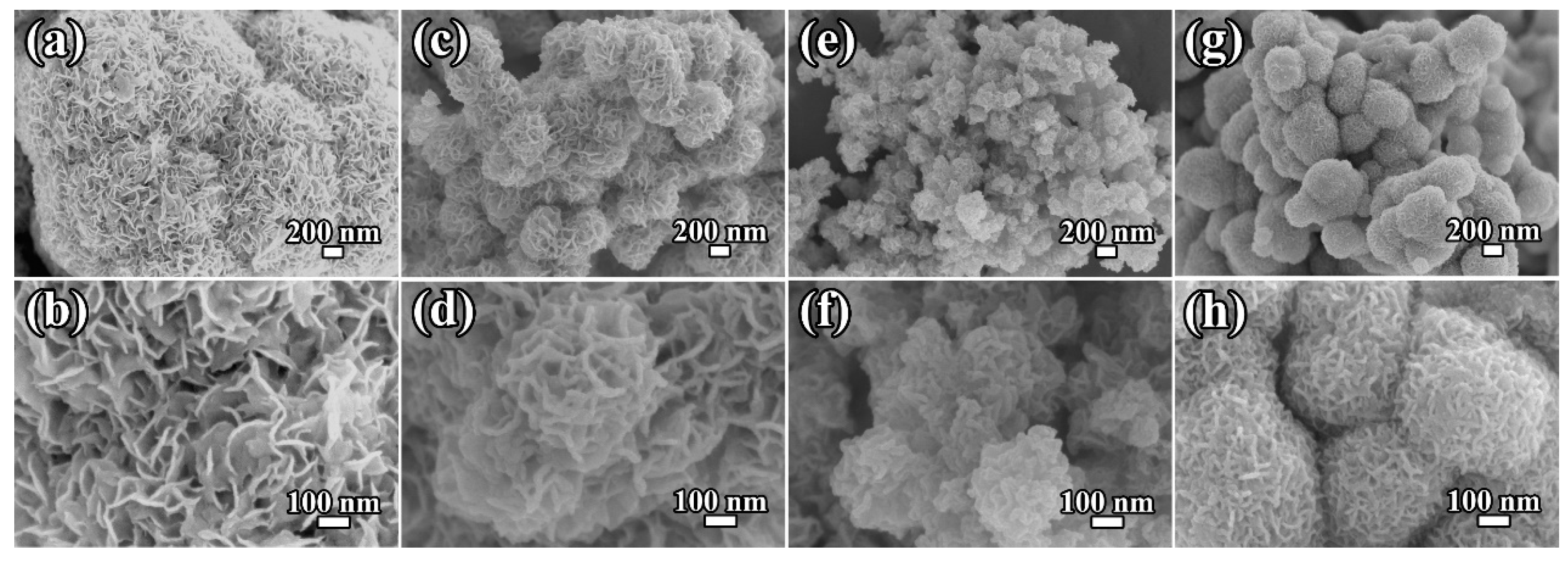
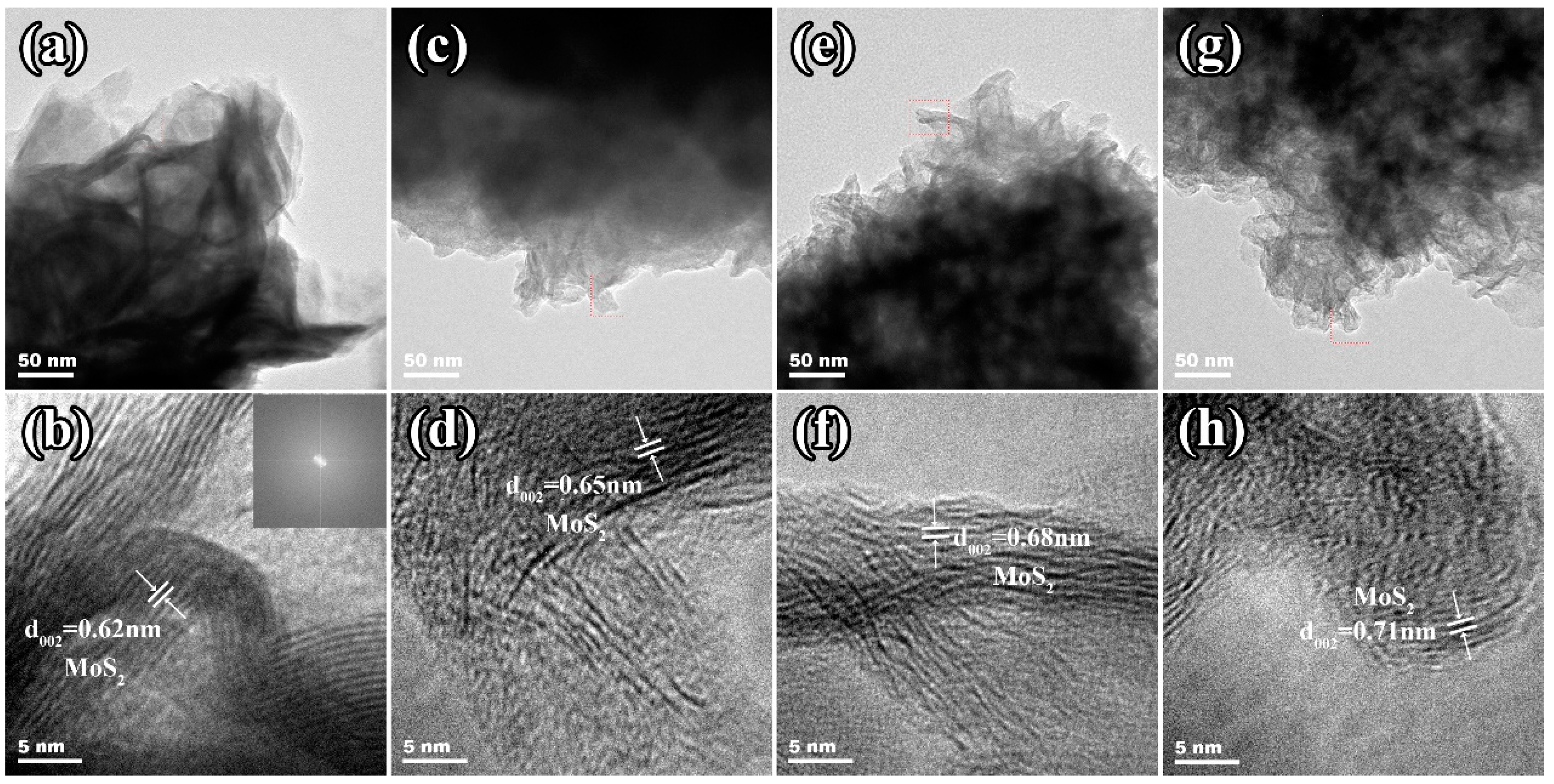
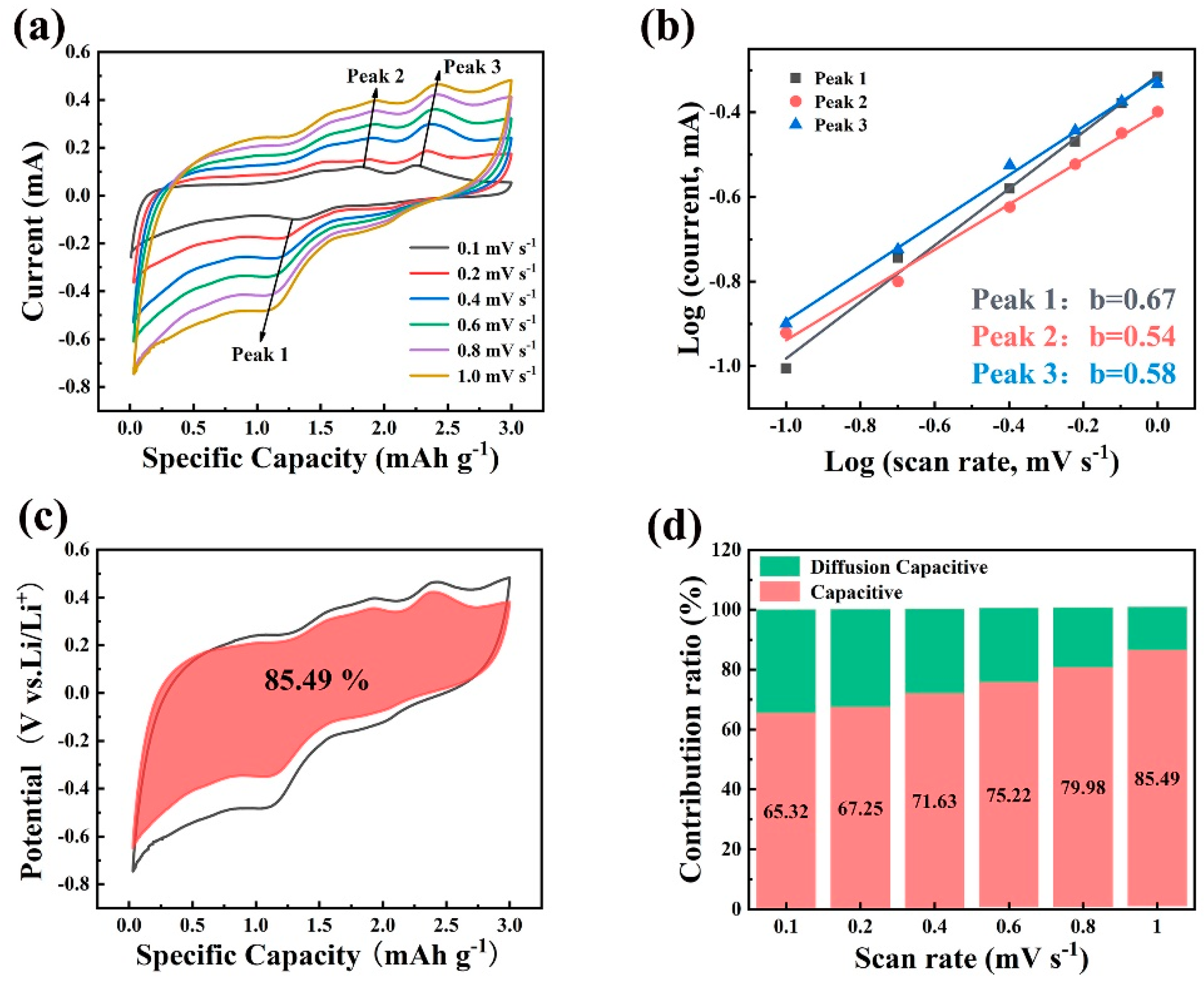
2. Results and Discussion
3. Experimental Section
3.1. Materials Synthesis
3.2. Material Characterization
3.3. Electrochemical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, G.; Shi, Y.; Jiang, S.; Pang, H. Recent Progress in MOF-Derived Porous Materials as Electrodes for High-Performance Lithium-Ion Batteries. Adv. Funct. Mater. 2023, 33, 2303121. [Google Scholar] [CrossRef]
- Liu, Q.; Su, X.; Lei, D.; Qin, Y.; Wen, J.; Guo, F.; Wu, Y.A.; Rong, Y.; Kou, R.; Xiao, X. Approaching the capacity limit of lithium cobalt oxide in lithium ion batteries via lanthanum and aluminium doping. Nat. Energy 2018, 3, 936–943. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, S.; Ma, J.; Das, P.; Zheng, S.; Wu, Z.-S. Recent status and future perspectives of 2D MXene for micro-supercapacitors and micro-batteries. Energy Storage Mater. 2022, 51, 500–526. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, K.; Zhang, B.; Wang, Z.; Wang, Y. Unveiling the effects of different component ratios on the structure and electrochemical properties of MoS2/TiO2 composites. Ceram. Int. 2024, 50, 26750–26759. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, S.; Luo, W.; Li, K.; Yang, X. N-Containing Porous Carbon-Based MnO Composites as Anode with High Capacity and Stability for Lithium-Ion Batteries. Molecules 2024, 29, 2939. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Yang, Y.; Lv, W.; Lian, G.; Golberg, D.; Wang, X.; Zhao, X.; Ding, Y. A MoS2/Carbon hybrid anode for high-performance Li-ion batteries at low temperature. Nano Energy 2020, 70, 104550. [Google Scholar] [CrossRef]
- Wu, L.; Feng, L.; Mao, X.; Niu, J.; Xin, W.; Wang, D. Construction of porous MoS2Mo2C@C aerogel for use as superior lithium-ion battery anode. J. Energy Storage 2023, 70, 108011. [Google Scholar] [CrossRef]
- Zhong, W.; Hong, J.; Wang, C.; Li, Z.; Chen, J.; Dmytro, S. MoS2/graphene nanosheet composites prepared by xylitol-assisted ball milling as high-performance anode materials for lithium-ion batteries. Ionics 2023, 29, 917–930. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Das, P.; Wu, Z.-S. Recent advances in interface engineering and architecture design of air-stable and water-resistant lithium metal anodes. Energy Fuels 2021, 35, 12902–12920. [Google Scholar] [CrossRef]
- Baheri, Y.T.; Maleki, M.; Karimian, H.; Javadpoor, J.; Masoudpanah, S.M. Well-distributed 1T/2H MoS2 nanocrystals in the N-doped nanoporous carbon framework by direct pyrolysis. Sci. Rep. 2023, 13, 7492. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, B.; Shi, C.; Cui, Y. 1T-MoS2/C composite as an efficient electrocatalyst for hydrogen evolution reaction under alkaline condition. J. Phys. Chem. Solids 2024, 185, 111796. [Google Scholar] [CrossRef]
- Fayed, M.G.; Attia, S.Y.; Barakat, Y.F.; El-Shereafy, E.; Rashad, M.; Mohamed, S.G. Carbon and nitrogen co-doped MoS2 nanoflakes as an electrode material for lithium-ion batteries and supercapacitors. Sustain. Mater. Technol. 2021, 29, e00306. [Google Scholar] [CrossRef]
- Zhu, W.; Shi, C.; Zhang, B.; Wang, Y.; Hu, Y.; Liu, K. 3D porous flower-like MoS2 grows on carbon cloth and used as anode material for lithium-ion batteries. Solid State Ion. 2023, 402, 116381. [Google Scholar] [CrossRef]
- He, X.; Wang, R.; Yin, H.; Zhang, Y.; Chen, W.; Huang, S. 1T-MoS2 monolayer as a promising anode material for (Li/Na/Mg)-ion batteries. Appl. Surf. Sci. 2022, 584, 152537. [Google Scholar] [CrossRef]
- Mi, Z.; Hu, D.; Lin, J.; Pan, H.; Chen, Z.; Li, Y.; Liu, Q.; Zhu, S. Anchoring nanoarchitectonics of 1T’-MoS2 nanoflakes on holey graphene sheets for lithium-ion batteries with outstanding high-rate performance. Electrochim. Acta 2022, 403, 139711. [Google Scholar] [CrossRef]
- Zhu, W.; Shi, C.; Wang, Y.; Hu, Y.; Liu, K. Glucose assisted synthesis of 1T-MoS2/C composite anode for high-performance lithium-ion batteries. Diam. Relat. Mater. 2022, 130, 109436. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Peng, L.; Cheng, L.; An, B.; Wang, Y.; Wei, Q.; Xia, X.; Zhou, H. Study on the construction of interlayer adjustable C@MoS2 fiber anode by biomass confining and its lithium/sodium storage mechanism. ChemSusChem 2023, 16, e202300576. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R.; Guo, Y.; Li, Y.; Li, K. A review on MoS2 structure, preparation, energy storage applications and challenges. J. Alloys Compd. 2024, 998, 174916. [Google Scholar] [CrossRef]
- Li, Z.; Han, M.; Zhang, Y.; Yuan, F.; Fu, Y.; Yu, J. Single-Layered MoS2 Fabricated by Charge-Driven Interlayer Expansion for Superior Lithium/Sodium/Potassium-Ion-Battery Anodes. Adv. Sci. 2023, 10, 2207234. [Google Scholar] [CrossRef]
- Dang, L.; Yuan, Y.; Wang, Z.; Li, H.; Yang, R.; Fu, A.; Liu, X.; Li, H. Carbon nanofibers decorated by MoS2 nanosheets with tunable quantity as self-supporting anode for high-performance lithium ion batteries. Nanomaterials 2023, 13, 2689. [Google Scholar] [CrossRef]
- Zhang, Q.; Yao, T.; He, Q.; Wang, H.; Liu, Z.; Wang, D.; Wang, H.; Meng, L. Enhancing lithium-ion storage performance of hollow CoS2/MoS2 nanospheres via N-doped carbon-coating. J. Energy Storage 2023, 72, 108639. [Google Scholar] [CrossRef]
- Baheri, Y.T.; Hedayati, M.A.; Maleki, M.; Karimian, H. A vapor-liquid-solid mechanism for in-situ deposition of ultra-small hollow MoS2 nanoparticles in N-doped carbon foam as an anode of lithium-ion batteries. J. Energy Storage 2023, 68, 107682. [Google Scholar] [CrossRef]
- Al-Ansi, N.; Salah, A.; Drmosh, Q.A.; Yang, G.D.; Hezam, A.; Al-Salihy, A.; Lin, J.; Wu, X.L.; Zhao, L.; Zhang, J.P. Carbonized Polymer Dots for Controlling Construction of MoS2 Flower-Like Nanospheres to Achieve High-Performance Li/Na Storage Devices. Small 2023, 19, 2304459. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Yu, M.; Liu, X.; Qian, J.; Qian, W.; Chen, Z.; Wu, Z. Plant-cell oriented few-layer MoS2/C as high performance anodes for lithium-ion batteries. Electrochim. Acta 2022, 424, 140685. [Google Scholar] [CrossRef]
- Li, B.; Chuan, X.; Yang, Y.; Liu, F.; Chen, S.; Li, X. MoS2/C nanotubes synthesized using halloysite as template through one-pot hydrothermal method for Li-ion batteries. J. Alloys Compd. 2022, 923, 166314. [Google Scholar] [CrossRef]
- Han, M.; Chen, J.; Cai, Y.; Wei, L.; Zhao, T. Magnetic-atom strategy enables unilamellar MoS2-C interoverlapped superstructure with ultrahigh capacity and ultrafast ion transfer capability in Li/Na/K-ion batteries. Chem. Eng. J. 2023, 454, 140137. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Huang, S.; Zhang, Y.; Chen, Z. Integrated design of sandwich-like C@MoS2@C nanospheres as active anode material for lithium-ion batteries. J. Mater. Sci. 2022, 57, 14948–14958. [Google Scholar] [CrossRef]
- Shaker, M.; Sadeghi Ghazvini, A.A.; Riahifar, R.; Mumtaz, A. On the relationship between the porosity and initial coulombic efficiency of porous carbon materials for the anode in lithium-ion batteries. Electron. Mater. Lett. 2022, 18, 400–406. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, K.; Zhang, H.; Wang, X.; Zhou, Y.; He, W.; Cui, J.; Sun, J. Constructing Biomass-Based Ultrahigh-Rate Performance SnOy@C/SiOx Anode for LIBs via Disproportionation Effect. Small 2023, 19, 2204867. [Google Scholar] [CrossRef]
- Zhu, W.; Shi, C.; Zhao, J.; Wang, Y.; Hu, Y. Structure and electrochemical performance of MoS2 based on different molybdenum-sulfur mole ratios. J. Phys. Chem. Solids 2022, 167, 110749. [Google Scholar] [CrossRef]
- Kumar, G.; Francis, M.K.; Bhargav, P.B.; Ahmed, N. Silicon infused Molybedenum di-selinide (MoSe2) nanosheets for enhanced hydrogen evolution reaction (HER) and lithium-ion battery (LIB) applications. Int. J. Hydrogen Energy 2024, 51, 1448–1461. [Google Scholar] [CrossRef]
- da Silva, A.L.; Pereira, H.d.L.; Sales, H.B.; Dionízio, J.K.; Alves, M.C.F.; Guedes, D.G.; Luna, C.B.B.; Costa, A.C.F.d.M. Optimization of Biodiesel Production Process Using MoO3 Catalysts and Residual Oil: A Comprehensive Experimental 23 Study. Molecules 2024, 29, 2404. [Google Scholar] [CrossRef] [PubMed]
- Matveev, A.T.; Konopatsky, A.S.; Leybo, D.V.; Volkov, I.N.; Kovalskii, A.M.; Varlamova, L.A.; Sorokin, P.B.; Fang, X.; Kulinich, S.A.; Shtansky, D.V. Amorphous MoSxOy/h-BNxOy nanohybrids: Synthesis and dye photodegradation. Nanomaterials 2021, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-U.; Park, J.; Son, Y.-W.; Cheong, H. Anomalous excitonic resonance Raman effects in few-layered MoS2. Nanoscale 2015, 7, 3229–3236. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhao, J.; Tao, X. MoS2-carbon based nanocomposites as anodes for lithium-ion batteries: A review. J. Energy Storage 2024, 84, 110934. [Google Scholar] [CrossRef]
- Sarkar, D.; Das, D.; Das, S.; Kumar, A.; Patil, S.; Nanda, K.K.; Sarma, D.; Shukla, A. Expanding interlayer spacing in MoS2 for realizing an advanced supercapacitor. ACS Energy Lett. 2019, 4, 1602–1609. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Khaidarov, A.A.; Pavlikov, A.V.; Minnebaev, K.F. The field-effect transistor based on a polyyne–polyene structure obtained via PVDC dehydrochlorination. J. Compos. Sci. 2023, 7, 264. [Google Scholar] [CrossRef]
- Du, J.; Yang, Z.; Wang, X.; Qi, C.; Li, Y.; Mao, W.; Qiao, H.; Yu, Z.; Ren, T.; Qiao, Q. Fabrication of multilayered-sandwich MoS2/c architectures with advanced lithium storage properties. Electrochim. Acta 2017, 250, 238–243. [Google Scholar] [CrossRef]
- Li, Z.; Liu, S.; Vinayan, B.P.; Zhao-Karger, Z.; Diemant, T.; Wang, K.; Behm, R.J.; Kuebel, C.; Klingeler, R.; Fichtner, M. Hetero-layered MoS2/C composites enabling ultrafast and durable Na storage. Energy Storage Mater. 2019, 21, 115–123. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Song, Z.; Gong, Z.; Guo, X.; Liu, J.; Zhang, Z.; Li, G. Carbon confinement synthesis of interlayer-expanded and sulfur-enriched MoS2+x nanocoating on hollow carbon spheres for advanced Li-S batteries. Nano Res. 2019, 12, 2908–2917. [Google Scholar] [CrossRef]
- Ni, X.; Chen, H.; Liu, C.; Zeng, F.; Yu, H.; Ju, A. A freestanding nitrogen-doped carbon nanofiber/MoS2 nanoflowers with expanded interlayer for long cycle-life lithium-ion batteries. J. Alloys Compd. 2020, 818, 152835. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, J.; Li, J.; Wu, H.; Zhang, Y.; Liu, H. A flexible three-dimensional MoS2/carbon architecture derived from melamine foam as free-standing anode for high performance lithium-ion batteries. Appl. Surf. Sci. 2018, 462, 337–343. [Google Scholar] [CrossRef]
- Yu, W.; Cui, B.; Han, J.; Zhu, S.; Xu, X.; Tan, J.; Xu, Q.; Min, Y.; Peng, Y.; Liu, H. In Situ Encapsulation of SnS2/MoS2 Heterojunctions by Amphiphilic Graphene for High-Energy and Ultrastable Lithium-Ion Anodes. Adv. Sci. 2024, e2405135. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, N.; Wang, S.; Li, Y.; Liang, C.; Yu, K. 3D nanoflower-like MoS2 grown on wheat straw cellulose carbon for lithium-ion battery anode material. J. Alloys Compd. 2023, 933, 167689. [Google Scholar] [CrossRef]
- Shi, X.; Liu, M.; Gu, T.; Han, J.; Ren, R.-P.; Lv, Y.-K.; Ren, J. Fe single atoms-nitrogen doped carbon modified separator with promoted catalytic conversion for MoS2 electrode in lithium-ion batteries. J. Alloys Compd. 2023, 960, 170938. [Google Scholar] [CrossRef]
- Chen, X.; Wang, X.; Fang, D. A review on C1s XPS-spectra for some kinds of carbon materials. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 1048–1058. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, H.; Huang, Z.; Li, S.; Wang, C.; Li, H.; Yan, Z.; Hou, T.; Kuang, Y. 3D hierarchical microspheres constructed by ultrathin MoS2-C nanosheets as high-performance anode material for sodium-ion batteries. J. Energy Chem. 2020, 49, 307–315. [Google Scholar] [CrossRef]
- Zhang, R.; Li, H.; Sun, D.; Luan, J.; Huang, X.; Tang, Y.; Wang, H. Facile preparation of robust porous MoS2/C nanosheet networks as anode material for sodium ion batteries. J. Mater. Sci. 2019, 54, 2472–2482. [Google Scholar] [CrossRef]
- Lu, Y.; Zhao, Q.; Zhang, N.; Lei, K.; Li, F.; Chen, J. Facile spraying synthesis and high-performance sodium storage of mesoporous MoS2/C microspheres. Adv. Funct. Mater. 2016, 26, 911–918. [Google Scholar] [CrossRef]
- Streletskiy, O.A.; Zavidovskiy, I.A.; Nuriahmetov, I.F.; Nishchak, O.Y.; Pavlikov, A.V.; Savchenko, N.F. Resistive Gas Sensors Based on Porous Sp-Containing Films Obtained by Dehydrohalogenation of PVDC and PVDC-PVC Copolymer. C 2023, 9, 82. [Google Scholar] [CrossRef]
- Rahmatinejad, J.; Liu, X.; Raisi, B.; Ye, Z. Synergistic Cathode Design for High-Performance Dual-Salt Magnesium/Lithium-Ion Batteries Using 2D/2D 1T/2H-MoS2@Ti3C2Tx MXene Nanocomposite. Small 2024, 20, 2401391. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, L.; Tan, H.; Xie, F.; Qu, Y.; Hu, J.; Gao, K.; Shi, X.; Wang, K.; Zhang, Y. Double-phase 1T/2H–MoS2 heterostructure loaded in N-doped carbon/CNT complex carbon for efficient and rapid lithium storage. Mater. Today Energy 2022, 29, 101103. [Google Scholar] [CrossRef]
- Guan, X.; Zhao, L.; Zhang, P.; Liu, J.; Song, X.; Gao, L. Electrode material of core-shell hybrid MoS2@C/CNTs with carbon intercalated few-layer MoS2 nanosheets. Mater. Today Energy 2020, 16, 100379. [Google Scholar] [CrossRef]
- Ye, S.; Yang, Z.; Ye, Y.; Cheng, Z.; Hong, H.; Zeng, Z.; Meng, Z.; Lan, Q.; Zhang, H.; Chen, Y. Forming SnS@C/MoS2 nanotubes with high specific surface area via self-sacrificing template method as superior performance anode for lithium-ion batteries. CrystEngComm 2024, 26, 1779–1788. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Jia, Q.-C.; Kong, L.-B. Multi-dimensional hybrid heterostructure MoS2@C nanocomposite as a highly reversible anode for high-energy lithium-ion capacitors. Appl. Surf. Sci. 2020, 531, 147222. [Google Scholar] [CrossRef]
- Liu, X.; Tan, J.; Li, X.; Zhang, C. Glucose-assisted one-pot hydrothermal synthesis of hierarchical-structured MoS2/C quasi-hollow microspheres for high-performance lithium ion battery. Polymers 2021, 13, 837. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, K.; Na, R.; Liu, X.; Shan, Z.; Wang, S. Preparation and Lithium Storage Properties of Hierarchical Hydrangea-Like MoS2/C Composites. Energy Technol. 2022, 10, 2101136. [Google Scholar] [CrossRef]
- Song, D.; Yu, J.; Wang, M.; Tan, Q.; Liu, K.; Li, J. Advancing recycling of spent lithium-ion batteries: From green chemistry to circular economy. Energy Storage Mater. 2023, 61, 102870. [Google Scholar] [CrossRef]
- Liu, J.; Yue, M.; Wang, S.; Zhao, Y.; Zhang, J. A review of performance attenuation and mitigation strategies of lithium-ion batteries. Adv. Funct. Mater. 2022, 32, 2107769. [Google Scholar] [CrossRef]
- Wu, W.; Wang, J.; Deng, Q.; Luo, H.; Li, Y.; Wei, M. Low crystalline 1T-MoS2@S-doped carbon hollow spheres as an anode material for Lithium-ion battery. J. Colloid Interface Sci. 2021, 601, 411–417. [Google Scholar] [CrossRef]
- Wang, L.; Han, J.; Kong, D.; Tao, Y.; Yang, Q.-H. Enhanced roles of carbon architectures in high-performance lithium-ion batteries. Nano-Micro Lett. 2019, 11, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, Y.; Qin, J.; Shi, C.; Liu, E.; Zhao, N. Capacitance controlled, hierarchical porous 3D ultra-thin carbon networks reinforced prussian blue for high performance Na-ion battery cathode. Nano Energy 2019, 58, 192–201. [Google Scholar] [CrossRef]
- Faizan, M.; Hussain, S.; Vikraman, D.; Ali, B.; Kim, H.-S.; Jung, J.; Nam, K.-W. MoS2@Mo2C hybrid nanostructures formation as an efficient anode material for lithium-ion batteries. J. Mater. Res. Technol. 2021, 14, 2382–2393. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Kim, I.T. Self-assembled few-layered MoS2 on SnO2 anode for enhancing lithium-ion storage. Nanomaterials 2020, 10, 2558. [Google Scholar] [CrossRef]


| Materials | Cyclic Performance (mAh g−1/A g−1) | Rate Performance (mAh g−1/A g−1) | Refs |
|---|---|---|---|
| MoS2 nanoflakes | 530/0.1 (after 100 cycles) | 1080/0.1, 260/1, 400/0.1 | [12] |
| MoS2@Mo2C | 145/0.05 (after 100 cycles) | 210/0.01, 89/0.2, 210/0.01 | [63] |
| MoS2@SnO2 | 277/0.1 (after 100 cycles) | 600/0.01, 290/0.1, 510/0.01 | [64] |
| MoS2/C | 790/0.1 (after 50 cycles) | 854.3/0.1, 140.9/3, 734.2/0.1 | [6] |
| MCM | 411.7/0.5 (after 100 cycles) | 1124/0.1, 585/1, 742/0.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Zhao, J.; Zhang, H.; Tian, J.; Cui, Y.; Zhu, W. Unveiling the Influences of In Situ Carbon Content on the Structure and Electrochemical Properties of MoS2/C Composites. Molecules 2024, 29, 4513. https://doi.org/10.3390/molecules29184513
Zhang B, Zhao J, Zhang H, Tian J, Cui Y, Zhu W. Unveiling the Influences of In Situ Carbon Content on the Structure and Electrochemical Properties of MoS2/C Composites. Molecules. 2024; 29(18):4513. https://doi.org/10.3390/molecules29184513
Chicago/Turabian StyleZhang, Bofeng, Junyao Zhao, He Zhang, Jian Tian, Yang Cui, and Wenjun Zhu. 2024. "Unveiling the Influences of In Situ Carbon Content on the Structure and Electrochemical Properties of MoS2/C Composites" Molecules 29, no. 18: 4513. https://doi.org/10.3390/molecules29184513







