Experimental and Theoretical Studies on the Adsorption of Bromocresol Green from Aqueous Solution Using Cucumber Straw Biochar
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Characterization of Biochar
2.1.1. Pore Structure Analysis
2.1.2. Powder X-ray Diffraction Studies
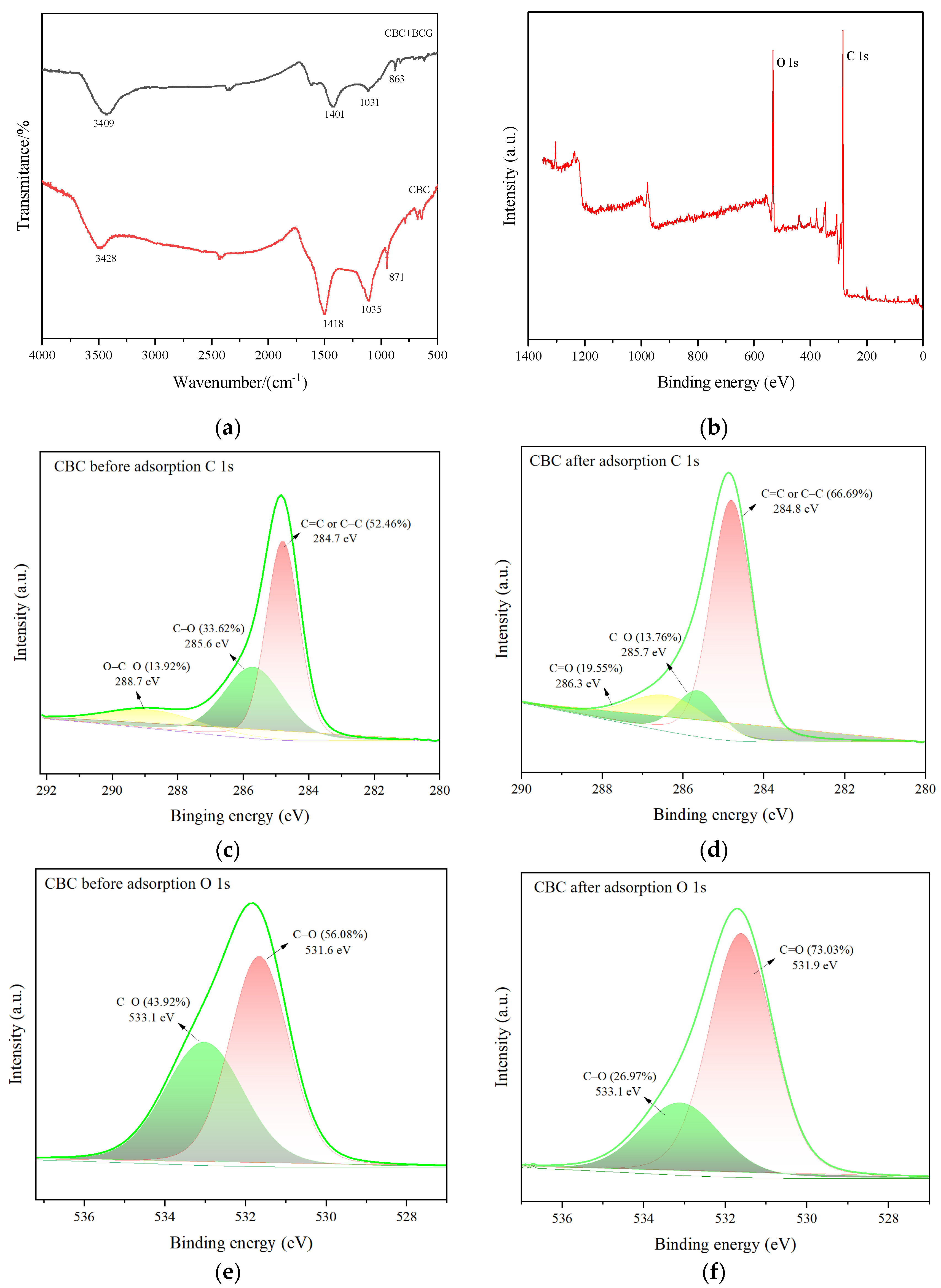
2.1.3. X-ray Photoelectron Spectroscopy and Fourier Transform Infrared Spectroscopy Analysis
2.2. Adsorption Characteristics of CBC for BCG
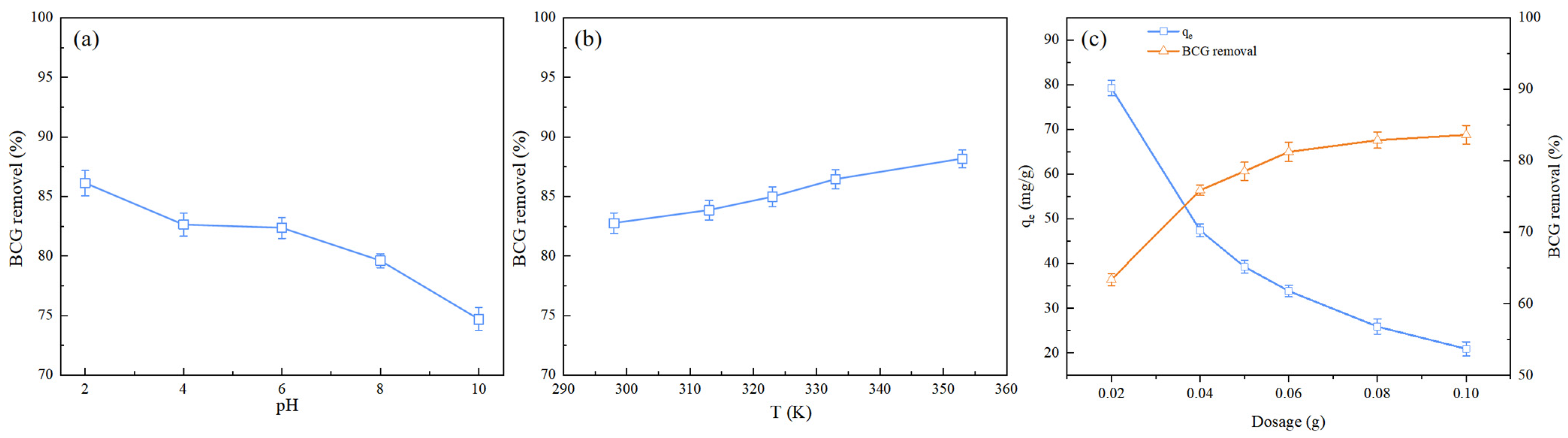
2.2.1. Effect of Initial Solution pH
2.2.2. Effect of Temperature
2.2.3. Effect of Biochar Dosage
2.3. Adsorption Model Analysis
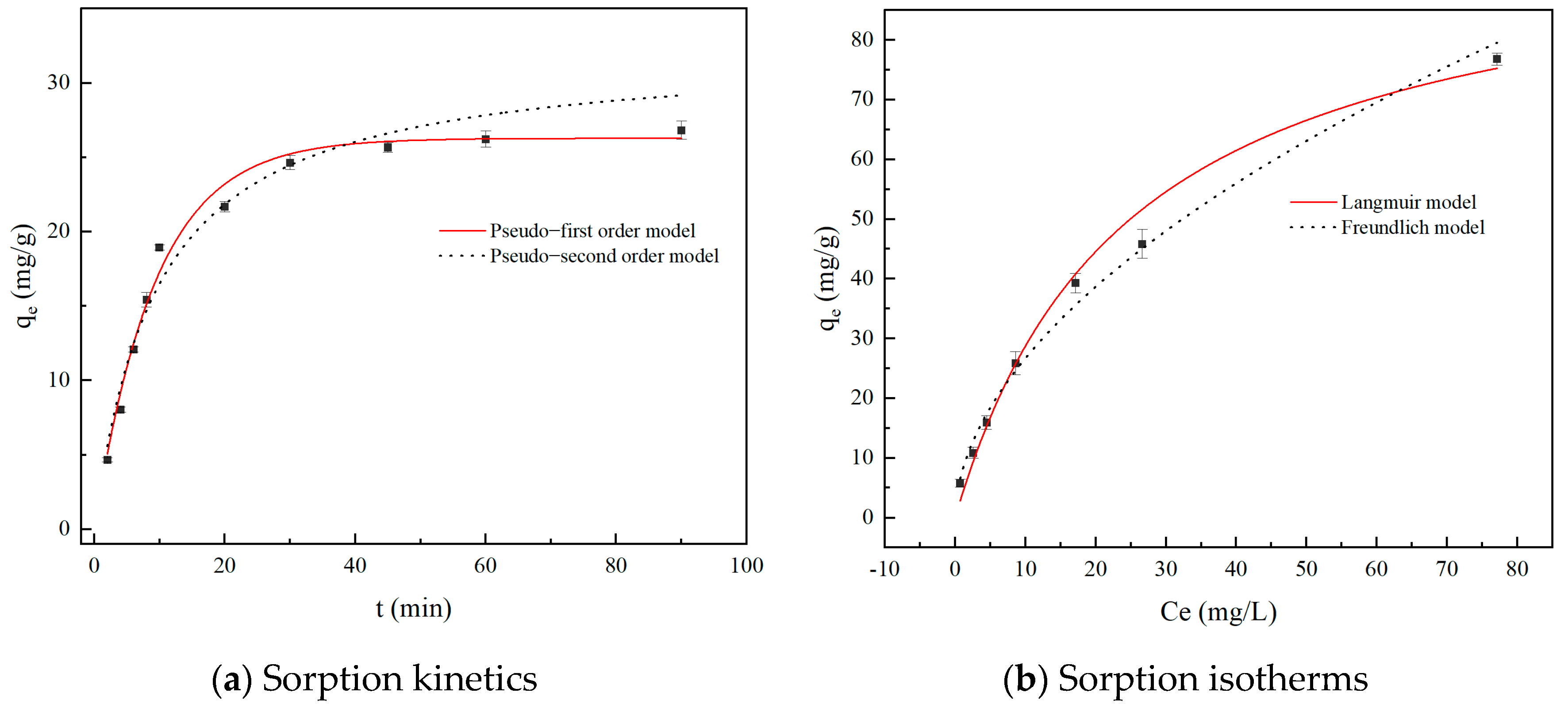
2.3.1. Adsorption Kinetics
2.3.2. Adsorption Isotherm
2.4. Possible Adsorption Mechanism
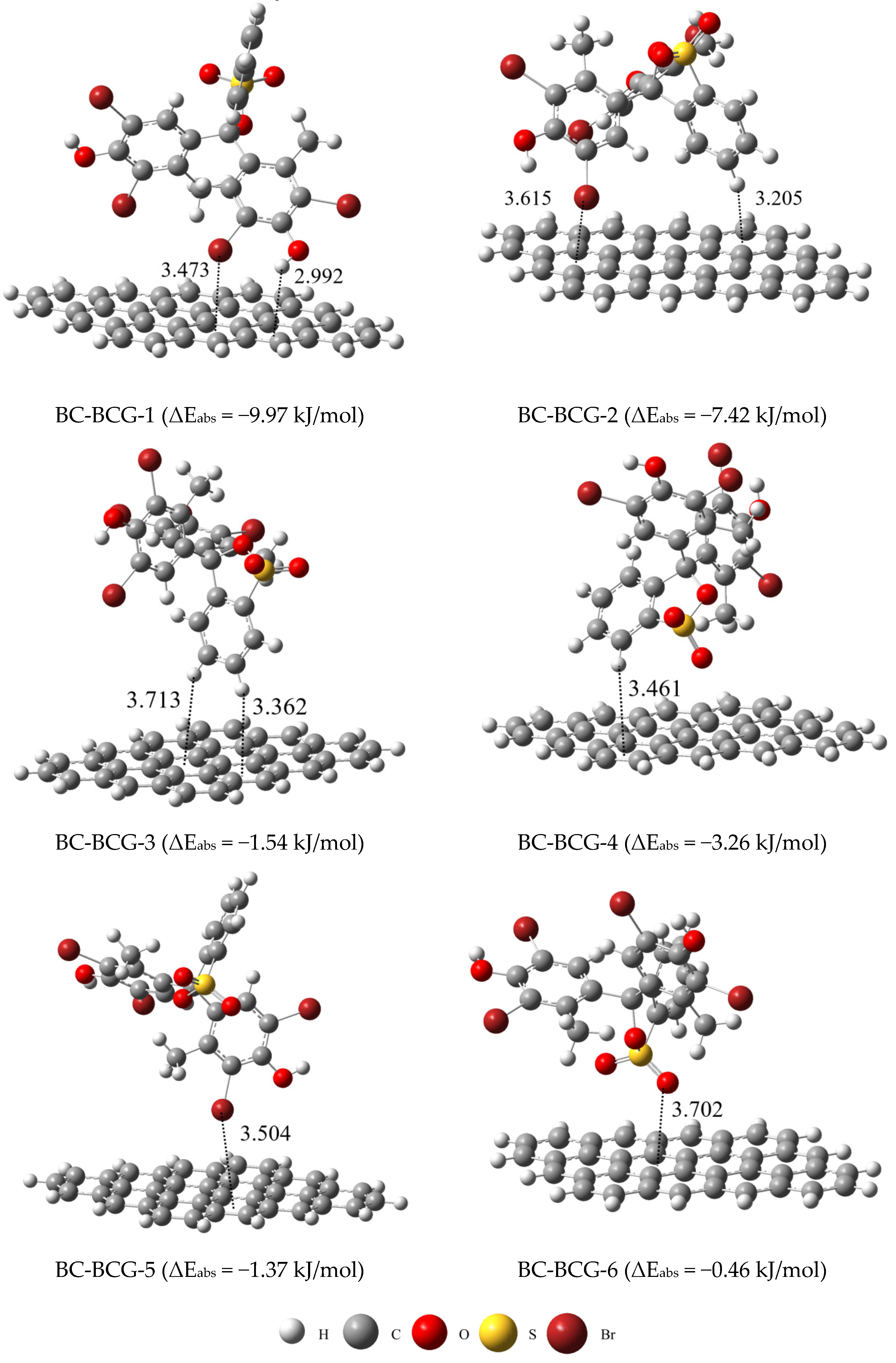
2.5. DFT Calculations
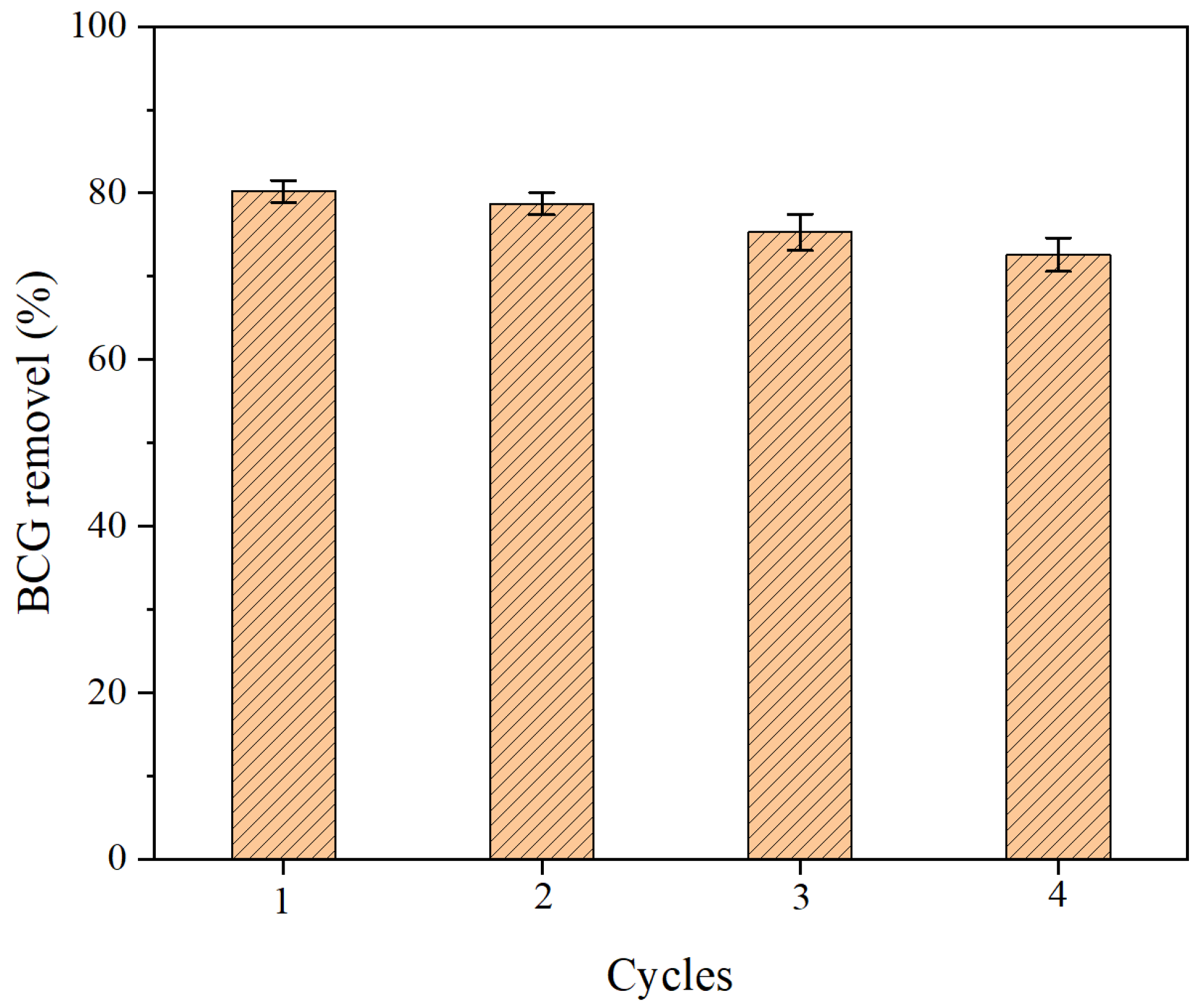
2.6. Cyclic Adsorption Experiment
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Preparation of CBC
3.3. Characterization of CBC
3.4. Batch Adsorption Experiments for BCG Solutions
3.5. Adsorption Models
3.6. DFT Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murmu, B.M.; Behera, S.S.; Das, S.; Mohapatra, R.K.; Bindhani, B.K.; Parhi, P.K. Extensive investigation on the study for the adsorption of bromocresol green (BCG) dye using activated Phragmites karka. Indian. J. Chem. Technol. 2018, 25, 409–420. [Google Scholar]
- Ghaedi, M.; Khajesharifi, H.; Hemmati Yadkuri, A.; Roosta, M.; Sahraei, R.; Daneshfar, A. Cadmium hydroxide nanowire loaded on activated carbon as efficient adsorbent for removal of Bromocresol Green. Spectrochim. Acta A 2012, 86, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Shair, A.S.; Dena, A.S.A.; El-Sherbiny, I.M. Matrix-dispersed PEI-coated SPIONs for fast and efficient removal of anionic dyes from textile wastewater samples: Applications to triphenylmethanes. Spectrochim. Acta A 2021, 249, 119301. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.M.; Carr, C.M. A critical review on recent advancements of the removal of reactive dyes from dyehouse effluent by ion-exchange adsorbents. Chemosphere 2018, 209, 201–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Yuan, J.; Li, J.; Zhang, G. Preparation of chitosan poly (methacrylate) composites for adsorption of bromocresol green. ACS Omega 2019, 4, 12680–12686. [Google Scholar] [CrossRef]
- Arica, T.A.; Kuman, M.; Gercel, O.; Ayas, E. Poly(dopamine) grafted bio-silica composite with tetraethylenepentamine ligands for enhanced adsorption of pollutants. Chem. Eng. Res. Des. 2019, 141, 317–327. [Google Scholar] [CrossRef]
- Biglari, H.; RodríguezíCouto, S.; Khaniabadi, Y.O.; Nourmoradi, H.; Khoshgoftar, M.; Amrane, A.; Vosoughi, M.; Esmaeili, S.; Heydari, R.; Mohammadi, M.J.; et al. Cationic surfactant-modified clay as an adsorbent for the removal of synthetic dyes from aqueous solutions. Int. J. Chen. React. Eng. 2018, 16, 20170064. [Google Scholar] [CrossRef]
- Naz, K.; Sayed, M.; Rehman, F.; Gul, I.; Noreen, S.; Khan, Q.; Gul, S.; Hussain, S. Photochemical degradation of bromocresol green dye by UV/Co2+ process via activation of peroxymonosulfate: A mechanistic approach. Water Pract. Technol. 2024, 19, 1003–1015. [Google Scholar] [CrossRef]
- Pacheco, C.R.; Cruz, M.R.R.; Garcia, J.C.; Hilares, R.T.; Andrade, G.D.J.C.; Tanaka, D.A.P.; Mogrovejo-Valdivia, A. Adsorption and degradation of rhodamine B and bromocresol green by FeOCl under advanced oxidation process. Arab. J. Chem. 2023, 16, 105049. [Google Scholar] [CrossRef]
- Bai, H.M.; He, P.; Chen, J.C.; Liu, K.L.; Lei, H.; Zhang, X.J.; Dong, F.Q.; Li, H. Electrocatalytic degradation of bromocresol green wastewater on Ti/SnO2-RuO2 electrode. Water Sci. Technol. 2017, 75, 220–227. [Google Scholar] [CrossRef]
- Ogoko, E.C.; Kelle, H.I.; Akintola, O.; Eddy, N.O. Experimental and theoretical investigation of Crassostrea gigas (gigas) shells based CaO nanoparticles as a photocatalyst for the degradation of bromocresol green dye (BCGD) in an aqueous solution. Biomass Convers. Bior. 2024, 14, 14859–14875. [Google Scholar] [CrossRef]
- Tabet, A.; Meneceur, S.; Laouini, S.E.; Salmi, C.; Mohammed, H.A.; Kir, I.; Hasan, G.G.; Alharthi, F.; Abdullah, J.A.A. One post biosynthesis of novel ternary nanocomposite ZnO/CuO/Cu2MgO3 for enhancing photocatalytic degradation of Bromocresol Green in wastewater. J. Clust. Sci. 2024, 35, 765–777. [Google Scholar] [CrossRef]
- Nguyen, K.D.; Tran, A.T.H.; Kaus, N.H.M. Preparation and characterization of red mud-based geopolymer composited with rice husk ash for the adsorption of Bromocresol Green in aqueous solution. Chem. Chem. Technol. 2023, 17, 857–869. [Google Scholar] [CrossRef]
- Hmoudah, M.; El-Qanni, A.; Abuhatab, S.; Marei, N.N.; El-Hamouz, A.; Tarboush, B.J.A.; Alsurakji, I.H.; Baniowda, H.M.; Russo, V.; Di Serio, M. Competitive adsorption of Alizarin Red S and Bromocresol Green from aqueous solutions using brookite TiO2 nanoparticles: Experimental and molecular dynamics simulation. Environ. Sci. Pollut. R. 2022, 29, 77992–78008. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef]
- Saad, H.; El-Dien, F.N.; El-Gamel, N.E.; Dena, A.S.A. Azo-functionalized superparamagnetic Fe3O4 nanoparticles: An efficient adsorbent for the removal of bromocresol green from contaminated water. RSC Adv. 2022, 12, 25487–25499. [Google Scholar] [CrossRef]
- Wang, J.; Su, Y.; Lv, S.W.; Sun, L.H. The efficient removal of diclofenac sodium and bromocresol green from aqueous solution by sea urchin-like Ni/Co-BTC bimetallic organic framework: Adsorption isotherms, kinetics and mechanisms. New J. Chem. 2022, 46, 18374–18383. [Google Scholar] [CrossRef]
- Crini, G. Non-conventional low-cost adsorbents for dye removal: A review. Bioresour. Technol. 2006, 97, 1061–1085. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Loc, N.X.; Miyanishi, T. Efficiency of dye adsorption by biochars produced from residues of two rice varieties, Japanese Koshihikari and Vietnamese IR50404. Desalin. Water Treat 2019, 165, 333–351. [Google Scholar] [CrossRef]
- Kaya, N.; Uzun, Z.Y. Experimental and modeling studies on the removal of bromocresol green from aqueous solutions by using pine cone-derived activated biochar. Biomass Convers. Bior. 2024. [Google Scholar] [CrossRef]
- Luo, Z.R.; Yao, B.; Yang, X.; Wang, L.Q.; Xu, Z.Y.; Yan, X.L.; Tian, L.; Zhou, H.; Zhou, Y.Y. Novel insights into the adsorption of organic contaminants by biochar: A review. Chemosphere 2022, 287, 132113. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, L.; Kang, X.; Song, J.; Guo, H.; Zhang, Q. Insight into atrazine removal by fallen leaf biochar prepared at different pyrolysis temperatures: Batch experiments, column adsorption and DFT calculations. Environ. Pollut. 2023, 317, 120832. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.X.; Huang, L.L.; Wu, L.G.; Zhang, C.T.; Zhu, S.H.; Xiao, X.Y.; Li, M.; Zou, X.M. Adsorption of sulfonamides on biochars derived from waste residues and its mechanism. J. Hazard. Mater. 2021, 406, 124291. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ji, Y.H.; Liu, X.M.; Mu, L.W.; Zhu, J.H. Sorption mechanism of organic dyes on a novel self-nitrogen-doped porous graphite biochar: Coupling DFT calculations with experiments. Chem. Eng. Sci. 2021, 242, 116739. [Google Scholar] [CrossRef]
- Zhou, P.; Li, X.Z.; Zhou, J.; Peng, Z.Y.; Shen, L.Q.; Li, W.S. Insights of the adsorption mechanism of methylene blue on biochar from phytoextraction residues of Citrus aurantium L.: Adsorption model and DFT calculations. J. Environ. Chem. Eng. 2023, 11, 110496. [Google Scholar] [CrossRef]
- Xu, Y.F.; Zhang, X.L.; Wu, S.; Chen, C.; Wang, J.Z.; Yuan, S.Q.; Chen, B.; Li, P.P.; Xu, R.J. Numerical simulation of particle motion at cucumber straw grinding process based on EDEM. Int. J. Agric. Biol. Eng. 2020, 13, 227–235. [Google Scholar] [CrossRef]
- Xu, Y.F.; Zhang, X.L.; Sun, X.J.; Wang, J.Z.; Liu, J.Z.; Li, Z.G.; Qian, G.; Li, P.P. Tensile mechanical properties of greenhouse cucumber cane. Int. J. Agric. Biol. Eng. 2016, 9, 1–8. [Google Scholar]
- Chang, R.; Guo, Q.; Chen, Q.; Bernal, M.P.; Wang, Q.; Li, Y. Effect of initial material bulk density and easily-degraded organic matter content on temperature changes during composting of cucumber stalk. J. Environ. Sci. 2019, 80, 306–315. [Google Scholar] [CrossRef]
- Xu, X.Y.; Zhao, Y.H.; Sima, J.K.; Zhao, L.; Masek, O.; Cao, X.D. Indispensable role of biochar-inherent mineral constituents in its environmental applications: A review. Bioresour. Technol. 2017, 241, 887–899. [Google Scholar] [CrossRef]
- Pipíška, M.; Krajčíková, E.K.; Hvostik, M.; Frišták, V.; Ďuriška, L.; Černičková, I.; Kaňuchová, M.; Conte, P.; Soja, G. Biochar from Wood Chips and Corn Cobs for Adsorption of Thioflavin T and Erythrosine B. Materials 2022, 15, 1492. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Zheng, H.L.; Li, H.; Zhang, Z.M.; Zhao, C.; Gou, Q.; Liu, Y.Y. Highly effective and selective adsorption of thorium (IV) from aqueous solution using mesoporous graphite carbon nitride prepared by sol-gel template method. Chem. Eng. J. 2021, 410, 128321. [Google Scholar] [CrossRef]
- Zhang, G.X.; Zhang, Q.; Sun, K.; Liu, X.T.; Zheng, W.J.; Zhao, Y. Sorption of simazine to corn straw biochars prepared at different pyrolytic temperatures. Environ. Pollut. 2011, 159, 2594–2601. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fan, S.S. Removal of cadmium in aqueous solution using wheat straw biochar: Effect of minerals and mechanism. Environ. Sci. Pollut. Res. 2018, 25, 8688–8700. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.N.; Hu, X.Y.; Liu, H.W. Influence of pyrolysis conditions on stability of biochar. Environ. Sci. Technol. 2013, 36, 11–14. [Google Scholar]
- Zhou, Y.R.; Wei, Z.H.; Yao, S.R.; Li, Z.L.; Zhang, Z.Y.; Ji, L.L.; Jing, H. Activated biochar derived from Enteromorpha with high specific surface area for efficient removal of phenanthrene: Experiments, mechanism and DFT calculations. Environ. Pollut. 2024, 340, 122709. [Google Scholar] [CrossRef]
- Zhu, Y.H.; Zhao, Q.; Li, D.Y.; Li, J.; Guo, W. Enhanced recovery of phosphorus in sewage sludge-derived biochar with CaCO3: Phosphorus speciation and slow-release phosphorus behavior. Sep. Purif. Technol. 2023, 311, 123325. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Liu, G.C.; Zheng, H.; Li, F.M.; Ngo, H.H.; Guo, W.S.; Liu, C.; Chen, L.; Xing, B.S. Investigating the mechanisms of biochar’s removal of lead from solution. Bioresour. Technol. 2015, 177, 308–317. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Liao, B.H.; Lin, L.N.; Qiu, W.W.; Song, Z.G. Adsorption of Cu (II) and Cd (II) from aqueous solutions by ferromanganese binary oxide−biochar composites. Sci. Total Environ. 2018, 615, 115–122. [Google Scholar] [CrossRef]
- Ma, S.Q.; Jing, F.Q.; Sohi, S.P.; Chen, J.W. New insights into contrasting mechanisms for PAE adsorption on millimeter, micron-and nano-scale biochar. Environ. Sci. Pollut. Res. 2019, 26, 18636–18650. [Google Scholar] [CrossRef]
- Radoor, S.; Jayakumar, A.; Karayil, J.; Kim, J.T.; Siengchin, S. Biodegradable polymeric green adsorbent for the highly efficient removal of crystal violet dye from aqueous solution. Chem. Eng. Res. Des. 2023, 199, 473–485. [Google Scholar] [CrossRef]
- Salleh, M.A.M.; Mahmoud, D.K.; Karim, W.A.W.A.; Idris, A. Cationic and anionic dye adsorption by agricultural solid wastes: A comprehensive review. Desalination 2011, 280, 1–13. [Google Scholar] [CrossRef]
- Sintakindi, A.; Ankamwar, B. Biosorption of Bromocresol green from aqueous solution by Earliella scabrosa fungal biomass in removal of environmental pollutants. Int. J. Environ. Sci. Technol. 2023, 20, 5253–5264. [Google Scholar] [CrossRef]
- Onu, C.E.; Asadu, C.O.; Ohale, P.E.; Nweke, C.N.; Nwokedi, I.C.; Musei, N.N.; Onu, C.P. Adsorptive removal of bromocresol green dye using activated corn cob. J. Eng. Appl. Sci. 2022, 21, 824–841. [Google Scholar]
- Samaraweera, H.; Rivera, A.; Carter, K.; Felder, T.; Nawalage, S.; Chui, I.; Perez, F.; Khan, A.H.; Mlsna, T. Green iron oxide-modified biochar for methylene blue removal from aqueous solutions. Groundw. Sustain. Dev. 2023, 21, 100945. [Google Scholar] [CrossRef]
- Akpomie, K.G.; Conradie, J. Banana peel as a bio sorbent for the decontamination of water pollutants. A review. Environ. Chem. Lett. 2020, 18, 1085–1112. [Google Scholar] [CrossRef]
- Ji, Y.M.; Ma, C.G.; Li, J.; Zhao, H.Y.; Chen, Q.Q.; Li, M.X.; Liu, H.L. A magnetic adsorbent for the removal of cationic dyes from wastewater. Nanomaterials 2018, 8, 710. [Google Scholar] [CrossRef]
- Tang, L.; Yu, J.F.; Pang, Y.; Zeng, G.M.; Deng, Y.C.; Wang, J.J.; Ren, X.Y.; Ye, S.J.; Peng, B.; Feng, H.P. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.W.; Xu, J.C.; Dang, Z.; Zhang, L.J. Insights into sulfamethazine adsorption interfacial interaction mechanism on mesoporous cellulose biochar: Coupling DFT/FOT simulations with experiments. Chem. Eng. J. 2019, 356, 341–349. [Google Scholar] [CrossRef]
- Zhang, X.T.; Hou, J.J.; Zhang, S.D.; Cai, T.; Liu, S.J.; Hu, W.J.; Zhang, Q.Z. Standardization and micromechanistic study of tetracycline adsorption by biochar. Biochar 2024, 6, 12. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, F.; Lu, Y.; Xue, X.; Li, N. Adsorption interaction of tetracyclines with porous synthetic resins. Ind. Eng. Chem. Res. 2011, 50, 13892–13898. [Google Scholar] [CrossRef]
- Youn, I.S.; Kim, D.Y.; Cho, W.J.; Madridejos, J.M.L.; Lee, H.M.; Kołaski, M.; Lee, J.; Baig, C.; Shin, S.K.; Filatov, M.; et al. Halogen−π interactions between benzene and X2/CX4 (X = Cl, Br): Assessment of various density functionals with respect to CCSD (T). J. Phys. Chem. A 2016, 120, 9305–9314. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Zou, J.W.; Wang, Y.H.; Yu, Q.S. Theoretical investigations of the C–X/π interactions between benzene and some model halocarbons. Chem. Phys. 2007, 334, 1–7. [Google Scholar] [CrossRef]
- Tehrani, A.Z.; Kim, K.S. Functional molecules and materials by π-interaction based quantum theoretical design. Int. J. Quantum Chem. 2016, 116, 622–633. [Google Scholar] [CrossRef]
- Fiori, A.T.M.; Nakahata, D.H.; Cuin, A.; Lustri, W.R.; Corbi, P.P. Synthesis, crystallographic studies, high resolution mass spectrometric analyses and antibacterial assays of silver(I) complexes with sulfisoxazole and sulfadimethoxine. Polyhedron 2017, 121, 172–179. [Google Scholar] [CrossRef]
- Khan, E.A.; Shahjahan, K.T.A. Adsorption of methyl red on activated carbon derived from custard apple (annona squamosa) fruit shell: Equilibrium isotherm and kinetic studies. J. Mol. Liq. 2018, 249, 1195–1211. [Google Scholar] [CrossRef]
- Özdemir, M.; Durmuş, Ö.; Şahin, Ö.; Saka, C. Removal of methylene blue, methyl violet, rhodamine B, alizarin red, and bromocresol green dyes from aqueous solutions on activated cotton stalks. Desalin. Water Treat. 2016, 57, 18038–18048. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Alizadeh, A.; Malekhosseini, Z.; Ranjbar, M. Removal of bromocresol green from aqueous solution via adsorption of Ziziphus nummularia as a new, natural and low cost adsorbent: Kinetic and thermodynamic study of removal process. J. Chem. Eng. Data 2011, 56, 3738–3746. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. A kinetic study of dye sorption by biosorbent waste product pith. Resour. Conserv. Recycl. 1999, 25, 171–193. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03; Gaussian: Wallingford, CT, USA, 2016. [Google Scholar]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]




| Materials | BET Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Size (nm) | C (%) | H (%) | O (%) | N (%) |
|---|---|---|---|---|---|---|---|
| CBC | 101.58 | 0.0942 | 1.85 | 71.23 | 3.12 | 25.19% | 0.27 |
| Kinetic Models | Parameters | Value |
|---|---|---|
| Pseudo-first order model | qe (mg/g) | 26.28 |
| k1 (min−1) | 0.1072 | |
| R2 | 0.9811 | |
| Pseudo-second order model | qe (mg/g) | 32.26 |
| k2 (mg·g−1·min−1) | 3.25 × 10−3 | |
| R2 | 0.9610 |
| Isotherm Models | Parameters | Value |
|---|---|---|
| Langmuir model | qm (mg/g) | 99.18 |
| KL (L/mg) | 0.0408 | |
| R2 | 0.9906 | |
| Freundlich model | Kf (mg/g·(L/mg)1/n) | 7.79 |
| 1/n | 0.5347 | |
| R2 | 0.9865 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Meng, L.; Fang, Z.; Xu, Y.; Zhou, Y.; Guo, H.; Wang, J.; Zhao, X.; Zang, S.; Shen, H. Experimental and Theoretical Studies on the Adsorption of Bromocresol Green from Aqueous Solution Using Cucumber Straw Biochar. Molecules 2024, 29, 4517. https://doi.org/10.3390/molecules29194517
Zhang C, Meng L, Fang Z, Xu Y, Zhou Y, Guo H, Wang J, Zhao X, Zang S, Shen H. Experimental and Theoretical Studies on the Adsorption of Bromocresol Green from Aqueous Solution Using Cucumber Straw Biochar. Molecules. 2024; 29(19):4517. https://doi.org/10.3390/molecules29194517
Chicago/Turabian StyleZhang, Chenxi, Lingbin Meng, Zhihao Fang, Youxin Xu, Yue Zhou, Hongsen Guo, Jinyu Wang, Xiaotian Zhao, Shuyan Zang, and Hailin Shen. 2024. "Experimental and Theoretical Studies on the Adsorption of Bromocresol Green from Aqueous Solution Using Cucumber Straw Biochar" Molecules 29, no. 19: 4517. https://doi.org/10.3390/molecules29194517
APA StyleZhang, C., Meng, L., Fang, Z., Xu, Y., Zhou, Y., Guo, H., Wang, J., Zhao, X., Zang, S., & Shen, H. (2024). Experimental and Theoretical Studies on the Adsorption of Bromocresol Green from Aqueous Solution Using Cucumber Straw Biochar. Molecules, 29(19), 4517. https://doi.org/10.3390/molecules29194517





