Hollow Gold–Silver Nanorods—A New, Very Efficient Nanomaterial for Surface-Enhanced Raman Scattering (SERS) Measurements
Abstract
:1. Introduction
2. Results and Discussion
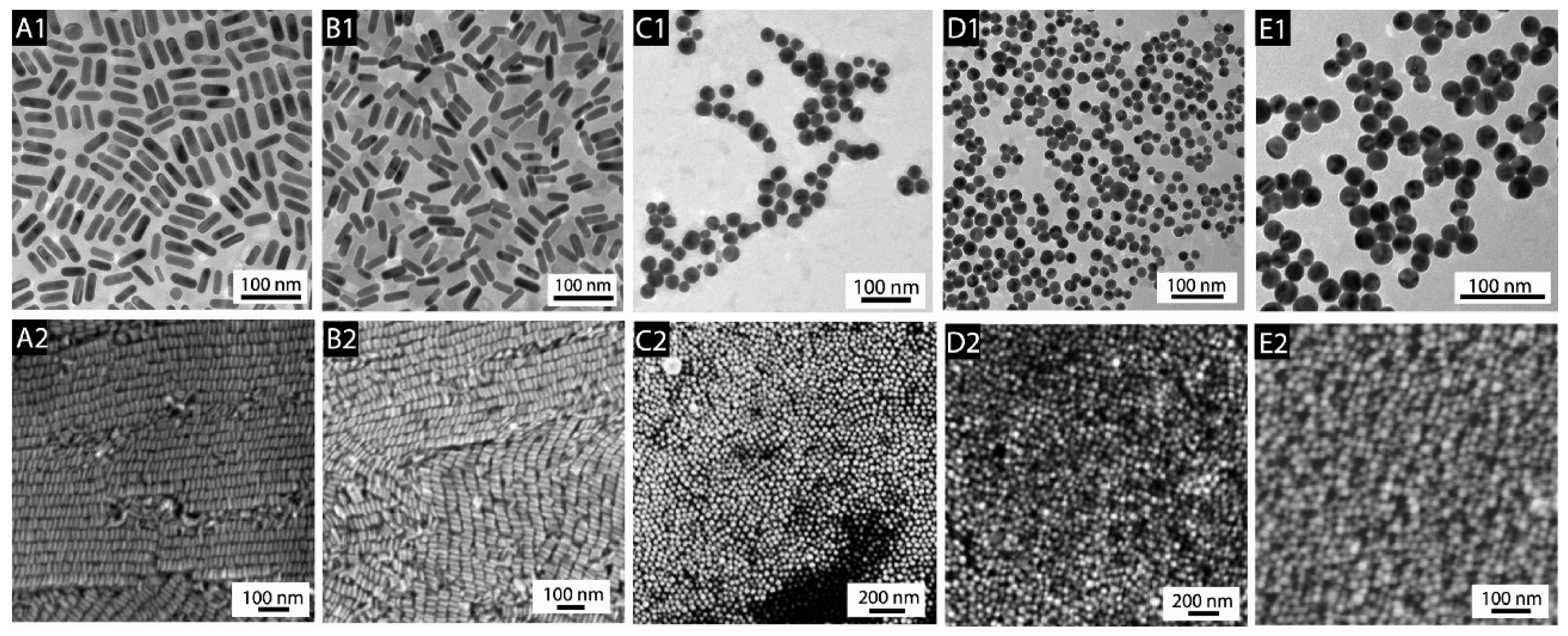
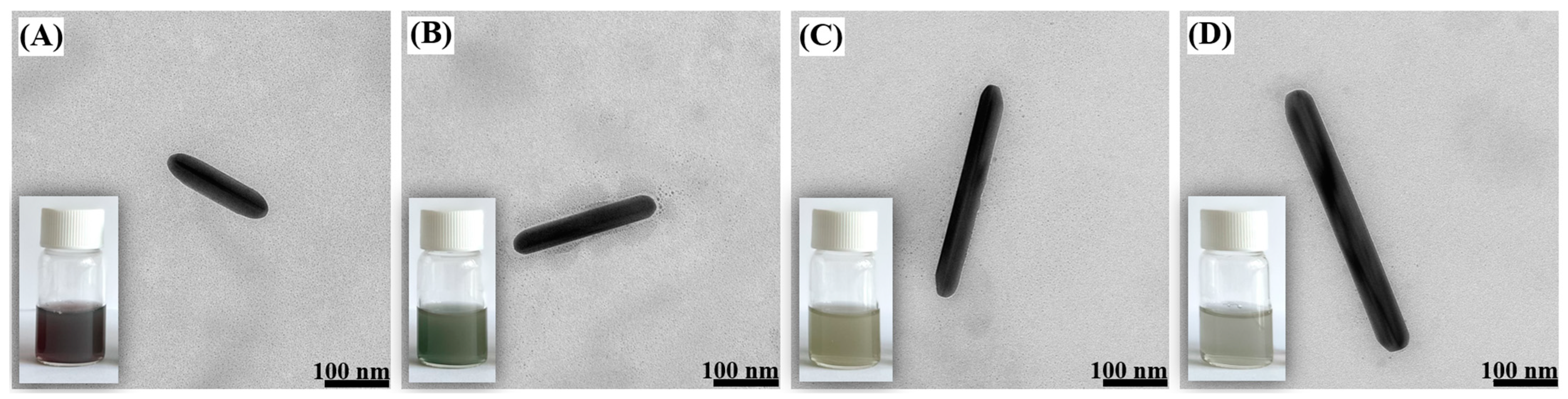
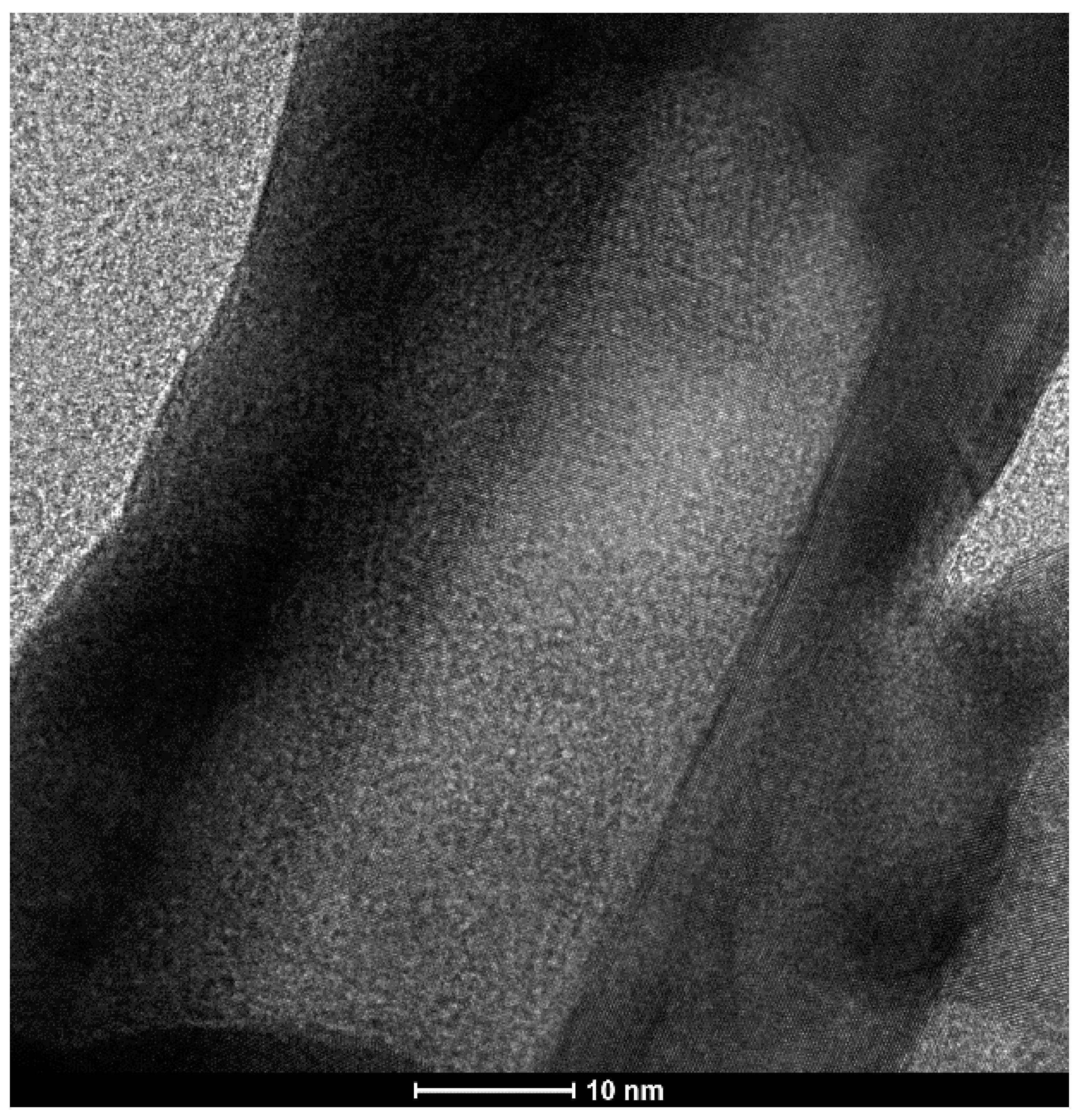
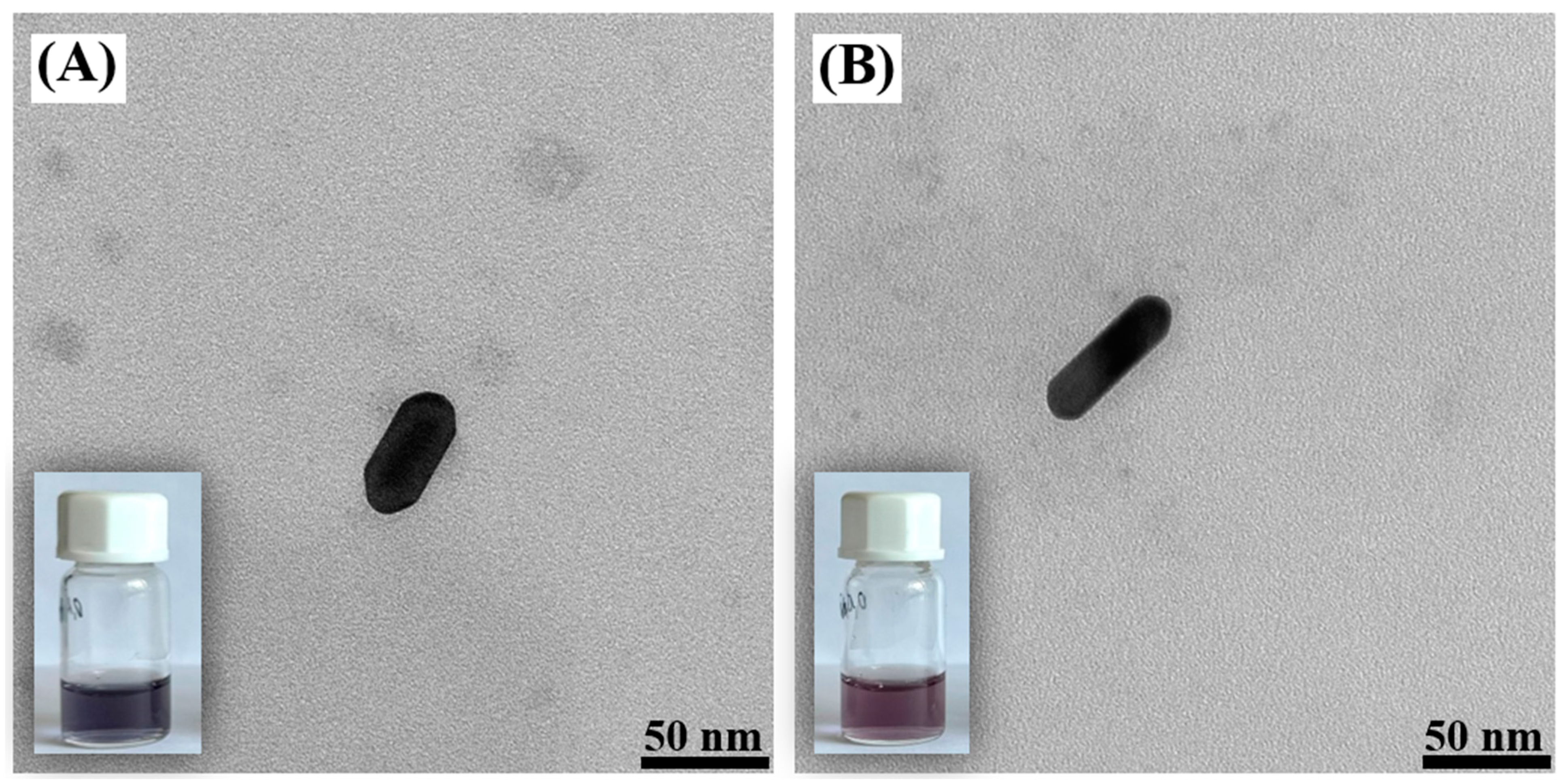
2.1. Structural Characterization of the Obtained Nanomaterials
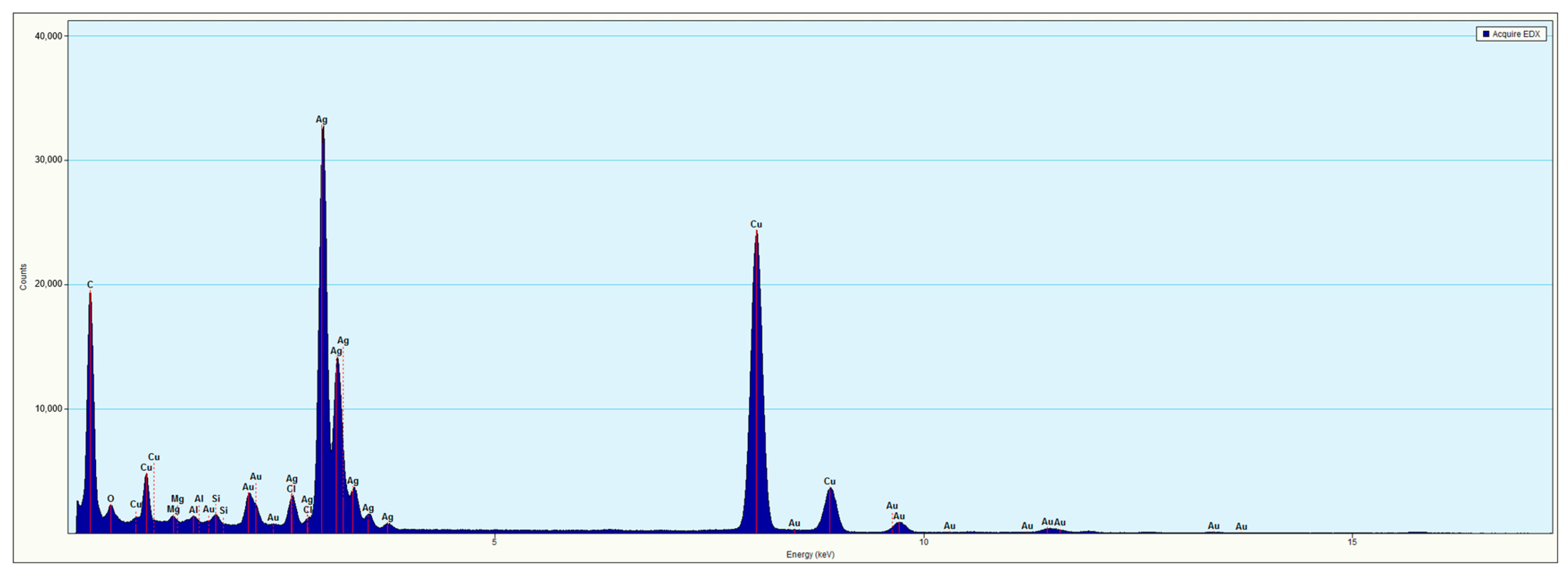
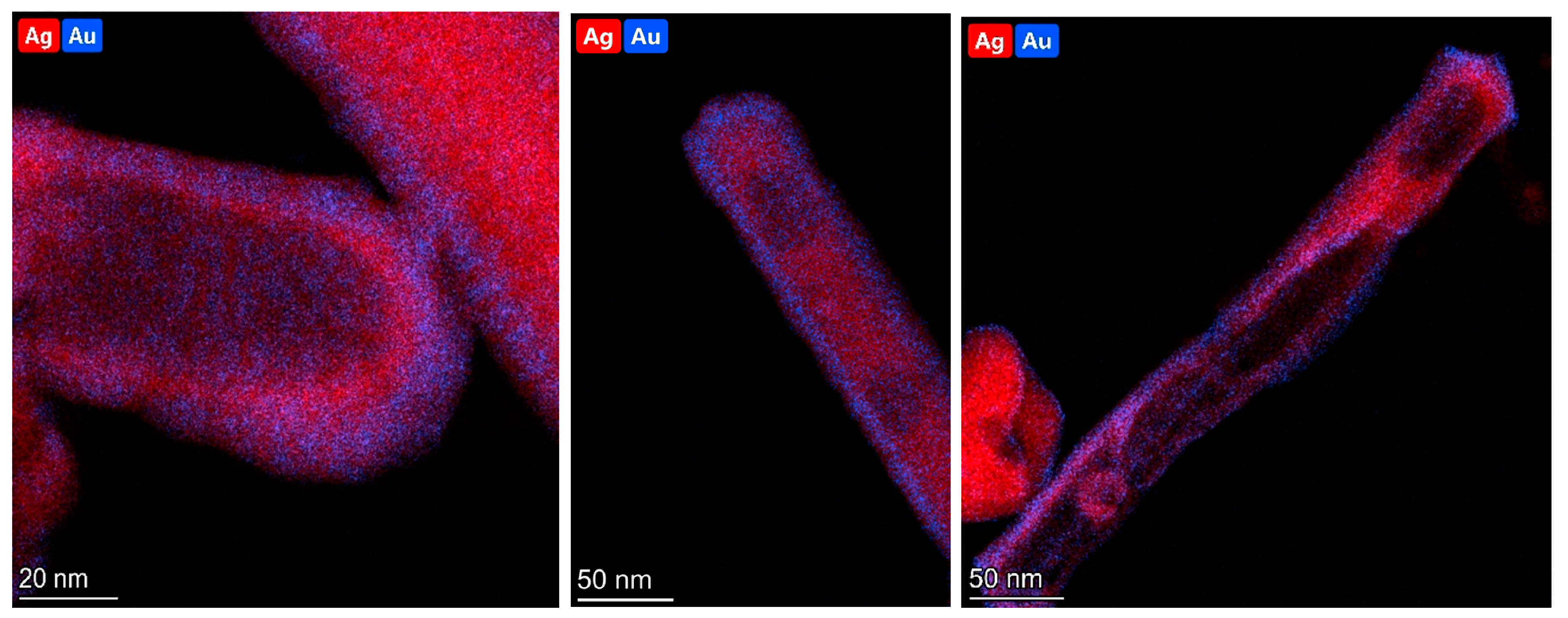
2.2. Elemental Characterization of AuAg Nanorods
2.3. Optical Characterization of the Nanorods
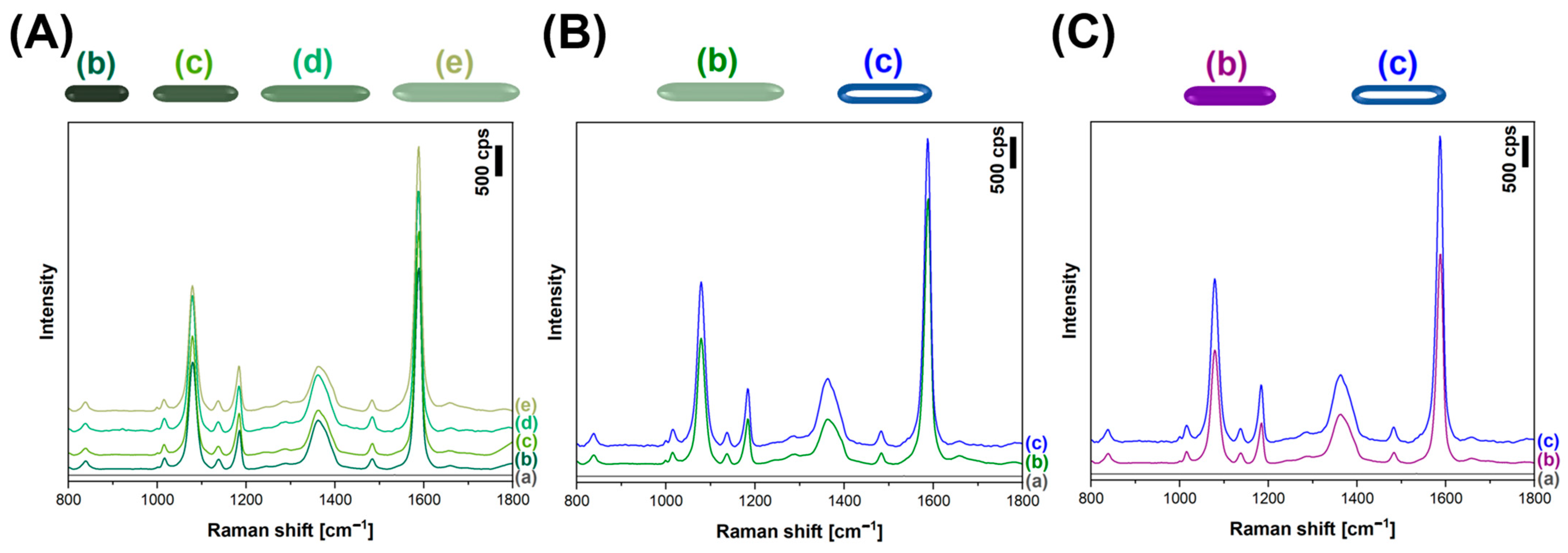
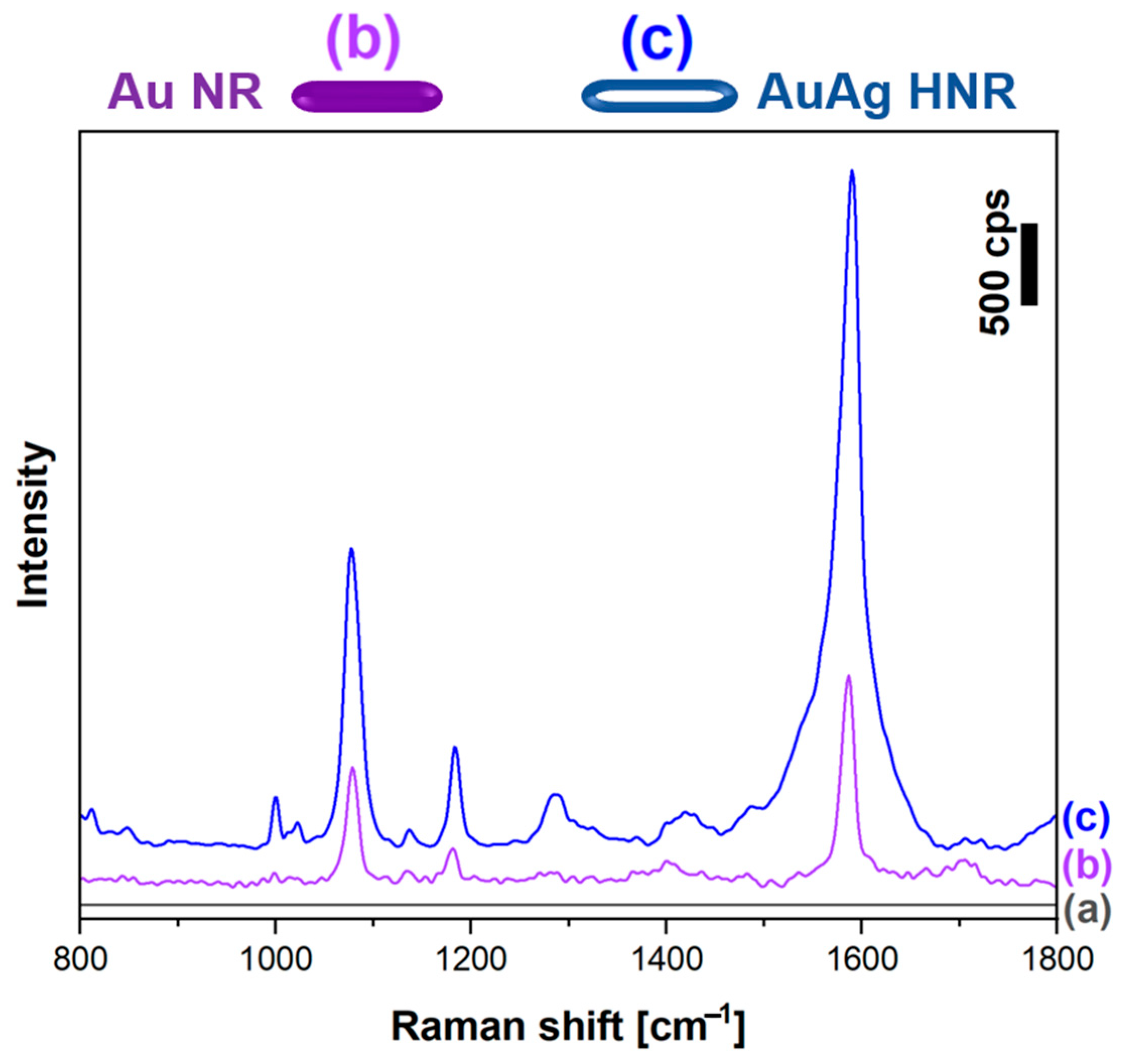
2.4. SERS Measurements
2.5. Stability Tests of Ag and AuAg Nanorods
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Silver Nanorods
3.3. Galvanic Exchange Reaction—Synthesis of AuAg Nanorods
3.4. Synthesis of Pure Gold Nanorods
3.5. Experimental Techniques
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Michaels, A.M.; Nirmal, M.; Brus, L.E. Surface enhanced Raman spectroscopy of individual rhodamine 6G molecules on large Ag nanocrystals. J. Am. Chem. Soc. 1999, 121, 9932–9939. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced Raman scattering (SERS). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, L.; Gou, L.; Wei, S.; Hou, X.; Wu, L. Au nanoparticles decorated CoP nanowire array: A highly sensitive, anticorrosive, and recyclable surface-enhanced Raman scattering substrate. Anal. Chem. 2023, 95, 11037–11046. [Google Scholar] [CrossRef]
- Michałowska, A.; Kudelski, A. Plasmonic substrates for biochemical applications of surface-enhanced Raman spectroscopy. Spectrochim. Acta A 2024, 308, 123786. [Google Scholar] [CrossRef]
- Kahraman, M.; Mullen, E.R.; Korkmaz, A.; Wachsmann-Hogiu, S. Fundamentals and applications of SERS-based bioanalytical sensing. Nanophotonics 2017, 6, 831–852. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Etchegoin, P.G.; Le Ru, E.C. Basic Electromagnetic Theory of SERS. In Surface Enhanced Raman Spectroscopy; Schlücker, S., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2010; pp. 1–37. [Google Scholar]
- Hao, E.; Schatz, G.C. Electromagnetic fields around silver nanoparticles and dimers. J. Chem. Phys. 2004, 120, 357–366. [Google Scholar] [CrossRef]
- McLellan, J.M.; Li, Z.-Y.; Siekkinen, A.R.; Xia, Y. The SERS activity of a supported Ag nanocube strongly depends on its orientation relative to laser polarization. Nano Lett. 2007, 7, 1013–1017. [Google Scholar] [CrossRef]
- Hu, H.-C.; Wu, S.-H.; Jin, L.-X.; Sun, J.-J. Plasmonic Au nanocube enhanced SERS biosensor based on heated electrode and strand displacement amplification for highly sensitive detection of Dam methyltransferase activity. Biosens. Bioelectron. 2022, 210, 114283. [Google Scholar] [CrossRef]
- Abdulrahman, H.B.; Krajczewski, J.; Kudelski, A. Modification of surfaces of silver nanoparticles for controlled deposition of silicon, manganese, and titanium dioxides. Appl. Surf. Sci. 2018, 427, 334–339. [Google Scholar] [CrossRef]
- Ciou, S.-H.; Cao, Y.-W.; Huang, H.-C.; Su, D.-Y.; Huang, C.-L. SERS enhancement factors studies of silver nanoprism and spherical nanoparticle colloids in the presence of bromide ions. J. Phys. Chem. C 2009, 113, 9520–9525. [Google Scholar] [CrossRef]
- Tiwari, V.S.; Oleg, T.; Darbha, G.K.; Hardy, W.; Singh, J.P.; Ray, P.C. Non-resonance SERS effects of silver colloids with different shapes. Chem. Phys. Lett. 2007, 446, 77–82. [Google Scholar] [CrossRef]
- Amin, M.U.; Fang, J. Self-assembled gold nano-bipyramids for solution-based surface-enhanced Raman spectroscopy detection. ACS Appl. Nano Mater. 2022, 5, 10421–10430. [Google Scholar] [CrossRef]
- Chen, J.; Guo, L.; Chen, L.; Qiu, B.; Hong, G.; Lin, Z. Sensing of hydrogen sulfide gas in the Raman-silent region based on gold nano-bipyramids (Au NBPs) encapsulated by zeolitic imidazolate framework-8. ACS Sens. 2020, 5, 3964–3970. [Google Scholar] [CrossRef] [PubMed]
- Kołątaj, K.; Krajczewski, J.; Kudelski, A. Dipyramidal-Au@SiO2 nanostructures: New efficient electromagnetic nanoresonators for Raman spectroscopy analysis of surfaces. Appl. Surf. Sci. 2018, 456, 932–940. [Google Scholar] [CrossRef]
- Kołątaj, K.; Krajczewski, J.; Kudelski, A. Silver nanoparticles with many sharp apexes and edges as efficient nanoresonators for shell-isolated nanoparticle-enhanced Raman spectroscopy. J. Phys. Chem. C 2017, 121, 12383–12391. [Google Scholar] [CrossRef]
- Garcia-Leis, A.; Garcia-Ramos, J.V.; Sanchez-Cortes, S. Silver nanostars with high SERS performance. J. Phys. Chem. C 2013, 117, 7791–7795. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, M.-J.; Li, J.-J.; Li, X.; Zhao, J.-W. Multi-branched gold nanostars with fractal structure for SERS detection of the pesticide thiram. Spectrochim. Acta A 2018, 189, 586–593. [Google Scholar] [CrossRef]
- Krajczewski, J.; Michałowska, A.; Kudelski, A. Star-shaped plasmonic nanostructures: New, simply synthetized materials for Raman analysis of surfaces. Spectrochim. Acta A 2020, 225, 117469. [Google Scholar] [CrossRef]
- Lin, K.-Q.; Yi, J.; Hu, S.; Liu, B.-J.; Liu, J.-Y.; Wang, X.; Ren, B. Size effect on SERS of gold nanorods demonstrated via single nanoparticle spectroscopy. J. Phys. Chem. C 2016, 120, 20806–20813. [Google Scholar] [CrossRef]
- Smitha, S.L.; Gopchandran, K.G.; Ravindran, T.R.; Prasad, V.S. Gold nanorods with finely tunable longitudinal surface plasmon resonance as SERS substrates. Nanotechnology 2011, 22, 265705. [Google Scholar] [CrossRef]
- Zhang, B.-Q.; Li, S.-B.; Xiao, Q.; Li, J.; Sun, J.-J. Rapid synthesis and characterization of ultra-thin shell Au@SiO2 nanorods with tunable SPR for shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS). J. Raman Spectrosc. 2013, 44, 1120–1125. [Google Scholar] [CrossRef]
- Li, S.-B.; Li, L.-M.; Anema, J.R.; Ren, B.; Sun, J.-J.; Tian, Z.-Q. Shell-isolated nanoparticle-enhanced Raman spectroscopy (SHINERS) based on gold-core silica-shell nanorods. Z. Phys. Chem. 2011, 225, 775–783. [Google Scholar] [CrossRef]
- Wang, S.; Xi, W.; Cai, F.; Zhao, X.; Xu, Z.; Qian, J.; He, S. Three-photon luminescence of gold nanorods and its applications for high contrast tissue and deep in vivo brain imaging. Theranostics 2015, 5, 251–266. [Google Scholar] [CrossRef]
- Smith, D.K.; Korgel, B.A. The importance of the CTAB surfactant on the colloidal seed-mediated synthesis of gold nanorods. Langmuir 2008, 24, 644–649. [Google Scholar] [CrossRef]
- Zeman, E.J.; Schatz, G.C. An accurate electromagnetic theory study of surface enhancement factors for silver, gold, copper, lithium, sodium, aluminum, gallium, indium, zinc, and cadmium. J. Phys. Chem. 1987, 91, 634–643. [Google Scholar] [CrossRef]
- West, P.R.; Ishii, S.; Naik, G.V.; Emani, N.K.; Shalaev, V.M.; Boltasseva, A. Searching for better plasmonic materials. Laser & Photon. Rev. 2010, 4, 795–808. [Google Scholar] [CrossRef]
- Jana, N.R.; Gearheart, L.; Murphy, C.J. Wet chemical synthesis of silver nanorods and nanowires of controllable aspect ratio. Chem. Commun. 2001, 7, 617–618. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michałowska, A.; Kudelski, A. Hollow Gold–Silver Nanorods—A New, Very Efficient Nanomaterial for Surface-Enhanced Raman Scattering (SERS) Measurements. Molecules 2024, 29, 4540. https://doi.org/10.3390/molecules29194540
Michałowska A, Kudelski A. Hollow Gold–Silver Nanorods—A New, Very Efficient Nanomaterial for Surface-Enhanced Raman Scattering (SERS) Measurements. Molecules. 2024; 29(19):4540. https://doi.org/10.3390/molecules29194540
Chicago/Turabian StyleMichałowska, Aleksandra, and Andrzej Kudelski. 2024. "Hollow Gold–Silver Nanorods—A New, Very Efficient Nanomaterial for Surface-Enhanced Raman Scattering (SERS) Measurements" Molecules 29, no. 19: 4540. https://doi.org/10.3390/molecules29194540
APA StyleMichałowska, A., & Kudelski, A. (2024). Hollow Gold–Silver Nanorods—A New, Very Efficient Nanomaterial for Surface-Enhanced Raman Scattering (SERS) Measurements. Molecules, 29(19), 4540. https://doi.org/10.3390/molecules29194540






