The Coordination of Aluminum Sulfate with a Water-Soluble Block Copolymer Containing Carboxyl, Amide, Sulfonic and Anhydride Groups Providing Both Accelerating and Hardening Effects in Cement Setting
Abstract
1. Introduction
2. Results and Discussion
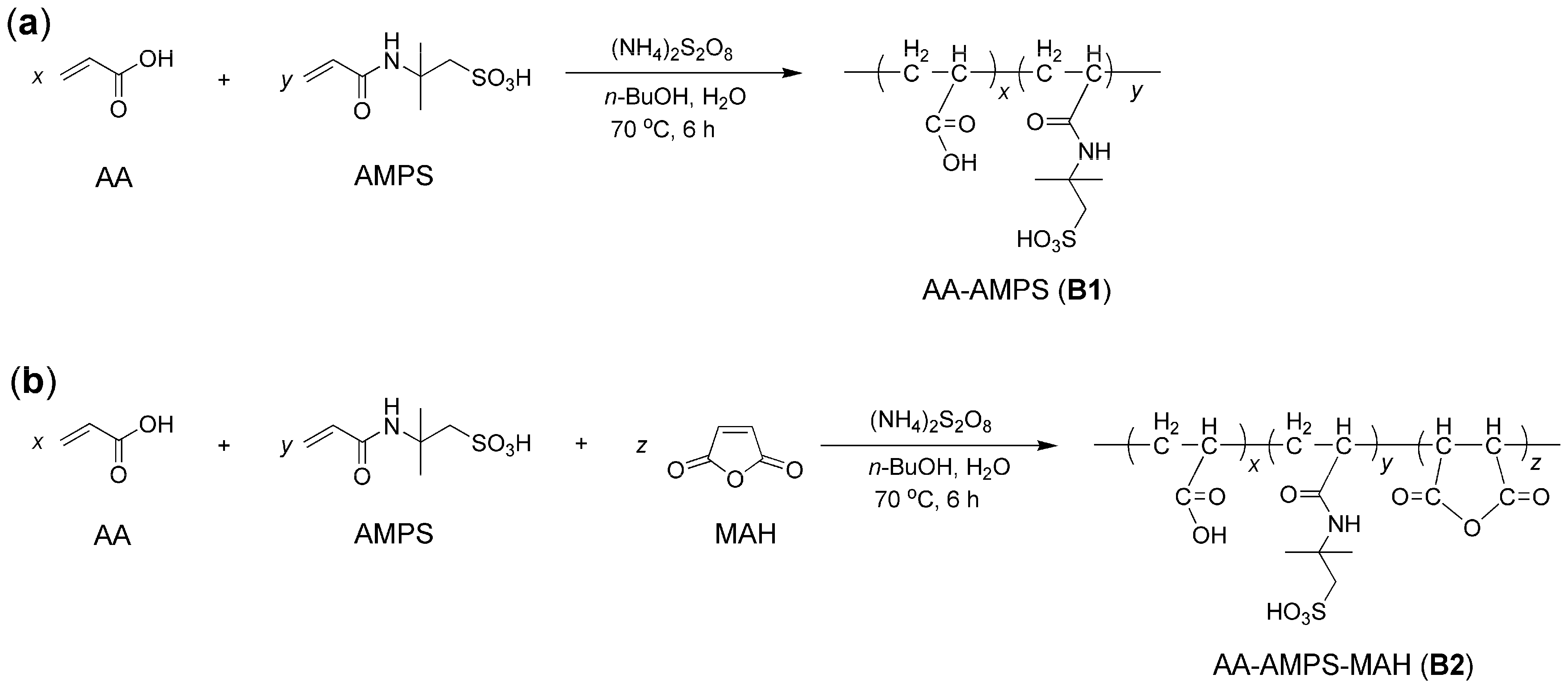
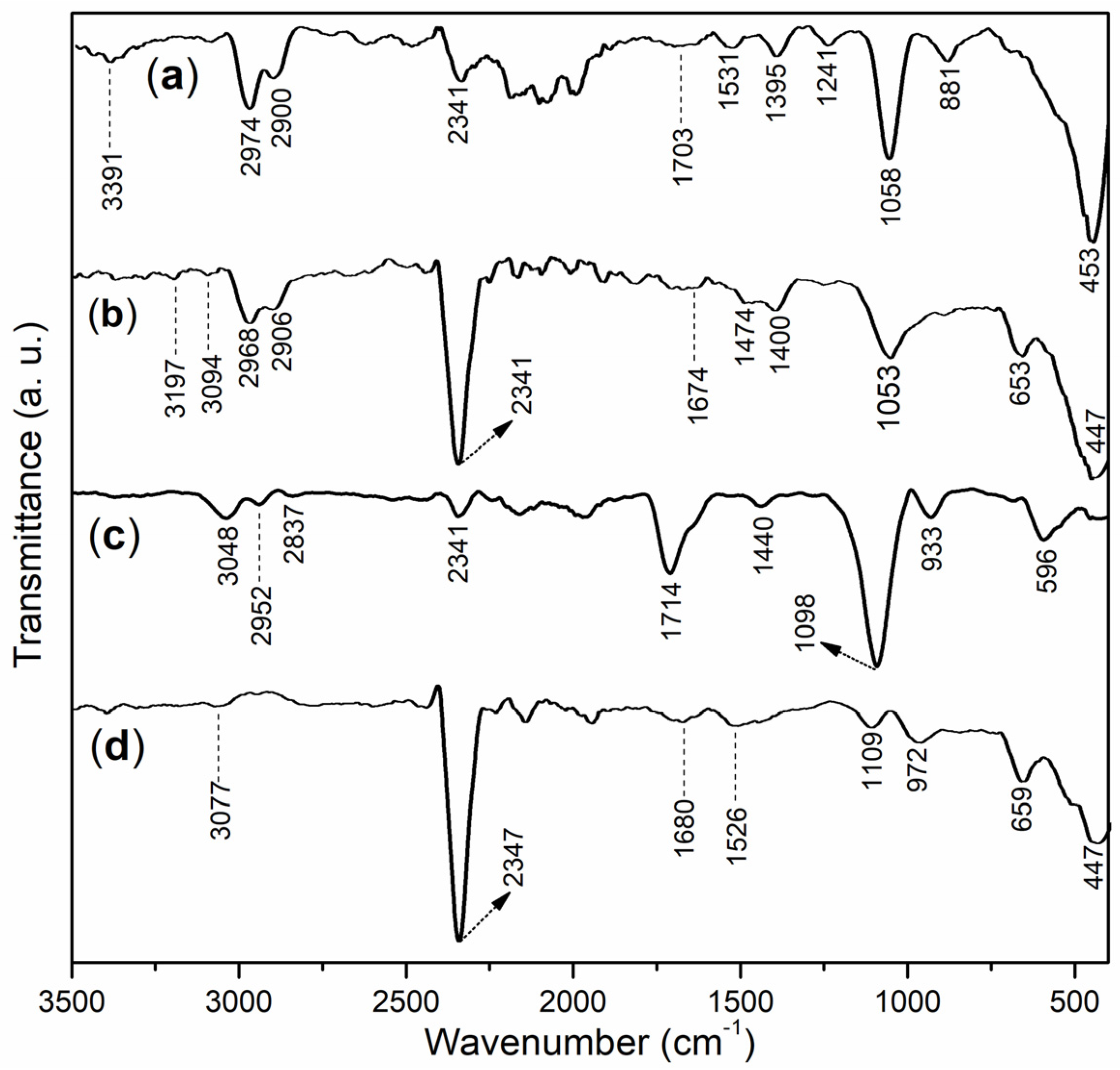
2.1. Characterization of Block Copolymers and Accelerators
2.2. Effects of Accelerators
2.3. Effects of Maleic Anhydride Monomer and Accelerator Dosage
2.4. Analytical Insights into Cementing Process Facilitated by Accelerator
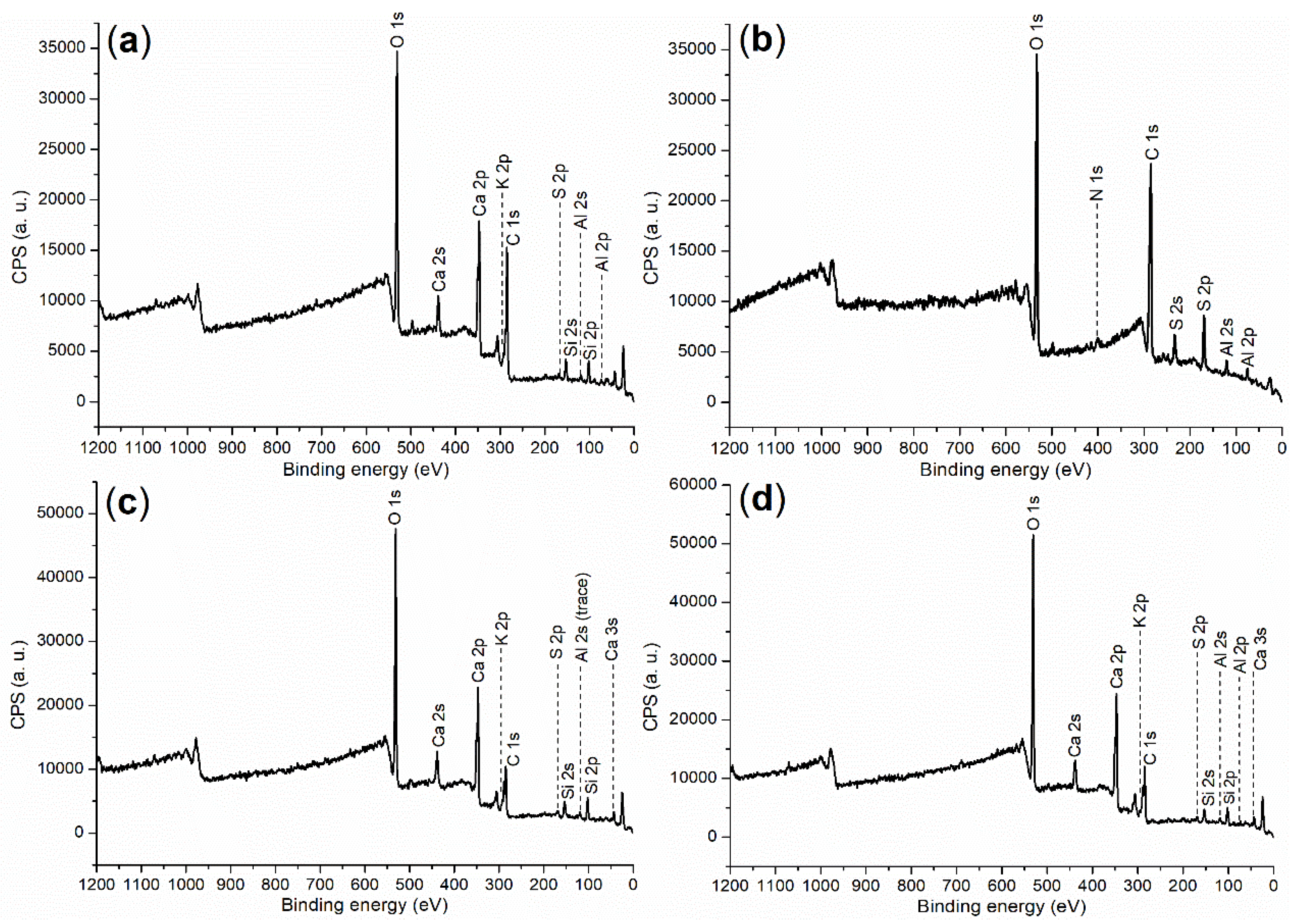
2.4.1. Composition of Cement and Accelerators
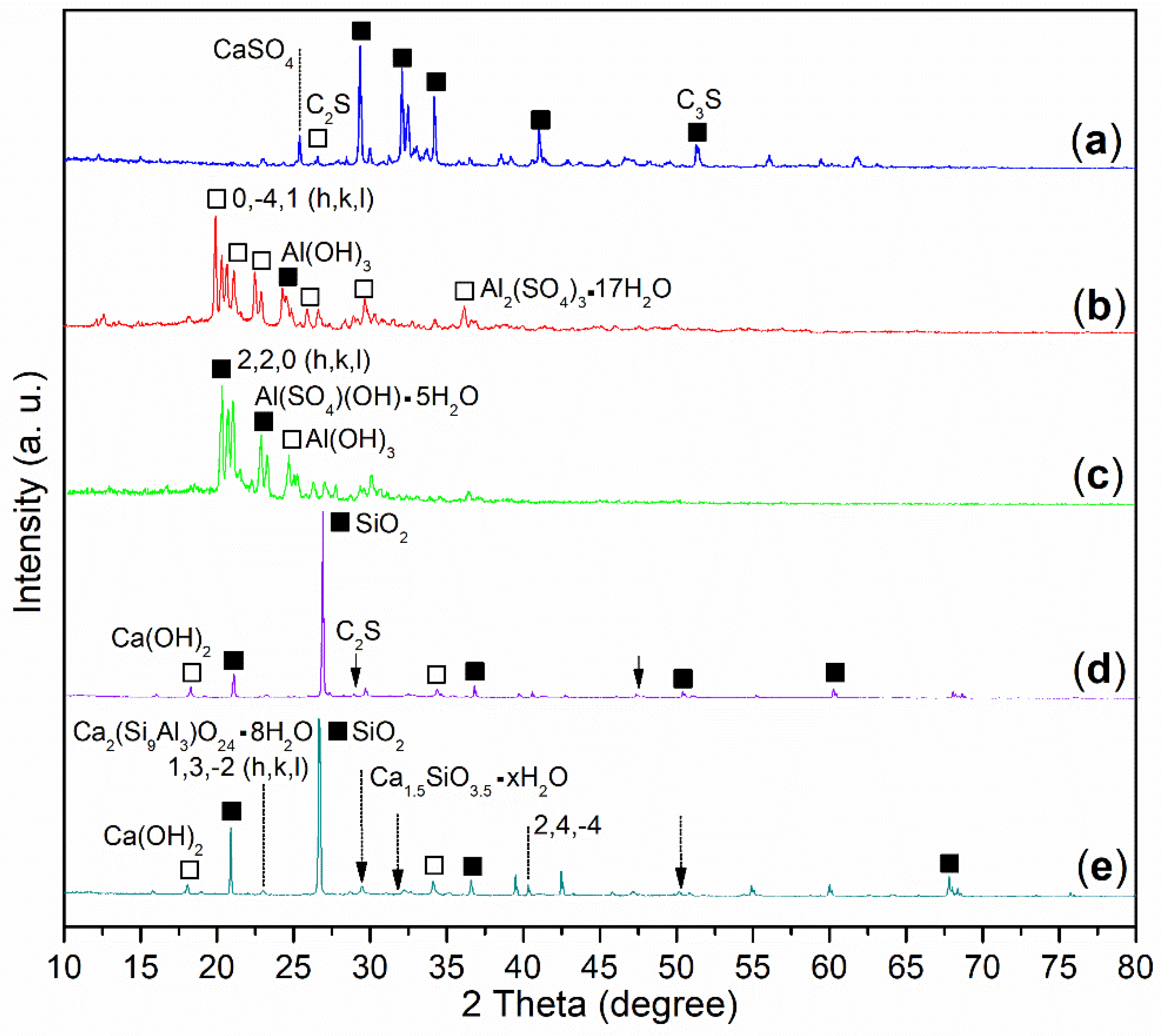
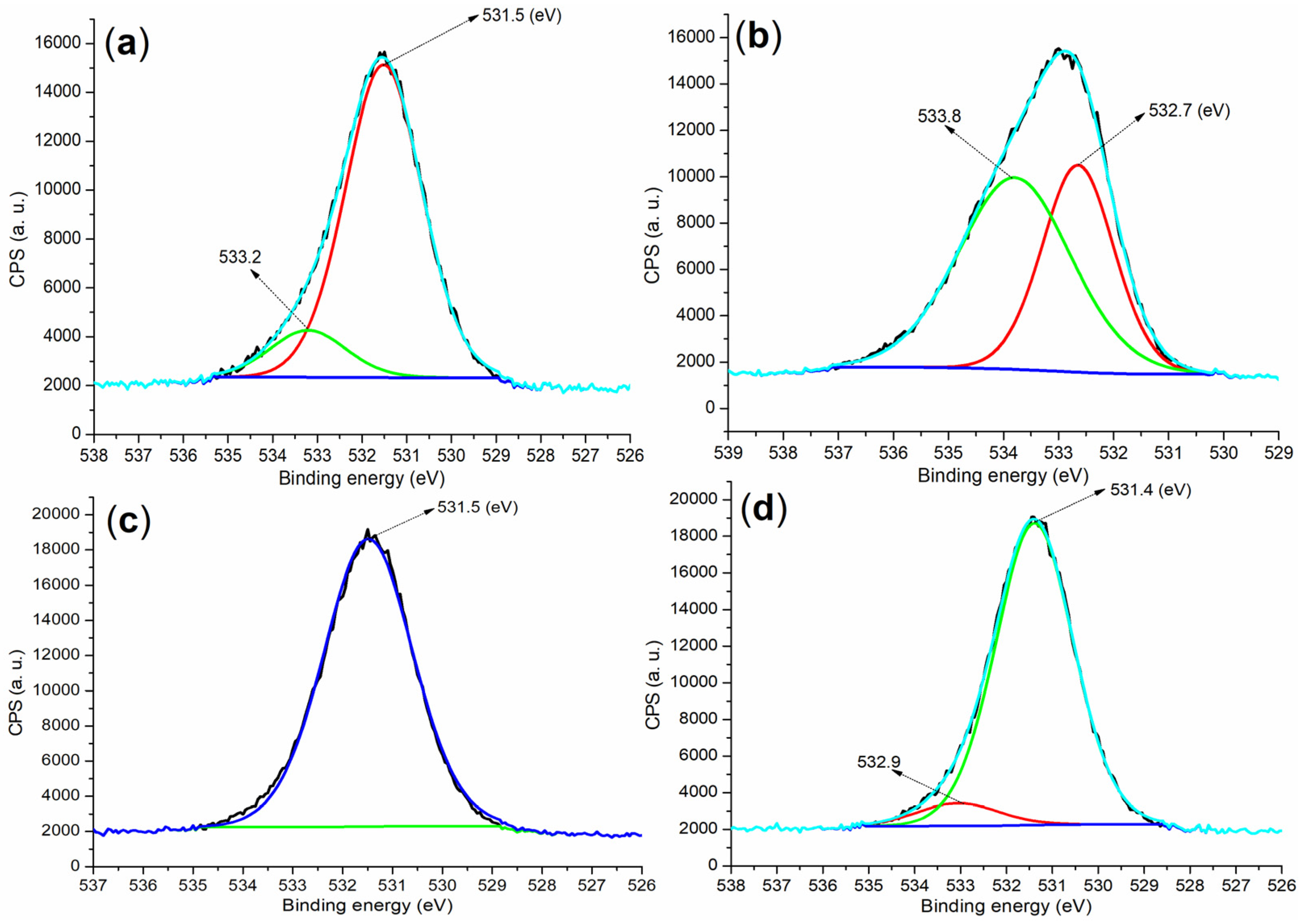
2.4.2. Composition of Hydrated Mortars
2.4.3. Morphology of Hydrated Mortars
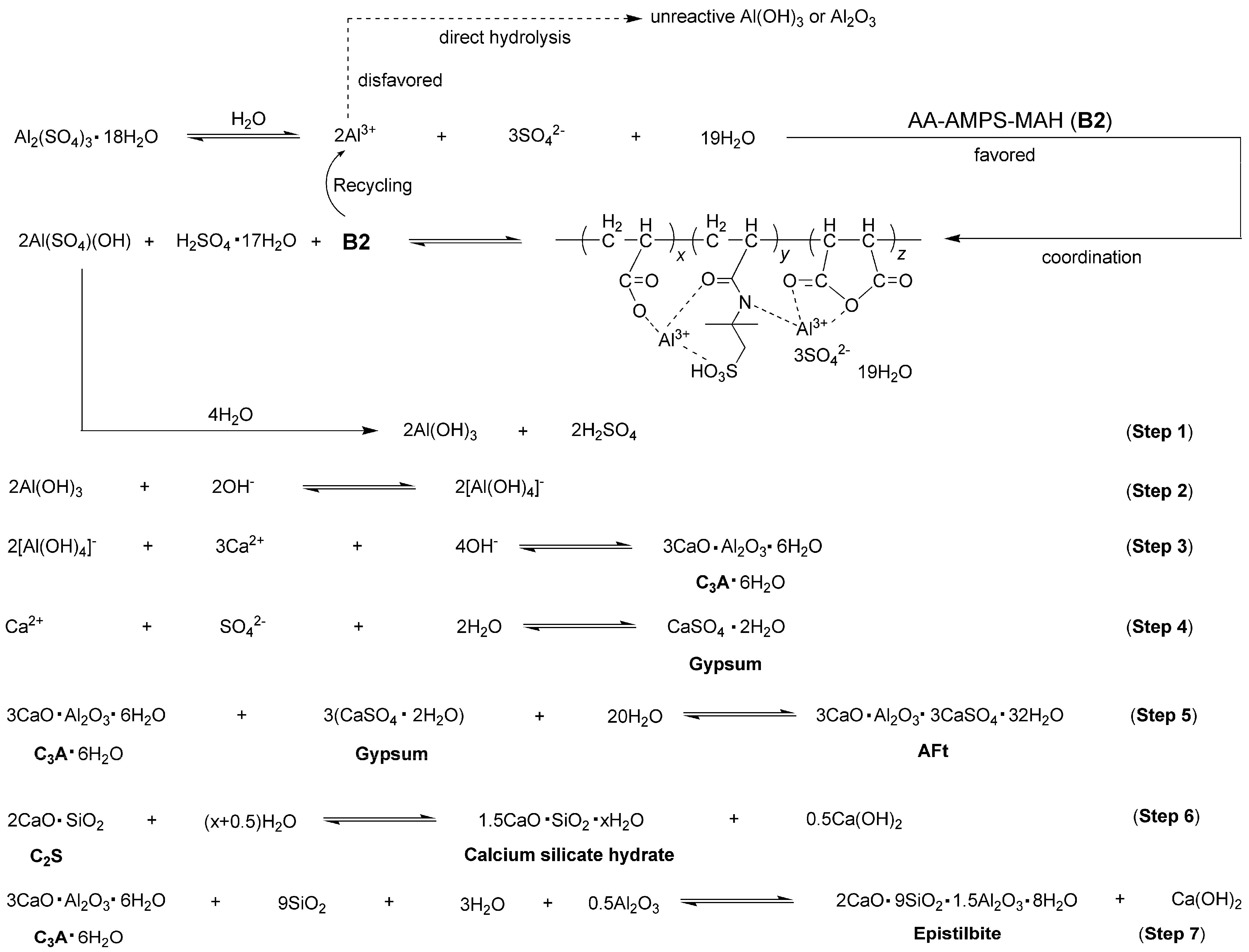
2.5. Proposed Mechanism for Cement Hydration Facilitated by Accelerator B2a
3. Experimental Section
3.1. Starting Materials
3.2. Instruments
3.3. Synthesis of Block Copolymers
3.4. Determination of Monomer Conversion of Block Copolymers
3.5. Synthesis of Setting Accelerators
3.6. Measurement of Setting Time
3.7. Measurement of Compressive and Flexural Strengths
4. Conclusions
- (1)
- The two block copolymers were prepared with high efficiency through ammonium persulfate-catalyzed free radical polymerization in an aqueous solution. The ternary block copolymer having monomers such as AA (acrylic acid), AMPS (2-acrylamido-2-methylpropane sulfonic acid), and MAH (maleic anhydride) showed higher monomer conversion than the binary copolymer featuring AA and AMPS units.
- (2)
- The combination of aluminum sulfate with two synthesized block copolymers can significantly shorten the initial and final setting times of the cement paste and improve the compressive and flexural strengths of the mortar. The conjunction of aluminum sulfate with a ternary block copolymer (AA–AMPS–MAH) performed even better than that with a binary copolymer (AA–AMPS) in cementing.
- (3)
- The role of synthesized block copolymers lay in the inhibition of Al3+ hydrolysis into unreactive Al(OH)3 or Al2O3. In particular, Al(SO4)(OH), derived from the mixing of Al3+ with AA–AMPS–MAH, was detected as an unexpected and highly active intermediate for optimizing cement hydration.
- (4)
- The use of aluminum sulfate associated with AA–AMPS–MAH would produce two new phases, including Ca1.5SiO3.5·xH2O and Ca2(Si9Al3)O24·8H2O in cement mortar after 24 h incubation compared to that with AA–AMPS, which actually acted as a great linker to enhance the mechanical strength of the mortar. Furthermore, the combination of aluminum sulfate with AA–AMPS–MAH produced a much denser mortar (28 d) with better mechanical strength than that with AA–AMPS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wei, T.; Xiao, J.; Cheng, X.; Gong, P.; Mei, K.; Hou, Z.; Wu, X. Improving the mechanical properties of cement-based materials under high temperature: Reducing the C3S/C2S ratio. Constr. Build. Mater. 2024, 421, 135741. [Google Scholar] [CrossRef]
- Phair, J.W. Green chemistry for sustainable cement production and use. Green Chem. 2006, 8, 763–780. [Google Scholar] [CrossRef]
- Trussell, N.; Nordtug, P.Ø.; Asadi, I.; Kristoffersen, M.; Jacobsen, S. Water transport in cracks controlled by digital image correlation in wet sprayed concrete with and without an EVA based co-polymer admixture. Constr. Build. Mater. 2023, 400, 132423. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, C.; Ma, Y.; Xiao, Y.; Liu, Y. Accelerators for shotcrete–Chemical composition and their effects on hydration, microstructure and properties of cement-based materials. Constr. Build. Mater. 2021, 281, 122557. [Google Scholar] [CrossRef]
- Shanahan, N.; Sedaghat, A.; Zayed, A. Effect of cement mineralogy on the effectiveness of chloride-based accelerator. Cement Concr. Comp. 2016, 73, 226–234. [Google Scholar] [CrossRef]
- Xu, Y.; He, T.; Ma, X. The influence of calcium nitrate/sodium nitrate on the hydration process of cement paste mixed with alkali free liquid accelerator. Constr. Build. Mater. 2022, 347, 128555. [Google Scholar] [CrossRef]
- Niu, M.; Li, G.; Zhang, J.; Cao, L. Preparation of alkali-free liquid accelerator based on aluminum sulfate and its accelerating mechanism on the hydration of cement pastes. Constr. Build. Mater. 2020, 253, 119246. [Google Scholar] [CrossRef]
- Xu, Y.; He, T.; Yang, R.; Ma, X. Effect of simulated different concentrations of sulfate on early properties of mineral admixture-cement-liquid accelerator system. J. Water Process. Eng. 2022, 47, 102829. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Zhang, J.; Deng, X.; Li, M.; Jian, S.; Yang, H.; Tan, H. Effects of AFt and steel slag on the performance of beta-hemihydrate phosphogypsum-based cementitious materials. Constr. Build. Mater. 2024, 434, 136749. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Stephan, D.; Elimbi, A. Setting and hardening behavior of volcanic ash phosphate cement. J. Build. Eng. 2020, 31, 101427. [Google Scholar] [CrossRef]
- Park, H.G.; Sung, S.K.; Park, C.G.; Won, J.P. Influence of a C12A7 mineral-based accelerator on the strength and durability of shotcrete. Cem. Concr. Res. 2008, 38, 379–385. [Google Scholar] [CrossRef]
- Rezania, M.; Moradnezhad, H.; Panahandeh, M.; Kami, M.J.R.; Rahmani, A.; Hosseini, B.V. Effects of Diethanolamine (DEA) and Glass Fibre Reinforced Polymer (GFRP) on setting time and mechanical properties of shotcrete. J. Build. Eng. 2020, 31, 101343. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, Z.; Zhang, Y.; Liu, Z.; Pan, Y.; Zhang, G.; He, Y.; Sun, Z. New insight into the combined effect of aluminum sulfate and triethanolamine on cement hydration. Cem. Concr. Res. 2024, 181, 107547. [Google Scholar] [CrossRef]
- Huang, J.; Ge, S.; Wang, H.; Chen, R. Study on the improvement of water resistance and water absorption of magnesium oxychloride cement using long-chain organosilane-nonionic surfactants. Constr. Build. Mater. 2021, 306, 124872. [Google Scholar] [CrossRef]
- Davoodia, S.; Al-Shargabi, M.; Wood, D.A.; Rukavishnikov, V.S. Recent advances in polymers as additives for wellbore cementing applications: A review. Fuel 2024, 357, 129692. [Google Scholar] [CrossRef]
- Tuffrey, J.; Siwseng, P.; Laksanakit, C.; Chusilp, N. Enhancing the performance of waste paper pulp-cement composites, through the incorporation of natural rubber latex: A sustainable approach for high-performance construction materials. Constr. Build. Mater. 2024, 430, 136345. [Google Scholar] [CrossRef]
- Lanka, S.T.; Moses, N.G.A.; Suppiah, R.R.; Maulianda, B.T. Physio-chemical interaction of Ethylene-Vinyl Acetate copolymer on bonding ability in the cementing material used for oil and gas well. Petrol. Res. 2022, 7, 341–349. [Google Scholar] [CrossRef]
- Wu, W.; Yu, X.; Hu, A.; Yan, H.; Dong, Z.; Yang, H.; Su, G. Amphoteric retarder for long-standing cementing: Preparation, properties and working mechanism. Geoenergy Sci. Eng. 2023, 223, 211524. [Google Scholar] [CrossRef]
- Zhu, W.; Feng, Q.; Luo, Q.; Bai, X.; Chen, K.; Lin, X. Effect of a specific PCE superplasticizer on the initial dissolution and early hydration of Portland cement. J. Build. Eng. 2022, 46, 103786. [Google Scholar] [CrossRef]
- Singh, N.B.; Singh, V.D.; Rai, S.; Chaturvedi, S. Effect of lignosulfonate, calcium chloride and their mixture on the hydration of RHA-blended portland cement. Cem. Concr. Res. 2002, 32, 387–392. [Google Scholar] [CrossRef]
- Cui, Y.; Tan, Z.; Zhou, Z.; Wu, J.; Wang, J. Preparation and application of low rebound liquid alkali-free accelerator for shotcrete. Constr. Build. Mater. 2023, 367, 130220. [Google Scholar] [CrossRef]
- Garba, M.J.; Tian, Y.; Xie, Z.; Yu, C.; Hu, C.; Chen, L.; Yuan, Q. Effect of accelerators on the long-term performance of shotcrete and its improvement strategies: A review. J. Build. Eng. 2024, 89, 109364. [Google Scholar] [CrossRef]
- Tiemeyer, C.; Plank, J. Synthesis, characterization, and working mechanism of a synthetic high temperature (200 °C) fluid loss polymer for oil well cementing containing allyloxy-2-hydroxy propane sulfonic (AHPS) acid monomer. J. Appl. Polym. Sci. 2013, 128, 851–860. [Google Scholar] [CrossRef]
- Chang, Q.; Liu, G.; Dong, Z.; Miao, X.; Hu, M.; Guo, J. Effect of poly(AMPS/DMAA/IA/SSS) intercalated Mg/Al layered double hydroxides on reducing fluid loss at 240 °C and improving early strength of oil well cement. Appl. Clay Sci. 2022, 229, 106658. [Google Scholar] [CrossRef]
- Jiang, B.; Chen, D.; Wang, L.; Yang, W. A novel approach to methoxy polyethylene glycol-grafted sulfonated poly(maleic anhydride-alt-styrene) as superplasticizers in cement pastes. Constr. Build. Mater. 2023, 370, 130598. [Google Scholar] [CrossRef]
- Bednarza, S.; Wesołowska-Piętaka, A.; Konefał, R.; Świergosz, T. Persulfate initiated free-radical polymerization of itaconic acid: Kinetics, end-groups and side products. Eur. Polym. J. 2018, 106, 63–67. [Google Scholar] [CrossRef]
- Shu, H.; Song, C.; Yang, L.; Wang, C.; Chen, D.; Zhang, X.; Ma, Y.; Yang, W. Self-stabilized precipitation polymerization of vinyl chloride and maleic anhydride. Ind. Eng. Chem. Res. 2023, 62, 3612–3621. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.M.; Zhang, L.; Gerritsen, G.; Abbenhuis, H.C.L.; van Santen, R.A.; Li, C. Enantioselective epoxidation epoxidation of β-methylstyrene catalyzed by immobilized Mn(salen) catalysts in different mesoporous silica supports. J. Catal. 2008, 256, 226–236. [Google Scholar] [CrossRef]
- Kara, Y.S.; Koparal, S.; Tekin, N.; Ömür, N. New approach: Solvent effects of benzaldehyde in aliphatic alcohol solvents with FT-IR spectroscopy and augmented vertex-adjacency matrices. J. Mol. Struct. 2024, 1321, 139815. [Google Scholar] [CrossRef]
- Huang, B.; Fan, C.; Yu, H.; Ma, J.; Pan, C.; Zhang, D.; Zheng, A.; Li, Y.; Sun, Y. Sol-gel preparation of helical silicate containing palladium oxide nanoparticles and the application for nitration of aromatic compound. Mol. Catal. 2018, 446, 140–151. [Google Scholar] [CrossRef]
- Zhao, F.; Yang, S.; Xiao, X.; Chen, L.; Wang, Y.; Li, Y.; E, T. Bovine hide collagen/tannin extract composite: A revelation of the selective structure-activity relationship between phenolic hydroxyls and Cu(II) and study on adsorption properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 682, 132886. [Google Scholar] [CrossRef]
- Dharani, S.; Kalaiarasi, G.; Lynch, V.M.; Shankar, R.; Prabhakaran, R. Unpredicted coordination of hydrogen chloride in organoruthenium(II) pyrazolone complex: Synthesis, spectral and structural characterization. Inorg. Chem. Commun. 2023, 153, 110849. [Google Scholar] [CrossRef]
- Li, S. Microstructure and composition characterisation of three 20-year-old GGBS-OPC blended pastes. Constr. Build. Mater. 2016, 123, 226–234. [Google Scholar] [CrossRef]
- Shivani; Sharma, N.; Kumar, M.; Kumar, M. Low resistance ohmic contact of multi-metallic Mo/Al/Au stack with ultra-wide bandgap Ga2O3 thin film with post-annealing and its in-depth interface studies for next-generation high-power devices. Surf. Interfaces 2024, 46, 103937. [Google Scholar] [CrossRef]
- Chubar, N. XPS determined mechanism of selenite (HSeO3−) sorption in absence/presence of sulfate (SO42−) on Mg-Al-CO3 Layered double hydroxides (LDHs): Solid phase speciation focus. J. Environ. Chem. Eng. 2023, 11, 109669. [Google Scholar]
- Haselbach, L.M.; Ma, S. Potential for carbon adsorption on concrete: Surface XPS Analyses. Environ. Sci. Technol. 2008, 42, 5329–5334. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhang, Y.; Ma, Y.; Gu, Q.; Low, Z.; Zhang, Z.; Wang, J. Recycling metal-organic framework residues (MOFRs) as bifunctional additives in fabricating porous ceramic membranes. J. Environ. Chem. Eng. 2024, 12, 114105. [Google Scholar] [CrossRef]
- Iatsunskyi, I.; Gottardi, G.; Micheli, V.; Canteri, R.; Coy, E.; Bechelany, M. Atomic layer deposition of palladium coated TiO2/Si nanopillars: ToF-SIMS, AES and XPS characterization study. Appl. Surf. Sci. 2021, 542, 148603. [Google Scholar] [CrossRef]
- Korin, E.; Froumin, N.; Cohen, S. Surface Analysis of nanocomplexes by x-ray photoelectron spectroscopy (XPS). ACS Biomater. Sci. Eng. 2017, 3, 882–889. [Google Scholar] [CrossRef]
- Imamura, M.; Matsubayashi, N.; Shimada, H. Catalytically active oxygen species in La1−xSrxCoO3−δ studied by XPS and XAFS spectroscopy. J. Phys. Chem. B 2000, 104, 7348–7353. [Google Scholar] [CrossRef]
- Rincon, J.; Camarillo, R.; Martín, A. Solubility of aluminum sulfate in near-critical and supercritical water. J. Chem. Eng. Data 2012, 57, 2084–2094. [Google Scholar] [CrossRef]
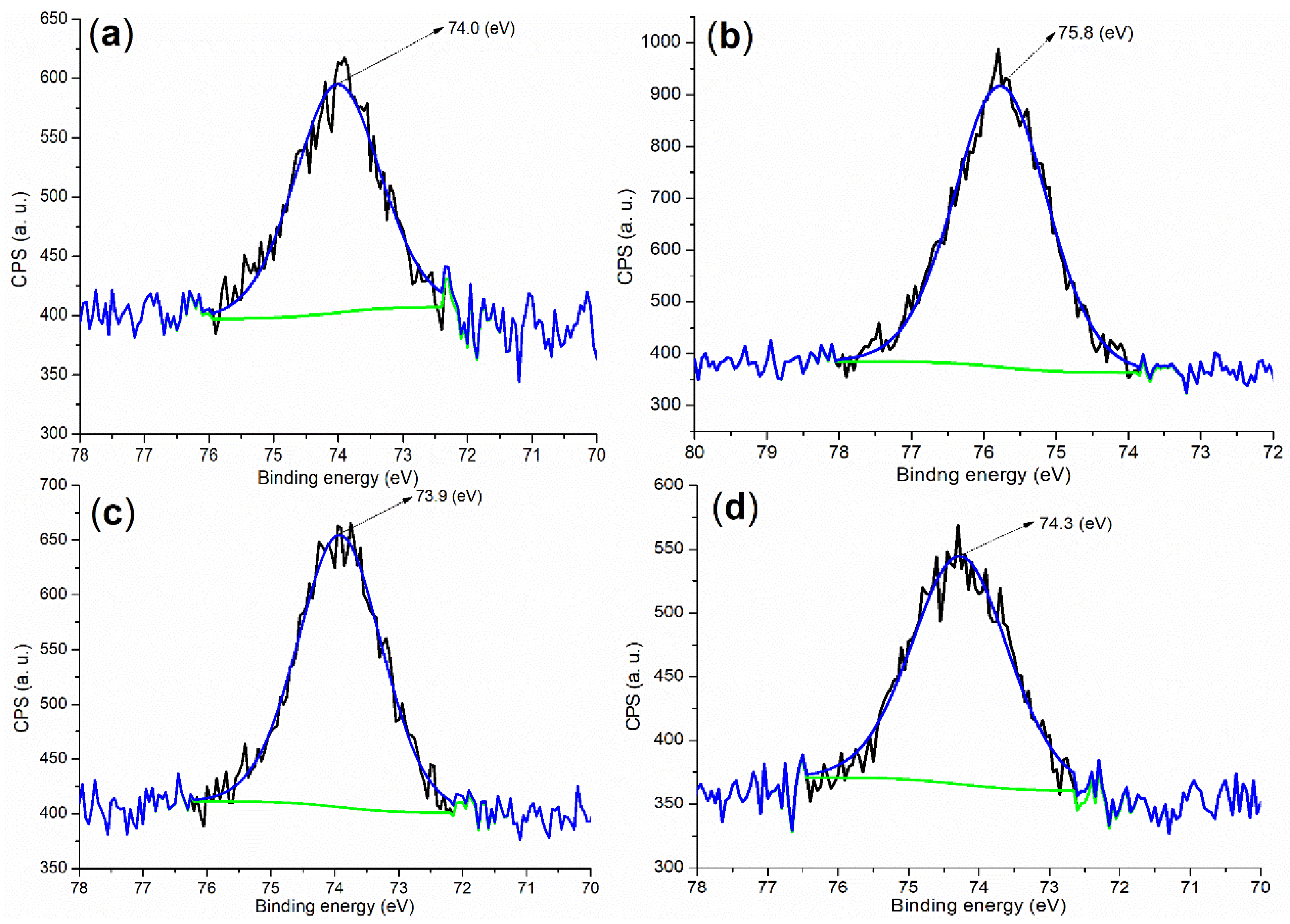

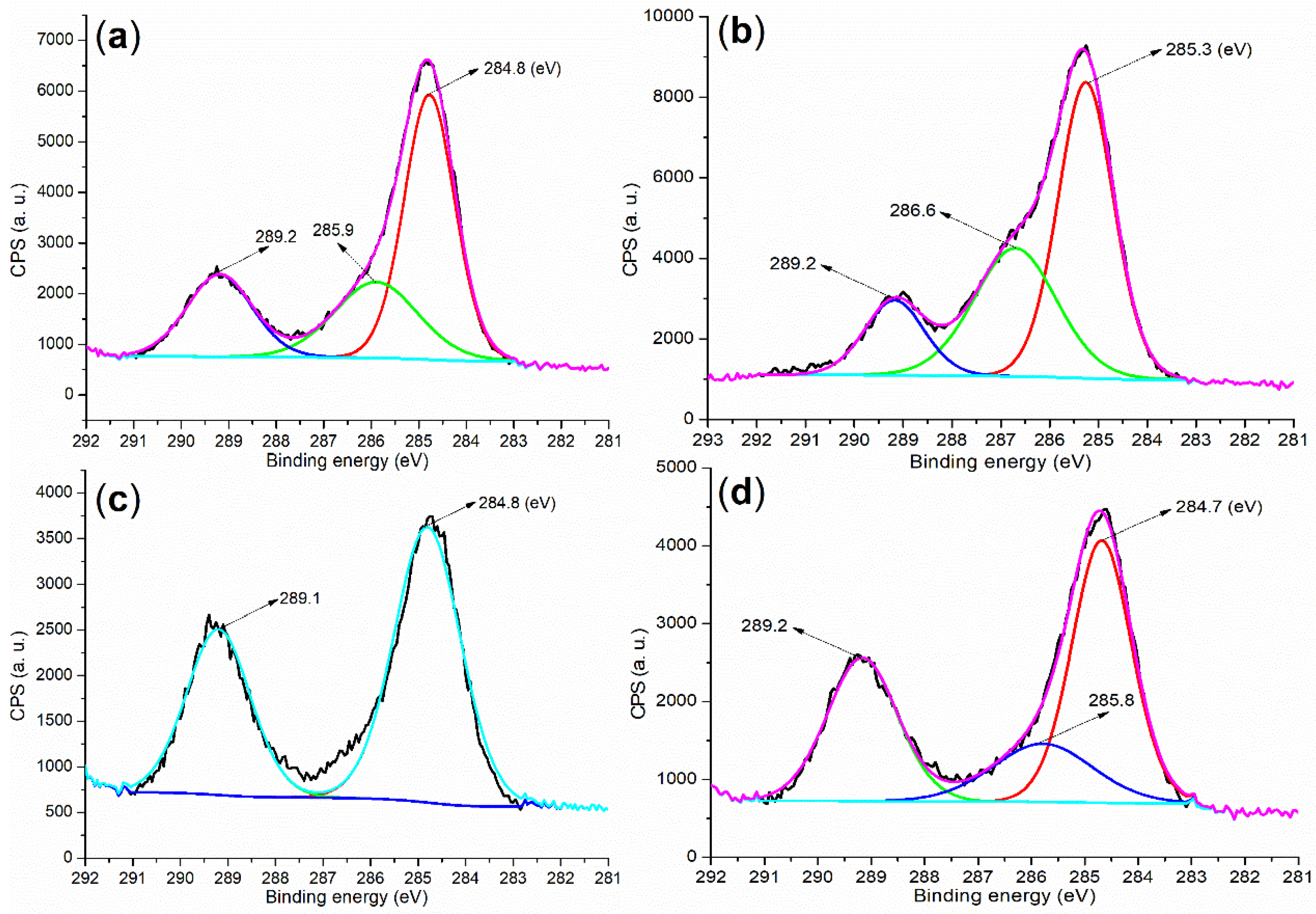
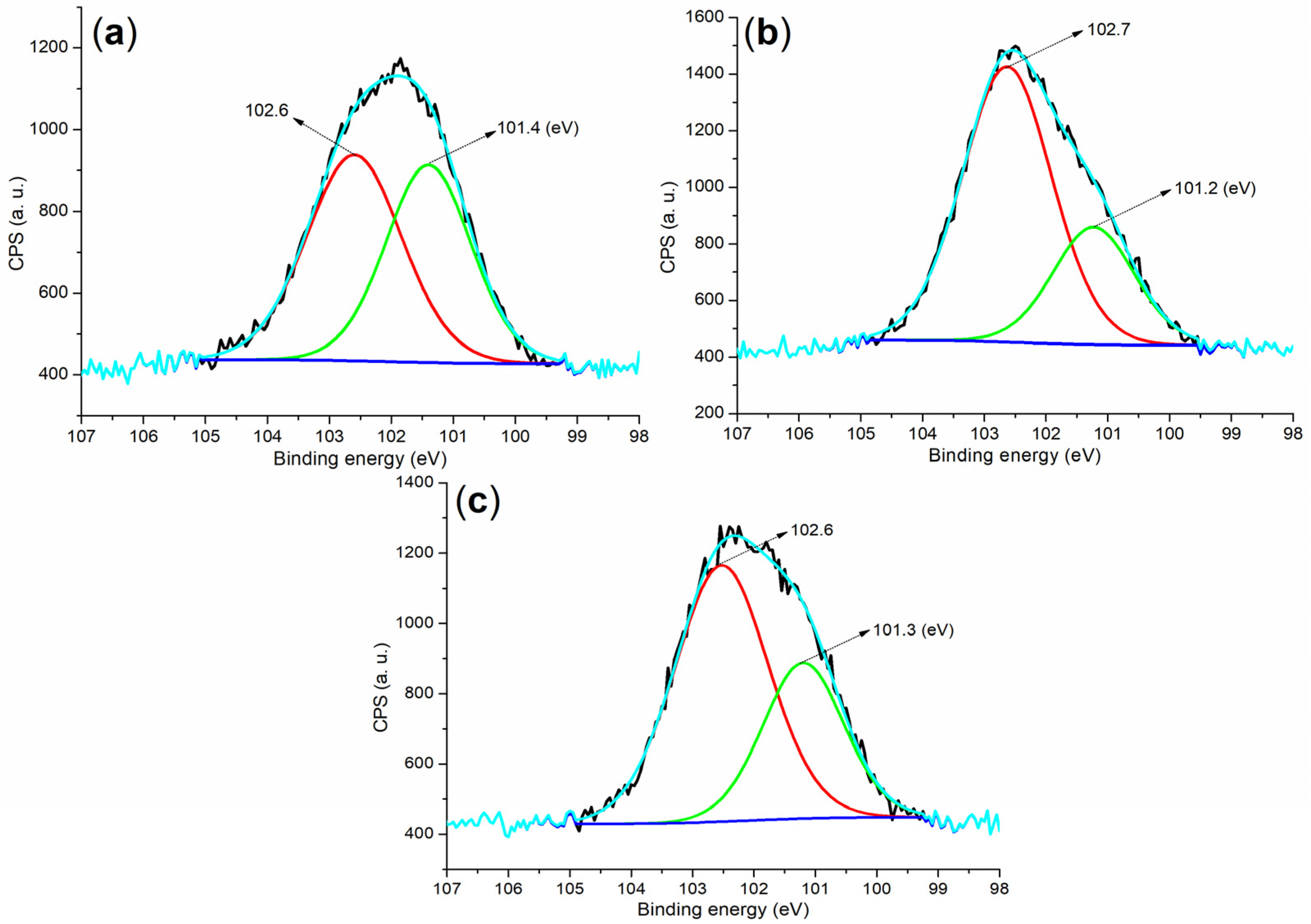
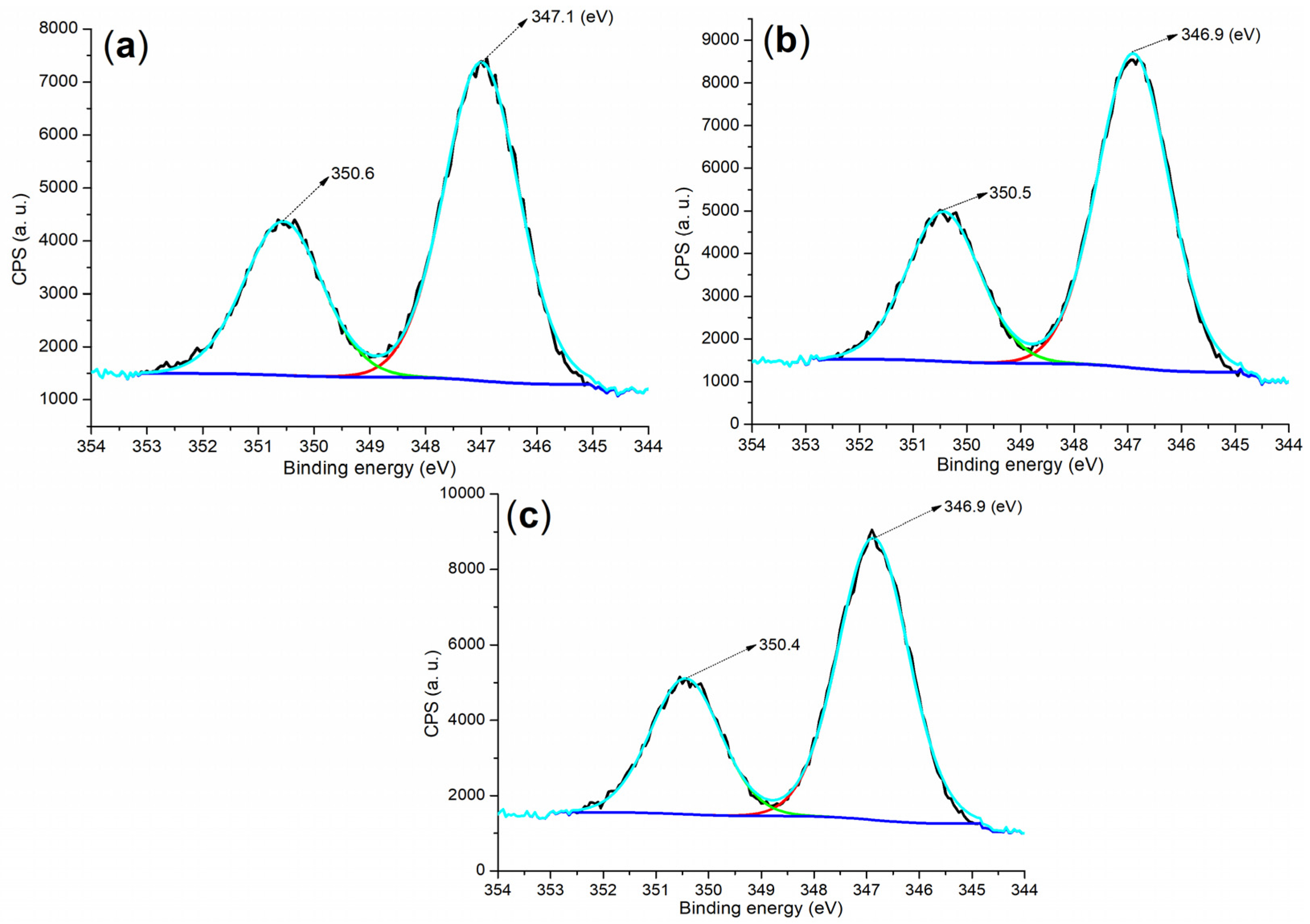

| Sample | m (g) b | V (mL) c | X (mg g−1) d | α (%) e |
|---|---|---|---|---|
| blank | - | 20.60 | - | - |
| B1 | 0.50 | 14.70 | 94.28 | 96.90 |
| B2 | 0.50 | 16.50 | 65.52 | 97.62 |
| Entry a | Accelerator (Dosage) b | Setting Time (Min, Cement Paste) c | Compressive Strength (MPa, Cement Mortar) d | Flexural Strength (MPa, Cement Mortar) d | |||||
|---|---|---|---|---|---|---|---|---|---|
| Initial (IST) | Final (FST) | 6 h | 24 h | 28 d | 6 h | 24 h | 28 d | ||
| 1 | blank | 28.50 ± 0.81 e | 39.75 ± 0.95 | 0.6 ± 0.02 | 4.5 ± 0.31 | 22.4 ± 0.45 | 0.3 ± 0.02 | 2.7 ± 0.15 | 10.9 ± 0.10 |
| 2 | AS (7%) | 18.75 ± 0.95 | 36.74 ± 1.87 | 1.1 ± 0.16 | 6.0 ± 0.25 | 24.9 ± 0.50 | 0.8 ± 0.11 | 2.9 ± 0.02 | 11.7 ± 0.22 |
| 3 | B1 (7%) | 26.77 ± 0.17 | 35.65 ± 0.27 | 0.9 ± 0.04 | 6.2 ± 0.30 | 23.9 ± 0.41 | 0.4 ± 0.03 | 2.9 ± 0.20 | 11.9 ± 0.17 |
| 4 | B1a (6%) | 4.75 ± 0.25 | 8.30 ± 0.30 | 1.3 ± 0.03 | 7.4 ± 0.20 | 25.9 ± 0.37 | 0.8 ± 0.01 | 3.6 ± 0.21 | 12.8 ± 0.19 |
| 5 | B1a (7%) | 1.03 ± 0.02 | 1.95 ± 0.04 | 1.3 ± 0.04 | 10.7 ± 0.23 | 27.9 ± 0.60 | 0.9 ± 0.15 | 4.0 ± 0.32 | 13.6 ± 0.28 |
| 6 | B1a (8%) | 1.35 ± 0.02 | 2.18 ± 0.05 | 1.6 ± 0.07 | 9.8 ± 0.30 | 27.0 ± 0.53 | 0.2 ± 0.07 | 3.8 ± 0.26 | 12.1 ± 0.31 |
| 7 | B2a (6%) | 2.01 ± 0.17 | 3.08 ± 0.09 | 1.7 ± 0.05 | 7.8 ± 0.26 | 26.2 ± 0.49 | 1.5 ± 0.07 | 4.3 ± 0.20 | 13.9 ± 0.09 |
| 8 | B2a (7%) | 1.01 ± 0.02 | 1.66 ± 0.02 | 1.8 ± 0.03 | 12.5 ± 0.55 | 30.5 ± 0.67 | 1.7 ± 0.03 | 4.6 ± 0.11 | 14.5 ± 0.12 |
| 9 | B2a (8%) | 1.45 ± 0.05 | 2.02 ± 0.06 | 1.5 ± 0.03 | 9.8 ± 0.19 | 27.3 ± 0.39 | 1.1 ± 0.06 | 4.1 ± 0.08 | 12.9 ± 0.28 |
| Entry a | R28 (%) b | Rr, 90 (%) c |
|---|---|---|
| 5 | 125 | 107 |
| 8 | 136 | 198 |
| Composition | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | MgO | K2O | Na2O | Ignition Loss b |
|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 60.66 | 18.26 | 5.95 | 4.01 | 4.75 | 1.76 | 1.35 | 0.41 | 2.85 |
| Entry | C (1s) | O (1s) | S (2p) | Si (2p) | K (2p) | Ca (2p) or N (1s) | Al (2p) |
|---|---|---|---|---|---|---|---|
| Cement a | 284.80 (40.84) b | 530.80 (30.70) | 167.80 (0.55) | 101.80 (6.54) | 292.80 (10.29) | 346.80 (9.36) | 73.80 (1.73) |
| B1a | 284.80 (56.59) | 531.80 (30.24) | 168.80 (9.14) | - c | - | 401.80 (0.11) d | 74.80 (3.92) |
| B2a | 284.80 (57.29) | 531.80 (31.76) | 168.80 (7.18) | - | - | 401.80 (1.59) d | 74.80 (2.17) |
| 5 (24 h) e | 284.80 (29.85) | 530.80 (40.12) | 167.80 (1.30) | 101.80 (9.55) | 292.80 (7.52) | 346.80 (11.51) | 73.80 (0.15) |
| 8 (24 h) e | 284.80 (29.27) | 531.80 (39.89) | 169.80 (0.89) | 102.80 (9.22) | 293.0 (7.37) | 346.80 (11.35) | 73.80 (2.01) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Z.; Chaudhary, S.; Bibi, Z.; Wu, Y.; Jia, Q.; Li, X.; Ouyang, W.; Sun, Y. The Coordination of Aluminum Sulfate with a Water-Soluble Block Copolymer Containing Carboxyl, Amide, Sulfonic and Anhydride Groups Providing Both Accelerating and Hardening Effects in Cement Setting. Molecules 2024, 29, 4543. https://doi.org/10.3390/molecules29194543
Song Z, Chaudhary S, Bibi Z, Wu Y, Jia Q, Li X, Ouyang W, Sun Y. The Coordination of Aluminum Sulfate with a Water-Soluble Block Copolymer Containing Carboxyl, Amide, Sulfonic and Anhydride Groups Providing Both Accelerating and Hardening Effects in Cement Setting. Molecules. 2024; 29(19):4543. https://doi.org/10.3390/molecules29194543
Chicago/Turabian StyleSong, Zhiyuan, Sidra Chaudhary, Zainab Bibi, Yong Wu, Qinxiang Jia, Xiaoyong Li, Weiyi Ouyang, and Yang Sun. 2024. "The Coordination of Aluminum Sulfate with a Water-Soluble Block Copolymer Containing Carboxyl, Amide, Sulfonic and Anhydride Groups Providing Both Accelerating and Hardening Effects in Cement Setting" Molecules 29, no. 19: 4543. https://doi.org/10.3390/molecules29194543
APA StyleSong, Z., Chaudhary, S., Bibi, Z., Wu, Y., Jia, Q., Li, X., Ouyang, W., & Sun, Y. (2024). The Coordination of Aluminum Sulfate with a Water-Soluble Block Copolymer Containing Carboxyl, Amide, Sulfonic and Anhydride Groups Providing Both Accelerating and Hardening Effects in Cement Setting. Molecules, 29(19), 4543. https://doi.org/10.3390/molecules29194543






