Discovery of Cinnamic Acid Derivatives as Potent Anti-H. pylori Agents
Abstract
:1. Introduction
2. Results and Discussion
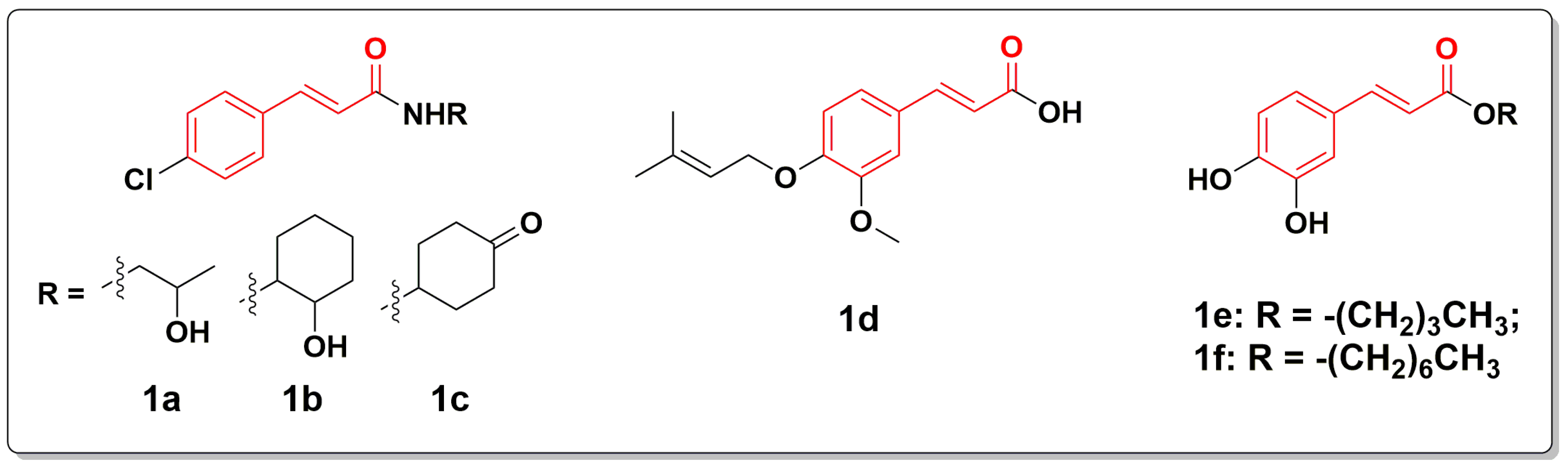
2.1. Chemistry
2.2. Inhibitory Effect of All Compounds against H. pylori
2.3. MIC and MBC
2.4. Inhibiting Kinetics and Killing Kinetics of Compound 23 against H. pylori
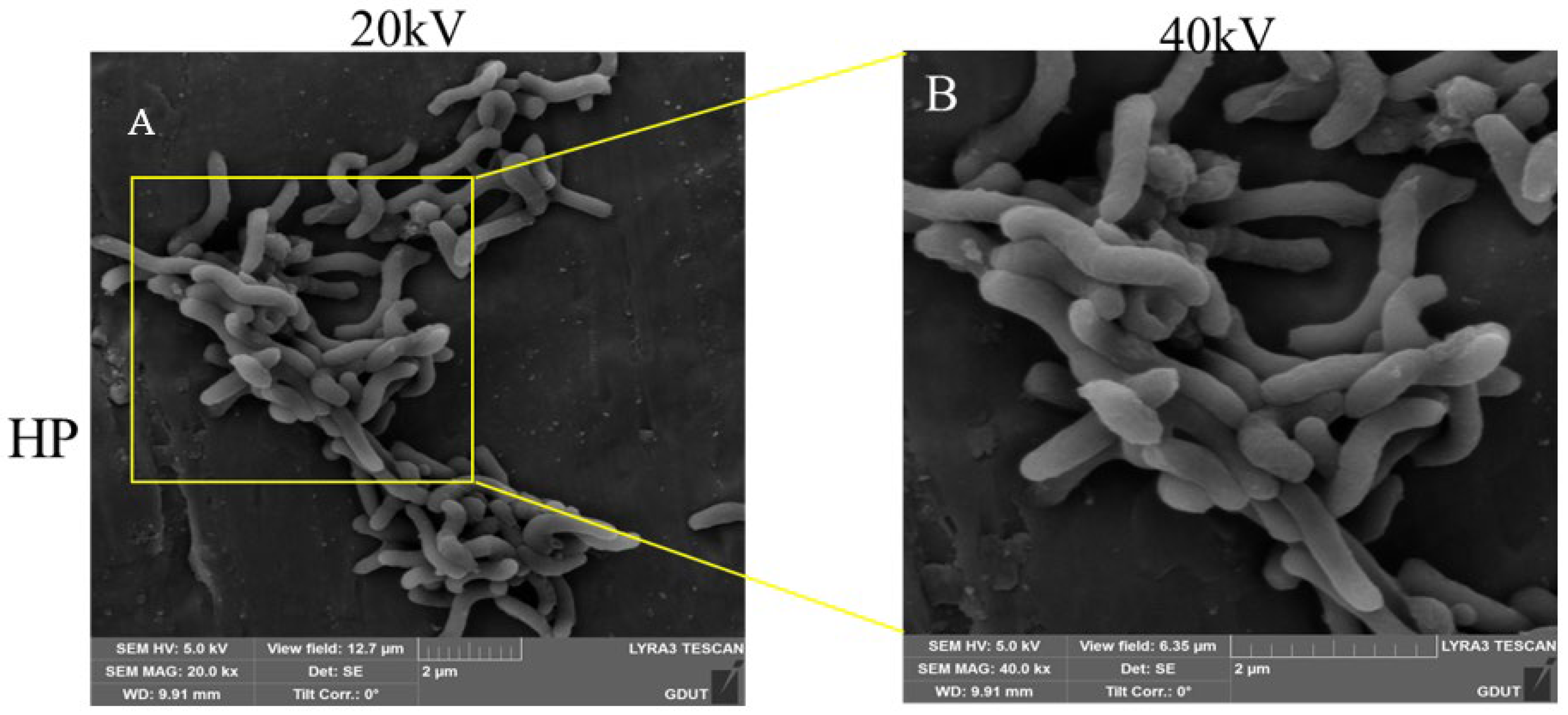
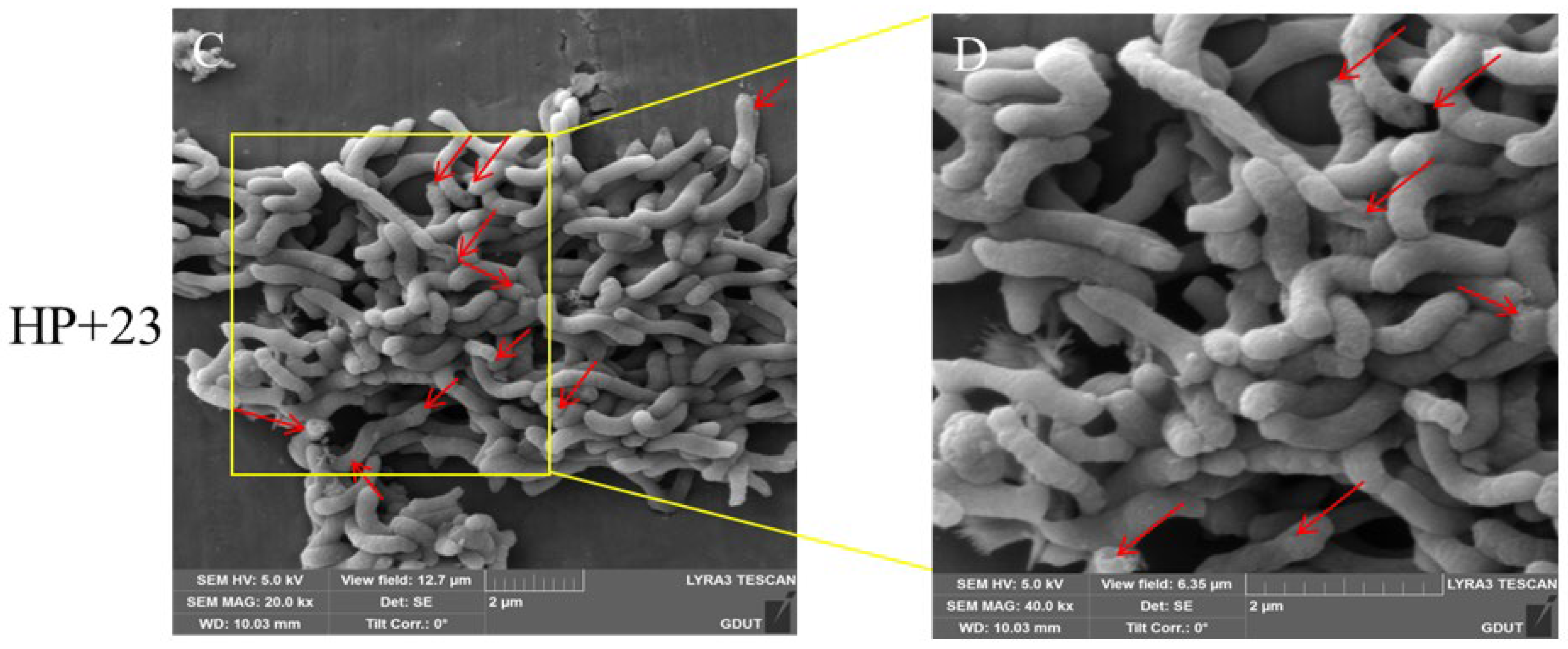
2.5. Effect of Compound 23 on the Morphology of H. pylori
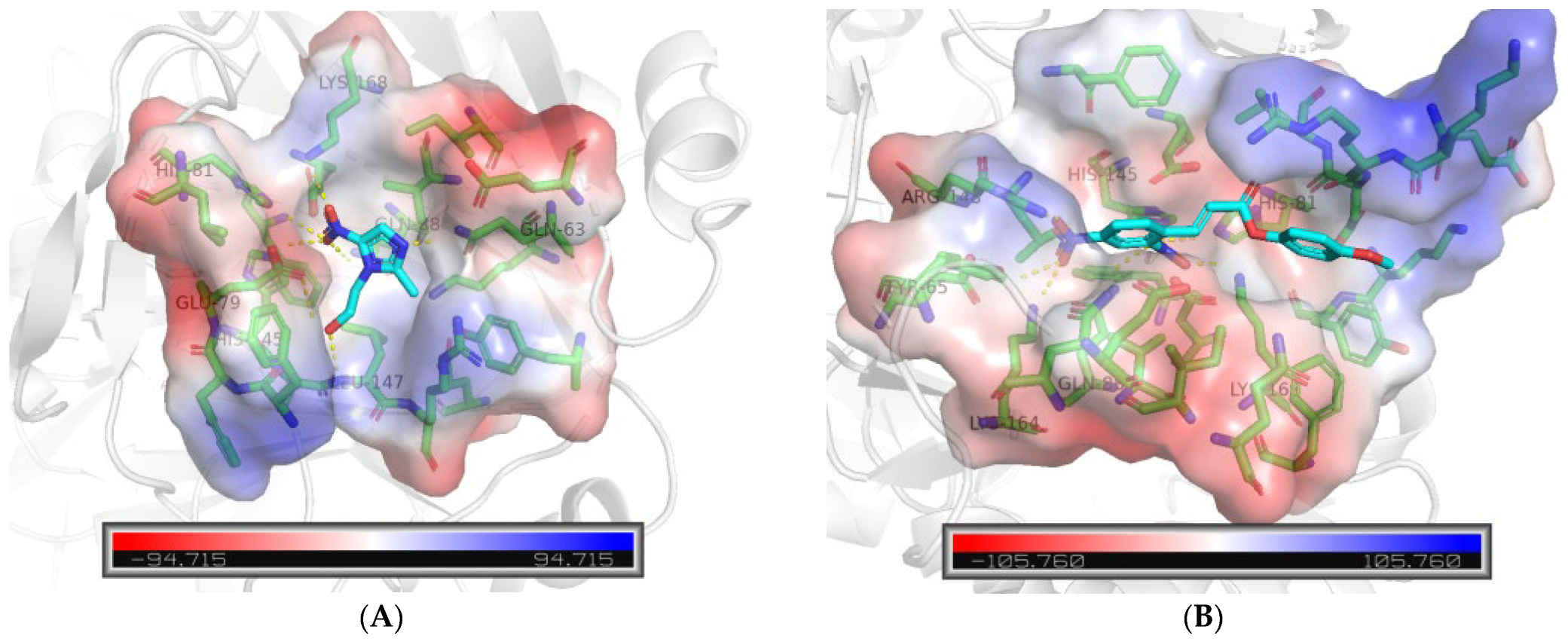
2.6. Molecular Docking Analysis
3. Materials and Methods
3.1. Materials
3.2. General Procedure for the Preparation of Compound 1–30
3.3. Bacterial Culture
3.4. Anti-Helicobacter pylori Activity
3.5. Inhibiting Kinetics and Killing Kinetics Assays
3.6. SEM
3.7. Molecular Docking
3.8. Data and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dunn, B.; Cohen, H.; Blaser, M. Helicobacter pylori. Clin. Microbiol. Rev. 1997, 10, 720–741. [Google Scholar] [CrossRef] [PubMed]
- Suerbaum, S.; Michetti, P. Helicobacter pylori infection. N. Engl. J. Med. 2002, 347, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Butcher, L.D.; den Hartog, G.; Ernst, P.B.; Crowe, S.E. Oxidative stress resulting from Helicobacter pylori infection contributes to gastric carcinogenesis. Cell. Mol. Gastroenterol. Hepatol. 2017, 3, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Toh, J.W.T.; Wilson, R.B. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. Int. J. Mol. Sci. 2020, 21, 6451. [Google Scholar] [CrossRef]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y.; Chen, Y.; Wang, J.B.; Du, Y.Q.; Lu, N.H. Fifth Chinese National consensus report on the management of Helicobacter pylori infection. Helicobacter 2018, 23, 12475. [Google Scholar] [CrossRef]
- Otto, R.G.; van Gorp, E.; Kloezen, W.; Meletiadis, J.; van den Berg, S.; Mouton, J.W. An alternative strategy for combination therapy: Interactions between polymyxin B and non-antibiotics. Int. J. Antimicrob. Agents 2019, 53, 34–39. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef]
- Thung, I.; Aramin, H.; Vavinskaya, V.; Gupta, S.; Park, J.Y.; Crowe, S.E.; Valasek, M.A. Review article: The global emergence of Helicobacter pylori antibiotic resistance. Aliment. Pharmacol. Ther. 2016, 43, 514–533. [Google Scholar] [CrossRef]
- Lyu, T.; Cheung, K.S.; Ni, L.; Guo, J.; Mu, P.; Li, Y.; Yang, Q.; Yu, X.; Lyu, Z.; Wu, J.; et al. High prevalence and risk factors of multiple antibiotic resistance in patients who fail first-line Helicobacter pylori therapy in southern China: A municipality-wide, multicentre, prospective cohort study. J. Antimicrob. Chemother. 2020, 75, 3391–3394. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, C.; Liu, Y.; Huang, Y.; Bai, Y.; Hang, X.; Zeng, L.; Zhu, D.; Bi, H. Armeniaspirol A: A novel anti-Helicobacter pylori agent. Microb. Biotechnol. 2020, 15, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Sweet, R.; Kroon, P.A.; Webber, M.A. Activity of antibacterial phytochemicals and their potential use as natural food preservatives. Crit. Rev. Food Sci. Nutr. 2024, 64, 2076–2087. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Pastor, R.; Carrera-Pacheco, S.E.; Zúñiga-Miranda, J.; Rodríguez-Pólit, C.; Mayorga-Ramos, A.; Guamán, L.P.; Barba-Ostria, C. Current landscape of methods to evaluate antimicrobial activity of natural extracts. Molecules 2023, 28, 1068. [Google Scholar] [CrossRef] [PubMed]
- Ghobadi, E.; Ghanbarimasir, Z.; Emami, S. A review on the structures and biological activities of anti-Helicobacter pylori agents. Eur. J. Med. Chem. 2021, 223, 113669. [Google Scholar] [CrossRef]
- Tshibangu-Kabamba, E.; Yamaoka, Y. Helicobacter pylori infection and antibiotic resistance-from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 613–629. [Google Scholar]
- Shreaz, S.; Wani, W.A.; Behbehani, J.M.; Raja, V.; Irshad, M.; Karched, M.; Ali, I.; Siddiqi, W.A.; Hun, L.T. Cinnamaldehyde and its constituents, a novel class of antifungal agents. Fitoterapia 2016, 12, 116–131. [Google Scholar]
- Melo, A.D.B.; Amaral, A.F.; Schaefer, G.; Luciano, F.B.; de Andrade, C.; Costa, L.B.; Rostagno, M.H. Antimicrobial effect against different bacterial strains and bacterial adaptation to essential oils used as feed additives. Can. J. Vet. Res. 2015, 79, 285–289. [Google Scholar]
- Lu, Z.; Jia, Q.; Wang, R.; Wu, X.; Wu, Y.; Huang, C.; Li, Y. Hypoglycemic activities of A- and B-type procyanidin oligomer-rich extracts from different Cinnamon barks. Phytomedicine 2011, 18, 298–302. [Google Scholar] [CrossRef]
- Tung, Y.T.; Chua, M.T.; Wang, S.Y.; Chang, S.T. Antiinflammation activities of essential oil and its constituents from indigenous cinnamon (Cinnamomum osmophloeum) twigs. Bioresour. Technol. 2008, 99, 3908–3913. [Google Scholar] [CrossRef]
- Koppikar, S.J.; Choudhari, A.S.; Suryavanshi, S.A.; Kumari, S.; Chattopadhyay, S.; Kaul-Ghanekar, R. Aqueous cinnamon rxtract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer 2010, 10, 210. [Google Scholar] [CrossRef]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, J.A. The occurrence, metabolism and toxicity of cinnamic acid and related compounds. J. Appl. Toxicol. 1984, 4, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic acid derivatives and their biological efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef] [PubMed]
- Klesiewicz, K.; Karczewska, E.; Nowak, P.; Skiba-Kurek, I.; Sito, E.; Panczyk, K.A.; Koczurkiewicz, P.; Żelaszczyk, D.; Pękala, E.; Waszkielewicz, A.M.; et al. Anti-Helicobacter pylori activities of selected N-substituted cinnamamide derivatives evaluated on reference and clinical bacterial strains. J. Antibiot. 2018, 71, 543–548. [Google Scholar] [CrossRef]
- Epifano, F.; Menghini, L.; Pagiotti, R.; Angelini, P.; Genovese, S.; Curini, M. In vitro inhibitory activity of boropinic acid against Helicobacter pylori. Bioorg. Med. Chem. Lett. 2006, 16, 5523–5525. [Google Scholar] [CrossRef]
- Paracatu, L.C.; Bonacorsi, C.; de Farias, C.M.Q.G.; Nazaré, A.C.; Petrônio, M.S.; Regasini, L.O.; Silva, D.H.S.; Raddi, M.S.G.; da Fonseca, L.M.; Ximenes, V.F. Alkyl caffeates as anti-helicobacter pylori and scavenger of oxidants produced by neutrophils. Med. Chem. 2014, 10, 74–80. [Google Scholar] [CrossRef]
- Faraci, W.S.; Yang, B.V.; O’Rourke, D.; Spencer, R.W. Inhibition of Helicobacter pylori urease by phenyl phosphorodiamidates: Mechanism of action. Bioorg. Med. Chem. 1995, 3, 605–610. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Sisto, F.; Carradori, S.; Guglielmi, P.; Traversi, C.B.; Spano, M.; Sobolev, A.P.; Secci, D.; Di Marcantonio, M.C.; Haloci, E.; Grande, R.; et al. Synthesis and biological evaluation of carvacrol-based derivatives as dual inhibitors of H. pylori strains and AGS cell proliferation. Pharmaceuticals 2020, 13, 405. [Google Scholar] [CrossRef]
- Yan, J.; Peng, C.; Chen, P.; Zhang, W.; Jiang, C.; Sang, S.; Zhu, W.; Yuan, Y.; Hong, Y.; Yao, M. In-Vitro anti-Helicobacter pylori activity and preliminary mechanism of action of Canarium album raeusch. Fruit extracts. J. Ethnopharmacol. 2021, 283, 114578. [Google Scholar] [CrossRef]
- Vallese, F.; Percudani, R.; Fischer, W.; Zanotti, G. The crystal structure of Helicobacter pylori HP 1029 highlights the functional diversity of the sialic acid-related DUF 386 family. FEBS J. 2015, 282, 3311–3322. [Google Scholar] [CrossRef] [PubMed]
- Kalivoda, K.A.; Steenbergen, S.M.; Vimr, E.R.; Plumbridge, J. Regulation of sialic acid catabolism by the DNA binding protein NanR in Escherichia coli. J. Bacteriol. 2003, 185, 4806–4815. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Liu, W.; Li, Y.; Zhang, K.; Zheng, X.; Wu, H.; Tang, G. Design, synthesis, and activity study of cinnamic acid derivatives as potent anti-neuroinflammatory agents. ACS Chem. Neurosci. 2021, 12, 419–429. [Google Scholar] [CrossRef] [PubMed]


| Compound | R | Inhibition (%) at 32 μM a | MIC (μM) a |
|---|---|---|---|
| Metronidazole | -- | 99.92 ± 0.10 | 8 |
| Cinnamic acid | -- | 57.30 ± 10.38 | >64 |
| 1 | Ph | 73.29 ± 1.20 | 64 |
| 2 | 2-CH3-Ph | 98.16 ± 0.73 | 32 |
| 3 | 3-CH3-Ph | 76.44 ± 4.57 | 64 |
| 4 | 4-CH3-Ph | 77.60 ± 0.23 | 64 |
| 5 | 2-CH3O-Ph | 82.03 ± 4.30 | 64 |
| 6 | 3-CH3O-Ph | 50.58 ± 3.82 | 64 |
| 7 | 4-CH3O-Ph | 83.16 ± 1.18 | 64 |
| 8 | 2-F-Ph | 67.92 ± 3.37 | 64 |
| 9 | 3-F-Ph | 69.00 ± 0.52 | 64 |
| 10 | 4-F-Ph | 86.33 ± 1.32 | 64 |
| 11 | 2-Cl-Ph | 55.91 ± 8.64 | 64 |
| 12 | 3-Cl-Ph | 81.11 ± 2.71 | 64 |
| 13 | 4-Cl-Ph | 18.52 ± 10.73 | 64 |
| 14 | 2-Br-Ph | 64.67 ± 1.21 | 64 |
| 15 | 3-Br-Ph | 62.24 ± 2.05 | 32 |
| 16 | 4-Br-Ph | 100.55 ± 0.30 | 16 |
| 17 | 2-CF3-Ph | 62.23 ± 0.66 | 64 |
| 18 | 3-CF3-Ph | 65.30 ± 3.55 | 64 |
| 19 | 4-CF3-Ph | 98.68 ± 0.19 | 16 |
| 20 | 2-NO2-Ph | 49.26 ± 2.05 | 64 |
| 21 | 3-NO2-Ph | 96.34 ± 0.59 | 32 |
| 22 | 4-NO2-Ph | 87.10 ± 0.32 | 64 |
| 23 | 2,4-diNO2-Ph | 95.70 ± 1.07 | 4 |
| 24 | pyridin | 96.48 ± 0.54 | 32 |
| 25 | quinolin | 97.95 ± 0.41 | 8 |
| 26 | naphthalen | 40.43 ± 3.12 | 64 |
| 27 | 6-CH3O-naphthalen | 54.48 ± 1.03 | >64 |
| 28 | thiophen | 82.17 ± 1.69 | 64 |
| 29 | furan | 71.53 ± 5.90 | 64 |
| 30 | 3,4,5-triCH3O-Ph | 74.98 ± 1.27 | 64 |
| Compound | MIC (μM) a | MBC (μM) | MBC/MIC |
|---|---|---|---|
| Metronidazole | 8 | 32 | 4 |
| 1–23 | 4 | 8 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, K.; Wu, Z.; Zheng, Y.; Yu, J.; Wu, S.; Wong, V.K.W.; Chen, M.; Liu, W.; Zhao, S. Discovery of Cinnamic Acid Derivatives as Potent Anti-H. pylori Agents. Molecules 2024, 29, 4548. https://doi.org/10.3390/molecules29194548
Li Y, Zhao K, Wu Z, Zheng Y, Yu J, Wu S, Wong VKW, Chen M, Liu W, Zhao S. Discovery of Cinnamic Acid Derivatives as Potent Anti-H. pylori Agents. Molecules. 2024; 29(19):4548. https://doi.org/10.3390/molecules29194548
Chicago/Turabian StyleLi, Yonglian, Kun Zhao, Zhidi Wu, Yujun Zheng, Jialin Yu, Sikun Wu, Vincent Kam Wai Wong, Min Chen, Wenfeng Liu, and Suqing Zhao. 2024. "Discovery of Cinnamic Acid Derivatives as Potent Anti-H. pylori Agents" Molecules 29, no. 19: 4548. https://doi.org/10.3390/molecules29194548
APA StyleLi, Y., Zhao, K., Wu, Z., Zheng, Y., Yu, J., Wu, S., Wong, V. K. W., Chen, M., Liu, W., & Zhao, S. (2024). Discovery of Cinnamic Acid Derivatives as Potent Anti-H. pylori Agents. Molecules, 29(19), 4548. https://doi.org/10.3390/molecules29194548





