High-Entropy and Component Stoichiometry Tuning Strategies Boost the Sodium-Ion Storage Performance of Cobalt-Free Prussian Blue Analogues Cathode Materials
Abstract
1. Introduction
2. Results and Discussion
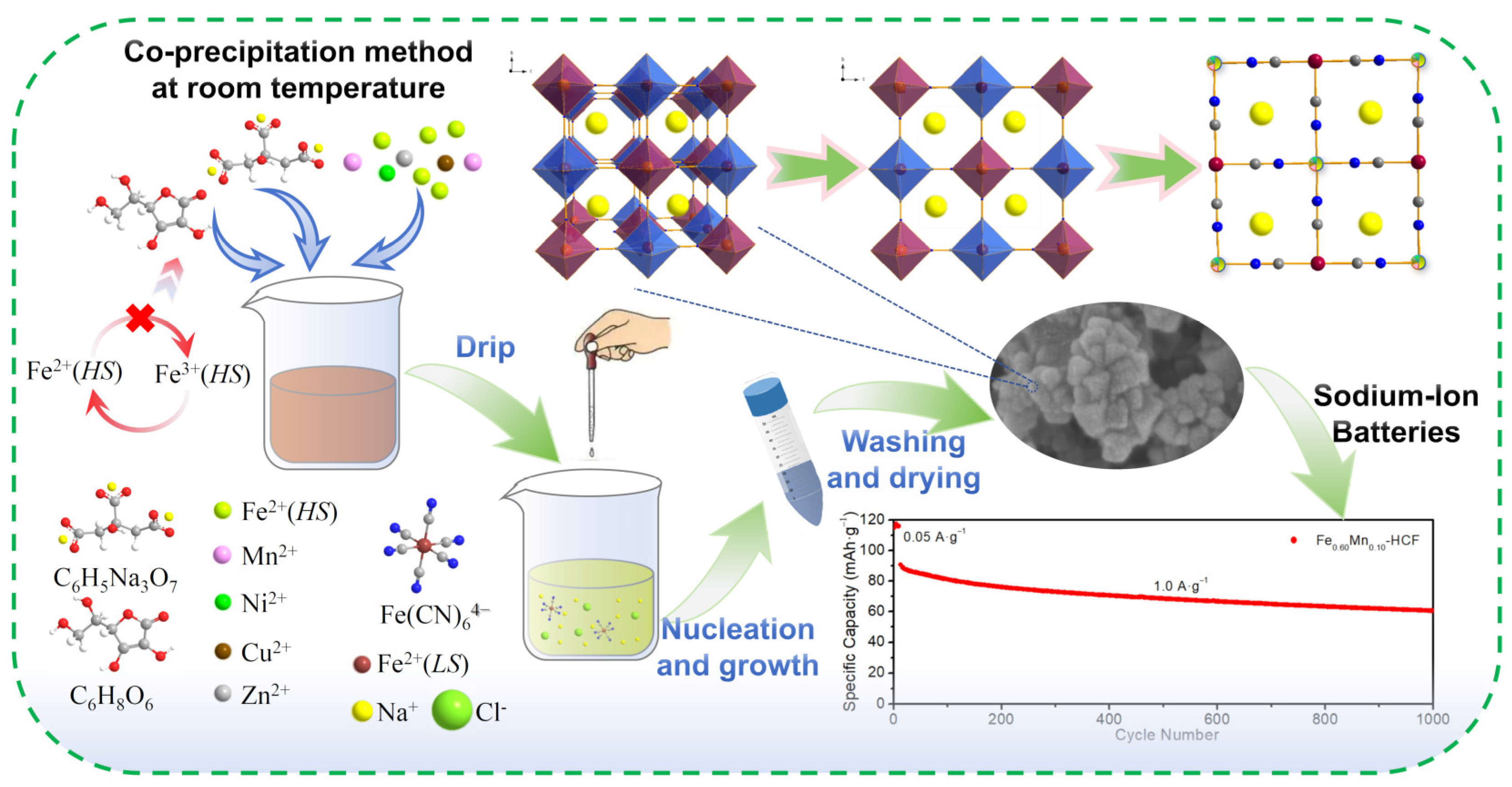
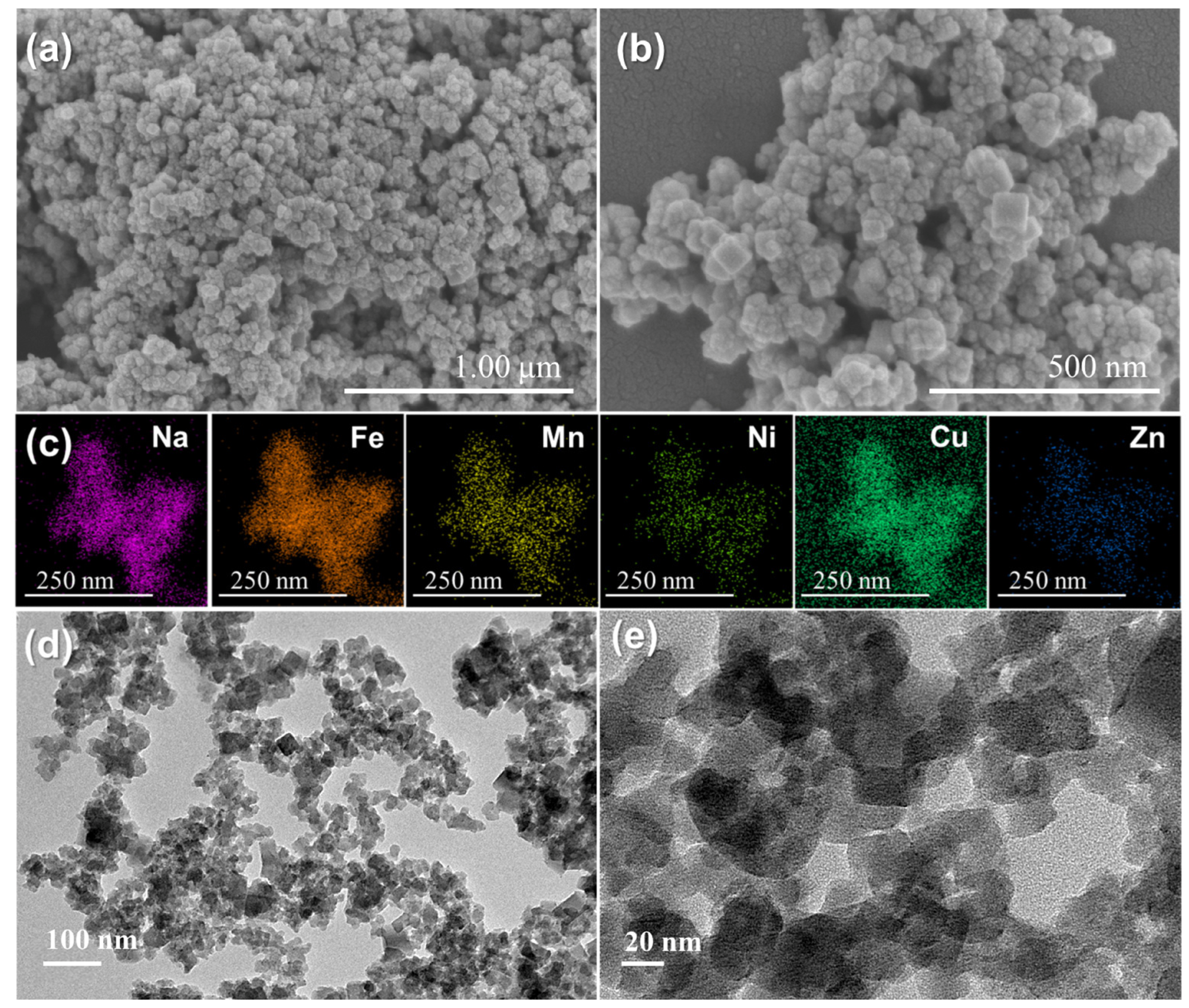
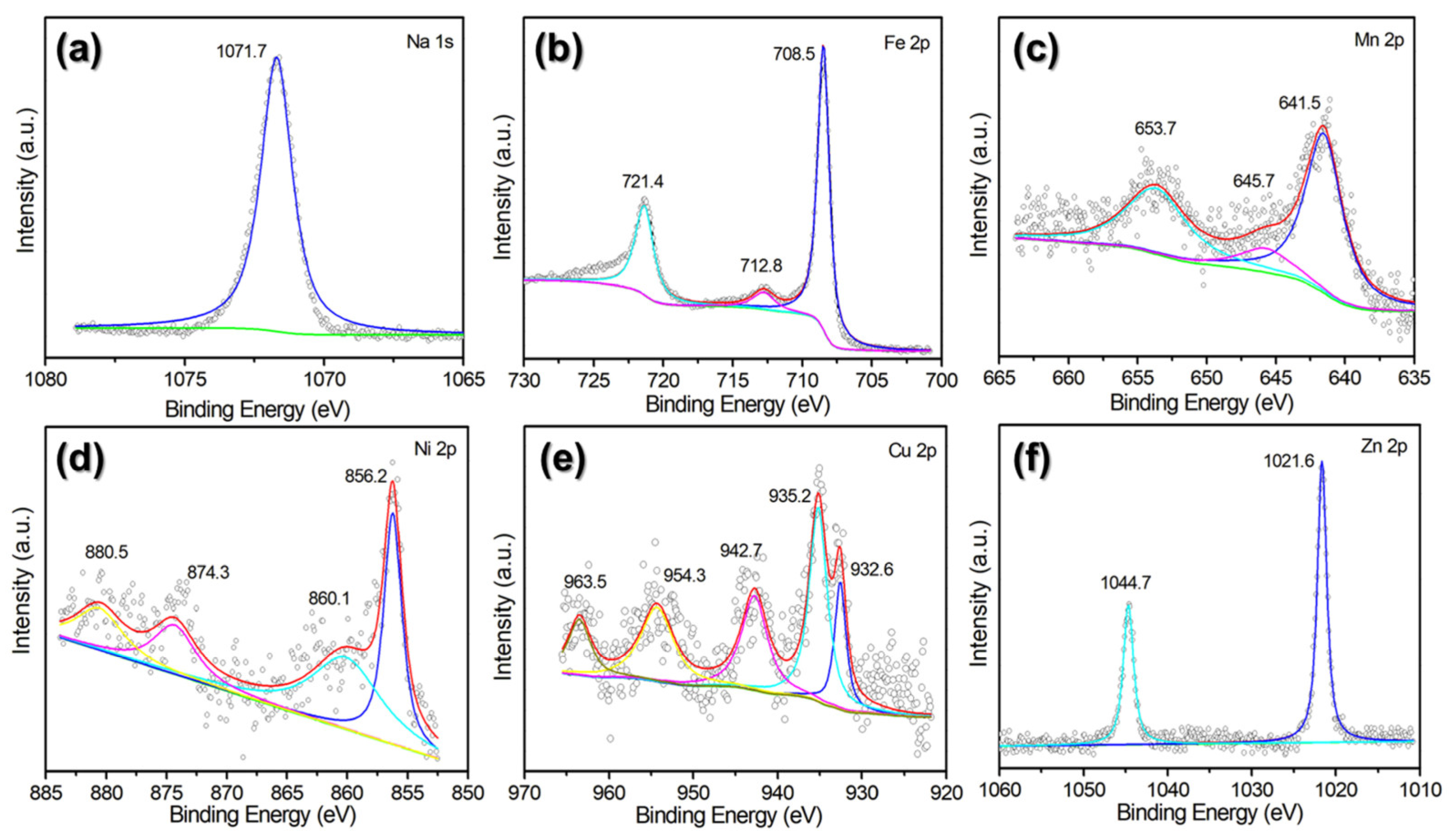
2.1. Physical and Chemical Characterizations
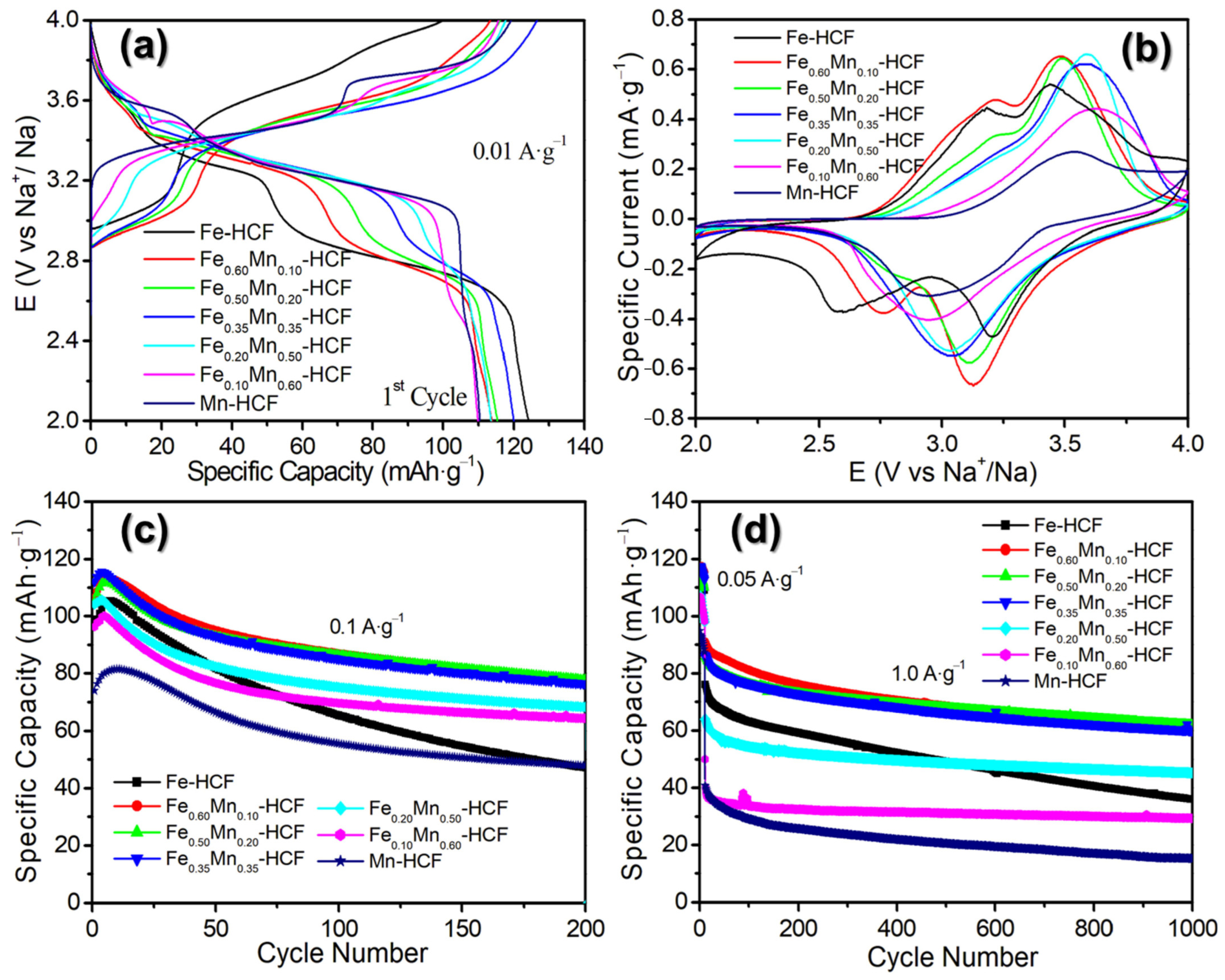
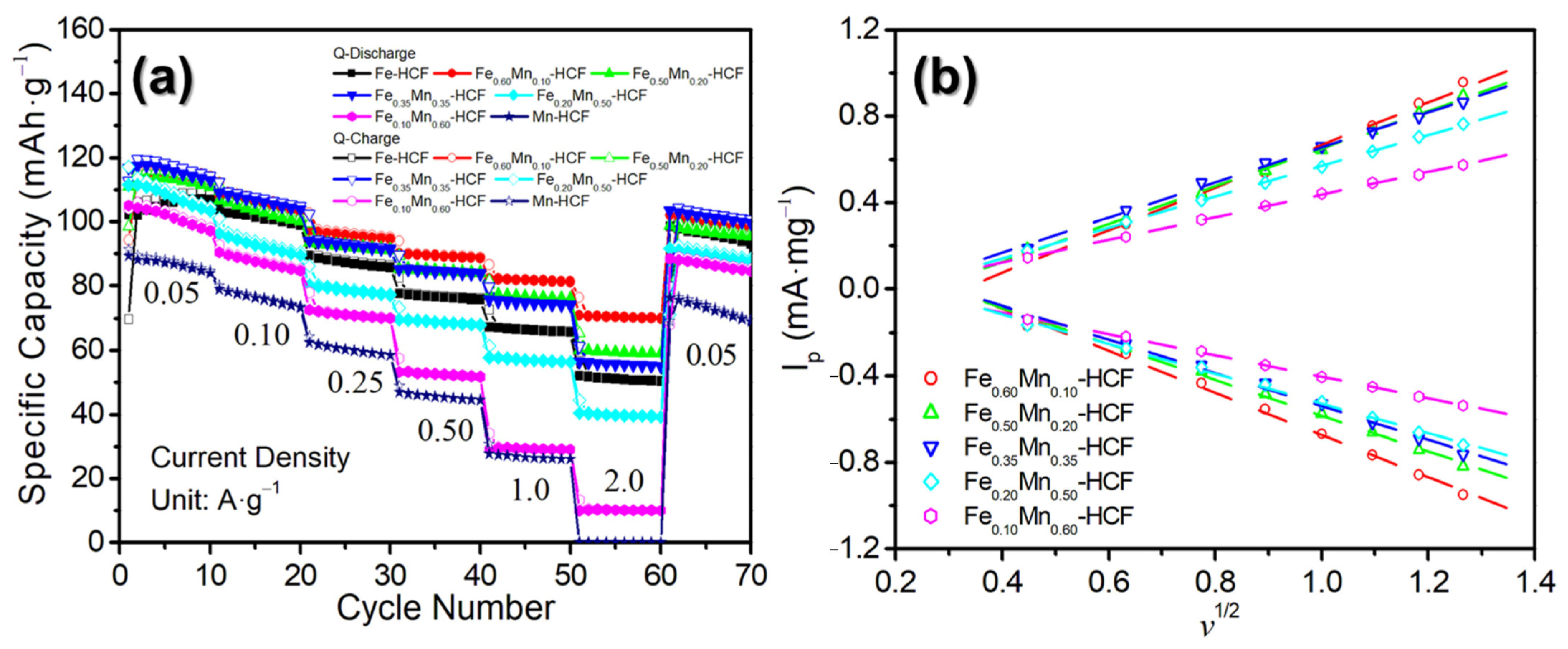
2.2. Sodium-Ion Storage Performance of the Synthesized PB and PBAs
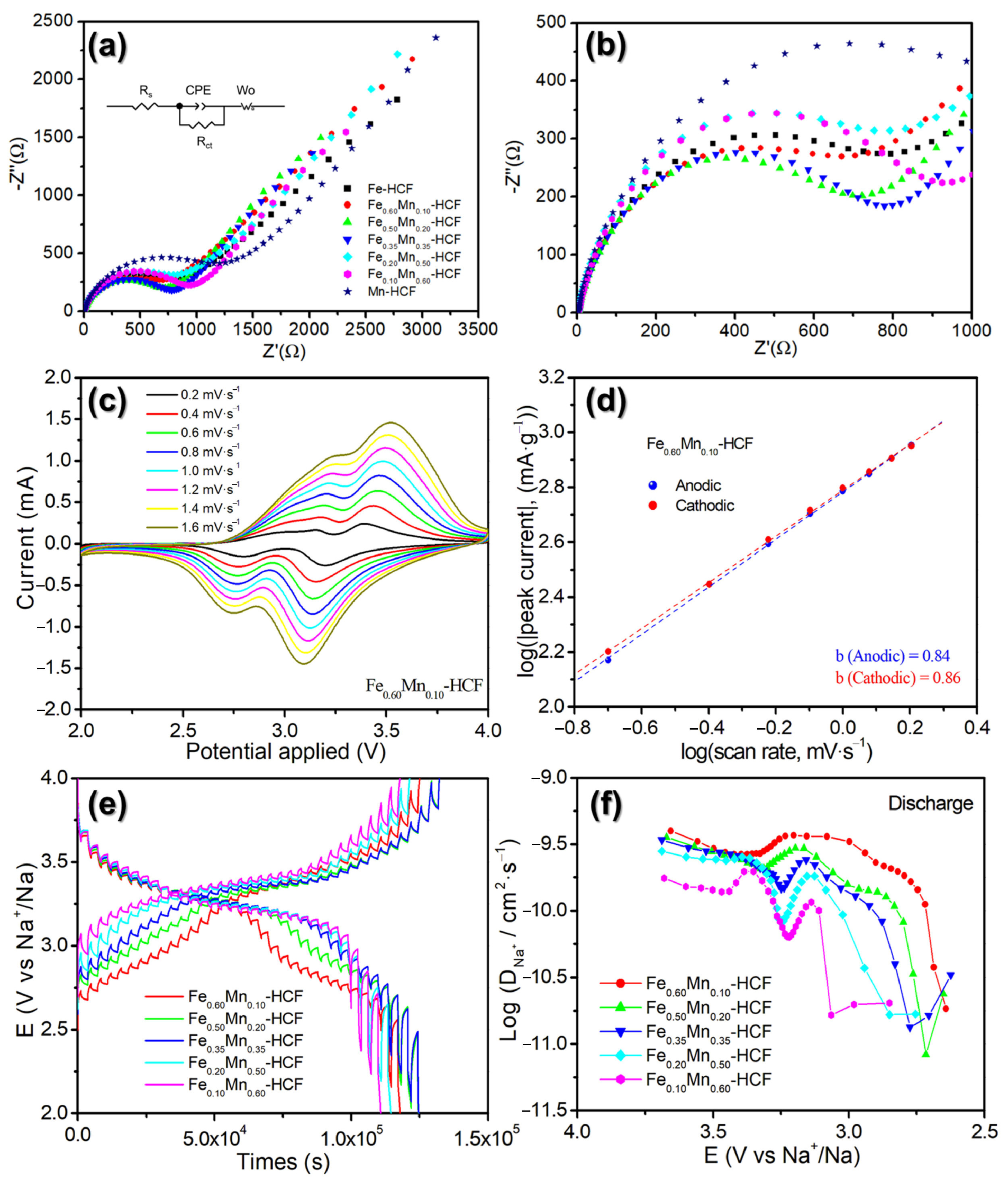
2.3. Exploring the Origin of the Sodium-Ion Storage Performance Improvement of the HE-HCFs
3. Materials and Methods
Synthesis of Metal Hexacyanoferrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, H.; Gao, Y.; Lin, X.-H.; Zhang, H.; Li, L.; Chou, S.-L. Prussian blue analogues for aqueous sodium-ion batteries: Progress and commercialization assessment. Adv. Energy Mater. 2024, 14, 2401984. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Peng, J.; Li, L.; Xiao, Y.; Li, L.; Liu, Y.; Qiao, Y.; Chou, S.-L. A 30-year overview of sodium-ion batteries. Carbon Energy 2024, 6, e464. [Google Scholar] [CrossRef]
- Wang, J.-Q.; Zhu, Y.-F.; Su, Y.; Guo, J.-X.; Chen, S.-Q.; Liu, H.-K.; Dou, S.-X.; Chou, S.-L.; Xiao, Y. Routes to high-performance layered oxide cathodes for sodium-ion batteries. Chem. Soc. Rev. 2024, 53, 4230–4301. [Google Scholar] [CrossRef] [PubMed]
- Jo, M.-R.; Kim, Y.; Yang, J.; Jeong, M.; Song, K.; Kim, Y.-I.; Lim, J.-M.; Cho, M.; Shim, J.-H.; Kim, Y.-M.; et al. Triggered reversible phase transformation between layered and spinel structure in manganese-based layered compounds. Nat. Commun. 2019, 10, 3385. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Lim, J.-M.; Park, M.; Lee, G.-H.; Lee, S.; Cho, M.; Kang, Y.-M. Thermally activated P2-O3 mixed layered cathodes toward synergistic electrochemical enhancement for Na ion batteries. Adv. Energy Mater. 2021, 11, 2102444. [Google Scholar] [CrossRef]
- Ahsan, Z.-S.; Cai, Z.-F.; Wang, S.; Moin, M.; Wang, H.-C.; Liu, D.-M.; Ma, Y.-Z.; Song, G.-S.; Wen, C. Recent development of phosphate based polyanion cathode materials for sodium-ion batteries. Adv. Energy Mater. 2024, 14, 2400373. [Google Scholar] [CrossRef]
- Hou, J.; Hadouchi, M.; Sui, L.; Liu, J.; Tang, M.; Hu, Z.; Lin, H.-J.; Kuo, C.-Y.; Chen, C.-T.; Pao, C.-W.; et al. Insights into reversible sodium intercalation in a novel sodium-deficient NASICON-type structure: Na3.40□0.60Co0.5Fe0.5V(PO4)3. Small 2023, 19, 2302726. [Google Scholar] [CrossRef]
- Hou, J.; Hadouchi, M.; Sui, L.; Liu, J.; Tang, M.; Kan, W.-H.; Avdeev, M.; Zhong, G.; Liao, Y.-K.; Lai, Y.-H.; et al. Unlocking fast and reversible sodium intercalation in NASICON Na4MnV(PO4)3 by fluorine substitution. Energy Storage Mater. 2021, 42, 307–316. [Google Scholar] [CrossRef]
- Fan, S.-W.; Liu, Y.-J.; Gao, Y.; Liu, Y.; Qiao, Y.; Li, L.; Chou, S.-L. The design and synthesis of prussian blue analogs as a sustainable cathode for sodium-ion batteries. SusMat 2023, 3, 749–780. [Google Scholar] [CrossRef]
- Zhang, Y.-C.; Zhou, X.; Yang, C.; Liu, X.-W.; Wang, M.-L.; Han, J.; Yan, H.; You, Y. Air-stable prussian white cathode materials for sodium-ion batteries enabled by ZnO surface modification. ACS Appl. Mater. Interfaces 2024, 16, 15649–15656. [Google Scholar] [CrossRef]
- Xing, F.-F.; Li, S.; Chen, L.; Dang, J.-S.; He, X.-M. Construction of naphthalene diimide derived nanostructured cathodes through selfassembly for high-performance sodium-organic batteries. ACS Nano 2023, 17, 21432–21442. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.-T.; Gao, S.; Wang, J.; Cao, S.-L.; Wang, K.-L.; Zhou, M.; Jiang, K. Electrochemical properties and kinetics of asymmetric sodium benzene-1,2,4-tricarboxylate as an anode material for sodium-organic batteries. ChemElectroChem 2020, 7, 3517–3521. [Google Scholar] [CrossRef]
- He, M.-L.; Davis, R.; Chartouni, D.; Johnson, M.; Abplanalp, M.; Troendle, P.; Suetterlin, R.-P. Assessment of the first commercial prussian blue based sodium-ion battery. J. Power Sources 2022, 548, 232036. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Cao, Y.; Sun, J. Defect engineering in prussian blue analogs for high-performance sodium-ion batteries. Adv. Energy Mater. 2022, 12, 2202532. [Google Scholar] [CrossRef]
- Ojwang, D.-O.; Häggström, L.; Ericsson, T.; Mogensen, R.; Brant, W.-R. Guest water hinders sodium-ion diffusion in low-defect berlin green cathode material. Dalton Trans. 2022, 51, 14712–14720. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-J.; Ma, Y.; Dreyer, S.-L.; Wang, Q.-S.; Wang, K.; Goonetilleke, D.; Omar, A.; Mikhailova, D.; Hahn, H.; Breitung, B.; et al. High-entropy metal-organic frameworks for highly reversible sodium storage. Adv. Mater. 2021, 33, 2101342. [Google Scholar] [CrossRef]
- Ma, Y.-J.; Hu, Y.; Pramudya, Y.; Diemant, T.; Wang, Q.-S.; Goonetilleke, D.; Tang, Y.-S.; Zhou, B.; Hahn, H.; Wenzel, W.; et al. Resolving the role of configurational entropy in improving cycling performance of multicomponent hexacyanoferrate cathodes for sodium-ion batteries. Adv. Funct. Mater. 2022, 32, 2202372. [Google Scholar] [CrossRef]
- Dai, J.-Y.; Tan, S.; Wang, L.-F.; Ling, F.-X.; Duan, F.-Q.; Ma, M.-Z.; Shao, Y.; Rui, X.-H.; Yao, Y.; Hu, E.; et al. High-voltage potassium hexacyanoferrate cathode via high-entropy and potassium incorporation for stable sodium-ion batteries. ACS Nano 2023, 17, 20949–20961. [Google Scholar] [CrossRef]
- He, Y.-Y.; Dreyer, S.-L.; Ting, Y.-Y.; Ma, Y.; Hu, Y.; Goonetilleke, D.; Tang, Y.-S.; Diemant, T.; Zhou, B.; Kowalski, P.-M.; et al. Entropy-mediated stable structural evolution of prussian white cathodes for long-life Na-ion batteries. Angew. Chem. Int. Ed. 2024, 63, e202315371. [Google Scholar] [CrossRef]
- Peng, J.; Zhang, B.; Hua, W.-B.; Liang, Y.-R.; Zhang, W.; Du, Y.-M.; Peleckis, G.; Indris, S.; Gu, Q.-F.; Cheng, Z.-X.; et al. A disordered rubik’s cube-inspired framework for sodium-ion batteries with ultralong cycle lifespan. Angew. Chem. Int. Ed. 2023, 62, e202215865. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Jiang, N.; Yang, C.; Liu, J.-H.; Sun, S.-Y.; Wang, X.-Y.; Yang, J.-H.; Liu, Y. High-entropy prussian blue analogs with 3D confinement effect for long-life sodium-ion batteries. J. Mater. Chem. A 2024, 12, 5170–5180. [Google Scholar] [CrossRef]
- Xie, B.; Su, B.; Gao, T.; Ma, Y.; Yin, G.; Zuo, P. Recent progress of Prussian blue analogues as cathode materials for nonaqueous sodium-ion batteries. Coord. Chem. Rev. 2022, 460, 214478. [Google Scholar] [CrossRef]
- Shen, Y.; Zou, J.; Zeng, M.; Fu, L. Atomic manufacturing in electrode materials for high-performance batteries. ACS Nano 2023, 17, 22167–22182. [Google Scholar] [CrossRef]
- Qiu, S.; Xu, Y.; Wu, X.; Ji, X. Prussian blue analogues as electrodes for aqueous monovalent ion batteries. Electrochem. Energy Rev. 2022, 5, 242–262. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, X.; Ji, L.; Wang, L.; Xu, B.-B.; Shahzad, M.-W.; Tang, Y.-X.; Zhu, Y.-F.; Yan, M.; Sun, G.-X.; et al. Boosting the sodium storage performance of prussian blue analogs by single-crystal and high-entropy approach. Energy Storage Mater. 2023, 58, 1–8. [Google Scholar] [CrossRef]
- Wang, Z.-N.; Nie, K.-Q.; Sougrati, M.-T.; Wang, C.; Liu, Z.-Q.; Wang, J.-O.; Ge, R.-L.; Zheng, Q.; Wang, J.-H. Charge transfer induced highly active low-spin iron of Prussian blue cathode through calcination strategy for high performance Sodium-ion batteries. Chem. Eng. J. 2024, 488, 151090. [Google Scholar] [CrossRef]
- Tang, C.; Lu, W.; Zhang, Y.-X.; Zhang, W.-W.; Cui, C.-C.; Liu, P.; Han, L.; Qian, X.-S.; Chen, L.-W.; Xu, F.-G. Toward ultrahigh rate and cycling performance of cathode materials of sodium ion battery by introducing a bicontinuous porous structure. Adv. Mater. 2024, 36, 2402005. [Google Scholar] [CrossRef]
- Hu, J.-W.; Tao, H.-W.; Chen, M.-L.; Zhang, Z.-C.; Cao, S.-L.; Shen, Y.; Jiang, K.; Zhou, M. Interstitial water improves structural stability of iron hexacyanoferrate for high-performance sodium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 12234–12242. [Google Scholar] [CrossRef]
- Peng, J.; Gao, Y.; Zhang, H.; Liu, Z.-G.; Zhang, W.; Li, L.; Qiao, Y.; Yang, W.-S.; Wang, J.-Z.; Dou, S.-X.; et al. Ball milling solid-state synthesis of highly crystalline prussian blue analogue Na2-xMnFe(CN)6 cathodes for all-climate sodium-ion batteries. Angew. Chem. Int. Ed. 2022, 61, e202205867. [Google Scholar] [CrossRef]
- Wang, W.-L.; Gang, Y.; Peng, J.; Hu, Z.; Yan, Z.-C.; Lai, W.-H.; Zhu, Y.-F.; Appadoo, D.; Ye, M.; Cao, Y.-L. Effect of eliminating water in prussian blue cathode for sodium-ion batteries. Adv. Funct. Mater. 2022, 32, 2111727. [Google Scholar] [CrossRef]
- Mao, Y.-J.; Chen, Y.-T.; Qin, J.; Shi, C.-S.; Liu, E.; Zhao, N.-Q. Capacitance controlled, hierarchical porous 3D ultra-thin carbon networks reinforced prussian blue for high performance Na-ion battery cathode. Nano Energy 2019, 58, 192–201. [Google Scholar] [CrossRef]
- You, Y.; Yu, X.-Q.; Yin, Y.-X.; Nam, K.-W.; Guo, Y.-G. Sodium iron hexacyanoferrate with high Na content as a Na-rich cathode material for Na-ion batteries. Nano Res. 2015, 8, 117–128. [Google Scholar] [CrossRef]
- He, H.-N.; Zeng, X.-G.; Wang, H.-Y.; Chen, N.; Sun, D.; Tang, Y.-G.; Huang, X.-B.; Pan, Y.-F. NaV6O15 nanoflakes with good cycling stability as a cathode for sodium ion battery. J. Electrochem. Soc. 2015, 162, A39–A43. [Google Scholar] [CrossRef]
- Tang, Y.; Li, W.; Feng, P.-Y.; Zhou, M.; Wang, K.-L.; Wang, Y.-S.; Zaghib, K.; Jiang, K. High-performance manganese hexacyanoferrate with cubic structure as superior cathode material for sodium-ion batteries. Adv. Funct. Mater. 2020, 30, 1908754. [Google Scholar] [CrossRef]
- Zhao, X.; Xing, Z.-H.; Huang, C.-D. Investigation of high-entropy prussian blue analog as cathode material for aqueous sodium-ion batteries. J. Mater. Chem. A 2023, 11, 22835–22844. [Google Scholar] [CrossRef]
- Chen, P.-P.; Wang, H.; Li, H.; Niu, B.-T.; Guo, H.-X.; Chen, Z.-X. A high-activity Fe-based MOFs fabricated through ultrasound strategy for electrochemical sensor of heavy metal ions and dopamine. J. Electroanal. Chem. 2024, 957, 118129. [Google Scholar] [CrossRef]
- Liu, Y.; He, D.-D.; Han, R.-M.; Wei, G.-Y.; Qiao, Y. Nanostructured potassium and sodium ion incorporated prussian blue frameworks as cathode materials for sodium-ion batteries. Chem. Commun. 2017, 53, 5569–5572. [Google Scholar] [CrossRef]
- Li, A.; Duan, L.-P.; Liao, J.-Y.; Sun, J.-L.; Man, Y.-H.; Zhou, X.-S. Formation of Mn-Ni prussian blue analogue spheres as a superior cathode material for potassium-ion batteries. ACS Appl. Energy Mater. 2022, 5, 11789–11796. [Google Scholar] [CrossRef]
- Biesinger, M.-C.; Payne, B.-P.; Grosvenor, A.-P.; Lau, L.-W.-M.; Gerson, A.-R.; Smart, R.-S.-C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Niu, B.-T.; Liu, M.-M.; Li, X.-L.; Guo, H.-X.; Chen, Z.-X. Vein-like Ni-BTC@Ni3S4 with sulfur vacancy and Ni3+ fabricated in situ etching vulcanization strategy for an electrochemical sensor of dop. ACS Appl. Mater. Interfaces 2023, 15, 13319–13331. [Google Scholar] [CrossRef]
- Zhang, X.-K.; Xia, M.-T.; Liu, T.-T.; Peng, N.; Yu, H.-X.; Zheng, R.-T.; Zhang, L.-Y.; Shui, M.; Shu, J. Copper hexacyanoferrate as ultra-high rate host for aqueous ammonium ion storage. Chem. Eng. J. 2021, 421, 127767. [Google Scholar] [CrossRef]
- Luo, Y.-F.; Shen, J.-L.; Yao, Y.; Dai, J.-Y.; Ling, F.-X.; Li, L.; Jiang, Y.; Wu, X.-J.; Rui, X.-H.; Yu, Y. Inhibiting the jahn-teller effect of manganese hexacyanoferrate via Ni and Cu codoping for advanced sodium-ion batteries. Adv. Mater. 2024, 36, 2405458. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-L.; Ling, R.; Yang, C.; Qi, W.-T. Structural regulation of Mn-based prussian blue induced by zinc-substitution for enhanced sodium storage performance. Electrochim. Acta 2023, 462, 142711. [Google Scholar] [CrossRef]
- Xie, C.-H.; Li, H.; Niu, B.-T.; Guo, H.-X.; Lin, X.-M. Comparison of ultrasonic vs mechanochemistry methods for fabrication of mixed-ligand Zn-based MOFs for electrochemical determination of luteolin. J. Alloys Compd. 2024, 989, 174363. [Google Scholar] [CrossRef]
- Wang, W.-L.; Xing, Z.; Ren, H.-P.; Wang, Q.-L.; Gao, X.-R.; Nie, C.-H.; Ju, Z.-C. MnFe prussian blue analogue open cages for sodium-ion batteries: Simultaneous evolution of structure, morphology, and energy storage properties. Small 2024, 20, 2402072. [Google Scholar] [CrossRef]
- Wang, W.; Gang, Y.; Hu, Z.; Yan, Z.; Li, W.; Li, Y.; Gu, Q.-F.; Wang, Z.; Chou, S.-L.; Liu, H.-K.; et al. Reversible structural evolution of sodium-rich rhombohedral Prussian blue for sodium-ion batteries. Nat. Commun. 2020, 11, 980. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-Y.; Xu, C.-M.; Liang, Y.-R.; Yang, Z.; LeGe, N.; Peng, J.; Chen, L.-J.; Lai, W.-H.; Wang, Y.-X.; Tao, Z.-L.; et al. Reforming magnet waste to Prussian blue for sustainable sodium-ion batteries. ACS Appl. Mater. Interfaces 2022, 14, 47747–47757. [Google Scholar] [CrossRef]
- Lin, K.; He, Z.-M.; Shen, L.; Su, J.-B.; Huang, Z.-Y.; Xia, Y.-M.; Wang, Y. Preparation high-performance cathode of vacancy-free Prussian blue analogues for sodium ion batteries by directional capture of free Mn-ion. J. Electroanal. Chem. 2024, 966, 118395. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.-P.; Liu, H.; Gao, J.-F.; Xu, Y.-T.; Chen, Z.; Li, X.-L.; Liu, Y. Synthesis of Fe-doped Mn-based Prussian blue hierarchical architecture for high-performance sodium ion batteries. Electrochim. Acta 2023, 448, 142183. [Google Scholar] [CrossRef]
- Quan, J.-J.; Xu, E.-Z.; Zhu, H.-W.; Chang, Y.-J.; Zhu, Y.; Li, P.-C.; Sun, Z.-J.; Yu, D.-B.; Jiang, Y. A Ni-doping-induced phase transition and electron evolution in cobalt hexacyanoferrate as a stable cathode for sodium-ion batteries. Phys. Chem. Chem. Phys. 2021, 23, 2491–2499. [Google Scholar] [CrossRef]
- Lee, J.; Baek, J.; Kim, Y.; Jeong, W.; Kim, H.; Oh, G.; Oh, Y.; Jeong, S.; Kansara, S.; Sambandam, B.; et al. Cu-substituted Prussian white with low crystal defects as high-energy cathode materials for sodium-ion batteries. Mater. Today Chem. 2023, 33, 101741. [Google Scholar] [CrossRef]
- El-Hady, D.-A.; Lyu, Y.-X.; Zhan, S.-K.; Yang, J.-X.; Wang, Y.; Yang, F.; Zhao, Q.-L.; Gu, M.; Shao, M.-H. Vacancy and composition engineering of manganese hexacyanoferrate for sodium-ion storage. ACS Appl. Energy Mater. 2022, 5, 8547–8553. [Google Scholar] [CrossRef]
- Yang, J.-W.; Yan, B.; Ye, J.; Li, X.; Liu, Y.; You, H.-P. Carbon-coated LiCrTiO4 electrode material promoting phase transition to reduce asymmetric polarization for lithium-ion batteries. Phys. Chem. Chem. Phys. 2014, 16, 2882–2891. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef]
- Peng, F.-W.; Yu, L.; Gao, P.-Y.; Liao, X.-Z.; Wen, J.-G.; He, Y.-S.; Tan, G.-Q.; Ren, Y.; Ma, Z.-F. Highly crystalline sodium manganese ferrocyanide microcubes for advanced sodium ion battery cathodes. J. Mater. Chem. A 2019, 7, 22248–22256. [Google Scholar] [CrossRef]
- Hou, X.-H.; Huang, Y.-L.; Ma, S.-M.; Zou, X.-L.; Hu, S.-J.; Wu, Y.-P. Facile hydrothermal method synthesis of coralline-like Li1.2Mn0.54Ni0.13Co0.13O2 hierarchical architectures as superior cathode materials for lithium-ion batteries. Mater. Res. Bull. 2015, 63, 256–264. [Google Scholar] [CrossRef]
- Fu, X.-Y.; Zhang, L.-L.; Chen, Z.-Y.; Xu, Y.-K.; Wu, J.-X.; Wang, C.-C.; Ding, X.-K.; Yang, X.-L.; Lu, J. Achieving a superior Na storage performance of Fe-based prussian blue cathode by coating perylene tetracarboxylic dianhydride amine. Carbon Energy 2024, 6, e446. [Google Scholar] [CrossRef]
- Ali, G.; Patil, S.A.; Mehboob, S.; Ahmad, M.; Ha, H.Y.; Kim, H.-S.; Chung, K.Y. Determination of lithium diffusion coefficient and reaction mechanism into ultra-small nanocrystalline SnO2 particles. J. Power Sources 2019, 419, 229–236. [Google Scholar] [CrossRef]
- Gebert, F.; Cortie, D.L.; Bouwer, J.C.; Wang, W.; Yan, Z.; Dou, S.-X.; Chou, S.-L. Epitaxial nickel ferrocyanide stabilizes Jahn-Teller distortions of manganese ferrocyanide for sodium-ion batteries. Angew. Chem. Int. Ed. 2021, 60, 18519–18526. [Google Scholar] [CrossRef]

| Sample | Chemical Formula |
|---|---|
| Fe-HCF | Na1.247Fe [Fe(CN)6]0.791·3.61H2O |
| Fe0.60Mn0.10-HCF | Na1.156Fe0.599Mn0.095Ni0.092Cu0.109Zn0.105 [Fe(CN)6]0.724·3.11H2O |
| Fe0.50Mn0.20-HCF | Na1.142Fe0.505Mn0.185Ni0.094Cu0.111Zn0.105 [Fe(CN)6]0.728·3.09H2O |
| Fe0.35Mn0.35-HCF | Na1.152Fe0.373Mn0.314Ni0.092Cu0.116Zn0.104 [Fe(CN)6]0.744·3.14H2O |
| Fe0.20Mn0.50-HCF | Na1.157Fe0.234Mn0.460Ni0.091Cu0.109Zn0.106 [Fe(CN)6]0.759·3.45H2O |
| Fe0.10Mn0.60-HCF | Na1.110Fe0.138Mn0.550Ni0.102Cu0.109Zn0.101 [Fe(CN)6]0.741·3.37H2O |
| Mn-HCF | Na0.706Mn [Fe(CN)6]0.857·2.83H2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-T.; Niu, B.-T.; Wang, Z.-H.; Li, Y.-X.; Xu, Y.-P.; Liu, S.-W.; Chen, Y.-X.; Lin, X.-M. High-Entropy and Component Stoichiometry Tuning Strategies Boost the Sodium-Ion Storage Performance of Cobalt-Free Prussian Blue Analogues Cathode Materials. Molecules 2024, 29, 4559. https://doi.org/10.3390/molecules29194559
Lin Y-T, Niu B-T, Wang Z-H, Li Y-X, Xu Y-P, Liu S-W, Chen Y-X, Lin X-M. High-Entropy and Component Stoichiometry Tuning Strategies Boost the Sodium-Ion Storage Performance of Cobalt-Free Prussian Blue Analogues Cathode Materials. Molecules. 2024; 29(19):4559. https://doi.org/10.3390/molecules29194559
Chicago/Turabian StyleLin, Yuan-Ting, Bai-Tong Niu, Zi-Han Wang, Yu-Xi Li, Yun-Peng Xu, Shi-Wei Liu, Yan-Xin Chen, and Xiu-Mei Lin. 2024. "High-Entropy and Component Stoichiometry Tuning Strategies Boost the Sodium-Ion Storage Performance of Cobalt-Free Prussian Blue Analogues Cathode Materials" Molecules 29, no. 19: 4559. https://doi.org/10.3390/molecules29194559
APA StyleLin, Y.-T., Niu, B.-T., Wang, Z.-H., Li, Y.-X., Xu, Y.-P., Liu, S.-W., Chen, Y.-X., & Lin, X.-M. (2024). High-Entropy and Component Stoichiometry Tuning Strategies Boost the Sodium-Ion Storage Performance of Cobalt-Free Prussian Blue Analogues Cathode Materials. Molecules, 29(19), 4559. https://doi.org/10.3390/molecules29194559







