Design and Optimization of Nanoporous Materials as Catalysts for Oxygen Evolution Reaction—A Review
Abstract
1. Introduction
2. Improving the Number and Utilization Rate of Active Sites in Nanoporous Materials
2.1. Regulating the Pore Size and Porosity of Nanoporous Materials
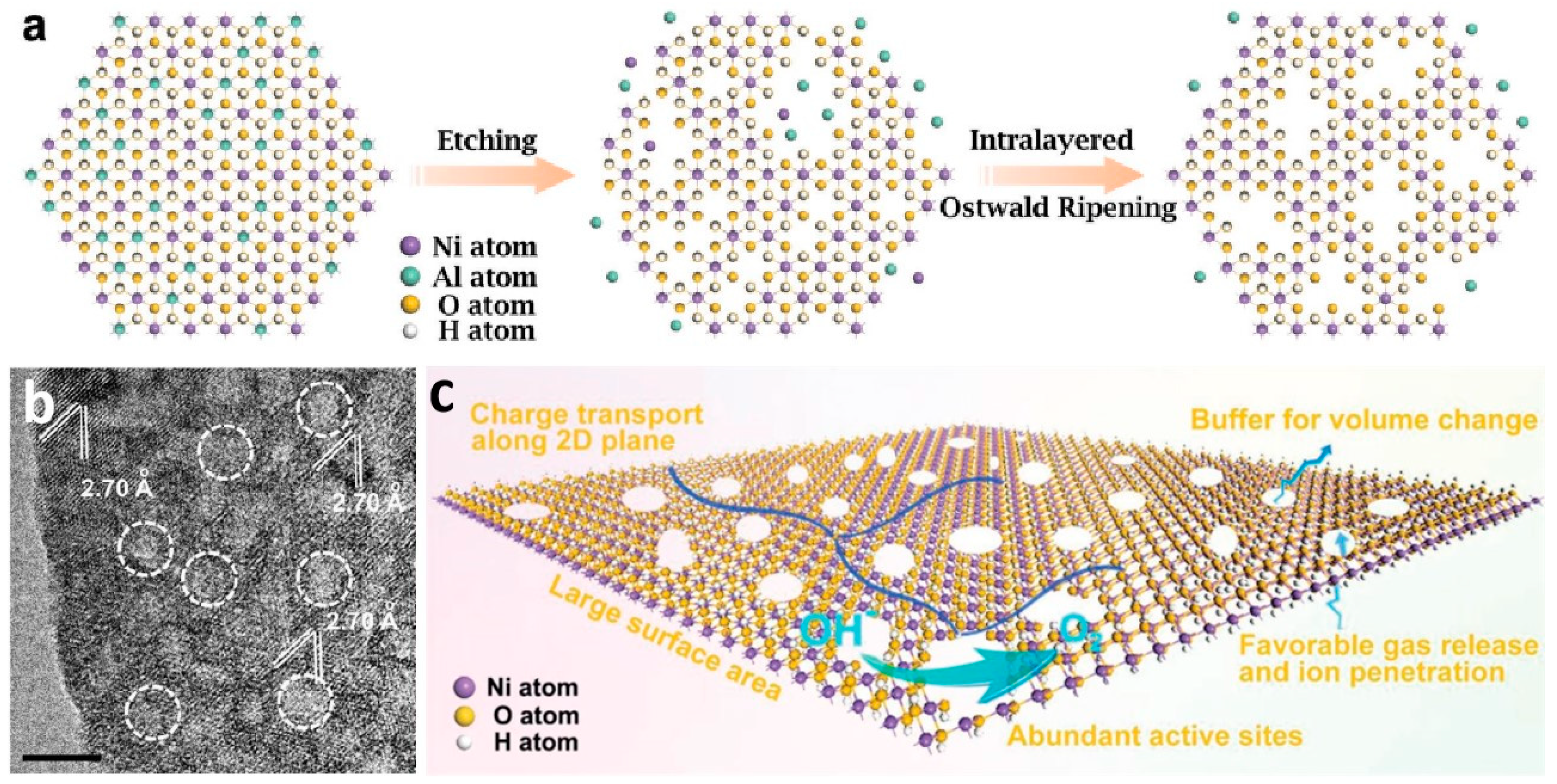
2.2. Construction of Hierarchical Nanoporous Materials
2.3. Improving the Conductivity of Nanoporous Materials
3. Improving the Intrinsic Activity of Nanoporous Materials
3.1. Metal Element Doping in Nanoporous Materials
3.2. Anion Tuning in Nanoporous Materials
3.3. Creating Vacancy Defects in Nanoporous Materials
3.4. Building Interface in Nanoporous Materials
3.5. Creating Boundary Active Sites in Nanoporous Materials
3.6. Other Methods for Improving the OER Activity of Nanoporous Materials
4. Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, N.S.; Nocera, D.G. Powering the planet: Chemical challenges in solar energy utilization. Proc. Natl. Acad. Sci. USA 2006, 103, 15729–15735. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Majumdar, A. Opportunities and challenges for a sustainable energy future. Nature 2012, 488, 294–303. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Zhu, X.; Gao, X.; An, C.; Wang, Z.; Zuo, C.; Dionysiou, D.D.; He, H.; Jiang, Z. Enhancing photocatalytic CO2 reduction with TiO2-based materials: Strategies, mechanisms, challenges, and perspectives. Environ. Sci. Ecotechnol. 2024, 20, 100368. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Deng, Y. Advances and Challenges for Hydrovoltaic Intelligence. ACS Nano 2023, 17, 14229–14252. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Chen, J.; Li, X.; Luo, K.; Xiong, Z.; Zhou, Z.; Zhu, W.; Luo, Z.; Huang, J.; Li, Y. Steering surface reconstruction of hybrid metal oxides for efficient oxygen evolution reaction in water splitting and zinc-air batteries. J. Energy Chem. 2024, 92, 383–393. [Google Scholar] [CrossRef]
- Quan, L.; Jiang, H.; Mei, G.; Sun, Y.; You, B. Bifunctional Electrocatalysts for Overall and Hybrid Water Splitting. Chem. Rev. 2024, 124, 3694–3812. [Google Scholar] [CrossRef]
- Aslam, S.; Rani, S.; Lal, K.; Fatima, M.; Hardwick, T.; Shirinfar, B.; Ahmed, N. Electrochemical hydrogen production: Sustainable hydrogen economy. Green Chem. 2023, 25, 9543–9573. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 44, 5148–5180. [Google Scholar] [CrossRef]
- You, B.; Sun, Y. Innovative Strategies for Electrocatalytic Water Splitting. Acc. Chem. Res. 2018, 51, 1571–1580. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, R.; Zou, J.-J.; Yang, H. Surface Design Strategy of Catalysts for Water Electrolysis. Small 2022, 18, 2202336. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Shan, B.; Wang, W.; Qi, Y.; Tai, X.; Wang, X.; Zheng, D.; Yan, H.; Ying, B. Recent advances in self-supported transition-metal-based electrocatalysts for seawater oxidation. Acta Phys. Chim. Sin 2023, 39, 2303012. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Z.; Gong, X.; Guo, Z. The intensification technologies to water electrolysis for hydrogen production—A review. Renew. Sustain. Energy Rev. 2014, 29, 573–588. [Google Scholar] [CrossRef]
- Ram, R.; Xia, L.; Benzidi, H.; Guha, A.; Golovanova, V.; Garzón Manjón, A.; Llorens Rauret, D.; Sanz Berman, P.; Dimitropoulos, M.; Mundet, B.; et al. Water-hydroxide trapping in cobalt tungstate for proton exchange membrane water electrolysis. Science 2024, 384, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, J.; Liu, Y.; Li, T.; Wang, T.; Li, X.; Ye, X.; Li, G.; Li, J.; Hu, W.; et al. Stability challenges and opportunities of NiFe-based electrocatalysts for oxygen evolution reaction in alkaline media. Carbon Neutralization 2024, 3, 172–198. [Google Scholar] [CrossRef]
- Lin, X.; Xu, J.; Peng, Z. Atomically dispersed catalysts toward the oxygen evolution reaction in electrochemical water splitting: From catalyst design, performance to catalytic mechanism. Next Sustain. 2024, 3, 100023. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, L.; Yan, X.; Yao, X. Recent Progress in Oxygen Electrocatalysts for Zinc–Air Batteries. Small Methods 2017, 1, 1700209. [Google Scholar] [CrossRef]
- Chen, F.-Y.; Wu, Z.-Y.; Adler, Z.; Wang, H. Stability challenges of electrocatalytic oxygen evolution reaction: From mechanistic understanding to reactor design. Joule 2021, 5, 1704–1731. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Q.; Sun, L.; Guo, H.; Tai, X.; Li, D.; Liu, L.; Ling, C.; Sun, X. Facilitating active species by decorating CeO2 on Ni3S2 nanosheets for efficient water oxidation electrocatalysis. Chin. J. Catal. 2021, 42, 482–489. [Google Scholar] [CrossRef]
- Danilovic, N.; Subbaraman, R.; Chang, K.-C.; Chang, S.H.; Kang, Y.J.; Snyder, J.; Paulikas, A.P.; Strmcnik, D.; Kim, Y.-T.; Myers, D.; et al. Activity–Stability Trends for the Oxygen Evolution Reaction on Monometallic Oxides in Acidic Environments. J. Phys. Chem. Lett. 2014, 5, 2474–2478. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, J.; Zhou, W.; Li, J. Catalyst-Support Interactions Promoted Acidic Electrochemical Oxygen Evolution Catalysis: A Mini Review. Molecules 2023, 28, 2262. [Google Scholar] [CrossRef]
- Hunter, B.M.; Gray, H.B.; Müller, A.M. Earth-Abundant Heterogeneous Water Oxidation Catalysts. Chem. Rev. 2016, 116, 14120–14136. [Google Scholar] [CrossRef] [PubMed]
- Abdelghafar, F.; Xu, X.; Guan, D.; Lin, Z.; Hu, Z.; Ni, M.; Huang, H.; Bhatelia, T.; Jiang, S.P.; Shao, Z. New Nanocomposites Derived from Cation-Nonstoichiometric Bax(Co, Fe, Zr, Y)O3−δ as Efficient Electrocatalysts for Water Oxidation in Alkaline Solution. ACS Mater. Lett. 2024, 6, 2985–2994. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Jian, T.; Ma, W.; Xu, C.; Zhou, Q.; Liu, H. Free-Standing Multiscale Porous High Entropy NiFeCoZn Alloy as the Highly Active Bifunctional Electrocatalyst for Alkaline Water Splitting. Chin. J. Chem. 2024, 42, 1465–1473. [Google Scholar] [CrossRef]
- Zhou, D.; Li, P.; Lin, X.; McKinley, A.; Kuang, Y.; Liu, W.; Lin, W.-F.; Sun, X.; Duan, X. Layered double hydroxide-based electrocatalysts for the oxygen evolution reaction: Identification and tailoring of active sites, and superaerophobic nanoarray electrode assembly. Chem. Soc. Rev. 2021, 50, 8790–8817. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, X.; Wang, D. Design concept for electrocatalysts. Nano Res. 2022, 15, 1730–1752. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Zhao, D. Mesoporous materials for energy conversion and storage devices. Nat. Rev. Mater. 2016, 1, 16023. [Google Scholar] [CrossRef]
- Luc, W.; Jiao, F. Nanoporous Metals as Electrocatalysts: State-of-the-Art, Opportunities, and Challenges. ACS Catal. 2017, 7, 5856–5861. [Google Scholar] [CrossRef]
- Jin, W.; Maduraiveeran, G. Recent advances of porous transition metal-based nanomaterials for electrochemical energy conversion and storage applications. Mater. Today Energy 2019, 13, 64–84. [Google Scholar] [CrossRef]
- Qiao, Y.; Peng, M.; Lan, J.; Jiang, K.; Chen, D.; Tan, Y. Active-site engineering in dealloyed nanoporous catalysts for electrocatalytic water splitting. J. Mater. Chem. A 2023, 11, 495–511. [Google Scholar] [CrossRef]
- Qi, J.; Zhang, W.; Cao, R. Porous materials as highly efficient electrocatalysts for the oxygen evolution reaction. ChemCatChem 2018, 10, 1206–1220. [Google Scholar] [CrossRef]
- Hall, A.S.; Yoon, Y.; Wuttig, A.; Surendranath, Y. Mesostructure-Induced Selectivity in CO2 Reduction Catalysis. J. Am. Chem. Soc. 2015, 137, 14834–14837. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Mellin, M.; Lauterbach, S.; Tian, C.; Dietz, C.; Hofmann, J.P.; Einert, M. Soft-templated, mesoporous Co3O4 thin films for electrocatalysis of the oxygen evolution reaction. Mater. Adv. 2024, 5, 2098–2109. [Google Scholar] [CrossRef]
- Sun, T.; Xu, L.; Yan, Y.; Zakhidov, A.A.; Baughman, R.H.; Chen, J. Ordered Mesoporous Nickel Sphere Arrays for Highly Efficient Electrocatalytic Water Oxidation. ACS Catal. 2016, 6, 1446–1450. [Google Scholar] [CrossRef]
- Feng, D.; Gao, T.-N.; Fan, M.; Li, A.; Li, K.; Wang, T.; Huo, Q.; Qiao, Z.-A. A general ligand-assisted self-assembly approach to crystalline mesoporous metal oxides. NPG Asia Mater. 2018, 10, 800–809. [Google Scholar] [CrossRef]
- Detsi, E.; Cook, J.B.; Lesel, B.K.; Turner, C.L.; Liang, Y.-L.; Robbennolt, S.; Tolbert, S.H. Mesoporous Ni60Fe30Mn10-alloy based metal/metal oxide composite thick films as highly active and robust oxygen evolution catalysts. Energy Environ. Sci. 2016, 9, 540–549. [Google Scholar] [CrossRef]
- Yu, L.; Wu, H.B.; Lou, X.W.D. Self-Templated Formation of Hollow Structures for Electrochemical Energy Applications. Acc. Chem. Res. 2017, 50, 293–301. [Google Scholar] [CrossRef]
- Tüysüz, H.; Hwang, Y.J.; Khan, S.B.; Asiri, A.M.; Yang, P. Mesoporous Co3O4 as an electrocatalyst for water oxidation. Nano Res. 2013, 6, 47–54. [Google Scholar] [CrossRef]
- Deng, X.; Schmidt, W.N.; Tüysüz, H. Impacts of Geometry, Symmetry, and Morphology of Nanocast Co3O4 on Its Catalytic Activity for Water Oxidation. Chem. Mater. 2014, 26, 6127–6134. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, H.; Liu, P.; Cheng, C.; Zhu, F.; Hirata, A.; Chen, M. 3D Nanoporous Metal Phosphides toward High-Efficiency Electrochemical Hydrogen Production. Adv. Mater. 2016, 28, 2951–2955. [Google Scholar] [CrossRef]
- Guo, D.; Kang, H.; Hao, Z.; Yang, Y.; Wei, P.; Zhang, Q.; Liu, L. Mesoporous cobalt-iron based materials as highly efficient electrocatalysts for oxygen evolution reaction. J. Electroanal. Chem. 2020, 873, 114443. [Google Scholar] [CrossRef]
- Jiang, J.; Kucernak, A. Oxygen Reduction Studies of Templated Mesoporous Platinum Catalysts. Electrochem. Solid-State Lett. 2000, 3, 559. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.M. Nanoporous metals: Fabrication strategies and advanced electrochemical applications in catalysis, sensing and energy systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tian, T.; Jiang, J.; Ai, L. Hierarchically porous Co3O4 architectures with honeycomb-like structures for efficient oxygen generation from electrochemical water splitting. J. Power Sources 2015, 294, 103–111. [Google Scholar] [CrossRef]
- Han, L.; Yu, X.-Y.; Lou, X.W. Formation of Prussian-Blue-Analog Nanocages via a Direct Etching Method and their Conversion into Ni–Co-Mixed Oxide for Enhanced Oxygen Evolution. Adv. Mater. 2016, 28, 4601–4605. [Google Scholar] [CrossRef]
- Lian, Y.; Sun, H.; Wang, X.; Qi, P.; Mu, Q.; Chen, Y.; Ye, J.; Zhao, X.; Deng, Z.; Peng, Y. Carved nanoframes of cobalt–iron bimetal phosphide as a bifunctional electrocatalyst for efficient overall water splitting. Chem. Sci. 2019, 10, 464–474. [Google Scholar] [CrossRef]
- Nai, J.; Guan, B.Y.; Yu, L.; Lou, X.W. Oriented assembly of anisotropic nanoparticles into frame-like superstructures. Sci. Adv. 2017, 3, e1700732. [Google Scholar] [CrossRef]
- Cao, Z.; Tingting Zhou, T.; Chen, Y.-L.; Liu, J.; Wang, D.; Zhang, W.; Pang, S.-S.; Zhao, Y. A Trimodal Porous Cobalt-Based Electrocatalyst for Enhanced Oxygen Evolution. Adv. Mater. Interfaces 2019, 6, 1900381. [Google Scholar] [CrossRef]
- Guan, B.Y.; Zhang, S.L.; Lou, X.W. Realization of Walnut-Shaped Particles with Macro-/Mesoporous Open Channels through Pore Architecture Manipulation and Their Use in Electrocatalytic Oxygen Reduction. Angew. Chem. Int. Ed. 2018, 57, 6176–6180. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Zhang, H.; Zhou, T.; Wang, J.; Wei, W.; Yang, Z.; Sun, X.; Cai, W.-B.; Zheng, G. Bio-Inspired Leaf-Mimicking Nanosheet/Nanotube Heterostructure as a Highly Efficient Oxygen Evolution Catalyst. Adv. Sci. 2015, 2, 1500003. [Google Scholar] [CrossRef]
- Sakamaki, A.; Yoshida-Hirahara, M.; Ogihara, H.; Kurokawa, H. One-Step Synthesis of Highly Active NiFe Electrocatalysts for the Oxygen Evolution Reaction. Langmuir 2022, 38, 5525–5531. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Wang, H.-S.; Wang, H.-F.; Zhang, Q.; Tian, G.-L.; Nie, J.-Q.; Wei, F. Spatially Confined Hybridization of Nanometer-Sized NiFe Hydroxides into Nitrogen-Doped Graphene Frameworks Leading to Superior Oxygen Evolution Reactivity. Adv. Mater. 2015, 27, 4516–4522. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Li, J.; Wu, T.; Ji, L.; Zhang, R.; Jiang, P.; Chen, H.; Zhao, R.; Asiri, A.M.; Sun, X. One-Step Preparation of Cobalt-Nanoparticle-Embedded Carbon for Effective Water Oxidation Electrocatalysis. ChemElectroChem 2019, 6, 1996–1999. [Google Scholar] [CrossRef]
- Lu, X.-F.; Gu, L.-F.; Wang, J.-W.; Wu, J.-X.; Liao, P.-Q.; Li, G.-R. Bimetal-Organic Framework Derived CoFe2O4/C Porous Hybrid Nanorod Arrays as High-Performance Electrocatalysts for Oxygen Evolution Reaction. Adv. Mater. 2017, 29, 1604437. [Google Scholar] [CrossRef]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal–Organic Framework Derived Hybrid Co3O4-Carbon Porous Nanowire Arrays as Reversible Oxygen Evolution Electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar] [CrossRef]
- Qiu, H.-J.; Johnson, I.; Chen, L.; Cong, W.; Ito, Y.; Liu, P.; Han, J.; Fujita, T.; Hirata, A.; Chen, M. Graphene-coated nanoporous nickel towards a metal-catalyzed oxygen evolution reaction. Nanoscale 2021, 13, 10916–10924. [Google Scholar] [CrossRef]
- Kim, H.; Kim, Y.; Noh, Y.; Kim, W.B. Ultrathin amorphous α-Co(OH)2 nanosheets grown on Ag nanowire surfaces as a highly active and durable electrocatalyst for oxygen evolution reaction. Dalton Trans. 2016, 45, 13686–13690. [Google Scholar] [CrossRef]
- Chen, H.; Gao, Y.; Sun, L. Highly Active Three-Dimensional NiFe/Cu2O Nanowires/Cu Foam Electrode for Water Oxidation. ChemSusChem 2017, 10, 1475–1481. [Google Scholar] [CrossRef]
- Sun, J.-S.; Zhou, Y.-T.; Yao, R.-Q.; Shi, H.; Wen, Z.; Lang, X.-Y.; Jiang, Q. Nanoporous gold supported chromium-doped NiFe oxyhydroxides as high-performance catalysts for the oxygen evolution reaction. J. Mater. Chem. A 2019, 7, 9690–9697. [Google Scholar] [CrossRef]
- Wang, J.; Zhong, H.-X.; Wang, Z.-L.; Meng, F.-l.; Zhang, X.-B. Integrated Three-Dimensional Carbon Paper/Carbon Tubes/Cobalt-Sulfide Sheets as an Efficient Electrode for Overall Water Splitting. ACS Nano 2016, 10, 2342–2348. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Hsu, Y.-Y.; Chen, R.; Chan, T.-S.; Chen, H.M.; Liu, B. Ni3+-Induced Formation of Active NiOOH on the Spinel Ni–Co Oxide Surface for Efficient Oxygen Evolution Reaction. Adv. Energy Mater. 2015, 5, 1500091. [Google Scholar] [CrossRef]
- Zhou, W.; Lu, X.-F.; Chen, J.-J.; Zhou, T.; Liao, P.-Q.; Wu, M.; Li, G.-R. Hierarchical Porous Prism Arrays Composed of Hybrid Ni–NiO–Carbon as Highly Efficient Electrocatalysts for Overall Water Splitting. ACS Appl. Mater. Interfaces 2018, 10, 38906–38914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Cao, Z.; Zhang, P.; Ma, H.; Gao, Z.; Wang, H.; Lu, Y.; He, J.; Zhao, Y. Transition metal ions regulated oxygen evolution reaction performance of Ni-based hydroxides hierarchical nanoarrays. Sci. Rep. 2017, 7, 46154. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xi, W.; Li, C.; Li, X.; Shi, J.; Shen, Y.; He, J.; Zhang, L.; Xie, L.; Sun, X.; et al. Nanoporous Zn-doped Co3O4 sheets with single-unit-cell-wide lateral surfaces for efficient oxygen evolution and water splitting. Nano Energy 2018, 44, 371–377. [Google Scholar] [CrossRef]
- An, Y.; Fei, H.; Zeng, G.; Xu, X.; Ci, L.; Xi, B.; Xiong, S.; Feng, J.; Qian, Y. Vacuum distillation derived 3D porous current collector for stable lithium–metal batteries. Nano Energy 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Zhou, T.; Liu, Z.; Yang, B.; Cao, Z.; Jiang, Z.; Cui, W.; Wang, K.; Yu, L.; Lu, J.; Zhang, L. Dealloying fabrication of hierarchical porous Nickel–Iron foams for efficient oxygen evolution reaction. Front. Chem. 2022, 10, 1047398. [Google Scholar] [CrossRef]
- Qin, R.; Hou, J.; Xu, C.; Yang, H.; Zhou, Q.; Chen, Z.; Liu, H. Self-supporting Co0.85Se nanosheets anchored on Co plate as highly efficient electrocatalyst for hydrogen evolution reaction in both acidic and alkaline media. Nano Res. 2020, 13, 2950–2957. [Google Scholar] [CrossRef]
- Zhou, Q.; Hao, Q.; Li, Y.; Yu, J.; Xu, C.; Liu, H.; Yan, S. Free-standing trimodal porous NiZn intermetallic and Ni heterojunction as highly efficient hydrogen evolution electrocatalyst in the alkaline electrolyte. Nano Energy 2021, 89, 106402. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, B.; Kim, H.; Kang, K. Recent Progress on Multimetal Oxide Catalysts for the Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1702774. [Google Scholar] [CrossRef]
- Gerken, J.B.; Shaner, S.E.; Massé, R.C.; Porubsky, N.J.; Stahl, S.S. A survey of diverse earth abundant oxygen evolution electrocatalysts showing enhanced activity from Ni–Fe oxides containing a third metal. Energy Environ. Sci. 2014, 7, 2376–2382. [Google Scholar] [CrossRef]
- Suntivich, J.; May, K.J.; Gasteiger, H.A.; Goodenough, J.B.; Shao-Horn, Y. A Perovskite Oxide Optimized for Oxygen Evolution Catalysis from Molecular Orbital Principles. Science 2011, 334, 1383–1385. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, I.; Yoon, G.; Kim, J.S.; Kim, H.; Kang, K. Atomistic Investigation of Doping Effects on Electrocatalytic Properties of Cobalt Oxides for Water Oxidation. Adv. Sci. 2018, 5, 1801632. [Google Scholar] [CrossRef] [PubMed]
- Grewe, T.; Deng, X.; Weidenthaler, C.; Schüth, F.; Tüysüz, H. Design of Ordered Mesoporous Composite Materials and Their Electrocatalytic Activities for Water Oxidation. Chem. Mater. 2013, 25, 4926–4935. [Google Scholar] [CrossRef]
- Li, Z.; Wu, R.; Duan, D.; Liu, X.; Li, R.; Wang, J.; Chen, H.; Chen, S.-W.; Wu, Y.; Wang, H.; et al. Empowering multicomponent alloys with unique nanostructure for exceptional oxygen evolution performance through self-replenishment. Joule 2024, 8, 1982–1998. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Feng, Y.; Guan, B.; Lou, X.W.; Paik, U. Carbon coated porous nickel phosphides nanoplates for highly efficient oxygen evolution reaction. Energy Environ. Sci. 2016, 9, 1246–1250. [Google Scholar] [CrossRef]
- Fu, S.; Zhu, C.; Song, J.; Engelhard, M.H.; Li, X.; Du, D.; Lin, Y. Highly Ordered Mesoporous Bimetallic Phosphides as Efficient Oxygen Evolution Electrocatalysts. ACS Energy Lett. 2016, 1, 792–796. [Google Scholar] [CrossRef]
- Cai, P.; Huang, J.; Chen, J.; Wen, Z. Oxygen-Containing Amorphous Cobalt Sulfide Porous Nanocubes as High-Activity Electrocatalysts for the Oxygen Evolution Reaction in an Alkaline/Neutral Medium. Angew. Chem. Int. Ed. 2017, 56, 4858–4861. [Google Scholar] [CrossRef]
- Nai, J.; Lu, Y.; Yu, L.; Wang, X.; Lou, X.W. Formation of Ni–Fe Mixed Diselenide Nanocages as a Superior Oxygen Evolution Electrocatalyst. Adv. Mater. 2017, 29, 1703870. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Y.; Li, Y.; Sun, J.; Qiu, Y.; Liu, C.; Zhou, G.; Cui, Y. In Situ Electrochemically Derived Nanoporous Oxides from Transition Metal Dichalcogenides for Active Oxygen Evolution Catalysts. Nano Lett. 2016, 16, 7588–7596. [Google Scholar] [CrossRef]
- Zu, M.Y.; Wang, C.; Zhang, L.; Zheng, L.R.; Yang, H.G. Reconstructing bimetallic carbide Mo6Ni6C for carbon interconnected MoNi alloys to boost oxygen evolution electrocatalysis. Mater. Horiz. 2019, 6, 115–121. [Google Scholar] [CrossRef]
- Nandi, S.; Singh, S.K.; Mullangi, D.; Illathvalappil, R.; George, L.; Vinod, C.P.; Kurungot, S.; Vaidhyanathan, R. Low Band Gap Benzimidazole COF Supported Ni3N as Highly Active OER Catalyst. Adv. Energy Mater. 2016, 6, 1601189. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Vasileff, A.; Qiao, S.Z. Anion and Cation Modulation in Metal Compounds for Bifunctional Overall Water Splitting. ACS Nano 2016, 10, 8738–8745. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent Advances in Novel Nanostructuring Methods of Perovskite Electrocatalysts for Energy-Related Applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, P.; Han, J.; Cheng, C.; Ning, S.; Hirata, A.; Fujita, T.; Chen, M. Engineering the internal surfaces of three-dimensional nanoporous catalysts by surfactant-modified dealloying. Nat. Commun. 2017, 8, 1066. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Shi, F.; Zhu, X.; Yang, W. The roles of oxygen vacancies in electrocatalytic oxygen evolution reaction. Nano Energy 2020, 73, 104761. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, T.; Jiang, K.; Da, P.; Peng, Z.; Tang, J.; Kong, B.; Cai, W.-B.; Yang, Z.; Zheng, G. Reduced Mesoporous Co3O4 Nanowires as Efficient Water Oxidation Electrocatalysts and Supercapacitor Electrodes. Adv. Energy Mater. 2014, 4, 1400696. [Google Scholar] [CrossRef]
- Zhou, T.; Cao, Z.; Tai, X.; Yu, L.; Ouyang, J.; Li, Y.; Lu, J. Hierarchical Co(OH)2 Dendrite Enriched with Oxygen Vacancies for Promoted Electrocatalytic Oxygen Evolution Reaction. Polymers 2022, 14, 1510. [Google Scholar] [CrossRef]
- Xu, L.; Jiang, Q.; Xiao, Z.; Li, X.; Huo, J.; Wang, S.; Dai, L. Plasma-Engraved Co3O4 Nanosheets with Oxygen Vacancies and High Surface Area for the Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2016, 55, 5277–5281. [Google Scholar] [CrossRef]
- Gong, M.; Zhou, W.; Tsai, M.-C.; Zhou, J.; Guan, M.; Lin, M.-C.; Zhang, B.; Hu, Y.; Wang, D.-Y.; Yang, J.; et al. Nanoscale nickel oxide/nickel heterostructures for active hydrogen evolution electrocatalysis. Nat. Commun. 2014, 5, 4695. [Google Scholar] [CrossRef]
- Jiang, Y.; Deng, Y.-P.; Fu, J.; Lee, D.U.; Liang, R.; Cano, Z.P.; Liu, Y.; Bai, Z.; Hwang, S.; Yang, L.; et al. Interpenetrating Triphase Cobalt-Based Nanocomposites as Efficient Bifunctional Oxygen Electrocatalysts for Long-Lasting Rechargeable Zn–Air Batteries. Adv. Energy Mater. 2018, 8, 1702900. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, F.; Xia, Y.; Li, J.; Tamirat, A.G.; Liu, Y.; Wang, L.; Wang, Y.; Xia, Y. In situ encapsulation of core–shell-structured Co@Co3O4 into nitrogen-doped carbon polyhedra as a bifunctional catalyst for rechargeable Zn–air batteries. J. Mater. Chem. A 2018, 6, 1443–1453. [Google Scholar] [CrossRef]
- Xu, H.; Feng, J.-X.; Tong, Y.-X.; Li, G.-R. Cu2O–Cu Hybrid Foams as High-Performance Electrocatalysts for Oxygen Evolution Reaction in Alkaline Media. ACS Catal. 2017, 7, 986–991. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, X.; Zhang, H.; Zhang, J.; Li, S.; Wang, R.; Pan, B.; Xie, Y. Intralayered Ostwald Ripening to Ultrathin Nanomesh Catalyst with Robust Oxygen-Evolving Performance. Adv. Mater. 2017, 29, 1604765. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Song, S.; He, M.; Li, C.; Wang, W.; Li, H.; Yuan, X.; Fang, Z.; Rinel, K.P.; Song, W.; et al. High-density defects in ordered macroporous-mesoporous CoNiFe-LDHs for efficient and robust oxygen evolution reaction. Chem Catal. 2023, 3, 100497. [Google Scholar] [CrossRef]
- Xie, J.; Xin, J.; Wang, R.; Zhang, X.; Lei, F.; Qu, H.; Hao, P.; Cui, G.; Tang, B.; Xie, Y. Sub-3 nm pores in two-dimensional nanomesh promoting the generation of electroactive phase for robust water oxidation. Nano Energy 2018, 53, 74–82. [Google Scholar] [CrossRef]
- Han, X.; Yu, C.; Zhou, S.; Zhao, C.; Huang, H.; Yang, J.; Liu, Z.; Zhao, J.; Qiu, J. Ultrasensitive iron-triggered nanosized Fe–CoOOH integrated with graphene for highly efficient oxygen evolution. Adv. Energy Mater. 2017, 7, 1602148. [Google Scholar] [CrossRef]
- Zhuang, Z.; Sheng, W.; Yan, Y. Synthesis of monodispere Au@Co3O4 core-shell nanocrystals and their enhanced catalytic activity for oxygen evolution reaction. Adv. Mater. 2014, 26, 3950–3955. [Google Scholar] [CrossRef]
- Liu, G.; Li, P.; Zhao, G.; Wang, X.; Kong, J.; Liu, H.; Zhang, H.; Chang, K.; Meng, X.; Kako, T.; et al. Promoting Active Species Generation by Plasmon-Induced Hot-Electron Excitation for Efficient Electrocatalytic Oxygen Evolution. J. Am. Chem. Soc. 2016, 138, 9128–9136. [Google Scholar] [CrossRef]
- Kaya, M.F.; Demir, N.; Albawabiji, M.S.; Taş, M. Investigation of alkaline water electrolysis performance for different cost effective electrodes under magnetic field. Int. J. Hydrogen Energy 2017, 42, 17583–17592. [Google Scholar] [CrossRef]
- Bayatsarmadi, B.; Zheng, Y.; Vasileff, A.; Qiao, S.-Z. Recent Advances in Atomic Metal Doping of Carbon-based Nanomaterials for Energy Conversion. Small 2017, 13, 1700191. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, Q.; Xue, H.; Pang, H. Metal-organic frameworks for direct electrochemical applications. Coord. Chem. Rev. 2018, 376, 292–318. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Y.; Dong, J.; He, C.-T.; Yin, H.; An, P.; Zhao, K.; Zhang, X.; Gao, C.; Zhang, L.; et al. Ultrathin metal–organic framework nanosheets for electrocatalytic oxygen evolution. Nat. Energy 2016, 1, 16184. [Google Scholar] [CrossRef]
- Sun, F.; Wang, G.; Ding, Y.; Wang, C.; Yuan, B.; Lin, Y. NiFe-Based Metal–Organic Framework Nanosheets Directly Supported on Nickel Foam Acting as Robust Electrodes for Electrochemical Oxygen Evolution Reaction. Adv. Energy Mater. 2018, 8, 1800584. [Google Scholar] [CrossRef]
- Rui, K.; Zhao, G.; Chen, Y.; Lin, Y.; Zhou, Q.; Chen, J.; Zhu, J.; Sun, W.; Huang, W.; Dou, S.X. Hybrid 2D Dual-Metal–Organic Frameworks for Enhanced Water Oxidation Catalysis. Adv. Funct. Mater. 2018, 28, 1801554. [Google Scholar] [CrossRef]
- Wu, L.; Li, Q.; Wu, C.H.; Zhu, H.; Mendoza-Garcia, A.; Shen, B.; Guo, J.; Sun, S. Stable Cobalt Nanoparticles and Their Monolayer Array as an Efficient Electrocatalyst for Oxygen Evolution Reaction. J. Am. Chem. Soc. 2015, 137, 7071–7074. [Google Scholar] [CrossRef]
- Cao, Z.; Zhou, T.; Ma, X.; Shen, Y.; Deng, Q.; Zhang, W.; Zhao, Y. Hydrogen Production from Urea Sewage on NiFe-Based Porous Electrocatalysts. ACS Sustain. Chem. Eng. 2020, 8, 11007–11015. [Google Scholar] [CrossRef]
- Dong, C.; Kou, T.; Gao, H.; Peng, Z.; Zhang, Z. Eutectic-Derived Mesoporous Ni-Fe-O Nanowire Network Catalyzing Oxygen Evolution and Overall Water Splitting. Adv. Energy Mater. 2018, 8, 1701347. [Google Scholar] [CrossRef]
- Trześniewski, B.J.; Diaz-Morales, O.; Vermaas, D.A.; Longo, A.; Bras, W.; Koper, M.T.M.; Smith, W.A. In Situ Observation of Active Oxygen Species in Fe-Containing Ni-Based Oxygen Evolution Catalysts: The Effect of pH on Electrochemical Activity. J. Am. Chem. Soc. 2015, 137, 15112–15121. [Google Scholar] [CrossRef]


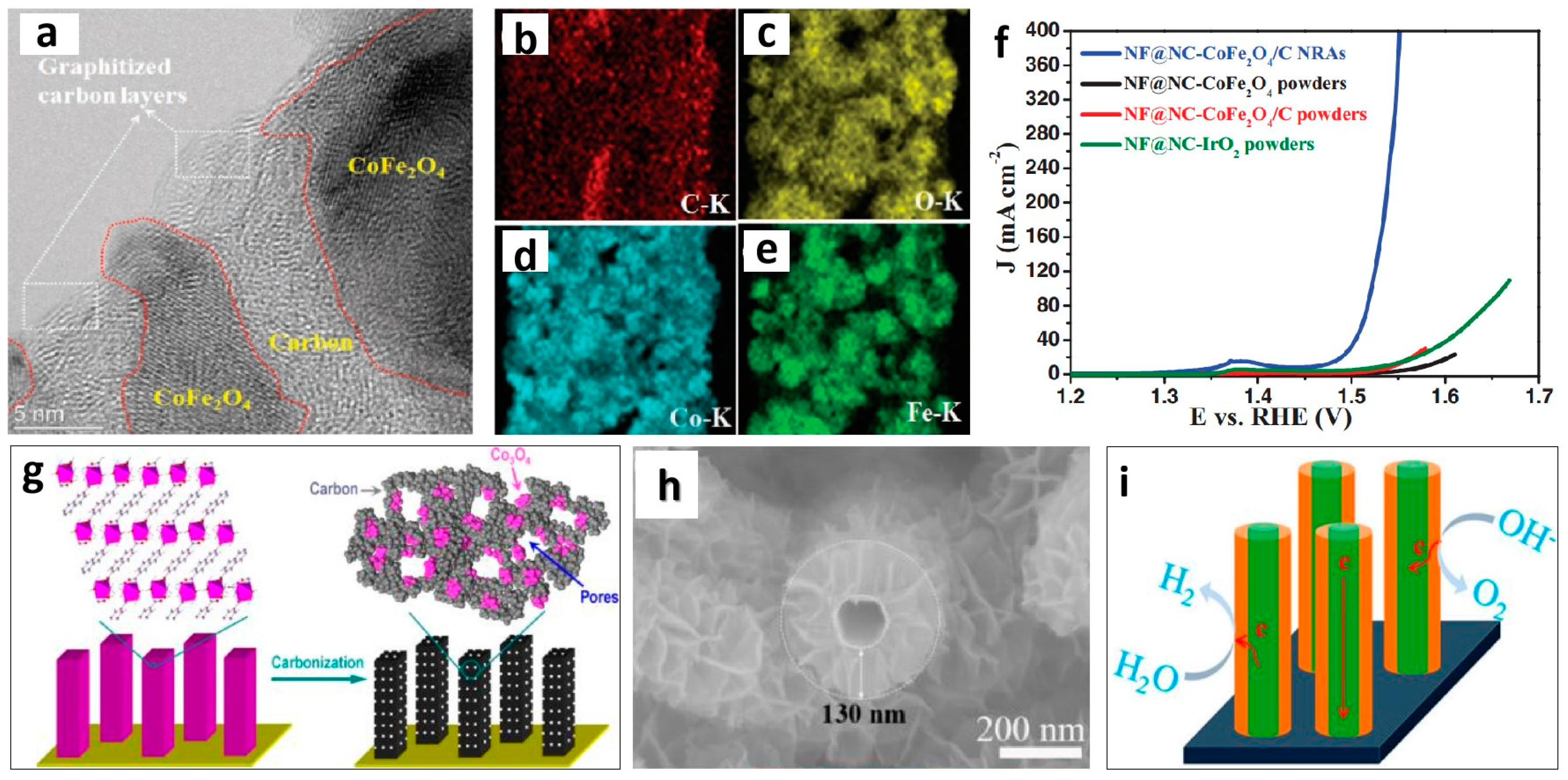
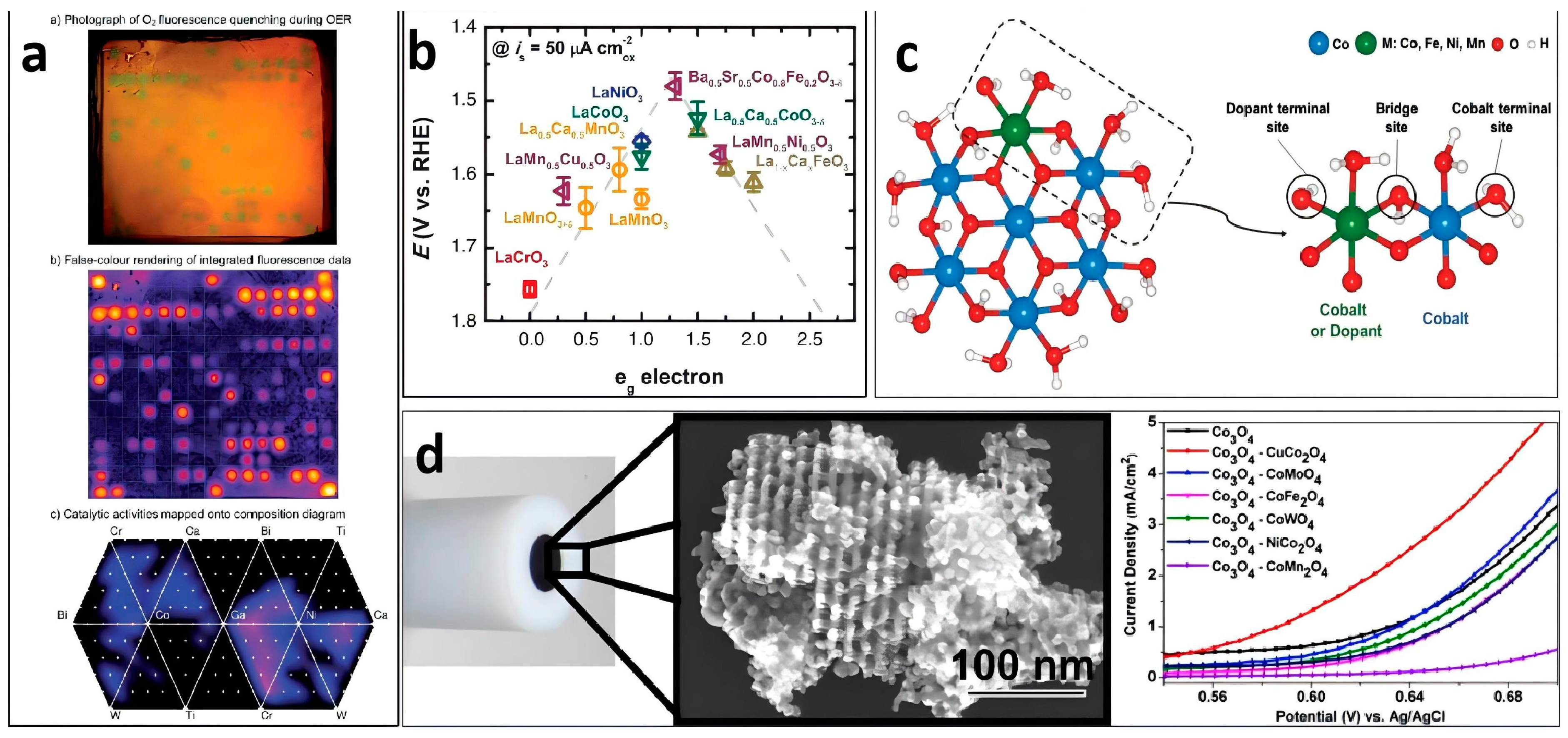
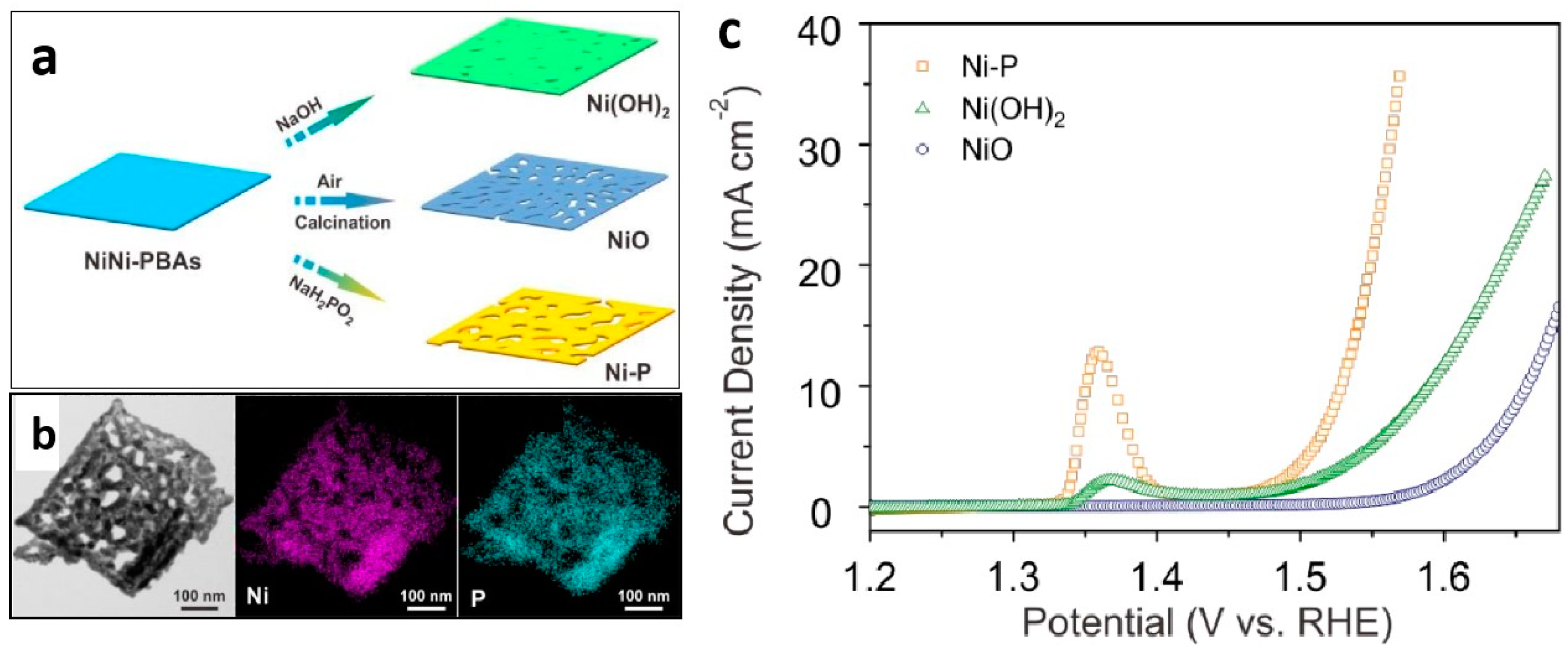
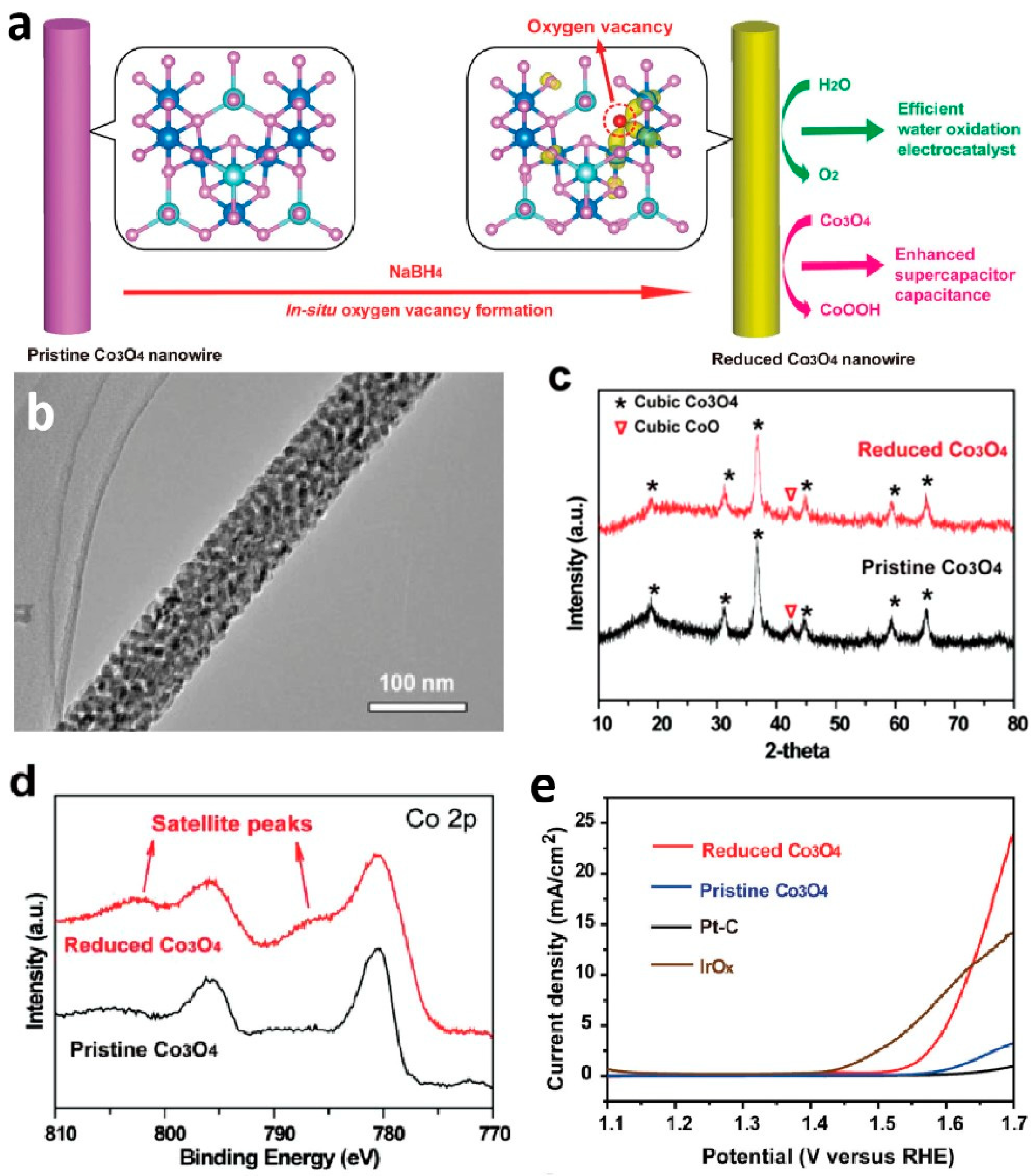

| Nanoporous Materials | Electrolyte | Overpotential ηmA cm−2 [mV] | Tafel [mV dec−1] | Extended Application Based on OER | Material Groups * | Ref. |
|---|---|---|---|---|---|---|
| meso-Co3O4 thin film | 1 M KOH | η10 = 340 | 57 | - | 1 | [33] |
| Ordered Mesoporous Ni Sphere Arrays | 1 M KOH | η10 = 254 | 39 | - | 1 | [34] |
| Co3O4/Fe3O4(4:1) | 1 M KOH | η10 = 320 | 78 | - | 1 | [35] |
| Mesoporous-Ni60Fe30Mn10 | 1 M KOH 0.5 M KOH | η500 = 360 η10 = 200 | 62 - | - | 1 | [36] |
| Co3O4-35 | 0.1 M KOH | η10 = 525 | - | - | 2.1 | [38] |
| c-Co3O4-5 | 0.1 M KOH 1 M KOH | η10 = 496 η10 = 410 | 96 59 | - | 2.1 | [39] |
| CoFe2O4@C nanomaterials | 1 M KOH | η10 = 248 | 58.7 | - | 2.1 | [41] |
| Hierarchically porous Co3O4 | 0.1 M KOH | η10 = 450 | 89 | - | 2.2 | [44] |
| CoNi Porous cages | 1 M KOH | η10 = 380 | 50 | - | 2.2 | [45] |
| Co0.6Fe0.4P-1.125 | 1 M KOH | η10 = 298 | 48 | Full water splitting | 2.2 | [46] |
| CoFe frame-like superstructure | 1 M KOH | η10 = 340 | 57 | - | 2.2 | [47] |
| HP-CoFe | 1 M KOH | η10 = 290 | 49.9 | - | 2.2 | [48] |
| hierarchical CoOx nanosheet/nanotube | 1 M KOH | η51.2 = 420 | 75 | Full water splitting (HER: Pt) | 2.2 | [50] |
| NF@NC-CoFe2O4/C | 1 M KOH | η10 = 240 | 45 | - | 2.3 | [54] |
| Co3O4C-NA | 0.1 M KOH | η10 = 290 | 70 | - | 2.3 | [55] |
| Ag NW@Co NS | 1 M KOH | η10 = 320 | 75.4 | - | 2.3 | [57] |
| NiFe/Cu2O NWs/CF | 1 M KOH | η400 = 310 | 42 | - | 2.3 | [58] |
| nanoporous Au/Cr–NiFe | 0.1 M KOH | η10 = 323 | 33 | - | 2.3 | [59] |
| CP/CTs/Co-S | 1 M KOH | η10 = 306 | 72 | Full water splitting | 2.3 | [60] |
| Ni-NiO/C HPPAs@NF | 1 M KOH | η10 = 295 | 52 | Full water splitting | 2.3 | [62] |
| Ni2.2Fe(OH)xHNAs | 1 M KOH | η100 = 298 | 64.3 | - | 2.3 | [63] |
| NP CoO-UCSs | 0.1 M KOH 1 M KOH | η10 = 182 ± 5 η10 = 132 ± 4 | 34 | Full water splitting | 2.3 | [64] |
| NP-NF@NFF | 1 M KOH | η10 = 210 η100 = 285 | 32.84 | - | 2.3 | [66] |
| CuxCoyO4 | 0.1 M KOH | η10 = 498 | - | - | 3.1 | [73] |
| NP-(FeCoNi)2Nb | 1 M KOH | η10 = 303 | 63.6 | Anion-exchange-membrane water electrolyzer | 3.1 | [74] |
| Ni-P | 1 M KOH | η10 = 300 | 64 | - | 3.2 | [75] |
| Co3Ni1 P | 1 M KOH | η10 = 280 | 66.5 | - | 3.2 | [76] |
| A-CoS4.6O0.6 PNCs | 1 M KOH 0.1 M PBS | η10 = 290 η4.59 = 570 | 67 164 | - | 3.2 | [77] |
| Ni-Fe-Se cages | 1 M KOH | η10 = 240 η100 = 270 | 24 | 3.2 | [78] | |
| CoFePO | 1 M KOH | η10 = 274.5 | 51.7 | Full water splitting | 3.2 | [82] |
| Reduced Co3O4 NWs | 1 M KOH | η13.1 = 420 | 72 | - | 3.3 | [87] |
| OV-Co(OH)2 | 1 M KOH | η10 = 350 | 64.9 | - | 3.3 | [88] |
| Ar-plasma engraved Co3O4 | 1 M KOH | η10 = 300 | 68 | - | 3.3 | [89] |
| Co/Co3O4@PGS | 0.1 M KOH | η10 = 350 | 52.6 | Rechargeable Zn–Air Batteries | 3.4 | [91] |
| Co@Co3O4@NC-900 | 1 M KOH | η10 = 370 | 94 | Rechargeable Zn–Air Batteries | 3.4 | [92] |
| Cu2O-Cu foams | 1 M KOH | η10 = 350 | 67.5 | - | 3.4 | [93] |
| β-Ni(OH)2 ultrathin nanomeshes | 1 M KOH | η20 = 236 | 132 (As a Ref.) | - | 3.5 | [95] |
| kh-CoNiFe-LDH | 1 M KOH | η10 = 197 | 58.3 | - | 3.5 | [96] |
| NiFe LDH nanomesh | 1 M KOH | η10 = 268 | 30 | - | 3.5 | [97] |
| Fe-CoOOH/G | 1 M KOH | η10 = 330 | 37 | - | 3.5 | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Z.; Zhang, W.; Zhou, T.; Yan, W.; Wang, K. Design and Optimization of Nanoporous Materials as Catalysts for Oxygen Evolution Reaction—A Review. Molecules 2024, 29, 4562. https://doi.org/10.3390/molecules29194562
Cao Z, Zhang W, Zhou T, Yan W, Wang K. Design and Optimization of Nanoporous Materials as Catalysts for Oxygen Evolution Reaction—A Review. Molecules. 2024; 29(19):4562. https://doi.org/10.3390/molecules29194562
Chicago/Turabian StyleCao, Zhen, Wenbin Zhang, Tingting Zhou, Wenhui Yan, and Kaili Wang. 2024. "Design and Optimization of Nanoporous Materials as Catalysts for Oxygen Evolution Reaction—A Review" Molecules 29, no. 19: 4562. https://doi.org/10.3390/molecules29194562
APA StyleCao, Z., Zhang, W., Zhou, T., Yan, W., & Wang, K. (2024). Design and Optimization of Nanoporous Materials as Catalysts for Oxygen Evolution Reaction—A Review. Molecules, 29(19), 4562. https://doi.org/10.3390/molecules29194562






