Structural Characterization, and Antioxidant, Hypoglycemic and Immunomodulatory Activity of Exopolysaccharide from Sanghuangporus sanghuang JM-1
Abstract
1. Introduction
2. Results
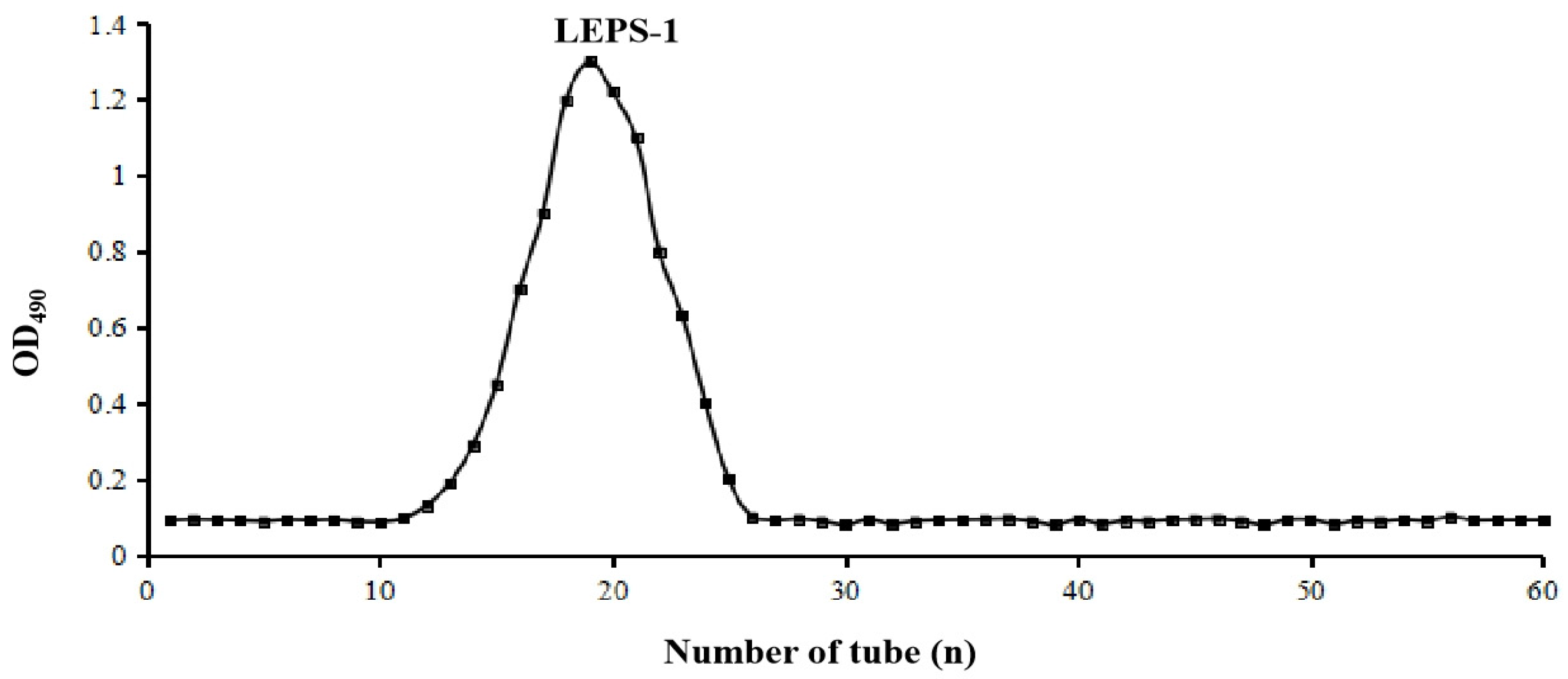
2.1. Extraction and Purification of EPS
2.2. Chemical Composition of LEPS-1
2.3. MW and Monosaccharide Composition of LEPS-1
2.4. Characterization of Chemical Structure of LEPS-1
2.4.1. Methylation and Gas Chromatography–Mass Spectrometry Analysis
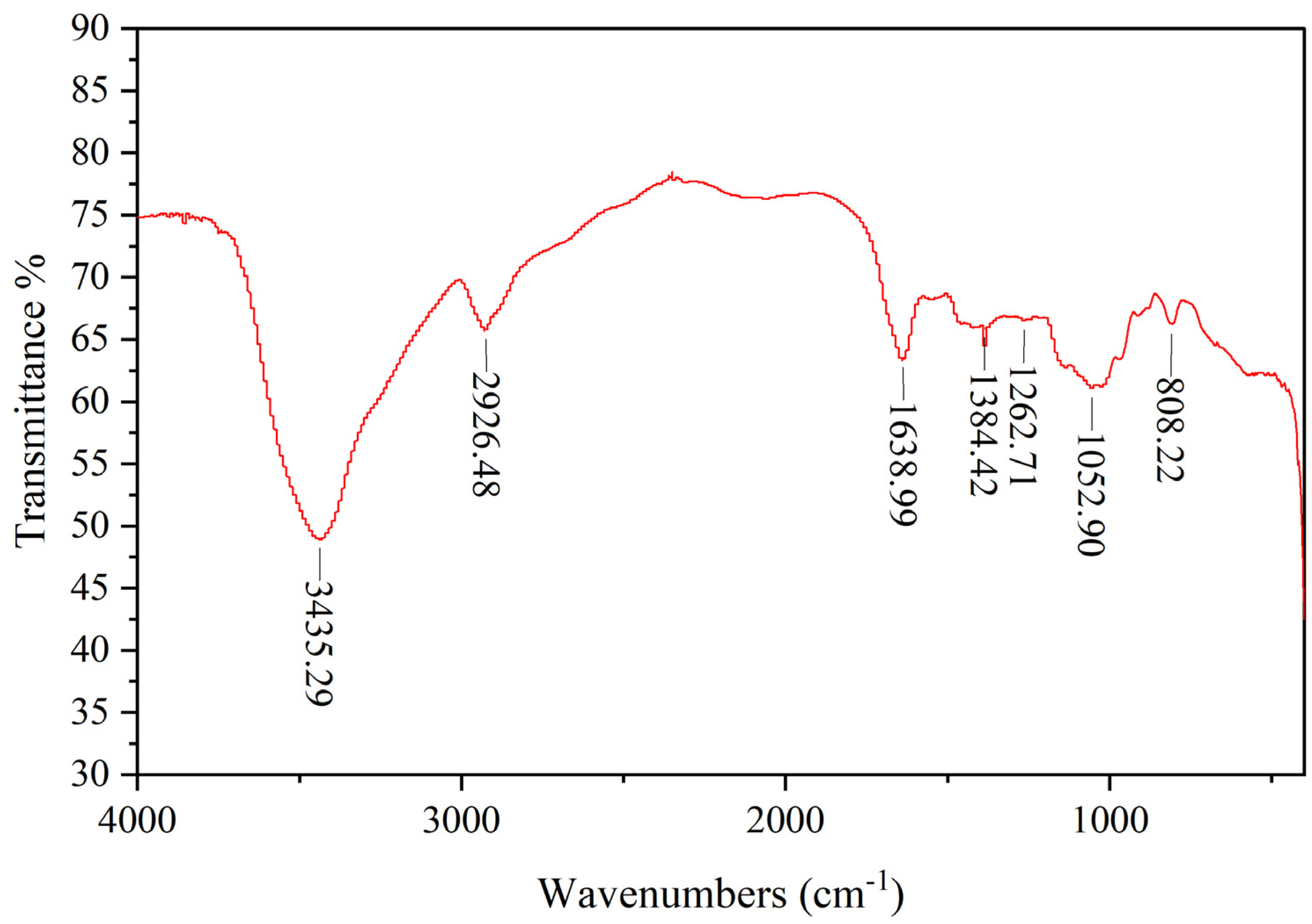
2.4.2. FT-IR Spectroscopy Measurement
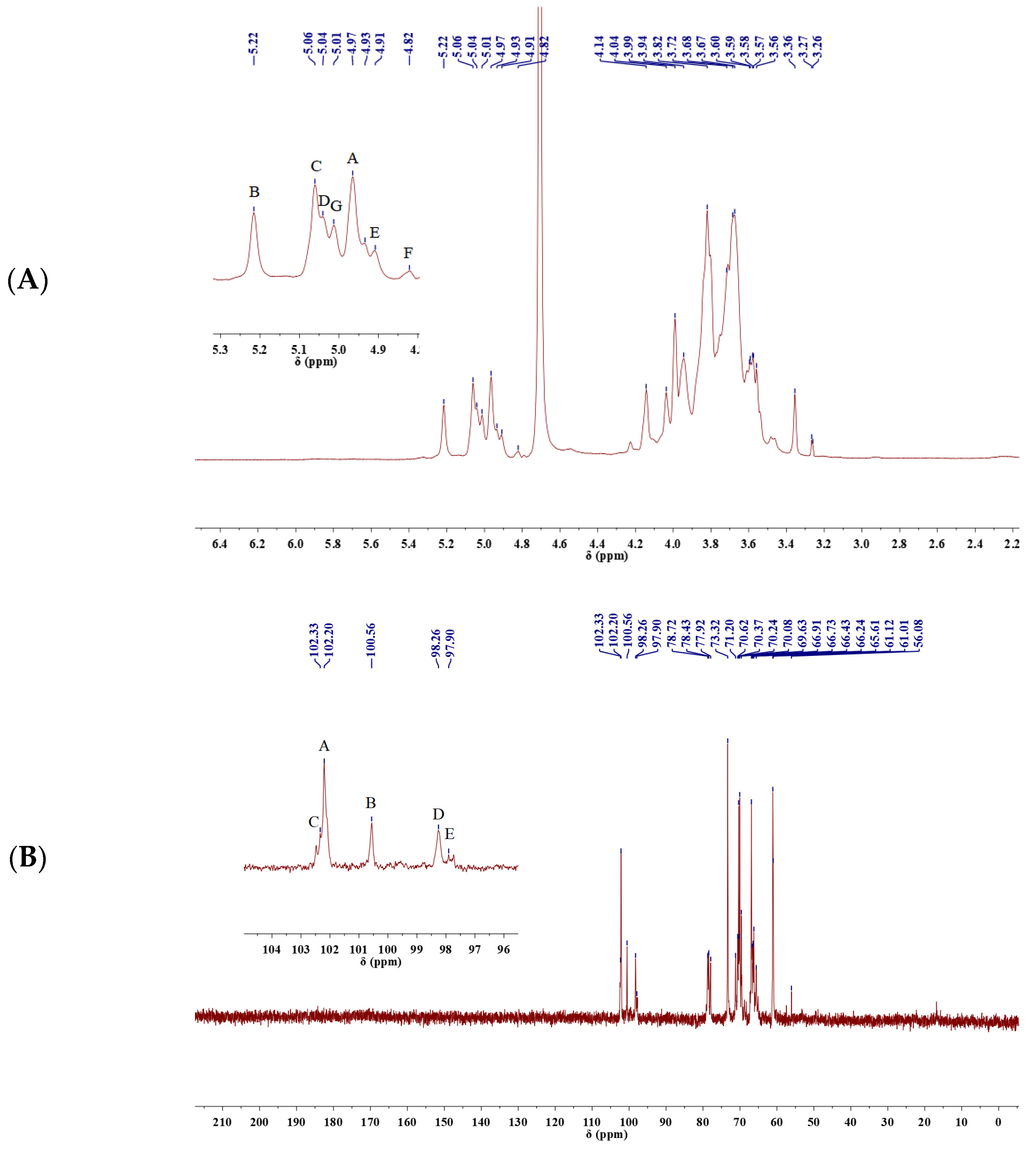
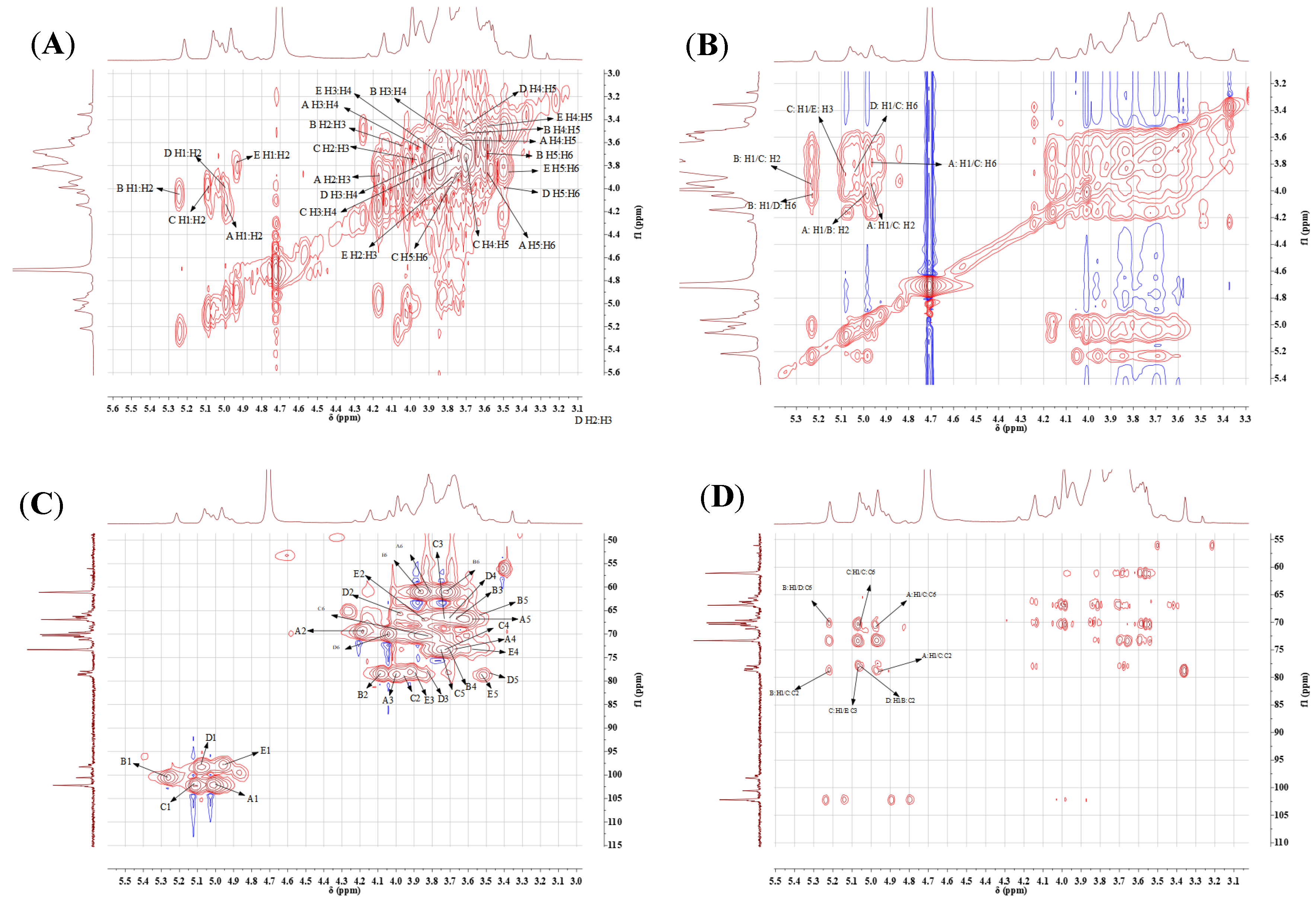
2.4.3. NMR Analysis
2.5. Evaluation of Biological Activities
2.5.1. Solubility of LEPS-1
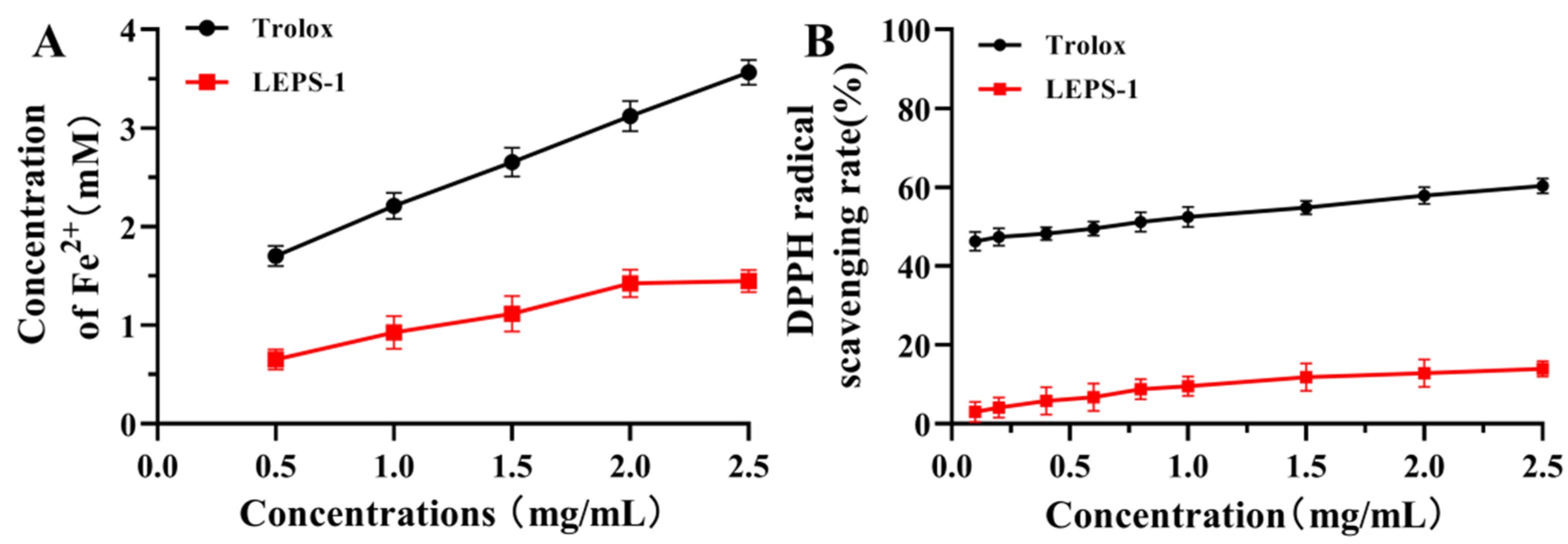
2.5.2. Antioxidant Activity
2.5.3. α-Amylase and α-Glucosidase Inhibitory Activity
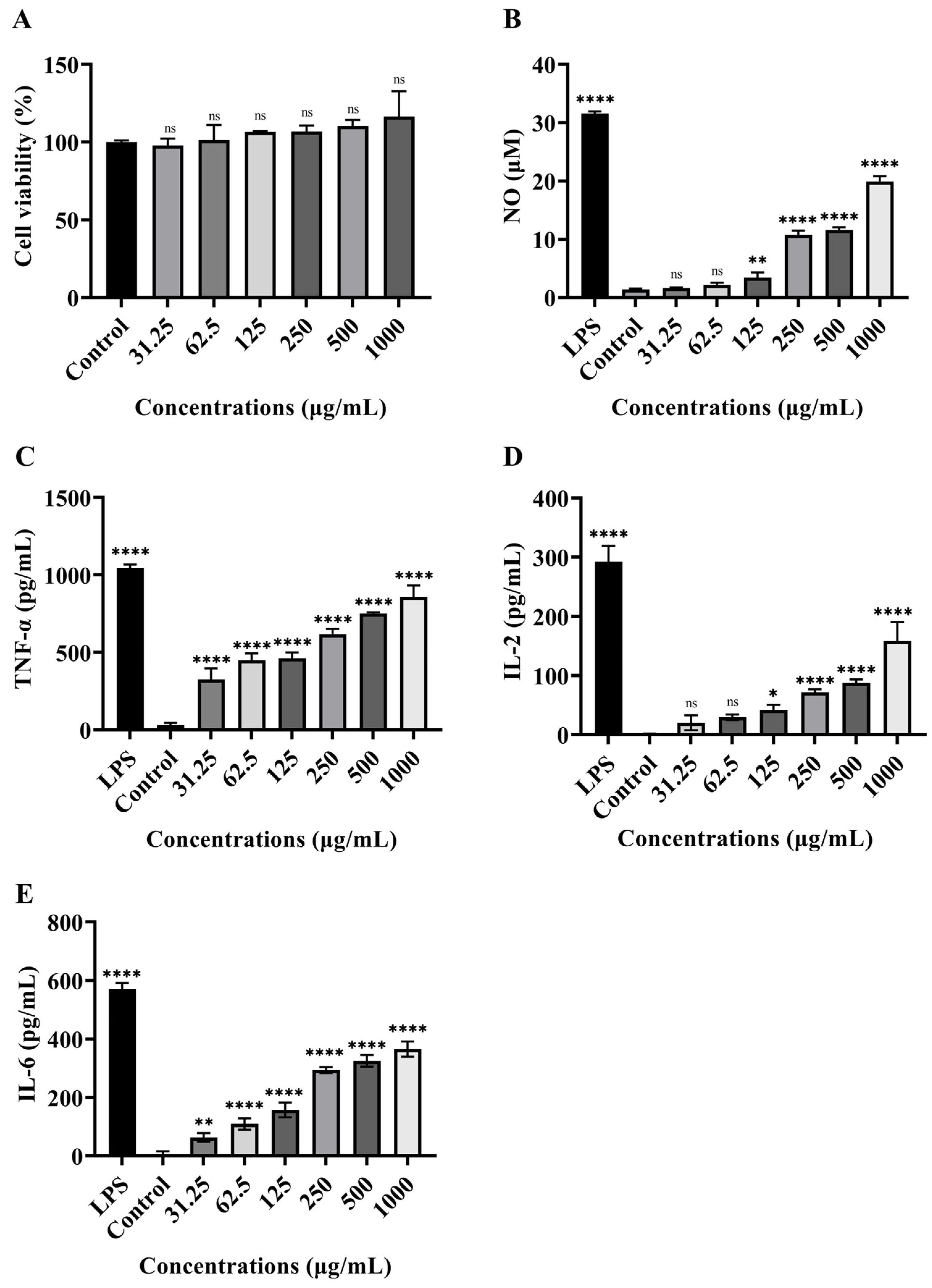
2.5.4. Immunomodulatory Activity
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Extraction and Purification of EPS
4.3. Chemical Composition Analysis of LEPS-1
4.4. Determination of Monosaccharide Composition of LEPS-1
4.5. Molecular Weight Determination of LEPS-1
4.6. Characterization of Chemical Structure of LEPS-1
4.6.1. Methylation and Gas Chromatography-Mass Spectrometry Analysis
4.6.2. Fourier Transform Infrared (FT-IR) Spectroscopy Measurement
4.6.3. Nuclear Magnetic Resonance (NMR) Spectroscopy Measurement
4.7. Bioactivity Analysis of LEPS-1
4.7.1. Solubility
4.7.2. Antioxidant Activity In Vitro
4.7.3. α-Amylase and α-Glucosidase Inhibitory Activity In Vitro
4.7.4. Immunomodulatory Assays
Cytotoxicity Assay
Quantification of NO, TNF-α, IL-2 and IL-6
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wan, X.; Jin, X.; Wu, X.; Yang, X.; Lin, D.; Li, C.; Fu, Y.; Liu, Y.; Liu, X.; Lv, J.; et al. Structural characterisation and antitumor activity against non-small cell lung cancer of polysaccharides from Sanghuangporus vaninii. Carbohydr. Polym. 2022, 276, 118798. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhou, L.; Vlasak, J.; Dai, Y. Global diversity and systematics of hymenochaetaceae with poroid hymenophore. Fungal Divers. 2022, 113, 1–192. [Google Scholar] [CrossRef]
- Wu, S.; Chang, C.; Wei, C.; Jiang, G.; Cui, B. Sanghuangporus toxicodendri sp. Nov. (Hymenochaetales, basidiomycota) from China. Mycokeys 2019, 57, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Dai, Y.; Hattori, T.; Yu, T.; Wang, D.; Parmasto, E.; Chang, H.; Shih, S. Species clarification for the medicinally valuable ‘sanghuang’ mushroom. Bot. Stud. 2012, 53, 135–149. [Google Scholar]
- Zhou, L.; Vlasak, J.; Decock, C.; Assefa, A.; Stenlid, J.; Abate, D.; Wu, S.; Dai, Y. Global diversity and taxonomy of the inonotus linteus complex (Hymenochaetales, basidiomycota): Sanghuangporus gen. Nov., Tropicoporus excentrodendri and T-guanacastensis gen. Et spp. Nov., and 17 new combinations. Fungal Divers. 2016, 77, 335–347. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, L.; Yang, Z.; Bau, T.; Li, T.; Dai, Y. Resource diversity of Chinese macrofungi: Edible, medicinal and poisonous species. Fungal Divers. 2019, 98, 1–76. [Google Scholar] [CrossRef]
- Lin, W.; Deng, J.; Huang, S.; Wu, S.; Chen, C.; Lin, W.; Lin, H.; Huang, G. Anti-inflammatory activity of Sanghuangporus sanghuang mycelium. Int. J. Mol. Sci. 2017, 18, 347. [Google Scholar] [CrossRef]
- Cai, C.; Ma, J.; Han, C.; Jin, Y.; Zhao, G.; He, X. Extraction and antioxidant activity of total triterpenoids in the mycelium of a medicinal fungus, Sanghuangporus sanghuang. Sci. Rep. 2019, 9, 7418. [Google Scholar] [CrossRef]
- Ma, J.X.; Cai, C.S.; Liu, J.J.; Gao, S.; Zhao, G.Z.; He, X.W. In vitro antibacterial and antitumor activity of total triterpenoids from a medicinal mushroom Sanghuangporus sanghuang (Agaricomycetes) in liquid fermentation culture. Int. J. Med. Mushrooms 2021, 23, 27–39. [Google Scholar] [CrossRef]
- Cheng, J.; Song, J.; Wei, H.; Wang, Y.; Huang, X.; Liu, Y.; Lu, N.; He, L.; Lv, G.; Ding, H.; et al. Structural characterization and hypoglycemic activity of an intracellular polysaccharide from Sanghuangporus sanghuang mycelia. Int. J. Biol. Macromol. 2020, 164, 3305–3314. [Google Scholar] [CrossRef]
- Wen, Y.; Wan, Y.; Qiao, C.; Xu, X.; Wang, J.; Shen, Y. Immunoregenerative effects of the bionically cultured sanghuang mushrooms (Inonotus sanghuagn) on the immunodeficient mice. J. Ethnopharmacol. 2019, 245, 112047. [Google Scholar] [CrossRef] [PubMed]
- Sulkowska-Ziaja, K.; Balik, M.; Muszynska, B. Selected species of the genus Phellinus-chemical composition, biological activity, and medicinal applications. Chem. Biodivers 2021, 18, e2100609. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Tian, T.; Miao, H.; Zhao, Y. Traditional uses, fermentation, phytochemistry and pharmacology of Phellinus linteus: A review. Fitoterapia 2016, 113, 6–26. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Zhang, H.; Di, W.; Feng, N.; Zhang, Z.; Zhang, J.; Feng, J.; Yang, Y. Kinetic models for the effect of temperature on flavonoid production in liquid submerged fermentation by Phellinus baumii. Biotechnol. Appl. Biochem. 2018, 65, 739–747. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ma, J.X.; Zhou, M.; Si, J.; Cui, B.K. Current advances and potential trends of the polysaccharides derived from medicinal mushrooms sanghuang. Front. Microbiol. 2022, 13, 965934. [Google Scholar] [CrossRef]
- Yang, S.; Yan, J.; Yang, L.; Meng, Y.; Wang, N.; He, C.; Fan, Y.; Zhou, Y. Alkali-soluble polysaccharides from mushroom fruiting bodies improve insulin resistance. Int. J. Biol. Macromol. 2019, 126, 466–474. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic potential of mushrooms in diabetes mellitus: Role of polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Lin, G.; Li, Y.; Chen, X.; Zhang, F.; Linhardt, R.J.; Zhang, A. Extraction, structure and bioactivities of polysaccharides from Sanghuangporus spp.: A review. Food Biosci. 2023, 53, 102587. [Google Scholar] [CrossRef]
- Liu, J.S.; Zeng, Y.X.; Bi, S.Y.; Zhou, J.W.; Cheng, R.; Li, J.; Jia, A.Q. Characterization and chemical modification of PLN-1, an exopolysaccharide from Phomopsis liquidambari NJUSTB1. Carbohydr. Polym. 2021, 253, 117197. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, Y.; Huang, J.; Qian, N.; Shen, G.; Chen, L. Anti-inflammatory activity of polysaccharides from Phellinus linteus by regulating the NF-κB translocation in LPS-stimulated RAW264.7 macrophages. Int. J. Biol. Macromol. 2019, 129, 61–67. [Google Scholar] [CrossRef]
- Yang, W.; Huang, G. Preparation and analysis of polysaccharide from Solanum tuberdsm. Ultrason. Sonochem. 2023, 98, 106520. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Han, M.; Song, Y.; Shin, B.; Shin, Y.; Lee, H.; Moon, D.; Lee, C.; Kwak, J.; Bae, Y.; et al. Proteoglycan isolated from Phellinus linteus induces toll-like receptors 2- and 4-mediated maturation of murine dendritic cells via activation of erk, p38, and NF-κB. Biol. Pharm. Bull. 2004, 27, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Song, J.; Liu, Y.; Lu, N.; Wang, Y.; Hu, C.; He, L.; Wei, H.; Lv, G.; Yang, S.; et al. Conformational properties and biological activities of β-D-mannan from Sanghuangporus sanghuang in liquid culture. Int. J. Biol. Macromol. 2020, 164, 3568–3579. [Google Scholar] [CrossRef]
- Ma, X.; She, X.; Peterson, E.C.; Wang, Y.Z.; Zheng, P.; Ma, H.; Zhang, K.; Liang, J. A newly characterized exopolysaccharide from Sanghuangporus sanghuang. J. Microbiol. 2019, 57, 812–820. [Google Scholar] [CrossRef]
- Mizrahy, S.; Peer, D. Polysaccharides as building blocks for nanotherapeutics. Chem. Soc. Rev. 2012, 41, 2623–2640. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Chen, B.S.; Dai, H.Q.; Ren, J.W.; Zhou, L.W.; Wu, S.H.; Liu, H.W. Sesquiterpenes and polyphenols with glucose-uptake stimulatory and antioxidant activities from the medicinal mushroom Sanghuangporus sanghuang. Chin. J. Nat. Med. 2021, 19, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Song, J.; Gao, F.; Chen, W.; Zong, Y.; Li, J.; He, Z.; Du, R. Extraction, purification, and structural characterization of polysaccharides from Sanghuangporus vaninii with anti-inflammatory activity. Molecules 2023, 28, 6081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Wang, J.; Wu, Y.; Hu, C.; Hao, X.; Jin, L.; Suo, H.; Li, Q. Purification, characterization, anti-ulcerative colitis activity of Sanghuangporus vaninii acidic polysaccharide a-3 (svp-a-3). Carbohydr. Polym. Technol. Appl. 2023, 6, 100387. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Q.; Chen, K.; Huang, Z.; Liu, Y.; Jia, R.; Liu, B. Sanghuangporus vaninii fruit body polysaccharide alleviates hyperglycemia and hyperlipidemia via modulating intestinal microflora in type 2 diabetic mice. Front. Nutr. 2022, 9, 1013466. [Google Scholar] [CrossRef]
- Guo, S.; Duan, W.; Wang, Y.; Chen, L.; Yang, C.; Gu, X.; Xue, Q.; Li, R.; Zhang, Z. Component analysis and anti-colorectal cancer mechanism via akt/mtor signalling pathway of Sanghuangporus vaninii extracts. Molecules 2022, 27, 1153. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Liu, G.; Li, Y.; Liang, L.; Liu, X.; Xu, X.; Wen, C. Advance in Morchella sp. polysaccharides: Isolation, structural characterization and structure-activity relationship: A review. Int. J. Biol. Macromol. 2023, 247, 125819. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Zhu, L.; Qu, Y.; Qu, X.; Mu, M.; Zhang, M.; Muneer, G.; Zhou, Y.; Sun, L. Analyses of active antioxidant polysaccharides from four edible mushrooms. Int. J. Biol. Macromol. 2019, 123, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Dong, Y.; Chen, G.; Hu, Q. Extraction, purification, characterization and antitumor activity of polysaccharides from Ganoderma lucidum. Carbohydr. Polym. 2010, 80, 783–789. [Google Scholar] [CrossRef]
- Chen, W.; Tan, H.; Liu, Q.; Zheng, X.; Zhang, H.; Liu, Y.; Xu, L. A review: The bioactivities and pharmacological applications of Phellinus linteus. Molecules 2019, 24, 1888. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Jin, X.; Xie, M.; Liu, J.; Gontcharov, A.A.; Wang, H.; Lv, R.; Liu, D.; Wang, Q.; Li, Y. Characterization of a polysaccharide from Sanghuangporus vaninii and its antitumor regulation via activation of the p53 signaling pathway in breast cancer MCF-7 cells. Int. J. Biol. Macromol. 2020, 163, 865–877. [Google Scholar] [CrossRef]
- Zhang, A.; Deng, J.; Liu, X.; He, P.; He, L.; Zhang, F.; Linhardt, R.J.; Sun, P. Structure and conformation of α-glucan extracted from Agaricus blazei murill by high-speed shearing homogenization. Int. J. Biol. Macromol. 2018, 113, 558–564. [Google Scholar] [CrossRef]
- Chi, Y.; Ye, H.; Li, H.; Li, Y.; Guan, H.; Mou, H.; Wang, P. Structure and molecular morphology of a novel moisturizing exopolysaccharide produced by Phyllobacterium sp. 921f. Int. J. Biol. Macromol. 2019, 135, 998–1005. [Google Scholar] [CrossRef]
- Ye, G.; Chen, Y.; Wang, C.; Yang, R.; Bin, X. Purification and characterization of exopolysaccharide produced by Weissella cibaria yb-1 from pickle chinese cabbage. Int. J. Biol. Macromol. 2018, 120, 1315–1321. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Y.; Men, Y.; Zhang, J.; Liu, H.; Sun, Y. Structural characterization and immunomodulatory activity of exopolysaccharides from submerged culture of Auricularia auricula-judae. Int. J. Biol. Macromol. 2018, 115, 978–984. [Google Scholar] [CrossRef]
- Thomas, M.J.; Bielski, B.H.J. Oxidation and reaction of trolox c, a tocopherol analog, in aqueous solution. A pulse-radiolysis study. J. Am. Chem. Soc. 1989, 111, 3315–3319. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in Premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Celik, E.E.; Rubio, J.M.A.; Gökmen, V. Behaviour of trolox with macromolecule-bound antioxidants in aqueous medium: Inhibition of auto-regeneration mechanism. Food Chem. 2018, 243, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Shafie, M.H.; Yusof, R.; Gan, C. Deep eutectic solvents (des) mediated extraction of pectin from Averrhoa bilimbi: Optimization and characterization studies. Carbohydr. Polym. 2019, 216, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Aftab, A.; Yousaf, Z.; Rashid, M.; Younas, A.; Yasin, H.; Riaz, N.; Mansoor, Q. Vegetative part of Nigella sativa L. potential antineoplastic sources against Hep2 and MCF7 human cancer cell lines. J. Taibah Univ. Sci. 2023, 17, 2161294. [Google Scholar] [CrossRef]
- Wu, J.; Shi, S.; Wang, H.; Wang, S. Mechanisms underlying the effect of polysaccharides in the treatment of type 2 diabetes: A review. Carbohydr. Polym. 2016, 144, 474–494. [Google Scholar] [CrossRef]
- Wasser, S.P. Current findings, future trends, and unsolved problems in studies of medicinal mushrooms. Appl. Microbiol. Biotechnol. 2011, 89, 1323–1332. [Google Scholar] [CrossRef]
- Lo, T.C.; Chang, C.A.; Chiu, K.; Tsay, P.; Jen, J. Correlation evaluation of antioxidant properties on the monosaccharide components and glycosyl linkages of polysaccharide with different measuring methods. Carbohydr. Polym. 2011, 86, 320–327. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78. [Google Scholar] [CrossRef]
- Chen, N.; Hsu, T.; Lin, F.; Lai, H.; Wu, J. Effects on cytokine-stimulating activities of eps from Tremella mesenterica with various carbon sources. Food Chem. 2006, 99, 92–97. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, X.; Li, J. Chemical properties and antioxidant activity of exopolysaccharides fractions from mycelial culture of Inonotus obliquus in a ground corn stover medium. Food Chem. 2012, 134, 1899–1905. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yan, M.; Zhu, J.; Xu, X. Enhancement of exo-polysaccharide production and antioxidant activity in submerged cultures of Inonotus obliquus by lignocellulose decomposition. J. Ind. Microbiol. Biotechnol. 2011, 38, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.O.; Park, H.W.; Kim, J.H.; Lee, J.Y.; Moon, S.H.; Shin, C.S. Anti-cancer effect and structural characterization of endo-polysaccharide from cultivated mycelia of Inonotus obliquus. Life Sci. 2006, 79, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y. Review of isolation, structural properties, chain conformation, and bioactivities of psyllium polysaccharides. Int. J. Biol. Macromol. 2019, 139, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, F.; Li, L.; Liu, X.; Yan, J. Recent advances in the bioactive polysaccharides and other key components from Phellinus spp. And their pharmacological effects: A review. Int. J. Biol. Macromol. 2022, 222, 3108–3128. [Google Scholar] [CrossRef]
- Pilarski, R.; Zielinski, H.; Ciesiolka, D.; Gulewicz, K. Antioxidant activity of ethanolic and aqueous extracts of Uncaria tomentosa (willd.) Dc. J. Ethnopharmacol. 2006, 104, 18–23. [Google Scholar] [CrossRef]
- Liao, C.; Wu, L.; Zhong, W.; Zheng, Q.; Tan, W.; Feng, K.; Feng, X.; Meng, F. Cellular antioxidant properties of Ischnoderma resinosum polysaccharide. Molecules 2022, 27, 7717. [Google Scholar] [CrossRef]
- Fidler, I.J.; Kleinerman, E.S. Therapy of cancer metastasis by systemic activation of macrophages: From the bench to the clinic. Res. Immunol. 1993, 144, 284. [Google Scholar] [CrossRef]
- Li, X.; Jiao, L.L.; Zhang, X.; Tian, W.M.; Chen, S.; Zhang, L.P. Anti-tumor and immunomodulating activities of proteoglycans from mycelium of Phellinus nigricans and culture medium. Int. Immunopharmacol. 2008, 8, 909–915. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal mushroom science: Current perspectives, advances, evidences, and challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, W.; Ren, Y.; Li, X.; Tang, Y.; Min, T.; Lai, F.; Wu, H. Structural characterization of a novel polysaccharide from Lepidium meyenii (maca) and analysis of its regulatory function in macrophage polarization In Vitro. J. Agric. Food. Chem. 2017, 65, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Bi, S.; Hu, X.; Yang, J.; Li, C.; Li, H.; Yu, D.B.; Zhu, J.; Song, L.; Yu, R. Structural characterization and immunomodulatory mechanisms of two novel glucans from Morchella importuna fruiting bodies. Int. J. Biol. Macromol. 2021, 183, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Li, P.; Luo, Q.; Yan, J.; Zhang, J.; Jin, X.; Huang, W. Effect of γ-irradiation on the structure and antioxidant activity of polysaccharide isolated from the fruiting bodies of Morchella sextelata. Biosci. Rep. 2020, 40, BSR20194522. [Google Scholar] [CrossRef] [PubMed]
- Akram, K.; Shahbaz, H.M.; Kim, G.R.; Farooq, U.; Kwon, J.H. Improved extraction and quality characterization of water-soluble polysaccharide from gamma-irradiated Lentinus edodes. J. Food Sci. 2017, 82, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, X.; Xiong, Q.; Yu, Y.; Peng, L. Sulfated modification, characterization, and potential bioactivities of polysaccharide from the fruiting bodies of Russula virescens. Int. J. Biol. Macromol. 2020, 154, 1438–1447. [Google Scholar] [CrossRef]
- Bi, S. Structure Characterization, Chemical Modification and Activity of Exopolysaccharides from Punica granatum L. Njustb1. Master’s Thesis, Nanjing University of Science & Technology, Nanjing, China, 2019. [Google Scholar]
- Xu, G.; Liao, A.; Huang, J.; Zhang, J.; Thakur, K.; Wei, Z. Evaluation of structural, functional, and anti-oxidant potential of differentially extracted polysaccharides from potatoes peels. Int. J. Biol. Macromol. 2019, 129, 778–785. [Google Scholar] [CrossRef]
- Zheng, C.; Dong, Q.; Chen, H.; Cong, Q.; Ding, K. Structural characterization of a polysaccharide from Chrysanthemum morifolium flowers and its antioxidant activity. Carbohydr. Polym. 2015, 130, 113–121. [Google Scholar] [CrossRef]
- Hendra, R.; Army, M.K.; Frimayanti, N.; Teruna, H.Y.; Abdulah, R.; Nugraha, A.S. A-glucosidase and α-amylase inhibitory activity of flavonols from Stenochlaena palustris (burm.f.) Bedd. Saudi Pharm. J. 2024, 32, 101940. [Google Scholar] [CrossRef]
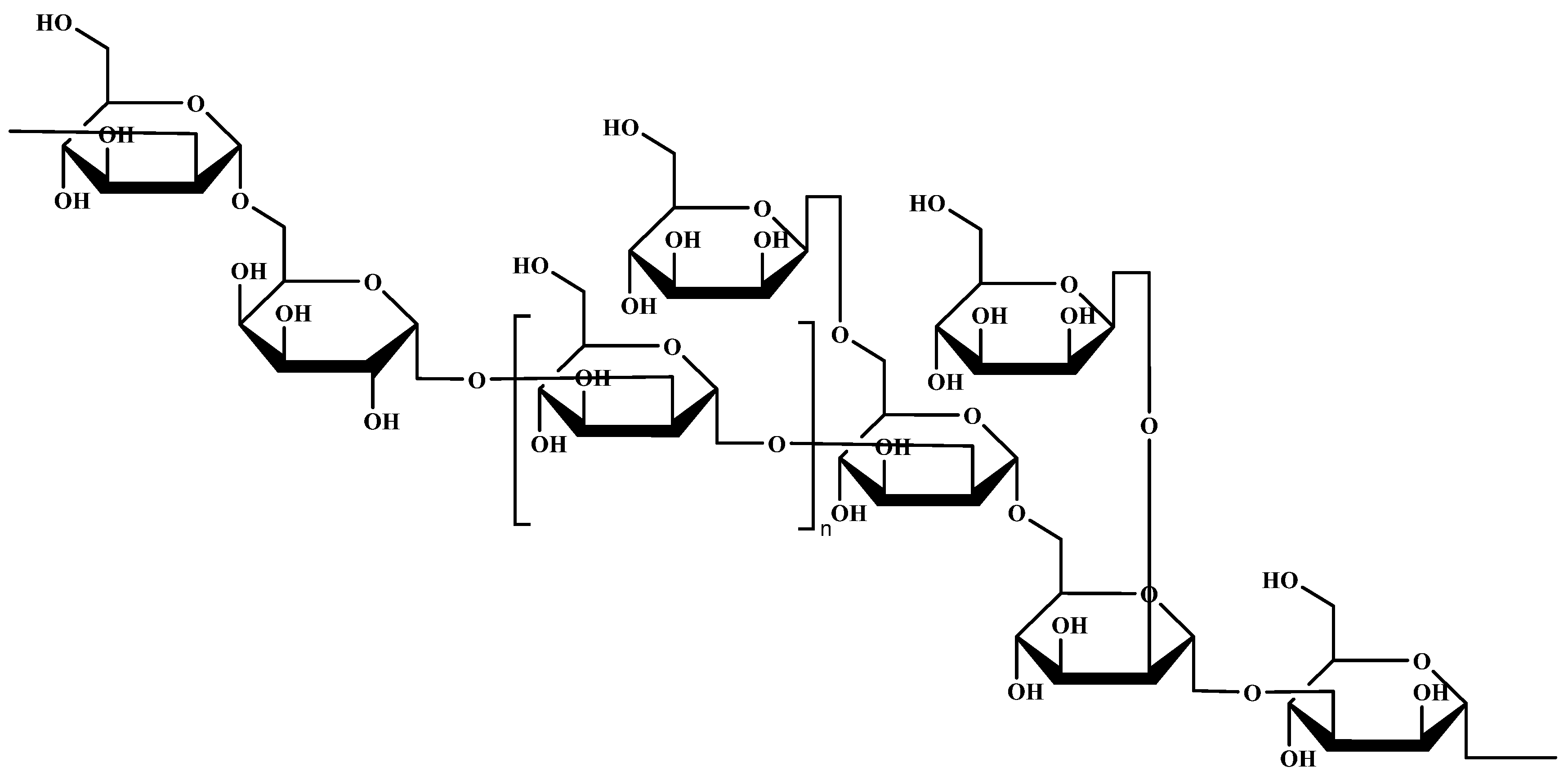

| Connection Mode | Derivative Name | Molecular Weight (MW) | Relative Molar Ratio (%) |
|---|---|---|---|
| t-Ara(f) | 1,4-di-O-acetyl-2,3,5-tri-O-methyl arabinitol | 279 | 1.970 |
| t-Fuc(p) | 1,5-di-O-acetyl-6-deoxy-2,3,4-tri-O-methyl fucitol | 293 | 0.61 |
| t-Man(p) | 1,5-di-O-acetyl-2,3,4,6-tetra-O-methyl mannitol | 323 | 35.70 |
| 3-Man(p) | 1,3,5-tri-O-acetyl-2,4,6-tri-O-methyl mannitol | 351 | 5.07 |
| 2-Man(p) | 1,2,5-tri-O-acetyl-3,4,6-tri-O-methyl mannitol | 351 | 24.41 |
| 6-Glc(p) | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl glucitol | 351 | 3.91 |
| 4-Glc(p) | 1,4,5-tri-O-acetyl-2,3,6-tri-O-methyl glucitol | 351 | 0.82 |
| 6-Gal(p) | 1,5,6-tri-O-acetyl-2,3,4-tri-O-methyl galactitol | 351 | 7.17 |
| 3,6-Man(p) | 1,3,5,6-tetra-O-acetyl-2,4-di-O-methyl mannitol | 379 | 0.82 |
| 2,6-Man(p) | 1,2,5,6-tetra-O-acetyl-3,4-di-O-methyl mannitol | 379 | 18.07 |
| 3,6-Gal(p) | 1,3,5,6-tetra-O-acetyl-2,4-di-O-methyl galactitol | 379 | 0.31 |
| 2,6-Gal(p) | 1,2,5,6-tetra-O-acetyl-3,4-di-O-methyl galactitol | 379 | 1.14 |
| Glycosyl Residues | Chemical Shifts (ppm) | |||||
|---|---|---|---|---|---|---|
| H1/C1 | H2/C2 | H3/C3 | H4/C4 | H5/C5 | H6/C6 | |
| A β-Manp(1→ | 4.97 | 4.14 | 3.96 | 3.68 | 3.58 | 3.82 |
| 102.21 | 69.66 | 78.67 | 73.40 | 66.91 | 61.14 | |
| B →2)-α-Manp(1→ | 5.22 | 4.04 | 3.67 | 3.69 | 3.55 | 3.68 |
| 100.57 | 78.51 | 66.69 | 73.42 | 67.02 | 61.15 | |
| C →2,6)-α-Manp(1→ | 5.06 | 3.95 | 3.74 | 3.70 | 3.75 | 3.82 |
| 102.34 | 78.76 | 66.71 | 73.36 | 73.42 | 70.47 | |
| D →6)-α-Galp(1→ | 5.02 | 3.95 | 3.81 | 3.71 | 3.48 | 3.99 |
| 98.26 | 65.93 | 78.87 | 66.35 | 78.87 | 70.18 | |
| E →3)-α-Manp(1→ | 4.91 | 3.80 | 3.87 | 3.58 | 3.47 | 3.84 |
| 97.89 | 67.22 | 77.97 | 73.36 | 78.76 | 61.27 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Cao, N.; Huang, L.; Tang, L.; Liu, X.; Zhang, W.; Huang, S.; Xie, X.; Yan, Y. Structural Characterization, and Antioxidant, Hypoglycemic and Immunomodulatory Activity of Exopolysaccharide from Sanghuangporus sanghuang JM-1. Molecules 2024, 29, 4564. https://doi.org/10.3390/molecules29194564
Luo Y, Cao N, Huang L, Tang L, Liu X, Zhang W, Huang S, Xie X, Yan Y. Structural Characterization, and Antioxidant, Hypoglycemic and Immunomodulatory Activity of Exopolysaccharide from Sanghuangporus sanghuang JM-1. Molecules. 2024; 29(19):4564. https://doi.org/10.3390/molecules29194564
Chicago/Turabian StyleLuo, Yanglan, Naixin Cao, Liling Huang, Lanlan Tang, Xuzhou Liu, Wenlong Zhang, Shilv Huang, Xiuchao Xie, and Yong Yan. 2024. "Structural Characterization, and Antioxidant, Hypoglycemic and Immunomodulatory Activity of Exopolysaccharide from Sanghuangporus sanghuang JM-1" Molecules 29, no. 19: 4564. https://doi.org/10.3390/molecules29194564
APA StyleLuo, Y., Cao, N., Huang, L., Tang, L., Liu, X., Zhang, W., Huang, S., Xie, X., & Yan, Y. (2024). Structural Characterization, and Antioxidant, Hypoglycemic and Immunomodulatory Activity of Exopolysaccharide from Sanghuangporus sanghuang JM-1. Molecules, 29(19), 4564. https://doi.org/10.3390/molecules29194564





