Vacancy Engineering of Selenium-Vacant NiCo2Se4 with Enhanced Electrochemical Performance for Supercapacitor
Abstract
:1. Introduction
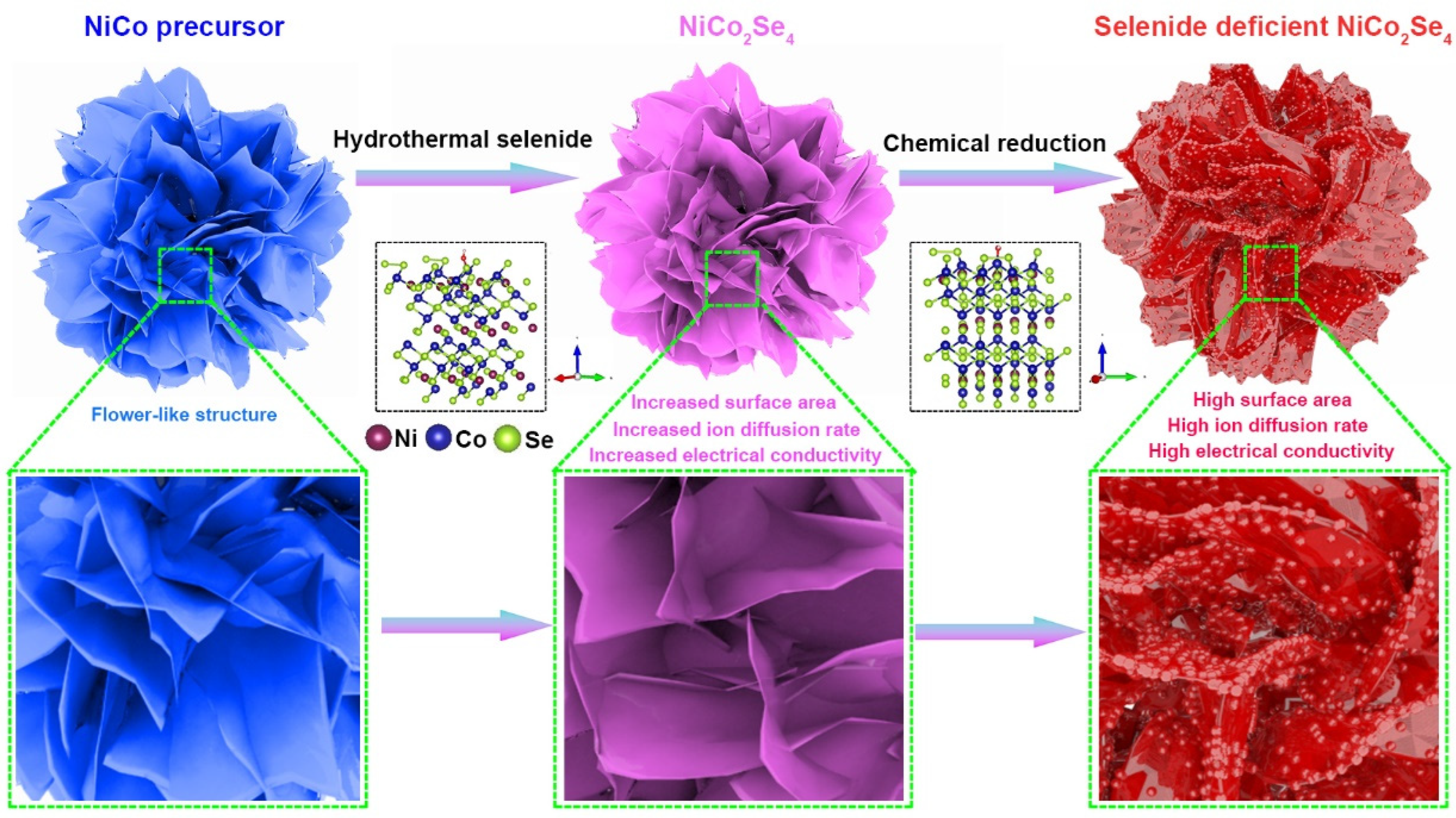
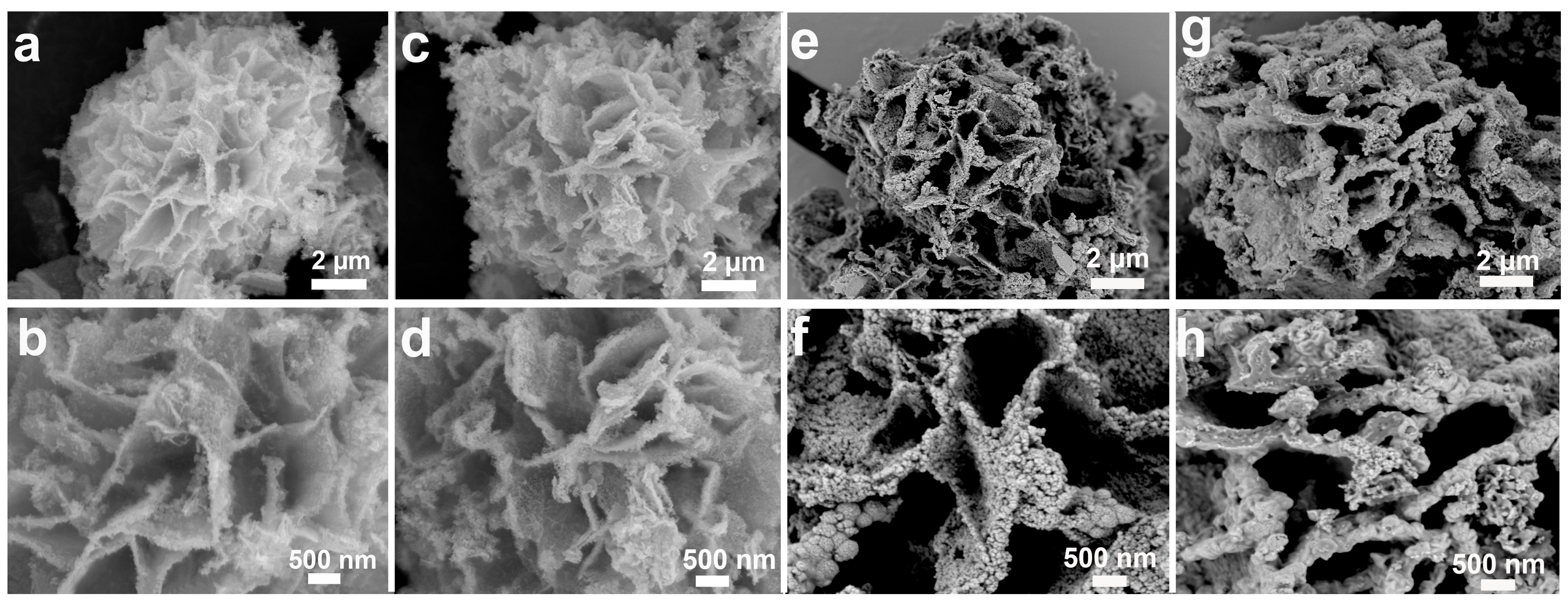
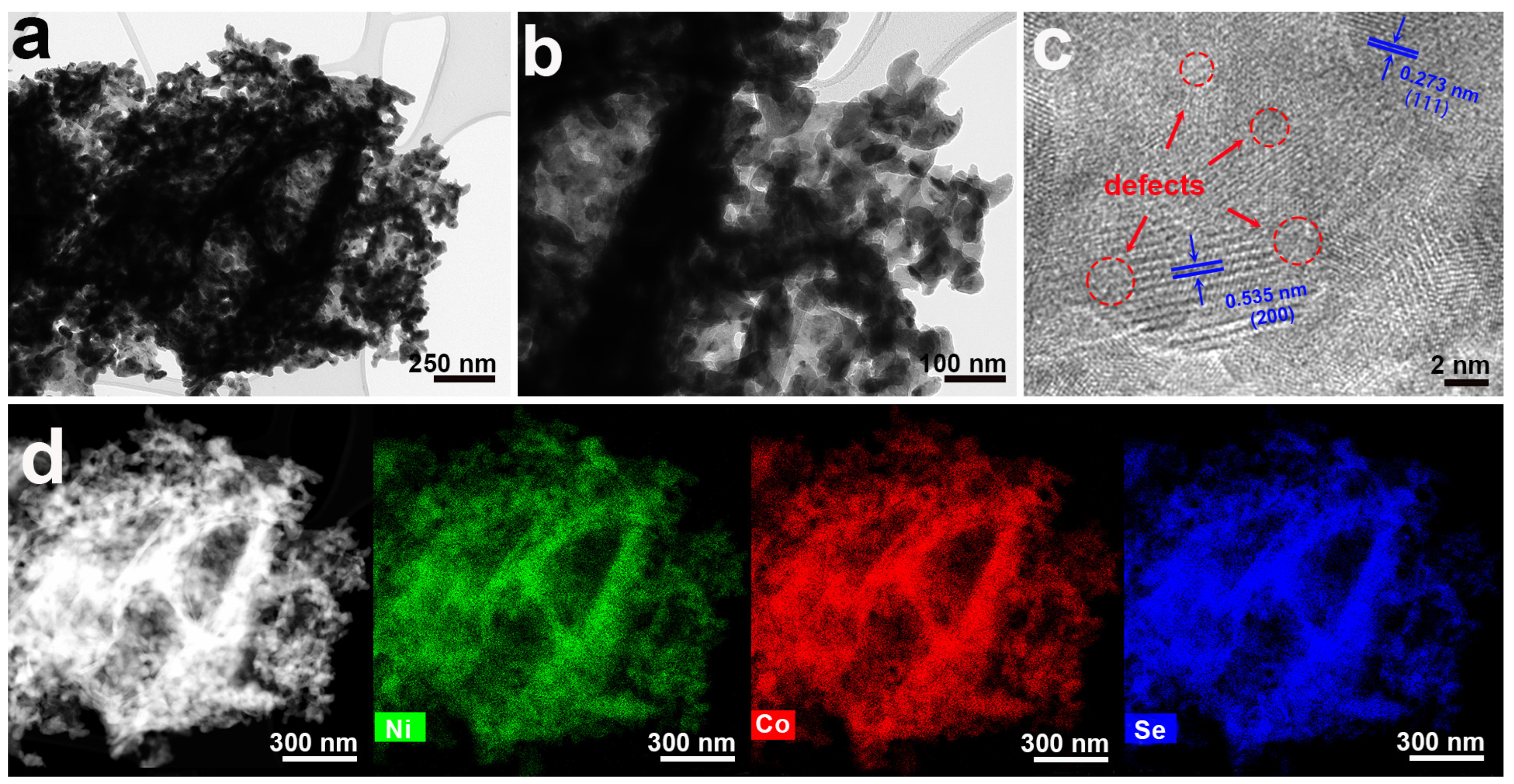
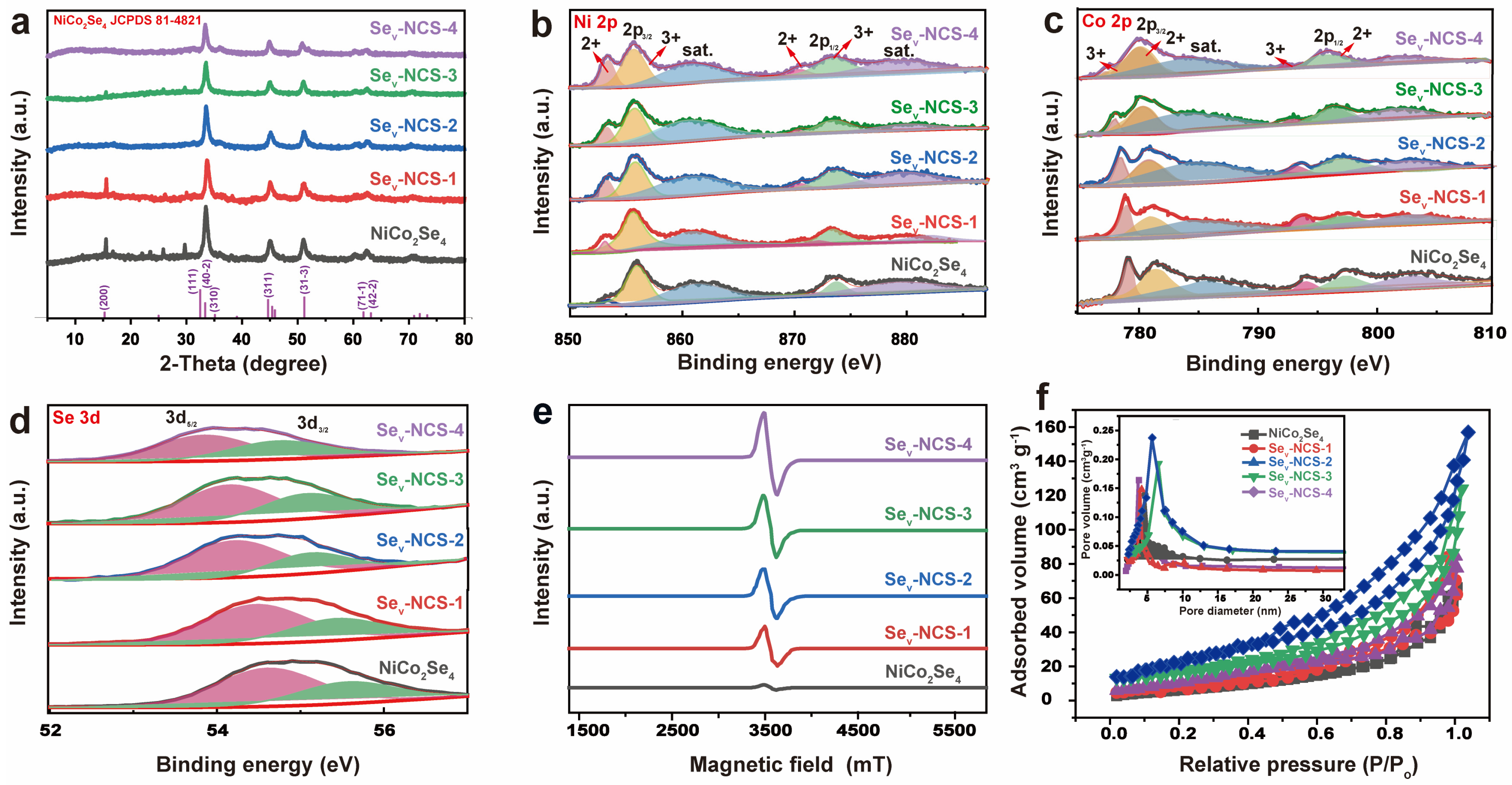
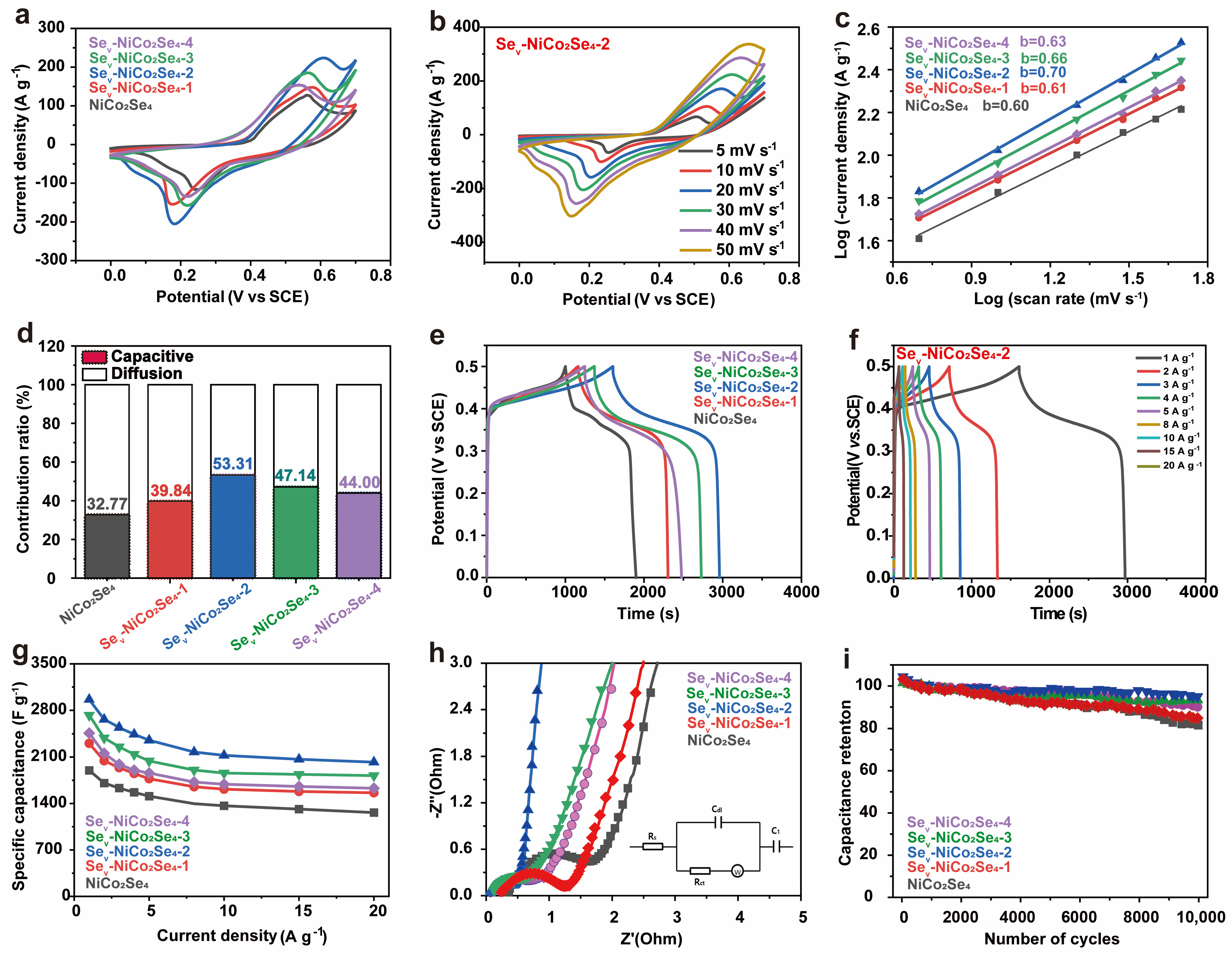
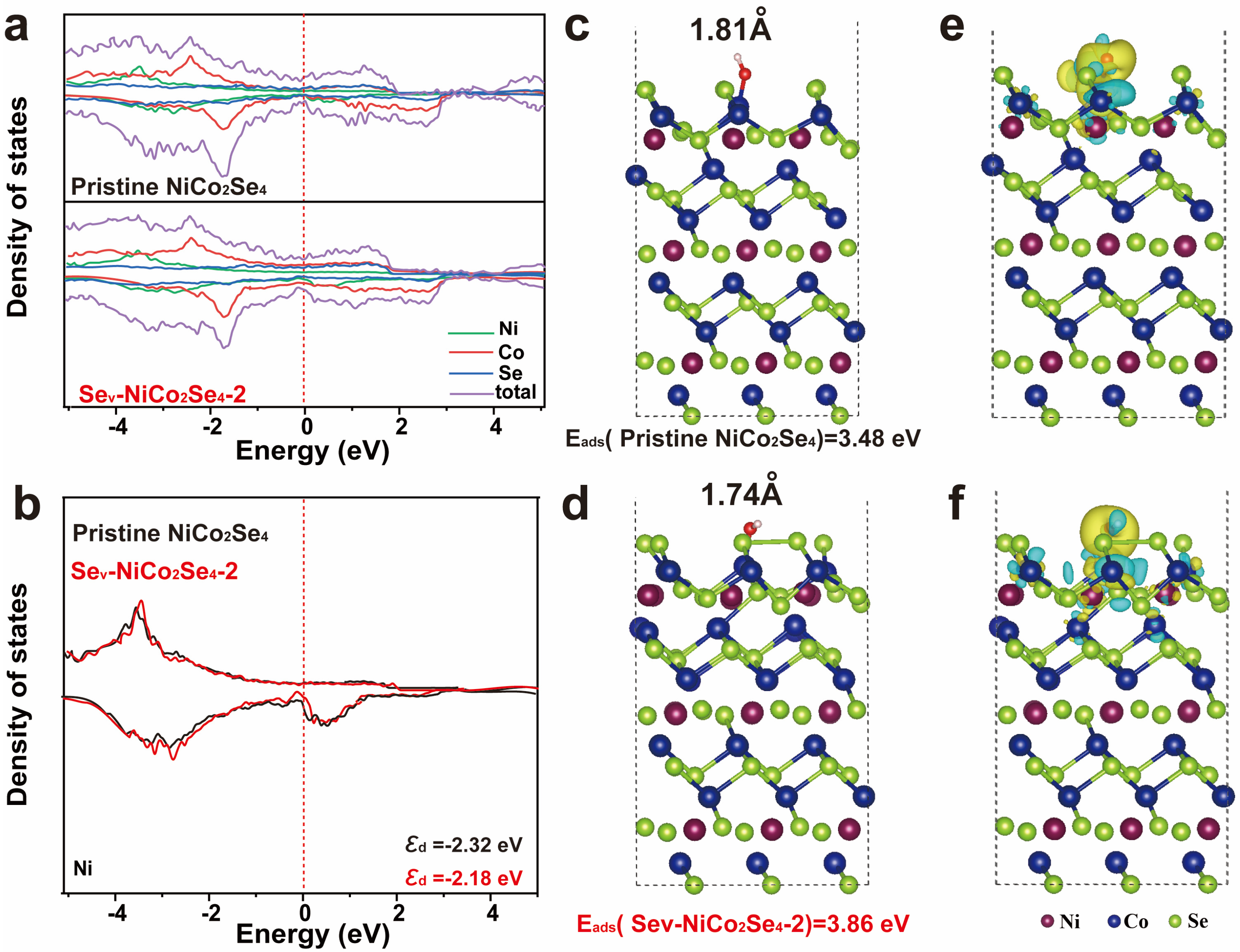
2. Results and Discussion
3. Experiment Details
3.1. Preparation of the NiCo2Se4
3.2. Preparation of the Defective Engineering of Sev-NCS-n (n = 1, 2, 3, and 4)
3.3. Characterization of Materials
3.4. Theoretical Computational Details
3.5. Electrochemical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lakshmi-Narayana, A.; Attarzadeh, N.; Shutthanandan, V.; Ramana, C.V. High-performance NiCo2O4/Graphene quantum dots for asymmetric and symmetric supercapacitors with enhanced energy efficiency. Adv. Funct. Mater. 2024, 2316379. [Google Scholar] [CrossRef]
- Lu, Z.; Kang, H.; Duan, Q.; Lv, C.; Liu, R.; Feng, F.; Zhao, H.J. The preparation of N, P-doped NiSe nanorod electrode materials on nickel foam using the microwave method for high-performance supercapacitors. Molecules 2024, 29, 3224. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Zhang, Y.; Ma, R.; Feng, X.; Yan, L.; Jia, D.; Xu, M.; Ai, L.; Guo, N.; Wang, L.J. Nitrogen-doped hierarchical porous carbon derived from coal for high-performance supercapacitor. Molecules 2023, 28, 3660. [Google Scholar] [CrossRef] [PubMed]
- Mo, T.; Peng, J.; Dai, W.; Chen, M.; Presser, V.; Feng, G. Horn-like pore entrance boosts charging dynamics and charge storage of nanoporous supercapacitors. ACS Nano 2023, 17, 14974–14980. [Google Scholar] [CrossRef] [PubMed]
- Al-Zohbi, F.; Ghamouss, F.; Jacquemin, J.; Schmaltz, B.; Tabcheh, M.F.; Abarbri, M.; Cherry, K.; Tran-Van, F.J. Non-substituted imidazolium-based electrolytes as potential alternatives to the conventional acidic electrolytes of polyaniline-based electrode materials for supercapacitors. Molecules 2024, 29, 2569. [Google Scholar] [CrossRef] [PubMed]
- Hong, R.; Zhao, X.; Lu, R.; You, M.; Chen, X.; Yang, X.J. Fabrication of polypyrrole hollow nanospheres by hard-template method for supercapacitor electrode material. Molecules 2024, 29, 2331. [Google Scholar] [CrossRef]
- Wang, T.; Pan, R.; Martins, M.L.; Cui, J.; Huang, Z.; Thapaliya, B.P.; Do-Thanh, C.-L.; Zhou, M.; Fan, J.; Yang, Z. Machine-learning-assisted material discovery of oxygen-rich highly porous carbon active materials for aqueous supercapacitors. Nat. Commun. 2023, 14, 4607. [Google Scholar] [CrossRef]
- Ahmad, S.A.; Shah, M.Z.; Arif, M.; Ullah, E.; Rahman, S.; Shah, M.S.; Eldin, S.M.; Song, P.; Sajjad, M.; Shah, A. High power aqueous hybrid asymmetric supercapacitor based on zero-dimensional ZnS nanoparticles with two-dimensional nanoflakes CuSe2 nanostructures. Ceram. Int. 2023, 49, 20007–20016. [Google Scholar] [CrossRef]
- Akbar, H.; Ali, A.; Mohammad, S.; Anjum, F.; Ahmad, A.; Afzal, A.M.; Albaqami, M.D.; Mohammad, S.; Choi, J.R. Exploring the potential of nitrogen-doped graphene in ZnSe-TiO2 composite materials for supercapacitor electrode. Molecules 2024, 29, 2103. [Google Scholar] [CrossRef]
- Park, T.H.; Kim, B.; Yu, S.; Park, Y.; Oh, J.W.; Kim, T.; Kim, N.; Kim, Y.; Zhao, D.; Khan, Z.U. Ionoelastomer electrolytes for stretchable ionic thermoelectric supercapacitors. Nano Energy 2023, 114, 108643. [Google Scholar] [CrossRef]
- Zhu, Y.; Deebansok, S.; Deng, J.; Wang, X.; Brousse, T.; Favier, F.; Fontaine, O. Electron delocalization and electrochemical potential distribution phenomena in faradaic electrode materials for understanding electrochemical behavior. Adv. Energy Mater. 2024, 2304317. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Y.; Liu, Z.; Feng, T.; Lin, H.; Li, G.; Wang, K.J. Uniform P-doped MnMoO4 nanosheets for enhanced asymmetric supercapacitors performance. Molecules 2024, 29, 1988. [Google Scholar] [CrossRef] [PubMed]
- Sukanya, R.; Karthik, R.; Hasan, M.; Breslin, C.; Shim, J.-J. Insight into the synergistic effect of 2D/2D layered metal selenides wrapped nickel boride nanoparticles based ternary heterostructure for constructing asymmetric supercapacitors with excellent energy density. Chem. Eng. J. 2023, 473, 145487. [Google Scholar] [CrossRef]
- Tang, G.; Liang, J.; Wu, W. Transition metal selenides for supercapacitors. Adv. Funct. Mater. 2024, 34, 2310399. [Google Scholar] [CrossRef]
- Ahmed, S.; Gondal, M.; Alzahrani, A.; Almessiere, M. Critical review on transition metal selenides/graphene composite as futuristic electrode material for high performance supercapacitors. J. Energy Storage 2023, 74, 109214. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, T.; Zi, B.; Zhao, J.; Li, D.; Chen, M.; Sun, H.; Zhang, J.; Zhang, Y.; Liu, Q. Dual supports by cation vacancies and surface optimization for CoNiSe2-based hybrid supercapacitors with high energy density. ACS Energy Lett. 2023, 8, 3420–3429. [Google Scholar] [CrossRef]
- Panah, T.S.; Shirvani, M.; Davarani, S.S. Bimetallic NiCo2Se4/Fe2CoSe4 chrysanthemum flowers assembled by nanosheets: An efficient electrode material for hybrid supercapacitor applications. J. Alloys Compd. 2024, 978, 173496. [Google Scholar] [CrossRef]
- Duan, L.; Fu, H.; Guo, H.; Sun, H.; Zhang, Q.; Xu, J.; Liu, J. Ni2CoS4@(NiCo)Se2 bimetallic selenides with core–shell heterostructure in-situ generated on Ni2CoS4 nanorods arrays for high performance supercapacitors. Appl. Surf. Sci. 2024, 648, 158966. [Google Scholar] [CrossRef]
- Zeshan, M.; Alharbi, F.; Alahmari, S.D.; Abdullah, M.; Al-Sehemi, A.G.; Henaish, A.; Ahmad, Z.; Waheed, M.S.; Aman, S.; Farid, H.M. Fabrication of niobium selenide-based rGO hybrid nanoparticles by hydrothermal method for supercapacitor applications. Ceram. Int. 2024, 50, 7110–7120. [Google Scholar] [CrossRef]
- Ali, M.; Alahmari, S.D.; Abdelmohsen, S.A.; Alanazi, M.M.; Al-Sehemi, A.G.; Abdullah, M.; Aman, S.; Farid, H.M. Fabrication of MnSe/WSe2 nanohybrid electrode prepared through hydrothermal method for supercapacitor applications. Ceram. Int. 2024, 50, 6931–6940. [Google Scholar]
- Zhang, Z.; Sun, S.; Xu, Z.; Yin, S. Multicomponent hybridization transition metal oxide electrode enriched with oxygen vacancy for ultralong-life supercapacitor. Small 2023, 19, 2302479. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, C.; Wang, J.; Zhang, Y.; Zhang, K.; Gates, I.D.; Li, H.; Huang, Z.-H.; Ma, T. Deep-implanting oxygen vacancy into VOx by alkylamine intercalation for life-oriented modular pouch supercapacitors. Chem. Eng. J. 2023, 453, 139948. [Google Scholar] [CrossRef]
- Dai, J.; Qi, X.; Xia, L.; Xue, Q.; Luo, L.; Wang, X.; Yang, C.; Li, D.; Xie, H.; Cabot, A. Aqueous ammonium-ion supercapacitors with unprecedented energy density and stability enabled by oxygen vacancy-enriched MoO3@C. Adv. Funct. Mater. 2023, 33, 2212440. [Google Scholar] [CrossRef]
- He, Y.; Liu, T.; Song, J.; Wang, Y.; Zhang, Y.; Feng, J.; Meng, A.; Li, G.; Wang, L.; Zhao, J. Lithiation-induced controllable vacancy engineering for developing highly active Ni3Se2 as a high-rate and large-capacity battery-type cathode in hybrid supercapacitors. J. Energy Chem. 2023, 78, 37–46. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Zhang, J.; Jun, S.C.; Yamauchi, Y. Sodium preintercalation-induced oxygen-deficient hydrated potassium manganese oxide for high-energy flexible Mg-ion supercapacitors. NPG Asia Mater. 2023, 15, 9. [Google Scholar] [CrossRef]
- Yan, C.; Han, E.; Yang, X.; Hu, K.; Xu, H.; Li, Y.; He, Y.; Lu, S. Engineering sulfur vacancies on Mo-doped nickel sulfide for enhanced electrochemical energy storage. Ceram. Int. 2023, 49, 14155–14165. [Google Scholar] [CrossRef]
- Cao, Y.; Ruan, P.; Xue, Y.; Cao, Y.; He, H.; Qiu, W. Performance enhancement of Hf-Ta-O nanofiber based energy storage materials using oxygen-vacancy and its application for supercapacitor. J. Alloys Compd. 2023, 958, 170542. [Google Scholar] [CrossRef]
- Jing, F.; Ma, Z.; Wang, J.; Fan, Y.; Qin, X.; Shao, G. Oxygen vacancy inducing phase transition during charge storage in MnOx@rGO supercapacitor electrode. Chem. Eng. J. 2022, 435, 135103. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.; Zhang, J.; Li, H.; Fang, K.; Wang, J.; Wang, Z.; Xu, M.; Zhao, B. Doping effect and oxygen vacancy engineering in nickel-manganese layered double hydroxides for high-performance supercapacitors. Nano Energy 2024, 126, 109690. [Google Scholar] [CrossRef]
- Liu, L.; Lv, W.; Wang, H. Synergetic surface coating and S-rich vacancy reconstruction NiCo2S4 electrode materials for high cycle stability asymmetric supercapacitor applications. J. Energy Storage 2023, 73, 109062. [Google Scholar] [CrossRef]
- Guo, X.; Lin, S.; Wang, Y.; Cai, W. Novel core–shell structure composite NiCo2O4–Vo@ZIF with amorphous ZIF shell and oxygen-vacancy-rich core for asymmetric supercapacitors. Electrochim. Acta 2024, 475, 143636. [Google Scholar] [CrossRef]
- Liu, S.; Kang, L.; Hu, J.; Jung, E.; Henzie, J.; Alowasheeir, A.; Zhang, J.; Miao, L.; Yamauchi, Y.; Jun, S.C. Realizing superior redox kinetics of hollow bimetallic sulfide nanoarchitectures by defect-induced manipulation toward flexible solid-state supercapacitors. Small 2022, 18, 2104507. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, H.; Liu, F.; Chu, X.; Yan, C.; Gu, B.; Huang, H.; Yang, T.; Chen, N.; Han, Y. Establishing highly-efficient surface faradaic reaction in flower-like NiCo2O4 nano-/micro-structures for next-generation supercapacitors. Electrochim. Acta 2019, 307, 302–309. [Google Scholar] [CrossRef]
- Chen, C.; Liu, M.; Liu, Z.; Xie, M.; Wan, L.; Chen, J.; Zhang, Y.; Du, C.; Li, D. Design of mesoporous Ni-Co hydroxides nanosheets stabilized by BO2-for pseudocapacitors with superior performance. J. Colloid Interface Sci. 2022, 614, 66–74. [Google Scholar] [CrossRef]
- Wen, J.; Li, S.; Chen, T.; Yue, Y.; Liu, N.; Gao, Y.; Li, B.; Song, Z.; Xiong, L.; Chen, Z. Three-dimensional hierarchical NiCo hydroxide@Ni3S2 nanorod hybrid structure as high performance positive material for asymmetric supercapacitor. Electrochim. Acta 2016, 222, 965–975. [Google Scholar] [CrossRef]
- Liu, S.; Yin, Y.; Ni, D.; San Hui, K.; Ma, M.; Park, S.; Hui, K.N.; Ouyang, C.-Y.; Jun, S.C. New insight into the effect of fluorine doping and oxygen vacancies on electrochemical performance of Co2MnO4 for flexible quasi-solid-state asymmetric supercapacitors. Energy Storage Mater. 2019, 22, 384–396. [Google Scholar] [CrossRef]
- Cai, Z.; Bi, Y.; Hu, E.; Liu, W.; Dwarica, N.; Tian, Y.; Li, X.; Kuang, Y.; Li, Y.; Yang, X.Q. Single-crystalline ultrathin Co3O4 nanosheets with massive vacancy defects for enhanced electrocatalysis. Adv. Energy Mater. 2018, 8, 1701694. [Google Scholar] [CrossRef]
- Zhang, C.; Biendicho, J.J.; Zhang, T.; Du, R.; Li, J.; Yang, X.; Arbiol, J.; Zhou, Y.; Morante, J.R.; Cabot, A. Combined high catalytic activity and efficient polar tubular nanostructure in urchin-like metallic NiCo2Se4 for high-performance lithium–sulfur batteries. Adv. Funct. Mater. 2019, 29, 1903842. [Google Scholar] [CrossRef]
- Wei, K.; Qiu, J.; Zhao, Y.; Ma, S.; Wei, Y.; Li, H.; Zeng, C.; Cui, Y. Tunable oxygen vacancies in MoO3 lattice with improved electrochemical performance for Li-ion battery thin film cathode. Ceram. Int. 2023, 49, 21729–21736. [Google Scholar] [CrossRef]
- Li, L.; Guo, Y.; Li, L.; Lai, C.; Tang, Z.; Lou, X.; Ju, L.; Fu, J. Self-assembled microflower-like NiCo2X4 (X= O, S, Se) as electrodes for asymmetric supercapacitors. J. Alloys Compd. 2024, 973, 172913. [Google Scholar] [CrossRef]
- Gao, M.; Huang, J.; Liu, Y.; Li, X.; Wei, P.; Yang, J.; Shen, S.; Cai, K. Electrochemically finely regulated NiCo-LDH/NiCoOOH nanostructured films for supercapacitors with record high mass loading, areal capacity, and energy density. Adv. Funct. Mater. 2023, 33, 2305175. [Google Scholar] [CrossRef]
- Huang, Q.; Su, W.; Zhong, G.; Xu, K.; Yang, C. Bimetal heterostructure NiCo2Se4 anode confined by carbon nano boxes for ultrafast and stable potassium storage. Chem. Eng. J. 2023, 460, 141875. [Google Scholar] [CrossRef]
- Pan, Z.; Zhan, Y.; Yaseen, M.; Shen, P.K. Self-supported Fe, Mn-co-doped NiCo2Se4 nanorods on nickel foam for enhanced electrocatalytic performance in wide pH range. Int. J. Hydrogen Energy 2024, 63, 265–273. [Google Scholar] [CrossRef]
- Kang, L.; Zhang, M.; Zhang, J.; Liu, S.; Zhang, N.; Yao, W.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C. Dual-defect surface engineering of bimetallic sulfide nanotubes towards flexible asymmetric solid-state supercapacitors. J. Mater. Chem. A 2020, 8, 24053–24064. [Google Scholar] [CrossRef]
- Rajesh, J.A.; Lee, Y.-H.; Yun, Y.-H.; Quy, V.H.V.; Kang, S.-H.; Kim, H.; Ahn, K.-S. Bifunctional NiCo2Se4 and CoNi2Se4 nanostructures: Efficient electrodes for battery-type supercapacitors and electrocatalysts for the oxygen evolution reaction. J. Ind. Eng. Chem. 2019, 79, 370–382. [Google Scholar] [CrossRef]
- Rathore, D.; Ghosh, S.; Chowdhury, J.; Pande, S. Fe-doped NiCo2Se4 nanorod arrays as electrocatalysts for overall electrochemical water splitting. ACS Appl. Nano Mater. 2023, 6, 3095–3110. [Google Scholar] [CrossRef]
- Kang, L.; Huang, C.; Zhang, J.; Zhang, M.; Zhang, N.; Liu, S.; Ye, Y.; Luo, C.; Gong, Z.; Wang, C. Effect of fluorine doping and sulfur vacancies of CuCo2S4 on its electrochemical performance in supercapacitors. Chem. Eng. J. 2020, 390, 124643. [Google Scholar] [CrossRef]
- Li, S.; Ruan, Y.; Xie, Q. Morphological modulation of NiCo2Se4 nanotubes through hydrothermal selenization for asymmetric supercapacitor. Electrochim. Acta 2020, 356, 136837. [Google Scholar]
- El Jaouhari, A.; Slassi, A.; Zhang, B.; Pershin, A.; Liu, W.; Cornil, D.; Liu, X.; Zhu, J. The role of selenium vacancies in the enhancement of electrocatalytic activity of CoNiSe2 for the oxygen evolution reaction. J. Power Sources 2021, 514, 230596. [Google Scholar] [CrossRef]
- Liang, J.; Li, S.; Li, F.; Zhang, L.; Jiang, Y.; Ma, H.; Cheng, K.; Qing, L. Defect engineering induces Mo-regulated Co9Se8/FeNiSe heterostructures with selenium vacancy for enhanced electrocatalytic overall water splitting in alkaline. J. Colloid Interface Sci. 2024, 655, 296–306. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Youk, J.H. Nitrogen-doped mesoporous carbon spheres decorated with NiCo alloy nanoparticles for high-performance electrochemical supercapacitors. J. Electroanal. Chem. 2024, 960, 118183. [Google Scholar] [CrossRef]
- Ikram, M.; Irshad, A.; Katubi, K.M.; Alrowaili, Z.; Al-Buriahi, M.; Warsi, M.F. Enhanced electrochemical activity of chemically engineered rGO-decorated NiO/CoFe2O4 for supercapacitor applications. Ceram. Int. 2024, 50, 19578–19591. [Google Scholar] [CrossRef]
- Fu, J.; Li, L.; Yun, J.M.; Lee, D.; Ryu, B.K.; Kim, K.H. Two-dimensional titanium carbide (MXene)-wrapped sisal-Like NiCo2S4 as positive electrode for High-performance hybrid pouch-type asymmetric supercapacitor. Chem. Eng. J. 2019, 375, 121939. [Google Scholar] [CrossRef]
- Manh, T.P.; Van, N.N.; Phung, V.B.T.; Thi, L.N.; Quy, Q.N.; Le The, S.; Tien, P.D.; Quang, D.T.; Van, T.N.; Van, N.T. One-step preparation of Ni–Co binary metal sulfides on reduced graphene oxide for all-solid-state supercapacitor devices with enhanced electrochemical performance. Ceram. Int. 2024, 50, 22757–22770. [Google Scholar] [CrossRef]
- Kumar, K.S.; Goud, J.P.; Roy, N.; Su, K.J.; Joo, S.W. In-situ synthesis of SnO2/CoFe2O4/Fe3O4 nanograss array composite: A redox-active electrode material for battery-type supercapacitors. Ceram. Int. 2024, 50, 20535–20546. [Google Scholar] [CrossRef]
- Li, Z.; Yu, J.; Hu, M.; Cai, X.; Chen, Y.; Cai, Y.; Wei, W. Construction of Zn–Co–Ni–Se nanosheet arrays on nickel foam for hybrid supercapacitors. Ceram. Int. 2021, 47, 29730–29738. [Google Scholar] [CrossRef]
- Yang, W.-D.; Xiang, J.; Zhao, R.-D.; Loy, S.; Li, M.-T.; Ma, D.-M.; Li, J.; Wu, F.-F. Nanoengineering of ZnCo2O4@CoMoO4 heterogeneous structures for supercapacitor and water splitting applications. Ceram. Int. 2023, 49, 4422–4434. [Google Scholar] [CrossRef]
- Liu, Z.; Tian, Y.; Yao, S.; Li, Y.; Fu, Y.; Zhou, Q. Interfacial interaction-induced shift of d-band center promotes photocatalytic antibiotics mineralization. Appl. Cataly. B Environ. Energy 2024, 352, 123998. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Y.; Yan, M.; Yang, Q.; Liu, X.; Shi, W. Lewis acid etched NixCo1-xSe2 derived from ZIF-L on CoO nanowires for hybrid-supercapacitors. Chem. Eng. J. 2022, 431, 133472. [Google Scholar] [CrossRef]
- Lu, Z.; Hu, Z.; Xiao, L.; Xie, Y.; Li, N.; Xi, L.; Chen, W.; Xiao, J.; Zhu, Y. Battery-type Ni-Co-Se hollow microspheres cathode materials enabled by bifunctional N-doped carbon quantum dots with ultrafast electrochemical kinetics for hybrid supercapacitors. Chem. Eng. J. 2022, 450, 138347. [Google Scholar] [CrossRef]
- Yin, X.; Han, L.; Fu, Y.; Lu, J.; Song, Q.; Li, H. (Ni, Co)Se2 nanoparticles on vertical graphene nanosheets@carbon microtubes for high-performance solid-state asymmetric supercapacitors. J. Energy Storage 2022, 53, 105205. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Y.; Deng, C.; Sun, L.; Shi, W. In-situ construction of heterostructure (Ni, Co) Se2 nanoarrays derived from cone-like ZIF-L for high-performance hybrid supercapacitors. J. Colloid Interface Sci. 2022, 608, 3049–3058. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Bai, X. Preparation of Ni-Co PBA-derived beaded NiSe2/CoSe2/CNT for high-performance supercapacitors. J. Alloys Compd. 2023, 944, 169110. [Google Scholar] [CrossRef]
- Jiang, B.; Liu, Y.; Zhang, J.; Wang, Y.; Zhang, X.; Zhang, R.; Huang, L.-L.; Zhang, D. Synthesis of bimetallic nickel cobalt selenide particles for high-performance hybrid supercapacitors. RSC advances 2022, 12, 1471–1478. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Physical review letters 1996, 77, 3865. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. Special points for Brillouin-zone integrations. Phys. Rev. B 1977, 16, 1748. [Google Scholar] [CrossRef]
- Guo, Z.; Diao, Y.; Han, X.; Liu, Z.; Ni, Y.; Zhang, L.J.C. Mesoporous NiCo2Se4 tube as an efficient electrode material with enhanced performance for asymmetric supercapacitor applications. CrystEngComm 2021, 23, 2099–2112. [Google Scholar] [CrossRef]
- Qu, G.; Zhang, X.; Xiang, G.; Wei, Y.; Yin, J.; Wang, Z.; Zhang, X.; Xu, X.J. ZIF-67 derived hollow Ni-Co-Se nano-polyhedrons for flexible hybrid supercapacitors with remarkable electrochemical performances. Chin. Chem. Lett. 2020, 31, 2007–2012. [Google Scholar] [CrossRef]
- Zhu, Y.; Huang, Z.; Hu, Z.; Xi, L.; Ji, X.; Liu, Y.J. 3D interconnected ultrathin cobalt selenide nanosheets as cathode materials for hybrid supercapacitors. Electrochim. Acta 2018, 269, 30–37. [Google Scholar] [CrossRef]
- Guo, K.; Cui, S.; Hou, H.; Chen, W.; Mi, L.J. Hierarchical ternary Ni–Co–Se nanowires for high-performance supercapacitor device design. Dalton Tran. 2016, 45, 19458–19465. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Samanta, P.; Samanta, P.; Murmu, N.C.; Kuila, T.J. Investigation of electrochemical charge storage efficiency of NiCo2Se4/RGO composites derived at varied duration and its asymmetric supercapacitor device. Energy Fuels 2020, 34, 13056–13066. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, J.; Li, L.; Xue, Q.; Li, L.; Guo, Z.; Meng, L.; Lai, C.; Guo, Y. Vacancy Engineering of Selenium-Vacant NiCo2Se4 with Enhanced Electrochemical Performance for Supercapacitor. Molecules 2024, 29, 4580. https://doi.org/10.3390/molecules29194580
Fu J, Li L, Xue Q, Li L, Guo Z, Meng L, Lai C, Guo Y. Vacancy Engineering of Selenium-Vacant NiCo2Se4 with Enhanced Electrochemical Performance for Supercapacitor. Molecules. 2024; 29(19):4580. https://doi.org/10.3390/molecules29194580
Chicago/Turabian StyleFu, Jianjian, Lei Li, Qian Xue, Lindong Li, Zhiying Guo, Lanxiang Meng, Changwei Lai, and Yao Guo. 2024. "Vacancy Engineering of Selenium-Vacant NiCo2Se4 with Enhanced Electrochemical Performance for Supercapacitor" Molecules 29, no. 19: 4580. https://doi.org/10.3390/molecules29194580





