The Synthesis of Green Palladium Catalysts Stabilized by Chitosan for Hydrogenation
Abstract
:1. Introduction
2. Results
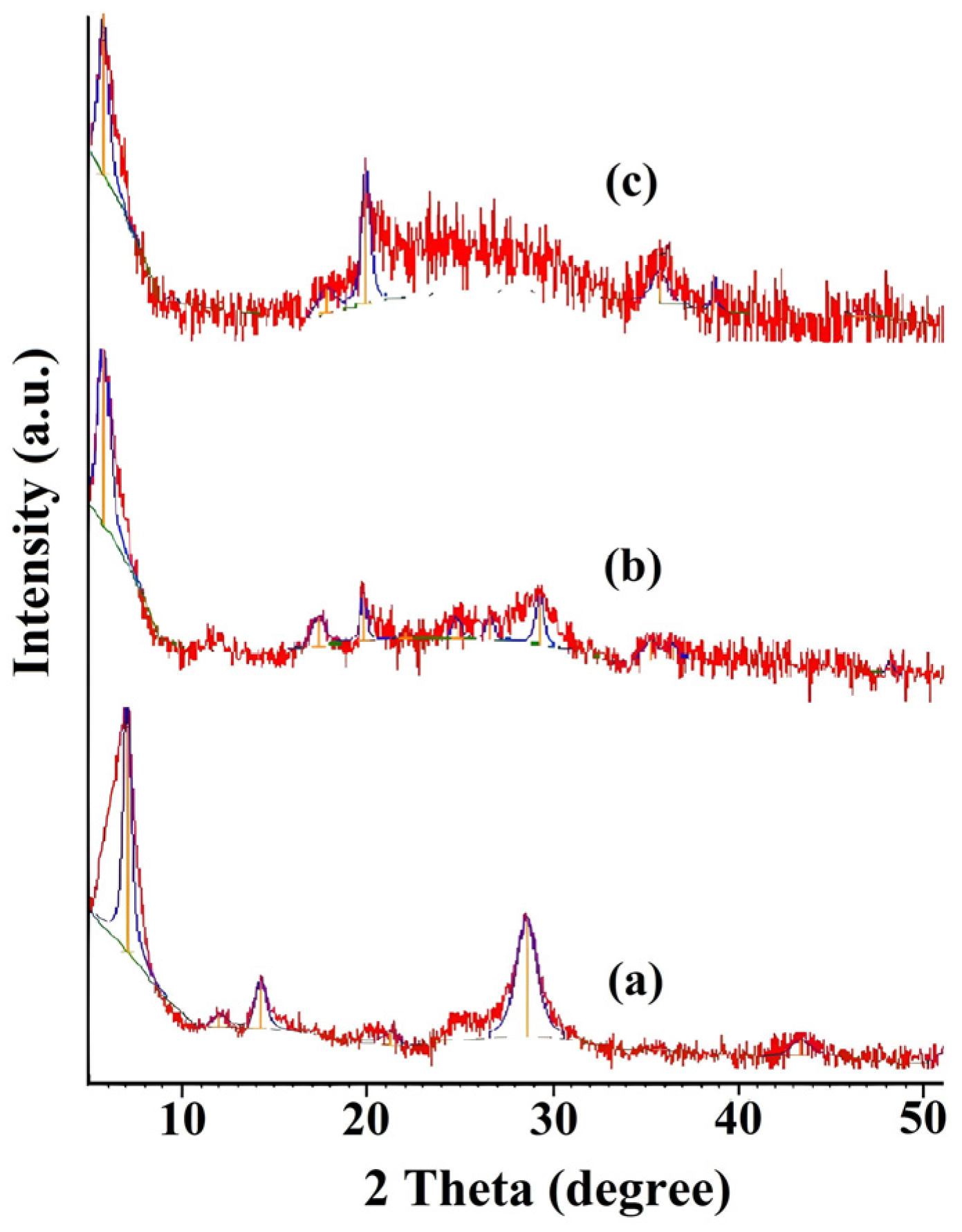
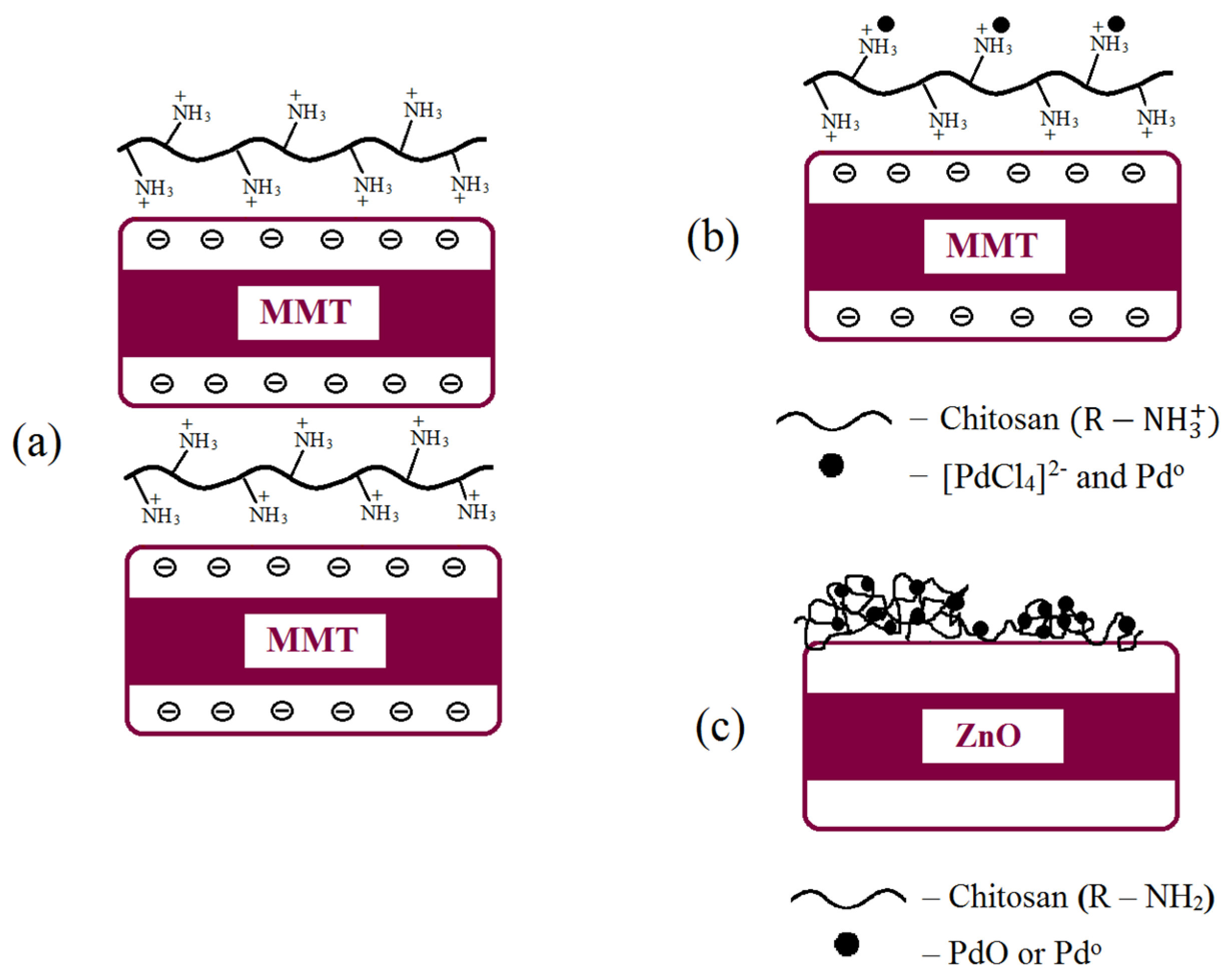
2.1. Modification of Inorganic Materials with Chitosan
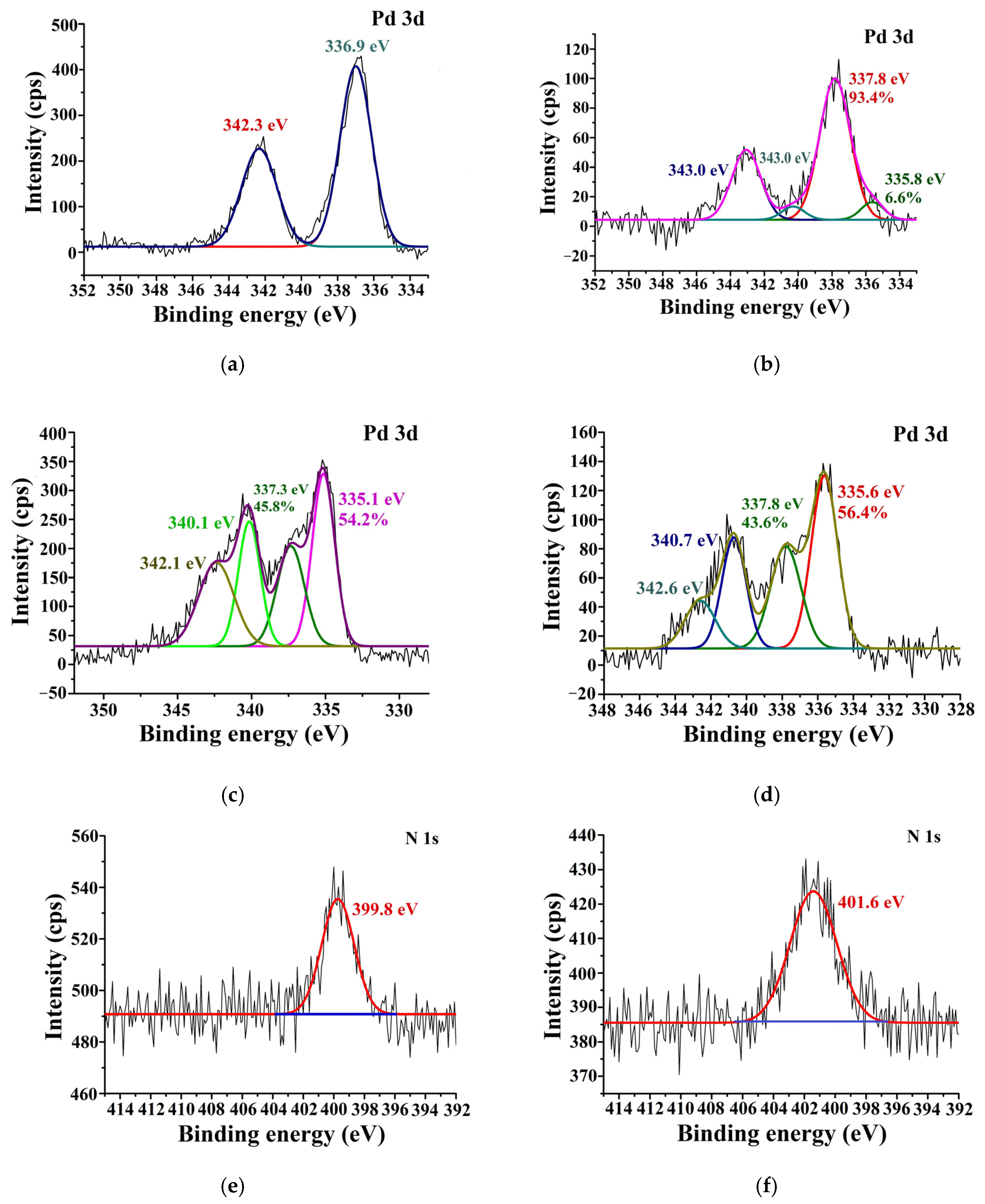
2.2. Synthesis of Chitosan–Metal Complexes Supported on Zinc Oxide and MMT
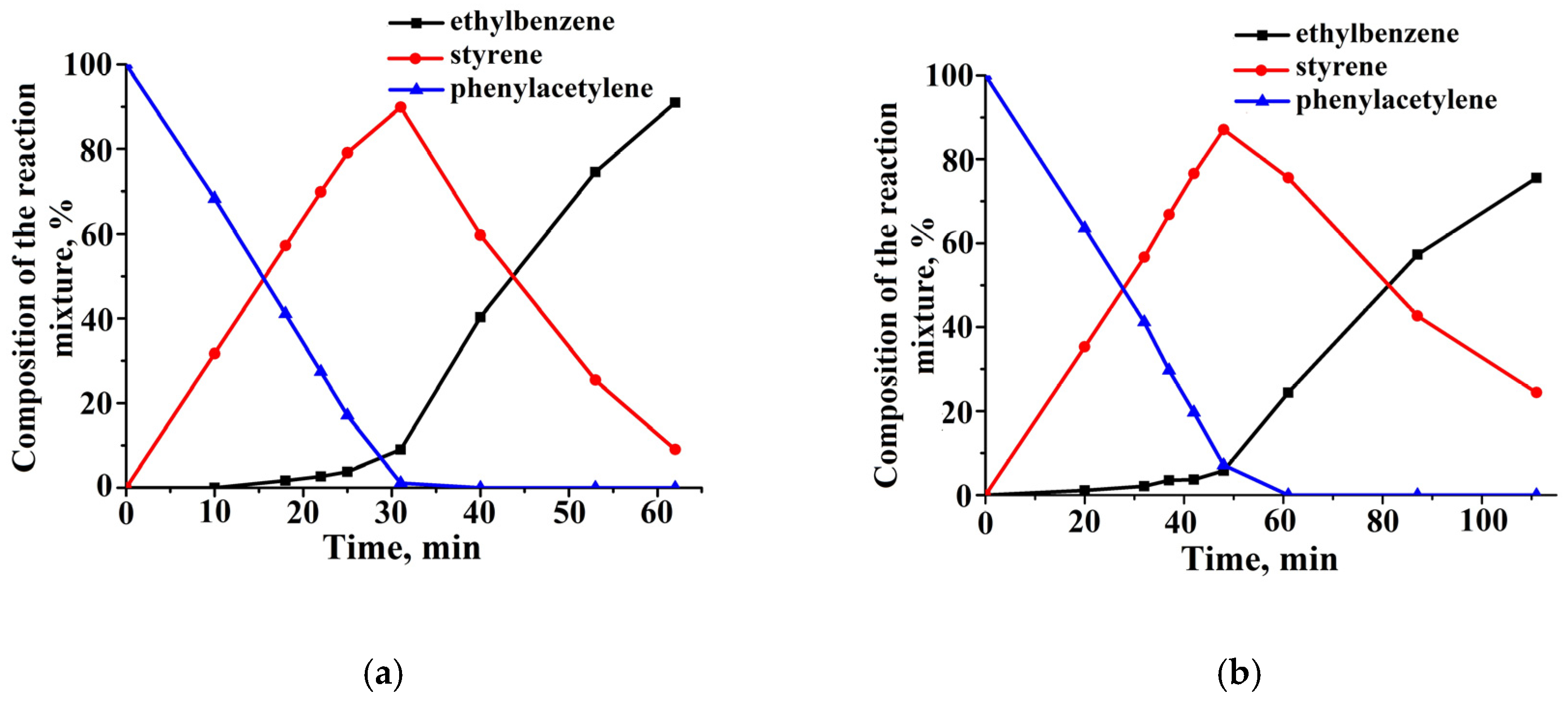
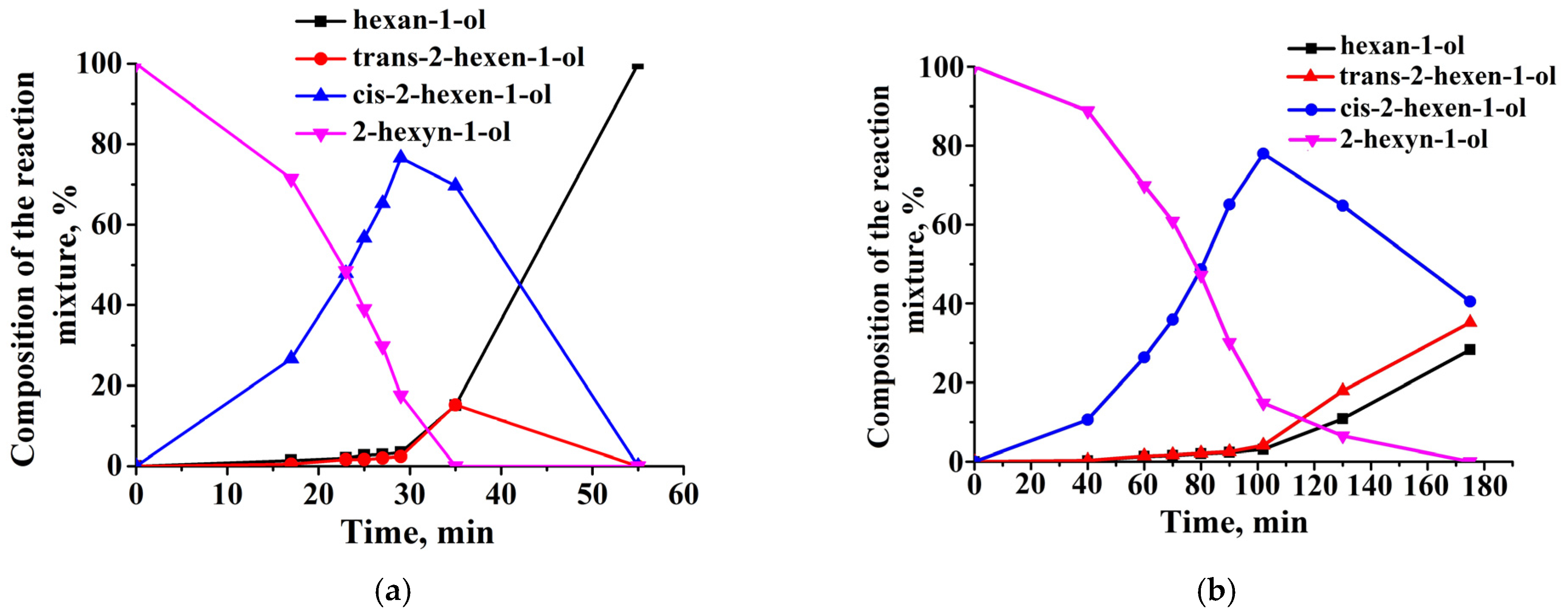
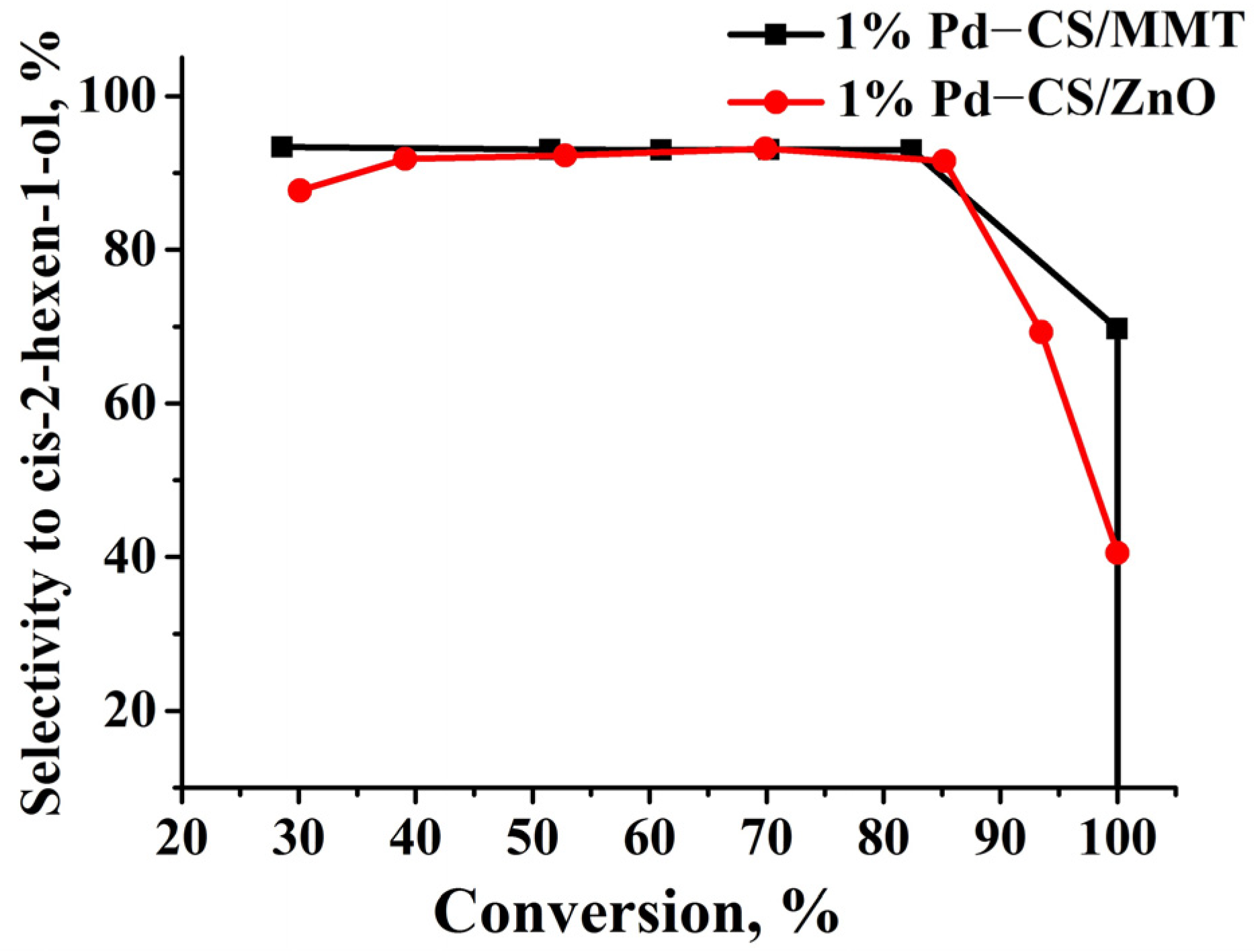
2.3. Catalytic Properties of Composites
3. Materials and Methods
3.1. Materials
3.2. Preparation of Composites
3.2.1. Preparation of CS/MMT(ZnO) Composites
3.2.2. Preparation of Composites Based on CS–Palladium Complexes Fixed on MMT (ZnO)
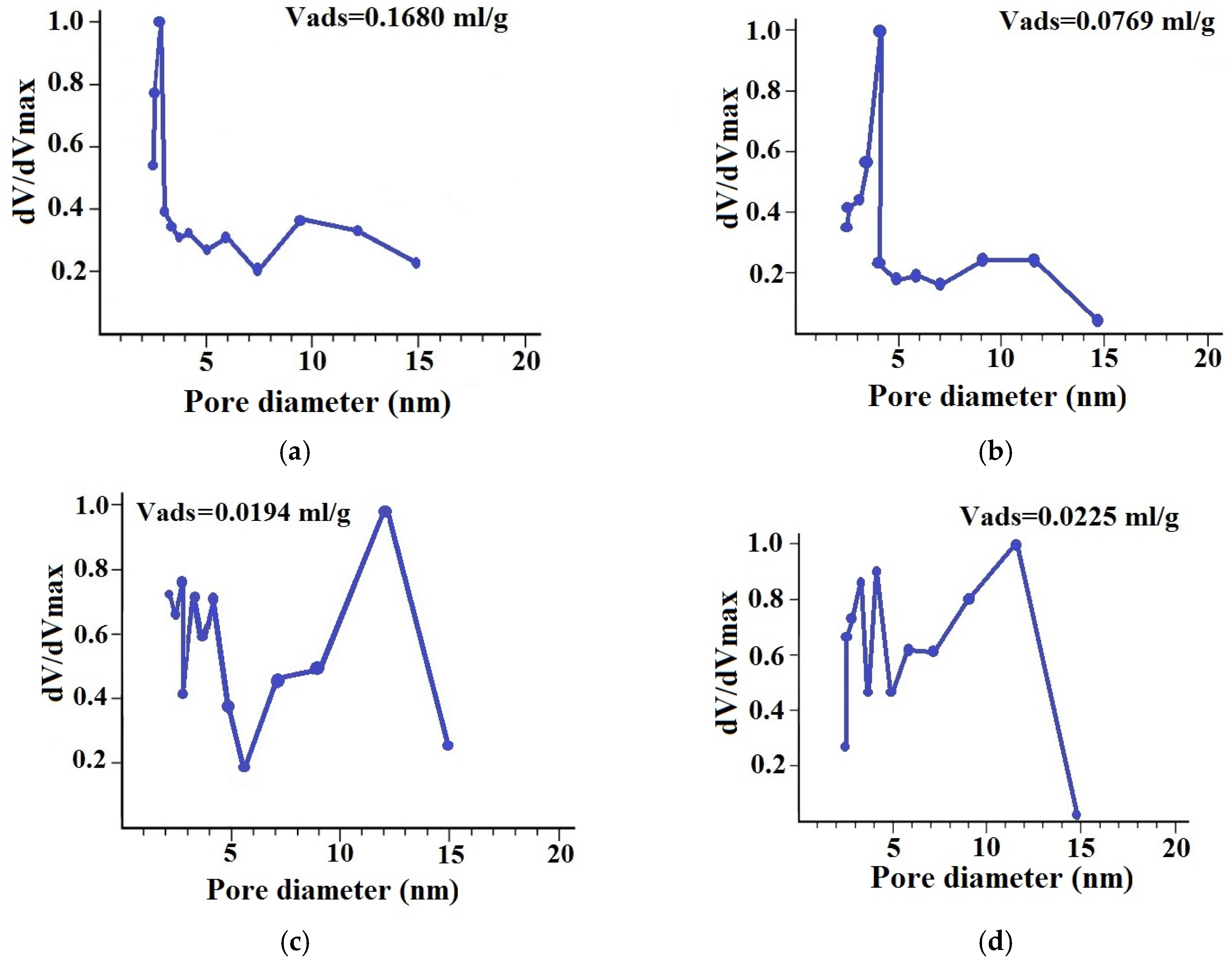
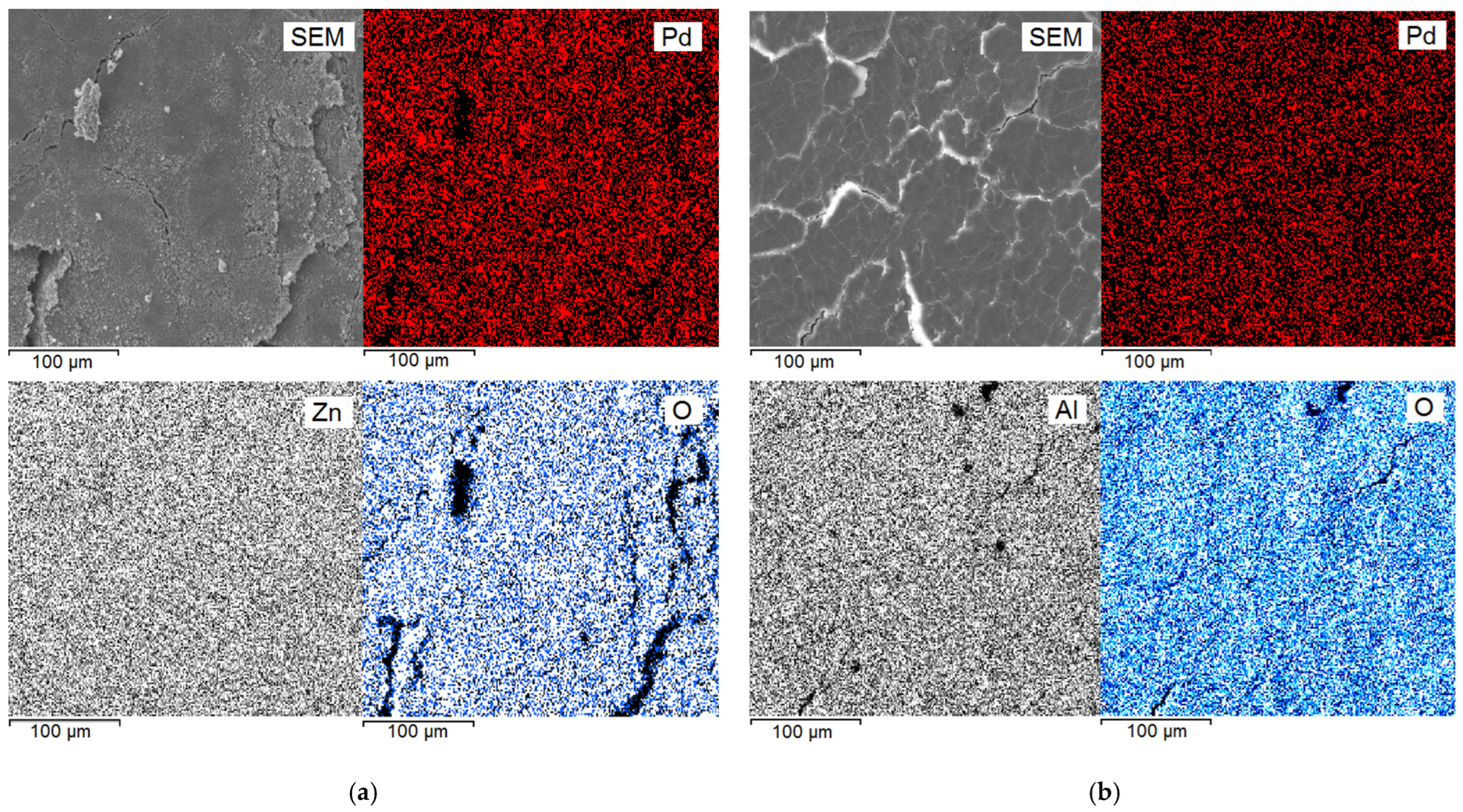
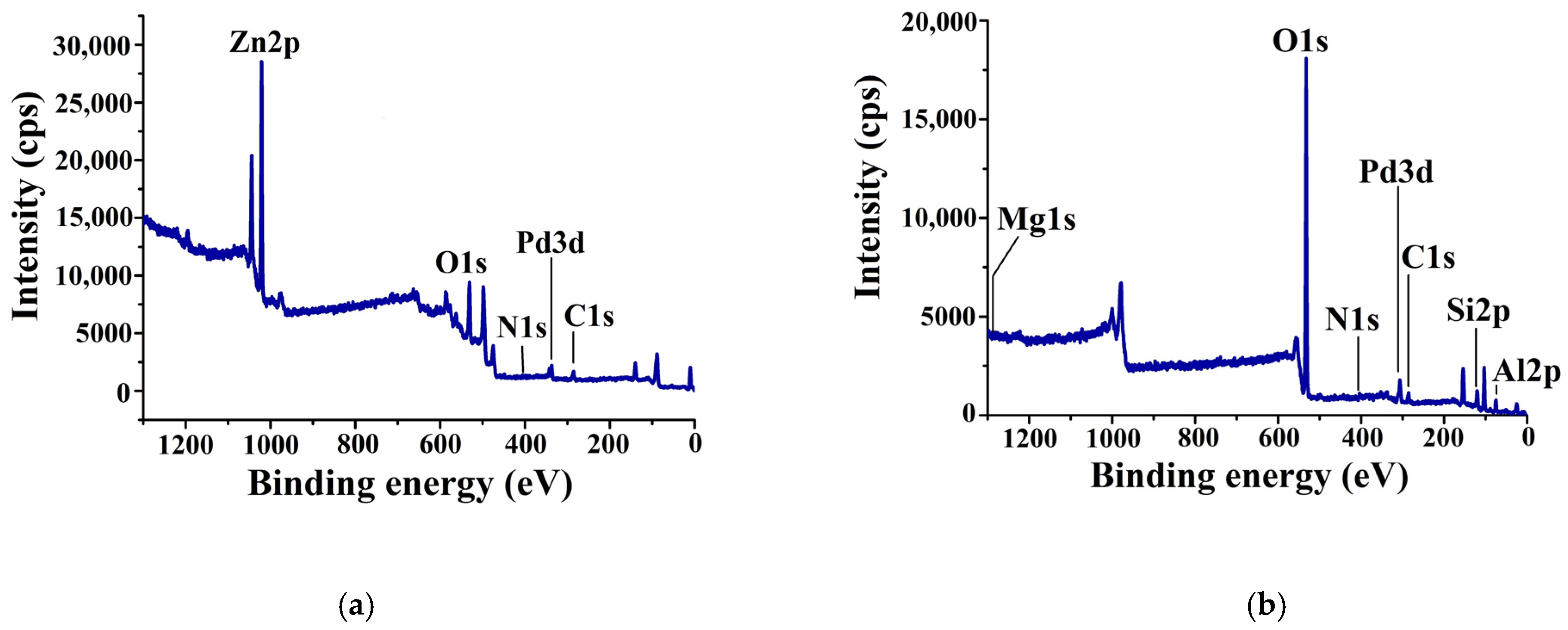
3.3. Characterization of the Composites by Physicochemical Methods
3.4. Methodology of Hydrogenation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Beyou, E.; Bourgeat-Lami, E. Organic-inorganic hybrid functional materials by nitroxide-mediated polymerization. Prog. Polym. Sci. 2021, 121, 101434. [Google Scholar] [CrossRef]
- Penelas, M.J.; Contreras, C.B.; Giussi, J.; Wolosiuk, A.; Azzaroni, O.; Soler Illia, G.J.A.A. Controlling dispersion, stability and polymer content on PDEGMA-functionalized core-brush silica colloids. Colloids Surf. A Physicochem. Eng. Asp. 2019, 574, 12–20. [Google Scholar] [CrossRef]
- Wang, X.; Wei, W.; Guo, Z.; Liu, X.; Liu, J.; Bing, T.; Yu, Y.; Yang, X.; Cai, Q. Organic–inorganic composite hydrogels: Compositions, properties, and applications in regenerative medicine. Biomater. Sci. 2024, 12, 1079–1114. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.R.; Nassar, E.J.; da Silva Barud, H.; Silva, J.M.; Rocha, L.A. Polymer and biopolymer organic-inorganic composites containing mixed oxides for application in energy up- and down-conversion. Opt. Mater. 2022, 134, 113189. [Google Scholar] [CrossRef]
- Kanaujia, P.K.; Adnan, M.; Dehury, K.M.; Prakash, G.V. Facile growth and re-crystallization of polymer-based inorganic-organic 2D hybrid composites and their applications. J. Alloys Compd. 2020, 820, 154550. [Google Scholar] [CrossRef]
- Ignat, M.; Samoila, P.; Cojocaru, C.; Soreanu, G.; Cretescu, I.; Harabagiu, V. Porous polymer/inorganic composite matrices as efficient desiccants for air dehumidification. Appl. Surf. Sci. 2019, 487, 1189–1197. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Seitkalieva, K.S.; Talgatov, E.T.; Auezkhanova, A.S.; Dzhardimalieva, G.I.; Pomogailo, A.D. Polymer-modified supported palladium catalysts for the hydrogenation of acetylene compounds. Kinet. Catal. 2016, 57, 360–367. [Google Scholar] [CrossRef]
- Kanwal, F.; Rani, I.; Batool, A.; Sandali, Y.; Li, C.; Shafique, S.; Irfan, A.; Sulaman, M. Enhanced dielectric and photocatalytic properties of TiO2-decorated rGO/PANI hybrid composites synthesized by in-situ chemical oxidation polymerization route. Mater. Sci. Eng. B 2023, 298, 116837. [Google Scholar] [CrossRef]
- Carrara, N.; Badano, J.M.; Betti, C.; Lederhos, C.; Busto, M.; Vera, C.; Quiroga, M. New Strategies for Obtaining Inorganic-Organic Composite Catalysts for Selective Hydrogenation. In New Advances in Hydrogenation Processes—Fundamentals and Applications; Licensee IntechOpen: London, UK, 2017; pp. 181–2017. [Google Scholar] [CrossRef]
- Erigoni, A.; Diaz, U. Porous Silica-Based Organic-Inorganic Hybrid Catalysts: A Review. Catalysts 2021, 11, 79. [Google Scholar] [CrossRef]
- Mahlambi, P.N.; Moloto, M.J. Starch-capped silver oxide (Ag2O) nanoparticles: Synthesis, characterization and antibacterial activity. Dig. J. Nanomater. Biostructures 2022, 17, 921–930. [Google Scholar] [CrossRef]
- de Almeida, D.A.; Sabino, R.M.; Souza, P.R.; Bonafé, E.G.; Venter, S.A.; Popat, K.C.; Martins, A.F.; Monteiro, J.P. Pectin-capped gold nanoparticles synthesis in-situ for producing durable, cytocompatible, and superabsorbent hydrogel composites with chitosan. Int. J. Biol. Macromol. 2020, 147, 138–149. [Google Scholar] [CrossRef]
- Molnár, A. The use of chitosan-based metal catalysts in organic transformations. Coord. Chem. Rev. 2019, 388, 126–171. [Google Scholar] [CrossRef]
- Khazaei, A.; Khazaei, M.; Rahmati, S. A green method for the synthesis of gelatin/pectin stabilized palladium nanoparticles as efficient heterogeneous catalyst for solvent-free Mizoroki–Heck reaction. J. Mol. Catal. A Chem. 2015, 398, 241–247. [Google Scholar] [CrossRef]
- Zauro, S.A.; Vishalakshi, B. Pectin graft copolymer-montmorillonite composite: Synthesis, swelling and divalent metal ion adsorption. Sep. Sci. Technol. 2018, 53, 2170–2185. [Google Scholar] [CrossRef]
- Nesic, A.; Meseldzija, S.; Cabrera-Barjas, G.; Onjia, A. Novel Biocomposite Films Based on High Methoxyl Pectin Reinforced with Zeolite Y for Food Packaging Applications. Foods 2022, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Talgatov, E.T.; Auezkhanova, A.S.; Kapysheva, U.N.; Bakhtiyrova, S.K.; Zharmagambetova, A.K. Synthesis and Detoxifying Properties of Pectin-Montmorillonite Composite. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1387–1391. [Google Scholar] [CrossRef]
- Wang, R.; Liang, R.; Dai, T.; Chen, J.; Shuai, X.; Liu, C. Pectin-based adsorbents for heavy metal ions: A review. Trends Food Sci. Technol. 2019, 91, 319–329. [Google Scholar] [CrossRef]
- Bok-Badura, J.; Jakóbik-Kolon, A.; Karoń, K.; Mitko, K. Sorption studies of heavy metal ions on pectin-nano-titanium dioxide composite adsorbent. Sep. Sci. Technol. 2017, 53, 1034–1044. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 11, 1599. [Google Scholar] [CrossRef]
- Lewandowska, K.; Sionkowska, A.; Furtos, G.; Grabska, S.; Michalska, M. Structure and Interactions in Chitosan Composites. Key Eng. Mater. 2016, 672, 257–260. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N. Chitin- and Chitosan-Based Composite Materials. Biomimetics 2022, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Chen, B.-Y.; Den, W. Chitosan as a Natural Polymer for Heterogeneous Catalysts Support: A Short Review on Its Applications. Appl. Sci. 2015, 5, 1272–1283. [Google Scholar] [CrossRef]
- Wang, W.; Xiao, Z.; Huang, C.; Zheng, K.; Luo, Y.; Dong, Y.; Shen, Z.; Li, W.; Qin, C. Preparation of Modified Chitosan Microsphere-Supported Copper Catalysts for the Borylation of α,β-Unsaturated Compounds. Polymers 2019, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Cunwei, Q.; Wenqian, Z.; Junlong, L.; Xuemin, W. Chitosan@Cu2O as A Facile, Efficient and Reusable Catalyst for Ligand Free C-O and C-N Coupling Chin. J. Org. Chem. 2019, 39, 1695–1703. [Google Scholar] [CrossRef]
- Reddy, K.P.; Swetha, C.; Murugadoss, A. Pd/Chitosan Nanoparticle Catalysts Prepared by Solid Mortar Grinding for Hydrogenation of Nitroarenes. ACS Sustain. Chem. Eng. 2023, 11, 1643–1654. [Google Scholar] [CrossRef]
- Shao, L.; Qi, C. Chitosan microspheres-supported palladium species as an efficient and recyclable catalyst for Mizoroki–Heck reaction. New J. Chem. 2017, 41, 8156–8165. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, M.; Wang, Y.; Feng, R.; Yang, Z.; Zuo, S.; Qi, C.; Zeng, M. Co-immobilization of Pd and Zn nanoparticles in chitosan/silica membranes for efficient, recyclable catalysts used in Ullmann reaction. Int. J. Biol. Macromol. 2017, 105, 575–583. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Shao, Y.; He, F.; Wu, M.; Ni, H.; Zheng, Y.; Sun, Y. Chitosan–silica nanoparticles catalyst (M@CS–SiO2) for the degradation of 1,1-dimethylhydrazine. Res. Chem. Intermed. 2019, 45, 1721–1735. [Google Scholar] [CrossRef]
- Ali, F.; Khan, S.B.; Kamal, T.; Alamry, K.A.; Bakhsh, E.M.; Asiri, A.M.; Sobahi, R.A. Synthesis and characterization of metal nanoparticles templated chitosan-SiO2 catalyst for the reduction of nitrophenols and dyes. Carbohydr. Polym. 2018, 192, 217–230. [Google Scholar] [CrossRef]
- Ma, L.; Su, Y.; Chen, J.; Xu, J. Silica/Chitosan Core−Shell Hybrid-Microsphere-Supported Pd Catalyst for Hydrogenation of Cyclohexene Reaction. Ind. Eng. Chem. Res. 2017, 56, 12655–12662. [Google Scholar] [CrossRef]
- Moura, D.; Mano, J.F.; Paiva, M.C.; Alves, N.M. Chitosan nanocomposites based on distinct inorganic fillers for biomedical applications. Sci. Technol. Adv. Mater. 2016, 17, 626–643. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Liu, Y.; Zhang, T.; Zhou, J.; Chen, J.; Ren, X.; Yang, Z.; Zeng, M. Modification of Pillared Intercalated montmorillonite Clay as Heterogeneous Pd Catalyst Supports. Molecules 2023, 28, 7638. [Google Scholar] [CrossRef]
- Talgatov, E.; Auyezkhanova, A.; Zharmagambetova, A.; Tastanova, L.; Bukharbayeva, F.; Jumekeyeva, A.; Aubakirov, T. The Effect of Polymer Matrix on the Catalytic Properties of Supported Palladium Catalysts in the Hydrogenation of Alkynols. Catalysts 2023, 13, 741. [Google Scholar] [CrossRef]
- Zharmagambetova, A.K.; Talgatov, E.T.; Auyezkhanova, A.S.; Bukharbayeva, F.U.; Jumekeyeva, A.I. Polysaccharide-Stabilized PdAg Nanocatalysts for Hydrogenation of 2-Hexyn-1-ol. Catalysts 2023, 13, 1403. [Google Scholar] [CrossRef]
- Zharmagambetova, A.; Auyezkhanova, A.; Talgatov, E.; Jumekeyeva, A.; Buharbayeva, F.; Akhmetova, S.; Myltykbayeva, Z.; Nieto, J.M.L. Synthesis of polymer protected Pd–Ag/ZnO catalysts for phenylacetylene hydrogenation. J. Nanoparticle Res. 2022, 24, 1–17. [Google Scholar] [CrossRef]
- Batista, M.K.S.; Pinto, L.F.; Gomes, C.A.R.; Gomes, P. Novel highly-soluble peptide–chitosan polymers: Chemical synthesis and spectral characterization. Carbohydr. Polym. 2006, 64, 299–305. [Google Scholar] [CrossRef]
- Aljawish, A.; Chevalot, I.; Jasniewski, J.; Scher, J.; Muniglia, L. Enzymatic synthesis of chitosan derivatives and their potential applications. J. Mol. Catal. B Enzym. 2015, 112, 25–39. [Google Scholar] [CrossRef]
- Abd El-kader, F.H.; Shehap, A.M.; Bakr, A.A.; Hussein, O.T. Characterization of Clay/Chitosan Nanocomposites and their Use for Adsorption On Mn(ΙΙ) from Aqueous Solution. Int. J. Sci. Eng. Appl. 2015, 4, 174–185. [Google Scholar] [CrossRef]
- Dziedzic, I.; Kertmen, A. Methods of Chitosan Identification: History and Trends. Lett. Appl. NanoBioScience 2023, 12, 94. [Google Scholar] [CrossRef]
- Cámara, C.I.; Quiroga, M.V.C.; Wilke, N.; Jimenez-Kairuz, A.; Yudi, L.M. Effect of chitosan on distearoylphosphatidylglycerol films at air/water and liquid/liquid interfaces. Electrochim. Acta 2013, 94, 124–133. [Google Scholar] [CrossRef]
- Devi, P.G.; Velu, A.S. Synthesis, structural and optical properties of pure ZnO and Co doped ZnO nanoparticles prepared by the co-precipitation method. J. Theor. Appl. Phys. 2016, 10, 233–240. [Google Scholar] [CrossRef]
- Al-Mulla, E.A.J.; Ibrahim, N.A. Poly(Lactic Acid) as a Biopolymer-Based Nano-Composite. In Products and Applications of Biopolymers; Part II; Intech Edition: London, UK, 2012; pp. 27–40. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Mansa, R.; Detellier, C. Preparation and Characterization of Guar-Montmorillonite Nanocomposites. Materials 2013, 6, 5199–5216. [Google Scholar] [CrossRef]
- Parambhath, V.B.; Nagar, R.; Ramaprabhu, S. Effect of Nitrogen Doping on Hydrogen Storage Capacity of Palladium Decorated Graphene. Langmuir 2012, 28, 7826–7833. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ren, D.; Tan, J.; Zhang, H.; Guo, Y.; Huang, L. Surface Modification by Amino Group Inducing for Highly Efficient Catalytic Oxidation of Toluene over a Pd/KIT-6 Catalyst. ACS Omega 2022, 7, 39950–39958. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Wei, W.; Lin, X.; Bediako, J.K.; Reddy, D.H.K.; Song, M.-H.; Yun, Y.-S. Pd(II)-Imprinted Chitosan Adsorbent for Selective Adsorption of Pd(II): Optimizing the Imprinting Process through Box–Behnken Experimental Design. ACS Omega 2021, 6, 13057–13065. [Google Scholar] [CrossRef] [PubMed]
- Baran, T. Pd(0) nanocatalyst stabilized on a novel agar/pectin composite and its catalytic activity in the synthesis of biphenyl compounds by Suzuki-Miyaura cross coupling reaction and reduction of o-nitroaniline. Carbohydr. Polym. 2018, 195, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.L.; Denicourt-Nowicki, A.; Mériadec, C.; Domingos, J.B.; Roucoux, A. Water soluble polymer–surfactant complexes-stabilized Pd(0) nanocatalysts: Characterization and structure–activity relationships in biphasic hydrogenation of alkenes and α,β-unsaturated ketones. J. Catal. 2016, 340, 144–153. [Google Scholar] [CrossRef]
- Di Pietrantonio, K.; Coccia, F.; Tonucci, L.; d’Alessandro, N.; Bressan, M. Hydrogenation of allyl alcohols catalyzed by aqueous palladium and platinum nanoparticles. RSC Adv. 2015, 5, 68493–68499. [Google Scholar] [CrossRef]
- Durin, G.; Lee, M.-Y.; Pogany, M.A.; Weyhermüller, T.; Kaeffer, N.; Leitner, W. Hydride-Free Hydrogenation: Unraveling the Mechanism of Electrocatalytic Alkyne Semihydrogenation by Nickel–Bipyridine Complexes. J. Am. Chem. Soc. 2023, 145, 17103–17111. [Google Scholar] [CrossRef] [PubMed]
- Zharmagambetova, A.K.; Ergozhin, E.E.; Sheludyakov, Y.L.; Mukhamedzhanova, S.G.; Kurmanbayeva, I.A.; Selenova, B.A.; Utkelov, B.A. 2-Propen-1-ol hydrogenation and isomerisation on polymer-palladium complexes—Effect of polymer matrix. J. Mol. Catal. A Chem. 2001, 177, 165–170. [Google Scholar] [CrossRef]
- IUPAC-NIST Solubility Data Series. Available online: https://srdata.nist.gov/solubility/IUPAC/iupac.aspx (accessed on 17 August 2007).






| m(CS) in the Initial Solution, mg | m(CS) in Solution after Sorption, mg | m(CS) Adsorbed, mg | Adsorption Degree, % | CS Content, % |
|---|---|---|---|---|
| CS/MMT | ||||
| 10.1 | 0.4 | 9.7 | 96.0 | 1.0 |
| 20.4 | 0.4 | 20.0 | 98.0 | 2.0 |
| 30.9 | 0.4 | 30.5 | 98.7 | 3.0 |
| 52.6 | 4.2 | 48.4 | 92.0 | 4.6 |
| CS/ZnO | ||||
| 10.1 | 0.3 | 9.8 | 97.0 | 1.0 |
| 20.4 | 0.3 | 20.1 | 98.5 | 2.0 |
| 30.9 | 0.3 | 30.6 | 99.0 | 3.0 |
| 52.6 | 0.4 | 52.2 | 99.2 | 5.0 |
| Sample | νOH νNH | νCH | δNH | νC–O–C ν(C–C)K ν(C–O)K | νZn–O νSi–O νAl–O |
|---|---|---|---|---|---|
| Chitosan | 3410 3160 | 2921 2853 | 1616 1564 | 1076 1037 | |
| Chitosanium chloride | 3425 3144 | 2935 2861 | 1622 1502 | 1074 1037 | |
| ZnO | 3444 | 494 446 | |||
| MMT | 3630 3430 | 1030 914 527 | |||
| CS/ZnO | 3569 3491 3226 3142 | 2925 2862 | 1621 1559 | 1153 1055 1031 | 478 442 |
| CS/MMT | 3636 3429 3179 | 2920 2842 | 1657 1512 | blocked by the MMT signal | 1034 920 530 |
| Sample | Specific Surface Area, m2/g | Blocked Surface, % |
|---|---|---|
| ZnO | 12.3 | - |
| 2.0%CS/ZnO | 10.2 | 17.1 |
| 5.0%CS/ZnO | 7.8 | 36.6 |
| MMT | 100.7 | - |
| 2.0%CS/MMT | 96.1 | 4.6 |
| 4.6%CS/MMT | 86.5 | 14.1 |
| PS Content, % | m(Pd) in Initial Solution, mg | m(Pd) in Solution after Sorption, mg | m(Pd) Adsorbed, mg | Ads. Degree, % | Pd Content, % | |
|---|---|---|---|---|---|---|
| FEC | Elem. Analysis | |||||
| 1 | 10.1 | 0.1 | 10.0 | 99.0 | 1.0 | 0.97 |
| 2 | 0.1 | 10.0 | 99.0 | 1.0 | 1.30 | |
| 3 | 0.1 | 10.0 | 99.0 | 1.0 | n.d.* | |
| 5 | 0.1 | 10.0 | 99.0 | 1.0 | n.d. | |
| PS Content, % | m(Pd) in Initial Solution, mg | m(Pd) in Solution after Sorption, mg | m(Pd) Adsorbed, mg | Ads. Degree, % | Pd Content, % | |
|---|---|---|---|---|---|---|
| FEC | Elem. Analysis | |||||
| 1 | 10.1 | 1.4 | 8.7 | 86.1 | 0.9 | 0.90 |
| 2 | 0.5 | 9.6 | 95.0 | 1.0 | 0.98 | |
| 3 | 0.1 | 10.0 | 99.0 | 1.0 | 1.10 | |
| 5 | 0.1 | 10.0 | 99.0 | 1.0 | 1.22 | |
| Chitosan Content,% | Reaction Rate(W)·10−6, mol/s | Selectivity, % | Conversion, % | |
|---|---|---|---|---|
| Propanal | Propanol | |||
| 1 | 3.6 | 19.6 | 80.4 | 100 |
| 2 | 4.3 | 17.2 | 82.8 | 100 |
| 3 | 3.3 | 17.0 | 83.0 | 100 |
| 5 | 2.7 | 15.6 | 84.4 | 100 |
| Support | Reaction Rate (W) · 10−6, mol/s | Selectivity, % | Conversion, % | |
|---|---|---|---|---|
| Propanal | Propanol | |||
| ZnO | 4.3 | 17.2 | 82.8 | 100 |
| MMT | 6.0 | 15.2 | 84.8 | 100 |
| Catalyst | Wmax·10−6, mol s−1 | Selectivity, % | Conversion, % |
|---|---|---|---|
| 1%Pd−CS/MMT | 1.5 | 90.9 | 98.9 |
| 1%Pd−CS/ZnO | 0.9 | 93.8 | 92.9 |
| Catalyst | W·10−6, mol s−1 | Selectivity to cis-2-hexen-1-ol, % | Conversion, % | |
|---|---|---|---|---|
| C≡C | C=C | |||
| 1%Pd−CS/MMT | 2.4 | 2.4 | 92.9 | 82.4 |
| 1%Pd−CS/ZnO | 0.3 | 0.3 | 91.5 | 85.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukharbayeva, F.; Zharmagambetova, A.; Talgatov, E.; Auyezkhanova, A.; Akhmetova, S.; Jumekeyeva, A.; Naizabayev, A.; Kenzheyeva, A.; Danilov, D. The Synthesis of Green Palladium Catalysts Stabilized by Chitosan for Hydrogenation. Molecules 2024, 29, 4584. https://doi.org/10.3390/molecules29194584
Bukharbayeva F, Zharmagambetova A, Talgatov E, Auyezkhanova A, Akhmetova S, Jumekeyeva A, Naizabayev A, Kenzheyeva A, Danilov D. The Synthesis of Green Palladium Catalysts Stabilized by Chitosan for Hydrogenation. Molecules. 2024; 29(19):4584. https://doi.org/10.3390/molecules29194584
Chicago/Turabian StyleBukharbayeva, Farida, Alima Zharmagambetova, Eldar Talgatov, Assemgul Auyezkhanova, Sandugash Akhmetova, Aigul Jumekeyeva, Akzhol Naizabayev, Alima Kenzheyeva, and Denis Danilov. 2024. "The Synthesis of Green Palladium Catalysts Stabilized by Chitosan for Hydrogenation" Molecules 29, no. 19: 4584. https://doi.org/10.3390/molecules29194584






