Seagrass Meadows: Prospective Candidates for Bioactive Molecules
Abstract
:1. Introduction
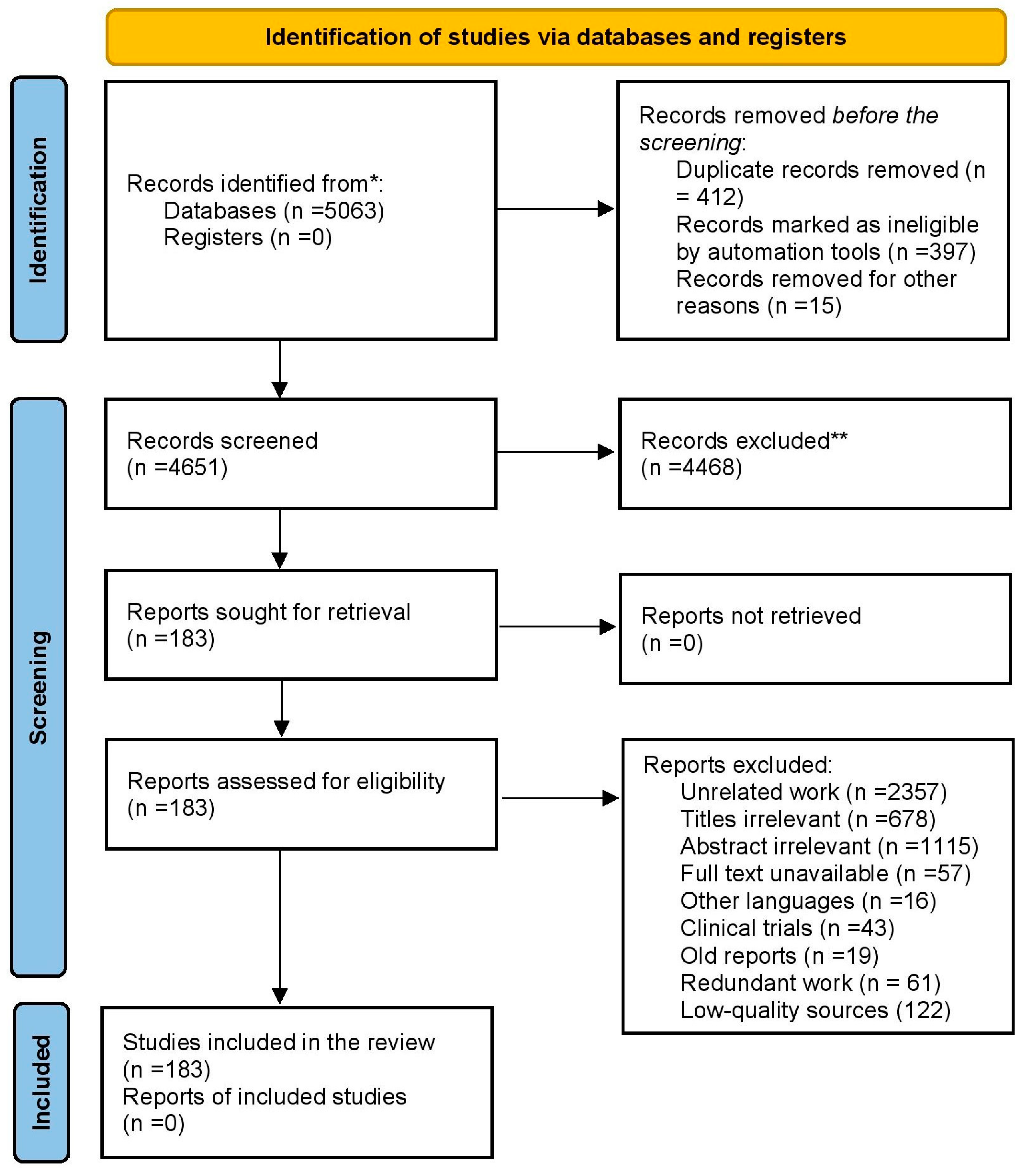
2. Methodology of Literature Retrieval
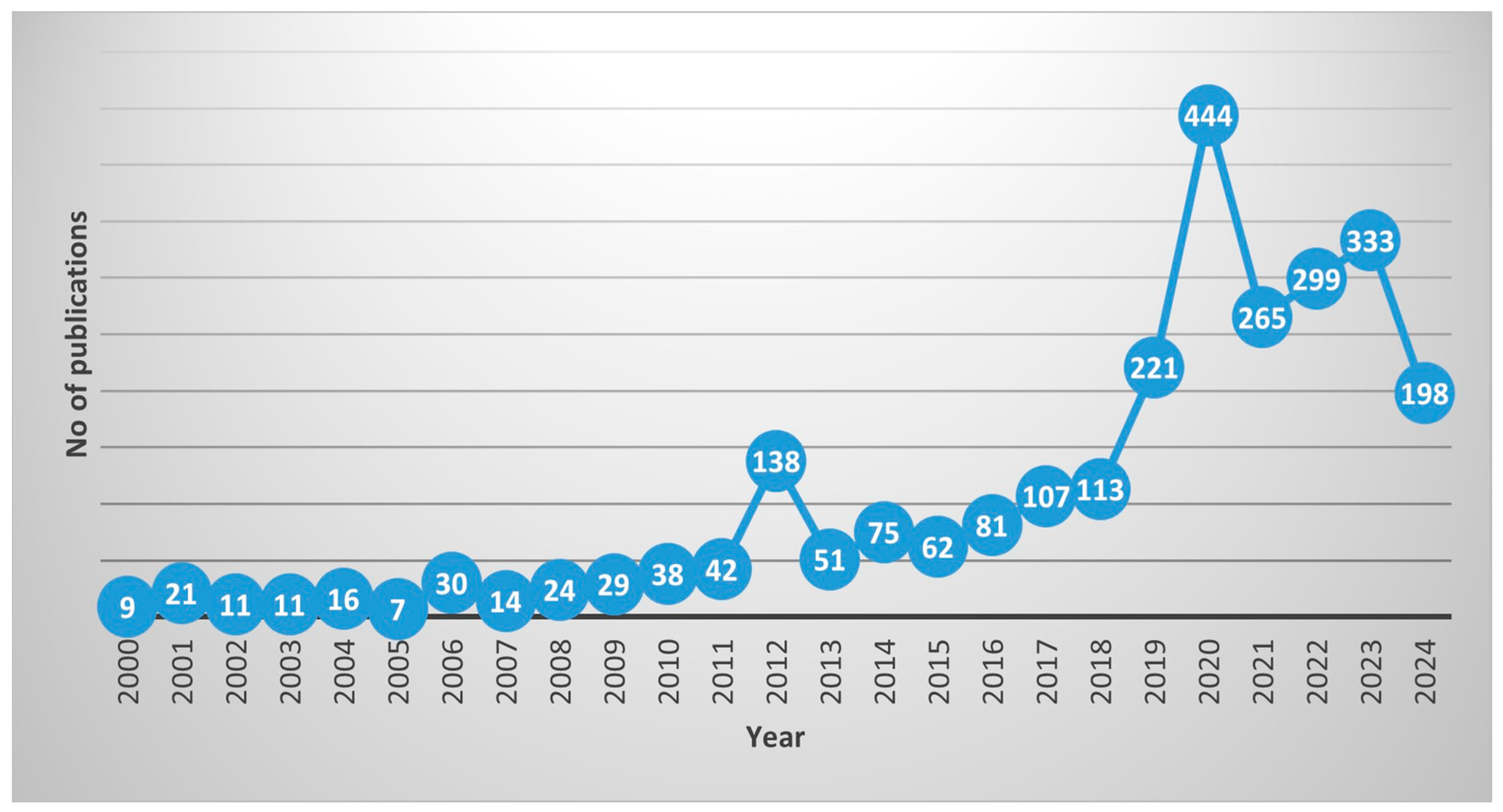
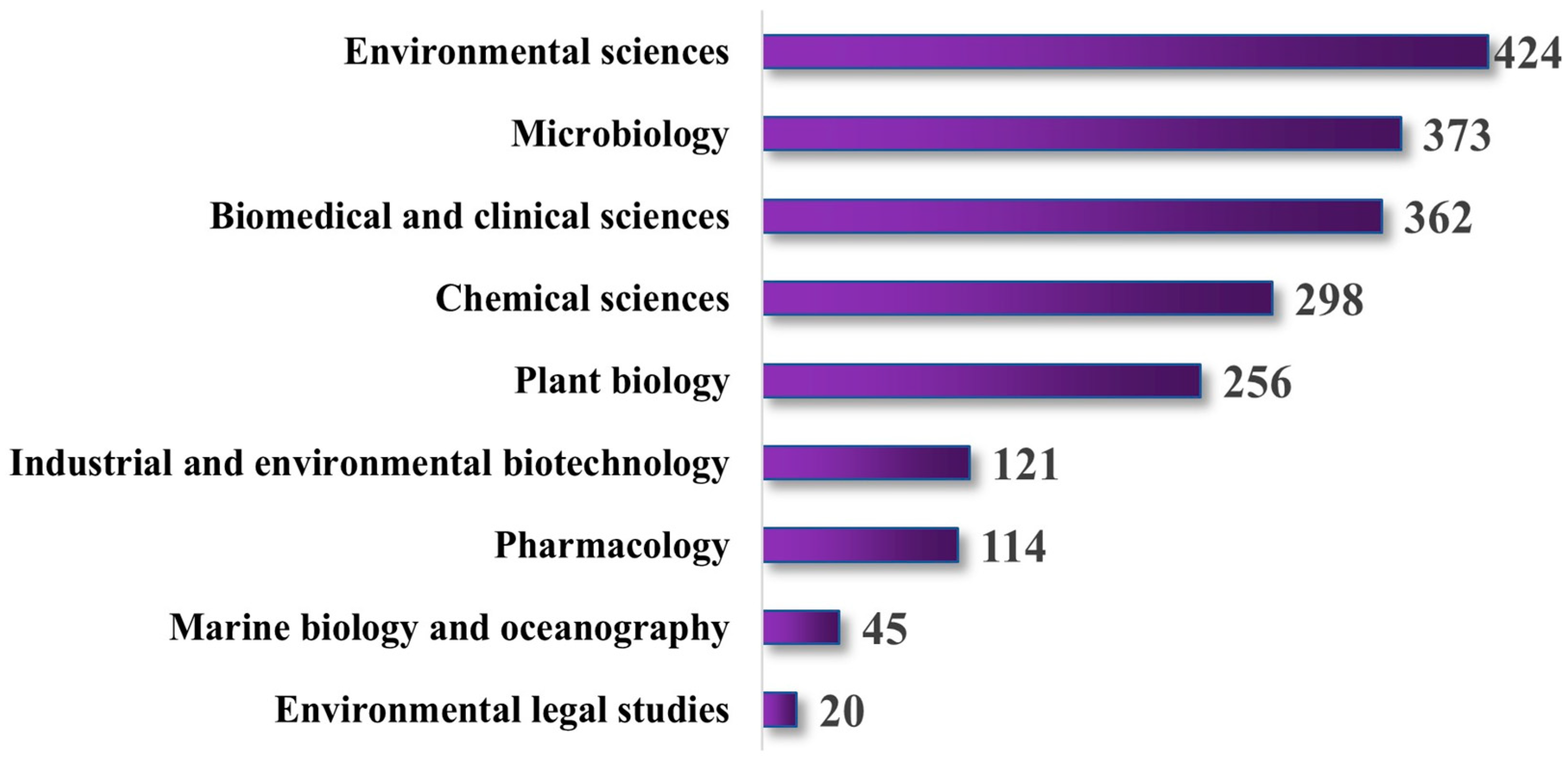
3. Trends in Seagrass Research
3.1. Seagrasses: The Marine Angiosperms
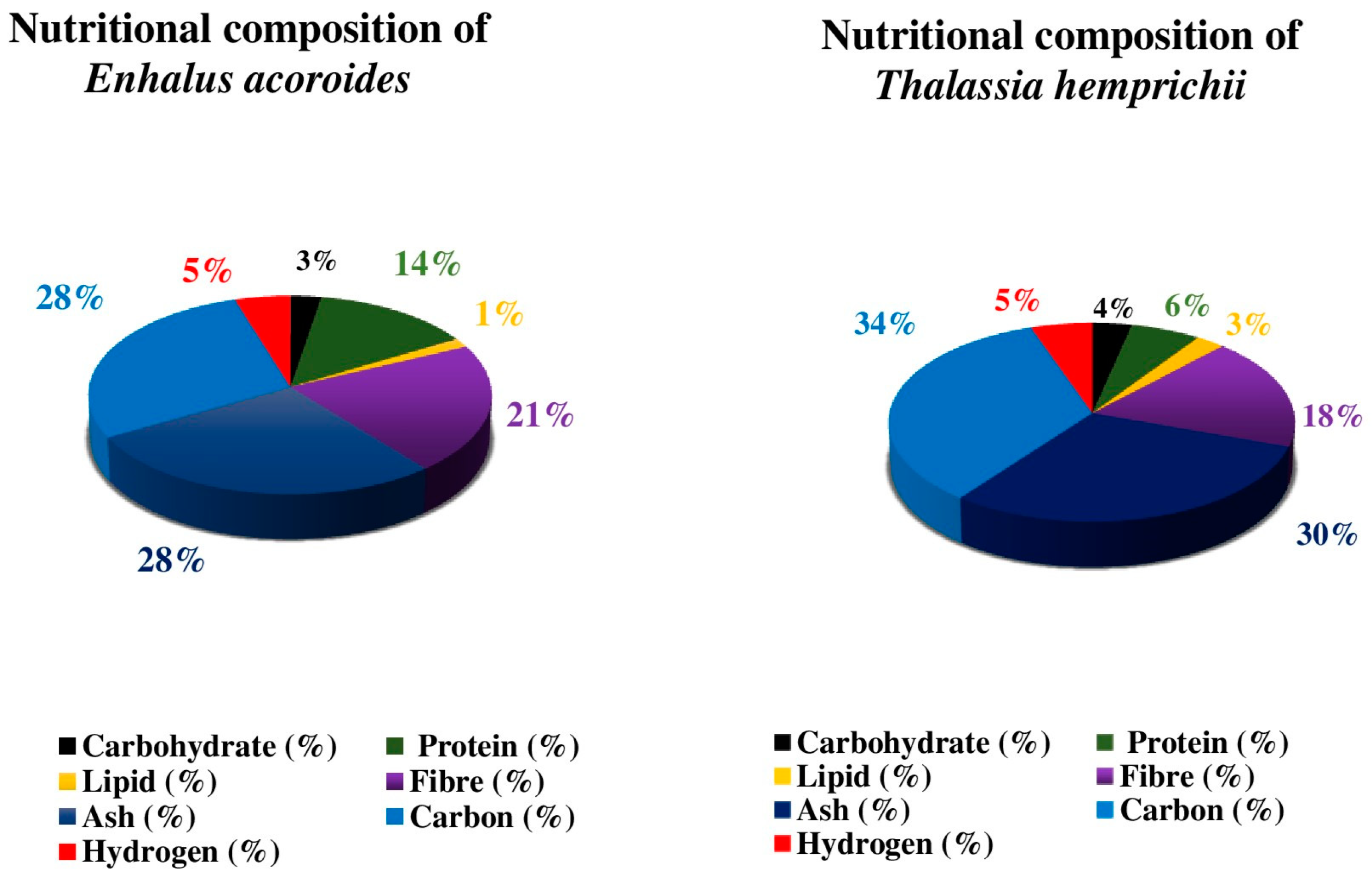
3.2. Nutritional Analysis of Seagrass
3.3. Phytochemical Analysis
3.4. Bioactivity of Seagrasses
3.5. Anti-Infectious and Antimicrobial Potential (Antibacterial, Antifungal and Antiviral)
3.6. Antioxidant and Anticancerous Potential
3.7. Anti-Inflammatory Potential
3.8. Anti-Diabetic, Anti-Larval, Anti-Aging and Anti-Tumor Potential
3.9. Bioprospecting Seagrass Microbiome
3.10. Comprehensive Methodologies for Assessing Bioactivity in Seagrasses: Chemical, Biological, and Ecological Approaches
4. Conclusions and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ODS column | Octadecyl-silica column |
| MIC | Minimum inhibitory concentration |
| DPPH | 2,2-diphenyl-1-picryl-hydrazyl-hydrate |
| MECS | Methanolic extract of Cymodocea serrulata |
| AIDS | Acquired Immuno Deficiency Syndrome |
References
- Steele, J.H.; Beet, A.R. Marine Protected Areas in “Nonlinear” Ecosystems. Proc. R. Soc. B Biol. Sci. 2003, 270, S230–S233. [Google Scholar] [CrossRef] [PubMed]
- The Oxygen Pumps in the Sea Seagrasses the Wealth of India Raw Materials Series (a Wealth of Information on Plants, Animals and Minerals of India). Available online: https://niscpr.res.in/includes/images/wealthofindia/WoI-Extension-Bulletin-Seagrasses-Jan13.pdf (accessed on 27 August 2024).
- Duffy, J.E.; Benedetti-Cecchi, L.; Trinanes, J.; Muller-Karger, F.E.; Ambo-Rappe, R.; Boström, C.; Buschmann, A.H.; Byrnes, J.; Coles, R.G.; Creed, J.; et al. Toward a Coordinated Global Observing System for Seagrasses and Marine Macroalgae. Front. Mar. Sci. 2019, 6, 317. [Google Scholar] [CrossRef]
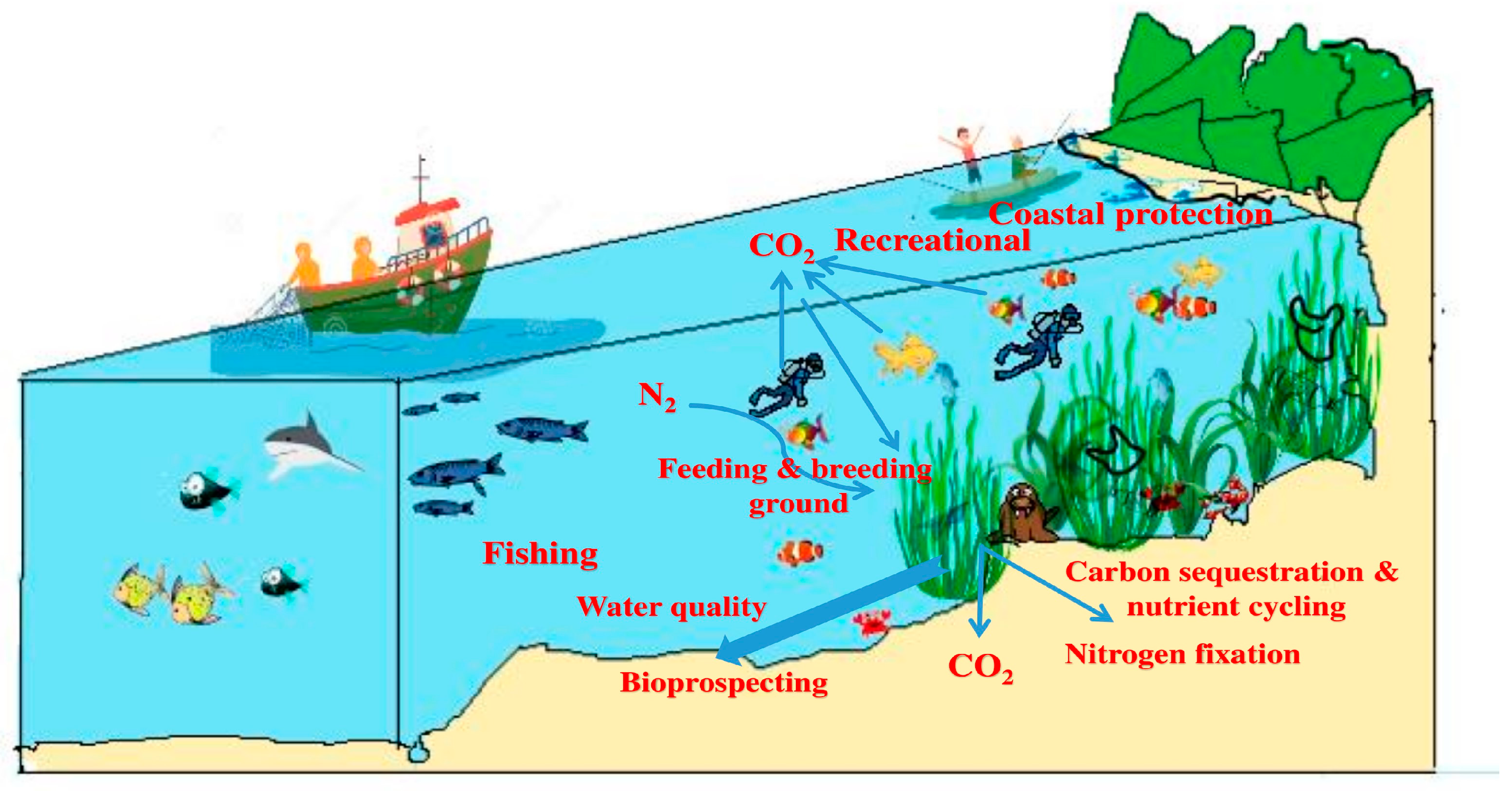
- Ruiz-Frau, A.; Gelcich, S.; Hendriks, I.E.; Duarte, C.M.; Marbà, N. Current State of Seagrass Ecosystem Services: Research and Policy Integration. Ocean Coast. Manag. 2017, 149, 107–115. [Google Scholar] [CrossRef]
- Papenbrock, J. Highlights in Seagrasses’ Phylogeny, Physiology, and Metabolism: What Makes Them Special? ISRN Bot. 2012, 2012, 103892. [Google Scholar] [CrossRef]
- James, R.K.; Keyzer, L.M.; van de Velde, S.J.; Herman, P.M.J.; van Katwijk, M.M.; Bouma, T.J. Climate Change Mitigation by Coral Reefs and Seagrass Beds at Risk: How Global Change Compromises Coastal Ecosystem Services. Sci. Total Environ. 2023, 857, 159576. [Google Scholar] [CrossRef]
- Reynolds, L.K.; Waycott, M.; McGlathery, K.J.; Orth, R.J. Ecosystem Services Returned through Seagrass Restoration. Restor. Ecol. 2016, 24, 583–588. [Google Scholar] [CrossRef]
- Raja, S.; Subhashini, P.; Thangaradjou, T. Differential Methods of Localisation of Fungal Endophytes in the Seagrasses. Mycology 2016, 7, 112–123. [Google Scholar] [CrossRef]
- Venkatachalam, A.; Thirunavukkarasu, N.; Suryanarayanan, T.S. Distribution and Diversity of Endophytes in Seagrasses. Fungal Ecol. 2015, 13, 60–65. [Google Scholar] [CrossRef]
- Supaphon, P.; Phongpaichit, S.; Rukachaisirikul, V.; Sakayaroj, J. Antimicrobial Potential of Endophytic Fungi Derived from Three Seagrass Species: Cymodocea serrulata, Halophila ovalis and Thalassia hemprichii. PLoS ONE 2013, 8, e72520. [Google Scholar] [CrossRef]
- Sakayaroj, J.; Preedanon, S.; Supaphon, O.; Jones, E.B.G.; Phongpaichit, S. Phylogenetic Diversity of Endophyte Assemblages Associated with the Tropical Seagrass Enhalus acoroides in Thailand. Fungal Divers. 2010, 42, 27–45. [Google Scholar] [CrossRef]
- Patil, R.; Mallya, R. A Mini Review on Biological Activities of Genus Thalassia: A Marine Seagrass. J. Pharmacogn. Phytochem. 2023, 12, 215–222. [Google Scholar] [CrossRef]
- Hu, G.-P.; Yuan, J.; Sun, L.; She, Z.-G.; Wu, J.-H.; Lan, X.-J.; Zhu, X.; Lin, Y.-C.; Chen, S.-P. Statistical Research on Marine Natural Products Based on Data Obtained between 1985 and 2008. Mar. Drugs 2011, 9, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The Re-Emergence of Natural Products for Drug Discovery in the Genomics Era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2010, 27, 165. [Google Scholar] [CrossRef]
- Capon, R.J. Marine bioprospecting—Trawling for treasure and pleasure. Eur. J. Org. Chem. 2001, 2001, 633–645. [Google Scholar] [CrossRef]
- de la Torre-Castro, M.; Rönnbäck, P. Links between humans and seagrasses—An example from tropical East Africa. Ocean. Coast. Manag. 2004, 47, 361–387. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. PRISMA2020: An R package and Shiny app for producing PRISMA 2020-compliant flow diagrams, with interactivity for optimised digital transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Burt, D.B. Evolutionary Stasis, Constraint and Other Terminology Describing Evolutionary Patterns. Biol. J. Linn. Soc. 2001, 72, 509–517. [Google Scholar] [CrossRef]
- Orth, R.J.; Carruthers, T.J.; Dennison, W.C.; Duarte, C.M.; Fourqurean, J.W.; Heck, K.L.; Williams, S.L. A global crisis for seagrass ecosystems. Bioscience 2006, 56, 987–996. [Google Scholar] [CrossRef]
- Nordlund, L.M.; Jackson, E.L.; Nakaoka, M.; Samper-Villarreal, J.; Beca-Carretero, P.; Creed, J.C. Seagrass Ecosystem Services—What’s Next? Mar. Pollut. Bull. 2018, 134, 145–151. [Google Scholar] [CrossRef]
- Mtwana Nordlund, L.; Koch, E.W.; Barbier, E.B.; Creed, J.C. Seagrass Ecosystem Services and Their Variability across Genera and Geographical Regions. PLoS ONE 2016, 11, e0163091. [Google Scholar] [CrossRef] [PubMed]
- Hemminga, M.A.; Duarte, C.M. Seagrass Ecology; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Fourqurean, J.W.; Duarte, C.M.; Kennedy, H.; Marbà, N.; Holmer, M.; Mateo, M.A.; Apostolaki, E.T.; Kendrick, G.A.; Krause-Jensen, D.; McGlathery, K.J.; et al. Seagrass Ecosystems as a Globally Significant Carbon Stock. Nat. Geosci. 2012, 5, 505–509. [Google Scholar] [CrossRef]
- Gillanders, B.M. Seagrasses, Fish, and Fisheries. In Seagrasses: Biology, Ecology and Conservation; Springer: Dordrecht, The Netherlands, 2007; pp. 503–505. [Google Scholar] [CrossRef]
- Lilley, R.J.; Unsworth, R.K.F. Atlantic Cod (Gadus morhua) Benefits from the Availability of Seagrass (Zostera marina) Nursery Habitat. Glob. Ecol. Conserv. 2014, 2, 367–377. [Google Scholar] [CrossRef]
- Bujang, J.S.; Zakaria, M.H.; Arshad, A. Distribution and Significance of Seagrass Ecosystems in Malaysia. Aquat. Ecosyst. Health Manag. 2006, 9, 203–214. [Google Scholar] [CrossRef]
- Maxwell, P.S.; Eklöf, J.S.; van Katwijk, M.M.; O’Brien, K.R.; de la Torre-Castro, M.; Boström, C.; van der Heide, T. The fundamental role of ecological feedback mechanisms for the adaptive management of seagrass ecosystems—A review. Biol. Rev. 2017, 92, 1521–1538. [Google Scholar] [CrossRef]
- Cullen-Unsworth, L.; Unsworth, R. Seagrass Meadows, Ecosystem Services, and Sustainability. Environ. Sci. Policy Sustain. Dev. 2013, 55, 14–28. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef]
- Prasad, G.; Ramesh, M.V.; Ramesh, T.; Thomas, G.M. Changing profile of natural organic matter in groundwater of a Ramsar site in Kerala implications for sustainability. Case Stud. Chem. Environ. Eng. 2023, 8, 100390. [Google Scholar] [CrossRef]
- Gayathri, N.; Prasad, G.; Prabhakaran, V.; Priya, V. Understanding the impact of microplastic contamination on soil quality and eco-toxicological risks in horticulture: A comprehensive review. Case Stud. Chem. Environ. Eng. 2024, 9, 100633. [Google Scholar] [CrossRef]
- Widiastuti, E.L.; Rima, K.; Busman, H. Anticancer Potency of Seagrass (Enhalus acoroides) Methanol Extract in the HeLa Cervical Cancer Cell Culture. In Advances in Engineering Research/Advances in Engineering Research; Atlantis Press: Dordrecht, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Shailaja, V.; Christina, V.; Mohanapriya, C.; Sneha, P.; Sundaram, R.L.; Magesh, R.; Doss, C.G.P.; Gnanambal, K.M.E. A Natural Anticancer Pigment, Pheophytin A, from a Seagrass Acts as a High Affinity Human Mitochondrial Translocator Protein (TSPO) Ligand, in Silico, to Reduce Mitochondrial Membrane Potential (∆ψ) in Adenocarcinomic A549 Cells. Phytomedicine 2019, 61, 152858. [Google Scholar] [CrossRef]
- Imran, M.; Talpur, F.N.; Jan, M.I.; Khan, A.; Khan, I. Analysis of nutritional components of some wild edible plants. J. -Chem. Soc. Pak. 2007, 29, 500. [Google Scholar]
- Kim, D.H.; Mahomoodally, M.F.; Sadeer, N.B.; Seok, P.G.; Zengin, G.; Palaniveloo, K.; Khalil, A.A.; Rauf, A.; Rengasamy, K.R. Nutritional and Bioactive Potential of Seagrasses: A Review. S. Afr. J. Bot. 2021, 137, 216–227. [Google Scholar] [CrossRef]
- Cui, L.; Jiang, Z.; Huang, X.; Liu, S.; Wu, Y. Identification of Food Sources in Tropical Seagrass Bed Food Web Using Triple Stable Isotopes and Fatty Acid Signatures. Front. Mar. Sci. 2023, 10, 1093181. [Google Scholar] [CrossRef]
- Clores, M.A. Food-Web Dynamics in Three Seagrass Systems in Caramoan Peninsula, Philippines. Environ. Ecol. Res. 2023, 11, 295–312. [Google Scholar] [CrossRef]
- Rondevaldova, J.; Quiao, M.A.; Drabek, O.; Dajcl, J.; Dela Pena-Galanida, G.D.; Leopardas, V.E.; Kokoska, L. Mineral Composition of Seaweeds and Seagrasses of the Philippines. Phycologia 2023, 62, 217–224. [Google Scholar] [CrossRef]
- Pradheeba, M.; Dilipan, E.; Nobi, E.P.; Thangaradjou, T.; Sivakumar, K. Evaluation of seagrasses for their nutritional value. Indian J. Geo-Mar. Sci. 2011, 45, 105–111. [Google Scholar]
- Immaculate, J.K.; Lilly, T.T.; Patterson, J. Macro and micro nutrients of seagrass species from Gulf of Mannar, India. MOJ Food Process Technol. 2018, 6, 391–398. [Google Scholar]
- Rengasamy, R.R.K.; Radjassegarin, A.; Perumal, A. Seagrasses as Potential Source of Medicinal Food Ingredients: Nutritional Analysis and Multivariate Approach. Biomed. Prev. Nutr. 2013, 3, 375–380. [Google Scholar] [CrossRef]
- Bharatharathna, P.; Santhanam, P. Analyses of phytochemical, biochemical, pigments and antioxidant activity of seagrass Syringodium isoetifolium. J. Adv. Sci. Res. 2019, 10 (Suppl. 2), 267–271. [Google Scholar]
- Ragupathi Raja Kannan, R.; Arumugam, R.; Thangaradjou, T.; Anantharaman, P. Phytochemical Constituents, Antioxidant Properties and P-Coumaric Acid Analysis in Some Seagrasses. Food Res. Int. 2013, 54, 1229–1236. [Google Scholar] [CrossRef]
- Bharathi, N.P.; Jayalakshmi, M.; Amudha, P.; Vanitha, V. Phytochemical screening and in vitro antioxidant activity of the seagrass Cymodocea serrulata. Indian J. Geo-Mar. Sci. 2019, 48, 1216–1221. [Google Scholar]
- De Vincenti, L.; Glasenapp, Y.; Cattò, C.; Villa, F.; Cappitelli, F.; Papenbrock, J. Hindering the Formation and Promoting the Dispersion of Medical Biofilms: Non-Lethal Effects of Seagrass Extracts. BMC Complement. Altern. Med. 2018, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Baby, L.; Sankar, T.V.; Chandramohanakumar, N. Changes in Phenolic Compounds in Seagrasses against Changes in the Ecosystem. J. Pharmacogn. Phytochem. 2017, 6, 742–747. [Google Scholar]
- Smadi, A.; Ciavatta, M.; Bitam, F.; Carbone, M.; Villani, G.; Gavagnin, M. Prenylated Flavonoids and Phenolic Compounds from the Rhizomes of Marine Phanerogam Cymodocea nodosa. Planta Medica 2017, 84, 704–709. [Google Scholar] [CrossRef]
- Kannan, R.R.R.; Arumugam, R.; Anantharaman, P. Chemical Composition and Antibacterial Activity of Indian Seagrasses against Urinary Tract Pathogens. Food Chem. 2012, 135, 2470–2473. [Google Scholar] [CrossRef]
- Setyoningrum, D.; Yamindago, A.; Hikmah Julinda Sari, S.; Maftuch, M. Phytochemical Analysis and in vitro Antibacterial Activities of Seagrass Enhalus acoroides against Staphylococcus aureus. Res. J. Life Sci. 2020, 7, 85–91. [Google Scholar] [CrossRef]
- Pharmawati, M.; Wrasiati, L.P. Phytochemical screening and FTIR spectroscopy on crude extract from Enhalus acoroides leaves. Malays. J. Anal. Sci. 2020, 24, 70–77. [Google Scholar]
- Noor, N.M.; Febriani, D.; Ali, M. Seagrass of Enhalus acoroides as a Traditional Body Scrubs in Preventing Malarial Bites by Pahawang Island Community in Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2022, 1012, 012037. [Google Scholar] [CrossRef]
- Kim, H.S.; Park, N.H.; Suk, H.Y.; You, S.G.; Woo, J.H. Identification of Polyphenol Substances (MP-1) from Seagrass, Phyllospadix japonica Makino. Korean J. Environ. Agric. 2022, 41, 50–54. [Google Scholar] [CrossRef]
- Das, D.; Arulkumar, A.; Paramasivam, S.; Lopez-Santamarina, A.; del Carmen Mondragon, A.; Miranda Lopez, J.M. Phytochemical Constituents, Antimicrobial Properties and Bioactivity of Marine Red Seaweed (Kappaphycus alvarezii) and Seagrass (Cymodocea serrulata). Foods 2023, 12, 2811. [Google Scholar] [CrossRef]
- Windyaswari, A.S.; Purba, J.P.; Nurrahmah, S.S.; Ayu, I.P.; Imran, Z.; Amin, A.A.; Kurniawan, F.; Pratiwi, N.T.M.; Iswantari, A. Phytochemical Profile of Sea Grass Extract (Enhalus acoroides): A New Marine Source from Ekas Bay, East Lombok. IOP Conf. Ser. Earth Environ. Sci. 2019, 278, 012081. [Google Scholar] [CrossRef]
- Qi, S.-H.; Huang, L.-S.; He, F.; Zhang, S.; Dong, J.-D. Phytochemical and Chemotaxonomic Investigation of Seagrass Thalassia hemprichii (Ehrenb.) Aschers (Hydrocharitaceae). Biochem. Syst. Ecol. 2012, 43, 128–131. [Google Scholar] [CrossRef]
- Bitam, F.; Ciavatta, M.L.; Carbone, M.; Manzo, E.; Mollo, E.; Gavagnin, M. Chemical Analysis of Flavonoid Constituents of the Seagrass Halophila stipulacea: First Finding of Malonylated Derivatives in Marine Phanerogams. Biochem. Syst. Ecol. 2010, 38, 686–690. [Google Scholar] [CrossRef]
- Yuvaraj, N.; Kanmani, P.; Satishkumar, R.; Paari, A.; Pattukumar, V.; Arul, V. Seagrass as a potential source of natural antioxidant and anti-inflammatory agents. Pharm. Biol. 2012, 50, 458–467. [Google Scholar] [CrossRef]
- Tangon, E.; Elvinia, R.A.; Jocelyn, A.P.; Kingpu, O.A. Phytochemical Screening and proximate composition of the seagrass Halodule pinifolia of the coastal waters of Carmen, Agusan Del Norte, Philippines. Int. J. Mod. Pharm. Res. 2021, 5, 75–80. [Google Scholar]
- Nazar, S.; Ravikumar, S.; Williams, G.P.; Ali, M.S.; Suganthi, P. Screening of Indian coastal plant extracts for larvicidal activity of Culex quinquefasciatus. Indian J. Sci. Technol. 2009, 2, 24–27. [Google Scholar] [CrossRef]
- Grignon-Dubois, M.; Rezzonico, B. Phenolic Fingerprint of the Seagrass Posidonia oceanica from Four Locations in the Mediterranean Sea: First Evidence for the Large Predominance of Chicoric Acid. Bot. Mar. 2015, 58, 379–391. [Google Scholar] [CrossRef]
- Blunt, J.W.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef]
- Kavitha, D.; Padmini, R.; Dhanaraju, M.D.; Gopi, C.; Thiyagarajan, D.; Veeramaneni, A. Syringodium isoetifolium Fosters an Antioxidant Defense System, Modulates Glycolytic Enzymes and Protects Membrane Integrity in DEN-induced Hepatocellular Carcinoma in Albino Wistar Rats. Ind. J. Pharm. Edu. Res. 2023, 57, s690–s700. [Google Scholar] [CrossRef]
- Haznedaroglu, M.Z.; Zeybek, U. HPLC Determination of Chicoric Acid in Leaves of Posidonia oceanica. Pharm. Biol. 2007, 45, 745–748. [Google Scholar] [CrossRef]
- Nuissier, G.; Rezzonico, B.; Grignon-Dubois, M. Chicoric Acid from Syringodium filiforme. Food Chem. 2010, 120, 783–788. [Google Scholar] [CrossRef]
- Qi, S.-H.; Zhang, S.; Qian, P.-Y.; Wang, B.-G. Antifeedant, Antibacterial, and Antilarval Compounds from the South China Sea Seagrass Enhalus acoroides. Bot. Mar. 2008, 51, 441–447. [Google Scholar] [CrossRef]
- Kontiza, I.; Stavri, M.; Zloh, M.; Vagias, C.; Gibbons, S.; Roussis, V. New Metabolites with Antibacterial Activity from the Marine Angiosperm Cymodocea nodosa. Tetrahedron 2008, 64, 1696–1702. [Google Scholar] [CrossRef]
- Kannan, R.R.R.; Arumugam, R.; Anantharaman, P. Antibacterial Potential of Three Seagrasses against Human Pathogens. Asian Pac. J. Trop. Med. 2010, 3, 890–893. [Google Scholar] [CrossRef]
- Rowley, D.C.; Hansen, M.S.T.; Rhodes, D.; Sotriffer, C.A.; Ni, H.; McCammon, J.A.; Bushman, F.D.; Fenical, W. Thalassiolins A–C: New Marine-Derived Inhibitors of HIV CDNA Integrase. Bioorganic Med. Chem. 2002, 10, 3619–3625. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, A.-H.A.; Mettwally, W.S.A.; El Fotouh, M.A.; Rodriguez, B.; El-Dewany, A.I.; El-Toumy, S.A.A.; Hussein, A.A. Bioactive Phenolic Compounds from the Egyptian Red Sea Seagrass Thalassodendron ciliatum. Z. Für Naturforschung C 2012, 67, 291–296. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.; Ibrahim, A.K.; Yamada, K.; Ahmed, S.A. Cytotoxic and Anti-Inflammatory Compounds from Red Sea Grass Thalassodendron ciliatum. Med. Chem. Res. 2018, 27, 1238–1244. [Google Scholar] [CrossRef]
- Bel Mabrouk, S.; Reis, M.; Sousa, M.L.; Ribeiro, T.; Almeida, J.R.; Pereira, S.; Antunes, J.; Rosa, F.; Vasconcelos, V.; Achour, L.; et al. The Marine Seagrass Halophila stipulacea as a Source of Bioactive Metabolites against Obesity and Biofouling. Mar. Drugs 2020, 18, 88. [Google Scholar] [CrossRef]
- Kjersti Hasle, E. Analysis of Polyphenolic Content in Marine and Aquatic Angiosperms from Norwegian Coastal Waters. Ph.D. Thesis, The University of Bergen, Bergen, Norway, 2018. Available online: https://hdl.handle.net/1956/17676 (accessed on 5 July 2024).
- Velika, B.; Kron, I. Antioxidant Properties of Benzoic Acid Derivatives against Superoxide Radical. Free Radic. Antioxid. 2012, 2, 62–67. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X1987417. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Jones, D.A. Rosacea, reactive oxygen species, and azelaic acid. J. Clin. Aesthetic Dermatol. 2009, 2, 26. [Google Scholar] [PubMed Central]
- Weimann, E.; Silva, M.B.B.; Murata, G.M.; Bortolon, J.R.; Dermargos, A.; Curi, R.; Hatanaka, E. Topical Anti-Inflammatory Activity of Palmitoleic Acid Improves Wound Healing. PLoS ONE 2018, 13, e0205338. [Google Scholar] [CrossRef]
- Tao, C.; Wu, J.; Liu, Y.; Liu, M.; Yang, R.; Lv, Z. Antimicrobial Activities of Bamboo (Phyllostachys heterocycla Cv. Pubescens) Leaf Essential Oil and Its Major Components. Eur. Food Res. Technol. 2017, 244, 881–891. [Google Scholar] [CrossRef]
- Yoon, B.; Jackman, J.; Valle-González, E.; Cho, N.-J. Antibacterial Free Fatty Acids and Monoglycerides: Biological Activities, Experimental Testing, and Therapeutic Applications. Int. J. Mol. Sci. 2018, 19, 1114. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A Review of Biomedical Activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Ahmed, N. Phytochemical screening, antioxidant potential, isolation and characterization of bioactive compound from Enhalus acoroides. J. Pharm. Negat. Results 2022, 13, 4118–4131. [Google Scholar] [CrossRef]
- Anzaku, A.A.; Akyala, J.I.; Juliet, A.; Obianuju, E.C. Antibacterial Activity of Lauric Acid on Some Selected Clinical Isolates. Ann. Clin. Lab. Res. 2017, 5, 170. [Google Scholar] [CrossRef]
- Zhang, H.; Dolan, H.L.; Ding, Q.; Wang, S.; Tikekar, R.V. Antimicrobial Action of Octanoic Acid against Escherichia coli O157:H7 during Washing of Baby Spinach and Grape Tomatoes. Food Res. Int. 2019, 125, 108523. [Google Scholar] [CrossRef]
- Sangeetha, J.; Asokan, S. Antibacterial activity of different sea grass Extracts against some human eye pathogens. World J. Pharm. Sci. 2015, 4, 677–683. [Google Scholar]
- Prabhakaran, S.; Rajaram, R.; Balasubramanian, V.; Mathivanan, K. Antifouling potentials of extracts from seaweeds, seagrasses and mangroves against primary biofilm forming bacteria. Asian Pac. J. Trop. Biomed. 2012, 2, S316–S322. [Google Scholar] [CrossRef]
- Ali, M.S.; Ravikumar, S.; Beula, J.M. Bioactivity of Seagrass against the Dengue Fever Mosquito Aedes aegypti Larvae. Asian Pac. J. Trop. Biomed. 2012, 2, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Kolenchenko, E.A.; Khotimchenko, M.Y.; Khozhaenko, E.V.; Khotimchenko, Y.S. Strontium Sorption by Pectins Isolated from the Sea Grasses Zostera marina and Phyllospadix iwatensis. Russ. J. Mar. Biol. 2012, 38, 346–350. [Google Scholar] [CrossRef]
- Athiperumalsami, T.; Kumar, V.; Jesudass, L.L. Survey and Phytochemical Analysis of Seagrasses in the Gulf of Mannar, Southeast Coast of India. Bot. Mar. 2008, 51, 269–277. [Google Scholar] [CrossRef]
- Kolsi, R.B.A.; Gargouri, B.; Sassi, S.; Frikha, D.; Lassoued, S.; Belghith, K. In Vitro Biological Properties and Health Benefits of a Novel Sulfated Polysaccharide Isolated from Cymodocea nodosa. Lipids Health Dis. 2017, 16, 252. [Google Scholar] [CrossRef] [PubMed]
- Purnomo, H.K.; Handayani, W.; Yasman, Y. Bioprospecting of Potential Seagrass Thalassia hemprichii (Ehrenb. Ex Solms) Asch. (Hydrocharitaceae) Extract from Pramuka Island against Aedes aegypti L. Larvae. In Proceedings of the 3rd International Symposium on Current Progress in Mathematics and Sciences 2017 (ISCPMS2017), Bali, Indonesia, 26–27 July 2017. [Google Scholar] [CrossRef]
- Bengen, D.G.; Khoeri, M.M.; Marhaeni, B.; Radjasa, O.K.; Sabdono, A.; Sudoyo, H. Antifouling Activity of Bacterial Symbionts of Seagrasses against Marine Biofilm-Forming Bacteria. J. Environ. Prot. 2011, 2, 1245–1249. [Google Scholar] [CrossRef]
- Hammami, S.; Ben Salem, A.; Ashour, M.L.; Cheriaa, J.; Graziano, G.; Mighri, Z. A Novel Methylated Sesquiterpene from Seagrass Posidonia oceanica (L.) Delile. Nat. Prod. Res. 2013, 27, 1265–1270. [Google Scholar] [CrossRef]
- Kalaivani, P.; Amudha, P. Identification of Bioactive Components in the Hydroalcoholic Extract of Syringodium isoetifolium and Assessment of Its Biological Activity by Gas Chromatography—Masspectrometry. Int. J. Res. Pharm. Sci. 2021, 12, 1168–1173. [Google Scholar] [CrossRef]
- Vijayalingam, T.A.; Rajesh, N.V. Seagrasses as Potential Source of Fodder for Livestock: Complete Proximate and Gas Chromatography-Mass Spectrometry (GCMS) Analysis. Ann. Phytomedicine Int. J. 2019, 8, 93–98. [Google Scholar] [CrossRef]
- Wisespongpand, P.; Khantavong, A.; Phothong, P.; Wanghom, W. Antimicrobial, Antioxidant, and Antifouling Activity from Extracts of Aboveground and Belowground Parts of Seagrasses Cymodocea rotundata and Cymodocea serrulata. J. Fish. Environ. 2022, 46, 37–53. [Google Scholar]
- Farid, M.M.; Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; Abdel-Hameed, E.S. Comparative study of Posidonia oceanica L.: LC/ESI/MS analysis, cytotoxic activity and chemosystematic significance. J. Mater. Environ. Sci. 2018, 9, 1676–1682. [Google Scholar] [CrossRef]
- Hawas, U.W.; Abou El-Kassem, L.T. Thalassiolin D: A New Flavone O-Glucoside Sulphate from the Seagrass Thalass. Hemprichii. Nat. Prod. Res. 2017, 31, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Abdelmohsen, M.; Hassanein, H.D.; Hassan, R.A.; Abreu, A.C.; Simões, M.; Nazif, N.M.; Abou-Setta, L.M. Phytochemical analysis, in vitro evaluation of antioxidant and antimicrobial activities of phenolic extracts from Posidonia oceanica (L.) Delile leaves. J. Chem. Pharm. Res. 2016, 8, 449–457. [Google Scholar]
- Alfattani, A.; Blanchet, E.; Da Silva, J.O.; Leoni, S.; Allard, P.; Queiroz, E.; Roy, M.; Chave, J.; Lami, R.; Perron, K.; et al. Bioactive Potential and Role of Secondary Metabolites within the Microorganism Community of the Sea Grass Posidonia oceanica. Planta Medica Int. Open 2017, 4, S1–S202. [Google Scholar] [CrossRef]
- Mettwally, W.S.; Ragab, T.I.; Hamdy, A.-H.A.; Helmy, W.A.; Hassan, S.A. Preliminary Study on the Possible Impact of Thalassodendron ciliatum (Forss.) Den Hartog Acidic Polysaccharide Fractions against TAA Induced Liver Failure. Biomed. Pharmacother. 2021, 138, 111502. [Google Scholar] [CrossRef]
- Piazzini, V.; Vasarri, M.; Degl’Innocenti, D.; Guastini, A.; Barletta, E.; Salvatici, M.C.; Bergonzi, M.C. Comparison of Chitosan Nanoparticles and Soluplus Micelles to Optimize the Bioactivity of Posidonia oceanica Extract on Human Neuroblastoma Cell Migration. Pharmaceutics 2019, 11, 655. [Google Scholar] [CrossRef]
- Delange, D.M.; Garcia, K.G.; Rivera, Y.H.; Suárez, Y.A.; Cuesta, R.G.; Riera-Romo, M.; Echemendia, O.; Dutra, L.M.; Almeida, J.R.G.D.S.; Pérez-Martínez, D.; et al. Chemical Composition and Biological Potential of a Chloroform Fraction from the Leaves of Marine Plant Syringodium filiforme Kützing. Pharmacogn. Mag. 2020, 16, 750. [Google Scholar] [CrossRef]
- Riera-Romo, M.; Marrero-Delange, D.; Hernandez-Balmaseda, I.; González, K.; Pérez-Martínez, D.; Manso, A.; Labrada, M.; Rodeiro, I. Chemical Characterization and Cytotoxic Potential of a Chloroform Fraction Obtained from Marine Plant Thalassia testudinum. J. Chromatogr. Sep. Tech. 2018, 9, 1–6. [Google Scholar] [CrossRef]
- Susilo, B.; Oktavianty, O.; Rahayu, F.; Handayani, M.L.W.; Rohim, A. Potential Transformation of Seagrass (Syringodium isoetifolium) into a Bioactive Food Ingredient Using Different Extraction Techniques. F1000Research 2023, 12, 1078. [Google Scholar] [CrossRef]
- Thinh, P.D.; Hang, C.T.T.; Trung, D.T.; Nguyen, T.-D. Pectin from Three Vietnamese Seagrasses: Isolation, Characterization and Antioxidant Activity. Processes 2023, 11, 1054. [Google Scholar] [CrossRef]
- Punginelli, D.; Vazzana, M.; Mauro, M.; Catania, V.; Arizza, V.; Schillaci, D. Antimicrobial activity from Polypeptide-rich extracts of the Seagrass Posidonia oceanica. J. Biol. Res. 2021, 94, 1826–8838. Available online: https://iris.unipa.it/handle/10447/523288 (accessed on 23 July 2024).
- Abruscato, G.; Chiarelli, R.; Lazzara, V.; Punginelli, D.; Sugár, S.; Mauro, M.; Librizzi, M.; Di Stefano, V.; Arizza, V.; Vizzini, A.; et al. In Vitro Cytotoxic Effect of Aqueous Extracts from Leaves and Rhizomes of the Seagrass Posidonia oceanica (L.) Delile on HepG2 Liver Cancer Cells: Focus on Autophagy and Apoptosis. Biology 2023, 12, 616. [Google Scholar] [CrossRef] [PubMed]
- Punginelli, D.; Catania, V.; Abruscato, G.; Luparello, C.; Vazzana, M.; Mauro, M.; Cunsolo, V.; Saletti, R.; Di Francesco, A.; Arizza, V.; et al. New Bioactive Peptides from the Mediterranean Seagrass Posidonia oceanica (L.) Delile and Their Impact on Antimicrobial Activity and Apoptosis of Human Cancer Cells. Int. J. Mol. Sci. 2023, 24, 5650. [Google Scholar] [CrossRef] [PubMed]
- Amiin, M.K.; Lahay, A.F. Anti-Bacterial Effectiveness of Cymodocea rotundata Extract and Assay for Primary Bioactive Composition. J. Aquatropica Asia 2023, 8, 6–12. [Google Scholar] [CrossRef]
- Kalaivani, P.; Amudha, P.; Chandramohan, A.; Vidya, R.; Prabhaharan, M.; Sasikumar, P.; Albukhaty, S.; Sulaiman, G.M.; Abomughaid, M.M.; Abu-Alghayth, M.H. Evaluation of Cytotoxic Activity of Syringodium isoetifolium against Human Breast Cancer Cell Line—An in Silico and in Vitro Study. Arab. J. Chem. 2023, 16, 105179. [Google Scholar] [CrossRef]
- Amutha, V.; Aiswarya, D.; Deepak, P.; Selvaraj, R.; Tamilselvan, C.; Perumal, P.; Balasubramani, G. Toxicity Potential Evaluation of Ethyl Acetate Extract of Cymodocea serrulata against the Mosquito Vectors Vis-a-Vis Zebrafish Embryos and Artemia salina Cysts. S. Afr. J. Bot. 2023, 152, 230–239. [Google Scholar] [CrossRef]
- Berfad, M.A.; Fahej, M.A.S.; Kumar, A.; Edrah, S. Preliminary phytochemical and antifungal studies of seagrass, Posidonia oceanica obtained from Mediterranean Sea of Libya. Int. J. Sci. Res. 2015, 4, 30–33. [Google Scholar]
- Phandee, S.; Buapet, P. Photosynthetic and Antioxidant Responses of the Tropical Intertidal Seagrasses Halophila ovalis and Thalassia hemprichii to Moderate and High Irradiances. Bot. Mar. 2018, 61, 247–256. [Google Scholar] [CrossRef]
- Sitania, M.; Kakisina, P.; Nindatu, M.; Moniharapon, M.; Kunda, R.M. Alveolar Performance of Mice (Mus musculus) Exposure to Cigarette Smoke After-Treatment of Ethanol Extract of Seagrass (Enhalus acoroides). In Proceedings of the 7th International Conference on Basic Sciences 2021 (ICBS 2021), Ambon, Indonesia, 23–24 November 2020. [Google Scholar] [CrossRef]
- Kavitha, D.; Padmini, R.; Chandravadivelu, G.; Magharla, D.D. Phytoconstituents Screening and Antioxidant Activity of Syringodium isoetifolium Leaf Extracts. Indian J. Pharm. Sci. 2022, 84, 1309–1322. [Google Scholar] [CrossRef]
- Dilipan, E.; Sivaperumal, P.; Kamala, K.; Ramachandran, M.; Vivekanandhan, P. Green Synthesis of Silver Nanoparticles Using Seagrass Cymodocea serrulata (R.Br.) Asch. & Magnus, Characterization, and Evaluation of Anticancer, Antioxidant, and Antiglycemic Index. Biotechnol. Appl. Biochem. 2023, 70, 1346–1356. [Google Scholar] [CrossRef]
- Narayanan, M.; Chanthini, A.; Devarajan, N.; Saravanan, M.; Sabour, A.; Alshiekheid, M.; Chi, N.T.L.; Brindhadevi, K. Antibacterial and Antioxidant Efficacy of Ethyl Acetate Extract of Cymodocea serrulata and Assess the Major Bioactive Components in the Extract Using GC-MS Analysis. Process Biochem. 2023, 124, 24–32. [Google Scholar] [CrossRef]
- Wagey, B.T.; Gunawan, W.B.; Lasabuda, R.; Mayulu, N.; Al Mahira, M.F.N.; Lailossa, D.G.; Nurkolis, F. New insight on antioxidants and anti-obesity properties of two Indonesian seagrass Thalassia hemprichii and Zostera marina: An integrated molecular docking simulation with in vitro study. F1000Research 2023, 12, 727. [Google Scholar] [CrossRef]
- Chaabani, E.; Rebey, I.B.; Wannes, W.A.; Ksouri, R.; Shili, A. Variability on the Phytochemical Composition, Antioxidant and Antimicrobial Activities of Ruppia cirrhosa Extract Using Two Different Methods of Extraction. Avicenna J. Clin. Microbiol. Infect. 2023, 10, 65–69. [Google Scholar] [CrossRef]
- Abd-Elraoof, W.A.; Tayel, A.A.; El-Far, S.W.; Mohamed, O.; Diab, A.M.; Abonama, O.M.; Assas, M.A.; Abdella, A. Characterization and Antimicrobial Activity of a Chitosan-Selenium Nanocomposite Biosynthesized Using Posidonia oceanica. RSC Adv. 2023, 13, 26001–26014. [Google Scholar] [CrossRef]
- Santoso, J.; Purwaningsih, S.; Ramadhan, W.; Noveliyana, Y. Bioactive Compounds, Phenol content and antioxidants activity of tropical seagrass Halodule pinifolia. Coast. Ocean. J. 2023, 7, 37–49. [Google Scholar] [CrossRef]
- Kevrekidou, A.; Assimopoulou, A.N.; Trachana, V.; Stagos, D.; Malea, P. Antioxidant Activity, Inhibition of Intestinal Cancer Cell Growth and Polyphenolic Compounds of the Seagrass Posidonia oceanica’s Extracts from Living Plants and Beach Casts. Mar. Drugs 2024, 22, 130. [Google Scholar] [CrossRef]
- Mabrouk, S.B.; Grami, B.; Ayache, S.B.; Kacem, A. Antioxidant, antimicrobial and analgesic activities of the invasive seagrass Halophila stipulacea leaf and stem extracts. Authorea Preprints 2024. [Google Scholar]
- Magdy, M.D.; Mohammed; Hamdy, A.-H.A.; El-Fiky, N.M.; Mettwally, W.S.A.; El-Beih, A.A.; Kobayashi, N. Anti-Influenza a Virus Activity of a New Dihydrochalcone Diglycoside Isolated from the Egyptian Seagrass Thalassodendron ciliatum (Forsk.) Den Hartog. Nat. Prod. Res. 2014, 28, 377–382. [Google Scholar] [CrossRef]
- Kumar, C.S.; Sarada, D.V.L.; Gideon, T.P.; Rengasamy, R. Antibacterial Activity of Three South Indian Seagrasses, Cymodocea serrulata, Halophila ovalis and Zostera capensis. World J. Microbiol. Biotechnol. 2008, 24, 1989–1992. [Google Scholar] [CrossRef]
- Hawas, U.W. A New 8-Hydroxy Flavone O-Xyloside Sulfate and Antibacterial Activity from the Egyptian Seagrass Thalassia hemprichii. Chem. Nat. Compd. 2014, 50, 629–632. [Google Scholar] [CrossRef]
- Berfad, M.A.; Alnour, T.M. Phytochemical analysis and antibacterial activity of the 5 different extract from the seagrasses Posidonia oceanica. J. Med. Plants Stud. 2014, 15, 15–18. [Google Scholar]
- Emmanuel Joshua Jebasingh, S.; Lakshmikandan, M.; Sivaraman, K.; Uthiralingam, M. Assessment of Antibacterial, Antifungal Property and Purification of Bioactive Compounds from Seagrass, Thalassia hemprichii. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 86, 905–910. [Google Scholar] [CrossRef]
- Mayavu, P.; Sugesh, S.; Ravindran, V.J. Antibacterial activity of seagrass species against biofilm forming bacteria. Res. J. Microbiol. 2009, 4, 314–319. [Google Scholar] [CrossRef]
- Kumar, S.R.; Ramanathan, G.; Subhakaran, M.; Inbaneson, S.J. Antimicrobial Compounds from Marine Halophytes for Silkworm Disease Treatment. Int. J. Med. Med. Sci. 2009, 1, 184–191. [Google Scholar]
- Krylova, N.V.; Leonova, G.N.; Maystrovskaya, O.S.; Popov, A.M.; Artyukov, A.A. Mechanisms of Antiviral Activity of the Polyphenol Complex from Seagrass of the Zosteraceae Family against Tick-Borne Encephalitis Virus. Bull. Exp. Biol. Med. 2018, 165, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Santoso, J.; Anwariyah, S.; Rumiantin, R.O.; Putri, A.P.; Ukhty, N.; Yoshie-Stark, Y. Phenol content, antioxidant activity and fibers profile of four tropical seagrasses from Indonesia. J. Coast. Dev. 2012, 15, 189–196. [Google Scholar]
- Jeyapragash, D.; Subhashini, P.; Raja, S.; Abirami, K.; Thangaradjou, T. Evaluation of In-Vitro Antioxidant Activity of Seagrasses: Signals for Potential Alternate Source. Free Radic. Antioxid. 2016, 6, 77–89. [Google Scholar] [CrossRef]
- Baehaki, A.; Widiastuti, I.; Nurul Jannah, H. Antioxidant Activity of Extracts of Halodule pinifolia Seagrass from Solvents with Different Polarities. Orient. J. Chem. 2017, 33, 181–185. [Google Scholar] [CrossRef]
- Girija, K.; Parthiban, C.; Hemalatha, A.; Saranya, C.; Anantharaman, P. Evaluation of antioxidant activities and preliminary phytochemical analysis of seagrasses Halodule pinifolia, Halophila ovalis and Syringodium isoetifolium. J. Phytochem. 2013, 114, 181–187. [Google Scholar]
- Ramalingam, N.; Ramakrishnan, K.; Krishnan, C.; Sankar, S. Phytochemical Analysis, HPTLC Finger Printing, in Vitro Antioxidant and Cytotoxic Activity of Cymodocea serrulata. Pharmacogn. J. 2013, 5, 238–241. [Google Scholar] [CrossRef]
- Messina, C.M.; Arena, R.; Manuguerra, S.; Pericot, Y.; Curcuraci, E.; Kerninon, F.; Renda, G.; Hellio, C.; Santulli, A. Antioxidant Bioactivity of Extracts from Beach Cast Leaves of Posidonia oceanica (L.) Delile. Mar. Drugs 2021, 19, 560. [Google Scholar] [CrossRef] [PubMed]
- El Shaffai, A.; Mettwally, W.S.A.; Mohamed, S.I.A. A Comparative Study of the Bioavailability of Red Sea Seagrass, Enhalus acoroides (L.f.) Royle (Leaves, Roots, and Rhizomes) as Anticancer and Antioxidant with Preliminary Phytochemical Characterization Using HPLC, FT-IR, and UPLC-ESI-TOF-MS Spectroscopic Analysis. Beni-Suef Univ. J. Basic Appl. Sci. 2023, 12, 1–12. [Google Scholar] [CrossRef]
- Prajoko, Y.W.; Qhabibi, F.R.; Gerardo, T.S.; Kizzandy, K.; Tanjaya, K.; Willyanto, S.E.; Permatasari, H.K.; Surya, R.; Mayulu, N.; Taslim, N.A.; et al. Revealing Novel Source of Breast Cancer Inhibitors from Seagrass Enhalus acoroides: In Silico and in Vitro Studies. Molecules 2024, 29, 1082. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Cheng, X.; Wink, M. Cytotoxicity of Arctigenin and Matairesinol against the T-Cell Lymphoma Cell Line CCRF-CEM. J. Pharm. Pharmacol. 2015, 67, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Vasarri, M.; Leri, M.; Barletta, E.; Ramazzotti, M.; Marzocchini, R.; Degl’Innocenti, D. Anti-Inflammatory Properties of the Marine Plant Posidonia oceanica (L.) Delile. J. Ethnopharmacol. 2020, 247, 112252. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.; Liu, Q.; Wu, Q.; Chen, S.; Wang, J.; Li, J.; Liu, L.; Gao, Z. Cyclohexenone Derivative and Drimane Sesquiterpenes from the Seagrass-Derived Fungus Aspergillus insuetus. Chem. Biodivers. 2023, 20, e202300424. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Nora Dahmash Al-Dahmash, G.K. Jhanani. Anti-Candida, Antioxidant and Antidiabetic Potential of Ethyl Acetate Extract Fraction-7a from Cymodocea serrulata and Its Bioactive Compound Characterization through FTIR and NMR. Environ. Res. 2023, 229, 115985. [Google Scholar] [CrossRef]
- Fatmawati, Y.; Sandrina, S.; Aina, R.N.; Narulita, E. Molecular Docking Analysis of Seagrass (Enhalus acoroides) Phytochemical Compounds as an Antidiabetic. J. Biol. Res. Boll. Della Soc. Ital. Biol. Sper. 2022, 95, 10224. [Google Scholar] [CrossRef]
- Baehaki, A.; Herpandi, H.H.; Lestari, S.; Hendri, M.; Ariska, F. Antidiabetic Activity with N-Hexane, Ethyl-Acetate and Ethanol Extract of Halodule uninervis Seagrass. Pharmacogn. J. 2020, 12, 805–808. [Google Scholar] [CrossRef]
- Ravikumar, S.; Vinoth, R.; Selvan, G.P. Bioactive Potential of a Seagrass Syringodium isoetifolium against Bacterial Fish Pathogens. J. Pharm. Res. 2011, 4, 1854–1856. [Google Scholar]
- Cornara, L.; Pastorino, G.; Borghesi, B.; Salis, A.; Clericuzio, M.; Marchetti, C.; Damonte, G.; Burlando, B. Posidonia oceanica (L.) Delile Ethanolic Extract Modulates Cell Activities with Skin Health Applications. Mar. Drugs 2018, 16, 21. [Google Scholar] [CrossRef] [PubMed]
- Vani, M.; Vasavi, T.; Uma, M.D.P. Evaluation of in Vitro Antidiabetic activity of Methanolic extract of Seagrass Halophila beccarii. Asian J. Pharm. Clin. Res. 2018, 11, 150. [Google Scholar] [CrossRef]
- Hua, K.-F.; Hsu, H.-Y.; Su, Y.-C.; Lin, I.-F.; Yang, S.-S.; Chen, Y.-M.; Chao, L.K. Study on the Antiinflammatory Activity of Methanol Extract from Seagrass Zostera japonica. J. Agric. Food Chem. 2005, 54, 306–311. [Google Scholar] [CrossRef]
- Hegazi, N.M.; Saad, H.; Marzouk, M.M.; Abdel, M.F.; El, H.; Zayed, A.; Ulber, R.; Ezzat, S.M. Molecular Networking Leveraging the Secondary Metabolomes Space of Halophila stipulaceae (Forsk.) Aschers. And Thalassia hemprichii (Ehrenb. Ex Solms) Asch. In Tandem with Their Chemosystematics and Antidiabetic Potentials. Mar. Drugs 2021, 19, 279. [Google Scholar] [CrossRef] [PubMed]
- Ghandourah, M.; Hawas, U.W.; Abou El-Kassem, L.T.; Shaher, F.M. Fatty Acids and Other Chemical Compositions of Some Seagrasses Collected from the Saudi Red Sea with Potential of Antioxidant and Anticancer Agents. Thalass. Int. J. Mar. Sci. 2020, 37, 13–22. [Google Scholar] [CrossRef]
- Sharma, H.; Stephen, N.M.; Gopal, S.S.; Udayawara Rudresh, D.; Kavalappa, Y.P.; Haranahalli Shivarudrappa, A.; Gavirangappa, H.; Ponesakki, G. Phenolic Extract of Seagrass, Halophila ovalis Activates Intrinsic Pathway of Apoptosis in Human Breast Cancer (MCF-7) Cells. Nutr. Cancer 2020, 73, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Balmaseda, I.; Guerra, I.R.; Declerck, K.; Isidrón, J.A.H.; Pérez-Novo, C.; Van Camp, G.; De Wever, O.; González, K.; Labrada, M.; Carr, A.; et al. Marine Seagrass Extract of Thalassia testudinum Suppresses Colorectal Tumor Growth, Motility and Angiogenesis by Autophagic Stress and Immunogenic Cell Death Pathways. Mar. Drugs 2021, 19, 52. [Google Scholar] [CrossRef]
- PPerumal, P.; Arthanari, U.; Sanniyasi, E. Phlorizin Isolated from Seagrass Syringodium isoetifolium Inhibits Diethylnitrosamine and Carbon Tetrachloride-Induced Hepatocellular Carcinoma in BALB/c Mice. S. Afr. J. Bot. 2023, 155, 1–15. [Google Scholar] [CrossRef]
- Narayanan, M.; Srinivasan, S.; Gnanasekaran, C.; Ramachandran, G.; Chelliah, C.K.; Rajivgandhi, G.; Maruthupandy, M.; Quero, F.; Li, W.-J.; Hayder, G.; et al. Synthesis and Characterization of Marine Seagrass (Cymodocea serrulata) Mediated Titanium Dioxide Nanoparticles for Antibacterial, Antibiofilm and Antioxidant Properties. Microb. Pathog. 2024, 189, 106595. [Google Scholar] [CrossRef]
- Hemmati, S.; Noveiry, B.B.; Keshavarz-Fathi, M. Cancer and Allergy; Molecular Association and Integrated Therapies. Immunol. Genet. J. 2021, 4, 171–185. [Google Scholar] [CrossRef]
- Bushehri, R.H.; Navabi, P.; Saeedifar, A.M.; Keshavarzian, N.; Rouzbahani, N.H.; Mosayebi, G.; Ghazavi, A.; Ghorban, K.; Ganji, A. Integration of phytotherapy and chemotherapy: Recent advances in anticancer molecular pathways. Iran. J. Basic Med. Sci. 2023, 26, 987–1000. [Google Scholar] [CrossRef]
- Mohammadi, A.; Rahbardar, M.G.; Hosseinzadeh, H. Antidotal and protective effects of mangosteen (Garcinia mangostana) against natural and chemical toxicities: A review. Iran. J. Basic Med. Sci. 2023, 26, 492. [Google Scholar] [CrossRef]
- Bertrand, B.; Morales-Martínez, A.; Hernández-Adame, P.L.; Muñoz-Garay, C. Multirresistencia antibióticos y alternativas para resolver esta crisis. Rev. Digit. Univ. 2023, 24, 3–10. [Google Scholar] [CrossRef]
- Varshney, M.; Kumar, B.; Rana, V.S.; Sethiya, N.K. An overview on therapeutic and medicinal potential of poly-hydroxy flavone viz. Heptamethoxyflavone, Kaempferitrin, Vitexin and Amentoflavone for management of Alzheimer’s and Parkinson’s diseases: A critical analysis on mechanistic insight. Crit. Rev. Food Sci. Nutr. 2023, 63, 2749–2772. [Google Scholar] [CrossRef]
- Javadi, B.; Sobhani, Z. Role of apigenin in targeting metabolic syndrome: A systematic review. Iran. J. Basic Med. Sci. 2024, 27, 524. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Thirunavukkarasu, N.; Govindarajulu, M.B.; Sasse, F.; Jansen, R.; Murali, T.S. Fungal Endophytes and Bioprospecting. Fungal Biol. Rev. 2009, 23, 9–19. [Google Scholar] [CrossRef]
- Nair, A.M.; Yesodharan, G.; Arun, K.; Prasad, G. Unveiling the factors influencing groundwater resources in a coastal environment-a review. Agron. Res. 2023, 21, 1487–1498. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Thirunavukkarasu, N.; Govindarajulu, M.B.; Gopalan, V. Fungal Endophytes: An Untapped Source of Biocatalysts. Fungal Divers. 2012, 54, 19–30. [Google Scholar] [CrossRef]
- Supaphon, P.; Phongpaichit, S.; Sakayaroj, J.; Rukachaisirikul, V.; Kobmoo, N.; Spatafora, J.W. Phylogenetic Community Structure of Fungal Endophytes in Seagrass Species. Bot. Mar. 2017, 60, 489–501. [Google Scholar] [CrossRef]
- Prasad, G.; Mamane, H.; Ramesh, M.V. Geogenic and anthropogenic contamination of groundwater in a fragile eco-friendly region of southern Kerala, India. Agron. Res. 2022, 20, 1072–1089. [Google Scholar] [CrossRef]
- Ravikumar, S.; Thajuddin, N.; Suganthi, P.; Jacob Inbaneson, S.; Vinodkumar, T. Bioactive potential of seagrass bacteria against human bacterial pathogens. J. Environ. Biol. 2010, 31, 387. [Google Scholar]
- Marhaeni, B.; Radjasa, O.K.; Bengen, D.G.; Kaswadji, R.F. Screening of Bacterial Symbionts of Seagrass Enhalus Sp. against biofilm-forming bacteria. J. Coast. Dev. 2024, 13, 126–132. [Google Scholar]
- Fitri, D.S.; Pangastuti, A.; Susilowati, A.; Sutarno, S. Endophytic Bacteria Producing Antibacterial against Methicillinresistant Staphylococcus aureus (MRSA) in Seagrass from Rote Ndao, East Nusa Tenggara, Indonesia. Biodiversitas J. Biol. Divers. 2017, 18, 733–740. [Google Scholar] [CrossRef]
- Cristianawati, O.; Sibero, M.T.; Ayuningrum, D.; Nuryadi, H.; Syafitri, E.; Riniarsih, I.O.; Radjasa, O.K. Screening of antibacterial activity of seagrass-associated bacteria from the North Java Sea, Indonesia against multidrug-resistant bacteria. AACL Bioflux 2019, 12, 1054–1064. [Google Scholar]
- Petersen, L.-E.; Marner, M.; Labes, A.; Tasdemir, D. Rapid Metabolome and Bioactivity Profiling of Fungi Associated with the Leaf and Rhizosphere of the Baltic Seagrass Zostera marina. Mar. Drugs 2019, 17, 419. [Google Scholar] [CrossRef]
- Ginting, E.L.; Maarisit, I.; Kemer, K.; Siby, M.S.; Tilaar, S.O.; Moko, E.M.; Tumbol, R.A. Isolation and Identification of Endophytic Bacteria from Seagrass Thalassia hemprichii as Antibacterial Producer. Appl. Biochem. Microbiol. 2023, 59, 673–678. [Google Scholar] [CrossRef]
- Ugarelli, K.; Jagels, A.; Choi, C.J.; Loesgen, S.; Stingl, U. Fungal Endophytes from Thalassia testudinum Show Bioactivity against the Seagrass Pathogen, Labyrinthula spp. Front. Mar. Sci. 2024, 11, 1359610. [Google Scholar] [CrossRef]
- Tasdemir, D.; Scarpato, S.; Utermann-Thüsing, C.; Jensen, T.; Blümel, M.; Wenzel-Storjohann, A.; Welsch, C.; Echelmeyer, V.A. Epiphytic and Endophytic Microbiome of the Seagrass Zostera marina: Do They Contribute to Pathogen Reduction in Seawater? Sci. Total. Environ. 2023, 908, 168422. [Google Scholar] [CrossRef]
- Goda, M.; Eltamany, E.E.; Habib, E.S.; Hassanean, H.; Ahmed, S.A.E.; Abdelhameed, R.F.; Ibrahim, A.K. B:Gas Chromatography-Mass Spectrometry Analysis of Marine Seagrass Thalassodendron ciliatum Collected from Red Sea. Rec. Pharm. Biomed. Sci./Rec. Pharm. Biomed. Sci. 2020, 4, 1–15. [Google Scholar] [CrossRef]
- Menaa, F.; Wijesinghe, U.; Thiripuranathar, G.; Althobaiti, N.A.; Albalawi, A.E.; Khan, B.A.; Menaa, B. Marine Algae-Derived Bioactive Compounds: A New Wave of Nanodrugs? Mar. Drugs 2021, 19, 484. [Google Scholar] [CrossRef] [PubMed]
- Grauso, L.; Li, Y.; Scarpato, S.; Shulha, O.; Rárová, L.; Strnad, M.; Teta, R.; Mangoni, A.; Zidorn, C. Structure and Conformation of Zosteraphenols, Tetracyclic Diarylheptanoids from the Seagrass Zostera marina: An NMR and DFT Study. Org. Lett. 2019, 22, 78–82. [Google Scholar] [CrossRef]
- Sarvesh, N.; Afeeza, K.; Suresh, V.; Dilipan, E. Development of the Antioxidant Property of Seagrass Extract-Based Hydrogel for Dental Application. Cureus 2024, 16, e54544. [Google Scholar] [CrossRef] [PubMed]
- Grauso, L.; Li, Y.; Scarpato, S.; Cacciola, N.A.; De Cicco, P.; Zidorn, C.; Mangoni, A. A Cytotoxic Heterodimeric Cyclic Diarylheptanoid with a Rearranged Benzene Ring from the Seagrass Zostera marina. J. Nat. Prod. 2022, 85, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Veettil, B.K.; Ward, R.D.; Lima, M.D.A.C.; Stankovic, M.; Hoai, P.N.; Quang, N.X. Opportunities for Seagrass Research Derived from Remote Sensing: A Review of Current Methods. Ecol. Indic. 2020, 117, 106560. [Google Scholar] [CrossRef]
- Davey, P.A.; Pernice, M.; Sablok, G.; Larkum, A.; Lee, H.T.; Golicz, A.; Edwards, D.; Dolferus, R.; Ralph, P. The Emergence of Molecular Profiling and Omics Techniques in Seagrass Biology; Furthering Our Understanding of Seagrasses. Funct. Integr. Genom. 2016, 16, 465–480. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Kuzhiumparambil, U.; Pernice, M.; Jiang, Z.; Ralph, P.J. Metabolomics: An Emerging Frontier of Systems Biology in Marine Macrophytes. Algal Res. 2016, 16, 76–92. [Google Scholar] [CrossRef]
- Griffiths, L.L.; Melvin, S.D.; Connolly, R.M.; Pearson, R.M.; Brown, C.J. Metabolomic Indicators for Low-Light Stress in Seagrass. Ecol. Indic. 2020, 114, 106316. [Google Scholar] [CrossRef]
- Arnold, T.M.; Targett, N.M. Marine tannins: The importance of a mechanistic framework for predicting ecological roles. J. Chem. Ecol. 2002, 28, 1919–1934. [Google Scholar] [CrossRef]
- Paul, V.J.; Arthur, K.E.; Ritson-Williams, R.; Ross, C.; Sharp, K. Chemical Defenses: From Compounds to Communities. Biol. Bull. 2007, 213, 226–251. [Google Scholar] [CrossRef]
- Sieg, R.D.; Kubanek, J. Chemical Ecology of Marine Angiosperms: Opportunities at the Interface of Marine and Terrestrial Systems. J. Chem. Ecol. 2013, 39, 687–711. [Google Scholar] [CrossRef] [PubMed]
- Zupo, V.; Maibam, C.; Buia, M.C.; Gambi, M.C.; Patti, F.P.; Scipione, M.B.; Lorenti, M.; Fink, P. Chemoreception of the Seagrass Posidonia oceanica by Benthic Invertebrates Is Altered by Seawater Acidification. J. Chem. Ecol. 2015, 41, 766–779. [Google Scholar] [CrossRef] [PubMed]
- Mutalipassi, M.; Fink, P.; Maibam, C.; Porzio, L.; Buia, M.C.; Gambi, M.C.; Patti, F.P.; Scipione, M.B.; Lorenti, M.; Zupo, V. Ocean Acidification Alters the Responses of Invertebrates to Wound-Activated Infochemicals Produced by Epiphytes of the Seagrass Posidonia oceanica. J. Exp. Mar. Biol. Ecol. 2020, 530–531, 151435. [Google Scholar] [CrossRef]




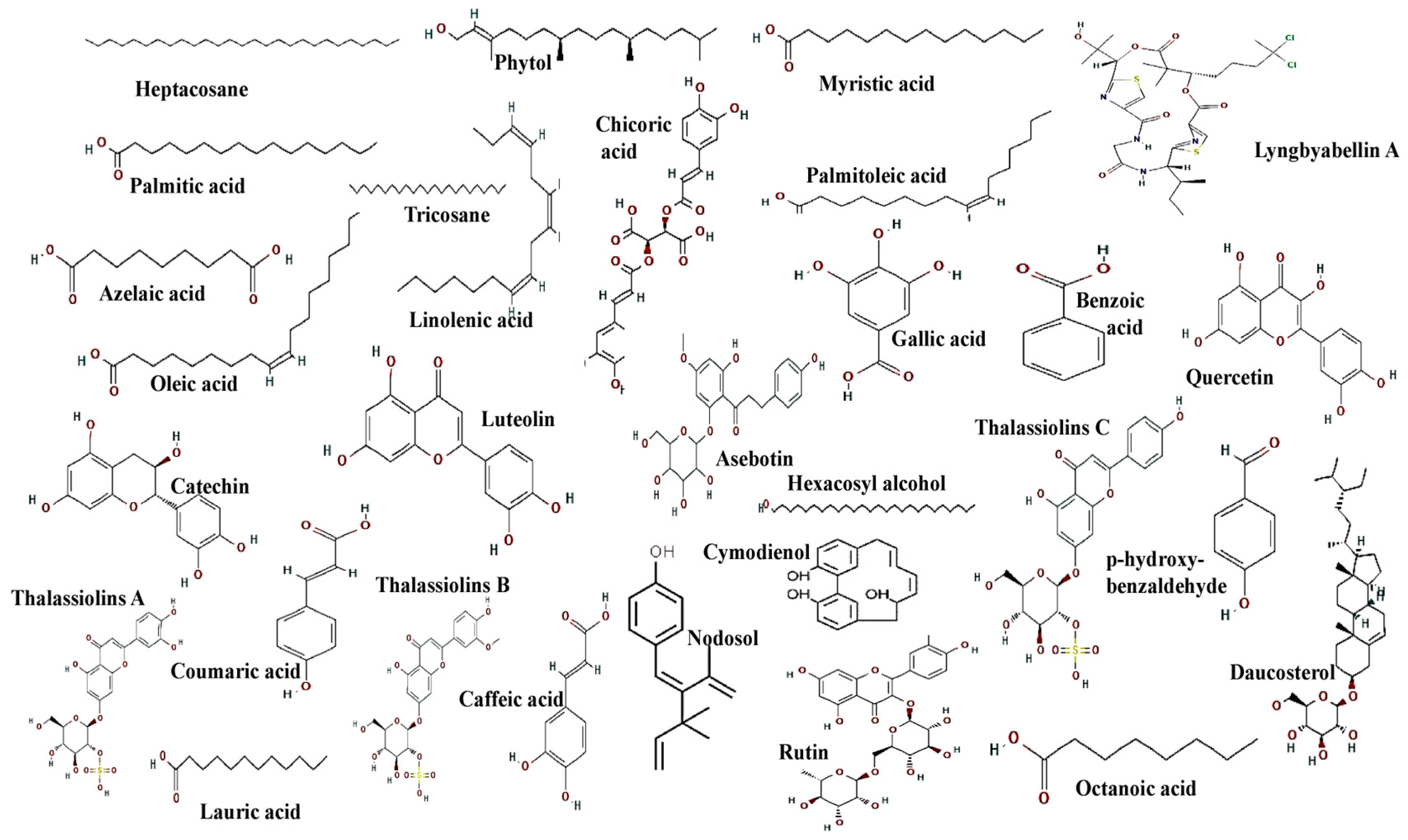

| Compound Name | Seagrass | Bioactivity | References |
|---|---|---|---|
| Caffeic acid | Posidonia oceanica, Zostera marina, Thalasia testudinum, Thalasia hemprichii, Thalassodendron ciliatum | Antioxidant, Antiviral and Cytotoxic | [64,65] |
| Enhalus acoroides | Antifeedant | [66] |
| Enhalus acoroides | Antibacterial and antilarval activities | [66] |
| Cymodocea nodosa | Antibacterial activity against methicillin-resistant (MRSA) strains | [67] |
| Coumaric acid | Posidonia oceanica, Zostera marina; Thalassia testudinum, Halodule pinifolia, Thalassia hemprichii | Antioxidant activity | [68] |
| Thalassiolins A (luteolin 7-O-ß-D-glucopyranosyl-2′-sulfate) | Thalassia hemprichii Thalasia testudinum | Anti-HIV activity and Antifouling activity | [56,69] |
| Thalassiolins B | Thallasia testudinum, Thallasia hemprichii | Anti-HIV activity, skin-regenerating activities and antioxidant | [56,69] |
| Thalassiolins C | Thallasia testudinum | Anti-HIV activity | [69] |
| Thalassodendron ciliatum | Antioxidant, Antiviral and Cytotoxicity against cancer cell lines | [70] |
| Thalassodendron ciliatum | Cytotoxic activity against HepG2 and MCF7 cells | [71] |
| Luteolin | Halophila stipulacea | Anticancerous activity | [72] |
| Quercetin | Zostera marina L., Zostera noltii | Antioxidant activity | [73] |
| Benzoic acid | Thallasia testudinum Syringodium filiforme | Antioxidant activity | [74] |
| Gallic acid | Posidonia oceanica | Radical scavenging activity | [75] |
| Chicoric acid | Posidonia oceanica | Antioxidant activity | [76] |
| Azelaic acid | Syringodium filiforme Thalassia testudinum | Antioxidant activity | [77] |
| Palmitoleic acid | Syringodium filiforme | Anti-inflammatory activity | [78] |
| Tricosane | Syringodium filiforme | Antibacterial | [79] |
| Oleic acid Linolenic acid Myristic acid Palmitic acid | Halophila ovalis | Antibacterial | [80] |
| Phytol | Thalassia hemprichii Enhalus acoroides Cymodocea serrulata Cymodocea rotundata | Antibacterial | [81] |
| Lyngbyabellin A | Halophila stipulacea | Anticancerous | [72] |
| Heptacosane | Enhalus acoroides | Antioxidant | [82] |
| Lauric acid | Syringodium filiforme Thalassia testudinum | Antibacterial | [83] |
| Octanoic acid | Syringodium filiforme Thalassia testudinum | Antibacterial | [84] |
| Solvent | Seagrass Species | Biological Activity | Reference |
|---|---|---|---|
| Chloroform, ethyl acetate, ethanol and hexane | Halophila ovalis, Cymodocea serrulata, Halodule pinifolia | Antimicrobial | [85] |
| Ethanol, methanol | Halodule pinifolia, Cymodocea serrulata | Antimicrobial | [86] |
| Methanol | Halophila ovalis | Antimicrobial | [58] |
| Aqueous ethanol | Syringodium isoetifolium, Cymodocea serrulata, Halophila beccarii | Larvicidal | [87] |
| Pectin | Zostera marina, Phyllospadix iwatensis | Cerium binding activity | [88] |
| Methanol | Halodule pinifolia, Halophila ovalis, Syringodium isoetifolium, Thallasia hemprichii, Cymodocea serrulata | Antioxidant | [89] |
| Ethanol | Enhalus acoroides, Halophila ovalis, Halophila ovata, Halophila stipulacea, Thalassia hemprichii, Syringodium isoetifolium, Cymodocea serrulata, Halodule pinifolia | Antibacterial | [49] |
| Methanol | Syringodium isoetifolium | Antioxidant | [43] |
| Sulfated polysaccharide | Cymodocea nodosa | Antioxidant, antimicrobial and cytotoxic properties | [90] |
| Methanol | Thalassia hemprichii | Larvicidal | [91] |
| n-hexane | Thalassia hemprichii, and Enhalus acoroides | Antifouling | [92] |
| Chloroform | Posidonia oceanica | Antimicrobial | [93] |
| Hydroalcoholic | Syringodium isoetifolium | Antibacterial, antifungal, antimicrobial, antifouling and anticancerous | [94] |
| Ethanol | Cymodocea serrulata, Syringodium isoetifolium and Enhalus acoroides | Nutritional supplements | [95] |
| 70% Acetone | Cymodocea rotundata and Cymodocea serrulata | Antimicrobial, antifouling and antioxidant | [96] |
| Methanol | Enhalus acoroides and Halophila ovalis | Antifungal | [46] |
| MeOH/H2O(7:3) | Posidonia oceanica | Antiviral (against H5N1) | [97] |
| Methanol | Thalassia hemprichii | Antiviral | [98] |
| Aqueous EtOH | Posidonia oceanica | Antioxidant and antimicrobial | [99] |
| Ethyl acetate | Posidonia oceanica | Antimicrobial | [100] |
| Hexane and methanol | Halophila stipulacea | Cytotoxic activity, lipid-reducing activity, and antifouling activity. | [72] |
| 85% Ethanol | Thalassodendron ciliatum | Antioxidant and hapatoprotective activity | [101] |
| EtOH/H2O (7:3) | Posidonia oceanica | Anticancerous | [102] |
| Chloroform | Syringodium filiforme | Anticancerous | [103] |
| Chloroform | Thalassia testudinum | Anticancerous | [104] |
| 50% Ethanol | Syringodium isoetifolium | Anti-inflammatory | [105] |
| Ethanol | Enhalus acoroides, Thalassia hemprichii, and Halophila ovalis. | Antioxidant | [106] |
| Acetic acid | Posidonia oceanica | Antimicrobial | [107] |
| Water | Posidonia oceanica | Anticancerous | [108] |
| Acetic acid | Posidonia oceanica | Antimicrobial and anticancerous | [109] |
| 96% Ethanol | Cymodocea Rotundata | Antibacterial | [110] |
| 70% Ethanol | Syringodium isoetifolium | Anticancerous | [111] |
| Ethyl acetate | Cymodocea serrulata | Larvicidal | [112] |
| Water | Posidonia oceanica | Antifungal | [113] |
| Hydroalcoholic | Enhalus acoroides | Antioxidant | [82] |
| Ethanol | Thalassia hemprichii and Halophila ovalis | Antioxidant | [114] |
| Ethanol | Enhalus acoroides | Antioxidant | [115] |
| Water, alcohol, hydro alcohol, acetone and n-hexane. | Syringodium isoetifolium | Antioxidant | [116] |
| Ethanol | Cymodocea serrulata | Antidiabetic, anticancerous and antioxidant activity | [117] |
| Ethyl acetate | Cymodocea serrulata | Antibacterial and antioxidant | [118] |
| Ethanol | Thalassia hemprichii and Zostera marina | Antioxidant and antiobesity activity | [119] |
| Hexane, petroleum ether and acetone | Ruppia cirrhosa | Antioxidant and antimicrobial | [120] |
| 70% ethanol | Posidonia oceanica | Antimicrobial activity | [121] |
| Ethyl acetate | Halodule pinifolia | Antioxidant activity | [122] |
| Methanol | Posidonia oceanica | Antioxidant activity and anticancerous activity | [123] |
| Ethyl acetate, hexane and methanol | Halophila stipulacea | Antioxidant, antimicrobial and analgesic activity | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ameen, H.M.; Jayadev, A.; Prasad, G.; Nair, D.I. Seagrass Meadows: Prospective Candidates for Bioactive Molecules. Molecules 2024, 29, 4596. https://doi.org/10.3390/molecules29194596
Ameen HM, Jayadev A, Prasad G, Nair DI. Seagrass Meadows: Prospective Candidates for Bioactive Molecules. Molecules. 2024; 29(19):4596. https://doi.org/10.3390/molecules29194596
Chicago/Turabian StyleAmeen, Hazeena M., Ayona Jayadev, Geena Prasad, and Deepa Indira Nair. 2024. "Seagrass Meadows: Prospective Candidates for Bioactive Molecules" Molecules 29, no. 19: 4596. https://doi.org/10.3390/molecules29194596





