Development of a Method for the Determination of Rifaximin and Rifampicin Residues in Foods of Animal Origin
Abstract
:1. Introduction
2. Results
2.1. Optimization of Chromatographic Conditions and Mass Spectrum Conditions
2.2. Optimization of Extraction Procedure
2.3. Method Validation
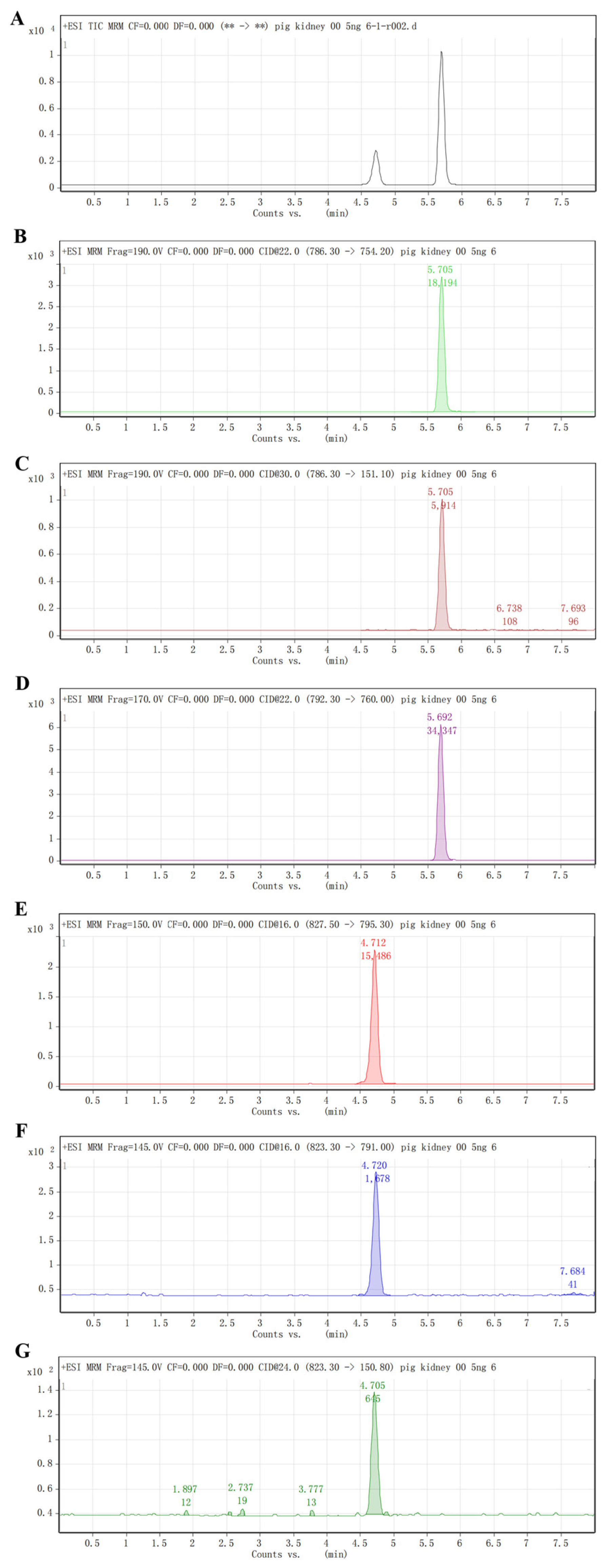
2.3.1. Specificity
2.3.2. Sensitivity
2.3.3. Matrix Effects
2.3.4. Linearity Range
2.3.5. Accuracy and Precision
2.3.6. Stability of the Stock Solution
3. Discussion
4. Method and Materials
4.1. Reagent and Apparatus
4.2. Solution Preparation
4.2.1. Standard Solution
4.2.2. Other Solution
4.3. Sample Preparation
4.4. HPLC–MS/MS Analysis
4.4.1. Chromatographic Condition
4.4.2. Mass Spectrometer Conditions
4.5. Investigation of Method
4.5.1. Investigation of Specificity
4.5.2. Investigation of Sensitivity
4.5.3. Investigation of Matrix Effects
4.5.4. Investigation of Linearity Range
4.5.5. Investigation of Accuracy and Precision
4.5.6. Investigation of Stability of Standard Stock Solution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- China National Veterinary Drug Basic Database/Veterinary Drug Product Approval Document Number Data. Available online: http://vdts.ivdc.org.cn:8081/cx/# (accessed on 23 September 2024).
- China Ministry of Agriculture Announcement No. 560. Veterinary Drugs Local Standard Abolition Catalogue, 2005.10.28. Available online: http://www.moa.gov.cn/gk/tzgg_1/gg/200511/t20051117_496523.htm (accessed on 23 September 2024).
- Huang, Y.; Deng, Y.; Jiang, L.; Tan, A.; Xue, H.; Wang, W.; Luo, L.; Zhao, F. Antimicrobial resistance and homology analysis in Aeromonas isolated from integrated fish farms. J. Fish. Sci. China 2014, 21, 777–785. [Google Scholar]
- Wu, Y.; Deng, Y.; Jiang, L.; Tan, A.; Xue, H.; Wang, W.; Luo, L.; Zhao, F. Antimicrobial susceptibilities of Aeromonas strains isolated from various aquatic animals in Guangdong Province. J. Shanghai Ocean Univ. 2013, 22, 219–224. [Google Scholar]
- Liang, R. Investigation on Antibacterial Drug Resistance of Three Swine Source Pathogens and Influences Factors in Guangdong Province. Ph.D. Thesis, South China Agricultural University, Chuangzhou, China, 2020. [Google Scholar]
- Tang, Y.; Lou, X.; Yang, G.; Tian, L.; Wang, Y.; Huang, X. Occurrence and human health risk assessment of antibiotics in cultured fish from 19 provinces in China. Front. Cell. Infect. Microbiol. 2022, 12, 964283. [Google Scholar] [CrossRef] [PubMed]
- SN/T 2224-2008; Determination of Rifaximin Residue in Foodstuffs of Animal Origin for Export and Import—LC-MS/MS Method. Implementation 2009-06-01. General Administration of Quality Supervision, Inspection and Quarantine of the People Republic of China: Beijing, China, 2008.
- GB 31659.5-2022; National Food Safety Standard—Determination of Rifaximin Residue in Milk by Liquid Chromatography-Tandem Mass Spectrometric Method. Implementation 2023-02-01. Ministry of Agriculture and Rural Affairs: Beijing, China, 2022.
- Zhang, Y.; Wang, Q.; Wu, Y. Rapid determination of rifaximin in milk by dispersive solid phase extraction coupled with liquid chromatography-tandem mass spectrometry. J. Food Saf. Qual. 2019, 10, 3049–3053. [Google Scholar]
- Li, P.; Zhang, X.; Zhang, S.; Yan, Z.; Chen, S.; Fang, Y.; Long, J. Screening and Confirmation of Mult-class of Veterinary Drug residues in Fish Muscle by Pass-Through SPE Purification and Ultra Performance Liquid Chromatography Coupled with Quadrupole/Time of Flight Mass Spectrometry. J. Zhejiang Ocean Univ. 2017, 36, 228–334. [Google Scholar]
- Zhu, S.; Zhang, Y.; Liu, Z.; Qian, B.C.; Li, B.; Miao, X.Q. Detection of Rifampicin in Fishery Products by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry with Dispersive Solid Phase extraction. Fish. Sci. 2020, 39, 425–430. [Google Scholar]
- Liu, J.; Jiang, L.; Zhang, J.; Huang, H.; Li, X.; Xu, F. Residue of Rifaximin in Milk of Lactating Dairy Cows Following Administration of Intrauterine Infusion. IOP Conf. Ser. Earth Environ. Sci. 2021, 697, 012001. [Google Scholar] [CrossRef]
- Li, Q.; Cao, J.; Han, G.; Liu, H.; Yan, J.; Wu, L.; Li, J. Quantitative determination of rifampicin in aquatic products by stable isotope-dilution high liquid chronatography-tandem mass spectrometry. Biomed. Chromatogr. 2020, 34, e4810. [Google Scholar] [CrossRef]
- (EU) No 37/2010 on Pharmacologically Active Substances and Their Classification Regarding Maximum Residue Limits in Foodstuffs of Animal Origin. Available online: http://down.foodmate.net/standard/sort/44/28726.html (accessed on 26 September 2024).
- Yu, X. Dynamic Study on the In Vivo Oxidation-Reduction Cycles of Polyphenols Rifampicin. Master’s Thesis, China Medical University, Shenyang, China, 2018. [Google Scholar]
- Tollefson, L.; Miller, M.A. Antibiotic use in food animals: Controlling the human health impact. J. AOAC Int. 2000, 83, 245–254. [Google Scholar] [CrossRef]
- Virto, M.; Santamarina-García, G.; Amores, G.; Hernández, I. Antibiotics in dairy production: Where is the problem? Dairy 2022, 3, 541–564. [Google Scholar] [CrossRef]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef] [PubMed]
- Mortier, L.; Daeseleire, E.; Van Peteghem, C. Determination of the ionophoric coccidiostats narasin, monensin, lasalocid and salinomycin in eggs by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass. Spectrom. 2005, 19, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Rama, A.; Lucatello, L.; Benetti, C.; Galina, G.; Bajraktari, D. Assessment of antibacterial drug residues in milk for consumption in Kosovo. J. Food Drug Anal. 2017, 25, 525–532. [Google Scholar] [CrossRef]
- Van Dresser, W.R.; Wilcke, J.R. Drug residues in food animals. J. Am. Vet. Med. Assoc. 1989, 194, 1700–1710. [Google Scholar] [PubMed]
- Guest, G.B.; Paige, J.C. The magnitude of the tissue residue problem with regard to consumer needs. J. Am. Vet. Med. Assoc. 1991, 198, 805–808. [Google Scholar] [CrossRef]
- Rana, M.S.; Lee, S.Y.; Kang, H.J.; Hur, S.J. Reducing Veterinary Drug Residues in Animal Products: A Review. Food Sci. Anim. Resour. 2019, 39, 687–703. [Google Scholar] [CrossRef]
- Lindemayr, H.; Knobler, R.; Kraft, D.; Baumgartner, W. Challenge of penicillin-allergic volunteers with penicillin-contaminated meat. Allergy 1981, 36, 471–478. [Google Scholar] [CrossRef]
- Kanny, G.; Puygrenier, J.; Beaudoin, E.; Moneret-Vautrin, D.A. Choc anaphylactique alimentaire: Implication des résidus de pénicilline [Alimentary anaphylactic shock: Implication of penicillin residues]. Allerg. Immunol. 1994, 26, 181–183. (In French) [Google Scholar]
- Molina, A.; Molina, M.P.; Althaus, R.L.; Gallego, L. Residue persistence in sheep milk following antibiotic therapy. Vet. J. 2003, 165, 84–89. [Google Scholar] [CrossRef]
- Payne, M.A.; Craigmill, A.; Riviere, J.E.; Webb, A.I. Extralabel use of penicillin in food animals. J. Am. Vet. Med. Assoc. 2006, 229, 1401–1403. [Google Scholar] [CrossRef]
- Berruga, M.I.; Molina, A.; Althaus, R.L.; Molina, M.P. Control and prevention of antibiotic residues and contaminants in sheep and goat’s milk. Small Rumin. Res. 2016, 142, 38–43. [Google Scholar] [CrossRef]
- Riffat, I.; Shah, A. Electrochemical and optical protocols for the detection and removal of an antibiotic drug rifaximin from wastewater. RSC Adv. 2024, 14, 22867–22876. [Google Scholar] [CrossRef]
- Reddy, V.K.; Babji, P.M.R.; Chitta, R. Validation of the HPLC–PDA method for detection of eluxadoline and rifaximin in rat plasma and application in a pharmacokinetic study. Future J. Pharm. Sci. 2022, 8, 14. [Google Scholar]
- Challa, B.; Kotaiah, M.; Chandu, B.; Chandrasekhar, K.B.; Kanchanamala, K.; Parveen SK, R.; Micheal, F. HPLC Method for Determination of Rifaximin in Human Plasma Using Tandem Mass Spectrometry Detection. East Cent. Afr. J. Pharm. Sci. 2010, 13, 3. [Google Scholar]
- Arumugam, J.; Shanmugam, G.; Venkatesan, M.; Sreedhar, S. Synergistic activity of copper and molybdenum based organic frameworks for the exceptional electrochemical detection of rifampicin in commercial dairy products. Microchem. J. 2024, 199, 110115. [Google Scholar] [CrossRef]
- Tang, X.; Zhao, Y.; Yu, H.; Cui, S.; Temple, H.; Amador, E.; Gao, Y.; Chen, M.-L.; Wang, S.; Hu, Z.; et al. Concentration-regulated multi-color fluorescent carbon dots for the detection of rifampicin, morin and Al3+. Mater. Today Adv. 2023, 18, 100383. [Google Scholar] [CrossRef]
- Pharmacopoeia of the People’s Republic of China. Guidelines for the Verification of Quantitative Analysis Methods of Biological Samples in General Rule 9012. Available online: https://ydz.chp.org.cn/#/search?content=9012 (accessed on 23 September 2024).
- Ministry of Agriculture and Rural Affairs of PRC. Guidelines for the Elimination of Veterinary Drug Residues; Notice No. 326 of the Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2020.
- GB/T 30891-2014; Practice of Sampling Plans for Aquatic Products: Appendix B. China. Implementation 2015-03-01. General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2014.
- 2002/657/EC, Official Journal of the European Communities L221 (2002) 8. Available online: http://down.foodmate.net/standard/sort/44/22814.html (accessed on 23 September 2024).
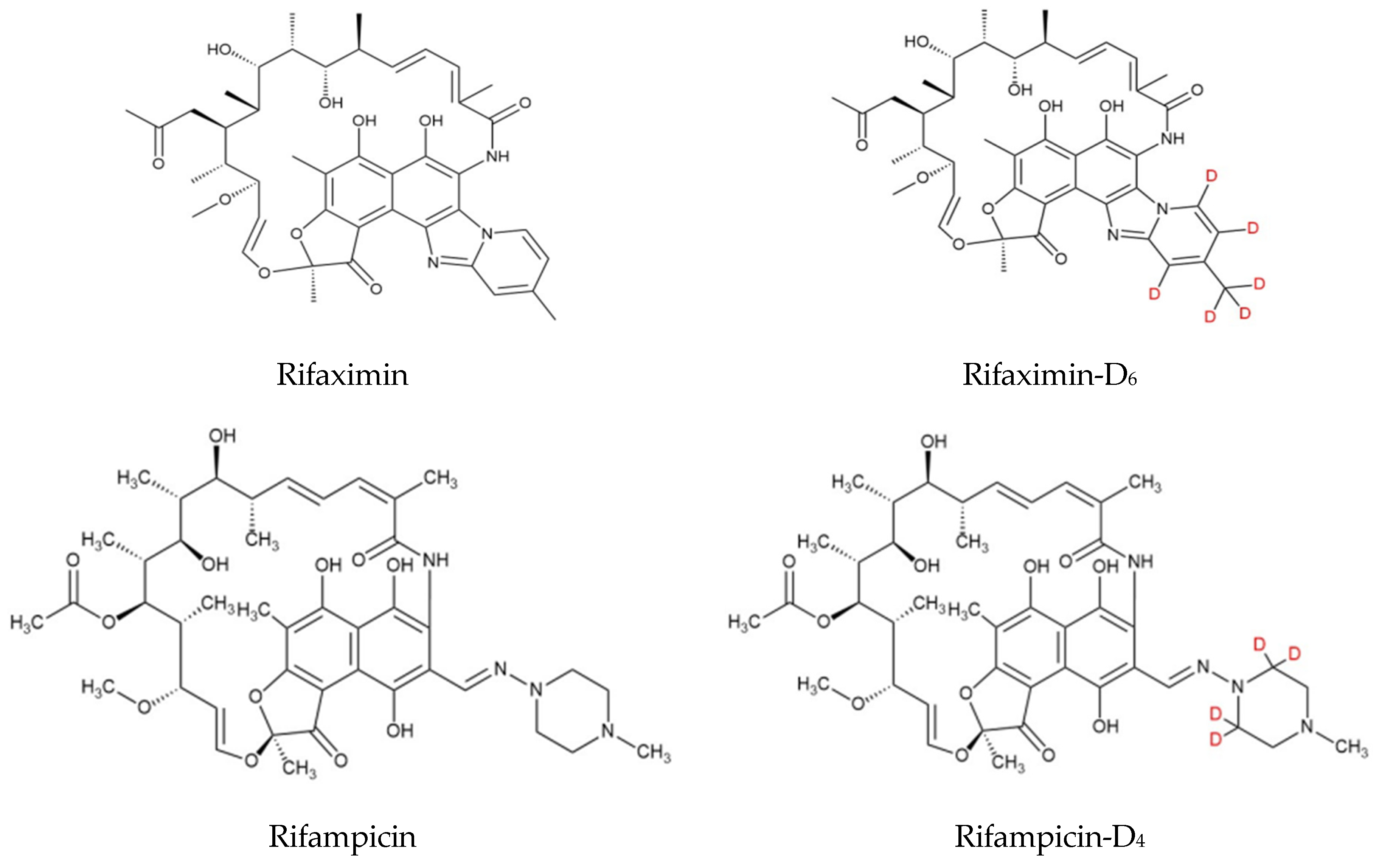
| Time (min) | A% | B% | Flow Rate (mL/min) |
|---|---|---|---|
| 0 | 10 | 90 | 0.4 |
| 1.0 | 10 | 90 | 0.4 |
| 2.0 | 70 | 30 | 0.4 |
| 4.0 | 70 | 30 | 0.4 |
| 5.5 | 10 | 90 | 0.4 |
| 8.0 | 10 | 90 | 0.4 |
| Compound | Prec Ion m/z | Prod Ion m/z | Fragmentor V | CE eV | Polarity |
|---|---|---|---|---|---|
| rifaximin | 786.3 | 754.2 * | 190 | 22 | + |
| 786.3 | 151.1 | 190 | 30 | + | |
| rifaximin-D6 | 792.3 | 760.0 * | 170 | 22 | + |
| rifampicin | 823.3 | 791.0 * | 145 | 16 | + |
| 823.3 | 150.8 | 145 | 24 | + | |
| rifampicin-D4 | 827.5 | 795.3 * | 150 | 16 | + |
| Dosage of VC | Method of Concentration | Recovery % | |
|---|---|---|---|
| Rifaximin | Rifampicin | ||
| 20 mg | dry with nitrogen in water bath | 96.7 | 95.3 |
| 50 mg | 101.2 | 95.7 | |
| 70 mg | 98.1 | 94.4 | |
| Tissue | Acetonitrile-Dichloromethane (6 + 4) | Acetonitrile | Methanol-Acetonitrile (3 + 7) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Dispersion | Protein Precipitation | Supernatant Extract Transfer | Dispersion | Protein Precipitation | Supernatant Extract Transfer | Dispersion | Protein Precipitation | Supernatant Extract Transfer | |
| fat | √ | √ | × | √ | √ | √ | √ | √ | × |
| egg | √ | + | √ | √ | √ | √ | √ | + | × |
| milk | √ | + | √ | √ | √ | √ | √ | + | × |
| shrimp | √ | √ | √ | × | √ | √ | × | + | × |
| fish | √ | √ | √ | + | √ | √ | √ | + | × |
| muscle | √ | √ | √ | + | √ | √ | √ | + | × |
| liver | √ | √ | √ | + | √ | √ | √ | + | × |
| kidney | √ | √ | √ | + | √ | √ | √ | + | × |
| Animal | Tissue | Result % | |||||
|---|---|---|---|---|---|---|---|
| LOD 5 μg/kg | LOQ 10 μg/kg | 2LOQ 20 μg/kg | MRL 60 μg/kg | 2MRL 120 μg/kg | 3MRL 180 μg/kg | ||
| cattle | muscle | 105.6 | 102.1 | 97.6 | 105.8 | 99.5 | 108.6 |
| fat | 105.2 | 97.4 | 95.3 | 102.0 | 99.7 | 102.1 | |
| liver | 98.5 | 99.0 | 93.8 | 96.0 | 108.0 | 101.1 | |
| kidney | 94.9 | 97.1 | 97.4 | 104.0 | 97.1 | 98.6 | |
| milk | 96.9 | 103.1 | 97.0 | 95.8 | 100.1 | 93.7 | |
| swine | muscle | 107.6 | 99.9 | 100.5 | 96.1 | 103.0 | —— |
| kidney | 101.5 | 90.8 | 97.6 | 104.0 | 92.6 | 92.8 | |
| skin fat | 103.0 | —— | 102.2 | 92.3 | 96.2 | 91.4 | |
| chicken | muscle | 101.2 | 104.7 | 98.3 | 102.7 | 100.7 | 117.6 |
| skin fat | 102.8 | 98.0 | 98.6 | 100.0 | 102.0 | 92.6 | |
| liver | 100.6 | 96.1 | 98.5 | 102.2 | 98.4 | 99.3 | |
| egg | 99.4 | 101.9 | 106.0 | 109.2 | 106.5 | 99.1 | |
| fish | skin muscle | 91.0 | 96.2 | 94.5 | 88.7 | 95.6 | 96.9 |
| shrimp | muscle | 99.2 | 98.3 | 101.4 | 101.7 | 102.4 | 100.4 |
| Animal | Tissue | Result % | |||||
|---|---|---|---|---|---|---|---|
| LOD 5 μg/kg | LOQ 10 μg/kg | 2LOQ 20 μg/kg | MRL 60 μg/kg | 2MRL 120 μg/kg | 3MRL 180 μg/kg | ||
| cattle | muscle | 118.3 | 111.1 | 104.1 | 111.9 | 94.9 | 101.6 |
| fat | 115.7 | 107.3 | 101.0 | 108.4 | 104.1 | 101.8 | |
| liver | 98.3 | 104.7 | 95.2 | 98.3 | 106.4 | 102.6 | |
| kidney | 99.0 | 105.9 | 101.1 | 105.0 | 95.4 | 99.5 | |
| milk | 109.5 | 106.3 | 103.1 | 95.1 | 103.1 | 96.1 | |
| swine | muscle | 118.8 | —— | 109.5 | 127.1 | 93.4 | 98.8 |
| kidney | 98.0 | 86.0 | 91.6 | 103.2 | 93.5 | 100.1 | |
| skin fat | 112.4 | —— | 113.8 | 105.7 | 97.9 | 89.5 | |
| chicken | muscle | 113.6 | 117.7 | 97.3 | 107.9 | 92.2 | 108.3 |
| skin fat | 107.9 | 112.3 | 96.5 | 95.4 | 97.9 | 85.9 | |
| liver | 95.1 | 90.5 | 91.1 | 103.7 | 94.9 | 99.2 | |
| egg | 110.2 | 113.1 | 113.7 | 113.7 | 105.7 | 101.0 | |
| fish | skin muscle | 104.0 | 98.3 | 99.7 | 87.6 | 98.1 | 89.0 |
| shrimp | muscle | 103.1 | 100.9 | 103.0 | 98.0 | 103.5 | 102.8 |
| Compound | Linearity Range | Weight | Standard Curve Equation | R2 |
|---|---|---|---|---|
| rifaximin | 10 μg/kg–300 μg/kg | 1/x | y = 1.7726x + 0.0457 | 0.9960 |
| y = 1.9007x − 0.0667 | 0.9991 | |||
| y = 1.6562x + 0.0244 | 0.9991 | |||
| y = 2.0479x + 0.0343 | 0.9990 | |||
| y = 2.4088x − 0.2063 | 0.9991 | |||
| y = 1.7781x − 0.0112 | 0.9901 | |||
| y = 2.5775x − 0.2717 | 0.9986 | |||
| rifampicin | 10 μg/kg–300 μg/kg | 1/x2 | y = 0.7500x − 0.0338 | 0.9981 |
| y = 0.8112x − 0.0561 | 0.9965 | |||
| y = 0.6285x − 0.0642 | 0.9967 | |||
| y = 0.6683x − 0.0490 | 0.9941 | |||
| y = 0.4351x − 0.0250 | 0.9961 | |||
| y = 0.6330x − 0.0061 | 0.9937 | |||
| y = 0.4885x − 0.1870 | 0.9920 |
| Concentration | Compound | 1 Month % | 2 Month % |
|---|---|---|---|
| 1 mg/mL | rifaximin | 102.5 | 93.8 |
| rifampicin | 97.6 | 98.0 | |
| 100 μg/mL | rifaximin | 94.1 | 109.3 |
| rifampicin | 94.0 | 97.6 |
| Concentration | Compound | 7 d % | 21 d % | 30 d % |
|---|---|---|---|---|
| 2 μg/mL | rifaximin | 98.6 | 101.5 | 100.1 |
| rifampicin | 101.0 | 100.0 | 94.7 | |
| 36 μg/mL | rifaximin | 98.8 | 98.8 | 97.7 |
| rifampicin | 100.6 | 97.7 | 93.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, L.-P.; Tao, Q.; Wang, X.-Q.; Xu, X.-H.; Yang, Y.-J.; Li, J.-Y. Development of a Method for the Determination of Rifaximin and Rifampicin Residues in Foods of Animal Origin. Molecules 2024, 29, 4599. https://doi.org/10.3390/molecules29194599
Fan L-P, Tao Q, Wang X-Q, Xu X-H, Yang Y-J, Li J-Y. Development of a Method for the Determination of Rifaximin and Rifampicin Residues in Foods of Animal Origin. Molecules. 2024; 29(19):4599. https://doi.org/10.3390/molecules29194599
Chicago/Turabian StyleFan, Li-Ping, Qi Tao, Xiao-Qiao Wang, Xiao-Hui Xu, Ya-Jun Yang, and Jian-Yong Li. 2024. "Development of a Method for the Determination of Rifaximin and Rifampicin Residues in Foods of Animal Origin" Molecules 29, no. 19: 4599. https://doi.org/10.3390/molecules29194599







