Unveiling the Exquisite Microstructural Details in Zebrafish Brain Non-Invasively Using Magnetic Resonance Imaging at 28.2 T
Abstract
:1. Introduction
2. Results and Discussion
2.1. Relaxation Times
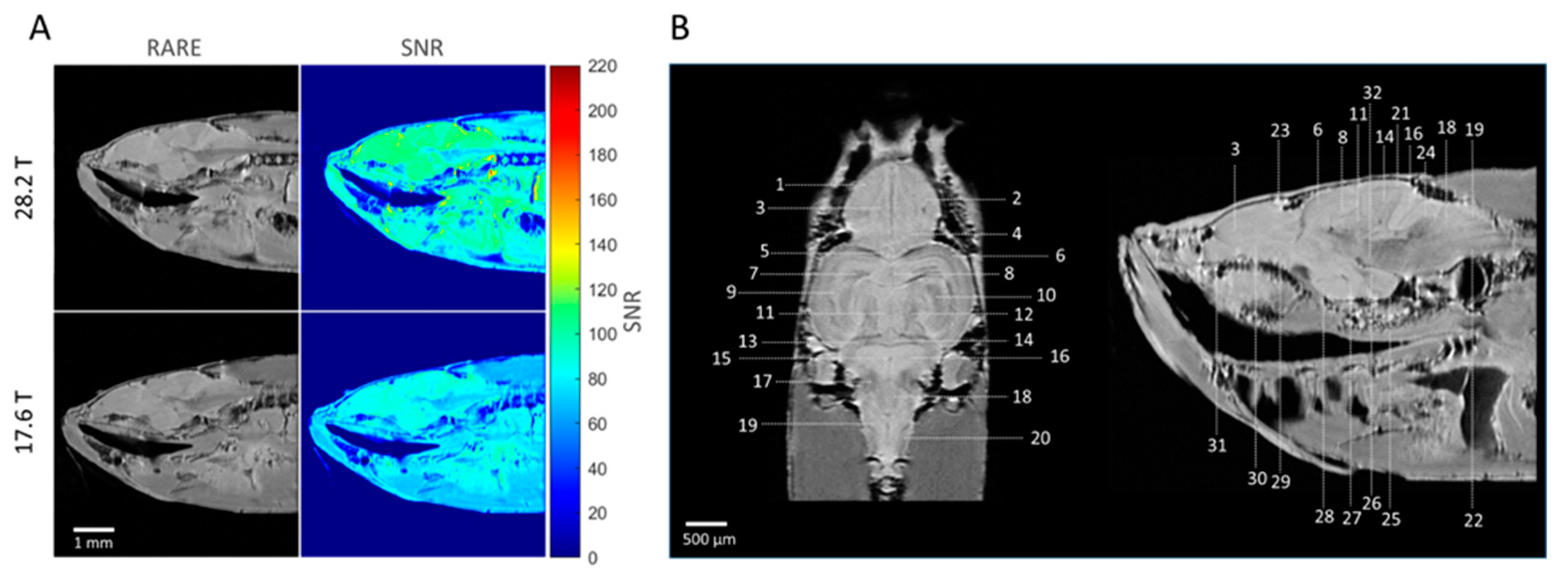
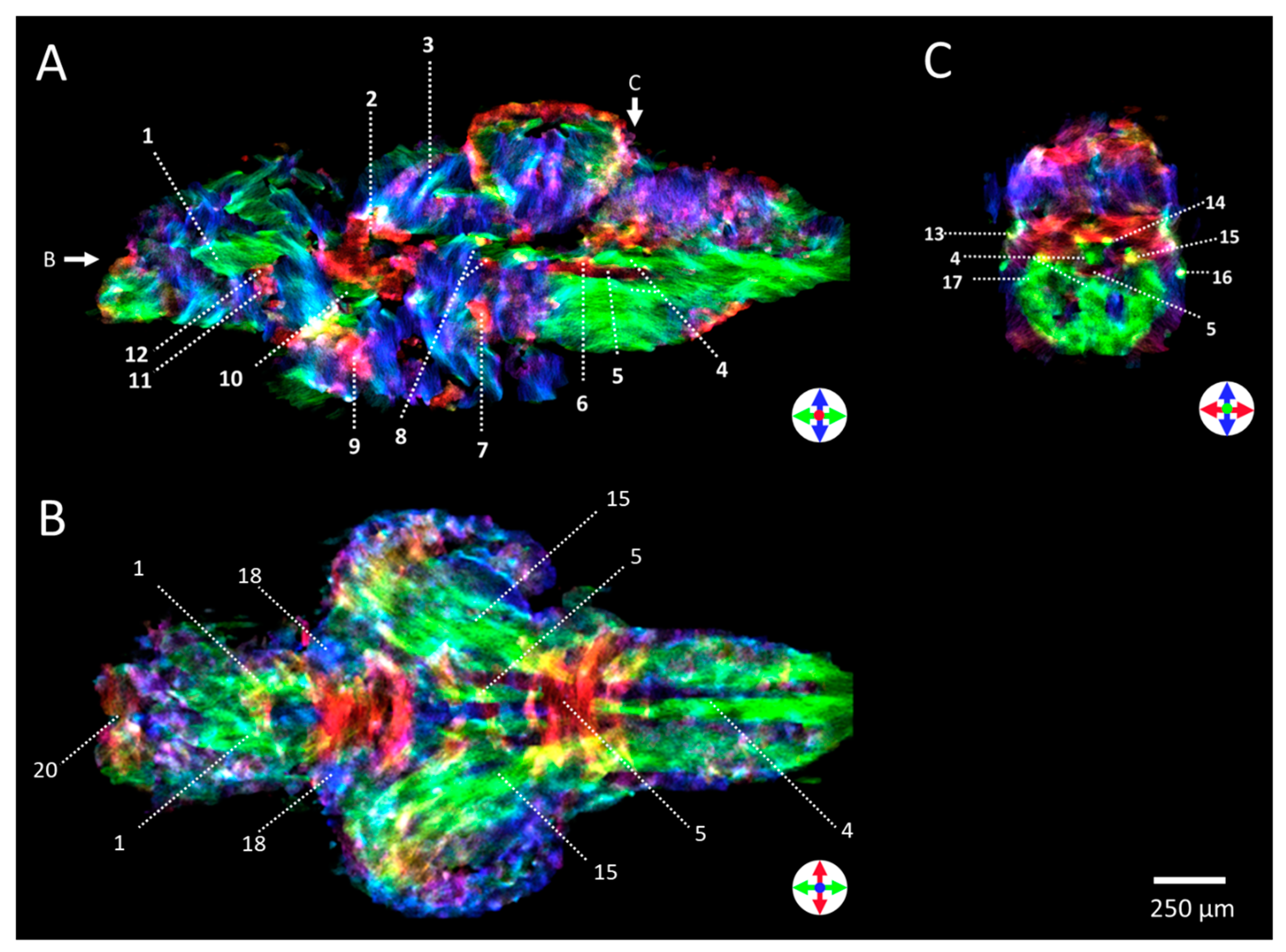
2.2. Anatomical Imaging
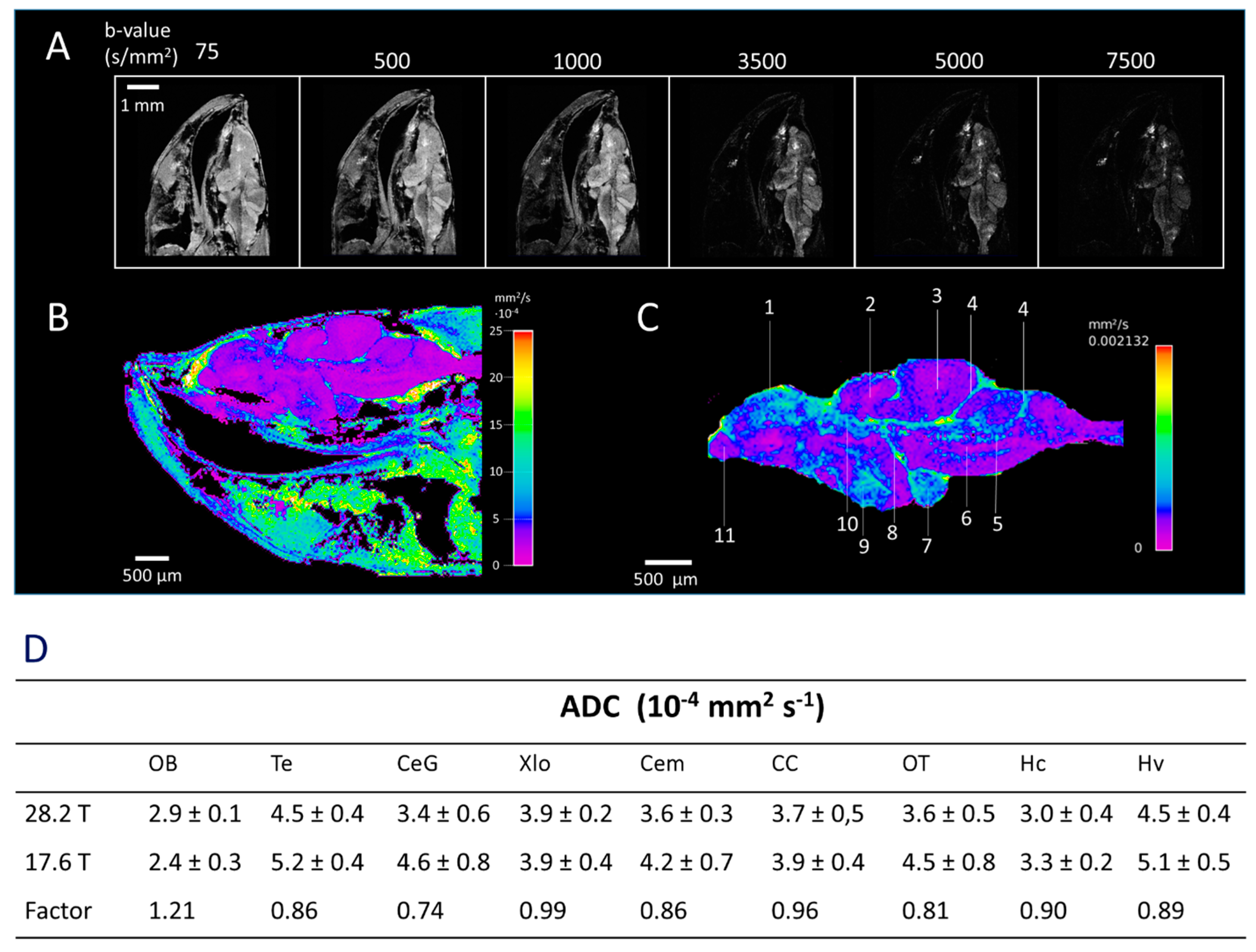
2.3. Diffusion Weighted Imaging (DWI)
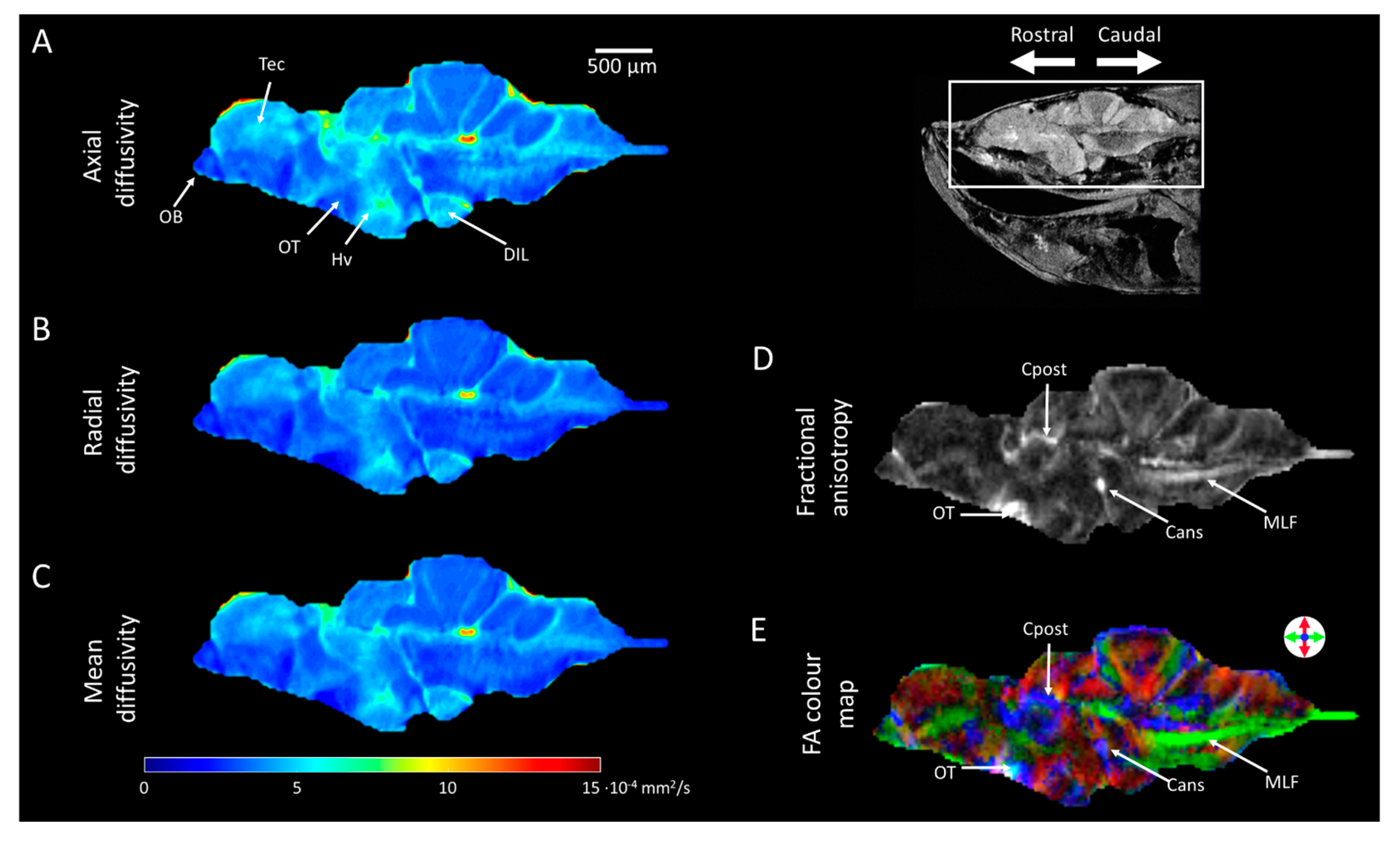
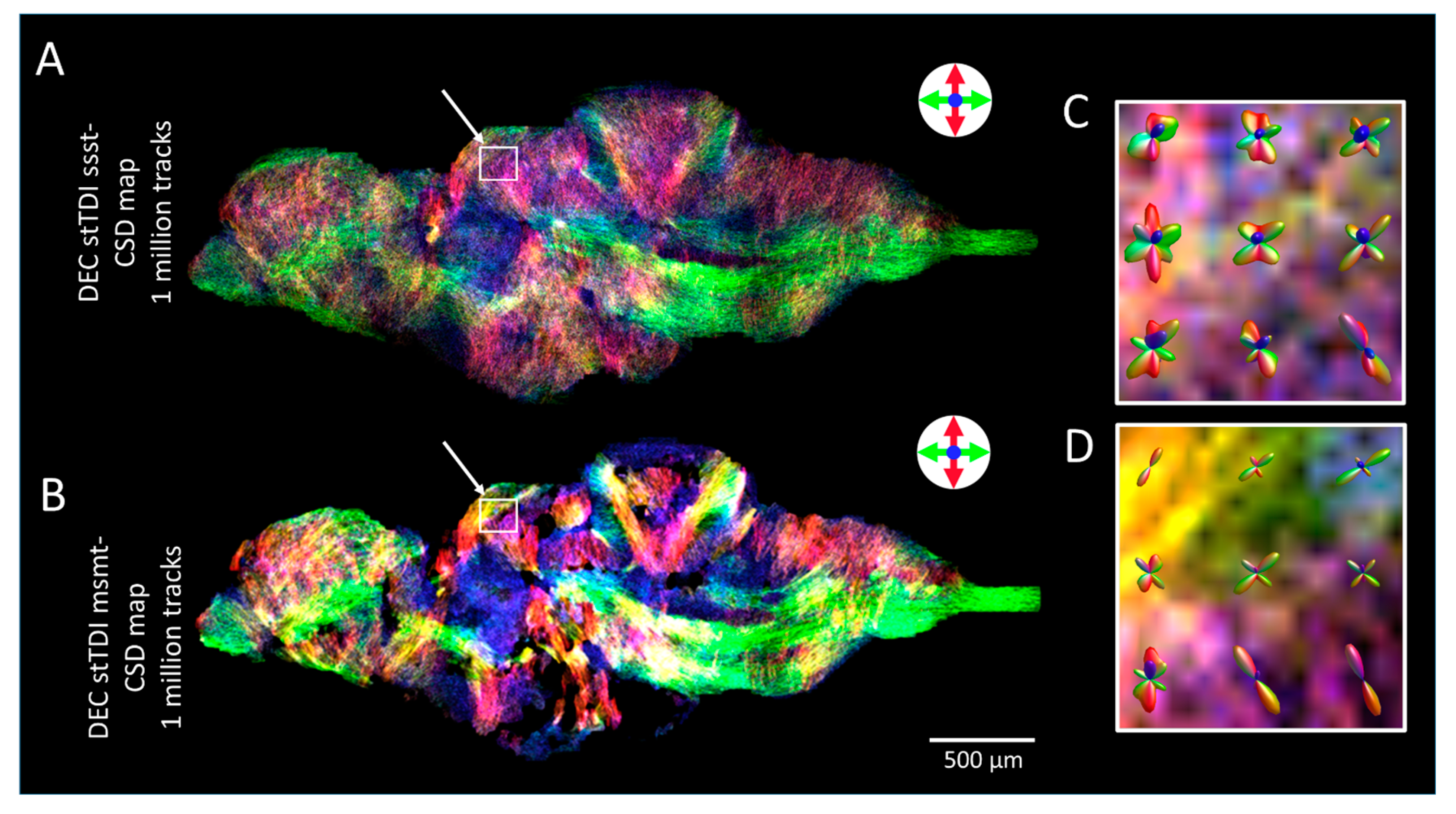
2.4. Diffusion Tensor Imaging (DTI)
3. Materials and Methods
3.1. Zebrafish Husbandry
3.2. Magnetic Resonance Imaging
3.3. Data Processing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailone, R.L.; Aguiar, L.K.d.; Roca, R.d.O.; Borra, R.C.; Corrêa, T.; Janke, H.; Fukushima, H.C.S. Zebrafish as an animal model for food safety research: Trends in the animal research. Food Biotechnol. 2019, 33, 283–302. [Google Scholar] [CrossRef]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Haks, M.C.; Forn-Cuni, G.; He, J.; Nowik, N.; Harms, A.C.; Hankemeier, T.; Eeza, M.N.H.; Matysik, J.; Alia, A.; et al. Metabolomic and transcriptomic profiling of adult mice and larval zebrafish leptin mutants reveal a common pattern of changes in metabolites and signaling pathways. Cell Biosci. 2021, 11, 126. [Google Scholar] [CrossRef]
- Bashirzade, A.A.; Zabegalov, K.N.; Volgin, A.D.; Belova, A.S.; Demin, K.A.; de Abreu, M.S.; Babchenko, V.Y.; Bashirzade, K.A.; Yenkoyan, K.B.; Tikhonova, M.A.; et al. Modeling neurodegenerative disorders in zebrafish. Neurosci. Biobehav. Rev. 2022, 138, 104679. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, J.B.; He, K.J.; Wang, F.; Liu, C.F. Advances of Zebrafish in Neurodegenerative Disease: From Models to Drug Discovery. Front. Pharmacol. 2021, 12, 713963. [Google Scholar] [CrossRef]
- Wang, J.; Cao, H. Zebrafish and Medaka: Important Animal Models for Human Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 10766. [Google Scholar] [CrossRef]
- Kabli, S.; Alia, A.; Spaink, H.P.; Verbeek, F.J.; De Groot, H.J. Magnetic resonance microscopy of the adult zebrafish. Zebrafish 2006, 3, 431–439. [Google Scholar] [CrossRef]
- Haud, N.; Kara, F.; Diekmann, S.; Henneke, M.; Willer, J.R.; Hillwig, M.S.; Gregg, R.G.; Macintosh, G.C.; Gartner, J.; Alia, A.; et al. rnaset2 mutant zebrafish model familial cystic leukoencephalopathy and reveal a role for RNase T2 in degrading ribosomal RNA. Proc. Natl. Acad. Sci. USA 2011, 108, 1099–1103. [Google Scholar] [CrossRef]
- Ramirez, I.B.; Pietka, G.; Jones, D.R.; Divecha, N.; Alia, A.; Baraban, S.C.; Hurlstone, A.F.; Lowe, M. Impaired neural development in a zebrafish model for Lowe syndrome. Hum. Mol. Genet. 2012, 21, 1744–1759. [Google Scholar] [CrossRef]
- Kabli, S.; Spaink, H.P.; De Groot, H.J.; Alia, A. In vivo metabolite profile of adult zebrafish brain obtained by high-resolution localized magnetic resonance spectroscopy. J. Magn. Reson. Imaging 2009, 29, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Eeza, M.N.H.; Singer, R.; Ding, Y.; He, J.; Zuberi, Z.; Baelde, H.J.; de Groot, H.J.M.; Matysik, J.; Spaink, H.P.; Alia, A. Probing microstructural changes in muscles of leptin-deficient zebrafish by non-invasive ex-vivo magnetic resonance microimaging. PLoS ONE 2023, 18, e0284215. [Google Scholar] [CrossRef]
- Kabli, S.; He, S.; Spaink, H.P.; Hurlstone, A.; Jagalska, E.S.; De Groot, H.J.; Alia, A. In vivo magnetic resonance imaging to detect malignant melanoma in adult zebrafish. Zebrafish 2010, 7, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Alexander, A.L.; Lee, J.E.; Lazar, M.; Field, A.S. Diffusion tensor imaging of the brain. Neurotherapeutics 2007, 4, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Auning, E.; Kjaervik, V.K.; Selnes, P.; Aarsland, D.; Haram, A.; Bjornerud, A.; Hessen, E.; Esnaashari, A.; Fladby, T. White matter integrity and cognition in Parkinson’s disease: A cross-sectional study. BMJ Open 2014, 4, e003976. [Google Scholar] [CrossRef]
- Bede, P.; Elamin, M.; Byrne, S.; McLaughlin, R.L.; Kenna, K.; Vajda, A.; Fagan, A.; Bradley, D.G.; Hardiman, O. Patterns of cerebral and cerebellar white matter degeneration in ALS. J. Neurol. Neurosurg. Psychiatry 2014, 86, 468–470. [Google Scholar] [CrossRef]
- de Schipper, L.J.; Hafkemeijer, A.; Bouts, M.; van der Grond, J.; Marinus, J.; Henselmans, J.M.L.; van Hilten, J.J. Age- and disease-related cerebral white matter changes in patients with Parkinson’s disease. Neurobiol. Aging 2019, 80, 203–209. [Google Scholar] [CrossRef]
- Gold, B.T.; Johnson, N.F.; Powell, D.K.; Smith, C.D. White matter integrity and vulnerability to Alzheimer’s disease: Preliminary findings and future directions. Biochim. Biophys. Acta 2012, 1822, 416–422. [Google Scholar] [CrossRef]
- Huang, P.; Xu, X.; Gu, Q.; Xuan, M.; Yu, X.; Luo, W.; Zhang, M. Disrupted white matter integrity in depressed versus non-depressed Parkinson’s disease patients: A tract-based spatial statistics study. J. Neurol. Sci. 2014, 346, 145–148. [Google Scholar] [CrossRef]
- Kantarci, K.; Murray, M.E.; Schwarz, C.G.; Reid, R.I.; Przybelski, S.A.; Lesnick, T.; Zuk, S.M.; Raman, M.R.; Senjem, M.L.; Gunter, J.L.; et al. White-matter integrity on DTI and the pathologic staging of Alzheimer’s disease. Neurobiol. Aging 2017, 56, 172–179. [Google Scholar] [CrossRef]
- Novak, M.J.; Seunarine, K.K.; Gibbard, C.R.; Hobbs, N.Z.; Scahill, R.I.; Clark, C.A.; Tabrizi, S.J. White matter integrity in premanifest and early Huntington’s disease is related to caudate loss and disease progression. Cortex 2014, 52, 98–112. [Google Scholar] [CrossRef] [PubMed]
- Reading, S.A.; Yassa, M.A.; Bakker, A.; Dziorny, A.C.; Gourley, L.M.; Yallapragada, V.; Rosenblatt, A.; Margolis, R.L.; Aylward, E.H.; Brandt, J.; et al. Regional white matter change in pre-symptomatic Huntington’s disease: A diffusion tensor imaging study. Psychiatry Res. 2005, 140, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Stricker, N.H.; Schweinsburg, B.C.; Delano-Wood, L.; Wierenga, C.E.; Bangen, K.J.; Haaland, K.Y.; Frank, L.R.; Salmon, D.P.; Bondi, M.W. Decreased white matter integrity in late-myelinating fiber pathways in Alzheimer’s disease supports retrogenesis. Neuroimage 2009, 45, 10–16. [Google Scholar] [CrossRef] [PubMed]
- van der Burgh, H.K.; Westeneng, H.J.; Walhout, R.; van Veenhuijzen, K.; Tan, H.H.G.; Meier, J.M.; Bakker, L.A.; Hendrikse, J.; van Es, M.A.; Veldink, J.H.; et al. Multimodal longitudinal study of structural brain involvement in amyotrophic lateral sclerosis. Neurology 2020, 94, e2592–e2604. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, X.; Zhao, L.; Evans, A.C.; Song, L.; Xie, B.; Li, H.; Luo, C.; Wang, J. Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. J. Neurol. 2014, 261, 412–421. [Google Scholar] [CrossRef]
- Abe, O.; Yamada, H.; Masutani, Y.; Aoki, S.; Kunimatsu, A.; Yamasue, H.; Fukuda, R.; Kasai, K.; Hayashi, N.; Masumoto, T.; et al. Amyotrophic lateral sclerosis: Diffusion tensor tractography and voxel-based analysis. NMR Biomed. 2004, 17, 411–416. [Google Scholar] [CrossRef]
- Agosta, F.; Pagani, E.; Petrolini, M.; Caputo, D.; Perini, M.; Prelle, A.; Salvi, F.; Filippi, M. Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: A diffusion tensor MR imaging tractography study. AJNR Am. J. Neuroradiol. 2010, 31, 1457–1461. [Google Scholar] [CrossRef]
- Kitamura, S.; Kiuchi, K.; Taoka, T.; Hashimoto, K.; Ueda, S.; Yasuno, F.; Morikawa, M.; Kichikawa, K.; Kishimoto, T. Longitudinal white matter changes in Alzheimer’s disease: A tractography-based analysis study. Brain Res. 2013, 1515, 12–18. [Google Scholar] [CrossRef]
- Kok, J.G.; Leemans, A.; Teune, L.K.; Leenders, K.L.; McKeown, M.J.; Appel-Cresswell, S.; Kremer, H.P.H.; de Jong, B.M. Structural Network Analysis Using Diffusion MRI Tractography in Parkinson’s Disease and Correlations With Motor Impairment. Front. Neurol. 2020, 11, 841. [Google Scholar] [CrossRef]
- Lo, C.Y.; Wang, P.N.; Chou, K.H.; Wang, J.; He, Y.; Lin, C.P. Diffusion tensor tractography reveals abnormal topological organization in structural cortical networks in Alzheimer’s disease. J. Neurosci. 2010, 30, 16876–16885. [Google Scholar] [CrossRef]
- Phillips, O.; Sanchez-Castaneda, C.; Elifani, F.; Maglione, V.; Di Pardo, A.; Caltagirone, C.; Squitieri, F.; Sabatini, U.; Di Paola, M. Tractography of the corpus callosum in Huntington’s disease. PLoS ONE 2013, 8, e73280. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, I.W.; Buckley, S.; Coffey, C.S.; Foster, E.; Mendick, S.; Seibyl, J.; Schuff, N. Diffusion tensor imaging of the nigrostriatal fibers in Parkinson’s disease. Mov. Disord. 2015, 30, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Freidlin, R.Z.; Morris, H.D.; Horkay, F.; Pierpaoli, C.; Toyama, R.; Dawid, I.B.; Basser, P.J. Diffusion Tensor MR Microscopy of Adult Zebrafish. Proc. Intl. Soc. Mag. Reson. Med. 2004, 11, 1755. [Google Scholar]
- Ullmann, J.F.; Calamante, F.; Collin, S.P.; Reutens, D.C.; Kurniawan, N.D. Enhanced characterization of the zebrafish brain as revealed by super-resolution track-density imaging. Brain Struct. Funct. 2015, 220, 457–468. [Google Scholar] [CrossRef]
- Dhollander, T.; Raffelt, D.; Connelly, A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. In Proceedings of the ISMRM Workshop on Breaking the Barriers of Diffusion MRI, Lisbon, Portugal, 11–16 September 2016. [Google Scholar]
- Dhollander, T.; Raffelt, D.; Mito, R.; Connelly, A. Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. In Proceedings of the 27th International Society of Magnetic Resonance in Medicine, Montréal, QC, Canada, 11–16 May 2019. [Google Scholar]
- Jeurissen, B.; Tournier, J.D.; Dhollander, T.; Connelly, A.; Sijbers, J. Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 2014, 103, 411–426. [Google Scholar] [CrossRef]
- Singer, R.; Oganezova, I.; Hu, W.; Liu, L.; Ding, Y.; de Groot, H.J.M.; Spaink, H.P.; Alia, A. Ultrahigh field diffusion magnetic resonance imaging uncovers intriguing microstructural changes in the adult zebrafish brain caused by Toll-like receptor 2 genomic deletion. NMR Biomed. 2024, 37, e5170. [Google Scholar] [CrossRef]
- Bottomley, P.A.; Foster, T.H.; Argersinger, R.E.; Pfeifer, L.M. A review of normal tissue hydrogen NMR relaxation times and relaxation mechanisms from 1-100 MHz: Dependence on tissue type, NMR frequency, temperature, species, excision, and age. Med. Phys. 1984, 11, 425–448. [Google Scholar] [CrossRef]
- de Graaf, R.A.; Brown, P.B.; McIntyre, S.; Nixon, T.W.; Behar, K.L.; Rothman, D.L. High magnetic field water and metabolite proton T1 and T2 relaxation in rat brain in vivo. Magn. Reson. Med. 2006, 56, 386–394. [Google Scholar] [CrossRef]
- Korb, J.P.; Bryant, R.G. Magnetic field dependence of proton spin-lattice relaxation times. Magn. Reson. Med. 2002, 48, 21–26. [Google Scholar] [CrossRef]
- Rooney, W.D.; Johnson, G.; Li, X.; Cohen, E.R.; Kim, S.-G.; Ugurbil, K.; Springer, C.S., Jr. Magnetic field and tissue dependencies of human brain longitudinal 1H2O relaxation in vivo. Magn. Reson. Med. 2007, 57, 308–318. [Google Scholar] [CrossRef]
- Wang, Y.; van Gelderen, P.; de Zwart, J.A.; Duyn, J.H. B0-field dependence of MRI T1 relaxation in human brain. NeuroImage 2020, 213, 116700. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, R.C.; Hogers, B.; van den Maagdenberg, A.M.; de Groot, H.J.; Ferrari, M.D.; Frants, R.R.; Poelmann, R.E.; van der Weerd, L.; Kiihne, S.R. T(1) relaxation in in vivo mouse brain at ultra-high field. Magn. Reson. Med. 2007, 58, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Kara, F.; Chen, F.; Ronen, I.; de Groot, H.J.; Matysik, J.; Alia, A. In vivo measurement of transverse relaxation time in the mouse brain at 17.6 T. Magn. Reson. Med. 2013, 70, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, N.; Allen, C.; Reynolds, S. Longitudinal brain studies in adult zebrafish by MRI. bioRxiv 2022. [Google Scholar] [CrossRef] [PubMed]
- Kenney, J.W.; Steadman, P.E.; Young, O.; Shi, M.T.; Polanco, M.; Dubaishi, S.; Covert, K.; Mueller, T.; Frankland, P.W. A 3D Adult Zebrafish Brain Atlas (AZBA) for the Digital Age. Available online: http://azba.wayne.edu/ (accessed on 1 September 2022).
- Wullimann, M.F.; Rupp, B.; Reichert, H. Neuroanatomy of the Zebrafish Brain: A Topological Atlas; Birkhäuser: Basel, Switzerland, 1996. [Google Scholar]
- Baliyan, V.; Das, C.J.; Sharma, R.; Gupta, A.K. Diffusion weighted imaging: Technique and applications. World J. Radiol. 2016, 8, 785–798. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, X.J. Diffusion MRI of cancer: From low to high b-values. J. Magn. Reson. Imaging 2019, 49, 23–40. [Google Scholar] [CrossRef]
- Ogura, A.; Tamura, T.; Ozaki, M.; Doi, T.; Fujimoto, K.; Miyati, T.; Ito, Y.; Maeda, F.; Tarewaki, H.; Takahashi, M. Apparent Diffusion Coefficient Value Is Not Dependent on Magnetic Resonance Systems and Field Strength Under Fixed Imaging Parameters in Brain. J. Comput. Assist. Tomogr. 2015, 39, 760–765. [Google Scholar] [CrossRef]
- Schilling, K.G.; Janve, V.; Gao, Y.; Stepniewska, I.; Landman, B.A.; Anderson, A.W. Histological validation of diffusion MRI fiber orientation distributions and dispersion. Neuroimage 2018, 165, 200–221. [Google Scholar] [CrossRef]
- Schilling, K.G.; Nath, V.; Hansen, C.; Parvathaneni, P.; Blaber, J.; Gao, Y.; Neher, P.; Aydogan, D.B.; Shi, Y.; Ocampo-Pineda, M.; et al. Limits to anatomical accuracy of diffusion tractography using modern approaches. NeuroImage 2019, 185, 1–11. [Google Scholar] [CrossRef]
- Basser, P.J.; Pajevic, S.; Pierpaoli, C.; Duda, J.; Aldroubi, A. In vivo fiber tractography using DT-MRI data. Magn. Reson. Med. 2000, 44, 625–632. [Google Scholar] [CrossRef]
- Jeurissen, B.; Leemans, A.; Jones, D.K.; Tournier, J.D.; Sijbers, J. Probabilistic fiber tracking using the residual bootstrap with constrained spherical deconvolution. Hum. Brain Mapp. 2011, 32, 461–479. [Google Scholar] [CrossRef] [PubMed]
- Jeurissen, B.; Leemans, A.; Tournier, J.D.; Jones, D.K.; Sijbers, J. Investigating the prevalence of complex fiber conFigureurations in white matter tissue with diffusion magnetic resonance imaging. Hum. Brain Mapp. 2013, 34, 2747–2766. [Google Scholar] [CrossRef] [PubMed]
- Jillings, S.; Morez, J.; Vidas-Guscic, N.; Van Audekerke, J.; Wuyts, F.; Verhoye, M.; Sijbers, J.; Jeurissen, B. Multi-tissue constrained spherical deconvolution in a murine brain. Proc. Intl. Soc. Mag. Reson. Med. 2020, 28, 900. [Google Scholar]
- He, J.; Ding, Y.; Nowik, N.; Jager, C.; Eeza, M.N.H.; Alia, A.; Baelde, H.J.; Spaink, H.P. Leptin deficiency affects glucose homeostasis and results in adiposity in zebrafish. J. Endocrinol. 2021, 249, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Carr, H.Y.; Purcell, E.M. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Krug, J.R.; van Schadewijk, R.; Vergeldt, F.J.; Webb, A.G.; de Groot, H.J.M.; Alia, A.; Van As, H.; Velders, A.H. Assessing spatial resolution, acquisition time and signal-to-noise ratio for commercial microimaging systems at 14.1, 17.6 and 22.3 T. J. Magn. Reson. 2020, 316, 106770. [Google Scholar] [CrossRef]
- Milford, D.; Rosbach, N.; Bendszus, M.; Heiland, S. Mono-Exponential Fitting in T2-Relaxometry: Relevance of Offset and First Echo. PLoS ONE 2015, 10, e0145255. [Google Scholar] [CrossRef]
- Tournier, J.D.; Smith, R.; Raffelt, D.; Tabbara, R.; Dhollander, T.; Pietsch, M.; Christiaens, D.; Jeurissen, B.; Yeh, C.H.; Connelly, A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 2019, 202, 116137. [Google Scholar] [CrossRef]
- Veraart, J.; Fieremans, E.; Novikov, D.S. Diffusion MRI noise mapping using random matrix theory. Magn. Reson. Med. 2016, 76, 1582–1593. [Google Scholar] [CrossRef]
- Basser, P.J.; Mattiello, J.; Lebihan, D. Estimation of the Effective Self-Diffusion Tensor from the NMR Spin Echo. J. Magn. Reson. 1994, 103, 247–254. [Google Scholar] [CrossRef]
- Veraart, J.; Sijbers, J.; Sunaert, S.; Leemans, A.; Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. Neuroimage 2013, 81, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.D.; Calamante, F.; Connelly, A. Determination of the appropriate b value and number of gradient directions for high-angular-resolution diffusion-weighted imaging. NMR Biomed. 2013, 26, 1775–1786. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.D.; Calamante, F.; Connelly, A. Robust determination of the fibre orientation distribution in diffusion MRI: Non-negativity constrained super-resolved spherical deconvolution. Neuroimage 2007, 35, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Tournier, J.D.; Calamante, F.; Connelly, A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 2012, 22, 53–66. [Google Scholar] [CrossRef]
- Calamante, F.; Tournier, J.D.; Jackson, G.D.; Connelly, A. Track-density imaging (TDI): Super-resolution white matter imaging using whole-brain track-density mapping. Neuroimage 2010, 53, 1233–1243. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singer, R.; Oganezova, I.; Hu, W.; Ding, Y.; Papaioannou, A.; de Groot, H.J.M.; Spaink, H.P.; Alia, A. Unveiling the Exquisite Microstructural Details in Zebrafish Brain Non-Invasively Using Magnetic Resonance Imaging at 28.2 T. Molecules 2024, 29, 4637. https://doi.org/10.3390/molecules29194637
Singer R, Oganezova I, Hu W, Ding Y, Papaioannou A, de Groot HJM, Spaink HP, Alia A. Unveiling the Exquisite Microstructural Details in Zebrafish Brain Non-Invasively Using Magnetic Resonance Imaging at 28.2 T. Molecules. 2024; 29(19):4637. https://doi.org/10.3390/molecules29194637
Chicago/Turabian StyleSinger, Rico, Ina Oganezova, Wanbin Hu, Yi Ding, Antonios Papaioannou, Huub J. M. de Groot, Herman P. Spaink, and A Alia. 2024. "Unveiling the Exquisite Microstructural Details in Zebrafish Brain Non-Invasively Using Magnetic Resonance Imaging at 28.2 T" Molecules 29, no. 19: 4637. https://doi.org/10.3390/molecules29194637






