Abstract
In the present report, we have described the synthesis of N-aminopolyhydroquinoline (N-PHQ) derivatives using highly efficient β-cyclodextrin (β-CD) as a catalyst by the Hantzsch condensation of substituted aromatic aldehydes, dimedone, and hydrazine hydrate in one pot. The reactions were completed in a shorter time without the generation of any other byproduct. The synthesized N-PHQs were washed thoroughly with distilled water and recrystallized with ethanol to get highly purified products (as crystals). The structure of the synthesized N-PHQs was established by using advanced spectroscopic techniques like FT-IR, NMR (1H, 13C, DEPT, COSY, and HSQC), ESI-MS, and Elemental Analyzer. The N-PHQs derivatives demonstrated moderate to excellent resistance against the tested strains (both fungal as well as bacterial). The presence of polar groups, which are able to form H-bonds, attached to the phenyl ring like -NO2 (4b and 4c), and -OMe (4i, 4j, and 4k) exhibits excellent activity, which is comparable to standard drugs, amoxicillin and fluconazole.
1. Introduction
Polyhydroquinoline (PHQ) derivatives belong to a significant group of heterocyclic compounds that have emerged as flexible biologically active compounds with various applications, such as anti-tumor [1], anti-atherosclerotic [2], bronchodilator [3], antidiabetic activity [4], and have the ability to modulate calcium channels [5], diuretics [6], vasodilators [7], tranquilizers [8], antibiotic [5,9], anti-defibrillatory [5], anticonvulsant [10], antihistaminic [11], herbicidal activity [12], plant growth regulation ability [13], anticancer [14,15], antihypertensive agents [16], and chemosensitizer in tumor therapy [3].
The synthesis of N-PHQ derivatives prominently features the Hantzsch reaction, a classical one-pot, multi-component reaction involving an aldehyde, β-ketoester, ammonium acetate, and active methylene compound. Despite its efficiency and ability to yield diverse N-PHQs, it often requires long reaction times and high temperatures. To improve this, microwave-assisted synthesis reduces reaction times and increases yields while enhancing simplicity and energy efficiency [17,18]. Green chemistry approaches, like using ionic liquids [19], TMSCl-NaI [20], HY-Zeolites [21], ceric ammonium nitrate (CAN) [22], p-TSA [10], polyacids [23], L-proline [24], ZrCl4 [25], Yb(OTf)3 [26], TiO2 and Fe3O4 nanoparticles [27], and Mont. K-10 [28]. The approaches to carry this reaction include conventional heating [29,30], microwave irradiation [31], and ultrasonic irradiation [32], among which microwave (MW) [33] and UV irradiation [34] are demonstrated to be more efficient and time-saving. Catalysis, employing Lewis’s acids [35], Brønsted acids [19,36], and metal-based catalysts, further enhances efficiency and selectivity, with notable success using lanthanide triflates and transition metals [26].
In the realm of green chemistry, the design and development of organic synthesis through multicomponent reactions (MCRs) have emerged as a critical research focus in organic chemistry. This approach aligns with the principles of sustainable chemistry, promoting environmentally friendly practices and resource conservation [36]. Therefore, MCRs often serve as efficient alternatives to traditional sequential multistep synthesis. The synergistic combination of β-CD with MCR enhances the synthesis of diverse nitrogen-containing heterocycles. One prominent method for preparing these compounds is the Hantzsch condensation reaction, which produces polyhydroquinolines [37]. By using β-CD as the catalyst, this approach improves reaction efficiency. The Hantzsch condensation is particularly valued for its ability to generate a wide variety of N-PHQ, compounds that are significant in pharmaceutical and materials science due to their diverse biological activities and functional properties [38].
β-CD, a cyclic oligosaccharide, possesses a unique molecular structure with a hydrophobic cavity. This cavity allows it to encapsulate hydrophobic guest molecules, including various organic compounds. Researchers have explored β-CD’s antimicrobial properties due to its ability to form inclusion complexes with lipophilic substances [39]. By trapping these substances, β-CD can inhibit microbial growth. Instead of solely investigating β-CD’s antimicrobial properties, we harnessed β-CD as a catalyst in the synthesis of N-aminopolyhydroquinoline derivatives. These derivatives hold immense promise in various applications, such as pharmaceuticals, dyes, and antioxidants. By using β-CD as a catalyst, we aimed to achieve efficient and selective transformations, leveraging its unique cavity for molecular interactions [40].
Therefore, the development of a clean and efficient synthesis method for N-PHQ derivatives is still timely. Understanding the importance of both N-PHQs and MCRs, in the present report, a novel and green protocol has been presented, which is much more efficient than existing procedures.
2. Result and Discussion
2.1. Optimization of Reaction Conditions
2.1.1. Effect of Catalyst and Solvent
The reaction conditions were standardized by numerous combinations of catalysts (5 mmol%) with solvent systems, which were examined on model reaction. The model reaction (Scheme 1) involved the condensation of 5,5-dimethylcyclohexane-1,3-dione 1 (2 eq.), benzaldehyde 2a (1 eq.), and hydrazine hydrate 3 (1 eq.) refluxed at 80 °C in the presence of various combinations of catalyst (5 mmol%) and solvent (5 mL) system to yield 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione 4a. We confirmed the reaction progress using TLC (hexane: ethyl acetate/8:2) and validated the product by comparing its melting point to literature data.
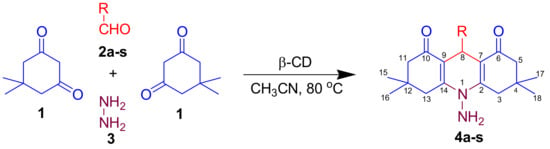
Scheme 1.
Synthesis of N-PHQ derivatives 4a–s using β-CD as a catalyst.
Initially, to get the product, the reaction was carried out without a catalyst with various solvents (ethanol, acetonitrile (ACN), and diethyl ether). To improve the reaction yields, a small quantity of catalyst, i.e., piperidine, was added to the reaction mixture, along with the above-mentioned solvents. Thereafter, the reaction was carried out in water using sodium dodecyl sulfate, Al(DS)3, Sc(DS)3, and 2-aminopyrazine as a catalyst. Among all, 2-aminopyrazine was found to be an inefficient catalyst, and no desired product was formed. However, using β-CD as a catalyst in ACN, ethanol, and diethyl ether not only improves the yield of the reaction but reduces the time of the reaction as well. Out of the various adopted methodologies, a combination of β-CD in ACN (Table 1) turns out to be the superlative catalyst–solvent combination for the synthesis of compound 4a in very high yield and purity (Table 1). The products were then purified by a simple recrystallization procedure.

Table 1.
Effect of Different Catalyst–Solvent Combinations on the Synthesis of Compound 4a.
2.1.2. Effect of Temperature
After optimization of the catalyst–solvent combination, the model reaction was further examined under different temperature ranges (50–95 °C) (Table 2). It was observed that, at lower temperatures, the yield of the produced product was very low, while, by increasing the temperature, the enhancement in yield was observed up to 96%. Conversely, elevating the temperature beyond temperature 80 °C led to a decline in product yield, attributed to the decomposition of products at higher temperatures. The results obtained using this methodology are given in Scheme 1.

Table 2.
Standardization of Temperature for the Synthesis of Compound 4a Using β-CD (5 mmol%) in ACN (5 mL).
Similarly, different aldehydes were reacted with dimedone and hydrazine hydrate using the same procedure, and their formation was monitored (Table 3). From Scheme 1, it was observed that the electron-withdrawing group (EWG) 4a–h substituent on the aromatic ring speeds up the reaction. A higher yield was obtained in a short period, while the electron-donating group (EDG) slowed down the rate of reaction.

Table 3.
Synthesis of N-PHQ Derivatives 4a–s Using β-CD (5 mmol%) in ACN (5 mL).
2.2. Chemistry
The various spectroscopic techniques, i.e., FT-IR spectroscopy (with diamond ATR), NMR (1H, 13C, and DEPT NMR), 2D NMR (COSY and HSQC), and mass spectroscopy were used to confirm the structure of the synthesized compounds (4a–s). The chemical structure of compound 4a is given in Figure 1.
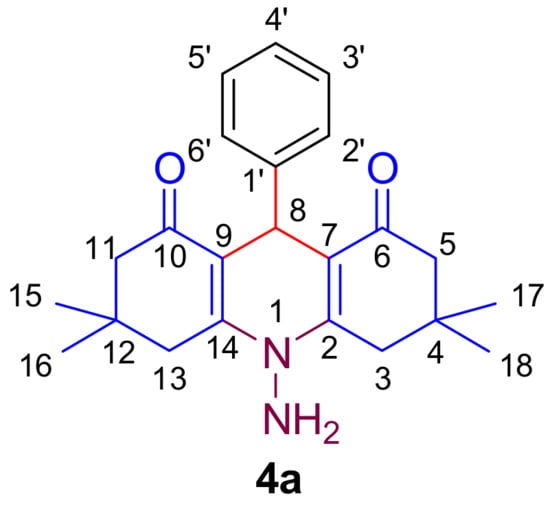
Figure 1.
Structure of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10 H)-dione (4a).
The functional group of the compounds was confirmed by IR spectra; the characteristic stretching peaks of compound 4a were observed at 3326.77 cm−1 for NH2, 3046 cm−1 for sp2 C-H bonds, 2959 and 2929 cm−1 for sp3 C-H bond, and 1615.16 cm−1 for C=O bond.
In 1H NMR spectra (500 MHz, DMSO) of compound 4a, triplet at δ 7.14–7.13 ppm and multiplet at δ 7.05–7.02 ppm was observed for protons of phenyl ring at C-4 position. Two singlets at δ 5.28 and δ 4.95 ppm were observed for the NH2 group and H-4 groups, respectively, whereas peaks of CH2 groups present in N-PHQs were observed at δ 2.91–2.87 (2H, H-11), δ 2.54 (2H, H-5), δ 2.17–2.14 (2H, H-13), and δ 2.02–1.99 (2H, H-3) ppm. The two singlet peaks of four methyl groups were observed at δ 1.02 (6H, 2*CH3) and δ 0.86 (6H, 2*CH3) ppm, respectively.
In 13C NMR spectra (125 MHz, DMSO) of molecule 4a, δ 194.44 ppm was observed for the C=O group, which was the most down-shielded peak. Peaks at δ 154.47 and δ 146.33 ppm were observed for C-2 and C-14 and C-7 and C-9 carbon atoms, whereas carbon belonging to the aromatic part was observed at δ 127.49, 127.20, and 125.38 ppm. The peaks for sp3 carbons were observed at 31.24 (C-8), 49.51 (C-3 and C-13), 38.50 (C-5 and C-11), 29.38 (CH3), and 26.57 (CH3) ppm, respectively. Further, these carbon peaks were confirmed by 13C DEPT 135.
Further to determine H-H coupling and C-H coupling, 2D NMR spectra COSY and HSQC, respectively, were taken. HSQC confirms that the aromatic carbon peaks observed at δ 127.49, and 127.20 couple with hydrogen at δ 7.14–7.13 (4H), and δ 125.38, couples with hydrogen at δ 7.05–7.02 (1H). These peaks remain sharp, suggesting that the aromatic part of the N-PHQ may not be deeply involved in the inclusion complex formation, or it may reside near the exterior of β-CD, not fully inside the hydrophobic cavity [43]. The CH2 peaks at 49.51 (C-3, C-13), and 38.50 (C-5, C-11) couples with hydrogen at δ 2.91–2.87 (2H, H-11), δ 2.54 (2H, H-5), δ 2.17–2.14 (2H, H-13), and δ 2.02–1.99 (2H, H-3), respectively. These aliphatic groups, particularly CH2 groups, may interact with the inner hydrophobic cavity of β-CD since it preferentially hosts nonpolar regions of guest molecules inside its cavity. The C-H (sp3) peak at 31.24 coupling with singlet at δ 4.95 is crucial because sp3 carbons are more likely to fit into the hydrophobic cavity of β-CD. The chemical shift changes observed here (sp3 carbons coupling with protons) may suggest that this region is involved in the inclusion process. The four CH3 of dimedone moiety gave carbon peaks at 29.37 and 26.57, which couples with hydrogen δ 1.0276 (6H, 2*CH3) and δ 0.8603 (6H, 2*CH3), respectively. These groups are relatively small and hydrophobic, making them ideal candidates to fit inside the β-CD cavity. Furthermore, COSY, EI-MS, and elemental analysis verified the synthesis of the desired compound.
2.3. Plausible Mechanism
The reasonable mechanism for the synthesis of N-PHQs is described in Scheme 2. The initial step proceeds with Knoevenagel condensation between aldehyde and dimedone to produce an enone system in the cavity of β-CD, and parallel in the same cavity, hydrazine undergoes a condensation reaction with the other molecule of dimedone, followed by the removal of water to form an enamine. Further, both the intermediate products were activated in the β-CD cavity by the formation of H-bonding and underwent Michael addition, followed by the removal of water to generate a final cyclic product. In the final step, the expulsion of final products (4a–s) takes place from the cavity of β-CD.
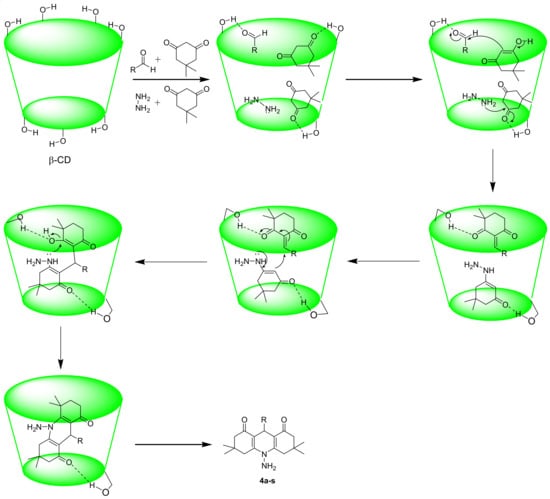
Scheme 2.
A plausible mechanism for the synthesis of N-PHQ derivatives 4a–s using β-CD as a catalyst.
The primary interaction between β-CD and N-PHQ derivatives occurs through the formation of inclusion complexes [44]. In this process, the hydrophobic moiety of the N-PHQ derivatives enters the hydrophobic cavity of β-CD, while the polar groups remain exposed to the aqueous environment, and this encapsulation stabilizes the N-PHQ derivatives.
The inclusion complex is stabilized by non-covalent interactions, such as hydrogen bonding, van der Waals forces, and hydrophobic interactions. Hydrogen bonds can form between the hydroxyl groups on the rim of β-CD and the functional groups (e.g., amines, carbonyls) on the N-PHQ derivatives, enhancing the strength and specificity of the complex. If the N-PHQ derivatives contain aromatic rings, π-π stacking interactions between the aromatic groups of the guest molecule and the glucose units of β-CD may also contribute to the stabilization of the complex [45].
2.4. Antimicrobial Evaluation
The minimum inhibitory concentration (MIC) method was employed to verify the antimicrobial characteristics of the synthesized compounds 4a–s. The MIC standards of the compounds were compared to those of the mentioned drugs, amoxicillin (4 μg/mL) and fluconazole (2 μg/mL), to assess their effectiveness. From Table 4, it is evident that compounds of series 4a–s show great interaction with numeral microbes (both fungal and bacterial). Among the entire tested compounds, only those compounds that have polar groups attached to the phenyl ring (4b, 4c, 4i, 4j, and 4k) were deemed to be more efficient against all the tested strains because they can form hydrogen bonding with the microbial’s protein. Compound 4k having two polar groups showed maximum activity among all the compounds, which is quite like the reference drug, amoxicillin, at MIC 4 μg/mL. It has also been observed that if the phenyl group were replaced by some alkyl group (4r and at MIC 4 μg/mL), the compounds become very less effective (MIC 64 and 128 μg/mL) against these strains.

Table 4.
Minimum Inhibitory Concentration (μg/mL) of Synthesized N-PHQ Derivatives 4a–s Against Various Microbial Agents.
The structure-activity relationship (SAR) in this series of compounds shows a clear correlation between the electronic nature of the substituents and their antimicrobial efficacy. In general, EWGs, such as nitro (-NO₂), chloro (-Cl), and bromo (-Br), tend to enhance antimicrobial activity in specific cases. For example, compound 4b (4-NO₂) demonstrates improved activity against Gram-positive bacteria, B. subtilis, and S. pyogenes, with MIC values of 8 and 4 μg/mL, respectively, compared to compound 4a, which lacks a strong EWG. Similarly, compound 4c (3-NO₂) displays potent activity across most tested strains, particularly E. coli, K. pneumoniae, and various fungi. The ability of the nitro group to withdraw electron density likely contributes to better binding interactions with microbial targets, enhancing overall efficacy [46].
On the other hand, EDGs, such as methoxy (-OCH₃) and methyl (-CH₃), appear to have mixed effects on antimicrobial activity. Compound 4k, with two methoxy groups (3,4-(OCH₃)₂), exhibits consistently strong activity against both Gram-positive and Gram-negative bacteria, with MIC values of 4 μg/mL across all tested strains. This suggests that certain EDGs can still promote favorable interactions with microbial enzymes or cell membranes. However, compounds with single methoxy or methyl groups, such as 4i (4-OCH₃) and 4l (4-CH₃), show moderate to lower activity compared to their electron-withdrawing counterparts, indicating that EDGs might not uniformly enhance antimicrobial properties [47].
The positional effects of these substituents also play a crucial role in determining activity. For instance, compound 4f (2-Cl) shows significantly reduced activity compared to 4e (4-Cl), with MIC values up to 64 μg/mL against Gram-positive bacteria. This highlights the importance of both the electronic nature and the spatial orientation of substituents in optimizing antimicrobial efficacy. Overall, the introduction of EWGs, particularly in para and meta positions, appears to strengthen antimicrobial activity, whereas EDGs require specific positioning to maintain or enhance biological effects.
2.5. Comparison of the Catalytical Activity β-CD with Previous Literature Reports for the Synthesis of 4a
Table 5 presents a comparison between previous studies and the current work. It clearly shows that the current research employs significantly milder reaction temperatures, unlike other reported methods that require higher temperatures or reflux conditions. (See entries 1–3 in Table 5) This highlights the improved efficiency of the present method under more favorable reaction conditions. Moreover, some earlier reports involve time-some procedures for preparing the catalyst (see Table 5, entry 3), whereas preparing β-CD is simpler. Consequently, this study offers notable benefits, including the use of a straightforward catalyst, eco-friendliness, milder conditions, and improvements over previous methods for synthesizing N-PHQ derivatives.

Table 5.
Comparison with Previous Literature Reports for the Synthesis of 4a.
3. Materials and Methods
The entire chemicals utilized in this research were procured from Sigma Aldrich (Mumbai, India) and were used without any additional refining, while the solvents were prearranged from Loba Chemie (Ludhiana, India). The melting point apparatus (digital) was used to measure the melting point via the open capillary method for all the synthesized products. IR spectra of the targeted compound were taken using ATR mode on Perkin Elmer Spectrum II (Chandigarh University, Gharuan, Punjab, India). NMR, such as 1H, 13C, DEPT, HSQC, and COSY, were collected using DMSO as a solvent on a Bruker Avance NEO 500 MHz NMR spectrometer (Punjab University, Chandigarh, India). Chemical shifts (δ) were accounted for in ppm relative to that of TMS as an internal standard. The mass spectroscopy was analyzed on LC-MS Spectrometer Model Q-ToF Micromass. For fundamental analysis, Thermo Scientific (FLASH 2000) CHN Elemental Analyzer was used (Punjab University, Chandigarh, India). The thin layer chromatographic (TLC) technique was used to observe the reaction time as well as to check the purity of the compound, and then with the help of a UV chamber, the visualization of TLC was done.
4. Experimental
4.1. Characterization of Synthesized Polyhydroquinolines Derivatives (4a–s)
- 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4a): Yield 96%, pale yellow crystalline solid, m.p. 260–261 °C. IR Spectrum (υ, cm−1): 3326.77 (N-H), 3046.1 (C=C-H), 2959.01, 2929.2 (sp3 C-H), and 1615.16 (C=O). 1H NMR spectrum (DMSO, 500 MHz): 7.14–7.13 (t, 4H, H-2′, H-3′ H-5′, H-6′), 7.05–7.0250 (m, 1H, H-4′), 5.28 (s, 2H, N-H2), 4.95 (s, 1H, H-8), 2.91-1.99 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.02 (s, 6H, 2*CH3), 0.86 (s, 6H, 2*CH3). 13C NMR spectrum (DMSO, 125 MHz): 194.44 (C=O), 154.47 (C-2, C-14), 146.33 (C-7, C-9), 127.49, 127.20, 125.38 (Ph), 110.95 (C-4, C-12), 49.51 (C-3, C-13), 38.50 (C-5, C-11), 31.24 (C-8), 29.37 (CH3), 26.57 (CH3). Anal. calcd. for C23H28N2O2: C, 75.79; H, 7.74; N, 7.69. Found; C, 75.5 6 H, 7.93; N, 7.56%. ESI-MS (m/z); M + 1 = 365.62.
- 10-amino-3,3,6,6-tetramethyl-9-(4-nitrophenyl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4b): Yield 95%, pale yellow crystalline solid, m.p. 270–272 °C. IR Spectrum (υ, cm−1): 3426.77 (N-H), 3146.1 (C=C-H), 2989, 2959 (sp3 C-H), and 1665.16 (C=O), 1510, 1342 (NO2). 1H NMR spectrum (CDCl3, 500 MHz): 8.11-8.14 (d, 2H, H-2′, H-6′), 7.24–7.28 (d, 2H, H-3′, H-5′), 5.34 (s, 2H, NH2), 5.01 (s, 1H, H-8), 2.32-2.51 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.23 (s, 6H, 2*CH3), 1.11 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 190.90 (C=O), 146.56 (C-2, C-14), 146.08 (C-7, C-9), 127.63, 123.47 (Ph), 114.87 (C-4, C-12), 46.96 (C-3, C-13), 33.23 (C-5, C-11), 31.45 (C-8), 29.46 (CH3), 27.44 (CH3). Anal. calcd. for C23H27N3O4: C, 67.46%; H, 6.65%; N, 10.26%. Found: C, 67.65%; H, 6.25%; N, 10.38%. ESI-MS (m/z); M + 1 = 410.20.
- 10-amino-3,3,6,6-tetramethyl-9-(3-nitrophenyl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4c): Yield 92%, pale yellow crystalline solid, m.p. 223–225 °C. IR Spectrum (υ, cm−1): 3356.75 (N-H), 3206.1 (C=C-H), 2979, 2945 (sp3 C-H), and 1635.16 (C=O), 1525, 1346 (NO2). 1H NMR spectrum (CDCl3, 500 MHz): 7.14 (d, 1H, H-6′), 7.13 (s, 1H, H-2′), 7.05 (t, 1H, H-5′), 7.02 (d, 1H, H-4′), 5.38 (s, 2H, NH2), 5.54 (s, 1H, H-8), 2.92–2.32 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.12 (s, 6H, 2*CH3), 1.07 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 191.06 (C=O), 148.40 (C-2, C-14), 140.71 (C-7, C-9), 132.87, 129.07, 122.20, 121.00 (Ph), 114.74 (C-4, C-12), 46.99 (C-3, C-13), 32.86 (C-5, C-11), 31.41 (C-8), 29.64 (CH3), 27.26 (CH3). Anal. calcd. for C23H27N3O4: C, 67.46%; H, 6.65%; N, 10.26%. Found: C, 67.65%; H, 6.25%; N, 10.38%. ESI-MS (m/z); M + 1 = 410.20.
- 10-amino-3,3,6,6-tetramethyl-9-(2-nitrophenyl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4d): Yield 89%, pale yellow crystalline solid, m.p. 210–212 °C. IR Spectrum (υ, cm−1): 3453.75 (N-H), 3215.1 (C=C-H), 2981, 2930 (sp3 C-H), and 1629.16 (C=O), 1530, 1349 (NO2). 1H NMR spectrum (CDCl3, 500 MHz): 7.29–7.23 (d, 1H, H-6′), 7.33 (t, 1H, H-4′), 7.21 (d, 1H, H-3′), 7.07 (t, 1H, H-5′), 5.76 (s, 2H, NH2), 4.47 (s, 1H, H-8), 2.67–2.29 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.05 (s, 6H, 2*CH3), 1.01 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 197.42 (C=O), 157.40 (C-2, C-14), 153.82 (C-7, C-9), 134.90, 134.01, 133.70 (Ph), 114.73 (C-4, C-12), 51.53 (C-3, C-13), 47.70 (C-5, C-11), 36.10 (C-8), 32.20 (CH3), 27.90 (CH3). Anal. calcd. for C23H27N3O4: C, 67.46%; H, 6.65%; N, 10.26%. Found: C, 67.46%; H, 6.65%; N, 10.26%, O = 15.63; ESI-MS (m/z); M + 1 = 410.20.
- 10-amino-9-(4-chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4e): Yield 94%, pale yellow crystalline solid, m.p. 274–275 °C. IR Spectrum (υ, cm−1): 3216.77 (N-H), 2982.1 (C=C-H), 2919, 2887 (sp3 C-H), and 1505.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.04–7.03 (d, 2H, H-2′, H-6′), 7.01–7.00 (d, 2H, H-3′, H-5′), 5.02 (s, 2H, NH2), 4.96 (s, 1H, H-8), 2.91–1.98 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.02 (s, 6H, 2*CH3), 0.85 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 192.44 (C=O), 151.47(C-2, C-14), 142.13 (C-7, C-9), 127.89, 126.12, 124.25 (Ph), 115.57 (C-4, C-12), 49.37 (C-3, C-13), 38.50 (C-5, C-11), 30.12 (C-8), 29.12 (CH3), 26.27 (CH3). Anal. calcd. for C23H27ClN2O2: C, 69.25; H, 6.82; N, 7.02%. Found; C, 69.15; H, 6.92; N, 7.23%. ESI-MS (m/z); M + 1 = 399.18, M + 2 = 400.17.
- 10-amino-9-(2-chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4f): Yield 91%, pale yellow crystalline solid, m.p. 203–204 °C. IR Spectrum (υ, cm−1): 3216.77 (N-H), 2982.1 (C=C-H), 2909, 2865 (sp3 C-H), and 1505.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 6.99–7.26 (d, 4H, H-3′,4′,5′,6′), 5.18 (s, 2H, NH2), 4.94 (s, 1H, H-8), 2.61–2.32 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.03 (s, 6H, 2*CH3), 0.99 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 200.89 (C=O), 169.14 (C-2, C-14), 151.04 (C-7, C-9), 127.98, 127.52, 124.57, 124.31, 118.32 (Ph), 115.74 (C-4, C-12), 49.93 (C-3, C-13), 32.29 (C-5, C-11), 30.95 (C-8), 29.16 (CH3), 27.77 (CH3). Anal. calcd. for C23H27ClN2O2: C, 69.25; H, 6.82; N, 7.02%. Found; C, 69.15; H, 6.92; N, 7.03%. ESI-MS (m/z); M + 1 = 399.18, M + 2 = 400.17.
- 10-amino-9-(4-bromophenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4g): Yield 95%, pale yellow crystalline solid, m.p. 221–223 °C. IR Spectrum (υ, cm−1): 3350.77 (N-H), 3086.1 (C=C-H), 2979, 2959 (sp3 C-H), and 1625.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 6.94–6.96 (d, 2H, H-3′, H-5′), 7.26–7.38 (d, 2H, H-2′, H-6′), 5.29 (s, 2H, NH2), 4.97 (s, 1H, H-8), 2.90–1.06 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 0.98 (s, 6H, 2*CH3), 0.86 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 197.45 (C=O), 157.40 (C-2, C-14), 137.29 (C-7, C-9), 131.21, 128.57, 119.54 (Ph), 115.18 (C-4, C-12), 46.99 (C-3, C-13), 32.42 (C-5, C-11), 31.36 (C-8), 28.00 (CH3), 27.38 (CH3). Anal. calcd. for C23H27BrN2O2: C, 62.31; H, 6.14; N, 6.32%. Found; C, 62.51; H, 6.04; N, 6.44%. ESI-MS (m/z); M + 1 = 443.14, M + 2 = 444.13.
- 10-amino-9-(4-florophenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4h): Yield 93%, pale yellow crystalline solid, m.p. 297–299 °C. IR Spectrum (υ, cm−1): 3339.23 (N-H), 3065.1 (C=C-H), 2929, 2909 (sp3 C-H), and 1616.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.14 (d, 2H, H-3′, H-5′), 7.01 (d, 2H, H-2′, H-6′), 5.29 (s, 2H, NH2), 4.94 (s, 1H, H-8), 2.90–2.01 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.02 (s, 6H, 2*CH3), 0.86 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 194.5 (C=O), 154.23 (C-2, C-14), 145.2 (C-7, C-9), 129.90, 128.49, 119.20, 111.38 (Ph), 115.15 (C-4, C-12), 49.47 (C-3, C-13), 31.50 (C-5, C-11), 30.26 (C-8), 29.38 (CH3), 26.57 (CH3). Anal. calcd. for C23H27FN2O2: C, 72.23; H, 7.12; N, 7.32%. Found; C, 72.43; H, 7.25; N, 7.13%. ESI-MS (m/z); M + 1 = 383.21, M + 2 = 384.21.
- 10-amino-9-(4-methoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4i): Yield 91%, pale yellow crystalline solid, m.p. 245–246 °C. IR Spectrum (υ, cm−1): 3327.77 (N-H), 3100.1 (C=C-H), 2945, 2920 (sp3 C-H), and 1591.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.24–7.20 (d, 4H, H-2′, H-3′, H-5′, H-6′), 5.39 (s, 2H, NH2), 4.81 (s, 1H, H-8), 2.91–1.98 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.10 (s, 6H, 2*CH3), 0.96 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 195.34 (C=O), 153.40 (C-2, C-14), 147.44 (C-7, C-9), 126.20, 126.05, 125.78 (Ph), 115.55 (C-4, C-12), 48.40 (C-3, C-13), 39.25 (C-5, C-11), 33.62 (C-8), 29.78 (CH3), 26.69 (CH3). Anal. calcd. for C24H30N2O3: C, 73.07; H, 7.66; N, 7.10%. Found; C, 73.5; H, 7.60; N, 7.13%. ESI-MS (m/z); M + 1 = 395.23.
- 10-amino-9-(3-methoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4j): Yield 93%, pale yellow crystalline solid, m.p. 229–231 °C. IR Spectrum (υ, cm−1): 3320.67 (N-H), 3105.1 (C=C-H), 2949, 2935 (sp3 C-H), and 1593.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.35–7.33 (d, 1H, H-4′), 7.17–7.13 (t, 1H, H-5′), 7.10–7.07 (d, 1H, H-6′), 7.05–7.020 (s, 1H, H-2′), 5.170 (s, 2H, NH2), 4.62 (s, 1H, H-8), 2.85–1.97 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.11 (s, 6H, 2*CH3), 0.97 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 195.29 (C=O), 153.49 (C-2, C-14), 147.39 (C-7, C-9), 126.67, 126.25, 125.81 (Ph), 115.13 (C-4, C-12), 48.54 (C-3, C-13), 39.56 (C-5, C-11), 33.25 (C-8), 29.81 (CH3), 26.79 (CH3). Anal. calcd. for C24H30N2O3: C, 73.07; H, 7.66; N, 7.10%. Found; C, 73.5; H, 7.60; N, 7.13%. ESI-MS (m/z); M + 1 = 395.23.
- 10-amino-9-(3,4-dimethoxyphenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4k): Yield 89%, pale yellow crystalline solid, m.p. 211–213 °C. IR Spectrum (υ, cm−1): 3326.77 (N-H), 3046.1 (C=C-H), 2959, 2929 (sp3 C-H), and 1615.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.14–7.13 (d, 4H, H-5′), 7.81–7.77 (s, 1H, H-2′), 7.05–7.0250 (d, 1H, H-6′), 5.28 (s, 2H, NH2), 4.95 (s, 1H, H-8), 2.91–1.99 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.02 (s, 6H, 2*CH3), 0.86 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 194.44 (C=O), 154.47 (C-2, C-14), 146.33 (C-7, C-9), 127.49, 127.20, 125.38 (Ph), 115.27 (C-4, C-12), 49.47 (C-3, C-13), 38.50 (C-5, C-11), 31.26 (C-8), 29.38 (CH3), 26.57 (CH3). Anal. calcd. for C25H32N2O4: C, 70.73; H, 7.60; N, 6.60%. Found; C, 70.78; H, 7.56; N, 6.56%. ESI-MS (m/z); M + 1 = 425.24.
- 10-amino-3,3,6,6-tetramethyl-9-p-tolyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4l): Yield 92%, pale yellow crystalline solid, m.p. 279–280 °C. IR Spectrum (υ, cm−1): 3316.77 (N-H), 3053.1 (C=C-H), 2945, 2927 (sp3 C-H), and 1601.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.72–7.82 (d, 2H, H-3′, H-5′), 7.26–7.35 (d, 2H, H-2′, H-6′), 5.29 (s, 1H, H-8), 4.90 (s, 2H, NH2), 2.31–2.51 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 2.32 (s, 3H, C-4′-CH3), 1.23 (s, 6H, 2*CH3), 1.11 (s, 6H, 2*CH3) ppm. 13C NMR spectrum (CDCl3, 125 MHz): 197.51 (C=O), 160.47 (C-2, C-14), 138.10 (C-7, C-9), 128.22, 126.80, 125.85, 119.69 (Ph), 115.60 (C-4, C-12), 47.08 (C-3, C-13), 46.46 (C-5, C-11), 31.43 (C-8), 29.66 (CH3), 25.95 (CH3). Anal. calcd. for C24H30N2O2: C, 76.16; H, 7.99; N, 7.40%. Found; C, 76.10; H, 7.93; N, 7.44. ESI-MS (m/z); M + 1 = 379.23.
- 10-amino-3,3,6,6-tetramethyl-9-m-tolyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4m): Yield 93%, pale yellow crystalline solid, m.p. 241–243 °C. IR Spectrum (υ, cm−1): 3310.65 (N-H), 3049.3 (C=C-H), 2950, 2931 (sp3 C-H), and 1604.23 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.07 (d, 1H, H-4′), 7.02 (t, 1H, H-5′), 7.01 (d, 1H, H-6′), 6.95 (s, J = 7.7 Hz, 1H, H-2′), 5.25 (s, 1H, H-8), 5.20 (s, 2H, NH2), 2.29 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 2.24 (s, 3H, C-3′-CH3), 1.29 (s, 6H, 2*CH3), 1.24 (s, 6H, 2*CH3) ppm. 13C NMR spectrum (CDCl3, 125 MHz): 195.44 (C=O), 159.47 (C-2, C-14), 142.33 (C-7, C-9), 129.49, 129.20, 128.38 (Ph), 115.53 (C-4, C-12), 46.29 (C-3, C-13), 45.39 (C-5, C-11), 30.43 (C-8), 29.73 (CH3), 20.43 (CH3). Anal. calcd. for C24H30N2O2: C, 76.16; H, 7.99; N, 7.40%. Found; C, 76.10; H, 7.93; N, 7.44%. ESI-MS (m/z); M + 1 = 379.23.
- 10-amino-3,3,6,6-tetramethyl-9-styryl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4n): Yield 81%, pale yellow crystalline solid, m.p. 211–213 °C. IR Spectrum (υ, cm−1): 3325.65 (N-H), 3040.3 (C=C-H), 2966, 2943 (sp3 C-H), and 1614.23 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.54 (t, 1H, H-4′), 7.12 (t, 2H, H-3′, H-5′), 7.07 (d, 1H, H-2′, H-6′), 7.03 (d, 1H, H-2′’), 6.70 (t, 1H, H-1′’), 5.75 (d, 1H, H-8), 5.21 (s, 2H, NH2), 2.29 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.29 (s, 6H, 2*CH3), 1.24 (s, 6H, 2*CH3) ppm. 13C NMR spectrum (CDCl3, 125 MHz): 200.44 (C=O), 162.47 (C-2, C-14), 145.33 (C-7, C-9), 130.49, 129.50, 129.38 (Ph), 113.67 (C-4, C-12), 46.29 (C-3, C-13), 46.39 (C-5, C-11), 31.43 (C-8), 20.73 (CH3), 20.20 (CH3). Anal. calcd. for C25H30N2O2: C, 76.89; H, 7.74; N, 7.17%. Found; C, 76.85; H, 7.70; N, 7.09%. ESI-MS (m/z); M + 1 = 391.23.
- 10-amino-9-(furan-2-yl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4o): Yield 93%, pale yellow crystalline solid, m.p. 265–266 °C. IR Spectrum (υ, cm−1): 3310.77 (N-H), 3025.1 (C=C-H), 2969, 2930 (sp3 C-H), and 1610.26 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.09–7.26 (d, 1H, H-3′), 6.85–6.87 (m, 1H, H-4′), 6.62–6.63 (d, 1H, H-5′), 5.29 (s, 2H, NH2), 4.97 (s, 1H, H-8), 2.22–2.43 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 2.54 (s, 2H, H-11), 2.17–2.14 (s, 2H, H-3), 2.02–1.99 (s, 2H, H-13), 1.02 (s, 6H, 2*CH3), 0.86 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 190.38 (C=O), 146.31 (C-2, C-14), 143.59 (C-7, C-9), 129.74, 119.51, 115.78, 114.08 (furyl–C), 109.82 (C-4, C-12), 55.66 (C-3, C-13), 47.09 (C-5, C-11), 46.39 (C-8), 29.84 (CH3), 27.10 (CH3). Anal. calcd. for C21H20N2O3: C, 71.16; H, 7.39; N, 7.90%. Found C, 71.19; H, 7.29; N, 7.75%. ESI-MS (m/z); M + 1 = 385.24.
- 10-amino-3,3,6,6-tetramethyl-9-(thiophen-2-yl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4p): Yield 95%, pale yellow crystalline solid, m.p. 243–244 °C. IR Spectrum (υ, cm−1): 3317.77 (N-H), 3029.1 (C=C-H), 2975, 2830 (sp3 C-H), and 1615.26 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.26 (d, 1H, H-3′), 6.79–6.80 (m, 1H, H-4′), 6.56–6.61 (d, 1H, H-5′), 5.31 (s, 2H, NH2), 4.98 (s, 1H, H-8), 2.29–2.43 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.23 (s, 6H, 2*CH3), 1.07 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 189.86 (C=O), 143.67 (C-2, C-14), 146.36 (C-7, C-9), 126.31, 124.51, 123.44 (thiolphenyl–C), 109.87 (C-4, C-12), 46.98 (C-3, C-13), 31.13 (C-5, C-11), 30.37 (C-8), 29.91 (CH3), 26.73 (CH3). Anal. calcd. for C21H26N2O2S: C, 68.08; H, 7.07; N, 7.56%. Found C, 68.19; H, 7.11; N, 7.48%. ESI-MS (m/z); M + 1 = 371.17.
- 10-amino-3,3,6,6-tetramethyl-9-(pyridin-2-yl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4q): Yield 89%, pale yellow crystalline solid, m.p. 278–280 °C. IR Spectrum (υ, cm−1): 3326.77 (N-H), 3046.1 (C=C-H), 2959, 2929 (sp3 C-H), and 1615.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 7.27 (d, J=7.4 Hz, 1H, H-6′), 7.20 (dd, 1H, H-4′), 7.14 (dd, 1H, H-5′), 7.08 (d, J=7.0 Hz, 1H, H-3′), 5.28 (s, 2H, NH2), 4.95 (s, 1H, H-8), 2.91–1.99 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.02 (s, 6H, 2*CH3), 0.86 (s, 6H, 2*CH3). 13C NMR spectrum (CDCl3, 125 MHz): 194.44 (C=O), 154.47(C-2, C-14), 146.33 (C-7, C-9), 127.49, 127.20, 125.38 (Ph), 112.47 (C-4, C-12), 49.47 (C-3, C-13), 38.50 (C-5, C-11), 31.26 (C-8), 29.38 (CH3), 26.57 (CH3). Anal. calcd. for C22H27N3O2: C, 72.30; H, 7.45; N, 11.50%. Found; C, 72.35; H, 7.39; N, 11.46%. ESI-MS (m/z); M + 1 = 366.24.
- 10-amino-3,3,6,6,9-pentamethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4r): Yield 91%, pale yellow crystalline solid, m.p. 295–297 °C. IR Spectrum (υ, cm−1): 3315.77 (N-H), 3046.1 (C=C-H), 2953, 2930 (sp3 C-H), and 1625.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 5.38 (s, 2H, NH2), 2.35 (m, 8H, 2*H-3, 2*H-5, 2*H-11, 2*H-13), 1.25 (s, 6H, 2*CH3), 1.21 (s, 6H, 2*CH3) ppm. 13C NMR spectrum (CDCl3, 125 MHz): 196.44 (C=O), 163.47 (C-2, C-14), 143.33 (C-7, C-9), 111.27 (C-4, C-12), 47.79 (C-3, C-13), 46.45 (C-5, C-11), 29.41 (CH3), 20.95 (CH3). Anal. calcd. for C19H28N2O2: C, 72.12; H, 8.93; N, 8.85%. Found; C, 72.16; H, 8.98; N, 8.83%. ESI-MS (m/z); M + 1 = 317.22.
- 10-amino-3,3,6,6-tetramethyl-9-propyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4s): Yield 89%, pale yellow crystalline solid, m.p. 216–217 °C. IR Spectrum (υ, cm−1): 3315.77 (N-H), 3046.1 (C=C-H), 2953, 2930 (sp3 C-H), and 1625.16 (C=O). 1H NMR spectrum (CDCl3, 500 MHz): 5.38 (s, 2H, NH2), 5.1 (s, 1H, H-4), 2.35 (m, 8H, H-3, H-5′, H-11, H-13), 1.25 (s, 6H, 2*CH3), 1.21 (s, 6H, 2*CH3) ppm, 1.01 (m, 2H, CH2), 0.92 (m, 2H, CH2), 0.73 (t, 3H, CH3). 13C NMR spectrum (CDCl3, 125 MHz): 196.44 (C=O), 163.47 (C-2, C-14), 143.33 (C-7, C-9), 113.34 (C-4, C-12), 47.79 (C-3, C-13), 46.45 (C-5, C-11), 29.41 (CH3), 20.95 (CH3). Anal. calcd. for C21H32N2O2: C, 73.22; H, 9.36; N, 8.13%. Found; C, 73.26; H, 9.41; N, 8.17%. ESI-MS (m/z); M + 1 = 345.25.
4.2. General Experimental Procedure for Preparation of 10-Amino-3,3,6,6-Tetramethyl-9-Phenyl-3,4,6,7-Tetrahydroacridine-1,8(2H,5H,9H,10H)-Dione (4a)
A mixture of 5,5-dimethylcyclohexane-1,3-dione 1 (10 mmol), benzaldehyde 2a (5 mmol), and hydrazine hydrate 3 (5 mmol) was added in a round bottom flask containing β-CD (5 mmol%) as a catalyst in ACN (5 mL). This mixture was stirred at 80 °C for constantly 5–6 h. The reaction was constantly observed by TLC (n-hexane: ethyl acetate/4:6). After the completion of the reaction, it was cooled to room temperature, followed by filtration of the obtained solid. The solid was then recrystallized with ethanol to obtain a pure compound 4a in 96% yield.
4.3. Antimicrobial Activity
The recently synthesized N-PHQ derivatives, namely 4a–s, were subjected to testing against three Gram-negative bacteria strains (Staphylococcus aureus MTCC 96, Klebsiella pneumonia MTCC 3384, and Escherichia coli MTCC 443), two Gram-positive bacteria strains (Streptococcus pyogenes MTCC 442 and Bacillus subtilis MTCC 441), and three fungal strains (Aspergillus Niger MTCC 281, Aspergillus Janus MTCC 2751, and Aspergillus sclerotium MTCC 1008). To prepare the bacterial samples, they were cultured at 37 °C for 24 h and then stored in nutrient broth. Before inoculation, at 28 °C for 72 h, the fungal strains were grown in malt extract. A sequential dilution protocol was employed to test triplicates of each synthesized chemical at concentrations of 2, 4, 8, 16, 32, 64, and 128 g/mL the chemicals were dissolved in DMSO.
5. Conclusions
The authors have described an effective method for synthesizing various N-PHQs through Hantzsch condensation using β-CD as an efficient catalyst. This process demonstrates flexibility and diversity while achieving high yields (up to 96%) using readily available starting materials. The synthesized N-PHQs derivatives (19 compounds) were tested against six bacterial and four fungal strains using the serial dilution method as compared to amoxicillin and fluconazole as standard drugs. All the synthesized N-PHQs showed good resistance against the tested strains. Interestingly, the presence of polar groups (-NO2 in compounds 4b and 4c and -OMe in compounds 4i, 4j, and 4k) on the phenyl ring, which can form hydrogen bonds with the microbe’s protein, displayed excellent antimicrobial activity, which is comparable to the standard drugs at MIC 4 μg/mL. It has also been noted that replacing the phenyl group with an alkyl group (as in compound 4r and at MIC 4 μg/mL) significantly reduces the effectiveness of the compound against these strains, with MIC values increasing to 64 and 128 μg/mL.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules29194655/s1, Figure S1. IR spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S2. 1H NMR spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S3. 13C NMR spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S4. DEPT-135 spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S5. DEPT-135 expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S6. DEPT-135 expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S7. HSQC spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S8. HSQC expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S9. HSQC expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S10. HSQC expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S11. COSY expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S12. COSY expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S13. COSY expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S14. COSY expanded spectrum of 10-amino-3,3,6,6-tetramethyl-9-phenyl-3,4,6,7,9,10-hexahydroacridine-1,8(2H,5H)-dione (4a), Figure S15. 1H NMR spectrum of 10-amino-3,3,6,6-tetramethyl-9-(4-nitrophenyl)-3,4,6,7-tetrahydroacridine-1,8(2H,5 H,9H,10H)- dione (4b), Figure S16. 1H NMR spectrum of 10-amino-3,3,6,6-tetramethyl-9-(3-nitrophenyl)-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4c), Figure S17. 1H NMR spectrum of 10-amino-9-(2-chlorophenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H, 5H,9H,10H)-dione (4f), Figure S18. 1H NMR spectrum of 10-amino-9-(4-bromophenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4g), Figure S19. 1H NMR spectrum of 10-amino-9-(4-florophenyl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H, 5H,9H,10H)-dione (4h), Figure S20. 1H NMR spectrum of 10-amino-3,3,6,6-tetramethyl-9-p-tolyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10 H)-dione (4l), Figure S21. 1H NMR spectrum of 10-amino-9-(furan-2-yl)-3,3,6,6-tetramethyl-3,4,6,7-tetrahydroacridine-1,8(2H,5H,9H,10H)-dione (4o), Figure S22. 1H NMR spectrum of 10-amino-3,3,6,6-tetramethyl-9-(thiophen-2-yl)-3,4,6,7-tetrahydroacridine-1,8(2H,5 H,9H,10H)-dione (4p), Table S1. Impact of the amount of catalyst on the model reaction.
Author Contributions
Methodology, P.K.B.; investigation, H.H., and F.M.H.; writing—original draft, S.G., and H.S.S.; writing—review and editing, M.K., H.H., and P.K.B.; supervision, M.K., and H.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank the Researchers Supporting Project Number (RSPD2024R729), King Saud University, Riyadh 11451, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors would like to thank the Researchers Supporting Project Number (RSPD2024R729), King Saud University, Riyadh, Saudi Arabia. P. K. B. sincerely acknowledges the financial support of an industrial sponsored project supported by Koshee Company, Las Vegas, USA. P. K. B. sincerely also acknowledges the Knowledge Fund that is administered by the Nevada Governor’s Office of Economic Development (GOED) and University of Nevada Las Vegas (UNLV). H. H. sincerely acknowledges the Faculty Opportunity Award (FOA) that is administered by the Office of Sponsored Programs (OSP) at UNLV. All authors are also thankful to Chandigarh University, Gharuan, Mohali, India, for their support and all other essential facilities for conducting this research.
Conflicts of Interest
The authors state that they do not have any conflicts of interest.
References
- Gong, G.H.; Bian, M.; Liu, C.Y.; Zhang, B. Retracted: Heterocyclic pyran and polyhydroquinoline derivatives to inhibit human breast cancer cells. Main Group Chem. 2019, 18, 15–22. [Google Scholar] [CrossRef]
- Kalaria, P.N.; Satasia, S.P.; Raval, D.K. Synthesis, characterization and pharmacological screening of some novel 5-imidazopyrazole incorporated polyhydroquinoline derivatives. Eur. J. Med. Chem. 2014, 78, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.M.; Pawar, R.P. Microwave Assisted Expeditious Synthesis of Bioactive Polyhydroquinoline Derivatives. Chem. Bull. 2013, 2, 679–682. Available online: https://www.researchgate.net/publication/284286952 (accessed on 14 May 2023).
- Kumar, A.; Sharma, S.; Tripathi, V.D.; Maurya, R.A.; Srivastava, S.P.; Bhatia, G.; Tamrakar, A.K.; Srivastava, A.K. Design and synthesis of 2,4-disubstituted polyhydroquinolines as prospective antihyperglycemic and lipid modulating agents. Bioorganic Med. Chem. 2010, 18, 4138–4148. [Google Scholar] [CrossRef] [PubMed]
- Zainab, H.; Yu, N.U.; Rehman, M.; Ali, A.; Alam, A.; Latif, N.; Shahab, I.; Amir Khan, A.; Jabbar Shah, M.; Khan, A. Novel Polyhydroquinoline-Hydrazide-Linked Schiff’s Base Derivatives: Multistep Synthesis, Antimicrobial, and Calcium-Channel-Blocking Activities. Antibiotics 2022, 11, 1568. [Google Scholar] [CrossRef]
- Hajjami, M.; Tahmasbi, B. Synthesis and characterization of glucosulfonic acid supported on Fe3O4 nanoparticles as a novel and magnetically recoverable nanocatalyst and its application in the synthesis of polyhydroquinoline and 2,3-dihydroquinazolin-4(1H)-one derivatives. RSC Adv. 2015, 5, 59194–59203. [Google Scholar] [CrossRef]
- Sapkal, S.B.; Shelke, K.F.; Shingate, B.B.; Shingare, M.S. Nickel nanoparticle-catalyzed facile and efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions. Tetrahedron Lett. 2009, 50, 1754–1756. [Google Scholar] [CrossRef]
- Chopra, P.K.P.G.; Lambat, T.L.; Mahmood, S.H.; Chaudhary, R.G.; Banerjee, S. Sulfamic Acid as Versatile Green Catalyst Used For Synthetic Organic Chemistry: A Comprehensive Update. ChemistrySelect 2021, 6, 6867–6889. [Google Scholar] [CrossRef]
- Venkatapathy, K.; Magesh, C.J.; Lavanya, G.; Perumal, P.T.; Prema, S. Design, synthesis, molecular docking, and spectral studies of new class of carbazolyl polyhydroquinoline derivatives as promising antibacterial agents with noncytotoxicity towards human mononuclear cells from peripheral blood. J. Heterocycl. Chem. 2020, 57, 1936–1955. [Google Scholar] [CrossRef]
- Shiri, L.; Heidari, L.; Kazemi, M. Magnetic Fe3O4 nanoparticles supported imine/Thiophene-nickel (II) complex: A new and highly active heterogeneous catalyst for the synthesis of polyhydroquinolines and 2,3-dihydroquinazoline-4(1H)-ones. Appl. Organomet. Chem. 2018, 32, e3943. [Google Scholar] [CrossRef]
- Ahmed, K.; Dubey, B.; Nadeem, S.; Shrivastava, B.; Sharma, P. p-TSA-catalyzed one-pot synthesis and docking studies of some 5H-indeno[1,2-b]quinoline-9,11 (6H,10H)-dione derivatives as anticonvulsant agents. Chin. Chem. Lett. 2016, 27, 721–725. [Google Scholar] [CrossRef]
- Hajjami, M.; Nejat, R.; Sharifirad, F.; Gholamian, F. Efficient Synthesis of Nickel (II) Complex Supported on Fe3O4@SiO2 Nanoparticles as a New and Facile Catalyst for Various Multicomponent Reactions. Org. Chem. Res. 2018, 4, 23–42. [Google Scholar] [CrossRef]
- Nezhad, S.M.; Zare, E.N.; Davarpanah, A.; Pourmousavi, S.A.; Ashrafizadeh, M.; Kumar, A.P. Ionic Liquid-Assisted Fabrication of Bioactive Heterogeneous Magnetic Nanocatalyst with Antioxidant and Antibacterial Activities for the Synthesis of Polyhydroquinoline Derivatives. Molecules 2022, 27, 1748. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Priya, A.; Sharma, A.; Singh, A.; Banerjee, B. Glycine and its derivatives catalyzed one-pot multicomponent synthesis of bioactive heterocycles. Synth. Commun. 2022, 52, 1635–1656. [Google Scholar] [CrossRef]
- Jadhvar, S.C.; Kasraliker, H.M.; Goswami, S.V.; Chakrawar, A.V.; Bhusare, S.R. One-pot synthesis and evaluation of anticancer activity of polyhydroquinoline derivatives catalyzed by [Msim]Cl. Res. Chem. Intermed. 2017, 43, 7211–7221. [Google Scholar] [CrossRef]
- Raslan, R.R.; Hessein, S.A.; Fouad, S.A.; Shmiess, N.A.M. Synthesis and antitumor evaluation of some new thiazolopyridine, nicotinonitrile, pyrazolopyridine, and polyhydroquinoline derivatives using ceric ammonium nitrate as a green catalyst. J. Heterocycl. Chem. 2022, 59, 832–846. [Google Scholar] [CrossRef]
- Brinkerhoff, R.C.; Santa-Helena, E.; Amaral, P.C.D.; Cabrera, D.D.C.; Ongaratto, R.F.; De Oliveira, P.M.; Da Ros Montes D’Oca, C.; Neves Gonçalves, C.A.; Maia Nery, L.E.; Montes D’Oca, M.G. Evaluation of the antioxidant activities of fatty polyhydroquinolines synthesized by Hantzsch multicomponent reactions. RSC Adv. 2019, 9, 24688–24698. [Google Scholar] [CrossRef] [PubMed]
- Dömling, A. Recent Developments in Isocyanide Based Multicomponent Reactions in Applied Chemistry. Chem. Rev. 2005, 106, 17–89. [Google Scholar] [CrossRef] [PubMed]
- Nair, V.; Rajesh, C.; Vinod, A.U.; Bindu, S.; Sreekanth, A.R.; Mathen, J.S.; Balagopal, L. Strategies for Heterocyclic Construction via Novel Multicomponent Reactions Based on Isocyanides and Nucleophilic Carbenes. Acc. Chem. Res. 2003, 36, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Li, B.L.; Zhong, A.G.; Ying, A.G. Novel SO3H-Functionalized Ionic Liquids–Catalyzed Facile and Efficient Synthesis of Polyhydroquinoline Derivatives via Hantzsch Condensation under Ultrasound Irradiation. J. Heterocycl. Chem. 2015, 52, 445–449. [Google Scholar] [CrossRef]
- Kumar, A.; Maurya, R.A. Bakers’ yeast catalyzed synthesis of polyhydroquinoline derivatives via an unsymmetrical Hantzsch reaction. Tetrahedron Lett. 2007, 48, 3887–3890. [Google Scholar] [CrossRef]
- Rama, V.; Kanagaraj, K.; Pitchumani, K. A multicomponent, solvent-free, one-pot synthesis of benzoxanthenones catalyzed by HY zeolite: Their anti-microbial and cell imaging studies. Tetrahedron Lett. 2012, 53, 1018–1024. [Google Scholar] [CrossRef]
- Ko, S.; Yao, C.F. Ceric Ammonium Nitrate (CAN) catalyzes the one-pot synthesis of polyhydroquinoline via the Hantzsch reaction. Tetrahedron 2006, 62, 7293–7299. [Google Scholar] [CrossRef]
- Ibrahim, A.A.; Salama, R.S.; El-Hakam, S.A.; Khder, A.S.; Ahmed, A.I. Synthesis of 12-tungestophosphoric acid supported on Zr/MCM-41 composite with excellent heterogeneous catalyst and promising adsorbent of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127753. [Google Scholar] [CrossRef]
- Budhewar, V.; Karade, N.N.; Budhewar, V.H.; Shinde, S.V.; Jadhav, W.N. L-Proline as an Efficient Organo-Catalyst for the Synthesis of Polyhydroquinoline via Multicomponent Hantzsch Reaction. Lett. Org. Chem. 2007, 4, 16–19. [Google Scholar] [CrossRef]
- Reddy, C.S.; Raghu, M. Facile ZrCl4 promoted four-component coupling one-pot synthesis of polyhydroquinoline derivatives through unsymmetric Hantzsch reaction. Indian J. Chem. 2008, 47, 1578–1582. [Google Scholar] [CrossRef]
- Wang, L.M.; Sheng, J.; Zhang, L.; Han, J.W.; Fan, Z.Y.; Tian, H.; Qian, C.T. Facile Yb(OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction. Tetrahedron 2005, 61, 1539–1543. [Google Scholar] [CrossRef]
- Leng, Y.; Guo, W.; Shi, X.; Li, Y.; Xing, L. Polyhydroquinone-Coated Fe3O4 Nanocatalyst for Degradation of Rhodamine B based on Sulfate Radicals. Ind. Eng. Chem. Res. 2013, 52, 13607–13612. [Google Scholar] [CrossRef]
- Kalbende, P.P.; Jadhav, N.B. K-10 Montmorillonite Catalysed Solvent Free Synthesis of Coumarins via Pechmann Condensation. Int. J. Sci. Res. Chem. Sci. 2020, 7, 15–21. Available online: https://www.researchgate.net/publication/346511710 (accessed on 14 May 2023).
- Mayurachayakul, P.; Pluempanupat, W.; Srisuwannaket, C.; Chantarasriwong, O. Four-component synthesis of polyhydroquinolines under catalyst- and solvent-free conventional heating conditions: Mechanistic studies. RSC Adv. 2017, 7, 56764–56770. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Foroughifar, N.; Fard, M.A.B.; Moghanian, H.; Ebrahimi, S.; Kalhor, M. Efficient One-Pot Synthesis of Polyhydroquinoline Derivatives Using Silica Sulfuric Acid as a Heterogeneous and Reusable Catalyst Under Conventional Heating and Energy-Saving Microwave Irradiation. Synth. Commun. 2009, 39, 1166–1174. [Google Scholar] [CrossRef]
- Zhang, X.-L.; Sheng, S.-R.; Liu, X.-L. Solvent-free liquid-phase synthesis of polyhydroquinoline derivatives under microwave irradiation. Arkivoc 2007, 7, 79–86. [Google Scholar] [CrossRef]
- Puri, S.; Kaur, B.; Parmar, A.; Kumar, H. Copper Perchlorate Hexahydrate: An Efficient Catalyst for the Green Synthesis of Polyhydroquinolines under Ultrasonication. ISRN Org. Chem. 2011, 2011, 948685. [Google Scholar] [CrossRef] [PubMed]
- Ahankar, H.; Ramazani, A.; Joo, S.W. Magnetic nickel ferrite nanoparticles as an efficient catalyst for the preparation of polyhydroquinoline derivatives under microwave irradiation in solvent-free conditions. Res. Chem. Intermed. 2016, 42, 2487–2500. [Google Scholar] [CrossRef]
- Deng, Y.; Liu, L.; Sarkisian, R.G.; Wheeler, K.; Wang, H.; Xu, Z. Arylamine-catalyzed enamine formation: Cooperative catalysis with arylamines and acids. Angew. Chem. Int. Ed. 2013, 52, 3663–3667. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Sarkisian, R.; Deng, Y.; Wang, H. Sc(OTf)3-Catalyzed Three-Component Cyclization of Arylamines, β,γ-Unsaturated α-Ketoesters, and 1,3-Dicarbonyl Compounds for the Synthesis of Highly Substituted 1,4-Dihydropyridines and Tetrahydropyridines. J. Org. Chem. 2013, 78, 5751–5755. [Google Scholar] [CrossRef]
- Kalydi, E.; Malanga, M.; Ujj, D.; Benkovics, G.; Szakács, Z.; Béni, S. Fully Symmetric Cyclodextrin Polycarboxylates: How to Determine Reliable Protonation Constants from NMR Titration Data. Int. J. Mol. Sci. 2022, 23, 14448. [Google Scholar] [CrossRef]
- Garibyan, A.; Delyagina, E.; Agafonov, M.; Ilya Khodov, I.T. Effect of PH, Temperature and Native Cyclodextrins on Aqueous Solubility of Baricitinib. J. Mol. Liq. 2022, 360, 119548. [Google Scholar] [CrossRef]
- Han, G.; Cui, B.; Chen, L.; Hu, X. Synthesis of 10-amino-9-aryl-2,3,4,5,6,7,9,10-octahydroacridine-1,8-dione derivatives. J. Heterocycl. Chem. 2012, 49, 195–199. [Google Scholar] [CrossRef]
- Sachar, A.; Gupta, P.; Gupta, S.; Sharma, R.L. A novel approach towards the synthesis of tricyclic systems based on pyridine, pyran, thiopyran, azepine, oxepin, thiepin, and pyrimidine rings under different solvent conditions. Can. J. Chem. 2010, 88, 478–484. [Google Scholar] [CrossRef]
- Madankumar, N.; Pitchumani, K. β-Cyclodextrin Monosulphonic Acid Promoted Multicomponent Synthesis of 1, 8-Dioxodecahydroacridines in Water. ChemistrySelect 2018, 3, 10886–10891. [Google Scholar] [CrossRef]
- Jiang, B.; Hao, W.J.; Zhang, J.P.; Tu, S.J.; Shi, F. New domino reaction for the selective synthesis of tetracyclic cinnolino[5,4,3-cde]cinnolines. Org. Biomol. Chem. 2009, 7, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Fifere, A.; Marangoci, N.; Maier, S.; Coroaba, A.; Maftei, D.; Pinteala, M. Theoretical Study on β-Cyclodextrin Inclusion Complexes with Propiconazole and Protonated Propiconazole. Beilstein J. Org. Chem. 2012, 8, 2191–2201. [Google Scholar] [CrossRef]
- Marques, C.S.F.; Barreto, N.S.; de Oliveira, S.S.C.; Santos, A.L.S.; Branquinha, M.H.; de Sousa, D.P.; Castro, M.; Andrade, L.N.; Pereira, M.M.; da Silva, C.F. β-Cyclodextrin/Isopentyl Caffeate Inclusion Complex: Synthesis, Characterization and Antileishmanial Activity. Molecules 2020, 25, 4181. [Google Scholar] [CrossRef] [PubMed]
- Sandilya, A.A.; Natarajan, U.; Priya, M.H. Molecular View into the Cyclodextrin Cavity: Structure and Hydration. ACS Omega 2020, 5, 25655–25667. [Google Scholar] [CrossRef] [PubMed]
- Khamkhenshorngphanuch, T.; Kulkraisri, K.; Janjamratsaeng, A.; Plabutong, N.; Thammahong, A.; Manadee, K.; Na Pombejra, S.; Khotavivattana, T. Synthesis and Antimicrobial Activity of Novel 4-Hydroxy-2-Quinolone Analogs. Molecules 2020, 25, 3059. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Cabrera, D.; Rosa, S.B.; De Oliveira, F.S.; Marinho, M.A.G.; Montes D’Oca, C.R.; Russowsky, D.; Horn, A.P.; Montes D’Oca, M.G. Synthesis and Antiproliferative Activity of Novel Hybrid 3-Substituted Polyhydroquinoline-Fatty Acids. Medchemcomm 2016, 7, 2167–2176. [Google Scholar] [CrossRef]
- Ramesh, K.B.; Pasha, M.A. Study on One-Pot Four-Component Synthesis of 9-Aryl-Hexahydro-Acridine-1,8-Diones Using SiO2-I as a New Heterogeneous Catalyst and Their Anticancer Activity. Bioorganic Med. Chem. Lett. 2014, 24, 3907–3913. [Google Scholar] [CrossRef] [PubMed]
- Yü, S.J.; Wu, S.; Zhao, X.M.; Lü, C.W. Green and Efficient Synthesis of Acridine-1,8-Diones and Hexahydroquinolines via a KH2PO4 Catalyzed Hantzsch-Type Reaction in Aqueous Ethanol. Res. Chem. Intermed. 2017, 43, 3121–3130. [Google Scholar] [CrossRef]
- Hong, M.; Xiao, G. FSG-Hf(NPf2)4 Catalyzed, Environmentally Benign Synthesis of 1,8-Dioxo-Decahydroaridines in Water-Ethanol. J. Fluor. Chem. 2012, 144, 7–9. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).