Abstract
Ageratum conyzoides L. is native to Tropical America, and it has naturalized in many other tropical, subtropical, and temperate countries in South America, Central and Southern Africa, South and East Asia, Eastern Austria, and Europe. The population of the species has increased dramatically as an invasive alien species, and it causes significant problems in agriculture and natural ecosystems. The life history traits of Ageratum conyzoides, such as its short life cycle, early reproductive maturity, prolific seed production, and high adaptive ability to various environmental conditions, may contribute to its naturalization and increasing population. Possible evidence of the molecules involved in the defense of Ageratum conyzoides against its natural enemies, such as herbivore insects and fungal pathogens, and the allelochemicals involved in its competitive ability against neighboring plant species has been accumulated in the literature. The volatiles, essential oils, extracts, residues, and/or rhizosphere soil of Ageratum conyzoides show insecticidal, fungicidal, nematocidal, and allelopathic activity. The pyrrolizidine alkaloids lycopsamine and echinatine, found in the species, are highly toxic and show insecticidal activity. Benzopyran derivatives precocenes I and II show inhibitory activity against insect juvenile hormone biosynthesis and trichothecene mycotoxin biosynthesis. A mixture of volatiles emitted from Ageratum conyzoides, such as β-caryophyllene, β-bisabolene, and β-farnesene, may work as herbivore-induced plant volatiles, which are involved in the indirect defense function against herbivore insects. Flavonoids, such as nobiletin, eupalestin, 5′-methoxynobiletin, 5,6,7,3′,4′,5′-hexamethoxyflavone, and 5,6,8,3,4′,5′-hexamethoxyflavone, show inhibitory activity against the spore germination of pathogenic fungi. The benzoic acid and cinnamic acid derivatives found in the species, such as protocatechuic acid, gallic acid, p-coumaric acid, p-hydroxybenzoic acid, and ferulic acid, may act as allelopathic agents, causing the germination and growth inhibition of competitive plant species. These molecules produced by Ageratum conyzoides may act as defense molecules against its natural enemies and as allelochemicals against neighboring plant species, and they may contribute to the naturalization of the increasing population of Ageratum conyzoides in new habitats as an invasive plant species. This article presents the first review focusing on the defense function and allelopathy of Ageratum conyzoides.
1. Introduction
Ageratum conyzoides L., belonging to the family Asteraceae, is an annual or subshrub and grows to 20–150 cm in height. The stems are erect and round, covered with villi, and they branch well. The opposite leaves are simple, ovate, serrate, pubescent, 2–8 cm long, and 1–5 cm wide, with long petioles. It has a fibrous root system. The capitula are 4–6 cm in diameter, generated in panicles at the ends of the twigs, and a single capitulum contains 30–50 tubular florets. The corollas of the florets are white to mauve. The fruits are black and liner achenes, having aristate pappi [1,2,3,4] (Figure 1).

Figure 1.
Ageratum conyzoides. Photos were kindly provided by Dr. Poonpaiboonpipat, T.
The native range of Ageratum conyzoides consists of Tropical America. The species is thought to have been introduced into different countries as an ornamental plant, but it has naturalized and spread in many tropical, subtropical, and temperate countries in South America, Central and Southern Africa, South and East Asia, Eastern Austria, and Europe [1,2,3,4,5]. Primary infestation may occur along road margins because the density of the species population is correlated with the distance from roads [2]. It was estimated that 40% of the geographical areas in the Eastern Ghats of India would be covered by Ageratum conyzoides by the end of 2100 [6].
The population of Ageratum conyzoides has been reported to have increased dramatically and it causes significant problems in agriculture in the introduced ranges. The infestation of Ageratum conyzoides has suppressed the production of more than 30 crops over 40 countries [6,7,8,9]. For example, the species reduced the production of direct-seed rice by 15–65%, soybean by 50–75%, maize by 15–65%, and groundnut by 45–70% [10]. Ageratum conyzoides also acts as a host for many crop diseases, such as okra enation leaf curl virus, capsicum chlorosis virus, cotton leaf curl virus, and tomato yellow leaf curl virus [11,12,13], and as a host of aphids that carry papaya ringspot virus [14]. The infestation of Ageratum conyzoides in grasslands reduced the production of grass fodder, causing a shortage in the fodder supply for livestock [15]. The infestation of Ageratum conyzoides has also been reported to significantly affect natural ecosystems. The species formed dense monocultural stands on forest floors and grasslands, reducing the species diversity by 32%, fresh biomass by 40%, and dry biomass by 49% in the introduced ranges [16]. Its infestation has been reported to threaten the survival of protective indigenous plant species on the Hawaiian islands, including Isodendrion longifolium and Brighamia insignis [4,17].
Its life history traits, such as its high growth rate, high reproduction rate, and high adaptivity, including phenotypic plasticity, contribute to the naturalization of this invasive plant species and to increasing its population in the introduced ranges [18,19,20,21,22]. Ageratum conyzoides has a short life cycle and early reproductive maturity. The species can complete its life cycle in less than 2 months, and it bears flowers when two leaves expand [23,24]. Ageratum conyzoides produces two generations a year under favorable growth conditions [24]. The species produces 40,000–95,000 seeds per plant [4,15,24]. The seeds are small and lightweight, and dispersed through water and wind, the attachment of the aristate pappus to stick to animals and human clothes, and the contaminant in crops and soil [3,4,5]. The average dispersal distance was recorded to be 2.4 km per year [25]. The seeds did not show any marked dormancy, and half of the seeds germinated [15,23,24,26,27,28,29].
Ageratum conyzoides thrives in open areas with high humidity and high soil fertility and at temperatures ranging between 20 °C and 25 °C [4,5]. Its chromosome number was reported to be 2n = 20 or 40 [1,4,30]. The species has great morphological variety and is highly adaptive to different moisture and temperature conditions and shade conditions [31]. The species has survived at temperatures between 15 °C and 30 °C [5]. The species was found in mountain areas at up to 1800 m elevation [28,32]. Ageratum conyzoides also maintains its dense population under dry and shaded conditions [33,34,35]. The species has infested protective forests, in which the forest floor was relatively dark, and destroyed the community of the native undergrowth species [34,35]. These observations suggest that the life history traits of Ageratum conyzoides, such as its short life cycle and early reproductive maturity, prolific seed production, and high adaptivity to various environmental conditions, may contribute to the invasiveness of the species.
Many of the invasive plant species are also reported to possess defense molecules, which are involved in defense functions against natural enemies, such as herbivores and pathogens, as well as allelochemicals involved in allelopathy against competitive plant species [19,20,36,37,38,39]. These compounds may also contribute to the invasiveness of Ageratum conyzoides. However, there has been no review article focusing on the defense molecules, including allelochemicals, of Ageratum conyzoides involved in such functions. This work provides an overview of the defense responses and allelopathy of the species, and the compounds involved in its defense functions. The action mechanisms of the molecules involved in the defense functions are also discussed. The literature has been searched using a combination of the predominant online search engines, i.e., Scopus, ScienceDirect, and Google Scholar, and all possible combinations of Ageratum conyzoides with the following terms: botany, biology, habitat, reproduction, adaptively, plasticity, invasiveness, impact, natural enemy, insecticidal activity, fungicidal activity, nematode, symbiosis, rhizobium, allelopathy, allelochemical, pharmacology, and second metabolite.
2. Defense Molecules against Herbivore Insects
One of the essential factors for plant species to survive invasion is their defense ability against herbivore insects as natural enemies. Herbivore insects sometimes cause significant damage to plant growth, development, and regeneration [40,41,42]. Therefore, some plant species have developed a chemical defense strategy against their natural enemies [19,20,43,44].
Aqueous extracts of Ageratum conyzoides stems and leaves increased the mortality of an adult polyphagous grasshopper (Zonocerus variegatus) [45]. Hexane extracts of Ageratum conyzoides leaves also increased the mortality of the adult insects of Diaphania hyalinata, Musca domestica, Periplaneta americana, and Rhyzopertha dominica [46]. The whole plant extracts of Ageratum conyzoides, using aqueous solutions, methanol, and other organic solvents, showed insecticidal activity against several crop pest insects, such as a stalk borer (Chilo partelus) [47], a rice weevil (Sitophilus oryza), a rice bug (Leptocorisa chinensis) [48], and a mosquito (Anopheles gambiae), which is the most important vector of malaria [49]. The essential oil of Ageratum conyzoides also showed insecticidal activity against a crop grain insect (Tribolium castaneum) [50] and inhibitory activity regarding the metamorphosis of a cowpea weevil (Callosobruche naculatus) [51]. The essential oil showed ovicidal activity and reduced the fertility of a cotton strainer (Dysdercus angulatus) [48].
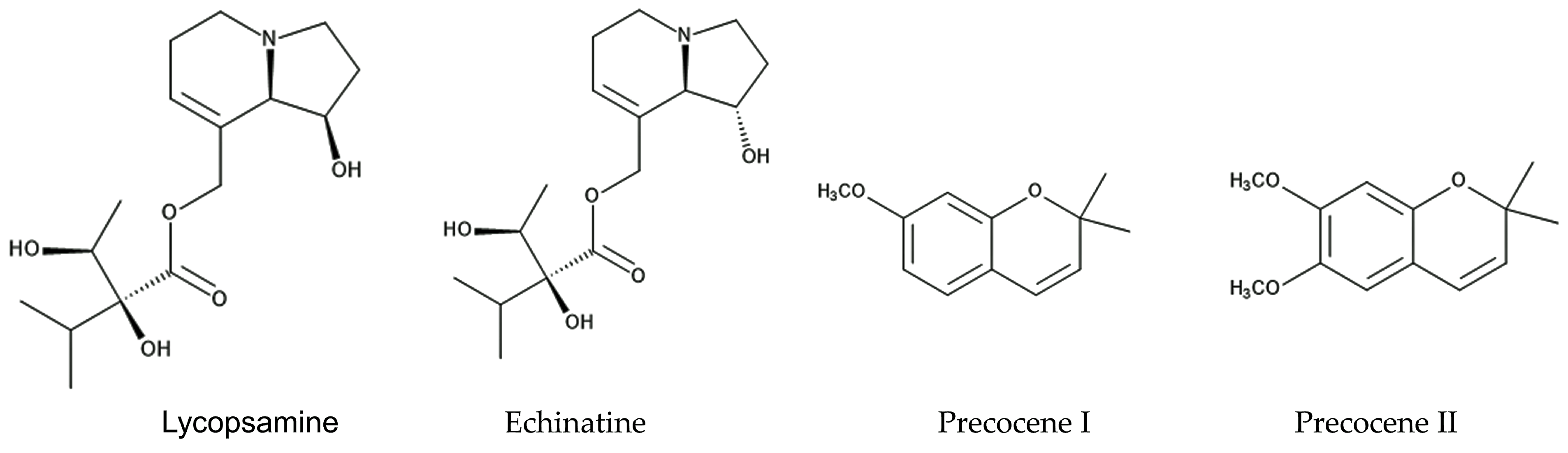
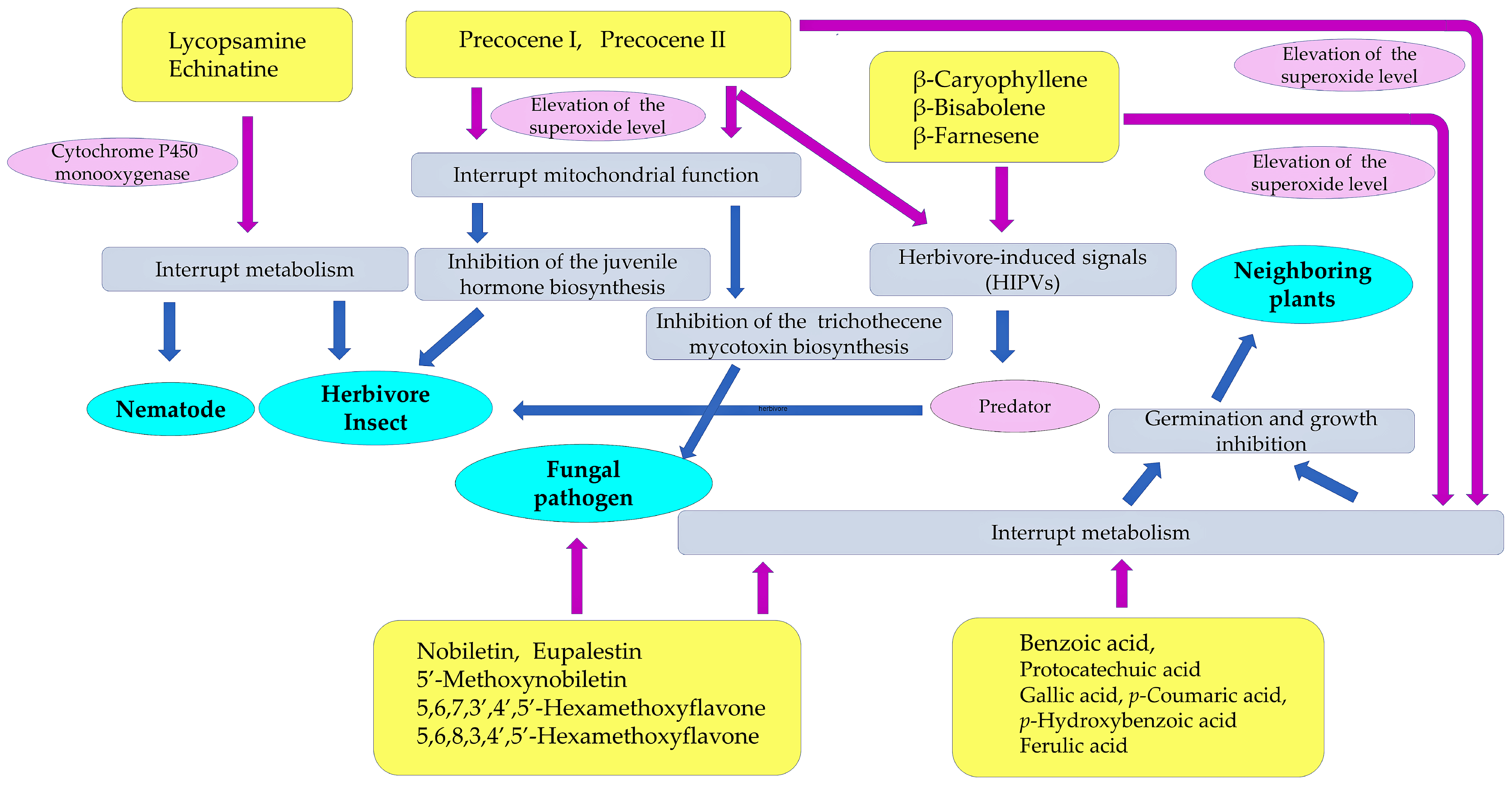
Two isomeric pyrrolizine alkaloids, lycopsamine and echinatine, were found in extracts of Ageratum conyzoides [52]. Pyrrolizidine alkaloids consist of a necine base and a double five-membered ring with a nitrogen atom in the middle, esterified with mono- or dicarboxylic acids, called a necic acid [53]. Pyrrolizidine alkaloids have been found in more than 300 different compounds in the plant families of Asteraceae, Boraginaceae, Fabaceae, and Orchidaceae [54]. These compounds are synthesized from L-arginine, and the specific intermediate is a homospermidine (polyamine). Pyrrolizidine alkaloid N-oxides are some of the primary products of pyrrolizidine alkaloid biosynthesis [55]. These plant species may produce these pyrrolizidine alkaloids as chemical defense agents against herbivores, such as insects and mammals [56,57,58,59]. The compounds are highly toxic, showing highly hepatotoxic, genotoxic, cytotoxic, tumorigenic, and neurotoxic activity. After absorption by insects and mammals, the first step in the activation of pyrrolizidine alkaloids is dehydrogenation catalyzed by cytochrome P450 monooxygenases [60,61], and the activated compounds interrupt several types of metabolism in the cell functions of these insects and mammals [62,63]. Therefore, the pyrrolizidine alkaloids in Ageratum conyzoides may be involved in the insecticidal activity caused by the extracts and essential oil of the species, as described above, and contribute to the protection of the species from herbivore attacks (Figure 2).
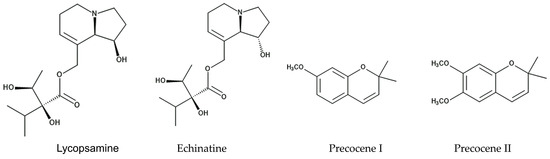
Figure 2.
The compounds involved in the insecticidal activity of Ageratum conyzoides.
However, certain specialist herbivores have evolved tolerance to pyrrolizidine alkaloids. These specialists accumulate and store pyrrolizidine alkaloids in certain organs. The accumulated pyrrolizidine alkaloids are used for protection from their predators as poison and as precursors to synthesize mating pheromones. Some of these insects also transfer the pyrrolizidine alkaloids to their eggs for the protection of their offspring [53,64,65]. However, Ageratum conyzoides may seldom meet these specialist insects in its introduced ranges, because there may be no such coevolutionary history between these insects and Ageratum conyzoides in the introduced ranges.
The extracts and essential oil of Ageratum conyzoides were reported to contain two benzopyran derivatives, precocene I and precocene II (formerly named ageratochromene) [48,66,67]. These compounds are toxic and have shown anti-juvenile hormone activity, such as the inhibition of the reproduction of a bean beetle (Epilachna varivestis), the induction of diapause in a potato beetle (Leptinotarsa decenlineata) [66], and the inhibition of the metamorphosis of a moth (Spodoptera manuritta) [68]. The juvenile hormone is known to control several aspects of insect development, such as reproduction, diapause, and metamorphosis [69]. Precocene II was reported to inhibit the biosynthesis of the juvenile hormone [70]. In addition, precocene II was reported to cause morphological abnormalities in the pupae development of a crop pest beetle (Epilachna vigintioctopunctata) [71] and to interrupt mitochondrial function in rat cells [72]. These observations suggest that precocene I and precocene II may suppress insect growth and development due to the interruption of juvenile hormone biosynthesis and contribute to protection from herbivore insect attacks as defense molecules (Figure 2).
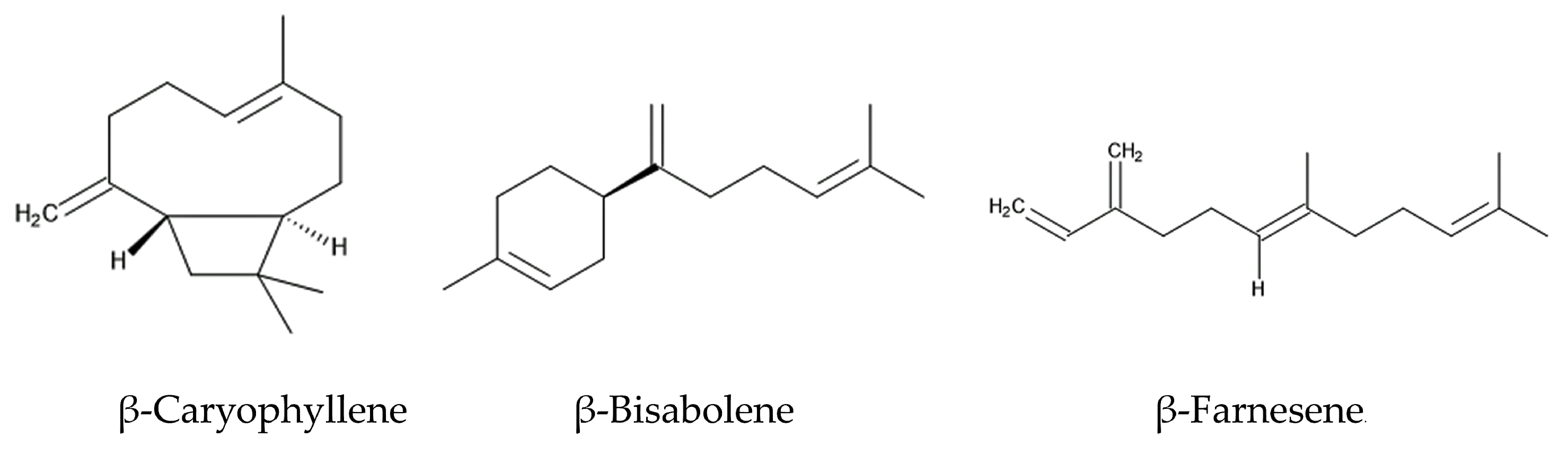
The intercropping of Ageratum conyzoides in citrus orchards increased the population of a predator mite, Amblyseius newsami, which hunts for a herbivore mite, Panonychus citri. Panonychus citri is the natural enemy of citrus and reduces citrus production significantly [68,73]. Ageratum conyzoides emits a mixture of volatiles, such as precocenes I and II, and three sesquiterpenes: β-caryophyllene, β-bisabolene, and β-farnesene (Figure 3). The concentrations of these volatiles in the air of Ageratum conyzoides-intercropping citrus orchards were greater than those in non-intercropping citrus orchards [67].
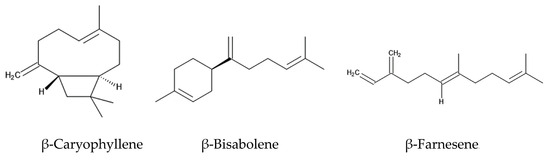
Figure 3.
The compounds that act as HIPVs involved in indirect defense function.
When herbivore insects attack, certain plants emit a mixture of volatiles consisting of different chemical classes, called herbivore-induced plant volatiles (HIPVs) [70]. HIPVs stimulate predators to hunt herbivores as their prey. The predator insects sense HIPVs via the olfactory sensilla located on their antennae [74]. The responses of predator insects to HIPVs vary among predator species, and only a particular mixture of HIPVs (chemical competition and concentration) serve as signals for specific insects [75]. Then, the sensorial functions trigger the hunting behavior of these insects against the herbivores. HIPVs are considered to be involved in the indirect defense function of plants against herbivores [74,75]. The essential oil of Ageratum conyzoides and a volatile mixture of precocenes I and II, β-caryophyllene, β-bisabolene, and β-farnesene attracted Amblyseius newsami [67]. Therefore, the volatile mixture emitted from Ageratum conyzoides may serve as HIPVs involved in indirect defense function. β-Farnesene is known to act as a HIPV in several other plant species [75].
3. Defense Molecules against Nematodes
Plant-parasitic nematodes, such as root-knot nematodes Meloidogyne spp., are some of the major plant pathogens [76,77]. The host range of Meloidogyne spp. is wide, and their parasitism causes significant growth retardation in the host plant species. The nematodes creates galls in the plant roots and reduce the photosynthates and nutrients available to their host plants, leading to the loss of plant vigor and defense capabilities against other pathogen attacks [78,79,80]. Aqueous extracts of Ageratum conyzoides leaves increased the mortality of Meloidogyne incognita [81] and Meloidogyne javanica [82]. Its aqueous leaf extracts also suppressed the parasitic gall formation of Meloidogyne incognita [83]. Although the active compounds in the extracts have not yet been determined, these observations suggest that Ageratum conyzoides may possess certain compounds that have nematicidal activity. As described in Section 2, Ageratum conyzoides contains pyrrolizidine alkaloids, which are highly toxic to insects and mammals [60,61]. Therefore, these pyrrolizidine alkaloids may be involved in the nematicidal activity of the species.
4. Defense Molecules against Fungal Pathogens
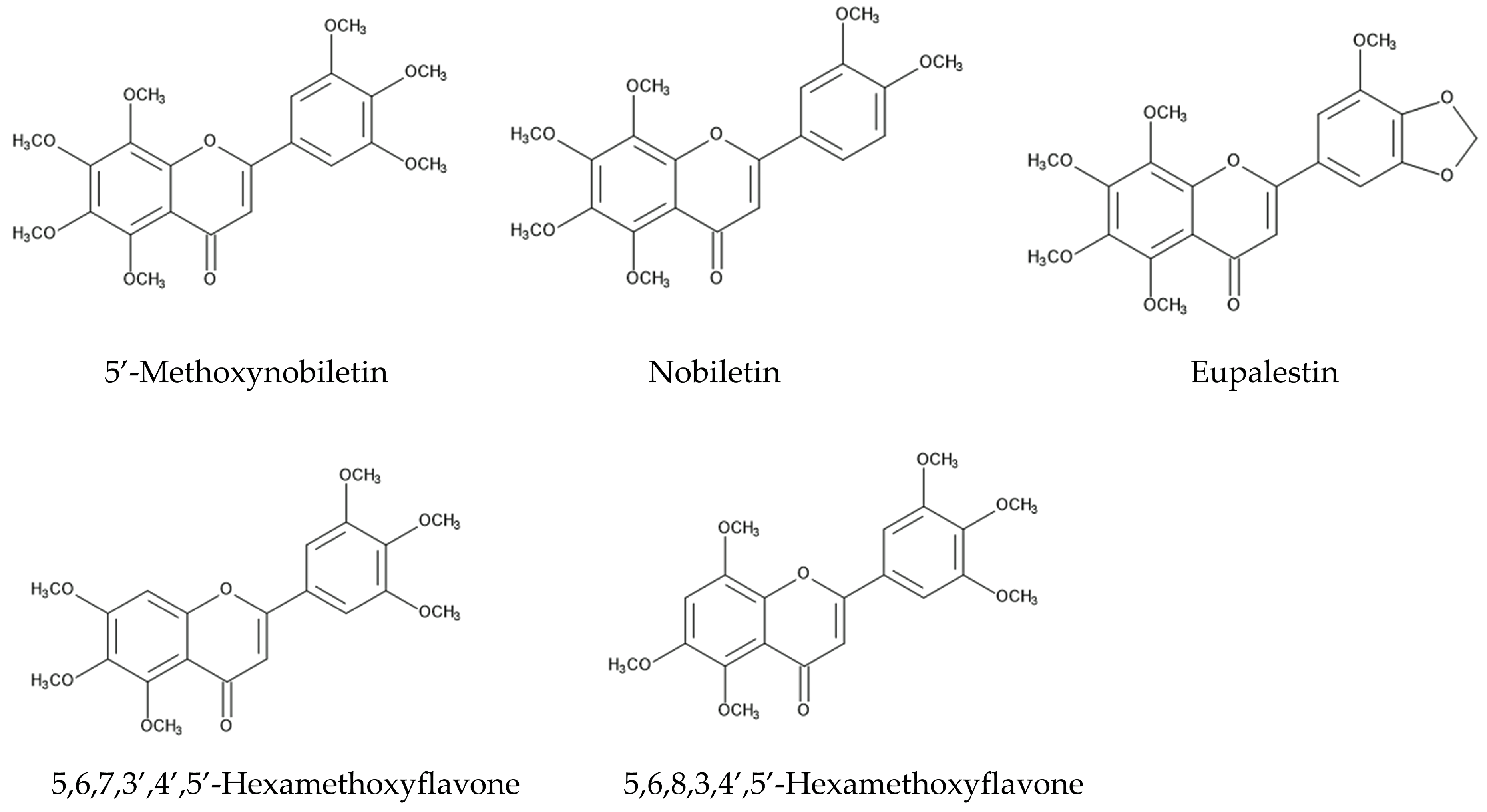
The defense ability against fungal pathogens is one of the essential factors for plants to survive an invasion. Some Fusarium spp. are fungal plant pathogens, causing diseases such as rot, blights, cankers, and wilts in the host plant tissue [84,85,86]. Fusarium also produces a number of mycotoxins, such as trichothecenes and fumonisins [87,88]. Aqueous n-hexane and methanol extracts of whole plants of Ageratum conyzoides suppressed the growth of Fusarium solani, which causes rot and wilt diseases [89]. Methanol extracts of the aboveground parts of Ageratum conyzoides suppressed the growth of Fusarium oxysporum, which causes blight and wilt diseases [90]. In addition, extracts of the aerial parts of Ageratum conyzoides inhibited the growth of a rice blast fungus, Pyricularia oryzae, and a sugar beet root rot fungus, Rhizoctonia solani. Precocene II and four flavonoids, nobiletin, 5′-methoxynobiletin, eupalestin, and 5,6,7,3′,4′,5′-hexamethoxyflavone, were identified in the extracts as the active compounds, and the inhibitory activity of precocene II was the highest among them [91] (Figure 4).
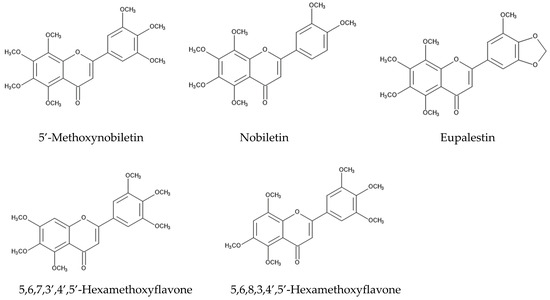
Figure 4.
The compounds involved in the fungicidal activity of Ageratum conyzoides.
The intercropping of Ageratum conyzoides in citrus orchards decreased the populations of the soil-pathogenic fungi Phytophthora citrophthora, Pythium aphanidermatum, and Fusarium solani. Precocenes I and II and three flavonoids, 5′-methoxynobiletin (5,6,7,8,3,4′,5′-heptamethoxyflavone), 5,6,7,3′,4′,5′-hexamethoxyflavone, and 5,6,8,3,4′,5′-hexamethoxyflavone, were found in the soil where Ageratum conyzoides was intercropped. These compounds inhibited the spore germination of these pathogenic fungi [92]. These observations suggest that Ageratum conyzoides possesses antifungal activity and precocenes I and II, as well as the mentioned flavonoids, may be involved in this activity.
Precocenes I and II were reported to inhibit the production of trichothecene mycotoxin in a pathogenic fungus, Fusarium graminearum. The inhibitory activity of precocene II was much greater than that of precocene I [93,94]. Trichothecene is synthesized from farnesyl pyrophosphate, which is produced through the mevalonate pathway, and its synthesis is regulated by the TRI6 (trichothecene biosynthesis positive transcription factor) protein encoded by Tri6 genes [95,96]. Precocenes II binds to a mitochondrial outer membrane protein and elevates the mitochondrial superoxide levels. The high levels of superoxide in mitochondria decrease the Tri6 gene levels and TRI6 protein, resulting in the suppression of trichothecene production [95,96,97]. In addition, the insect juvenile hormone is also synthesized from farnesyl pyrophosphate in the corpus allatum cells of insects [98]. As described in Section 2, precocene II was reported to interrupt mitochondrial function [72] and to inhibit the biosynthesis of the juvenile hormone [70]. Therefore, precocene II may bind to the mitochondrial membrane proteins of the corpus allatum cells and interrupt juvenile hormone biosynthesis.
These observations suggest that precocenes I and II and these flavonoids may work as defense molecules against fungal pathogen attacks and help the invasion of Ageratum conyzoides into the introduced ranges.
5. Inhibitors for Symbiosis
When the whole plant residues of Ageratum conyzoides were mixed with soil, the soil suppressed the growth and nodulation of a leguminous plant chickpea (Cicer arietinum) [99]. Leguminous plants generally coexist with symbiotic rhizobia [100,101,102]. Rhizobium nodulation enhances the host plant’s performance through the supply of nitrogen and ammonium to the host plant [103,104]. Ageratum conyzoides may possess certain compounds that degrade the nodulation of nearby legume plants. A reduction in rhizobium nodulation weakens the ability of these legumes to perform nitrogen and ammonium acquisition, which may cause the growth suppression of these plant species. Some other invasive plant species were also reported to suppress the colonization of the rhizobia and arbuscular mycorrhiza of native plant species [105,106]. Certain flavonoids released from leguminous plant species are known to act as signals for the induction of the nodulation genes in rhizobia and the initiation of symbiosis [103,104]. The compounds in Ageratum conyzoides may reduce the rhizobium population and interfere with the flavonoid signals and/or nodulation, resulting in the interruption of the symbiosis between the legumes and rhizobia. However, there is no information available on the compounds involved in the interruption of this symbiosis. The identification of these compounds is necessary.
6. Defense Molecules against Neighboring Plants
Allelopathy is the plant-to-plant interaction in the local plant community, occurring through certain secondary metabolites defied as allelochemicals. The donor plant species produce and release allelochemicals into their neighboring environments, and these released allelochemicals suppress the germination, growth, development, and/or regeneration process of the receiver plant species. Subsequently, the donor plants gain a relatively large quantity of resources, such as light, water, and nutrients, in the local plant community [107,108,109,110]. The competitive ability of invasive plant species against indigenous plant species for resource acquisition is one of the most important factors for their success in the introduced ranges [19,20,111,112]. The allelopathic potential of invasive plant species against indigenous plant species is often reported to be high [113,114,115].
The inhibitory effects of certain allelochemicals in invasive plant species against competitive plant species are considered to be greater in the introduced ranges than in the native ranges of the invasive plant species. In their native ranges, the competitive plant species may have developed tolerance to these allelochemicals because of their coevolutionary history. However, in their introduced ranges, the competitive plant species may not have had an opportunity to acquire tolerance to these allelochemicals because they had not existed together before. Therefore, according to the novel weapons hypothesis, the allelochemicals released from invasive plant species are more effective on indigenous plant species in the introduced ranges and contribute to their invasiveness [36,111,112].
Allelochemicals are synthesized, stored in certain plant organs, and released into the neighboring environment through volatilization, root exudation, and the decomposition of plant residues in the rhizosphere soil [107,108,109,110]. Therefore, allelochemicals have been identified in the extracts of plant organs (leaves, stems, and roots), essential oils, volatiles, root exudates, and rhizosphere soil [116,117,118].
Aqueous extracts of Ageratum conyzoides leaves inhibited the germination and growth of Parthenium hysterophorus [119]. Acetone extracts of Ageratum conyzoides leaves and roots inhibited the germination and growth of Oryza sativa [120]. Acetone extracts of Ageratum conyzoides shoots (leaves and stems) inhibited the germination and growth of Amaranthus caudatus, Digitaria sanguinalis, and Lactuca sativa in an extract concentration-dependent manner [121]. Meanwhile, n-hexane and ethyl acetate extracts of Ageratum conyzoides leaves inhibited the growth of Amaranthus spinosus, and a major constituent in both extracts was precocene II [122,123]. These observations suggest that Ageratum conyzoides contains certain extractable allelochemicals, including precocene II.
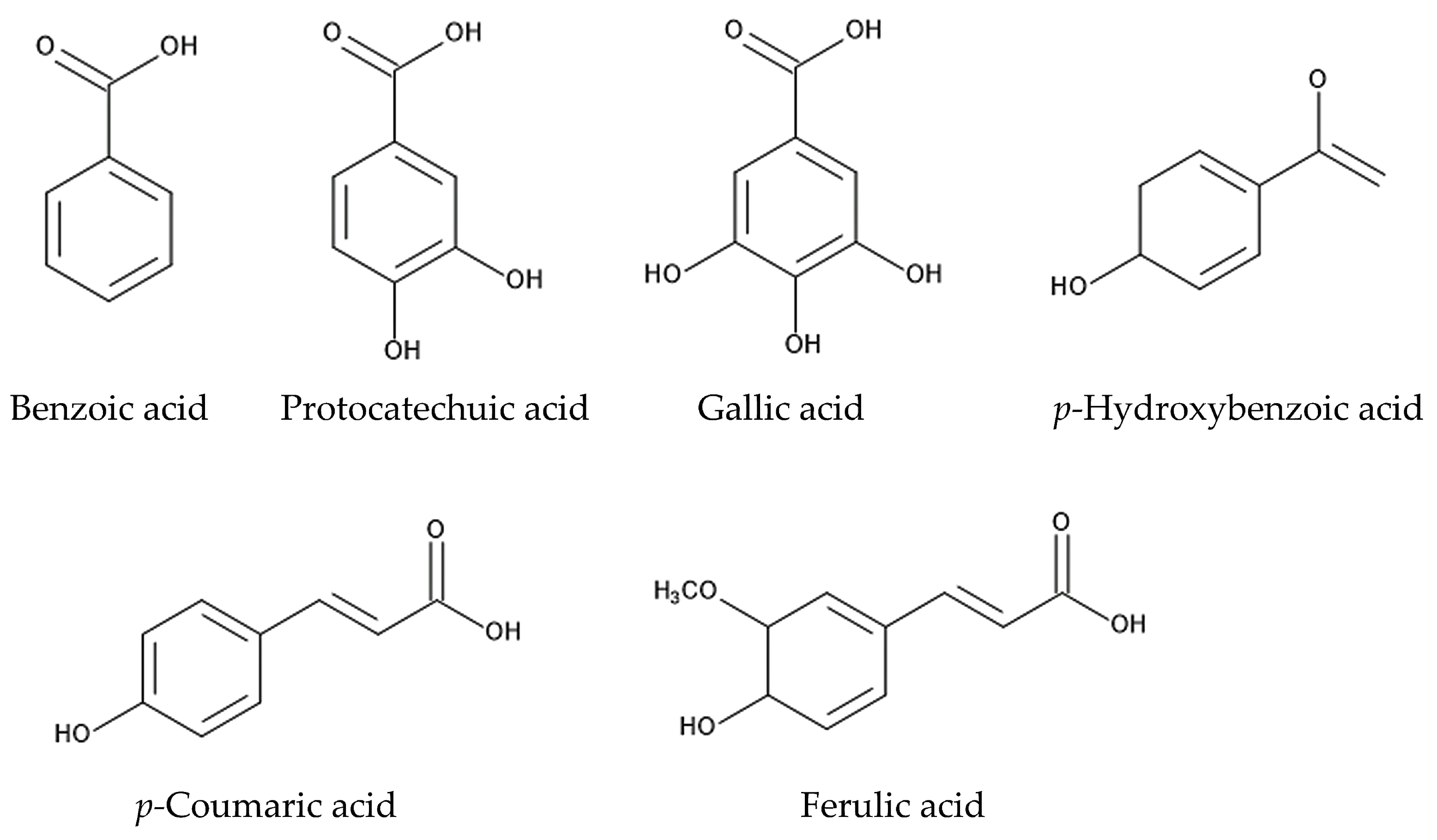
The whole plant powder of Ageratum conyzoides incorporated into the soil inhibited the germination and growth of Echinochloa crus-galli, Monochoria vaginalis, and Aeschynomene indica. Coumalic acid, gallic acid, and benzoic acid were major constituents in the aqueous methanol extracts of Ageratum conyzoides whole plants [124]. When the root residues of Ageratum conyzoides were incorporated into soil, the soil suppressed the growth of Oryza sativa [125], and protocatechuic acid, p-coumaric acid, gallic acid, ferulic acid, and p-hydroxybenzoic acid were identified in the aqueous extracts of the soil as allelopathic agents [8]. The aqueous extracts of soil previously infested by Ageratum conyzoides inhibited the growth of Triticum aestivum [126]. The root exudates of Ageratum conyzoides suppressed the germination and growth of Abelmoschus esculentus, Solanum lycopersicum, Phaseolus vulgaris, Zea mays, Cicer arietinum, and Cucumis sativus [127]. These observations suggest that Ageratum conyzoides may contain certain allelochemicals, which are released into the rhizosphere soil through the root exudation and decomposition processes of plant residues. Protocatechuic acid, p-coumaric acid, gallic acid, ferulic acid, and p-hydroxybenzoic acid may be some of these allelochemicals.
The intact fresh leaves of Ageratum conyzoides and its essential oil inhibited the growth of Cucumis sativus, Lolium ultiforum, Raphanus sativus, Phaseolus aureus, Triticum aestivum, and Lycapesicon spp. in sealed bottles. Precocenes I and II and β-caryophyllene were found as active compounds [128]. This observation suggests that certain allelochemicals, including precocenes I and II and β-caryophyllene, may be released into the air through volatilization from Ageratum conyzoides.
Precocenes I and II elevate the mitochondrial superoxide levels in the cells of insects and fungi, leading to insecticidal activity and fungicidal activity, as described in Section 4. High levels of superoxide were also reported to cause allelopathic activity, such as germination and growth inhibition against several plant and alga species [129,130,131]. Therefore, the allelopathic activity of precocenes I and II may also be caused by the elevation of the superoxide levels in plant cells. Three sesquiterpenes, β-caryophyllene, β-bisabolene, and β-farnesene, emitted from Ageratum conyzoides showed allelopathic activity [132]. In addition, β-caryophyllene was identified in another invasive plant species, Mikania micrantha [133], and was considered to act as a allelochemical due to the elevation of the superoxide levels in the cells of the receiver plant species [134,135,136]. Therefore, these sesquiterpenes emitted from Ageratum conyzoides may induce allelopathic activity due to the elevation of the superoxide levels in the cells of the receiver plant species.
Soil samples obtained from Ageratum conyzoides-intercropped citrus orchards suppressed the growth of Bidens pilosa, Digitaria sanguinalis, and Cyperus difformis. Precocene II and three flavonoids, 5′-methozynobiletin, 5,6,7,3′,4′,5′-heptamethoxyflavone, and 5,6,8,3,4′,5-hexamethoxyflavone, were identified in citrus orchard soil as allelopathic agents [92] (Figure 4). Flavonoids are polyphenolic secondary metabolites synthesized from phenylalanine through chalcone. Many flavonoids are reported to have anti-fungal, anti-herbivore, anti-bacterial, and allelopathic activity [137,138,139,140]. Ageratum conyzoides is rich in polyoxygenated flavonoids [141]. Therefore, some of these identified flavonoids and precocene II in Ageratum conyzoides may be released into the rhizosphere soil through the decomposition of plant residues and act as allelopathic agents. However, the molecular targets of the flavonoids in plant cells are unknown.
Benzoic acid and cinnamic acid derivatives, such as protocatechuic acid, gallic acid, p-coumaric acid, p-hydroxybenzoic acid, and ferulic acid, were identified in extracts of the roots, leaves, and stems of Ageratum conyzoides and/or in soil mixed with its root residues [8,124,125]. Cinnamic acid and its derivatives are synthesized via the shikimic acid pathway from phenylalanine [142,143]. These compounds have been identified in a wide range of plant extracts, plant residues, and plant rhizosphere soil. The involvement of benzoic acid and cinnamic acid derivatives in allelopathy and the mechanisms of their allelopathic action have been investigated in other plant species [144,145,146]. These compounds cause structural alterations in the plasma membrane lipids and proteins of plant cells and reduce the transmembrane electrochemical potential, which causes the depolarization of the membranes. The depolarization of the membranes induces the nonspecific efflux of both cations and anions, including phosphate, potassium, magnesium, and nitrate ions, and affects the water balance in the cells. These compounds also interrupt the activity of various enzymes involved in several types of metabolism, such as photosynthesis, respiration, phytohormone synthesis, protein synthesis, and the synthesis of other secondary metabolites, and they affect plant cell division, growth, and development [144,145,146,147]. Therefore, benzoic acid, protocatechuic acid, gallic acid, p-coumaric acid, p-hydroxybenzoic acid, and ferulic acid may affect the plasma membrane structure and certain enzymes’ activity and act as allelopathic agents (Figure 5).
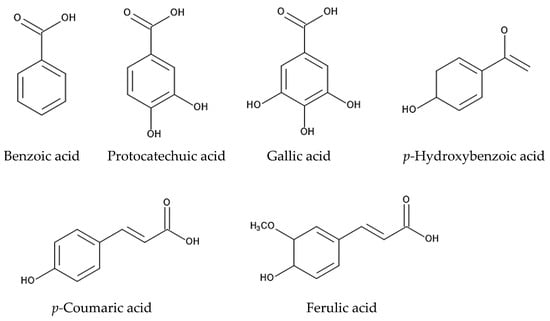
Figure 5.
The compounds involved in the allelopathy of Ageratum conyzoides.
These allelochemicals identified in Ageratum conyzoides may contribute to the suppression of the germination and growth of indigenous plant species and increase the competitive ability of Ageratum conyzoides for the acquisition of nutrients, water, and light in the introduced ranges.
7. Contributions of Defense Molecules to Invasive Traits of Ageratum conyzoides
Ageratum conyzoides produces several defense molecules against insects, nematodes, fungal pathogens, and competitive neighboring plant species. Among them, precocenes I and II showed insecticidal activity through the inhibition of insect juvenile hormone biosynthesis [70]. Precocenes I and II also have fungicidal activity [92], and they exhibit inhibitory activity regarding trichothecene mycotoxin biosynthesis through the elevation of the mitochondrial superoxide levels [93,94]. Both compounds have shown allelopathic activity and suppressed the germination and growth of several plant species [122,123,128]. In addition, a mixture of volatiles, including precocenes I and II and three sesquiterpenes, namely β-caryophyllene, β-bisabolene, and β-farnesene, emitted from Ageratum conyzoides, may work as HIPVs involved in the indirect defense function against herbivore insects [67] (Figure 6).
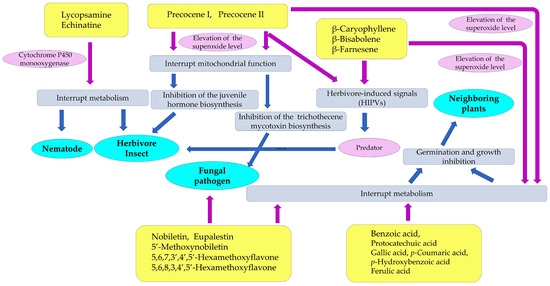
Figure 6.
Defense molecules involved in the invasive abilities of Ageratum conyzoides. These compounds act as nematicidal, insecticidal, fungicidal, and allelopathic agents of Ageratum conyzoides. Purple arrow: direct action; blue arrow: secondary and tertiary action.
Pyrrolizidine alkaloids, such as lycopsamine and echinatine, are highly toxic and possess insecticidal activity through the interruption of metabolism in insect cells, resulting in protection from herbivore insect attacks. These compounds may also work as defense agents and be involved in nematicidal activity. Flavonoids, such as nobiletin, 5′-methoxynobiletin, eupalestin, 5,6,7,3′,4′,5′-hexamethoxyflavone, and 5,6,8,3,4′,5′-hexamethoxyflavone, found in Ageratum conyzoides were reported to show inhibitory activity regarding the spore germination of pathogenic fungi [91,92]. Benzoic acid, protocatechuic acid, gallic acid, p-coumaric acid, p-hydroxybenzoic acid, and ferulic acid may act as allelopathic agents, causing the inhibition of the germination and growth of other plant species [124,125,126]. The sesquiterpene β-caryophyllene and the flavonoids found in Ageratum conyzoides may also act as allelopathic agents [128] (Figure 6).
Pharmacological and phytochemical investigations have shown that Ageratum conyzoides contains many other secondary metabolites in several chemical classes, such as monoterpenes, sesquiterpenes, flavonoids, and sterols. Some of these compounds are related to pharmacological activity, such as anti-pyretic, anti-inflammatory, cardiovascular, and analgesic activity, which is exploited in medicinal treatment, and anti-microbial activity, which benefits food security [94,148,149,150,151,152,153]. Although the identified compounds have not yet been related to the invasiveness of Ageratum conyzoides, some of them may act as defense molecules for unknown functions.
In conclusion, the defense responses of invasive plants to their natural enemies, such as herbivores and pathogens, is one of the essential functions for their naturalization and population expansion in their introduced ranges. The allelopathy of invasive plants against indigenous plant species is also one of these functions. As described above, Ageratum conyzoides produces several compounds that act as defense molecules against its natural enemies, such as herbivore insects, parasitic nematodes, and fungal pathogens, and act as allelochemicals against neighboring plant species. Therefore, these compounds may contribute to the naturalization and expanding population of Ageratum conyzoides in new habitats as an invasive plant species. These compounds may be used in the development of insecticides, fungicides, and/or herbicides.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ming, L.C. Ageratum conyzoides: A tropical source of medicinal and agricultural products. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 469–473. [Google Scholar]
- Global Invasive Species Database. Species Profile: Ageratum conyzoides. Available online: https://www.iucngisd.org/gisd/species.php?sc=1493 (accessed on 12 August 2024).
- Royal Botanical Gardens, Kew, Ageratum conyzoides. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:7086-2 (accessed on 12 August 2024).
- CABI Copmpendium. Ageratum conyzoides (Billy Goat Weed). Available online: https://www.cabidigitallibrary.org/doi/10.1079/cabicompendium.3572 (accessed on 12 August 2024).
- Kaur, A.; Kaur, S.; Singh, H.P.; Datta, A.; Chauhan, B.S.; Ullah, H.; Kohli, R.K.; Batish, D.R. Ecology, biology, environmental impacts, and management of an agro-environmental weed Ageratum conyzoides. Plants 2023, 12, 2329. [Google Scholar] [CrossRef] [PubMed]
- Paraseth, P.; Banerjee, K. Goat weed (Ageratum conyzoides L.): A biological threat to plant diversity in Eastern Ghats of India. J. Biosci. 2024, 49, 72. [Google Scholar] [CrossRef]
- Manandhar, S.; Shrestha, B.B.; Lekhak, H.D. Weeds of paddy field at Kirtipur, Kathmandu. Sci. World 2007, 5, 100–106. [Google Scholar] [CrossRef]
- Batish, D.R.; Kaur, S.; Singh, H.P.; Kohli, R.K. Role of root-mediated interactions in phytotoxic interference of Ageratum conyzoides with rice (Oryza sativa). Flora 2009, 204, 388–395. [Google Scholar] [CrossRef]
- Devi, C.; Khwairakpam, M. Feasibility of vermicomposting for the management of terrestrial weed Ageratum conyzoides using earthworm species Eisenia fetida. Environ. Technol. Innov. 2020, 18, 100696. [Google Scholar] [CrossRef]
- Gharde, Y.; Singh, P.K.; Dubey, R.P.; Gupta, P.K. Assessment of yield and economic losses in agriculture due to weeds in India. Crop Prot. 2018, 107, 12–18. [Google Scholar] [CrossRef]
- Anwar, I.; Bukhari, H.A.; Nahid, N.; Rashid, K.; Amin, I.; Shaheen, S.; Hussain, K.; Mansoor, S. Association of cotton leaf curl Multan betasatellite and Ageratum conyzoides symptomless alphasatellite with tomato leaf curl New Delhi virus in Luffa cylindrica in Pakistan. Australas. Plant Pathol. 2020, 49, 25–29. [Google Scholar] [CrossRef]
- Sharman, M.; Thomas, J.E.; Tree, D.; Persley, D.M. Natural host range and thrips transmission of capsicum chlorosis virus in Australia. Australas. Plant Pathol. 2020, 49, 45–51. [Google Scholar] [CrossRef]
- Serfraz, S.; Amin, I.; Akhtar, K.P.; Mansoor, S. Recombination among Begomoviruses on Malvaceous plants leads to the evolution of okra enation leaf curl virus in Pakistan. J. Phytopathol. 2015, 163, 764–776. [Google Scholar] [CrossRef]
- Martins, D.D.S.; Ventura, J.A.; Paula, R.D.C.A.L.; Fornazier, M.J.; Rezende, J.A.M.; Culik, M.P.; Ferreira, P.S.F.; Peronti, A.L.B.G.; de Carvalho, R.C.Z.; Sousa-Silva, C.R. Aphid vectors of Papaya ringspot virus and their weed hosts in orchards in the major papaya producing and exporting region of Brazil. Crop Prot. 2016, 90, 191–196. [Google Scholar] [CrossRef]
- Kohli, R.K.; Batish, D.R.; Singh, H.P.; Dogra, K.S. Status, invasiveness and environmental threats of three tropical American invasive weeds (Parthenium hysterophorus L., Ageratum conyzoides L., Lantana camara L.) in India. Biol. Invasions 2006, 8, 1501–1510. [Google Scholar] [CrossRef]
- Dogra, K.S.; Kohli, R.K.; Sood, S.K.; Dobhal, P.K. Impact of Ageratum conyzoides L. on the diversity and composition of vegetation in the Shivalik hills of Himachal Pradesh (Northwestern Himalaya), India. Int. J. Biodivers. Conser. 2009, 1, 135–145. [Google Scholar]
- US Fish and Wildlife Service. 5-Year Review: Summary and Evaluation. In Lsodendrion longifolium (aupaka); US Fish and Wildlife Service: Falls Church, VA, USA, 2011. Available online: http://ecos.fws.gov/docs/five_year_review/doc3809.pdf (accessed on 12 August 2024).
- Thompson, J.D.; McNeilly, T.; Gray, A.J. Population variation in Spartina anglica C.E. Hubbard. I. Evidence from a common garden experiment. New Phytol. 1991, 117, 115–128. [Google Scholar] [CrossRef]
- Mack, R.M. Predicting the identity and fate of plant invaders: Emergent and emerging approaches. Biol. Conserv. 1996, 78, 107–121. [Google Scholar] [CrossRef]
- Chengxu, W.; Mingxing, Z.; Xuhui, C.; Bo, Q. Review on allelopathy of exotic invasive plants. Procedia Engin. 2011, 18, 240–246. [Google Scholar] [CrossRef]
- Theoharides, K.A.; Dukes, J.S. Plant invasion across space and time: Factors affecting nonindigenous species success during four stages of invasion. New Phytol. 2007, 176, 256–273. [Google Scholar] [CrossRef]
- Warren, R.J.; Matt Candeias, M.; Labatore, A.; Olejniczak, M.; Yang, L. Multiple mechanisms in woodland plant species invasion. J. Plant Ecol. 2019, 12, 201–209. [Google Scholar] [CrossRef]
- Holm, L.G.; Plucknett, D.L.; Pancho, J.V.; Herberger, J.P. The World’s Worst Weeds. Distribution and Biology; University Press of Hawaii: Honolulu, HI, USA, 1977; pp. 1–609. [Google Scholar]
- Kaur, S.; Batish, D.R.; Kohli, R.K.; Singh, H.P. Ageratum conyzoides: An alien invasive weed in India. In Invasive Alien Plants: An Ecological Appraisal for the Indian Subcontinent; Bhatt, J.R., Singh, J.S., Singh, S.P., Tripathi, R.S., Kohli, R.K., Eds.; CABI: Wallingford, UK, 2012; pp. 57–76. [Google Scholar]
- Horvitz, N.; Wang, R.; Wan, F.H.; Nathan, R. Pervasive human-mediated large-scale invasion: Analysis of spread patterns and their underlying mechanisms in 17 of China’s worst invasive plants. J. Ecol. 2017, 105, 85–94. [Google Scholar] [CrossRef]
- Marks, M.K.; Nwachuku, A.C. Seed-bank characteristics in a group of tropical weeds. Weed Res. 1986, 26, 151–157. [Google Scholar] [CrossRef]
- Sauerborn, J.; Koch, W.; Krage, J. On the influence of light, temperature, depth of burial and water stress on the germination of selected weed species. Z. Pflanzenkr. Pflanzenschutz Sonderh. 1988, 11, 47–53. [Google Scholar]
- PIER. Pacific Island Ecosystems at Risk, Ageratum conyzoides. Available online: http://www.hear.org/pier/species/ageratum_conyzoides.htm (accessed on 12 August 2024).
- PROTA. PROTA4U Web Database. Plant Resources of Tropical Africa. 2018. Available online: https://www.prota4u.org/database/ (accessed on 12 August 2024).
- Gupta, R.C. Meiotic abnormalities in Ageratum conyzoides from hot desert of India (Rajasthan). Chromosome Bot. 2015, 10, 67–74. [Google Scholar]
- do Rosário, C.J.R.M.; Lima, A.S.; de Mendonça, C.; Soares, I.S.; Júnior, E.B.A.; Gomes, M.N.; Costa-Junior, L.M.; Maia, J.G.S.; da Rocha, C.Q. Essential oil Ageratum conyzoides chemotypes and anti-tick activities. Vet. Parasitol. 2023, 319, 109942. [Google Scholar] [CrossRef] [PubMed]
- Dogra, K.S.; Kohli, R.K.; Sood, S.K. An assessment and impact of three invasive species in the Shivalik hills of Himachal Pradesh, India. Int. J. Biodiv. Conserv. 2009, 1, 4–10. [Google Scholar]
- Chaudhary, N.; Narayan, R. The advancing dominance of Ageratum conyzoides L. and Lantana camara L. in dry tropical peri-urban vegetation in India. Int. Res. J. Environ. Sci. 2013, 2, 88–95. [Google Scholar]
- Sengupta, R.; Dash, S.S. A comprehensive inventory of alien plants in the protected forest areas of Tripura and their ecological consequences. Nelumbo 2021, 63, 163–182. [Google Scholar] [CrossRef]
- Semy, K.; Singh, M.R. Changes in plant diversity and community attributes of coal mine affected forest in relation to a community reserve forest of Nagaland, Northeast India. Trop. Ecol. 2024, 65, 16–25. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Meiners, S.J.; Kong, C.H.; Ladwig, L.M.; Pisula, N.L.; Lang, K.A. Developing an ecological context for allelopathy. Plant Ecol. 2012, 213, 1861–1867. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Involvement of allelopathy in the invasive potential of Tithonia diversifolia. Plants 2020, 9, 766. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy and allelochemicals of Leucaena leucocephala as an invasive plant species. Plants 2022, 11, 1672. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.L. Exotic plant invasions and the enemy release hypothesis. Trend Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Blossey, B.; Notzold, R. Evolution of increased competitive ability in invasive nonindigenous plants—A hypothesis. J. Ecol. 1995, 83, 887–889. [Google Scholar] [CrossRef]
- Muller-Scharer, H.; Schaffner, U.; Steinger, T. Evolution in invasive plants: Implications for biological control. Trends Ecol. Evol. 2004, 19, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. Allelopathy of Lantana camara as an invasive plant. Plants 2021, 10, 1028. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 15, 241. [Google Scholar] [CrossRef]
- Ingrid, D.T.; Akwanjoh, S.R.; Yacouba, M. Insecticidal activity of Ageratum conyzoides (Asteraceae) aqueous extracts against the grasshopper Zonocerus variegatus (Orthoptera: Pyrgomorphidae). J. Agric. Ecol. Res. Int. 2020, 21, 29–36. [Google Scholar] [CrossRef]
- Moreira, M.D.; Picanco, M.C.; Barbosa, L.C.A.; Guedes, R.N.C.; Barros, E.C.; Campos, M.R. Compounds from Ageratum conyzoides: Isolation, structural elucidation and insecticidal activity. Pest Manag. Sci. 2007, 63, 615–621. [Google Scholar] [CrossRef]
- Raja, S.S.; Singh, A.; Rao, S. Effect of Ageratum conyzoides on Chilo partelus swinhoe (Lepidoptera: Pyralidae). J. Anim. Morphol. Physiol. 1987, 34, 35–37. [Google Scholar]
- Fagoonee, I.; Umrit, G. Antigonadotropic hormones from the goatweed, Ageratum conyzoides. Insect Sci. Appl. 1981, 4, 373–376. [Google Scholar]
- Suwaiba, H.; Barde, A.A.; Mao, P.S.; Aliyu, O.A. Larvicidal activity of Ageratum conyzoides L. extracts on Anopheles gambiae complex. GSC Biol. Pharm. Sci. 2018, 3, 1–5. [Google Scholar]
- Singh, P.J.; Prakash, B.; Dubey, N.K. Insecticidal activity of Ageratum conyzoides L., Coleus aromaticus Benth. and Hyptis suaveolens (L.) Poit essential oils as fumigant against storage grain insect Tribolium castaneum Herbst. J. Food Sci. Technol. 2014, 51, 2210–2215. [Google Scholar]
- Gbolade, A.A.; Onayade, O.A.; Ayinde, B.A. Insecticidal activity of Ageratum conyzoides L. volatile oil against Callosobruchus chinensis F in seed treatment and fumigation laboratory tests. Insect Sci. Appl. 1999, 19, 237. [Google Scholar]
- Wiedenfeld, H.; Roder, E. Pyrrozidine alkaloids form Ageratum conyzoides. Planta Med. 1991, 57, 578–579. [Google Scholar] [CrossRef] [PubMed]
- Boppré, M. Lepidoptera and pyrrolizidine alkaloids. Exemplification of complexity in chemical ecology. J. Chem. Ecol. 1990, 16, 165–185. [Google Scholar] [CrossRef]
- Ober, D.; Hartmann, T. Homospermidine synthase, the first pathway-specific enzyme of pyrrolizidine alkaloid biosynthesis, evolved from deoxyhypusine synthase. Proc. Natl. Acad. Sci. USA 1999, 96, 14777–14782. [Google Scholar] [CrossRef]
- Schramm, S.; Köhler, N.; Rozhon, W. Pyrrolizidine alkaloids: Biosynthesis, biological activities and occurrence in crop plants. Molecules 2019, 24, 498. [Google Scholar] [CrossRef]
- van Dam, N.M.; Vuister, L.W.; Bergshoeff, C.; de Vos, H.; van der Meijden, E.D. The “Raison D’être” of pyrrolizidine alkaloids in Cynoglossum officinale: Deterrent effects against generalist herbivores. J. Chem. Ecol. 1995, 21, 507–523. [Google Scholar] [CrossRef]
- Joshi, J.; Vrieling, K. The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecol. Lett. 2005, 8, 704–714. [Google Scholar] [CrossRef]
- Gardner, D.R.; Thorne, M.S.; Molyneux, R.J.; Pfister, J.A.; Seawright, A.A. Pyrrolizidine alkaloids in Senecio madagascariensis from Australia and Hawaii and assessment of possible livestock poisoning. Biochem. Syst. Ecol. 2006, 34, 736–744. [Google Scholar] [CrossRef]
- Stegelmeier, B.L.; Colegate, S.M.; Brown, A.W. Dehydropyrrolizidine alkaloid toxicity, cytotoxicity, and carcinogenicity. Toxins 2016, 8, 356. [Google Scholar] [CrossRef]
- Gordon, G.J.; Coleman, W.B.; Grisham, J.W. Induction of cytochrome P450 enzymes in the livers of rats treated with the pyrrolizidine alkaloid retrorsine. Exp. Mol. Pathol. 2000, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Wang, W.; Yang, X.; Xiong, A.; Yang, L.; Wang, Z. Pyrrolizidine alkaloids: An update on their metabolism and hepatotoxicity mechanism. Liver Res. 2019, 3, 176–184. [Google Scholar] [CrossRef]
- Cheeke, P.R. Toxicity and metabolism of pyrrolizidine alkaloids. J. Anim. Sci. 1988, 66, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Tamariz, J.; Burgueño-Tapia, E.; Vázquez, M.A.; Delgado, F. Pyrrolizidine Alkaloids. In The Alkaloids: Chemistry and Biology; Hans-Joachim Knölker, H.J., Ed.; Academic Press: Cambridge, UK, 2018; pp. 1–314. [Google Scholar]
- Macel, M. Attract and deter: A dual role for pyrrolizidine alkaloids in plant-insect interactions. Phytochem. Rev. 2011, 10, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Livshultz, T.; Kaltenegger, E.; Straub, S.C.; Weitemier, K.; Hirsch, E.; Koval, K.; Mema, L.; Liston, A. Evolution of pyrrolizidine alkaloid biosynthesis in Apocynaceae: Revisiting the defense de-escalation hypothesis. New Phytol. 2018, 218, 762–773. [Google Scholar] [CrossRef]
- Bowers, W.S.; Ohta, T.; Cleere, J.S.; Marsella, P.A. Discovery of insect anti-juvenile hormones in plants. Science 1976, 193, 542–547. [Google Scholar] [CrossRef]
- Kong, C.H.; Hu, F.; Xu, X.H.; Zhang, M.X.; Liang, W.J. Volatile allelochemicals in the Ageratum conyzoides intercropped citrus orchard and their effects on mites Amblyseius newsami and Panonychus citri. J. Chem. Ecol. 2005, 31, 2193–2203. [Google Scholar] [CrossRef]
- Mathai, S.; Nair, V.S.K. Effects of precocene II on last instar larvae of Spodoptera mauritia (Lepidoptera: Noctuidae). Curr. Sci. 1983, 52, 376–377. [Google Scholar]
- Oi, C.A.; Ferreira, H.M.; da Silva, R.C.; Bienstman, A.; do Nascimento, F.S.; Wenseleers, T. Effects of juvenile hormone in fertility and fertility-signaling in workers of the common wasp Vespula vulgaris. PLoS ONE 2021, 16, e0250720. [Google Scholar] [CrossRef]
- Amsalem, E.; Teal, P.; Grozinger, C.M.; Hefetz, A. Precocene-I inhibits juvenile hormone biosynthesis, ovarian activation, aggression and alters sterility signal production in bumble bee (Bombus terrestris) workers. J. Exp. Biol. 2014, 217, 3178–3185. [Google Scholar] [CrossRef]
- Gupta, P.R.; Dogra, G.S. Bioactivity of Precocene II against the potato beetle Epilachna vigintioctopunctata. In Presented in National Symposium on Problems and Prospects of Botanical Pesticides in Integrated Pest Management; CTRI Press: Rajahmundry, India, 1990; pp. 21–22. [Google Scholar]
- Hammond, A.H.; Garle, M.J.; Fry, J.R. Mechanism of toxicity of precocene II in rat hepatocyte cultures. J. Biochem. Toxicol. 1995, 10, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.G.; Huang, M.D. Influence of citrus orchard ground cover plants on arthropod communities in China: A review. Agric. Ecosyst. Environ. 1994, 50, 29–37. [Google Scholar] [CrossRef]
- Dicke, M.; Baldwin, I.T. The evolutionary context for herbivore-induced plant volatiles: Beyond the ‘cry for help’. Trends Plant Sci. 2010, 15, 167–175. [Google Scholar] [CrossRef]
- Aartsma, Y.; Bianchi, F.J.; van der Werf, W.; Poelman, E.H.; Dicke, M. Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol. 2017, 216, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Seid, A.; Fininsa, C.; Mekete, T.; Decraemer, W.; Wesemael, W.M. Tomato (Solanum lycopersicum) and root-knot nematodes (Meloidogyne spp.)—A century-old battle. Nematology 2015, 17, 995–1009. [Google Scholar] [CrossRef]
- Sikandar, A.; Zhang, M.Y.; Wang, Y.Y.; Zhu, X.F.; Liu, X.Y.; Fan, H.Y.; Xuan, Y.H.; Chen, L.J.; Duan, Y.X. Meloidogyne incognita (root-knot nematode) a risk to agriculture. Appl. Ecol. Environ. Res. 2020, 18, 1679–1690. [Google Scholar] [CrossRef]
- Lambert, K.; Bekal, S. Introduction to Plant-Parasitic Nematodes. The Plant Health Instructor. Available online: https://www.apsnet.org/edcenter/disandpath/nematode/intro/Pages/IntroNematodes.aspx (accessed on 12 August 2024).
- den Akker, S.E. Plant–nematode interactions. Curr. Opin. Plant Biol. 2021, 62, 102035. [Google Scholar]
- Pires, D.; Vicente, C.S.L.; Menéndez, E.; Faria, J.M.S.; Rusinque, L.; Camacho, M.J.; Inácio, M.L. The fight against plant-parasitic nematodes: Current status of bacterial and fungal biocontrol agents. Pathogens 2022, 11, 1178. [Google Scholar] [CrossRef]
- Akhter, G.; Zafar, A.; Khan, W.; Jamshed, M. In vitro nemato-toxic potential of some leaf extracts on juvenile mortality of Meloidogyne incognita race-3. Arch. Phytopathol. Plant Prot. 2018, 51, 399–407. [Google Scholar] [CrossRef]
- Mamman, A. Nematicidal activity of Ageratum conyzoides leaf extract against root-knot nematode (Meloidogyne javanica) on eggplant in Jalingo, Nigeria. Niger. Dutse J. Pure Appl. Sci. 2023, 9, 120–128. [Google Scholar] [CrossRef]
- Pavaraj, M.; Karthikairaj, K.; Rajan, M.K. Effect of leaf extract of Ageratum conyzoides on the biochemical profile of blackgram Vigna mungo infected by root-knot nematode, Meloidogyne incognita. J. Biopest. 2010, 3, 313–316. [Google Scholar]
- Levine, J.M.; Adler, P.B.; Yelenik, S.G. A meta-analysis of biotic resistance to exotic plant invasions. Ecol. Lett. 2004, 7, 975–989. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Callaway, R.M. Soil biota and invasive plants. New Phytol. 2006, 170, 445–457. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G. Ecosystem consequences of biological invasions. Ann. Rev. Ecol. Evol. Syst. 2010, 41, 59–80. [Google Scholar] [CrossRef]
- Desmond, O.J.; Manners, J.M.; Stephens, A.E.; Maclean, D.J.; Schenk, P.M.; Gardiner, D.M.; Munn, A.L.; Kazan, K. The Fusarium mycotoxin deoxynivalenol elicits hydrogen peroxide production, programmed cell death and defense responses in wheat. Mol. Plant Pathol. 2008, 9, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium Pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Javed, S.; Basir, U. Antifungal activity of different extracts of Ageratum conyzoides for the management of Fusarium solani. Afr. J. Biotechnol. 2012, 11, 11022–11029. [Google Scholar]
- Hazirah, M.F.; Hamizah, O.; Natasya, W.A.W. Antifungal activity of Ageratum conyzoides extract against Fusarium oxysporum in Musa spp. IOP Conf. Ser. Earth Environ. Sci. 2023, 1182, 012074. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Nguyen, T.Q.C.; Kanaori, K.; Binh, T.D.; Dao, X.H.T.; Vang, L.V.; Kamei, K. Antifungal activities of Ageratum conyzoides L. extract against rice pathogens Pyricularia oryzae Cavara and Rhizoctonia solani Kühn. Agriculture 2021, 11, 1169. [Google Scholar] [CrossRef]
- Kong, C.H.; Liang, W.J.; Hu, F.; Xu, X.H.; Wang, P.; Jiang, Y. Allelochemicals and their transformations in A. conyzoides intercropped citrus orchard soil. Plant Soil 2004, 264, 149–157. [Google Scholar] [CrossRef]
- Yaguchi, A.; Yoshinari, T.; Tsuyuki, R.; Takahashi, H.; Nakajima, T.; Sugita-Konishi, Y. Isolation and identification of precocenes and piperitone from essential oils as specific inhibitors of trichothecene production by Fusarium graminearum. J. Agric. Food. Chem. 2009, 57, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Chahal, R.; Nanda, A.; Akkol, E.K.; Sobarzo-Sánchez, E.; Arya, A.; Kaushik, D.; Dutt, R.; Bhardwaj, R.; Rahman, M.H.; Mittal, V. Ageratum conyzoides L. and its secondary metabolites in the management of different fungal pathogens. Molecules 2021, 26, 2933. [Google Scholar] [CrossRef] [PubMed]
- Nasmith, C.G.; Walkowiak, S.; Wang, L.; Leung, W.Y.Y.; Gong, Y.; Johnston, A.; Harris, L.J.; Guttman, D.S.; Subramaniamet, R. Tri6 is a global transcription regulator in the phytopathogen Fusarium graminearum. PLoS Pathog. 2011, 7, e1002266. [Google Scholar] [CrossRef] [PubMed]
- Sakuda, S.; Yoshinari, T.; Furukawa, T.; Jermnak, U.; Takagi, K.; Kimura, M.; Yamamoto, T.; Suzuki, M.; Nagasawa, H. Search for aflatoxin and trichothecene production inhibitors and analysis of their modes of action. Biosci. Biotech. Biochem. 2016, 80, 43–54. [Google Scholar] [CrossRef]
- Furukawa, T.; Sakamoto, N.; Suzuki, M.; Kimura, M.; Nagasawa, H.; Sakuda, S. Precocene II, a trichothecene production inhibitor, binds to voltage-dependent anion channel and increases the superoxide level in mitochondria of Fusarium graminearum. PLoS ONE 2015, 10, e0135031. [Google Scholar] [CrossRef]
- Noriega, F.G. Juvenile hormone biosynthesis in insects: What is new, what do we know, and what questions remain? Int. Sch. Res. Notices 2014, 1, 967361. [Google Scholar] [CrossRef]
- Batish, D.R.; Singh, H.P.; Kaur, S.; Kohli, R.K. Phytotoxicity of Ageratum conyzoides residues towards growth and nodulation of Cicer arietinum. Agric. Ecosyst. Environ. 2006, 113, 399–401. [Google Scholar] [CrossRef]
- Barrett, C.F.; Parker, M.A. Coexistence of Burkholderia, Cupriavidus and Rhizobium sp. nodule bacteria on two Mimosa species in Costa Rica. Appl. Environ. Microbiol. 2006, 72, 1198–1206. [Google Scholar] [CrossRef]
- Chen, W.M.; James, E.K.; Coenye, T.; Chou, J.H.; Barrios, E.; de Faria, S.M.; Ellio, N.; Sheu, S.Y.; Sprent, J.I.; Vandamme, P. Burkholderia mimosarum sp. nov., isolated from root nodules of Mimosa spp. from Taiwan and South America. Int. J. Syst. Evol. Microbiol. 2006, 56, 1847–1851. [Google Scholar] [CrossRef]
- Elliott, G.N.; Chou, J.H.; Chen, W.M.; Bloemberg, G.V.; Bontemps, C.; Martínez-Romero, E.; Velázquez, E.; Young, J.P.W.; Sprent, J.I.; James, E.K. Burkholderia spp. are the most competitive symbionts of Mimosa, particularly under N-limited conditions. Environ. Microbiol. 2009, 11, 762–778. [Google Scholar] [CrossRef]
- Tsyganova, A.V.; Brewin, N.J.; Tsyganov, V.E. Structure and development of the legume-rhizobial symbiotic interface in infection threads. Cells 2021, 10, 1050. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathy of knotweeds as invasive plants. Plants 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Allelopathy and allelochemicals of Imperata cylindrica as an invasive plant species. Plants 2022, 11, 2551. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.L. Allelopathy, 2nd ed.; Academic Press: Orlando, FL, USA, 1984; pp. 1–422. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Bonanomi, G.; Sicurezza, M.G.; Caporaso, S.; Esposito, A.; Mazzoleni, S. Phytotoxicity dynamics of decaying plant materials. New Phytol. 2006, 169, 571–578. [Google Scholar] [CrossRef]
- Belz, R.G. Allelopathy in crop/weed interactions—An update. Pest. Manag. Sci. 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 419–426. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. The impact and invasive mechanisms of Pueraria montana var. lobata, one of the world’s worst alien species. Plants 2023, 12, 3066. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kato, M. Evolution of the secondary metabolites in invasive plant species Chromolaena odorata for the defense and allelopathic functions. Plants 2023, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Isolation and identification of allelochemicals and their activities and functions. J. Pesti. Sci. 2024, 49, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kato, M. Allelopathy and allelochemicals of Solidago canadensis L. and S. altissima L. for their naturalization. Plants 2022, 11, 3235. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Invasive mechanisms of one of the world’s worst alien plant species Mimosa pigra and its management. Plants 2023, 12, 1960. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Kurniadie, D. The invasive mechanisms of the noxious alien plant species Bidens pilosa. Plants 2024, 13, 356. [Google Scholar] [CrossRef]
- Javaid, N.; Shah, M.H.; Khan, I.H.; Javaid, A.; Waleed, S.M. Herbicidal activity of Ageratum conyzoides against Parthenium. Pak. J. Weed Sci. Res. 2020, 26, 137–146. [Google Scholar] [CrossRef]
- Negi, B.; Bargali, S.S.; Bargali, K.; Khatri, K. Allelopathic interference of Ageratum conyzoides L. against rice varieties. Curr. Agric. Res. J. 2020, 8, 69–76. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Assessment of the allelopathic potential of Ageratum conyzoides. Biol. Plant. 2001, 44, 309–311. [Google Scholar] [CrossRef]
- Erida, G.; Saidi, N.; Hasanuddin, H.; Sampietro, D.A.; Amist, N. Herbicidal effects of n-hexane, ethyl acetate and methanol extracts of billygoat weed (Ageratum conyzoides L.) leaves on Amaranthus spinosus L. growth. Allelopath. J. 2021, 54, 211–220. [Google Scholar] [CrossRef]
- Erida, G.; Saidi, N.; Hasanuddin, H.; Syafruddin, S. Herbicidal effects of ethyl acetate extracts of billygoat weed (Ageratum conyzoides L.) on spiny amaranth (Amaranthus spinosus L.) growth. Agronomy 2021, 11, 1991. [Google Scholar] [CrossRef]
- Xuan, T.D.; Shinkichi, T.; Hong, N.H.; Khanh, T.D.; Min, C.I. Assessment of phytotoxic action of Ageratum conyzoides L. (Billy goat weed) on weeds. Crop Prot. 2004, 23, 915–922. [Google Scholar] [CrossRef]
- Batish, D.R.; Harminder, P.S.; Shalinder, K.; Kohli, R.K. Nature of interference potential of leaf debris of Ageratum conyzoides L. Plant Grow Regul. 2008, 57, 137–144. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K. Phytotoxic interference of Ageratum conyzoides with wheat (Triticum aestivum). J. Agron. Crop Sci. 2003, 189, 341–346. [Google Scholar] [CrossRef]
- Akter, P.; Begum, R. Allelopathic effects of Ageratum conyzoides root exudates on germinability of selected crops: A comparative analysis. Middle East Res. J. Biol. Sci. 2024, 4, 22–29. [Google Scholar] [CrossRef]
- Kong, C.; Hu, F.; Xu, T.; Lu, Y. Allelopathic potential and chemical constituents of volatile oil from Ageratum conyzoides. Allelopath. J. 1999, 25, 2347–2356. [Google Scholar]
- Zheng, H.; Wei, N.; Wang, L.; He, P. Effects of Lantana camara leaf extract on the activity of superoxide dismutase and accumulation of H2O2 in water hyacinth leaf. J. Plant Physiol. Mol. Biol. 2006, 32, 189. [Google Scholar]
- Li, C.; Yang, X.; Tian, Y.; Yu, M.; Shi, S.; Qiao, B.; Zhao, C.; Mao, L. The effects of fig tree (Ficus carica L.) leaf aqueous extract on seed germination and seedling growth of three medicinal plants. Agronomy 2021, 11, 2564. [Google Scholar] [CrossRef]
- Mao, X.T.; Xu, R.X.; Gao, Y.; Li, H.Y.; Liu, J.S.; Yang, W.D. Allelopathy of Alexandrium pacificum on Thalassiosira pseudonana in laboratory cultures. Ecotoxicol. Environ. Saf. 2021, 215, 112123. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.; Fei, F.; Wenju, L.; Weng, P.; Yong, J. Allelopathic potential of Ageratum conyzoides at various growth stages in different habitats. Allelopath. J. 2004, 13, 233–240. [Google Scholar]
- Wang, R.; Peng, S.L.; Zeng, R.S.; Ding, L.W.; Xu, Z.F. Cloning, expression and wounding induction of β-caryophyllene synthase gene from Mikania micrantha HBK and allelopathic potential of β-caryophyllene. Allelopathy J. 2009, 24, 35–44. [Google Scholar]
- Richter, A.; Seidl-Adams, I.; Köllner, T.G.; Schaff, C.; Tumlinson, J.H.; Degenhardt, J. A small, differentially regulated family of farnesyl diphosphate synthases in maize (Zea mays) provides farnesyl diphosphate for the biosynthesis of herbivore-induced sesquiterpenes. Planta 2015, 241, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Balasubramani, S.; Kumari, B.D.R.; Moola, A.K.; Sathish, D.; Kumar, G.P.; Srimurali, S.; Rajendran, R.B. Enhanced production of β-caryophyllene by farnesyl diphosphate precursor-treated callus and hairy root cultures of Artemisia vulgaris L. Front. Plant Sci. 2021, 12, 634178. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zong, C.; Bai, A.; Yuan, S.; Li, Y.; Yu, Z.; Tian, R.; Liu, T.; Hou, X.; Li, Y. Transcriptome sequencing and gas chromatography-mass spectrometry analyses provide insights into β-caryophyllene biosynthesis in Brassica campestris. Food Chem. Mol. Sci. 2022, 5, 100129. [Google Scholar] [CrossRef] [PubMed]
- Treutter, D. Significance of flavonoids in plant resistance: A review. Environ. Chem. Lett. 2006, 4, 147. [Google Scholar] [CrossRef]
- Weston, L.A.; Mathesius, U. Flavonoids: Their structure, biosynthesis and role in the rhizosphere, including allelopathy. J. Chem. Ecol. 2013, 39, 283–297. [Google Scholar] [CrossRef]
- Mierziak, J.; Kostyn, K.; Kulma, A. Flavonoids as important molecules of plant interactions with the environment. Molecules 2014, 19, 16240–16265. [Google Scholar] [CrossRef]
- Fernández-Aparicio, M.; Masi, M.; Cimmino, A.; Vilariño, S.; Evidente, A. Allelopathic effect of quercetin, a flavonoid from Fagopyrum esculentum roots in the radicle growth of Phelipanche ramosa: Quercetin natural and semisynthetic analogues were used for a structure-activity relationship investigation. Plants 2021, 10, 543. [Google Scholar] [CrossRef]
- Okunade, A.L. Ageratum conyzoides L. (asteraceae). Fitoterapia 2002, 73, 1–16. [Google Scholar] [CrossRef]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef]
- Dalton, B.R. The occurrence and behavior of plant phenolic acids in soil environments and their potential involvement in allelochemical interference interactions: Methodological limitations in establishing conclusive proof of allelopathy. In Principals and Practices in Plant Ecology: Allelochemical Interactions; Inderjit, Dakshini, K.M.M., Foy, C.L., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 57–74. [Google Scholar]
- Inderjit. Plant phenolics in allelopathy. Bot. Rev. 1996, 62, 186–202. [Google Scholar] [CrossRef]
- Einhellig, F.A. Mode of action of allelochemical action of phenolic compounds. In Chemistry and Mode of Action of Allelochemicals; Macías, F.A., Galindo, J.C.G., Molino, J.M.G., Cutler, H.G., Eds.; CRC Press: Boca Raton, FL, USA; London, UK; New York, NY, USA; Washington, DC, USA, 2004; pp. 217–238. [Google Scholar]
- Šoln, K.; Koce, J.D. Allelopathic root inhibition and its mechanisms. Allelopath. J. 2021, 52, 181–198. [Google Scholar] [CrossRef]
- Paul, S.; Datta, B.K.; Ratnaparkhe, M.B.; Dholakia, B.B. Turning waste into beneficial resource: Implication of Ageratum conyzoides L. in sustainable agriculture, environment and biopharma sectors. Mol. Biotechnol. 2022, 64, 221–244. [Google Scholar] [CrossRef] [PubMed]
- Sundufu, A.J.; Shoushan, H. Chemical composition of the essential oils of Ageratum conyzoides L. occurring in South China. Flavour Fragr. J. 2004, 19, 6–8. [Google Scholar] [CrossRef]
- Kamboj, A.; Saluja, A.K. Ageratum conyzoides L.: A review on its phytochemical and pharmacological profile. Int. J. Green Pharm. 2008, 2, 59–68. [Google Scholar] [CrossRef]
- Singh, S.B.; Devi, W.R.; Marina, A.; Devi, W.I.; Swapana, N.; Singh, C.B. Ethnobotany, phytochemistry and pharmacology of Ageratum conyzoides Linn (Asteraceae). J. Med. Plants Res. 2013, 7, 371–385. [Google Scholar]
- Rioba, N.B.; Stevenson, P.C. Ageratum conyzoides L. for the management of pests and diseases by small holder farmers. Ind. Crop Prod. 2017, 110, 22–29. [Google Scholar] [CrossRef]
- Yadav, N.; Ganie, S.A.; Singh, B.; Chhillar, A.K.; Yadav, S.S. Phytochemical constituents and ethnopharmacological properties of Ageratum conyzoides L. Phytother. Res. 2019, 33, 2163–2178. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).