Encapsulating Transition Metal Nanoparticles inside Carbon (TM@C) Chainmail Catalysts for Hydrogen Evolution Reactions: A Review
Abstract
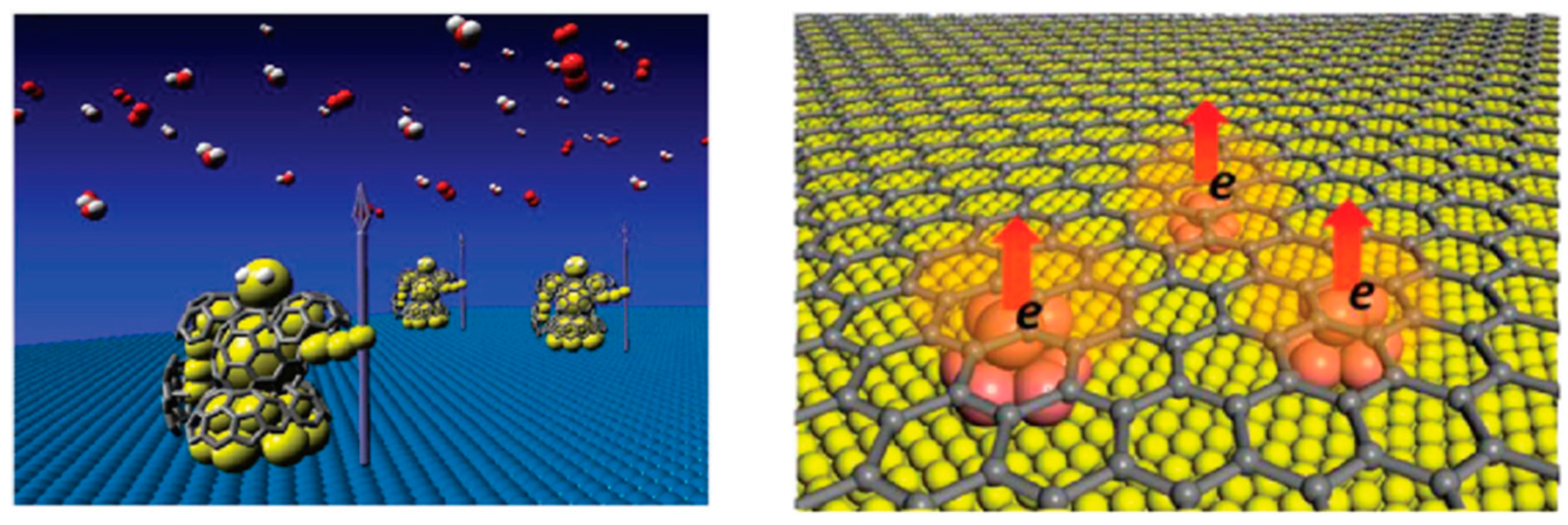
:1. Introduction
2. Preparation Methods of TM@C Chainmail Catalysts
2.1. Chemical Vapor Deposition
2.2. Pyrolysis Method
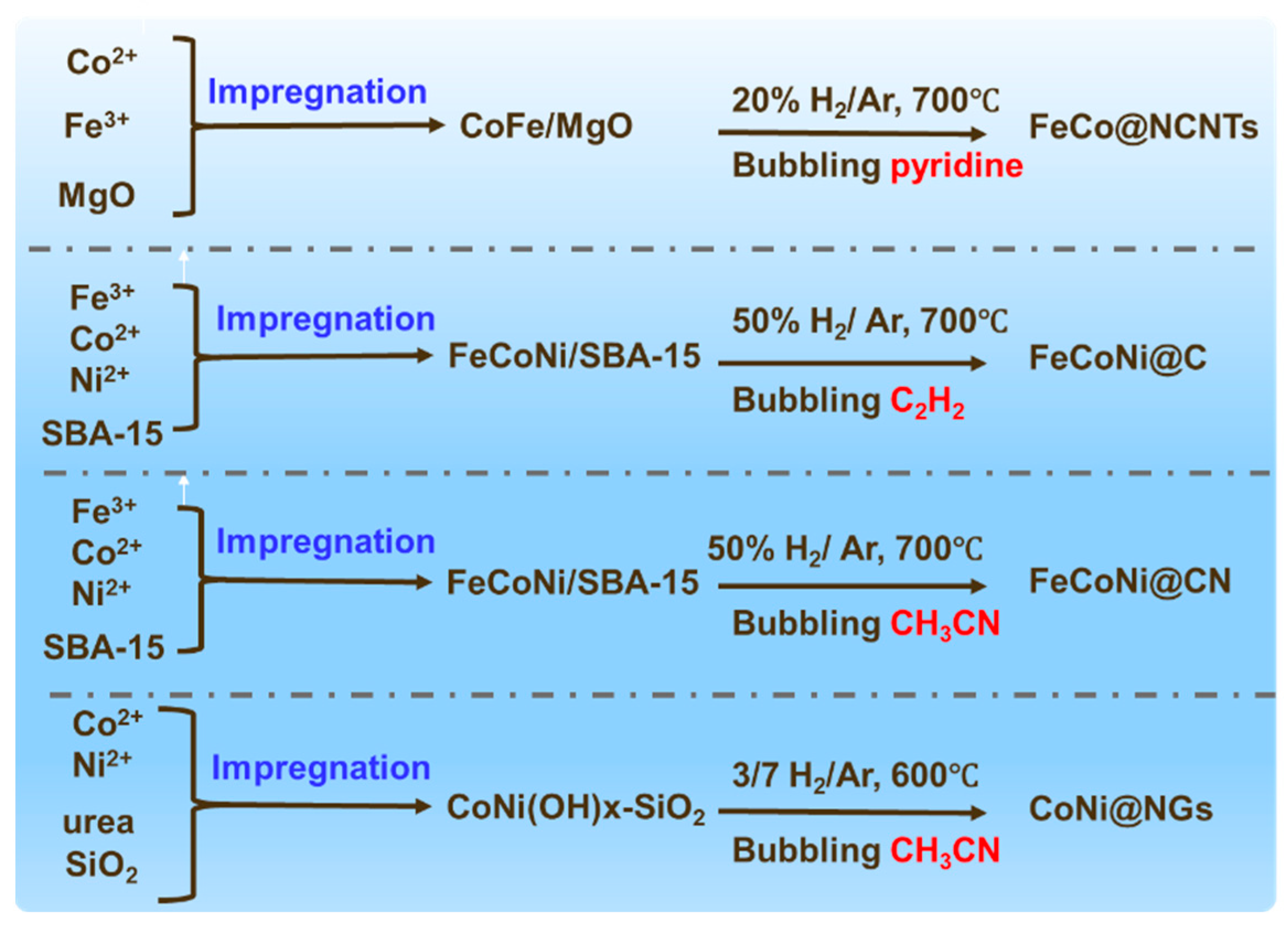
2.2.1. Metal–Organic Complex-Assisted Pyrolytic Carbonization Method
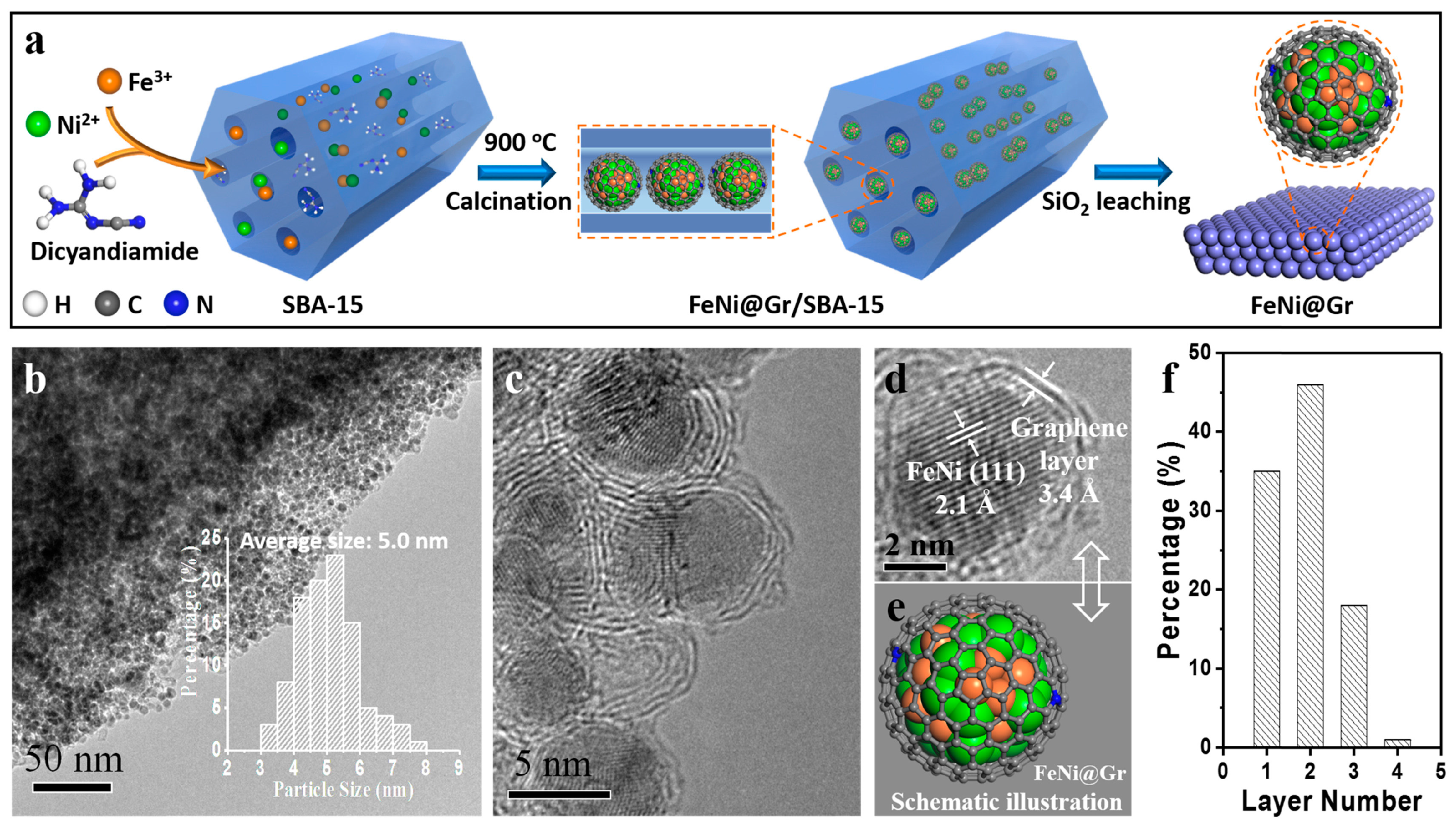
2.2.2. Pyrolytic Carbonization of Metal–Organic Framework Materials
3. Application in HER
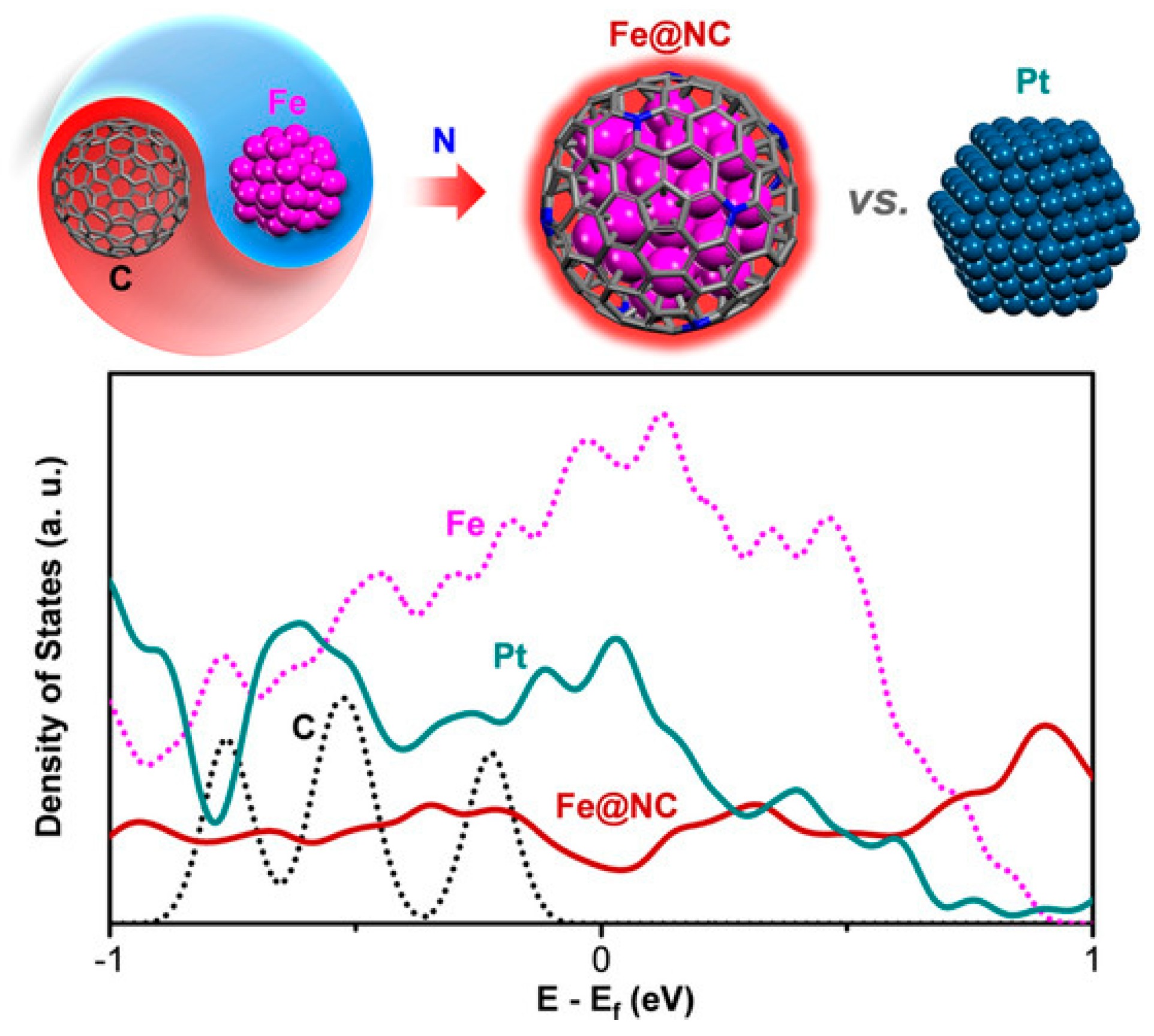
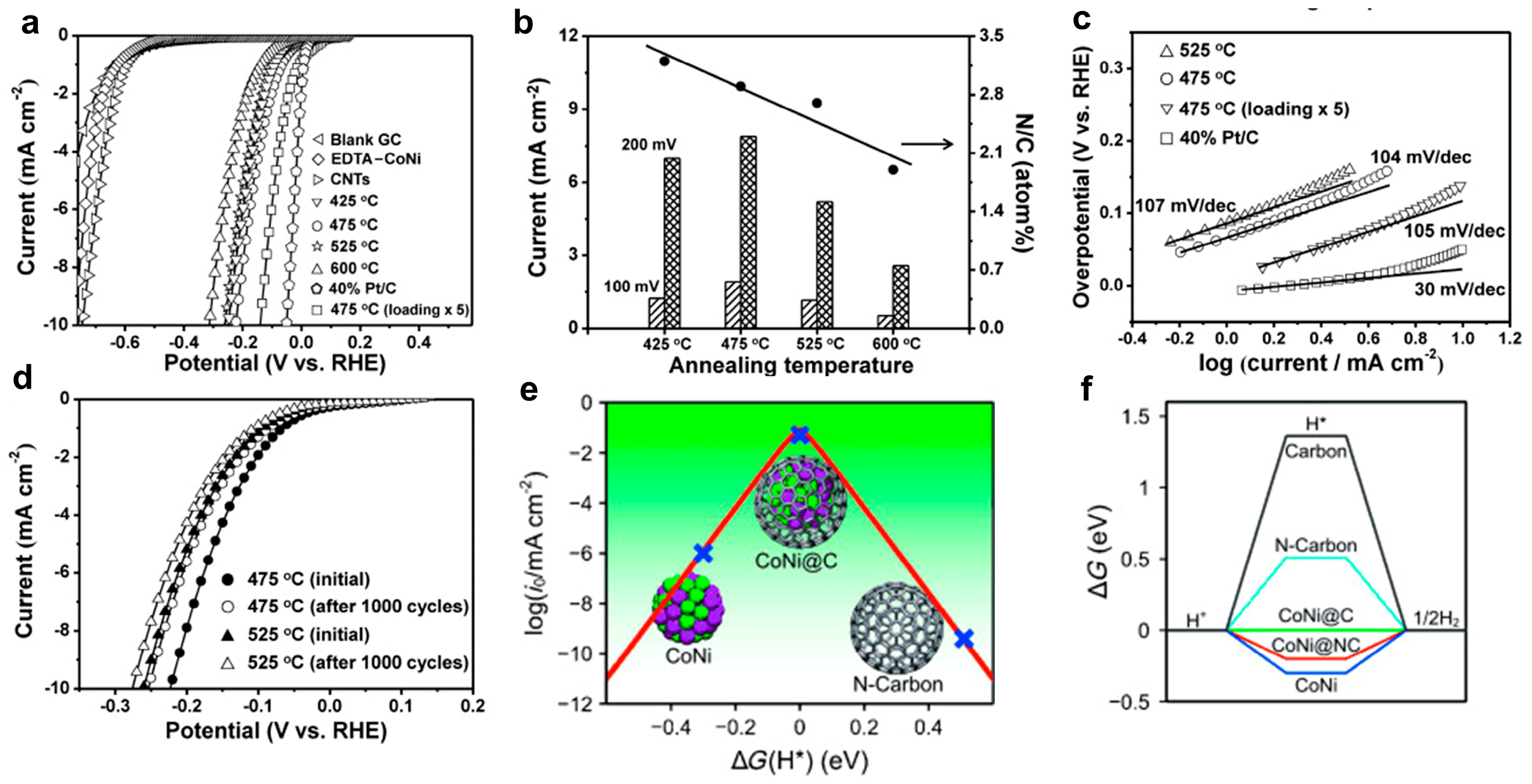
3.1. Transition Metal Alloy Chainmail Catalysts
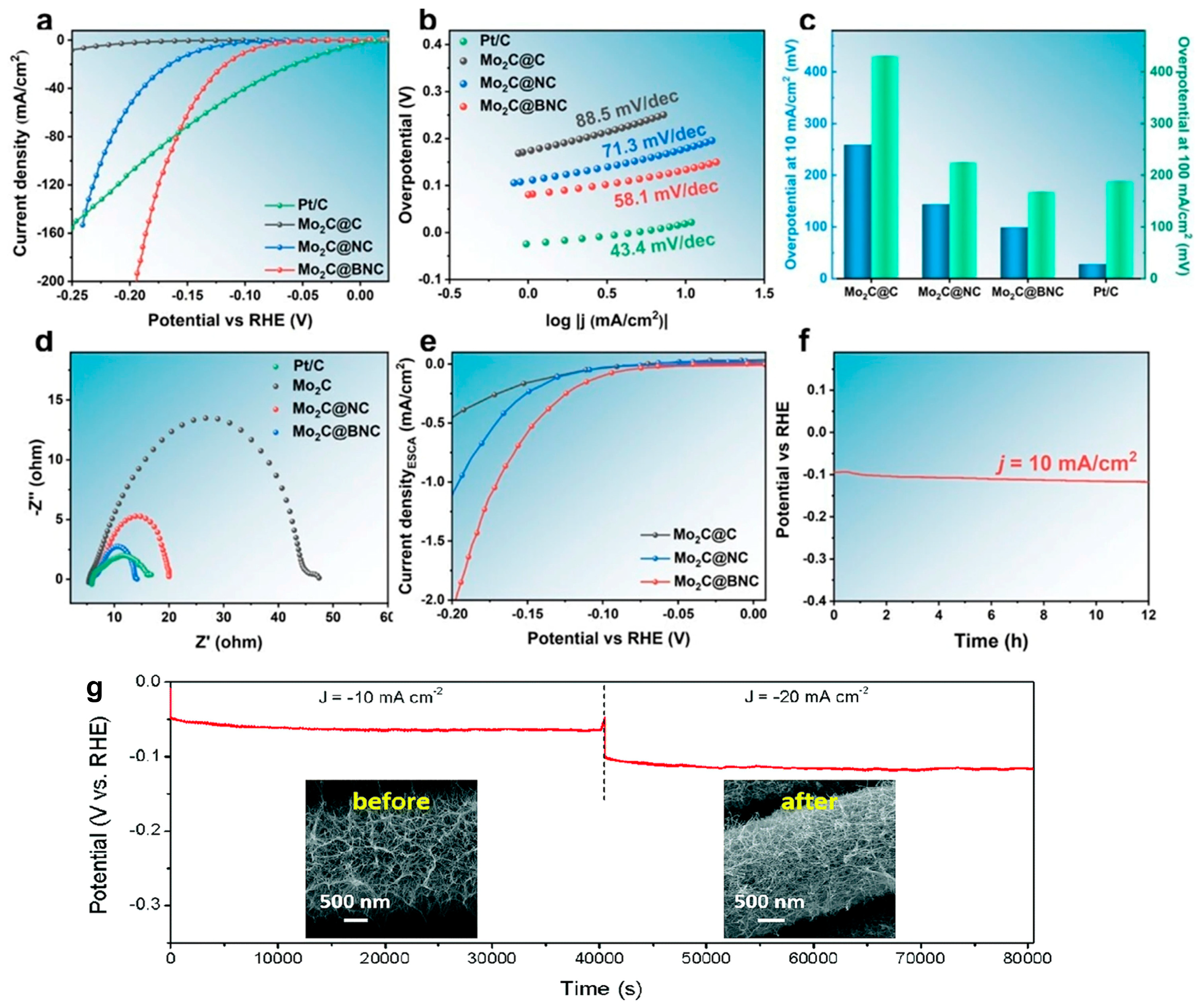
3.2. Transition Metal Carbides Chainmail Catalysts
3.3. Noble Metal Doping: Transition Metal Chainmail Catalysts
3.4. Transition Metal Phosphide-Based Chainmail Catalysts
3.5. Carbon-Based Single Atom Catalysts
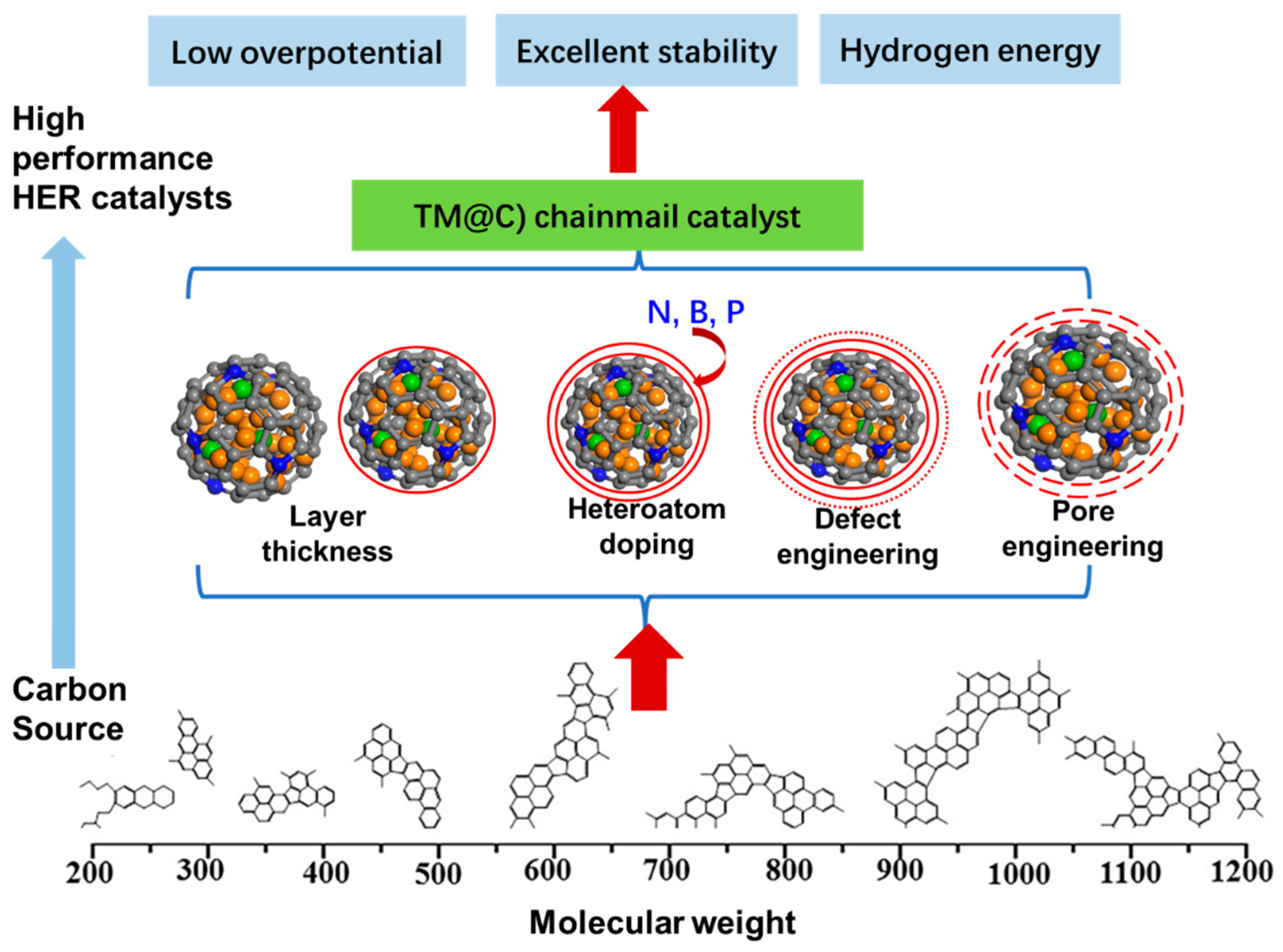
4. Summary and Outlook
4.1. The Thickness of the Chainmail Layer
4.2. Heteroatom Doping in the Carbon Layer
4.3. Defects Engineering
4.4. Pore Engineering: Multiple Active Site Designs
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zuttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philosophical Transactions of the Royal Society A: Mathematical. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar]
- Zou, X.; Zhang, Y. Noble metal-free hydrogen evolution catalysts for water splitting. Chem. Soc. Rev. 2015, 46, 5148–5180. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kraft, M.; Xu, R. Metal-free carbonaceous electrocatalysts and photocatalysts for water splitting. Chem. Soc. Rev. 2016, 45, 3039–3052. [Google Scholar] [CrossRef]
- Niu, K.Y.; Lin, F.; Jung, S.; Fang, L.; Nordlund, D.; Mccrory, C.C.L.; Weng, T.C.; Ercius, P.; Doeff, M.M.; Zheng, H. Tuning complex transition metal hydroxide nanostructures as active catalysts for water oxidation by a Laser–Chemical route. Nano. Lett. 2015, 15, 2498–2503. [Google Scholar] [CrossRef]
- Xu, X.; Zhong, Y.; Wajrak, M.; Bhatelia, T.; Jiang, S.P.; Shao, Z. Grain boundary engineering: An emerging pathway toward efficient electrocatalysis. InfoMat 2024, 6, e12608. [Google Scholar] [CrossRef]
- Sheng, W.; Myint M N, Z.; Chen J, G.; Yan, Y. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6, 1509–1512. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, H.; Li, Y.; Liao, F.; Lifshitz, Y.; Sheng, M.; Lee, S.T.; Shao, M. A rhodium/silicon co-electrocatalyst design concept to surpass platinum hydrogen evolution activity at high overpotentials. Nat. Commun. 2016, 7, 12272. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Ren, P.; Deng, D.; Bao, X. Enhanced electron penetration through an ultrathin graphene layer for highly efficient catalysis of the hydrogen evolution reaction. Angew. Chem. Int. Edit. 2015, 127, 2128–2132. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, C. Electrodeposition of hierarchically structured three-dimensional nickel–iron electrodes for efficient oxygen evolution at high current densities. Nat. Commun. 2015, 6, 6616. [Google Scholar] [CrossRef]
- Zhou, W.; Wu, X.J.; Cao, X.; Huang, X.; Tan, C.; Tian, J.; Liu, H.; Wang, J.; Zhang, H. Ni3S2 nanorods/Ni foam composite electrode with low overpotential for electrocatalytic oxygen evolution. Energy Environ. Sci. 2013, 6, 2921–2924. [Google Scholar] [CrossRef]
- Cui, X.; Ren, P.; Deng, D.; Deng, J.; Bao, X. Single layer graphene encapsulating non-precious metals as high-performance electrocatalysts for water oxidation. Energy Environ. Sci. 2016, 9, 123–129. [Google Scholar] [CrossRef]
- Pruchyathamkorn, J.; Yang, M.; Amin, H.M.A.; Batchelor-McAuley, C.; Compton, R.G. Imaging electrode heterogeneity using chemically confined fluorescence electrochemical microscopy. J. Phys. Chem. Lett. 2017, 8, 6124–6127. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yin, S.; Li, C.; Deng, K.; Xue, H.; Li, X.; Wang, H.; Wang, L. Low-ruthenium-content NiRu nanoalloys encapsulated in nitrogen-doped carbon as highly efficient and pH-universal electrocatalysts for the hydrogen evolution reaction. J. Mater. Chem. A 2018, 6, 1376–1381. [Google Scholar] [CrossRef]
- Mahmood, J.; Li, F.; Jung, S.M.; Okyay, M.S.; Ahmad, I.; Kim, S.J.; Park, N.; Jeong, H.Y.; Baek, J.B. An efficient and pH-universal ruthenium-based catalyst for the hydrogen evolution reaction. Nat. Nanotech. 2017, 12, 441–446. [Google Scholar] [CrossRef]
- Jiao, Y.; Zheng, Y.; Davey, K.; Qiao, S.Z. Activity origin and catalyst design principles for electrocatalytic hydrogen evolution on heteroatom-doped graphene. Nat. Energy 2016, 1, 1–9. [Google Scholar] [CrossRef]
- Wang, T.; Wang, X.; Liu, Y.; Zheng, J.; Li, X. A highly efficient and stable biphasic nanocrystalline Ni–Mo–N catalyst for hydrogen evolution in both acidic and alkaline electrolytes. Nano Energy 2016, 22, 111–119. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, Z.; Cao, X.; Hu, J.; Wu, X.; Ng, A.Y.R.; Lu, G.-P.; Chen, Z. Elucidating the sources of activity and stability of FeP electrocatalyst for hydrogen evolution reactions in acidic and alkaline media. Appl. Catal. B 2020, 260, 118156. [Google Scholar] [CrossRef]
- Tetzlaff, D.; Pellumbi, K.; Baier, D.M.; Hoof, L.; Shastry Barkur, H.; Smialkowski, M.; Amin, H.M.A.; Grätz, S.; Siegmund, D.; Borchardt, L.; et al. Sustainable and rapid preparation of nanosized Fe/Ni-pentlandite particles by mechanochemistry. Chem. Sci. 2020, 11, 12835–12842. [Google Scholar] [CrossRef]
- Zan, L.; Amin, H.M.A.; Mostafa, E.; Abd-El-Latif, A.A.; Iqbal, S.; Baltruschat, H. Electrodeposited cobalt nanosheets on smooth silver as a bifunctional catalyst for OER and ORR: In situ structural and catalytic characterization. ACS Appl. Mater. Interfaces 2022, 14, 55458–55470. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-supported perovskite as an efficient bifunctional electrocatalyst for oxygen reduction and evolution: Substrate effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Sun, H.; Kim, H.; Song, S.; Jung, W. Copper foam-derived electrodes as efficient electrocatalysts for conventional and hybrid water electrolysis. Mater. Rep. Energy 2022, 2, 100092. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Qian, H.; Wang, Z.; Yamauchi, Y. One-pot synthesis of zeolitic imidazolate framework 67-derived hollow Co3S4@ MoS2 heterostructures as efficient bifunctional catalysts. Chem. Mater. 2017, 29, 5566–5573. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, Y.; Yu, Y.; Zhang, J.; Zhuo, S.; Zhang, B. Sub-1.1 nm ultrathin porous CoP nanosheets with dominant reactive {200} facets: A high mass activity and efficient electrocatalyst for the hydrogen evolution reaction. Chem. Sci. 2017, 8, 2769–2775. [Google Scholar] [CrossRef]
- Wan, C.; Regmi, Y.N.; Leonard, B.M. Multiple phases of molybdenum carbide as electrocatalysts for the hydrogen evolution reaction. Angew. Chem. 2014, 53, 6407–6410. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.P.; Wu, G.; Chung, H.T.; Johnston, C.M.; Zelenay, P. Recent advances in non-precious metal catalysis for oxygen-reduction reaction in polymer electrolyte fuel cells. Energ. Environ. Sci 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Borup, R.; Meyers, J.; Pivovar, B.; Kim, Y.S.; Mukundan, R.; Garland, N.; Myers, D.; Wilson, M.; Garzon, F.; Wood, D. Scientific aspects of polymer electrolyte fuel cell durability and degradation. Chem. Rev. 2007, 107, 3904–3951. [Google Scholar] [CrossRef]
- Yang, G.; Guo, Y.; Bo, X. Laser-assisted coupling of nitrogen-doped carbon-coated molybdenum/molybdenum dioxide rods for efficient pH-universal hydrogen evolution electrocatalysis. J. Colloid Interface Sci. 2022, 608, 1696–1706. [Google Scholar] [CrossRef]
- Deng, J.; Yu, L.; Deng, D.; Chen, X.; Yang, F.; Bao, X. Highly active reduction of oxygen on a FeCo alloy catalyst encapsulated in pod-like carbon nanotubes with fewer walls. J. Mater. Chem. A 2013, 1, 14868–14873. [Google Scholar] [CrossRef]
- Chen, X.; Deng, D.; Pan, X.; Bao, X. Iron catalyst encapsulated in carbon nanotubes for CO hydrogenation to light olefins. Chinese J. Catal. 2015, 36, 1631–1637. [Google Scholar] [CrossRef]
- Deng, D.; Yu, L.; Chen, X.; Wang, G.; Jin, L.; Pan, X.; Deng, J.; Sun, G.; Bao, X. Iron encapsulated within pod-like carbon nanotubes for oxygen reduction reaction. Angew. Chem. 2013, 125, 389–393. [Google Scholar] [CrossRef]
- Zheng, X.; Deng, J.; Wang, N.; Deng, D.; Zhang, W.H.; Bao, X.; Li, C. Podlike N-doped carbon nanotubes encapsulating FeNi alloy nanoparticles: High-performance counter electrode materials for dye-sensitized solar cells. Angew. Chem. Int. Edit. 2014, 53, 7023–7027. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, T.; Wang, J.; Liu, H.; Dao, T.D.; Li, M.; Liu, G.; Meng, X.; Chang, K.; Shi, L. Surface-plasmon-enhanced photodriven CO2 reduction catalyzed by metal–organic-framework-derived iron nanoparticles encapsulated by ultrathin carbon layers. Adv. Mater. 2016, 28, 3703–3710. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Novoselov, K.S.; Fu, Q.; Zheng, N.; Tian, Z.; Bao, X. Catalysis with two-dimensional materials and their heterostructures. Nat. Nanotech. 2016, 11, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Li, H.; Deng, D.; Xiao, J.; Cui, X.; Ding, D.; Chen, M.; Bao, X. Low charge overpotential of lithium-oxygen batteries with metallic Co encapsulated in single-layer graphene shell as the catalyst. Nano Energy 2016, 30, 877–884. [Google Scholar] [CrossRef]
- Ruoff, R.S.; Lorents, D.C.; Chan, B.; Malhotra, R.; Subramoney, S. Single crystal metals encapsulated in carbon nanoparticles. Science 1993, 259, 346–348. [Google Scholar] [CrossRef]
- Bao, X.; Ren, P.; Deng, D.; Yang, F.; Deng, J.; Yu, L. Highly active and durable non-precious-metal catalysts encapsulated in carbon nanotubes for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 1919–1923. [Google Scholar]
- Deng, J.; Deng, D.; Bao, X. Robust catalysis on 2D materials encapsulating metals: Concept, application, and perspective. Adv. Mater. 2017, 29, 1606967. [Google Scholar] [CrossRef]
- Wang, M.; Chen, D.; Li, Z.; Wang, Z.; Huang, S.; Hai, P.; Tan, Y.; Zhuang, X.; Liu, P. Epitaxial growth of two-dimensional nonlayered AuCrS2 materials via Au-assisted chemical vapor deposition. Nano Lett. 2024, 24, 2308–2314. [Google Scholar] [CrossRef]
- Matsuoka, S.; Sharma, K.P.; Saida, T.; Kusada, K.; Kitagawa, H.; Maruyama, T. Single-walled carbon nanotube synthesis with RuRhPdIrPt high entropy alloy catalysts. Chem. Phys. Lett. 2024, 841, 141178. [Google Scholar] [CrossRef]
- Gakis, G.P.; Chrysoloras, T.A.; Aviziotis, I.G.; Charitidis, C.A. Towards a mechanistic understanding of the floating catalyst CVD of CNTs: Interplay between catalyst particle nucleation, growth and deactivation. Chem. Eng. Sci. 2024, 295, 120204. [Google Scholar] [CrossRef]
- Glauber, J.P.; Lorenz, J.; Liu, J.; Müller, B.; Bragulla, S.; Kostka, A.; Rogalla, D.; Wark, M.; Nolan, M.; Harms, C.; et al. A sustainable CVD approach for ZrN as a potential catalyst for nitrogen reduction reaction. Dalton T 2024, 53, 15454–45464. [Google Scholar] [CrossRef] [PubMed]
- Chitriv, S.P.; Saini, V.; Ratna, D. Carbon nanotubes synthesis over coal ash based catalysts using polypropylene waste via CVD process: Influence of catalyst and reaction temperature. J. Environ. Manag. 2024, 366, 121881. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Zhou, Y.; Wang, D.; Wu, X.; Li, J.; Xi, J. Nickel–Copper alloy encapsulated in graphitic carbon shells as electrocatalysts for hydrogen evolution reaction. Adv. Energy Mater. 2018, 8, 1701759. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.; He, Y.; Huang, H.; Wu, Y.; Hou, L.; Liu, X.; Yang, T.; Zou, J.; Huang, B. Synthesis, growth mechanism and thermal stability of copper nanoparticles encapsulated by multi-layer graphene. Carbon 2012, 50, 2119–2125. [Google Scholar] [CrossRef]
- Wang, J.; Cui, X.; Li, H.; Xiao, J.; Yang, J.; Mu, X.; Liu, H.; Sun, Y.M.; Xue, X.; Liu, C. Highly efficient catalytic scavenging of oxygen free radicals with graphene-encapsulated metal nanoshields. Nano Res. 2018, 11, 2821–2835. [Google Scholar] [CrossRef]
- Otor, H.O.; Steiner, J.B.; García-Sancho, C.; Alba-Rubio, A.C. Encapsulation methods for control of catalyst deactivation: A review. ACS Catal. 2020, 10, 7630–7656. [Google Scholar] [CrossRef]
- Tu, Y.; Ren, P.; Deng, D.; Bao, X. Structural and electronic optimization of graphene encapsulating binary metal for highly efficient water oxidation. Nano. Energy 2018, 52, 494–500. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, G.; Li, G.D.; Sun, Y.; Asefa, T.; Chen, W.; Zou, X. Coupling Mo2C with nitrogen-rich nanocarbon leads to efficient hydrogen-evolution electrocatalytic sites. Angew. Chem. Int. Edit. 2015, 127, 10902–11090. [Google Scholar] [CrossRef]
- Zou, X.; Huang, X.; Goswami, A.; Silva, R.; Sathe, B.B.R.R.; Mikmeková, E.; Asefa, T. Cobalt-embedded nitrogen-rich carbon nanotubes efficiently catalyze hydrogen evolution reaction at all pH values. Angew. Chem. 2014, 126, 4461–4465. [Google Scholar] [CrossRef]
- Liu, P.; Gao, D.; Xiao, W.; Ma, L.; Sun, K.; Xi, P.; Xue, D.; Wang, J. Self-powered water-splitting devices by core-shell NiFe@N-graphite-based Zn-Air batteries. Adv. Funct. Mater. 2018, 28, 1706928. [Google Scholar] [CrossRef]
- Mohanty, B.; Kumari, S.; Yadav, P.; Kanoo, P.; Chakraborty, A. Metal-organic frameworks (MOFs) and MOF composites based biosensors. Coordin. Chem. Rev. 2024, 519, 216102. [Google Scholar] [CrossRef]
- Khalil, I.E.; Fonseca, J.; Reithofer, M.R.; Eder, T.; Chin, J.M. Tackling orientation of metal-organic frameworks (MOFs): The quest to enhance MOF performance. Coordin. Chem. Rev. 2023, 481, 215043. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Liu, J.; Zhang, Z.; Gao, R.; Wu, Y.; Li, H.; Feng, M.; Chen, Z. Promises of MOF-based and MOF-derived materials for electrocatalytic CO2 reduction. Adv. Energy Mater. 2024, 2402278. [Google Scholar] [CrossRef]
- Xu, Y.; Tu, W.; Zhang, B.; Yin, S.; Huang, Y.; Kraft, M.; Xu, R. Nickel nanoparticles encapsulated in few-layer nitrogen-doped graphene derived from metal-organic frameworks as efficient bifunctional electrocatalysts for overall water splitting. Adv. Mater. 2017, 29, 1605957. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, Z.; Duan, J.; Liu, H.; Liu, G.; Wang, T.; Chang, K.; Li, M.; Shi, L.; Meng, X. Active sites implanted carbon cages in core-shell architecture: Highly active and durable electrocatalyst for hydrogen evolution reaction. Acs Nano 2016, 10, 684–694. [Google Scholar] [CrossRef]
- Yu, L.; Deng, D.; Bao, X. Chain mail for catalysts. Angew. Chem. Int. Ed. 2020, 59, 15294–15297. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, J.; Tu, Y.; Chen, S.; Wang, Y.; Wang, S.; Yu, L.; Ma, C.; Deng, D.; Bao, X. Highly efficient H2 production from H2S via a robust graphene-encapsulated metal catalyst. Energy Environ. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Asefa, T.; Huang, X. Heteroatom-doped carbon materials for electrocatalysis. Chem. Eur. J. 2017, 23, 10703–10713126. [Google Scholar] [CrossRef]
- Gao, Q.; Wang, Z.; Gao, W.; Yin, H. NiFeCo-based high entropy alloys nanoparticles coated with N-doped graphene layers as hydrogen evolution catalyst in alkaline solution. Chem. Eng. J. 2024, 489, 151370. [Google Scholar] [CrossRef]
- Zhou, W.; Zhou, J.; Zhou, Y.; Lu, J.; Zhou, K.; Yang, L.; Tang, Z.; Li, L.; Chen, S. N-doped carbon-wrapped cobalt nanoparticles on N-doped graphene nanosheets for high-efficiency hydrogen production. Chem. Mater. 2015, 27, 2026–2032. [Google Scholar] [CrossRef]
- Faber, M.S.; Song, J. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energy Environ. Sci 2014, 7, 3519–3542. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Stern, L.A.; Hu, X. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014, 43, 6555–6569. [Google Scholar] [CrossRef]
- Jin, D.; Johnson, L.R.; Raman, A.S.; Ming, X.; Gao, Y.; Du, F.; Wei, Y.; Chen, G.; Vojvodic, A.; Gogotsi, Y.; et al. Computational Screening of 2D ordered double transition-metal carbides (MXenes) as electrocatalysts for hydrogen evolution reaction. J. Phys. Chem. C 2020, 124, 10584–10592. [Google Scholar] [CrossRef]
- Ma, R.; Zhou, Y.; Chen, Y.; Li, P.; Liu, Q.; Wang, J. Ultrafine molybdenum carbide nanoparticles composited with carbon as a highly active hydrogen-evolution electrocatalyst. Angew. Chem. Int. Edit. 2015, 127, 14936–14940. [Google Scholar] [CrossRef]
- Zhou, M.; Jiang, X.; Kong, W.; Li, H.; Lu, F.; Zhou, X.; Zhang, Y. Synergistic effect of dual-doped carbon on Mo2C nanocrystals facilitates alkaline hydrogen evolution. Nano-Micro Lett. 2023, 15, 166. [Google Scholar] [CrossRef]
- Liu, X.; Ni, K.; Wen, B.; Niu, C.; Meng, J.; Guo, R.; Li, Q.; Li, J.; Zhu, Y.; Wu, X.; et al. Polyoxomolybdate-derived carbon-encapsulated multicomponent electrocatalysts for synergistically boosting hydrogen evolution. J. Mater. Chem. A 2018, 6, 17874–17881. [Google Scholar] [CrossRef]
- Qian, G.; Chen, J.; Yu, T.; Liu, J.; Luo, L.; Yin, S. Three-phase heterojunction NiMo-based nano-needle for water splitting at industrial alkaline condition. Nano-Micro. Lett. 2021, 14, 20. [Google Scholar] [CrossRef]
- Li, J.S.; Wang, Y.; Liu, C.H.; Li, S.L.; Wang, Y.G.; Dong, L.Z.; Dai, Z.H.; Li, Y.F.; Lan, Y.Q. Coupled molybdenum carbide and reduced graphene oxide electrocatalysts for efficient hydrogen evolution. Nat. Commun. 2016, 7, 11204. [Google Scholar] [CrossRef]
- Su, J.; Yang, Y.; Xia, G.; Chen, J.; Jiang, P.; Chen, Q. Ruthenium-cobalt nanoalloys encapsulated in nitrogen-doped graphene as active electrocatalysts for producing hydrogen in alkaline media. Nat. Commun. 2017, 8, 14969. [Google Scholar] [CrossRef]
- Si, Z.; Lv, Z.; Lu, L.; Liu, M.; Chen, Y.; Jin, H.; Tian, X.; Dai, K.; Liu, J.; Song, W. Nitrogen-doped graphene chainmail wrapped IrCo alloy particles on nitrogen-doped graphene nanosheet for highly active and stable full water splitting. ChemCatChem. 2019, 11, 5457–5465. [Google Scholar] [CrossRef]
- Yang, K.; Fu, H.; Duan, Y.; Wang, M.; Tran, M.X.; Lee, J.K.; Yang, W.; Liu, G. Uniform metal sulfide@N-doped carbon nanospheres for sodium storage: Universal synthesis strategy and superior performance. Energy Environ. Mater. 2023, 6, e12380. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Huang, X.; Zhu, Y.; Wang, D. Bimetallic phosphide heterostructure coupled with ultrathin carbon layer boosting overall alkaline water and seawater splitting. Small 2023, 19, 2206533. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xu, X.; Li, C.; Wu, D.; Cheng, D.; Cao, D. Vertical CoP nanoarray wrapped by N,P-doped carbon for hydrogen evolution reaction in both acidic and alkaline conditions. Adv. Energy Mater. 2019, 9, 1803970. [Google Scholar] [CrossRef]
- Wang, Y.-C.; Ma, X.-B.; Ayeza; Wang, C.-X.; Li, Y.; Yang, C.-L.; Wang, Z.F.; Wang, C.; Hu, C.; Zhang, Y.-T. A review of carbon-supported single-atom catalysts for electrochemical reactions. New Carbon Mater. 2024, 39, 407–438. [Google Scholar] [CrossRef]
- Yin, X.-P.; Wang, H.-J.; Tang, S.-F.; Lu, X.-L.; Shu, M.; Si, R.; Lu, T.-B. Engineering the coordination environment of single-atom platinum anchored on graphdiyne for optimizing electrocatalytic hydrogen evolution. Angew. Chem. Int. Ed. 2018, 57, 9382–9386. [Google Scholar] [CrossRef]
- Wang, M.J.; Ji, M.; Zheng, X.; Jiang, C.; Zhao, H.; Mao, Z.X.; Zhang, M.; Zhu, C.; Xu, J. Enhanced hydrogen evolution of single-atom Ru sites via geometric and electronic engineering: N and S dual coordination. Appl. Surface Sci. 2021, 551, 148742. [Google Scholar] [CrossRef]
- Moore, G.W.K.; Howell, S.E.L.; Brady, M.; Xu, X.; McNeil, K. Anomalous collapses of Nares Strait ice arches leads to enhanced export of Arctic sea ice. Nat. Commun. 2021, 12, 1. [Google Scholar] [CrossRef]
- Chen, H.A.; Hsin, C.L.; Huang, Y.T.; Tang, M.L.; Dhuey, S.; Cabrini, S.; Wu, W.W.; Leone, S.R. Measurement of interlayer screening length of layered graphene by plasmonic nanostructure resonances. J. Phys. Chem. C 2013, 117, 22211–22217. [Google Scholar] [CrossRef]
- Li, Z.; Wu, X.; Jiang, X.; Shen, B.; Teng, Z.; Sun, D.; Fu, G.; Tang, Y. Surface carbon layer controllable Ni3Fe particles confined in hierarchical N-doped carbon framework boosting oxygen evolution reaction. Adv. Powder Mater. 2022, 1, 100020. [Google Scholar] [CrossRef]
- Dolganov, A.V.; Klimaeva, L.A.; Kostryukov, S.G.; Yudina, A.D.; Zagorodnova, A.S.; Tankova, A.V. N-methyl ortho-bipyridines as a new member of metal-free electrocatalysts for hydrogen evolution reaction (HER): The influence of the substituent on a heteroatom on the pathway (mechanism) HER. Int. J. Hydrogen Energy 2024, 51, 85–97. [Google Scholar] [CrossRef]
- Lin, H.; Liu, N.; Shi, Z.; Guo, Y.; Gao, Q. Cobalt-doping in molybdenum-carbide nanowires toward efficient electrocatalytic hydrogen evolution. Adv. Funct. Mater. 2016, 26, 5581. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Zhang, Y.; Jiang, W.J.; Zhang, X.; Dai, Z.; Wan, L.J.; Hu, J.S. Pomegranate-like N, P-Doped Mo2C@C nanospheres as highly active electrocatalysts for alkaline hydrogen evolution. ACS Nano 2016, 10, 8851–8860. [Google Scholar] [CrossRef]
- Ozden, S.; Bawari, S.; Vinod, S.; Martinez, U.; Susarla, S.; Narvaez, C.; Joyner, J.; Tiwary, C.S.; Narayanan, T.N.; Ajayan, P.M. Interface and defect engineering of hybrid nanostructures toward an efficient HER catalyst. Nanoscale 2019, 11, 12489–12496. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Chakraborty, S.; Sen, P. Complementary effects of functionalization, vacancy defects and strain engineering in activating the basal plane of monolayer FePS3 for HER. Sustain. Energy Fuels 2022, 6, 5621–5630. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Zhang, K.; Hu, W.; Cheng, Z.; Chen, H.; Feng, X.; Peng, T.; Kou, Z. Monodispersed ruthenium nanoparticles interfacially bonded with defective nitrogen-and-phosphorus-doped carbon nanosheets enable pH-universal hydrogen evolution reaction. Appl. Catal. B 2022, 306, 121095. [Google Scholar] [CrossRef]
- Singh, T.I.; Rajeshkhanna, G.; Pan, U.N.; Kshetri, T.; Lin, H.; Kim, N.H.; Lee, J.H. Alkaline water splitting enhancement by MOF-Derived Fe–Co–Oxide/Co@NC-mNS heterostructure: Boosting OER and HER through defect engineering and in situ oxidation. Small 2021, 17, 2101312. [Google Scholar] [CrossRef]
- Tao, L.; Qiao, M.; Jin, R.; Li, Y.; Xiao, Z.; Wang, Y.; Zhang, N.; Xie, C.; He, Q.; Jiang, D.; et al. Bridging the surface charge and catalytic activity of a defective carbon electrocatalyst. Angew. Chem. Int. Edit. 2019, 58, 1019–1024. [Google Scholar] [CrossRef]
- Gu, L.F.; Chen, J.J.; Zhou, T.; Lu, X.F.; Li, G.R. Engineering cobalt oxide by interfaces and pore architectures for enhanced electrocatalytic performance for overall water splitting. Nanoscale 2020, 12, 11201–11208. [Google Scholar] [CrossRef]
- He, H.; Zhang, Y.; Zhang, W.; Li, Y.; Zhu, X.; Wang, P.; Hu, D. Porous carbon nanofibers derived from silk fibroin through electrospinning as N-doped metal-free catalysts for hydrogen evolution reaction in acidic and alkaline solutions. ACS Appl. Mater. Interf. 2022, 14, 834–849. [Google Scholar] [CrossRef]
- Li, H.; Ma, W.; Ma, X.; Guo, M.; Li, G. Multifunctional salt-assisted construction of lignin-derived Ru–Co bimetal/carbon composites with rich nanointerface for electrocatalytic water splitting. ACS Sustain. Chem. Eng. 2022, 10, 16214–16224. [Google Scholar] [CrossRef]
- Jena, H.S.; Krishnaraj, C.; Parwaiz, S.; Lecoeuvre, F.; Schmidt, J.; Pradhan, D.; Van Der Voort, P. Illustrating the role of quaternary-N of BINOL covalent triazine-based frameworks in oxygen reduction and hydrogen evolution reactions. ACS Appl. Mater. Interfaces 2020, 12, 44689–44699. [Google Scholar] [CrossRef]
- Ding, M.; Xu, H.; Liu, M.; Wang, Y.; Wang, A.; Lin, T.; Zhang, L.; Zhang, K. Interface construction and porosity engineering to trigger efficient hydrogen evolution of two-dimensional porous molybdenum phosphide/dioxide heterojunction nanosheets in acidic and alkaline electrolytes. Chem. Eng. J. 2022, 430, 132674. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Zhou, T.; Zhang, H.; Sun, X.; Tang, J.; Zhang, L.; Al-Enizi, A.M.; Yang, Z.; Zheng, G. Nanoparticle Superlattices as Efficient Bifunctional Electrocatalysts for Water Splitting. J. Am. Chem. Soc. 2015, 137, 14305–14312. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, M.; Ou, X.; Dou, Y.; Zhang, L.; Zhang, Q.; Wu, R.; Ding, Y.; Shao, M.; Luo, Z. Polymer-Embedded Fabrication of Co2P Nanoparticles Encapsulated in N,P-Doped Graphene for Hydrogen Generation. Nano Lett. 2016, 16, 4691–4698. [Google Scholar] [CrossRef]
- Dai, X.; Li, Z.; Ma, Y.; Liu, M.; Du, K.; Su, H.; Zhuo, H.; Yu, L.; Sun, H.; Zhang, X. Metallic Cobalt Encapsulated in Bamboo-Like and Nitrogen-Rich Carbonitride Nanotubes for Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 6439–6448. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Kou, M.; Yuan, Q.; Yuan, Y.; Zhao, J. Encapsulating Transition Metal Nanoparticles inside Carbon (TM@C) Chainmail Catalysts for Hydrogen Evolution Reactions: A Review. Molecules 2024, 29, 4677. https://doi.org/10.3390/molecules29194677
Zhao J, Kou M, Yuan Q, Yuan Y, Zhao J. Encapsulating Transition Metal Nanoparticles inside Carbon (TM@C) Chainmail Catalysts for Hydrogen Evolution Reactions: A Review. Molecules. 2024; 29(19):4677. https://doi.org/10.3390/molecules29194677
Chicago/Turabian StyleZhao, Jiamin, Meimei Kou, Qing Yuan, Ying Yuan, and Jinsheng Zhao. 2024. "Encapsulating Transition Metal Nanoparticles inside Carbon (TM@C) Chainmail Catalysts for Hydrogen Evolution Reactions: A Review" Molecules 29, no. 19: 4677. https://doi.org/10.3390/molecules29194677
APA StyleZhao, J., Kou, M., Yuan, Q., Yuan, Y., & Zhao, J. (2024). Encapsulating Transition Metal Nanoparticles inside Carbon (TM@C) Chainmail Catalysts for Hydrogen Evolution Reactions: A Review. Molecules, 29(19), 4677. https://doi.org/10.3390/molecules29194677






