Essential Oil-Based Soap with Clove and Oregano: A Promising Antifungal and Antibacterial Alternative against Multidrug-Resistant Microorganisms
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activity
2.2. Antifungal Activity
2.3. Antimicrobial Activity and Synergism of the Oils
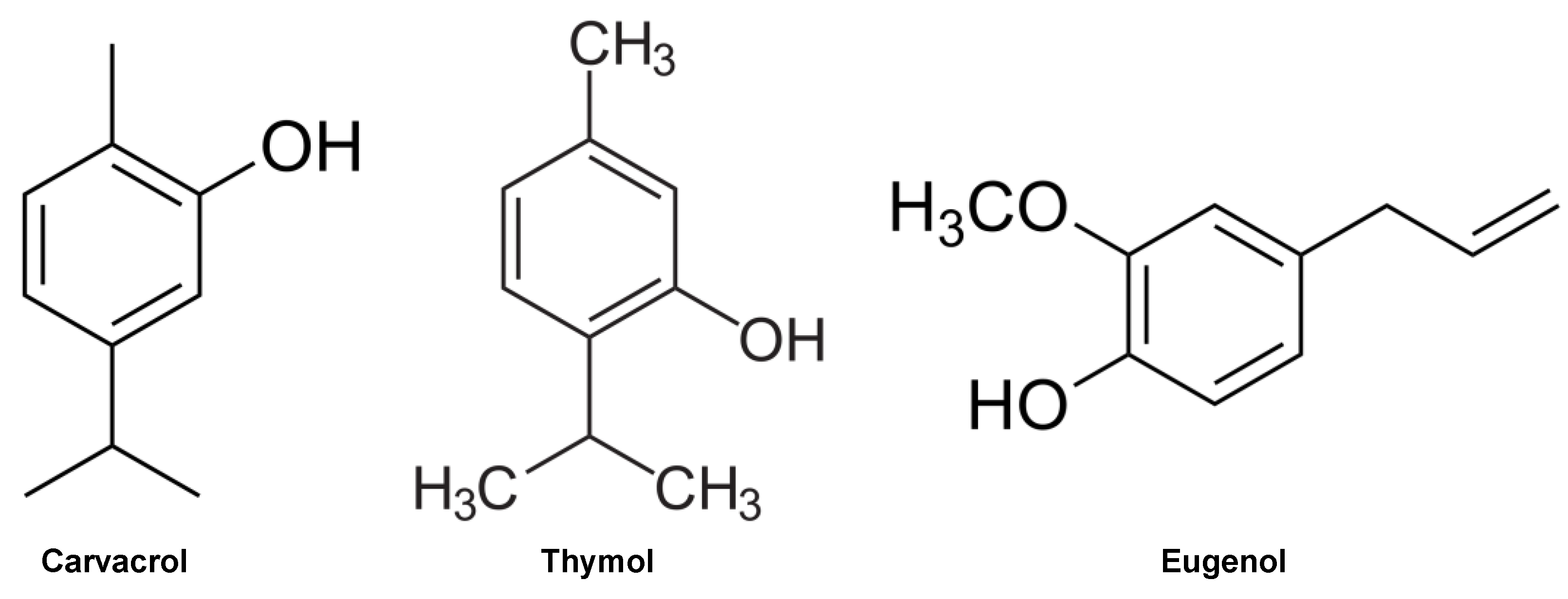
2.4. GC-MS
2.5. Evaluation of In Vivo Antimicrobial Activity
3. Discussion
4. Materials and Methods
4.1. Essential Oils
4.2. Bacterial Strains
4.3. Fungal Strains
4.4. Minimal Inhibitory Concentration
4.5. Minimal Bactericidal Concentration and Minimal Fungicidal Concentration
4.6. Evaluation of Synergistic Effect of Essential Oils for In Vitro Antimicrobial Activity
4.7. Soap Formulation
4.8. In Vivo Assay
4.8.1. Preparation of Bacterial Culture for Artificial Contamination of Hands
4.8.2. Analysis of In Vivo Assay Results
4.9. Analysis of Essential Oil Constituents by Gas Chromatography–Mass Spectrometry (GC–MS)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arda, O.; Göksügür, N.; Tüzün, Y. Basic Histological Structure and Functions of Facial Skin. Clin. Derm. 2014, 32, 3–13. [Google Scholar] [CrossRef] [PubMed]
- ANVISA Segurança Do Paciente Em Serviços de Saúde: Higienização Das Mãos 2009. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/seguranca_paciente_servicos_saude_higienizacao_maos.pdf (accessed on 24 September 2024).
- Anvisa Programa Nacional De Prevenção E Controle De Infecções Relacionadas À Assistência À Saúde (Pnpciras) 2021 a 2025 2021. Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/publicacoes/pnpciras_2021_2025.pdf (accessed on 24 September 2024).
- Fracarolli, I.F.L.; De Oliveira, S.A.; Marziale, M.H.P. Colonização Bacteriana e Resistência Antimicrobiana Em Trabalhadores de Saúde: Revisão Integrativa. Acta Paul. Enferm. 2017, 30, 651–657. [Google Scholar] [CrossRef]
- García-Salinas, S.; Elizondo-Castillo, H.; Arruebo, M.; Mendoza, G.; Irusta, S. Evaluation of the Antimicrobial Activity and Cytotoxicity of Different Components of Natural Origin Present in Essential Oils. Molecules 2018, 23, 1399. [Google Scholar] [CrossRef] [PubMed]
- Santos, N. de Q. A Resistência Bacteriana No Contexto Da Infecção Hospitalar. Texto Contexto–Enferm. 2004, 13, 64–70. [Google Scholar] [CrossRef]
- Gnatta, J.R.; Pinto, F.M.G.; Bruna, C.Q. de M.; de Souza, R.Q.; Graziano, K.U.; da Silva, M.J.P. Comparison of Hand Hygiene Antimicrobial Efficacy: Melaleuca Alternifolia Essential Oil versus Triclosan. Rev. Lat. Am. Enferm. 2013, 21, 1212–1219. [Google Scholar] [CrossRef]
- Halden, R.U. On the Need and Speed of Regulating Triclosan and Triclocarban in the United States. Environ. Sci. Technol. 2014, 48, 3603–3611. [Google Scholar] [CrossRef]
- Yueh, M.F.; Tukey, R.H. Triclosan: A Widespread Environmental Toxicant with Many Biological Effects. Annu. Rev. Pharm. Toxicol 2016, 56, 251–272. [Google Scholar] [CrossRef]
- Toholka, R.; Nixon, R. Allergic Contact Dermatitis to Chlorhexidine. Australas. J. Dermatol. 2013, 54, 303–306. [Google Scholar] [CrossRef]
- Food and Drug Adminnistration, H.H.S. Safety and effectiveness of consumer antiseptics; topical antimicrobial drug products for over-the-counter human use. Final. Rule. Fed. Regist. 2016, 81, 61106–61130. [Google Scholar]
- Babosa, A.S.; Évora, B.R.; Campos, J.S.; Oliveira, E.S.; Oliveira, J.N. Diminuição Da Susceptibilidade à Clorexidina: Revisão Sistemática. J. Infect. Control. 2019, 8. [Google Scholar]
- Yap, P.S.X.; Yiap, B.C.; Ping, H.C.; Lim, S.H.E. Essential Oils, A New Horizon in Combating Bacterial Antibiotic Resistance. Open Microbiol. J. 2014, 8, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Aziz, Z.A.A.; Ahmad, A.; Setapar, S.H.M.; Karakucuk, A.; Azim, M.M.; Lokhat, D.; Rafatullah, M.; Ganash, M.; Kamal, M.A.; Ashraf, G.M. Essential Oils: Extraction Techniques, Pharmaceutical And Therapeutic Potential–A Review. Curr. Drug Metab. 2018, 19, 1100–1110. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological Effects of Essential Oils–A Review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef] [PubMed]
- Leigh-de Rapper, S.; van Vuuren, S.F. Odoriferous Therapy: A Review Identifying Essential Oils against Pathogens of the Respiratory Tract. Chem. Biodivers. 2020, 17, e2000062. [Google Scholar] [CrossRef]
- Alanazi, A.K.; Alqasmi, M.H.; Alrouji, M.; Kuriri, F.A.; Almuhanna, Y.; Joseph, B.; Asad, M. Antibacterial Activity of Syzygium Aromaticum (Clove) Bud Oil and Its Interaction with Imipenem in Controlling Wound Infections in Rats Caused by Methicillin-Resistant Staphylococcus Aureus. Molecules 2022, 27, 8551. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, Antioxidant, and Immunomodulatory Properties of Essential Oils: A Systematic Review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Wińska, K.; Mączka, W.; Łyczko, J.; Grabarczyk, M.; Czubaszek, A.; Szumny, A. Essential Oils as Antimicrobial Agents—Myth or Real Alternative? Molecules 2019, 24, 2130. [Google Scholar] [CrossRef]
- Puškárová, A.; Bučková, M.; Kraková, L.; Pangallo, D.; Kozics, K. The Antibacterial and Antifungal Activity of Six Essential Oils and Their Cyto/Genotoxicity to Human HEL 12469 Cells. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Ben Arfa, A.; Combes, S.; Preziosi-Belloy, L.; Gontard, N.; Chalier, P. Antimicrobial Activity of Carvacrol Related to Its Chemical Structure. Lett. Appl. Microbiol. 2006, 43, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Vale, L.; de Paula, L.G.F.; Vieira, M.S.; Alves, S.D.G.A.; Junior, N.R.M.; Gomes, M.D.F.; Teixeira, W.F.P.; Rizzo, P.V.; Freitas, F.M.C.; Ferreira, L.L.; et al. Binary Combinations of Thymol, Carvacrol and Eugenol for Amblyomma Sculptum Control: Evaluation of in Vitro Synergism and Effectiveness under Semi-Field Conditions. Ticks Tick Borne Dis. 2021, 12, 101816. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Al-Holy, M.A.; Osaili, T.M.; Abughoush, M.H.; Al-Nabulsi, A.A.; Alawneh, M.; Deseh, L.; Abazeed, B.; Shqair, R.; Mutlaq, S.; et al. Antimicrobial Activity of Eugenol and Carvacrol against Salmonella Enterica and E. Coli O157:H7 in Falafel Paste at Different Storage Temperatures. Int. J. Food. Microbiol. 2024, 415, 110648. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.; Rhee, M.S. Synergism between Carvacrol or Thymol Increases the Antimicrobial Efficacy of Soy Sauce with No Sensory Impact. Int J Food Microbiol 2016, 217, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Naveen, K.V.; Saravanakumar, K.; Sathiyaseelan, A.; MubarakAli, D.; Wang, M.H. Human Fungal Infection, Immune Response, and Clinical Challenge–a Perspective During COVID-19 Pandemic. Appl. Biochem. Biotechnol. 2022, 194, 4244–4257. [Google Scholar] [CrossRef]
- Brown, G.D.; Denning, D.W.; Gow, N.A.R.; Levitz, S.M.; Netea, M.G.; White, T.C. Hidden Killers: Human Fungal Infections. Sci. Transl. Med. 2012, 4. [Google Scholar] [CrossRef]
- Petrucelli, M.F.; de Abreu, M.H.; Cantelli, B.A.M.; Segura, G.G.; Nishimura, F.G.; Bitencourt, T.A.; Marins, M.; Fachin, A.L. Epidemiology and Diagnostic Perspectives of Dermatophytoses. J. Fungi 2020, 6, 310. [Google Scholar] [CrossRef]
- Schroder, T.; Gaskin, S.; Ross, K.; Whiley, H. Antifungal Activity of Essential Oils against Fungi Isolated from Air. Int. J. Occup. Environ. Health 2017, 23, 181–186. [Google Scholar] [CrossRef]
- Kumari, P.; Mishra, R.; Arora, N.; Chatrath, A.; Gangwar, R.; Roy, P.; Prasad, R. Antifungal and Anti-Biofilm Activity of Essential Oil Active Components against Cryptococcus Neoformans and Cryptococcus Laurentii. Front. Microbiol. 2017, 8, 291228. [Google Scholar] [CrossRef]
- Parker, R.A.; Gabriel, K.T.; Graham, K.D.; Butts, B.K.; Cornelison, C.T. Antifungal Activity of Select Essential Oils against Candida Auris and Their Interactions with Antifungal Drugs. Pathogens 2022, 11, 821. [Google Scholar] [CrossRef]
- De Rapper, S.L.; Viljoen, A.; Van Vuuren, S. Optimizing the Antimicrobial Synergism of Melaleuca Alternifolia (Tea Tree) Essential Oil Combinations for Application against Respiratory Related Pathogens. Planta Med. 2023, 89, 454–463. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; De Sá Queiroz, J.H.F.; Da Silva, K.E.; De Paula Vasconcelos, P.C.; Croda, J.; Simionatto, S. Synergistic Effects of Cinnamomum Cassia, L. Essential Oil in Combination with Polymyxin B against Carbapenemase-Producing Klebsiella Pneumoniae and Serratia Marcescens. PLoS ONE 2020, 15, e0236505. [Google Scholar] [CrossRef] [PubMed]
- Buxser, S.; Al-Bakri, A. Has Resistance to Chlorhexidine Increased among Clinically-Relevant Bacteria? A Systematic Review of Time Course and Subpopulation Data. PLoS ONE 2021, 16, e0256336. [Google Scholar] [CrossRef]
- Chapman, J.S. Biocide Resistance Mechanisms. Int. Biodeterior. Biodegrad. 2003, 51, 133–138. [Google Scholar] [CrossRef]
- Nde, C.W.; Jang, H.J.; Toghrol, F.; Bentley, W.E. Global Transcriptomic Response of Pseudomonas Aeruginosa to Chlorhexidine Diacetate. Environ Sci Technol 2009, 43, 8406–8415. [Google Scholar] [CrossRef]
- Poole, K. Mechanisms of Bacterial Biocide and Antibiotic Resistance. J. Appl. Microbiol. 2002, 92, 55S–64S. [Google Scholar] [CrossRef]
- Gupta, A.; Jeyakumar, E.; Lawrence, R. Journey of Limonene as an Antimicrobial Agent. J. Pure Appl. Microbiol. 2021, 15, 1094–1110. [Google Scholar] [CrossRef]
- Han, Y.; Chen, W.; Sun, Z. Antimicrobial Activity and Mechanism of Limonene against Staphylococcus Aureus. J. Food Saf. 2021, 41, e12918. [Google Scholar] [CrossRef]
- Guo, F.; Chen, Q.; Liang, Q.; Zhang, M.; Chen, W.; Chen, H.; Yun, Y.; Zhong, Q.; Chen, W. Antimicrobial Activity and Proposed Action Mechanism of Linalool Against Pseudomonas Fluorescens. Front. Microbiol. 2021, 12, 562094. [Google Scholar] [CrossRef]
- Liu, X.; Cai, J.; Chen, H.; Zhong, Q.; Hou, Y.; Chen, W.; Chen, W. Antibacterial Activity and Mechanism of Linalool against Pseudomonas Aeruginosa. Microb. Pathog. 2020, 141, 103980. [Google Scholar] [CrossRef]
- Keykhasalar, R.; Tabrizi, M.H.; Ardalan, P. Antioxidant Property and Bactericidal Activity of Linum Usitatissimum Seed Essential Oil Nanoemulsion (LSEO-NE) on Staphylococcus Aureus. Int. J. Infect. 2020, 7, 101639. [Google Scholar] [CrossRef]
- Braz, V.S.; Furlan, J.P.R.; Passaglia, J.; Falcão, J.P.; Stehling, E.G. Genotypic Diversity and Presence of β-Lactamase Encoding Genes in Pseudomonas Aeruginosa Isolated from Brazilian Soils. Appl. Soil Ecol. 2018, 129, 94–97. [Google Scholar] [CrossRef]
- Furlan, J.P.R.; Savazzi, E.A.; Stehling, E.G. Widespread High-Risk Clones of Multidrug-Resistant Extended-Spectrum β-Lactamase-Producing Escherichia Coli B2-ST131 and F-ST648 in Public Aquatic Environments. Int. J. Antimicrob Agents 2020, 56, 106040. [Google Scholar] [CrossRef]
- Cruz, T.A.; Torres, F.R.; Petrucelli, M.F.; de Abreu, M.H.; Silva, S.S.; Marins, M.M.; Beleboni, R.O.; Fachin, A.L. Antimicrobial and synergistic activity of essential oils facing isolated bacteria from surgical staff’s. Rev. Prevenção Infecção Saúde 2019, 5, 8970. [Google Scholar] [CrossRef]
- da Fonseca, L.M.M.; Braga, V.F.; Tonani, L.; Grizante Barião, P.H.; Nascimento, E.; Martinez, R.; von Zeska Kress, M.R. Surveillance of Amphotericin B and Azole Resistance in Aspergillus Isolated from Patients in a Tertiary Teaching Hospital. J. Fungi 2023, 9, 1070. [Google Scholar] [CrossRef]
- Wayne, P.A. CSLI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Wayne, P.A. CSLI Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi, 11th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2017. [Google Scholar]
- Wayne, P.A. CSLI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Karpanen, T.J.; Worthington, T.; Hendry, E.R.; Conway, B.R.; Lambert, P.A. Antimicrobial Efficacy of Chlorhexidine Digluconate Alone and in Combination with Eucalyptus Oil, Tea Tree Oil and Thymol against Planktonic and Biofilm Cultures of Staphylococcus Epidermidis. J. Antimicrob. Chemother. 2008, 62, 1031–1036. [Google Scholar] [CrossRef]
- Andreyeva, I.N.; Ogorodnikova, T.I. Pigmentation of Serratia Marcescens and Spectral Properties of Prodigiosin. Microbiology 2015, 84, 28–33. [Google Scholar] [CrossRef]
- Bhagwat, A.; Padalia, U. Optimization of Prodigiosin Biosynthesis by Serratia Marcescens Using Unconventional Bioresources. J. Genet. Eng. Biotechnol. 2020, 18, 26. [Google Scholar] [CrossRef]
- EN 1500; European Committee for Standardization Chemical Disinfectants and Antiseptics Hygienic Handrub Test Method and Requirements (Phase 2/Step 2). Genorma: Madrid, Spain, 1997.
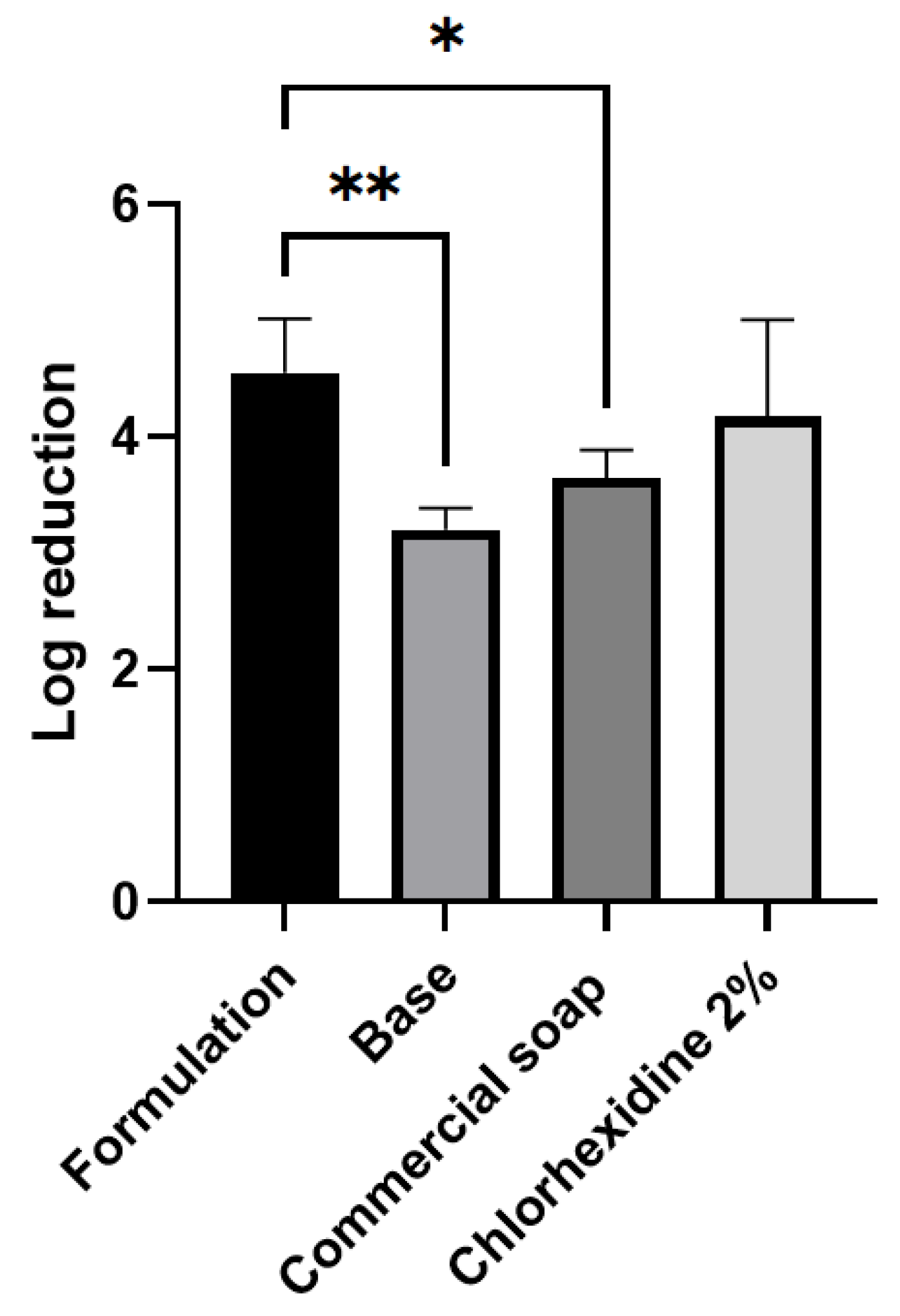
| CLO | ORE | THY | GEN | CHLO | |
|---|---|---|---|---|---|
| E. coli ATCC 25922 | 12.5 | 6.25 | 12.5 | 3.125 | 3.05 × 10−5 |
| E. coli EW222 | 3.125 | 6.25 | 25 | 1.562 | 2.44 × 10−4 |
| E. coli EW239 | 12.5 | 3.125 | 12.5 | >50 | 3.05 × 10−5 |
| P. aeruginosa ATCC 27853 | 25 | 25 | 50 | 6.25 | 1.95 × 10−3 |
| P. aeruginosa S15 | 12,5 | 12.5 | 50 | 0.39 | 9.76 × 10−4 |
| S. aureus ATCC 6538 | 25 | 6.25 | 12.5 | 6.25 | 1.22 × 10−4 |
| S. epidermidis ATCC 12228 | 12.5 | 6.25 | 12.5 | 0.096 | 7.63 × 10−6 |
| S. marcescens ATCC | 1.56 | 3.125 | 6.25 | 0.78 | 1.9 × 10−4 |
| P. vulgaris ATCC 6380 | 12.5 | 6.25 | 12.5 | 1.562 | 1.95 × 10−3 |
| S. Choleraesuis ATCC 10708 | 6.25 | 3.125 | 6.25 | 12.5 | 9.76 × 10−4 |
| CLO | ORE | THY | GEN | CHLO | |
|---|---|---|---|---|---|
| E. coli ATCC 25922 | BC | BC | BC | BS | BS |
| E. coli EW222 | BC | BC | BC | BS | BC |
| E. coli EW239 | BC | BC | BC | * | BC |
| P. aeruginosa ATCC 27853 | BC | BC | BC | BC | BS |
| P. aeruginosa S15 | BS | BC | BC | BS | BS |
| S. aureus ATCC 6538 | BC | BC | BC | BC | BS |
| S. epidermidis ATCC 12228 | BS | BS | BS | BS | BS |
| S. marcescens ATCC | BC | BC | BC | BC | BC |
| P. vulgaris ATCC 6380 | BC | BC | BC | BC | BS |
| S. choleraesuis ATCC 10708 | BS | BS | BS | BC | BS |
| CLO | ORE | THY | FLU | CHLO | |
|---|---|---|---|---|---|
| A. fumigatus ATCC 46645 | 3.125 | 0.003 | 0.195 | 250 | <2.3 × 10−5 |
| A. fumigatus LMC 9015.01 | 1.562 | 6.25 | 6.25 | 250 | <2.3 × 10−5 |
| T. rubrum ATCC MYA 3108 | 1.562 | 0.390 | 3.125 | 15.62 | <2.3 × 10−5 |
| CLO | ORE | THY | FLU | CLHO | |
|---|---|---|---|---|---|
| C. neoformans ATCC 90112 | 6.25 | 12.5 | 12.5 | 1.562 | 9.76 × 10−4 |
| C. albicans ATCC 10231 | 12.5 | 3.125 | 12.5 | 7.81 | 2.44 × 10−4 |
| C. auris CDC 811903 | 1.562 | 0.0976 | 0.781 | 3.9 | 9.76 × 10−4 |
| CLO/ORE | CLO/THY | THY/ORE | |
|---|---|---|---|
| S. aureus ATCC 6538 | 0.781 | 3.125 | 1.562 |
| S. epidermidis ATCC 12228 | 3.125 | 6.25 | 3.125 |
| S. marcescens ATCC | 0.781 | 3.125 | 1.56 |
| E. coli ATCC 25922 | 1.562 | 0.781 | 1.562 |
| E. coli EW239 | 1.562 | 3.125 | 3.125 |
| CLO/ORE | CLO/THY | THY/ORE | |
|---|---|---|---|
| S. aureus ATCC 6538 | BS | BS | BS |
| S. epidermidis ATCC 12228 | BC | BS | BS |
| S. marcescens ATCC | BC | BC | BC |
| E. coli ATCC 25922 | BC | BS | BC |
| E. coli EW239 | BC | BC | BC |
| CLO/ORE | CLO/THY | THY/ORE | |
|---|---|---|---|
| S. aureus ATCC 6538 | 0.156 (Synergistic) | 0.375 (Synergistic) | 0.375 (Synergistic) |
| S. epidermidis ATCC 12228 | 0.75 (Additive) | 1.00 (Additive) | 0.75 (Additive) |
| S. marcescens ATCC | 0.75 (Additive) | 2.5 (Indifferent) | 0.75 (Additive) |
| E. coli ATCC 25922 | 0.375 (Synergistic) | 0.125 (Synergistic) | 0.375 (Synergistic) |
| E. coli EW239 | 0.625 (Additive) | 0.50 (Synergistic) | 1.25 (Indifferent) |
| Identified Substance | KI | Clove (%) | Oregano (%) | Thyme (%) |
|---|---|---|---|---|
| tricyclene | 926 | - | - | 2.42 |
| p-cymene | 1011 | - | 5.90 | 24.51 |
| 1.8-cineole | 1020 | - | 1.80 | 1.49 |
| trans-ocimeno | 1048 | - | 2.41 | 4.49 |
| terpinoleno | 1085 | - | 1.95 | 5.25 |
| camphor | 1120 | - | 0.62 | 1.61 |
| neoisotujol | 1142 | - | - | 0.54 |
| neotujol | 1150 | - | 0.97 | 1.30 |
| terpinen-4-ol | 1162 | - | 0.54 | 0.91 |
| thujanol | 1174 | - | 0.57 | - |
| thymol | 1279 | - | 2.29 | 49 |
| carvacrol | 1287 | - | 72.63 | 2.83 |
| eugenol | 1336 | 84.36 | - | - |
| trans-caryophyllene | 1413 | 9.33 | 2.64 | - |
| humulene | 1446 | 1.46 | - | - |
| caryophyllene oxide | 1566 | 0.93 | 0.46 | 0.89 |
| Microorganism | Reference | Origin |
|---|---|---|
| Escherichia coli | ATCC 25922 | |
| Escherichia coli | EW222 | public aquatic environments |
| Escherichia coli | EW239 | public aquatic environments |
| Pseudomonas aeruginosa | ATCC 27853 | |
| Pseudomonas aeruginosa | S15 | soil |
| Staphylococcus aureus | ATCC 6538 | |
| Staphylococcus epidermidis | ATCC 12228 | |
| Serratia marcescens | ATCC 13880 | |
| Proteus vulgaris | ATCC 6380 | |
| Salmonella choleraesuis | ATCC 10708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, A.P.M.; Nishimura, F.G.; Santos, V.C.O.d.; Steling, E.G.; Von Zeska Kress, M.R.; Marins, M.; Fachin, A.L. Essential Oil-Based Soap with Clove and Oregano: A Promising Antifungal and Antibacterial Alternative against Multidrug-Resistant Microorganisms. Molecules 2024, 29, 4682. https://doi.org/10.3390/molecules29194682
Cruz APM, Nishimura FG, Santos VCOd, Steling EG, Von Zeska Kress MR, Marins M, Fachin AL. Essential Oil-Based Soap with Clove and Oregano: A Promising Antifungal and Antibacterial Alternative against Multidrug-Resistant Microorganisms. Molecules. 2024; 29(19):4682. https://doi.org/10.3390/molecules29194682
Chicago/Turabian StyleCruz, Ana Paula Merino, Felipe Garcia Nishimura, Vinícius Cristian Oti dos Santos, Eliana Guedes Steling, Marcia Regina Von Zeska Kress, Mozart Marins, and Ana Lucia Fachin. 2024. "Essential Oil-Based Soap with Clove and Oregano: A Promising Antifungal and Antibacterial Alternative against Multidrug-Resistant Microorganisms" Molecules 29, no. 19: 4682. https://doi.org/10.3390/molecules29194682
APA StyleCruz, A. P. M., Nishimura, F. G., Santos, V. C. O. d., Steling, E. G., Von Zeska Kress, M. R., Marins, M., & Fachin, A. L. (2024). Essential Oil-Based Soap with Clove and Oregano: A Promising Antifungal and Antibacterial Alternative against Multidrug-Resistant Microorganisms. Molecules, 29(19), 4682. https://doi.org/10.3390/molecules29194682






