Synthesis of an Ethylenediaminetetraacetic Acid-like Ligand Based on Sucrose Scaffold and Complexation and Proton Relaxivity Studies of Its Gadolinium(III) Complex in Solution
Abstract
:1. Introduction
2. Results and Discussion
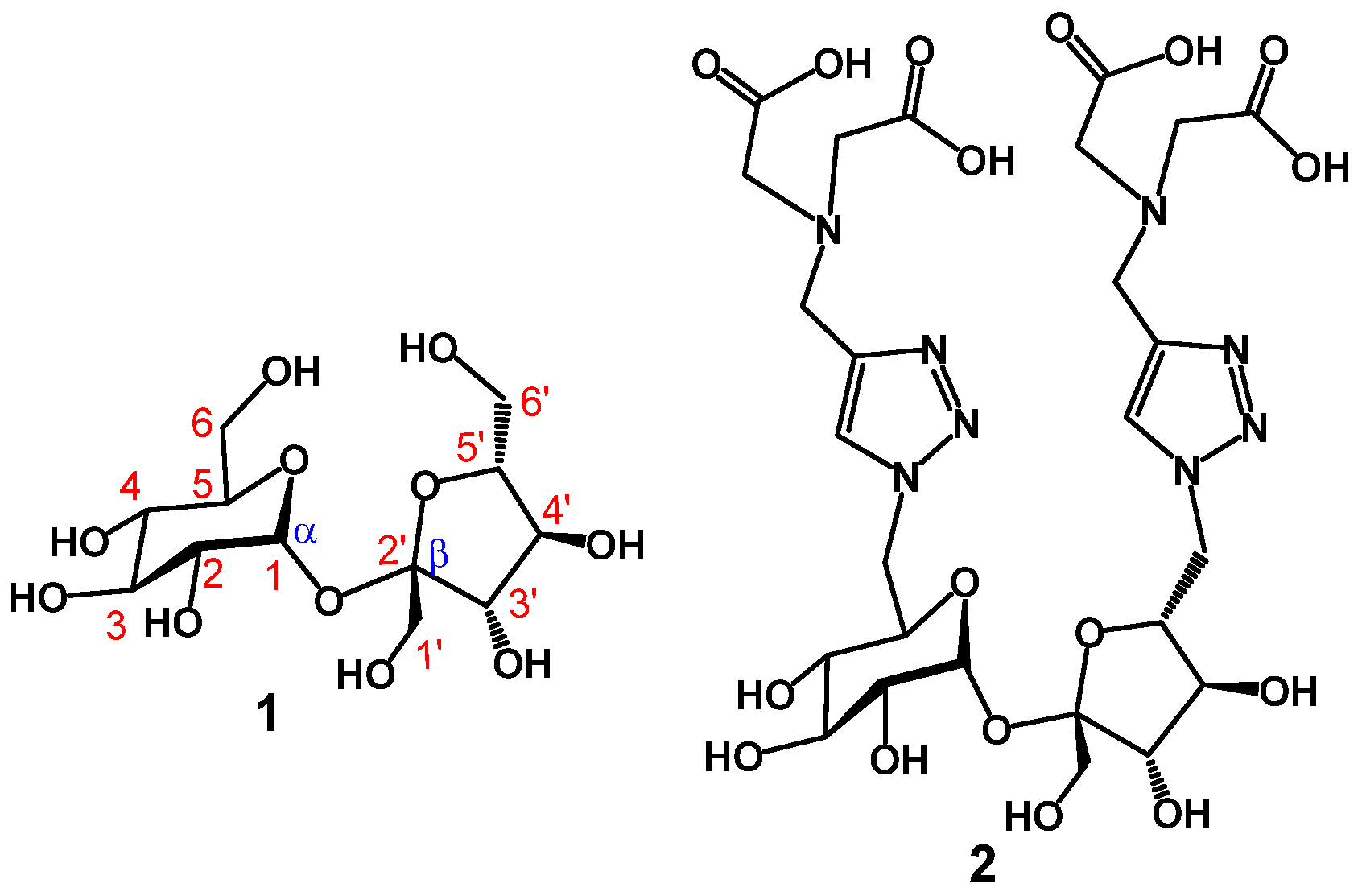
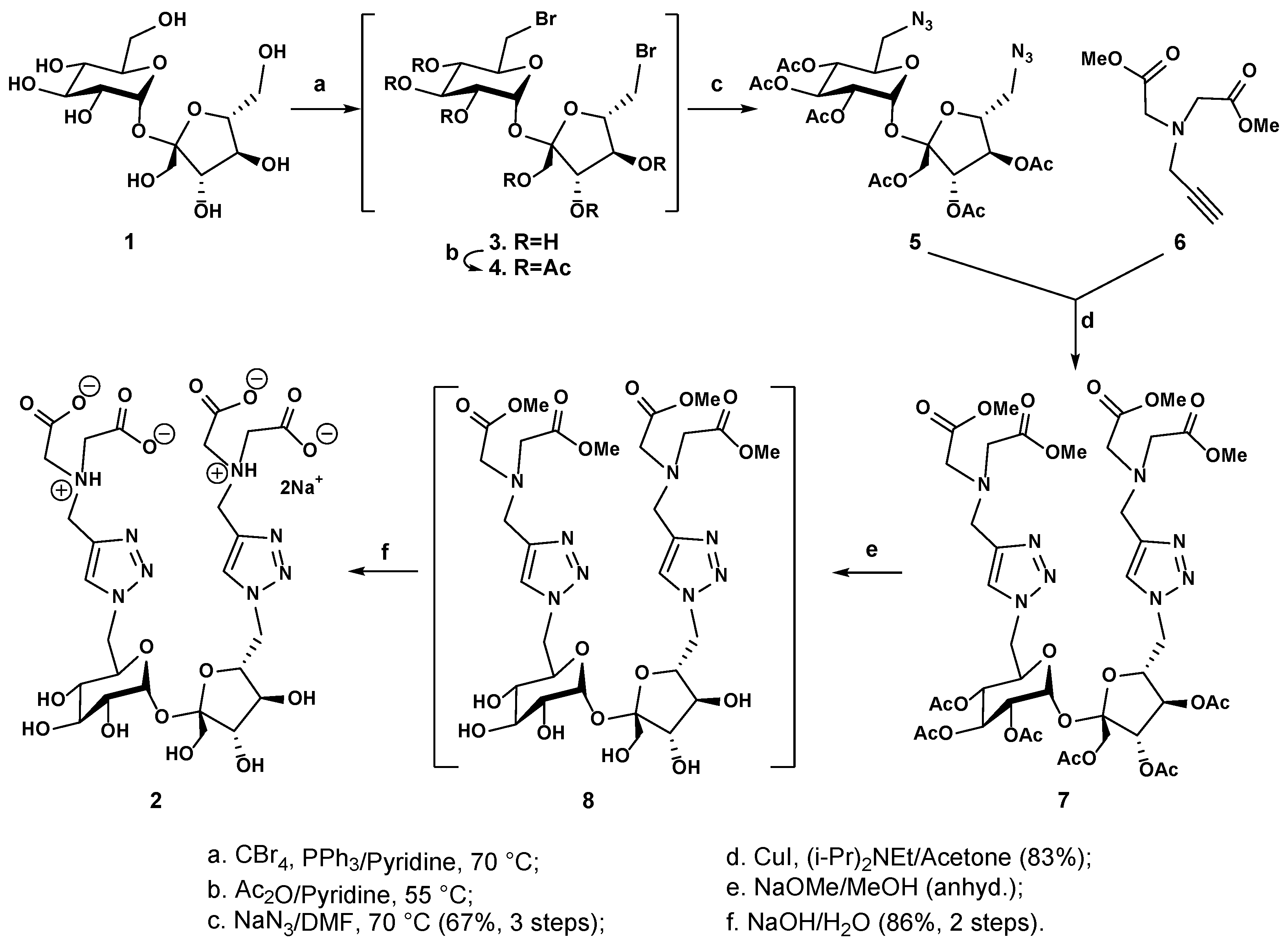
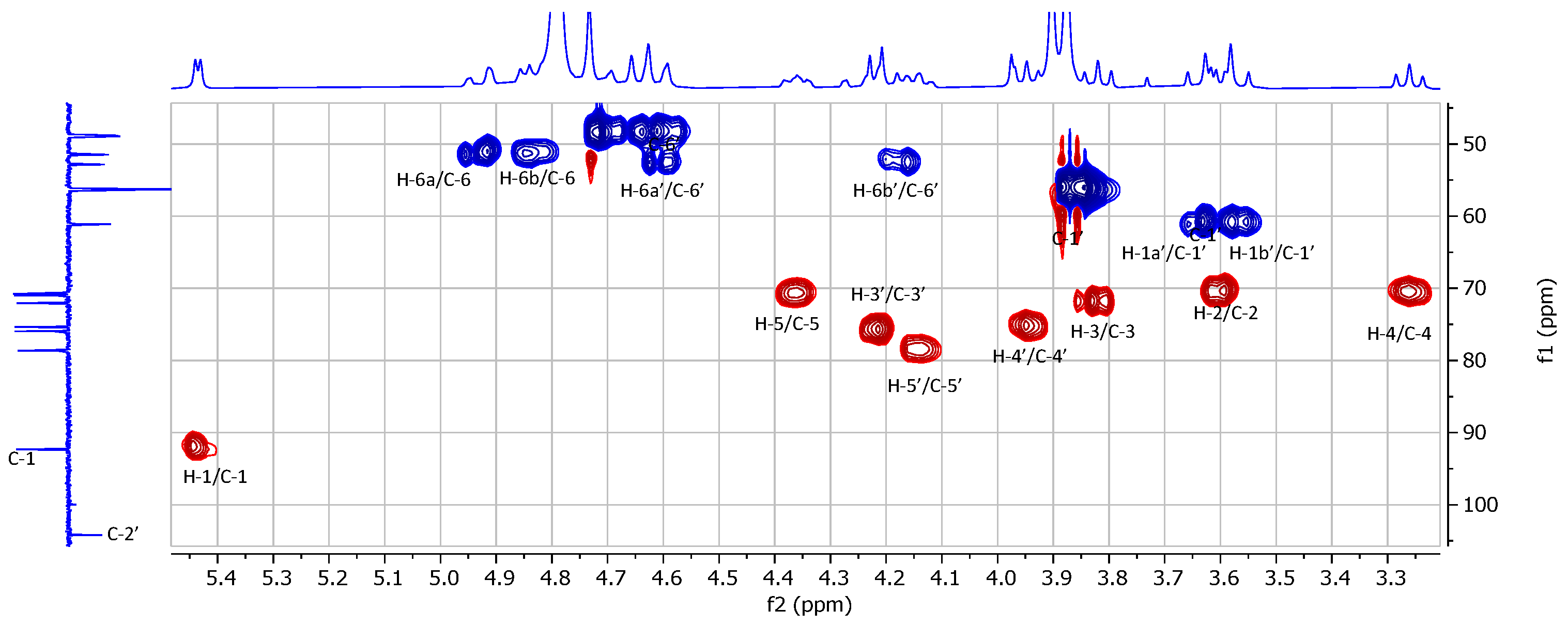
2.1. Chemical Synthesis and Characterization
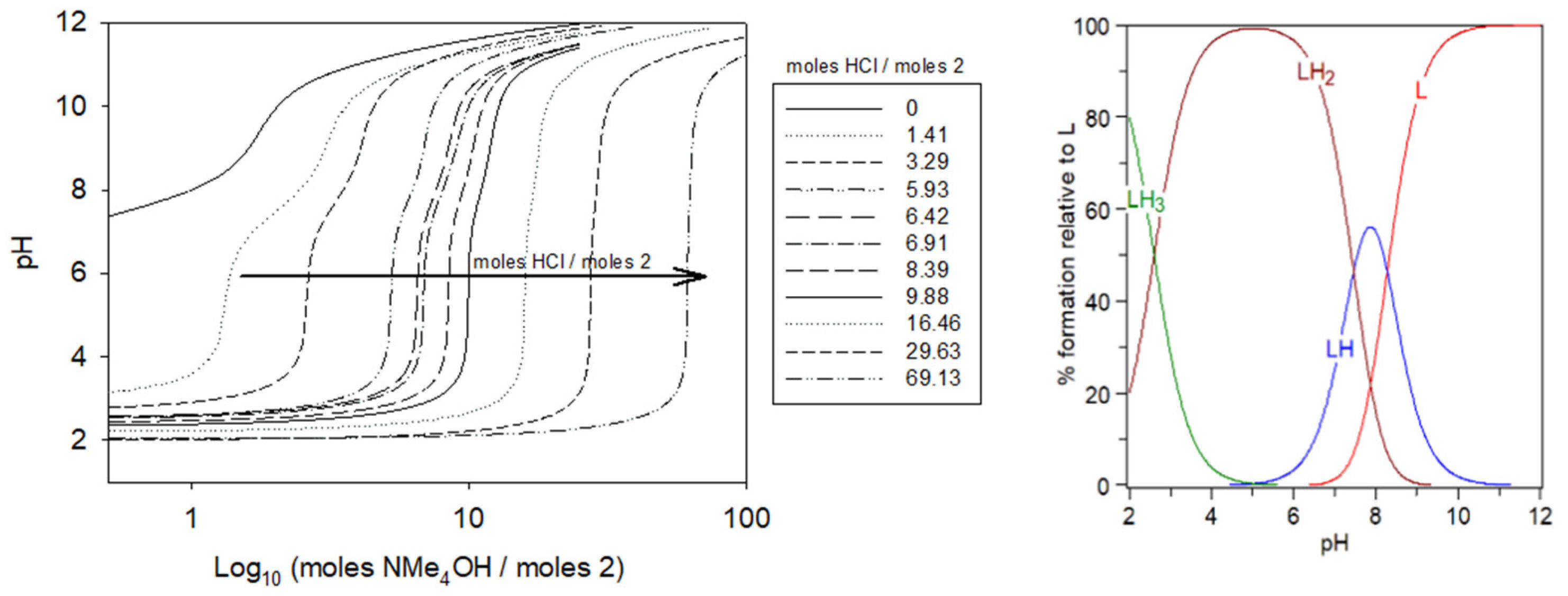
2.2. Protonation Studies of Compound 2 by Potentiometric Titration
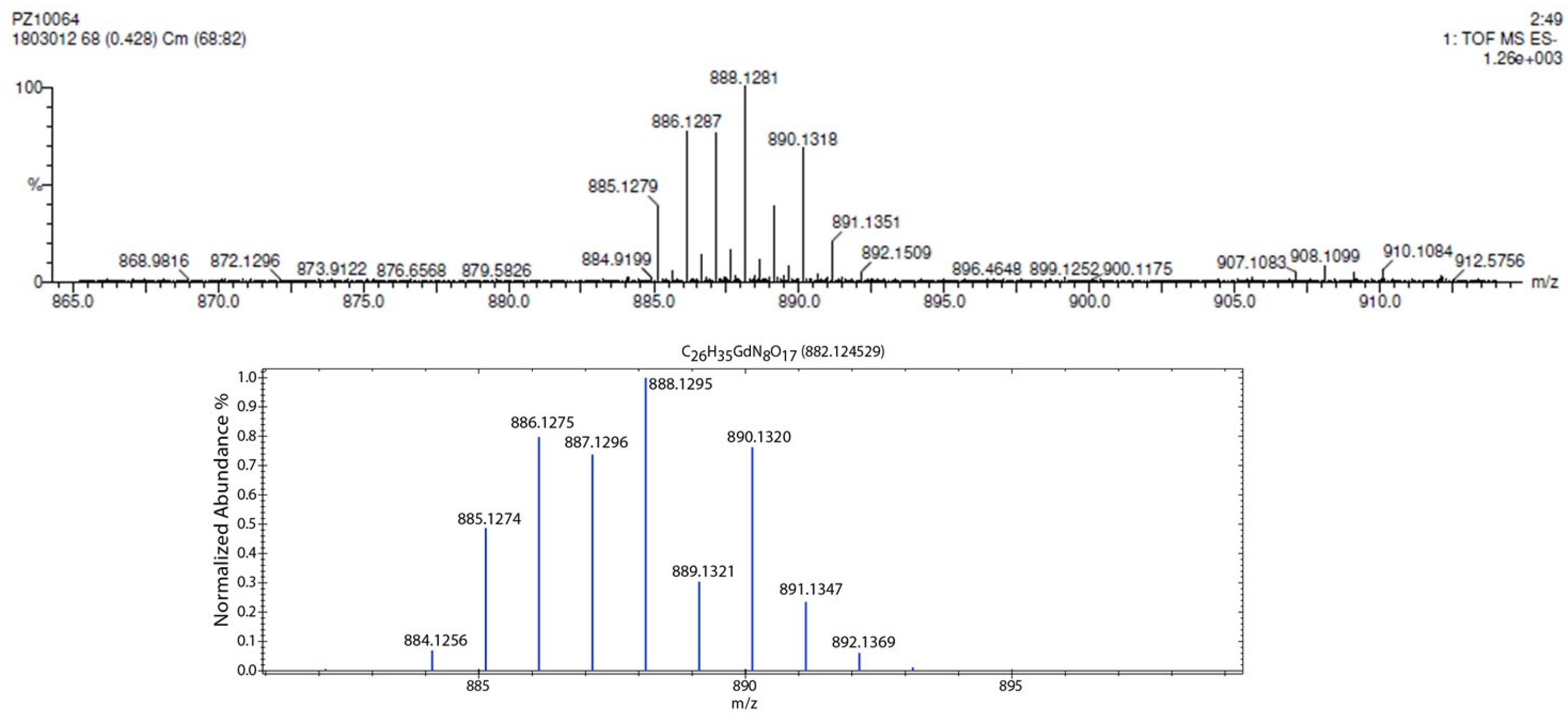
2.3. Complexation Studies of Gadolinium(III) with Compound 2 by High-Resolution Mass Spectrometry
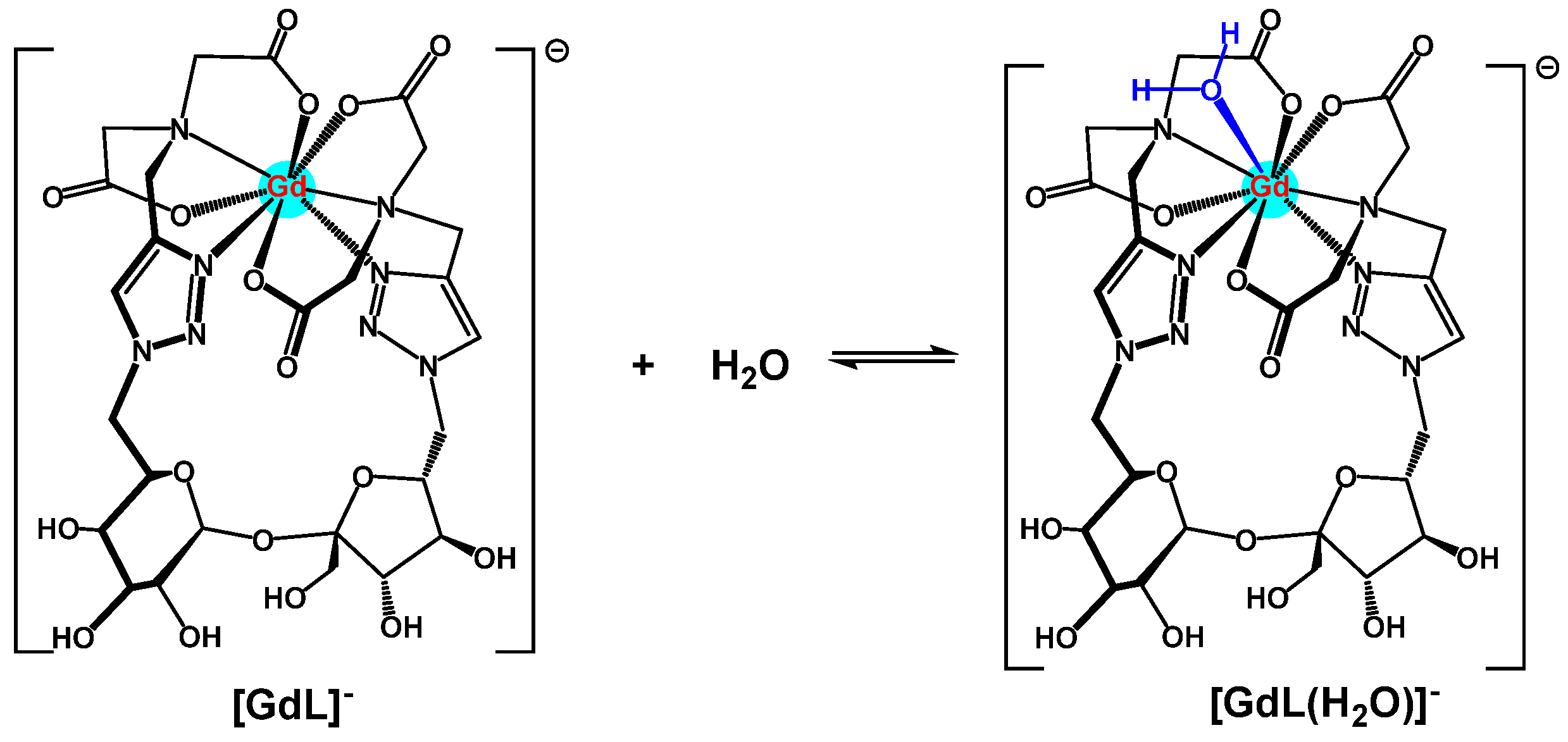
2.4. Studies of Ligand 2/Gd(III) Complex by Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
2.5. Complexation Studies of Gadolinium(III) with Ligand 2 by Potentiometric Titration
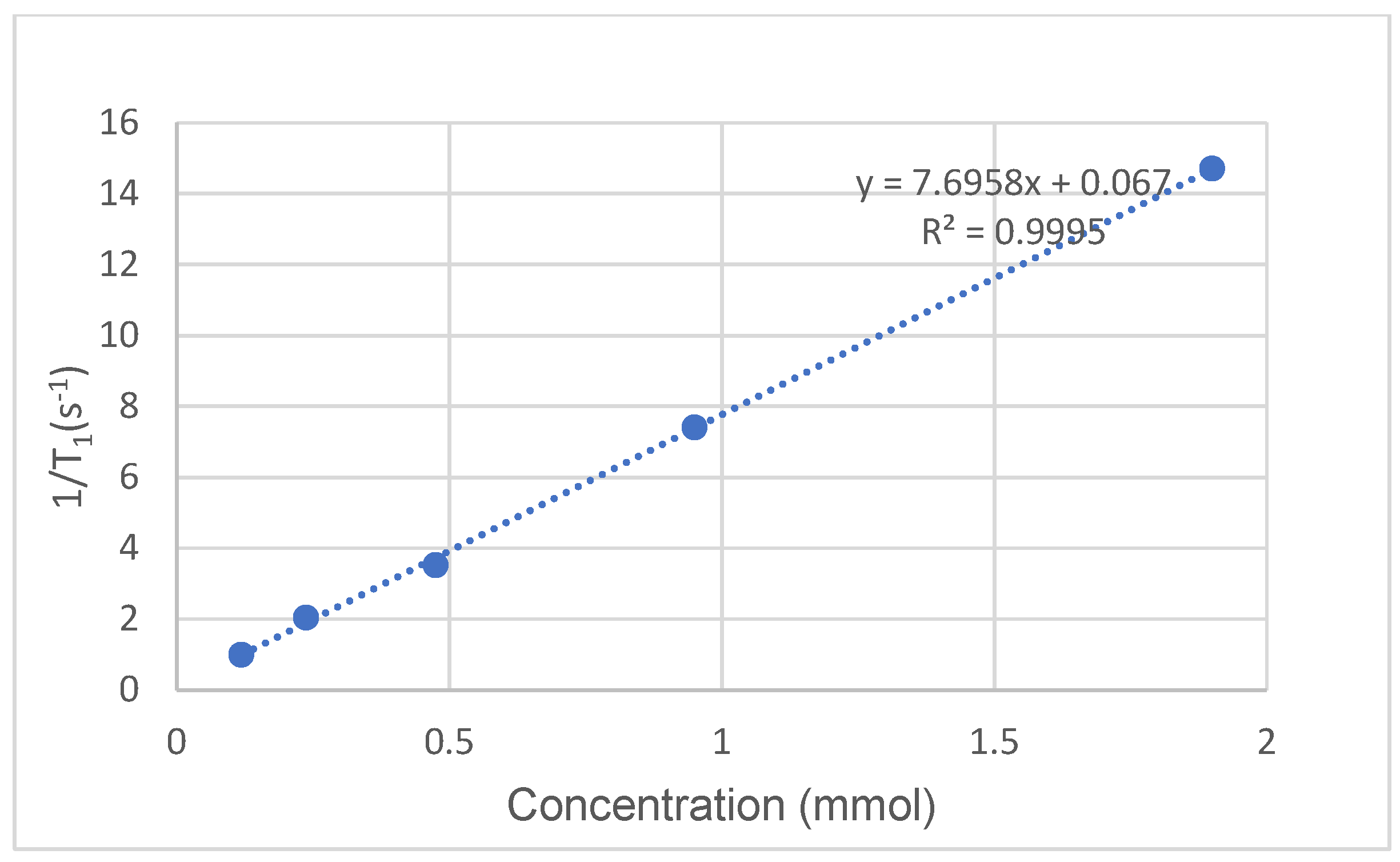
2.6. Relaxivity Measurement of Ligand 2/Gd(III) Complex
3. Conclusions
4. Materials and Methods
4.1. Chemical Synthesis
4.2. Preparation of Ligand 2/Gd(III) Complex
4.3. Ligand 2 Protonation Studies
4.4. Stability Constant Measurement
4.5. Relaxivity Measurements
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Queneau, Y.; Jarosz, S.; Lewandowski, B.; Fitremann, J. Sucrose Chemistry and Applications of Sucrochemicals. Adv. Carbohydr. Chem. Biochem. 2007, 61, 217–292. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, F.W.; Peters, S. Carbohydrates as Green Raw Materials for the Chemical Industry. C. R. Chim. 2004, 7, 65–90. [Google Scholar] [CrossRef]
- Jarosz, S.; Sokołowska, P.; Szyszka, Ł. Synthesis of Fine Chemicals with High Added Value from Sucrose: Towards Sucrose-Based Macrocycles. Tetrahedron Lett. 2020, 61, 151888–151902. [Google Scholar] [CrossRef]
- Petrova, K.T.; Correia-da-Silva, P.; Crucho, C.I.C.; Barros, M.T. Chemoselective Synthesis of Sucrose Building Blocks and Their Polymerization. Curr. Org. Chem. 2014, 18, 1788–1802. [Google Scholar] [CrossRef]
- Kordován, M.Á.; Hegedűs, C.; Czifrák, K.; Lakatos, C.; Kálmán-Szabó, I.; Daróczi, L.; Zsuga, M.; Kéki, S. Novel Polyurethane Scaffolds Containing Sucrose Crosslinker for Dental Application. Int. J. Mol. Sci. 2022, 23, 7904. [Google Scholar] [CrossRef] [PubMed]
- Nagtode, V.S.; Cardoza, C.; Yasin, H.K.A.; Mali, S.N.; Tambe, S.M.; Roy, P.; Singh, K.; Goel, A.; Amin, P.D.; Thorat, B.R.; et al. Green Surfactants (Biosurfactants): A Petroleum-Free Substitute for Sustainability—Comparison, Applications, Market, and Future Prospects. ACS Omega 2023, 8, 11674–11699. [Google Scholar] [CrossRef] [PubMed]
- Allen, D.K.; Tao, B.Y. Carbohydrate-Alkyl Ester Derivatives as Biosurfactants. J. Surfactants Deterg. 1999, 2, 383–390. [Google Scholar] [CrossRef]
- Plat, T.; Linhardt, R.J. Syntheses and Applications of Sucrose-Based Esters. J. Surfactants Deterg. 2001, 4, 415–421. [Google Scholar] [CrossRef]
- Bimal, C.; Guonong, Z. Olestra: A Solution to Food Fat? Food Rev. Int. 2006, 22, 245–258. [Google Scholar] [CrossRef]
- Shankar, P.; Ahuja, S.; Sriram, K. Non-Nutritive Sweeteners: Review and Update. Nutrition 2013, 29, 1293–1299. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Rother, K.I. Sucralose, A Synthetic Organochlorine Sweetener: Overview Of Biological Issues. J. Toxicol. Environ. Health Part B 2013, 16, 399–451. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Lee, T.S. Converting Sugars to Biofuels: Ethanol and Beyond. Bioengineering 2015, 2, 184–203. [Google Scholar] [CrossRef] [PubMed]
- Galkin, K.I.; Ananikov, V.P. When Will 5-Hydroxymethylfurfural, the “Sleeping Giant” of Sustainable Chemistry, Awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef] [PubMed]
- Lattuada, L.; Barge, A.; Cravotto, G.; Giovenzana, G.B.; Tei, L. The Synthesis and Application of Polyamino Polycarboxylic Bifunctional Chelating Agents. Chem. Soc. Rev. 2011, 40, 3019–3049. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Thirumurugan, P.; Matosiuk, D.; Jozwiak, K. Click Chemistry for Drug Development and Diverse Chemical–Biology Applications. Chem. Rev. 2013, 113, 4905–4979. [Google Scholar] [CrossRef]
- Sembo-Backonly, B.S.; Estour, F.; Gouhier, G. Cyclodextrins: Promising Scaffolds for MRI Contrast Agents. RSC Adv. 2021, 11, 29762–29785. [Google Scholar] [CrossRef]
- Champagne, P.-L.; Barbot, C.; Zhang, P.; Han, X.; Gaamoussi, I.; Hubert-Roux, M.; Bertolesi, G.E.; Gouhier, G.; Ling, C.-C. Synthesis and Unprecedented Complexation Properties of β-Cyclodextrin-Based Ligand for Lanthanide Ions. Inorg. Chem. 2018, 57, 8964–8977. [Google Scholar] [CrossRef]
- Abboud, J.-L.M.; Foces-Foces, C.; Notario, R.; Trifonov, R.E.; Volovodenko, A.P.; Ostrovskii, V.A.; Alkorta, I.; Elguero, J. Basicity of N-H- and N-Methyl-1,2,3-Triazoles in the Gas Phase, in Solution, and in the Solid State − An Experimental and Theoretical Study. Eur. J. Org. Chem. 2001, 2001, 3013–3024. [Google Scholar] [CrossRef]
- Arzik, S.; Ayan, E.M.; Celebi, A.S. Potentiometric Determination of the Stability Constants of Lanthanide Complexes with Iminodiacetic Acid in Water and Dioxane-Water Mixtures. Turk. J. Chem. 2008, 32, 721–729. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 6th ed.; Wiley-Interscience: Hoboken, NJ, USA, 2009; ISBN 978-0-471-74493-1. [Google Scholar]
- Deacon, G.B.; Phillips, R.J. Relationships between the Carbon-Oxygen Stretching Frequencies of Carboxylato Complexes and the Type of Carboxylate Coordination. Coord. Chem. Rev. 1980, 33, 227–250. [Google Scholar] [CrossRef]
- Kumar, K.; Chang, C.A.; Francesconi, L.C.; Dischino, D.D.; Malley, M.F.; Gougoutas, J.Z.; Tweedle, M.F. Synthesis, Stability, and Structure of Gadolinium(III) and Yttrium(III) Macrocyclic Poly(Amino Carboxylates). Inorg. Chem. 1994, 33, 3567–3575. [Google Scholar] [CrossRef]
- Davies, J.; Siebenhandl-Wolff, P.; Tranquart, F.; Jones, P.; Evans, P. Gadolinium: Pharmacokinetics and Toxicity in Humans and Laboratory Animals Following Contrast Agent Administration. Arch. Toxicol. 2022, 96, 403–429. [Google Scholar] [CrossRef] [PubMed]
- Fatin-Rouge, N.; Bünzli, J.-C.G. Thermodynamic and Structural Study of Inclusion Complexes between Trivalent Lanthanide Ions and Native Cyclodextrins. Inorganica Chim. Acta 1999, 293, 53–60. [Google Scholar] [CrossRef]
- Gamov, G.A.; Zavalishin, M.N.; Pimenov, O.A.; Klochkov, V.V.; Khodov, I.A. La(III), Ce(III), Gd(III), and Eu(III) Complexation with Tris(Hydroxymethyl)Aminomethane in Aqueous Solution. Inorg. Chem. 2020, 59, 17783–17793. [Google Scholar] [CrossRef]
- Nonat, A.; Giraud, M.; Gateau, C.; Fries, P.H.; Helm, L.; Mazzanti, M. Gadolinium(III) Complexes of 1,4,7-Triazacyclononane Based Picolinate Ligands: Simultaneous Optimization of Water Exchange Kinetics and Electronic Relaxation. Dalton Trans. 2009, 38, 8033–8046. [Google Scholar] [CrossRef]
- Caravan, P.; Ellison, J.J.; McMurry, T.J.; Lauffer, R.B. Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications. Chem. Rev. 1999, 99, 2293–2352. [Google Scholar] [CrossRef]
- Dai, L.; Jones, C.M.; Chan, W.T.K.; Pham, T.A.; Ling, X.; Gale, E.M.; Rotile, N.J.; Tai, W.C.-S.; Anderson, C.J.; Caravan, P.; et al. Chiral DOTA Chelators as an Improved Platform for Biomedical Imaging and Therapy Applications. Nat. Commun. 2018, 9, 857. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of Equilibria in Solution. Determination of Equilibrium Constants with the HYPERQUAD Suite of Programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Barge, A.; Cravotto, G.; Gianolio, E.; Fedeli, F. How to Determine Free Gd and Free Ligand in Solution of Gd Chelates. A Technical Note. Contrast Media Mol. Imaging 2006, 1, 184–188. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad Simulation and Speciation (HySS): A Utility Program for the Investigation of Equilibria Involving Soluble and Partially Soluble Species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]






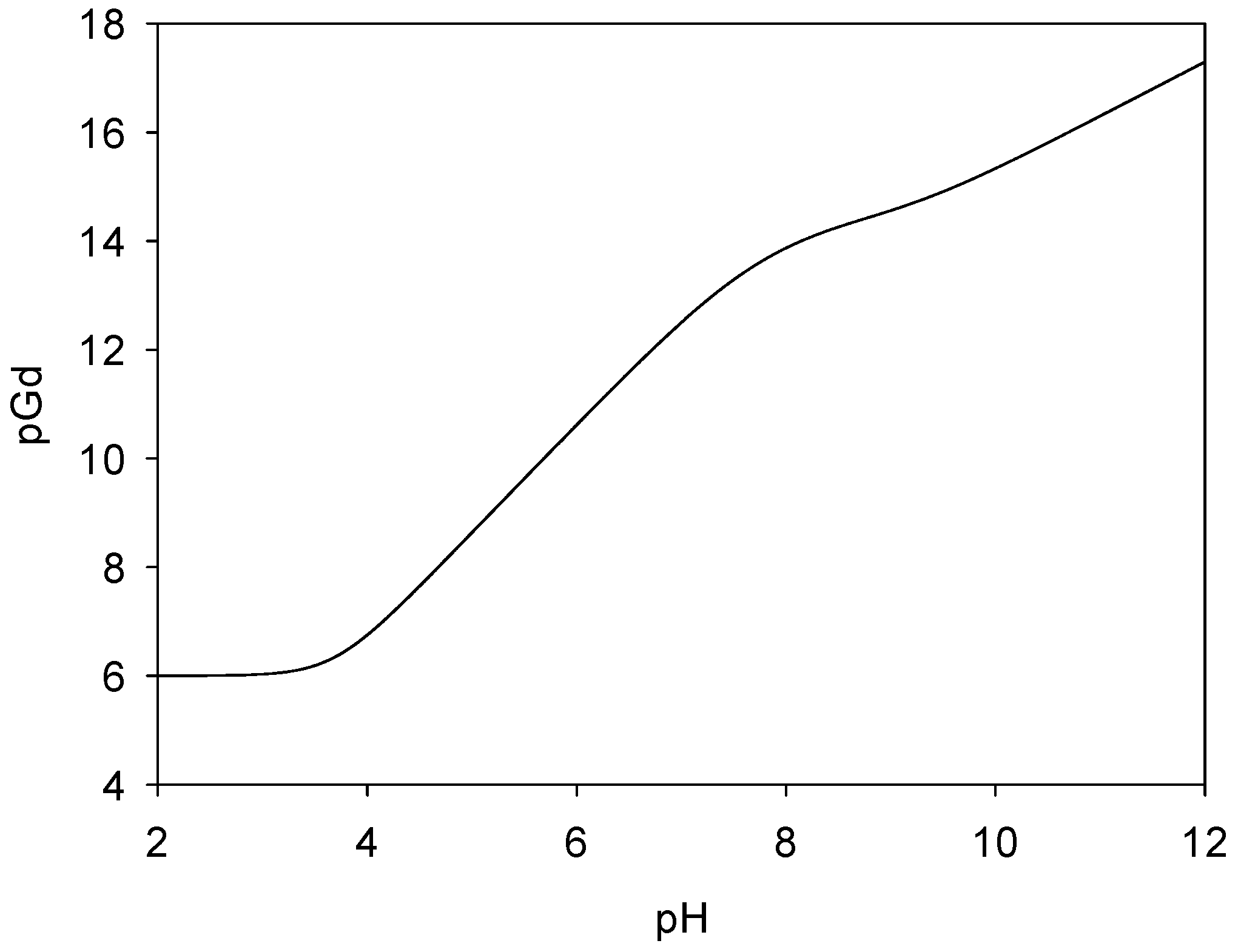
| Species | logβLHh | pKa |
|---|---|---|
| LH | 8.28 | 8.28 |
| LH2 | 15.74 | 7.46 |
| LH3 | 18.34 | 2.60 |
| mlh | logβSpecies | pKa | |
|---|---|---|---|
| GdLH−1 | 11−1 | 4.34 | - |
| GdL | 110 | 13.42 | 9.08 |
| GdLH | 111 | 16.44 | 3.02 |
| Concentration (mmol) | T1 (s) | 1/T1 (s−1) |
|---|---|---|
| 1.9 | 0.068 | 14.70588235 |
| 0.95 | 0.135 | 7.407407407 |
| 0.475 | 0.284 | 3.521126761 |
| 0.2375 | 0.49 | 2.040816327 |
| 0.11875 | 1.01 | 0.99009901 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Barbot, C.; Gandikota, R.; Li, C.; Gouriou, L.; Gouhier, G.; Ling, C.-C. Synthesis of an Ethylenediaminetetraacetic Acid-like Ligand Based on Sucrose Scaffold and Complexation and Proton Relaxivity Studies of Its Gadolinium(III) Complex in Solution. Molecules 2024, 29, 4688. https://doi.org/10.3390/molecules29194688
Zhang P, Barbot C, Gandikota R, Li C, Gouriou L, Gouhier G, Ling C-C. Synthesis of an Ethylenediaminetetraacetic Acid-like Ligand Based on Sucrose Scaffold and Complexation and Proton Relaxivity Studies of Its Gadolinium(III) Complex in Solution. Molecules. 2024; 29(19):4688. https://doi.org/10.3390/molecules29194688
Chicago/Turabian StyleZhang, Ping, Cécile Barbot, Ramakrishna Gandikota, Cenxiao Li, Laura Gouriou, Géraldine Gouhier, and Chang-Chun Ling. 2024. "Synthesis of an Ethylenediaminetetraacetic Acid-like Ligand Based on Sucrose Scaffold and Complexation and Proton Relaxivity Studies of Its Gadolinium(III) Complex in Solution" Molecules 29, no. 19: 4688. https://doi.org/10.3390/molecules29194688







