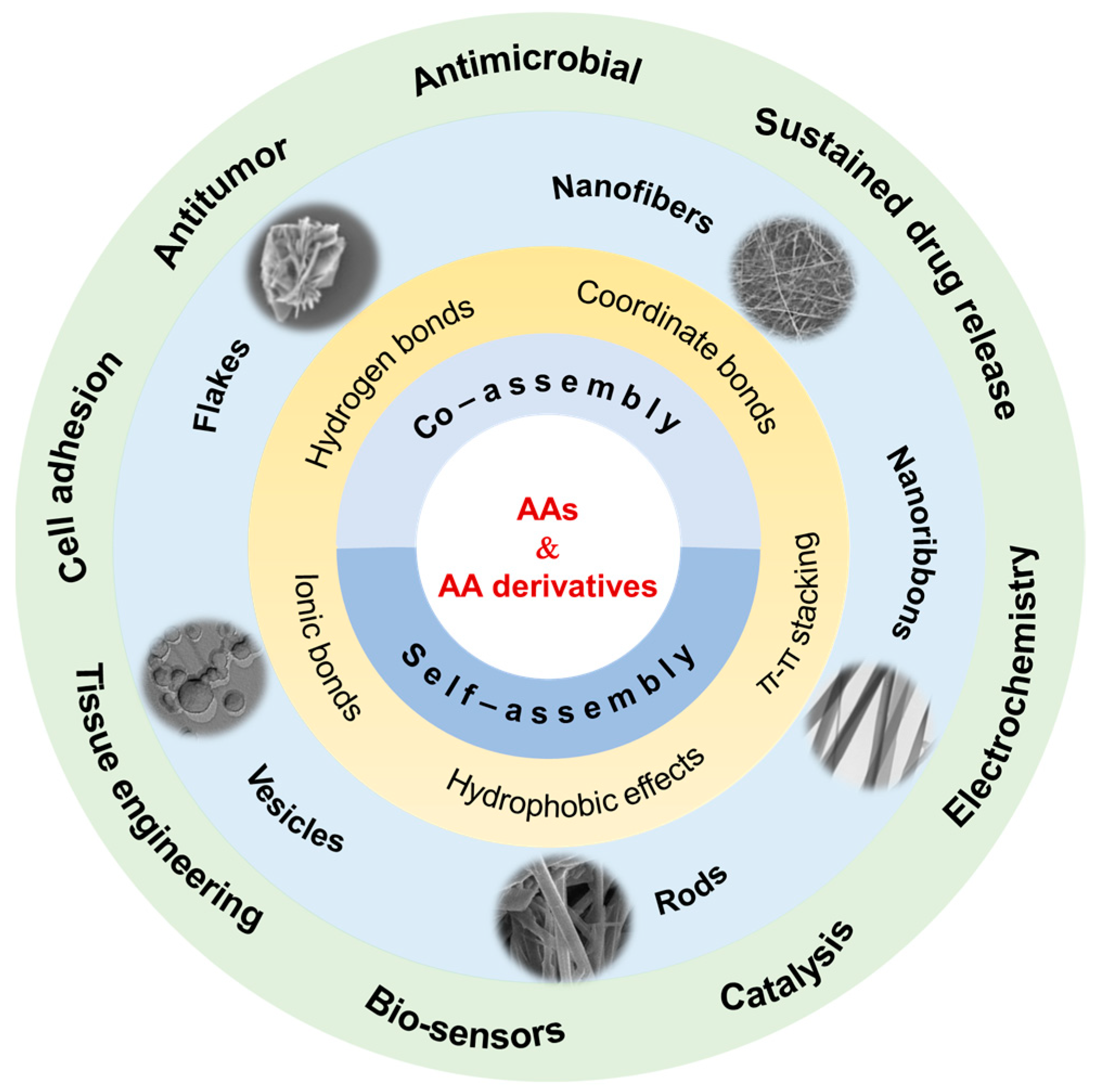
Amino Acid-Derived Supramolecular Assembly and Soft Materials
Abstract
1. Introduction
2. Supramolecular Assembly of Natural Amino Acids
2.1. Self-Assembly of Natural Amino Acids
2.2. Co-Assembly of Natural Amino Acids and Organic Species
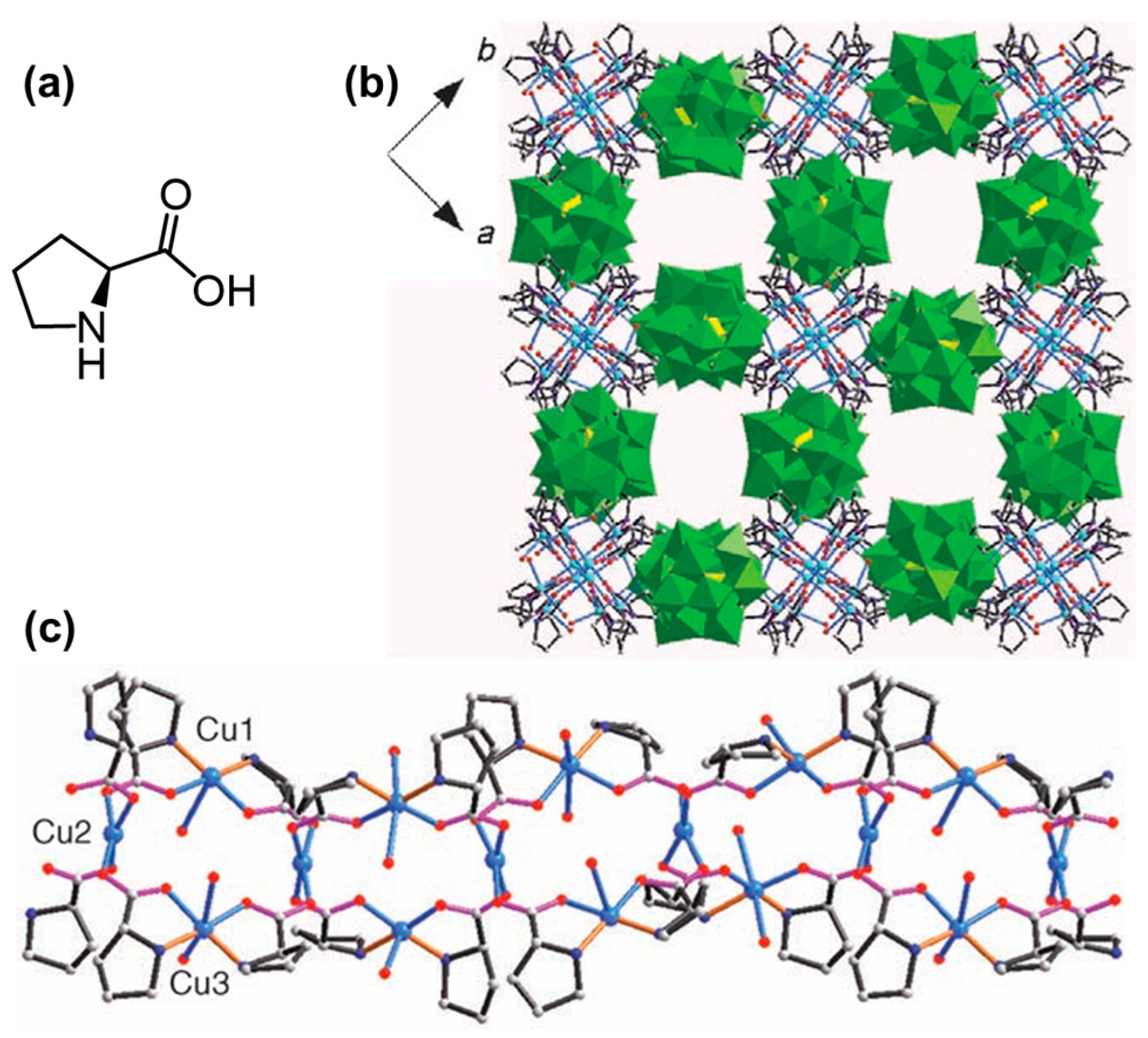
2.3. Assembly of Natural Amino Acids/Metal Ion Complexes
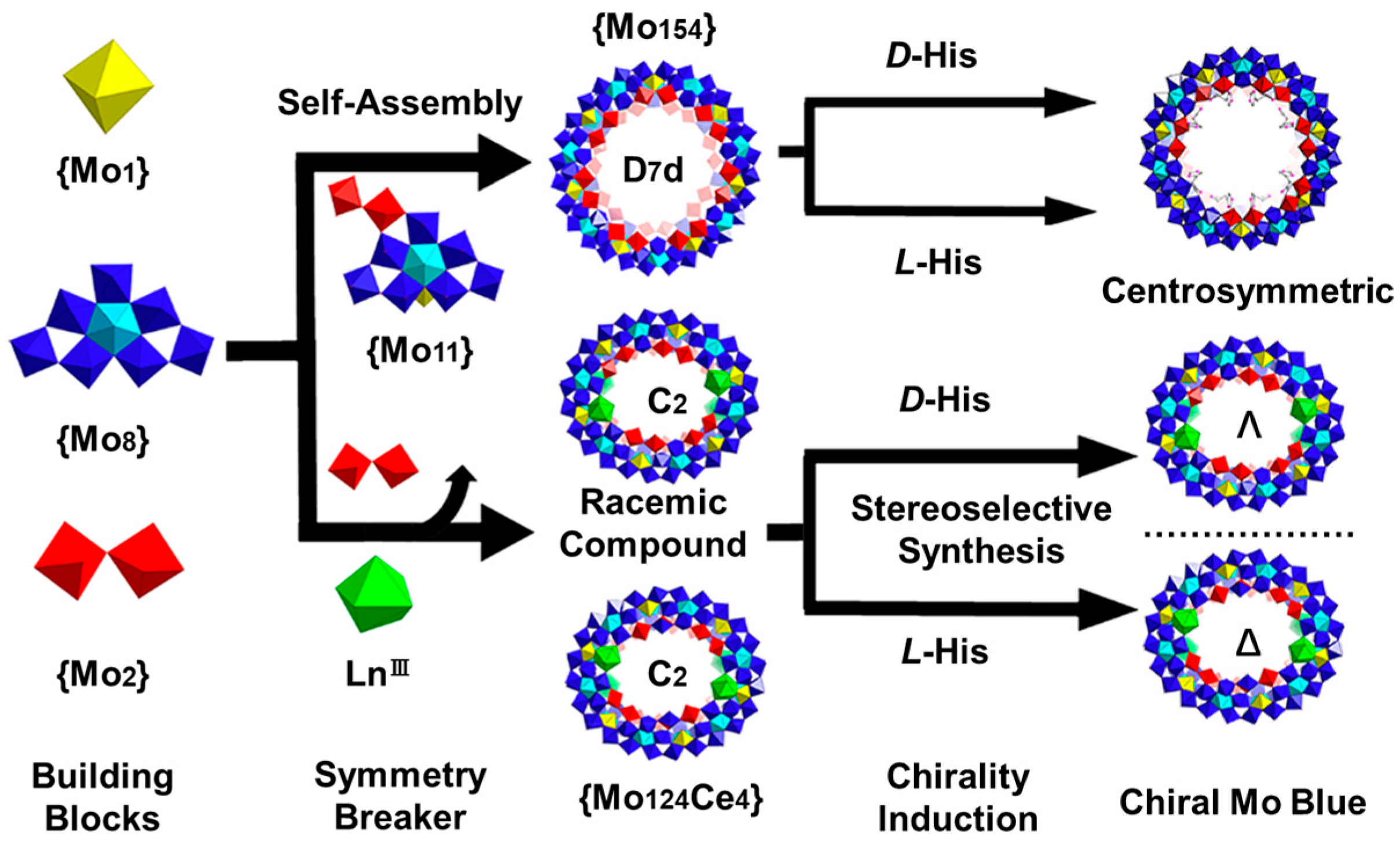
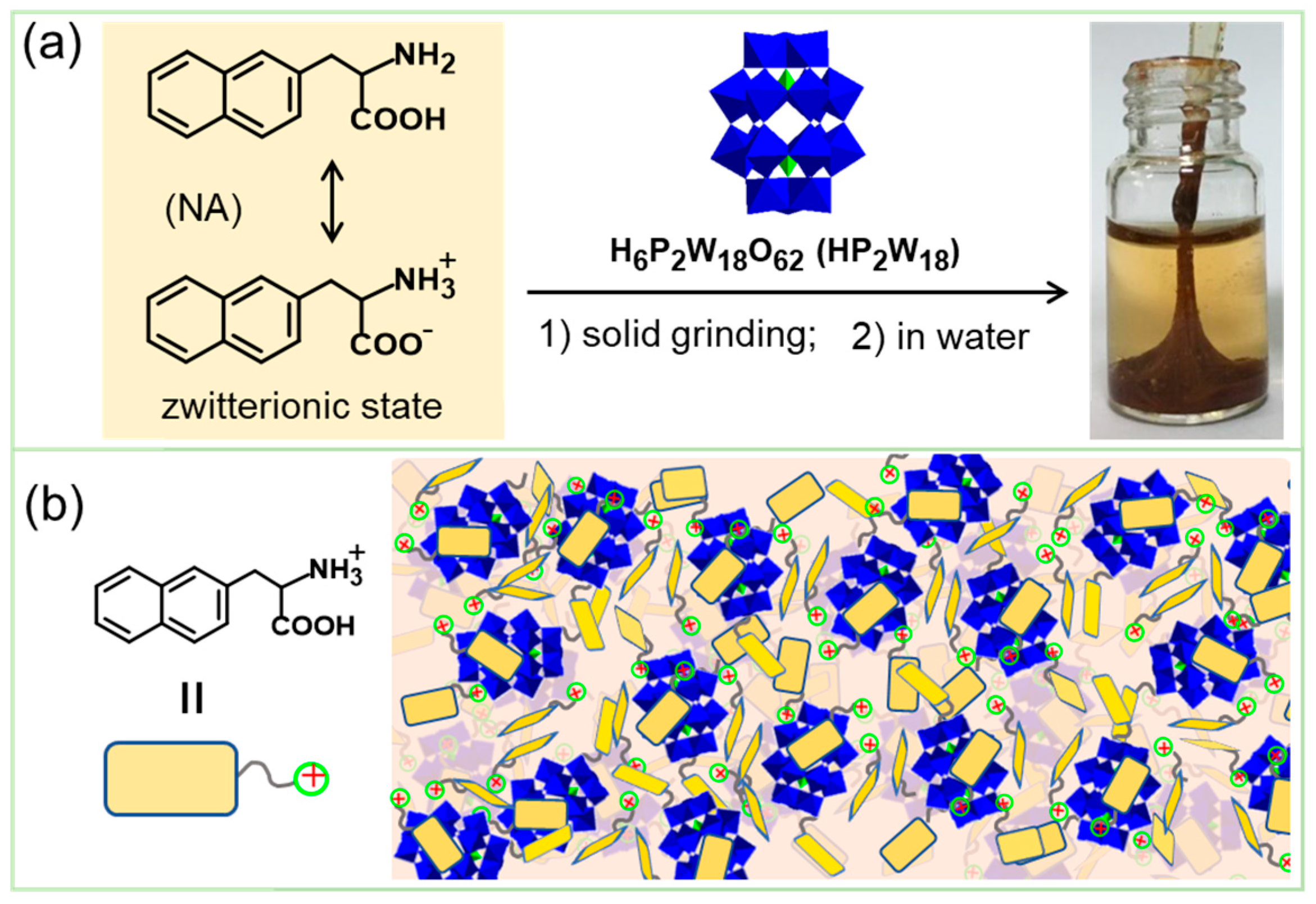
2.4. Co-Assembly of Natural Amino Acids and Inorganic Nanoclusters
3. Self-Assembly of Amino Acid Derivatives
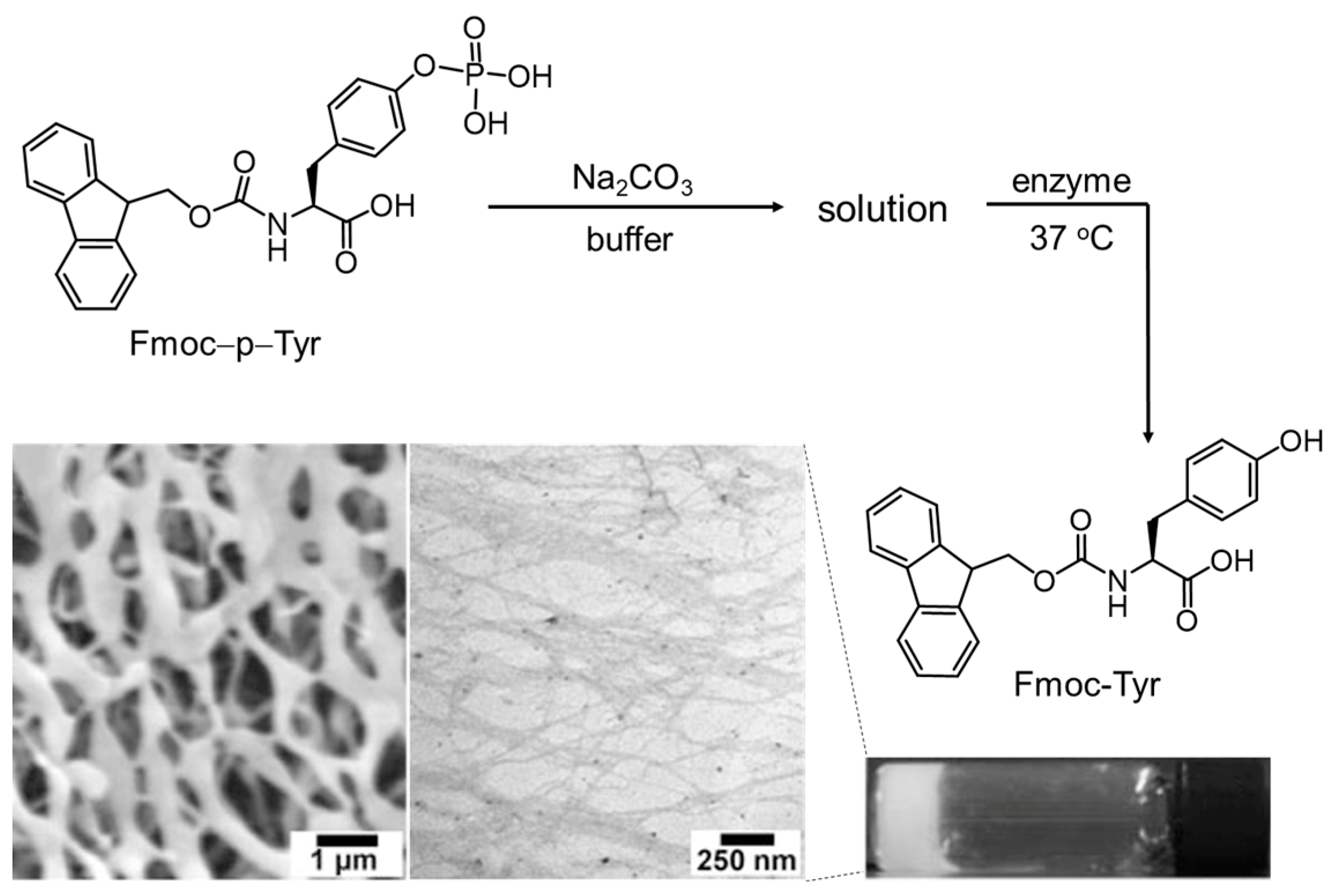
3.1. Self-Assembly of Aromatic Ring-Modified Amino Acid Derivatives
3.2. Self-Assembly of Alkylated Amino Acid Derivatives
4. Co-Assembly of Amino Acid Derivatives and Various Objects
4.1. Co-Assembly of Amino Acid Derivatives and Their Counterparts
4.2. Co-Assembly of Amino Acid Derivatives and Organic Objects
4.3. Co-Assembly of Amino Acid Derivatives and Inorganic Objects
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications. Chem. Rev. 2023, 123, 3976–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Avakyan, N.; Kakkis, A.; Hoffnagle, A.M.; Han, K.; Li, Y.; Zhang, Z.; Choi, T.S.; Na, Y.; Yu, C.-J.; et al. Protein Assembly by Design. Chem. Rev. 2021, 121, 13701–13796. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Zhao, L.; Xing, R.; Li, J.; Yan, X. Functional Chromopeptide Nanoarchitectonics: Molecular Design, Self-Assembly and Biological Applications. Chem. Soc. Rev. 2023, 52, 2688–2712. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jiang, Q.; Zhao, X.; Zhao, R.; Wang, Y.; Wang, Y.; Liu, J.; Shang, Y.; Zhao, S.; Wu, T.; et al. A DNA Nanodevice-Based Vaccine for Cancer Immunotherapy. Nat. Mater. 2021, 20, 421–430. [Google Scholar] [CrossRef]
- Tian, J.; Fu, D.; Liu, Y.; Guan, Y.; Miao, S.; Xue, Y.; Chen, K.; Huang, S.; Zhang, Y.; Xue, L.; et al. Rectifying Disorder of Extracellular Matrix to Suppress Urethral Stricture by Protein Nanofilm-Controlled Drug Delivery from Urinary Catheter. Nat. Commun. 2023, 14, 2816. [Google Scholar] [CrossRef]
- Wang, H.; Song, Y.; Wang, W.; Chen, N.; Hu, B.; Liu, X.; Zhang, Z.; Yu, Z. Organelle-Mediated Dissipative Self-Assembly of Peptides in Living Cells. J. Am. Chem. Soc. 2024, 146, 330–341. [Google Scholar] [CrossRef]
- Jana, B.; Jin, S.; Go, E.M.; Cho, Y.; Kim, D.; Kim, S.; Kwak, S.K.; Ryu, J.-H. Intra-Lysosomal Peptide Assembly for the High Selectivity Index against Cancer. J. Am. Chem. Soc. 2023, 145, 18414–18431. [Google Scholar] [CrossRef]
- Li, Q.; Min, J.; Zhang, J.; Reches, M.; Shen, Y.; Su, R.; Wang, Y.; Qi, W. Enzyme-Driven, Switchable Catalysis Based on Dynamic Self-Assembly of Peptides. Angew. Chem. Int. Ed. 2023, 62, e202309830. [Google Scholar] [CrossRef]
- Ge, L.; Xu, H.; Jiang, X.; Yu, J. Oligopeptide Self-Assembly: Mechanisms, Stimuli-Responsiveness, and Biomedical Applications. CCS Chem. 2024, 6, 69–90. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, X.; Sun, Y.; Nie, J.; Cheng, M.; Li, W.; Zhao, J. Peptide/Glycyrrhizic Acid Supramolecular Polymer: An Emerging Medical Adhesive for Dural Sealing and Repairing. Biomaterials 2023, 301, 122239. [Google Scholar] [CrossRef]
- Xie, X.; Gao, B.; Ma, Z.; Liu, J.; Zhang, J.; Liang, J.; Chen, Z.; Wu, L.; Li, W. Host–Guest Interaction Driven Peptide Assembly into Photoresponsive Two-Dimensional Nanosheets with Switchable Antibacterial Activity. CCS Chem. 2021, 3, 1949–1962. [Google Scholar] [CrossRef]
- Meng, R.; Zheng, T.; Nie, J.; Fu, L.; Li, W. Multivalent Interactions Enable the Natural Alkaline Amino Acids to Transmute into Macroscopic Adhesive Materials. Langmuir 2023, 39, 10047–10055. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Yuan, C.; Fan, W.; Ren, X.; Yan, X. Biomolecular Glass with Amino Acid and Peptide Nanoarchitectonics. Sci. Adv. 2023, 9, eadd8105. [Google Scholar] [CrossRef] [PubMed]
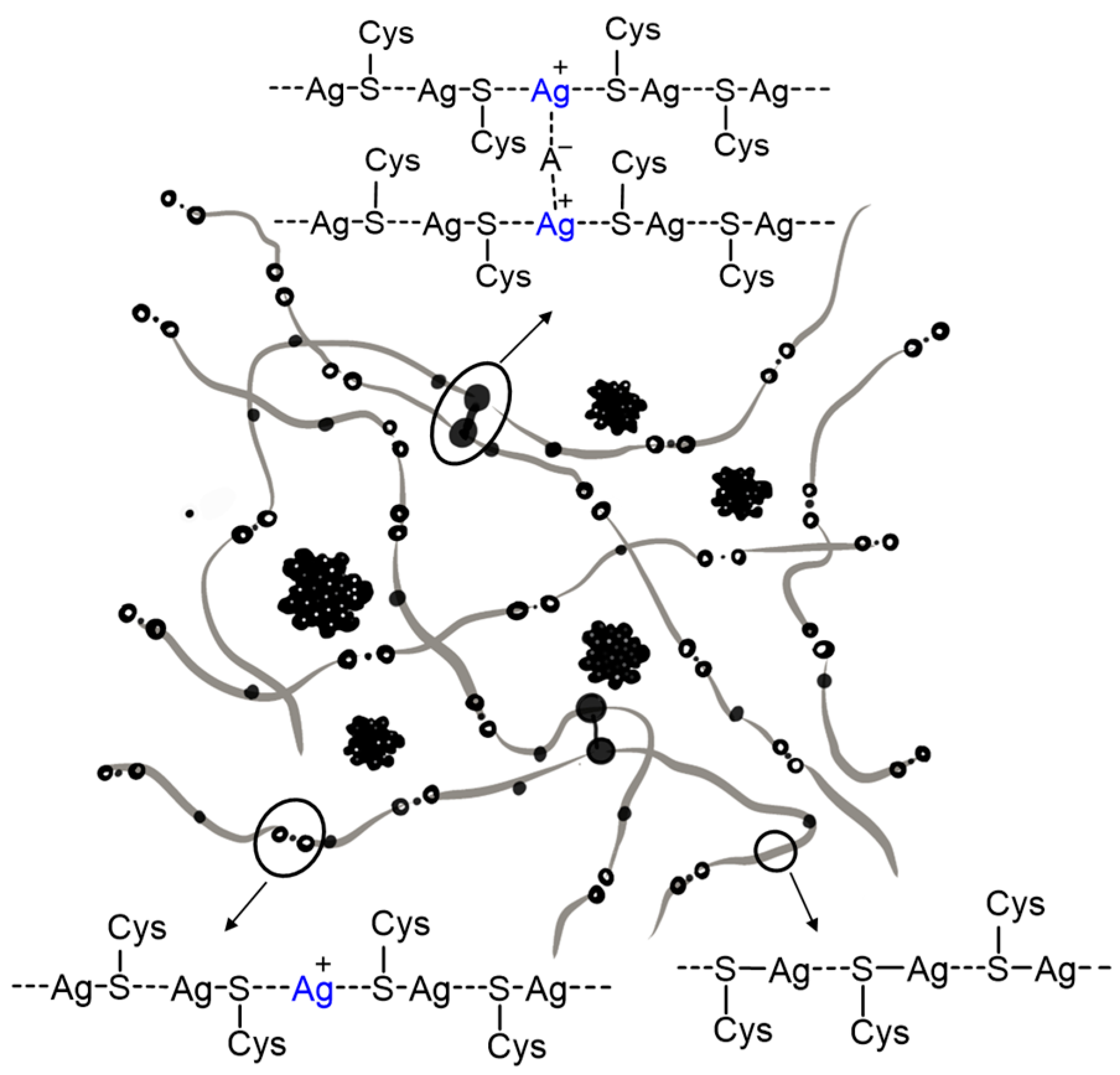
- Pakhomov, P.M.; Ovchinnikov, M.M.; Khizhnyak, S.D.; Roshchina, O.A.; Komarov, P.V. A Supramolecular Medical Hydrogel Based on L-Cysteine and Silver Ions. Polym. Sci. Ser. A 2011, 53, 820–826. [Google Scholar] [CrossRef]
- Singh, P.; Narang, N.; Sharma, R.K.; Wangoo, N. Interplay of Self-Assembling Aromatic Amino Acids and Functionalized Gold Nanoparticles Generating Supramolecular Structures. ACS Appl. Bio Mater. 2020, 3, 6196–6203. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, A. Amino Acid Based Smart Hydrogel: Formation, Characterization and Fluorescence Properties of Silver Nanoclusters within the Hydrogel Matrix. Soft Matter 2011, 7, 5300. [Google Scholar] [CrossRef]
- Singh, V.; Snigdha, K.; Singh, C.; Sinha, N.; Thakur, A.K. Understanding the Self-Assembly of Fmoc–Phenylalanine to Hydrogel Formation. Soft Matter 2015, 11, 5353–5364. [Google Scholar] [CrossRef]
- Wang, T.; Ménard-Moyon, C.; Bianco, A. Self-Assembly of Amphiphilic Amino Acid Derivatives for Biomedical Applications. Chem. Soc. Rev. 2022, 51, 3535–3560. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L. Ultrasound-Induced Amino Acid-Based Hydrogels with Superior Mechanical Strength for Controllable Long-Term Release of Anti-Cercariae Drug. Front. Bioeng. Biotechnol. 2021, 9, 703582. [Google Scholar]
- Srinivasulu, G.; Sridhar, B.; Ravi Kumar, K.; Sreedhar, B.; Ramesh, V.; Srinivas, R.; Kunwar, A.C. Molecular Self Assembly of Benzene-1,3,5-Tricarbonyl Phenylalanine. J. Mol. Struct. 2011, 1006, 180–184. [Google Scholar] [CrossRef]
- Liu, G.-F.; Ji, W.; Wang, W.-L.; Feng, C.-L. Multiresponsive Hydrogel Coassembled from Phenylalanine and Azobenzene Derivatives as 3D Scaffolds for Photoguiding Cell Adhesion and Release. ACS Appl. Mater. Inter. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Liu, G.-F.; Ji, W.; Feng, C.-L. Installing Logic Gates to Multiresponsive Supramolecular Hydrogel Co-Assembled from Phenylalanine Amphiphile and Bis(Pyridinyl) Derivative. Langmuir 2015, 31, 7122–7128. [Google Scholar] [CrossRef]
- Li, H.; Han, L.; Li, Q.; Lai, H.; Fernández-Trillo, P.; Tian, L.; He, F. Hierarchical Chiral Supramolecular Nanoarchitectonics with Molecular Detection: Helical Structure Controls upon Self-Assembly and Coassembly. Macromol. Rapid Commun. 2022, 43, 2100690. [Google Scholar] [CrossRef]
- Xia, Y.; Hao, A.; Xing, P. Chiroptical Coassemblies between Organic Carboxylic Acids and Amino Acid Derivatives with C3-Symmetry. Chin. Chem. Lett. 2023, 34, 107955. [Google Scholar] [CrossRef]
- Singh, P.; Pandey, S.K.; Grover, A.; Sharma, R.K.; Wangoo, N. Understanding the Self-Ordering of Amino Acids into Supramolecular Architectures: Co-Assembly-Based Modulation of Phenylalanine Nanofibrils. Mater. Chem. Front. 2021, 5, 1971–1981. [Google Scholar] [CrossRef]
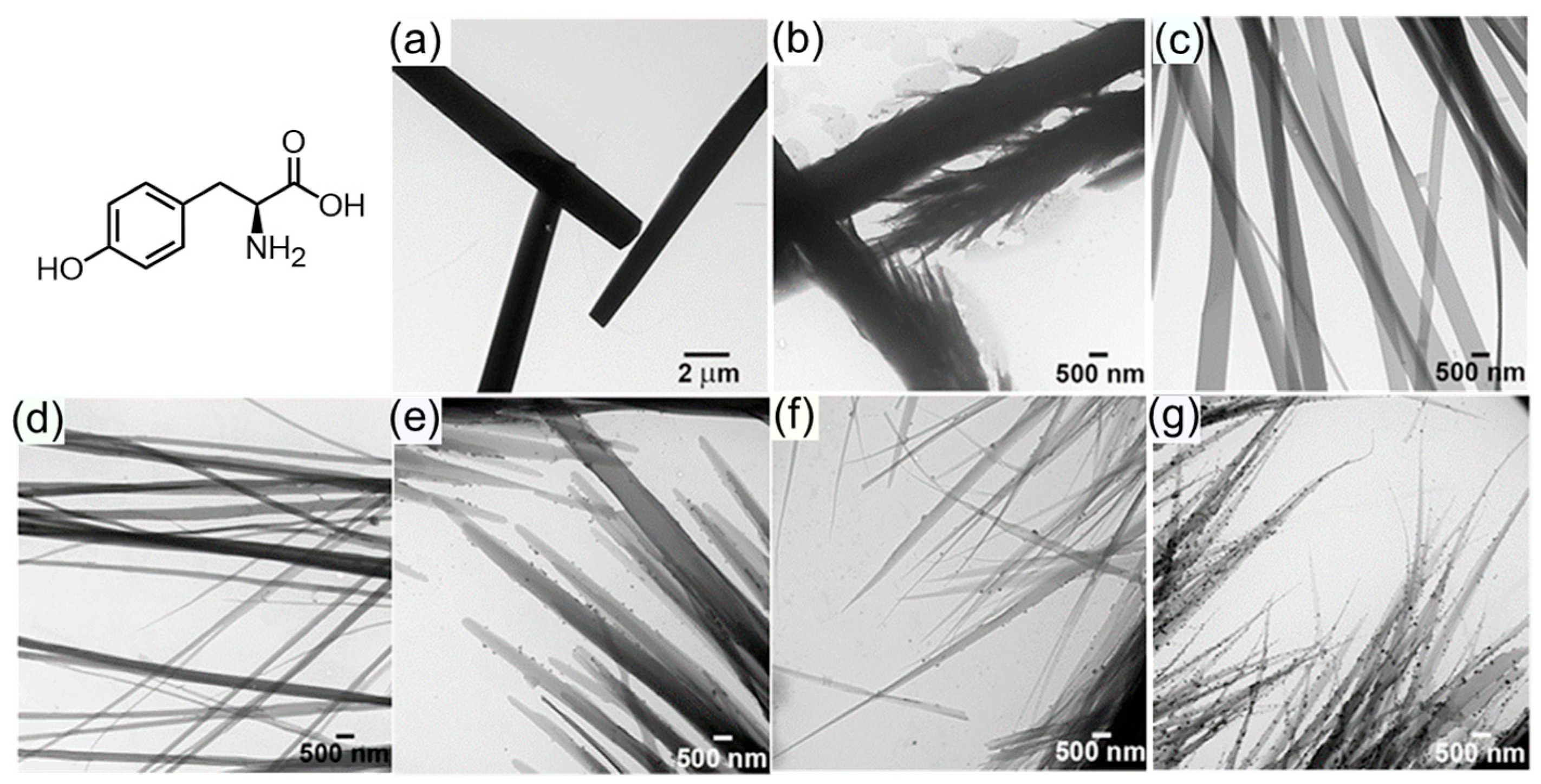
- Ménard-Moyon, C.; Venkatesh, V.; Krishna, K.V.; Bonachera, F.; Verma, S.; Bianco, A. Self-Assembly of Tyrosine into Controlled Supramolecular Nanostructures. Chem. Eur. J. 2015, 21, 11681–11686. [Google Scholar] [CrossRef]
- Babar, D.G.; Sarkar, S. Self-Assembled Nanotubes from Single Fluorescent Amino Acid. Appl. Nanosci. 2017, 7, 101–107. [Google Scholar] [CrossRef]
- Hsu, W.-P.; Koo, K.-K.; Myerson, A.S. The Gel-Crystallization of 1-Phenylalanine and Aspartame from Aqueous Solutions. Chem. Eng. Commun. 2002, 189, 1079–1090. [Google Scholar] [CrossRef]
- Ramalhete, S.M.; Nartowski, K.P.; Sarathchandra, N.; Foster, J.S.; Round, A.N.; Angulo, J.; Lloyd, G.O.; Khimyak, Y.Z. Supramolecular Amino Acid Based Hydrogels: Probing the Contribution of Additive Molecules Using NMR Spectroscopy. Chem. Eur. J. 2017, 23, 8014–8024. [Google Scholar] [CrossRef]
- Singh, V.; Rai, R.K.; Arora, A.; Sinha, N.; Thakur, A.K. Therapeutic Implication of L-Phenylalanine Aggregation Mechanism and Its Modulation by D-Phenylalanine in Phenylketonuria. Sci. Rep. 2014, 4, 3875. [Google Scholar] [CrossRef]
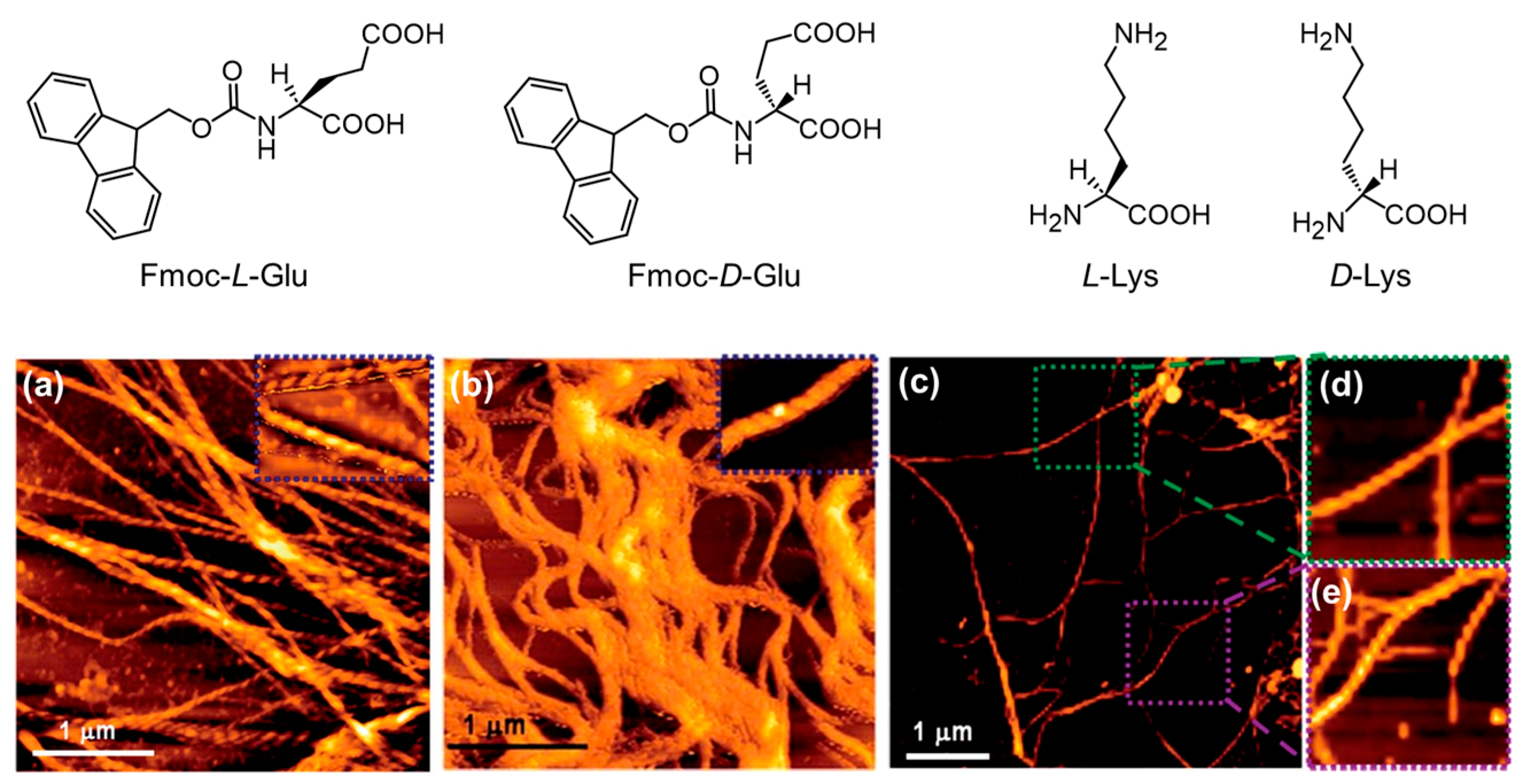
- Bera, S.; Xue, B.; Rehak, P.; Jacoby, G.; Ji, W.; Shimon, L.J.W.; Beck, R.; Král, P.; Cao, Y.; Gazit, E. Self-Assembly of Aromatic Amino Acid Enantiomers into Supramolecular Materials of High Rigidity. ACS Nano 2020, 14, 1694–1706. [Google Scholar] [CrossRef] [PubMed]
- Bera, S.; Mondal, S.; Tang, Y.; Jacoby, G.; Arad, E.; Guterman, T.; Jelinek, R.; Beck, R.; Wei, G.; Gazit, E. Deciphering the Rules for Amino Acid Co-Assembly Based on Interlayer Distances. ACS Nano 2019, 13, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.G.; Prajapati, K.P.; Ansari, M.; Yadav, D.K.; Temgire, M.; Kar, K. Genesis of Neurotoxic Hybrid Nanofibers from the Coassembly of Aromatic Amino Acids. ACS Appl. Mater. Interfaces 2021, 13, 36722–36736. [Google Scholar] [CrossRef]
- Hirai, A.; Kawasaki, H.; Tanaka, S.; Nemoto, N.; Suzuki, M.; Maeda, H. Effects of L-Arginine on Aggregates of Fatty-Acid/Potassium Soap in the Aqueous Media. Colloid. Polym. Sci. 2006, 284, 520–528. [Google Scholar] [CrossRef]
- Li, G.; Yang, Q.; Song, A.; Hao, J. Self-Assembled Structural Transition from Vesicle Phase to Sponge Phase and Emulsifying Properties in Mixtures of Arginine and Fatty Acids. Colloids Surf. A 2015, 487, 198–206. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Shen, Q.; Shen, J.; Han, Y.; Zhang, H. Investigation on the Self-Assembled Behaviors of C 18 Unsaturated Fatty Acids in Arginine Aqueous Solution. RSC Adv. 2017, 7, 41561–41572. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Zhen, D.; Liu, F.; Peng, Q.; Sun, J. Fatty Acid-Arginine Vesicles with Prominent Encapsulation Efficiency and Substantial Transdermal Delivery of Sinomenine Hydrochloride. Colloids Surf. A 2024, 698, 134514. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Wei, C.; Zhang, H. Phase Behaviors and Self-Assembled Properties of Ion-Pairing Amphiphile Molecules Formed by Medium-Chain Fatty Acids and l-Arginine Triggered by External Conditions. New J. Chem. 2017, 41, 14486–14497. [Google Scholar] [CrossRef]
- Fan, P.; Wang, Y.; Shen, J.; Jiang, L.; Zhuang, W.; Han, Y.; Zhang, H. Self-Assembly Behaviors of C18 Fatty Acids in Arginine Aqueous Solution Affected by External Conditions. Colloids Surf. A 2019, 577, 240–248. [Google Scholar] [CrossRef]
- Tong, J.; Zhou, H.; Zhou, J.; Chen, Y.; Shi, J.; Zhang, J.; Liang, X.; Du, T. Design and Evaluation of Chitosan-amino Acid Thermosensitive Hydrogel. Mar. Life Sci. Tech. 2022, 4, 74–87. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, D.; Wang, M.; Yu, C.; Han, Z.; Xu, M.; Yue, W.; Nie, G. β-Alanine Enhancing the Crosslink of Chitosan/Poly-(γ-Glutamic Acid) Hydrogel for a Potential Alkaline-Adapted Wound Dressing. Int. J. Biol. Macromol. 2023, 231, 123157. [Google Scholar] [CrossRef] [PubMed]
- Baranova, O.A.; Khizhnyak, S.D.; Pakhomov, P.M. Supramolecular Hydrogel Based on L-Cysteine and Silver Nanoparticles. J. Struct. Chem. 2014, 55, 169–174. [Google Scholar] [CrossRef]
- Vishnevetskii, D.V.; Mekhtiev, A.R.; Perevozova, T.V.; Averkin, D.V.; Ivanova, A.I.; Khizhnyak, S.D.; Pakhomov, P.M. l -Cysteine/AgNO 2 Low Molecular Weight Gelators: Self-Assembly and Suppression of MCF-7 Breast Cancer Cells. Soft Matter 2020, 16, 9669–9673. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Singh, A.; Sarma, T.K.; Sardana, N.; Pal, A. Chirality Control of Multi-Stimuli Responsive and Self-Healing Supramolecular Metallo-Hydrogels. New J. Chem. 2018, 42, 6427–6432. [Google Scholar] [CrossRef]
- Shen, J.-S.; Mao, G.-J.; Zhou, Y.-H.; Jiang, Y.-B.; Zhang, H.-W. A Ligand-Chirality Controlled Supramolecular Hydrogel. Dalton Trans. 2010, 39, 7054. [Google Scholar] [CrossRef][Green Version]
- Ma, M.; Qiu, S.; Qiao, Y.; Xu, Z.; Du, X.; Ding, D.; Yuan, Y.; Xing, P.; Shang, W. A Stable Green Amino Acid Hydrogel. ChemistrySelect 2023, 8, e202301151. [Google Scholar] [CrossRef]
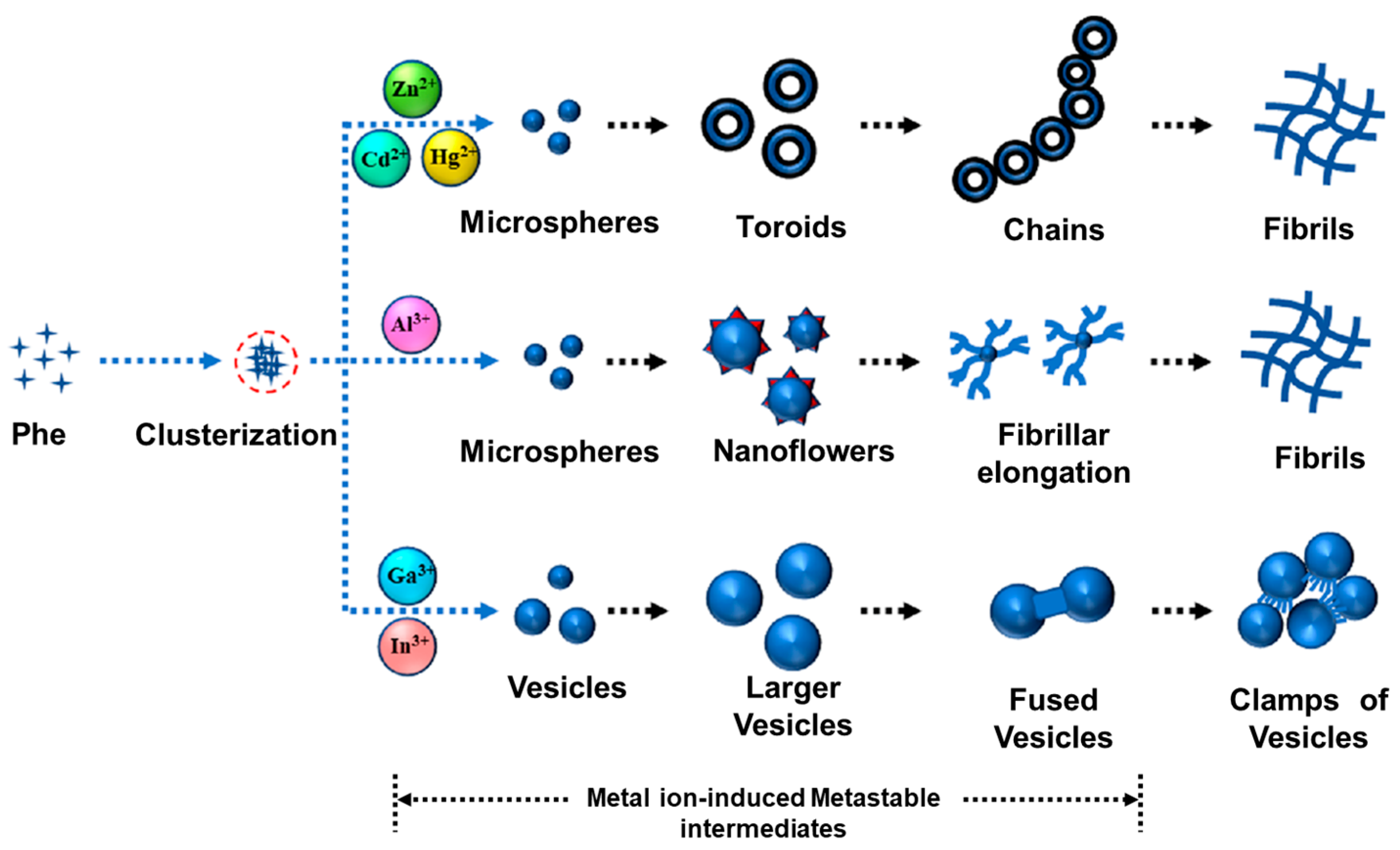
- Bagchi, D.; Maity, A.; De, S.K.; Chakraborty, A. Effect of Metal Ions on the Intrinsic Blue Fluorescence Property and Morphology of Aromatic Amino Acid Self-Assembly. J. Phys. Chem. B 2021, 125, 12436–12445. [Google Scholar] [CrossRef]
- Bagchi, D.; Maity, A.; De, S.K.; Chakraborty, A. Metal-Ion-Induced Evolution of Phenylalanine Self-Assembly: Structural Polymorphism of Novel Metastable Intermediates. J. Phys. Chem. Lett. 2022, 13, 10409–10417. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.; Zhang, L.; Yu, L.; Song, A.; Hu, J. pH-Responsive Foams Triggered by Particles from Amino Acids with Metal Ions. J. Mol. Liq. 2022, 367, 120374. [Google Scholar] [CrossRef]
- Wei, G.; Wang, C.; Ma, L.; Li, F.; Gao, Z.; Yan, M. Multi-Dimensional Architecture Materials of Amino Acids and Metal Ions. New J. Chem. 2018, 42, 17447–17452. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Wu, L. Ionic Complexes of Metal Oxide Clusters for Versatile Self-Assemblies. Acc. Chem. Res. 2017, 50, 1391–1399. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Shi, X.; Wu, L. Polyoxometalate-Based Frameworks for Photocatalysis and Photothermal Catalysis. Nanoscale 2023, 15, 9242–9255. [Google Scholar] [CrossRef] [PubMed]
- Judd, D.A.; Nettles, J.H.; Nevins, N.; Snyder, J.P.; Liotta, D.C.; Tang, J.; Ermolieff, J.; Schinazi, R.F.; Hill, C.L. Polyoxometalate HIV-1 Protease Inhibitors. A New Mode of Protease Inhibition. J. Am. Chem. Soc. 2001, 123, 886–897. [Google Scholar] [CrossRef]
- Zhang, T.; Li, H.-W.; Wu, Y.; Wang, Y.; Wu, L. Self-Assembly of an Europium-Containing Polyoxometalate and the Arginine/Lysine-Rich Peptides from Human Papillomavirus Capsid Protein L1 in Forming Luminescence-Enhanced Hybrid Nanoparticles. J. Phys. Chem. C 2015, 119, 8321–8328. [Google Scholar] [CrossRef]
- Yan, X.; Zhu, P.; Fei, J.; Li, J. Self-Assembly of Peptide-Inorganic Hybrid Spheres for Adaptive Encapsulation of Guests. Adv. Mater. 2010, 22, 1283–1287. [Google Scholar] [CrossRef]
- Li, J.; Chen, Z.; Zhou, M.; Jing, J.; Li, W.; Wang, Y.; Wu, L.; Wang, L.; Wang, Y.; Lee, M. Polyoxometalate-Driven Self-Assembly of Short Peptides into Multivalent Nanofibers with Enhanced Antibacterial Activity. Angew. Chem. Int. Ed. 2016, 55, 2592–2595. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Li, X.; Li, B.; Wu, L.; Li, W.; Xie, X.; Xue, R. Supramolecular Copolymerization of Short Peptides and Polyoxometalates: Toward the Fabrication of Underwater Adhesives. Biomacromolecules 2017, 18, 3524–3530. [Google Scholar] [CrossRef]
- Ji, F.; Li, Y.; Zhao, H.; Wang, X.; Li, W. Solvent-Exchange Triggered Solidification of Peptide/POM Coacervates for Enhancing the On-Site Underwater Adhesion. Molecules 2024, 29, 681. [Google Scholar] [CrossRef] [PubMed]
- Cindric, M.; Strukan, N.; Devčic, M.; Kamenar, B. Synthesis and Structure of K2[HMo6VIVVO22(NH3CH2COO)3]P·8H2O: A New Example of a Polyoxomolybdovanadate Coordinated by a Glycinato Ligand. Inorg. Chem. Commun. 1999, 2, 558–560. [Google Scholar] [CrossRef]
- Nikoloudakis, E.; Karikis, K.; Laurans, M.; Kokotidou, C.; Solé-Daura, A.; Carbó, J.J.; Charisiadis, A.; Charalambidis, G.; Izzet, G.; Mitraki, A.; et al. Self-Assembly Study of Nanometric Spheres from Polyoxometalate-Phenylalanine Hybrids, an Experimental and Theoretical Approach. Dalton Trans. 2018, 47, 6304–6313. [Google Scholar] [CrossRef]
- An, H.; Wang, E.; Xiao, D.; Li, Y.; Su, Z.; Xu, L. Chiral 3D Architectures with Helical Channels Constructed from Polyoxometalate Clusters and Copper–Amino Acid Complexes. Angew. Chem. Int. Ed. 2006, 45, 904–908. [Google Scholar] [CrossRef] [PubMed]
- An, H.; Wang, E.; Li, Y.; Zhang, Z.; Xu, L. A Functionalized Polyoxometalate by Hexanuclear Copper–Amino Acid Coordination Complexes. Inorg. Chem. Commun. 2007, 10, 299–302. [Google Scholar] [CrossRef]
- Ren, S.; Xie, Z.; Cao, L.; Xie, X.; Qin, G.; Wang, J. Clean Synthesis of Adipic Acid Catalyzed by Complexes Derived from Heteropoly Acid and Glycine. Catal. Commun. 2009, 10, 464–467. [Google Scholar] [CrossRef]
- Che, W.-S.; Gai, H.-H.; Hao, W.-B.; Ma, R.-H. Synthesis, Characterization, and Properties of a New Type of Heteropoly Acids and Supramolecular Compounds. Synth. React. Inorg. M. 2014, 44, 649–655. [Google Scholar] [CrossRef]
- Zhang, X.; Ouyang, K.; Liang, J.; Chen, K.; Tang, X.; Han, X. Optimization of Process Variables in the Synthesis of Butyl Butyrate Using Amino Acid-Functionalized Heteropolyacids as Catalysts. Green Process. Synth. 2016, 5, 321–329. [Google Scholar] [CrossRef]
- Masteri-Farahani, M.; Shahbazi, S. Facile Synthesis of Rod-like Nanostructured Histidine-Phosphomolybdate Hybrid Material with Microemulsion Method. Inorg. Nano-Met. Chem. 2017, 47, 543–548. [Google Scholar] [CrossRef]
- Arefian, M.; Mirzaei, M.; Eshtiagh-Hosseini, H. Structural Insights into Two Inorganic-Organic Hybrids Based on Chiral Amino Acids and Polyoxomolybdates. J. Mol. Struct. 2018, 1156, 550–558. [Google Scholar] [CrossRef]
- Bagherjeri, F.A.; Vonci, M.; Nagul, E.A.; Ritchie, C.; Gable, R.W.; Taylor, M.B.; Bryant, G.; Guo, S.-X.; Zhang, J.; Aparicio, P.A.; et al. Mixed-Metal Hybrid Polyoxometalates with Amino Acid Ligands: Electronic Versatility and Solution Properties. Inorg. Chem. 2016, 55, 12329–12347. [Google Scholar] [CrossRef]
- Heravi, M.M.; Momeni, T.; Mirzaei, M.; Zadsirjan, V.; Tahmasebi, M. An Amino Acid@isopolyoxometalate Nanoparticles Catalyst Containing Aspartic Acid and Octamolybdate for the Synthesis of Functionalized Spirochromenes. Inorg. Nano-Met. Chem. 2021, 51, 896–909. [Google Scholar] [CrossRef]
- Xuan, W.; Pow, R.; Watfa, N.; Zheng, Q.; Surman, A.J.; Long, D.-L.; Cronin, L. Stereoselective Assembly of Gigantic Chiral Molybdenum Blue Wheels Using Lanthanide Ions and Amino Acids. J. Am. Chem. Soc. 2019, 141, 1242–1250. [Google Scholar] [CrossRef]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Arefian, M.; Akbarnia, F.; Miri, F.; Edalatkar-Moghadam, S.; Shamsipur, M. Spectroscopic Studies on Tungstoheteropolyanions Functionalized by Amino Acids. J. Iran. Chem. Soc. 2015, 12, 1191–1198. [Google Scholar] [CrossRef]
- Farhadipour, A.; Alizadeh, M.H.; Eshghi, H. Synthesis of Amino Acid Polyoxometalate Hybrid Nanotubes under Solid-State Chemical Reaction at Room Temperature. Inorg. Chem. Commun. 2014, 41, 37–42. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, L.; Xie, Z.; Xin, X.; Sun, D.; Yuan, S. Tunable Aggregation-Induced Emission of Polyoxometalates via Amino Acid-Directed Self-Assembly and Their Application in Detecting Dopamine. Langmuir 2016, 32, 13736–13745. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.-T.; Xuan, Z.-X.; Wang, S.; Zhang, X.; Luo, F. Facile One-Pot Construction of Polyoxometalate-Based Lanthanide-Amino Acid Coordination Polymers for Proton Conduction. Inorg. Chem. Commun. 2019, 105, 147–150. [Google Scholar] [CrossRef]
- Li, S.; Wang, E.; Tian, C.; Mao, B.; Song, Y.; Wang, C.; Xu, L. In Situ Fabrication of Amino Acid-Polyoxometalate Nanoparticle Functionalized Ultrathin Films and Relevant Electrochemical Property Study. Mater. Res. Bull. 2008, 43, 2880–2886. [Google Scholar] [CrossRef]
- Fan, D.; Deng, Y.; Hao, J. In Situ Fabrication and Electrochemical Behavior of Amino Acid Polyoxometalate Nanoparticles-Embedded Microcapsules. Amino Acids 2010, 39, 1363–1367. [Google Scholar] [CrossRef]
- Daima, H.K.; Selvakannan, P.R.; Kandjani, A.E.; Shukla, R.; Bhargava, S.K.; Bansal, V. Synergistic Influence of Polyoxometalate Surface Corona towards Enhancing the Antibacterial Performance of Tyrosine-Capped Ag Nanoparticles. Nanoscale 2014, 6, 758–765. [Google Scholar] [CrossRef]
- Tian, N.; Chu, D.; Wang, H.; Yan, H. Synthesis and Anti-HIV-1 Activity Evaluation of Keggin-Type Polyoxometalates with Amino Acid as Organic Cations. Bioorg. Med. Chem. Lett. 2023, 91, 129380. [Google Scholar] [CrossRef]
- Xu, J.; Li, X.; Li, J.; Li, X.; Li, B.; Wang, Y.; Wu, L.; Li, W. Wet and Functional Adhesives from One-Step Aqueous Self-Assembly of Natural Amino Acids and Polyoxometalates. Angew. Chem. Int. Ed. 2017, 56, 8731–8735. [Google Scholar] [CrossRef]
- Liu, X.; Xie, X.; Du, Z.; Li, B.; Wu, L.; Li, W. Aqueous Self-Assembly of Arginine and K8SiW11O39: Fine-Tuning the Formation of a Coacervate Intended for Sprayable Anticorrosive Coatings. Soft Matter 2019, 15, 9178–9186. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Ma, Z.; Mu, C.; Li, W. Photochromic and Photothermal Hydrogels Derived from Natural Amino Acids and Heteropoly Acids. Soft Matter 2021, 17, 10140–10148. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Du, Z.; Song, Z.; Wu, L.; Liu, Q.; Zhang, H.; Li, W. Bringing Hetero-Polyacid-Based Underwater Adhesive as Printable Cathode Coating for Self-Powered Electrochromic Aqueous Batteries. Adv. Funct. Mater. 2018, 28, 1800599. [Google Scholar] [CrossRef]
- Mu, C. Redox and Conductive Underwater Adhesive: An Innovative Electrode Material for Convenient Construction of Flexible and Stretchable Supercapacitors. J. Mater. Chem. A 2022, 10, 7207–7217. [Google Scholar] [CrossRef]
- Mu, C.; Du, Z.; Li, W. Taming of Heteropoly Acids into Adhesive Electrodes Using Amino Acids for the Development of Flexible Two-Dimensional Supercapacitors. Polyoxometalates 2024, 3, 9140062. [Google Scholar] [CrossRef]
- Yang, Z.; Gu, H.; Fu, D.; Gao, P.; Lam, J.K.; Xu, B. Enzymatic Formation of Supramolecular Hydrogels. Adv. Mater. 2004, 16, 1440–1444. [Google Scholar] [CrossRef]
- Sahoo, J.K.; Pappas, C.G.; Sasselli, I.R.; Abul-Haija, Y.M.; Ulijn, R.V. Biocatalytic Self-Assembly Cascades. Angew. Chem. Int. Ed. 2017, 56, 6828–6832. [Google Scholar] [CrossRef] [PubMed]
- Sasselli, I.R. CHARMM Force Field Parameterization Protocol for Self-Assembling Peptide Amphiphiles: The Fmoc Moiety. Phys. Chem. Chem. Phys. 2016, 18, 4659–4667. [Google Scholar] [CrossRef]
- Sutton, S.; Campbell, N.L.; Cooper, A.I.; Kirkland, M.; Frith, W.J.; Adams, D.J. Controlled Release from Modified Amino Acid Hydrogels Governed by Molecular Size or Network Dynamics. Langmuir 2009, 25, 10285–10291. [Google Scholar] [CrossRef] [PubMed]
- Rajbhandary, A.; Brennessel, W.W.; Nilsson, B.L. Comparison of the Self-Assembly Behavior of Fmoc-Phenylalanine and Corresponding Peptoid Derivatives. Cryst. Growth Des. 2018, 18, 623. [Google Scholar] [CrossRef]
- Ryan, D.M.; Anderson, S.B.; Nilsson, B.L. The Influence of Side-Chain Halogenation on the Self-Assembly and Hydrogelation of Fmoc-Phenylalanine Derivatives. Soft Matter 2010, 6, 3220–3231. [Google Scholar] [CrossRef]
- Ryan, D.M.; Anderson, S.B.; Senguen, F.T.; Youngman, R.E.; Nilsson, B.L. Self-Assembly and Hydrogelation Promoted by F5-Phenylalanine. Soft Matter 2010, 6, 475–479. [Google Scholar] [CrossRef]
- Duraisamy, D.K.; Sureshbhai, P.D.; Saveri, P.; Deshpande, A.P.; Shanmugam, G. A ‘“Self-Shrinking”’ Supramolecular Hydrogel with a 3D Shape Memory Performance from an Unnatural Amino Acid Derivative. Chem. Commun. 2022, 58, 13377. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-Y. Structure-Dependent Antibacterial Activity of Amino Acid-Based Supramolecular Hydrogels. Colloids Surf. B 2020, 193, 111099. [Google Scholar] [CrossRef]
- Draper, E.R.; Morris, K.L.; Little, M.A.; Raeburn, J.; Colquhoun, C.; Cross, E.R.; McDonald, T.O.; Serpell, L.C.; Adams, D.J. Hydrogels Formed from Fmoc Amino Acids. CrystEngComm 2015, 17, 8047–8057. [Google Scholar] [CrossRef]
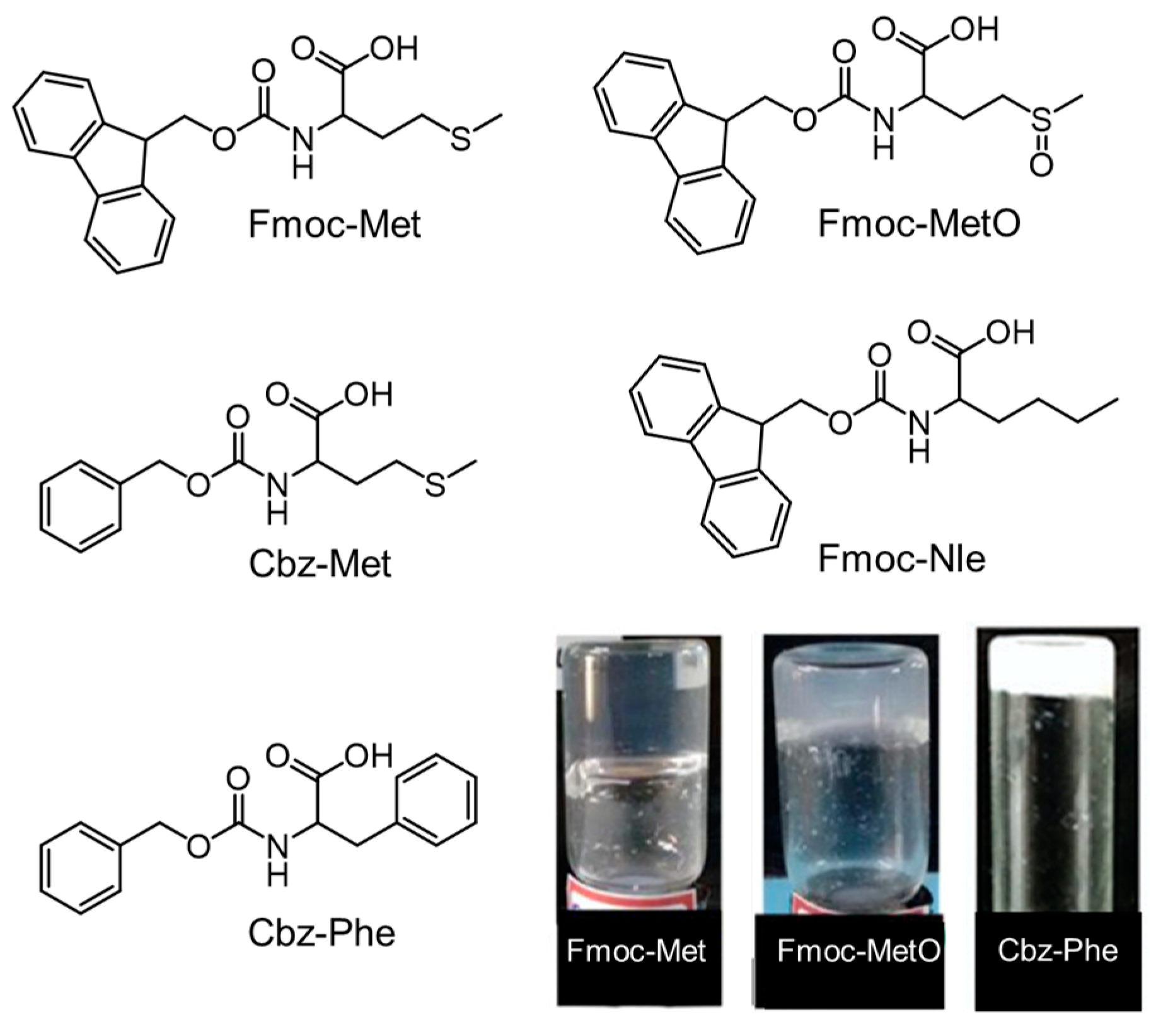
- Reddy, S.M.M.; Dorishetty, P.; Deshpande, A.P.; Shanmugam, G. Hydrogelation Induced by Change in Hydrophobicity of Amino Acid Side Chain in Fmoc-Functionalised Amino Acid: Significance of Sulfur on Hydrogelation. ChemPhysChem 2016, 17, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Petropoulou, K.; Platania, V.; Chatzinikolaidou, M.; Mitraki, A. A Doubly Fmoc-Protected Aspartic Acid Self-Assembles into Hydrogels Suitable for Bone Tissue Engineering. Materials 2022, 15, 8928. [Google Scholar] [CrossRef]
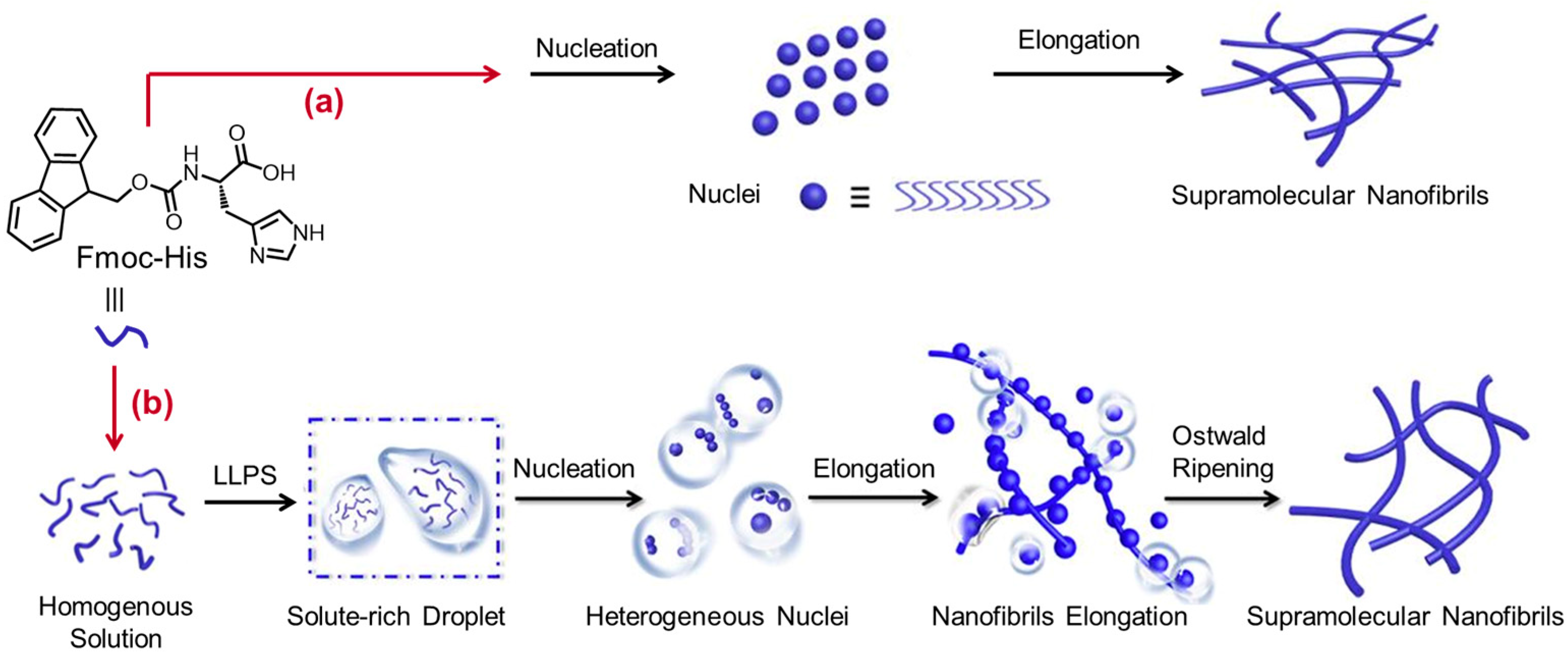
- Yuan, C.; Levin, A.; Chen, W.; Xing, R.; Zou, Q.; Herling, T.W.; Kumar, P.; Knowles, T.P.J.; Yan, X. Nucleation and Growth of Amino-acid and Peptide Supramolecular Polymers through Liquid-liquid Phase Separation. Angew. Chem. Int. Ed. 2019, 58, 18116–18123. [Google Scholar] [CrossRef]
- Abraham, B.L.; Liyanage, W.; Nilsson, B.L. Strategy to Identify Improved N-Terminal Modifications for Supramolecular Phenylalanine-Derived Hydrogelators. Langmuir 2019, 35, 14939–14948. [Google Scholar] [CrossRef]
- Tiwari, P. Can Self-Assembled Hydrogels Composed of Aromatic Amino Acid Derivatives Function as Drug Delivery Carriers? New J. Chem. 2017, 41, 308–315. [Google Scholar] [CrossRef]
- Mondal, S. Acid-Responsive Fibrillation and Urease-Assisted Defibrillation of Phenylalanine: A Transient Supramolecular Hydrogel. Soft Matter 2020, 16, 10115–10121. [Google Scholar] [CrossRef]
- Song, X.; He, S.; Zheng, J.; Yang, S.; Li, Q.; Zhang, Y. One-Step Construction of Tryptophan-Derived Small Molecule Hydrogels for Antibacterial Materials. Molecules 2023, 28, 3334. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.M.; Lavendomme, R.; Kralj, S.; Kurbasic, M.; Bellotto, O.; Cringoli, M.C.; Semeraro, S.; Bandiera, A.; Zorzi, R.D.; Marchesan, S. Self-Assembly of an Amino Acid Derivative into an Antimicrobial Hydrogel Biomaterial. Chem. Eur. J. 2020, 26, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, Y.; Yang, Z.; Xu, B. Exceptionally Small Supramolecular Hydrogelators Based on Aromatic–Aromatic Interactions. Beilstein J. Org. Chem. 2011, 7, 167–172. [Google Scholar] [CrossRef]
- Nanda, J.; Biswas, A.; Banerjee, A. Single Amino Acid Based Thixotropic Hydrogel Formation and pH-Dependent Morphological Change of Gel Nanofibers. Soft Matter 2013, 9, 4198–4208. [Google Scholar] [CrossRef]
- Zhou, M. Application of Hydrogel Prepared from Ferrocene Functionalized Amino Acid in the Design of Novel Electrochemical Immunosensing Platform. Biosens. Bioelectron. 2013, 49, 243–248. [Google Scholar] [CrossRef]
- Mohanty, A.; Dey, J. Spontaneous Formation of Vesicles and Chiral Self-Assemblies of Sodium N-(4-Dodecyloxybenzoyl)-L-Valinate in Water. Langmuir 2004, 20, 8452–8459. [Google Scholar] [CrossRef]
- Tan, C.; Lu, R.; Xue, P.; Bao, C.; Zhao, Y. Synthesis of CuS Nanoribbons Templated by Hydrogel. Mater. Chem. Phys. 2008, 112, 500–503. [Google Scholar] [CrossRef]
- Jung, S.H.; Lee, H.; Han, W.S.; Jung, J.H. The Effect of Hydrogen-Bonds of Amino Acid-Derived Diacetylene by Photopolymerization in Supramolecular Hydrogels. J. Nanosci. Nanotechnol. 2011, 11, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Patra, T.; Pal, A.; Dey, J. A Smart Supramolecular Hydrogel of Nα-(4-n-Alkyloxybenzoyl)-L-Histidine Exhibiting pH-Modulated Properties. Langmuir 2010, 26, 7761–7767. [Google Scholar] [CrossRef]
- Noponen, V.; Valkonen, A.; Lahtinen, M.; Salo, H.; Sievänen, E. Self-Assembly Properties of Bile Acid Derivatives of l-Cysteine, l-Valine and l-Serine Alkyl Esters. Supramol. Chem. 2012, 25, 133–145. [Google Scholar] [CrossRef]
- Maity, M.; Maitra, U. Supramolecular Gels from Conjugates of Bile Acids and Amino Acids and Their Applications. Eur. J. Org. Chem. 2017, 13, 1713–1720. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, F.; Wen, Y.; Liu, K.; Chen, L.; Mao, Y.; Yang, S.; Yi, T. (À)-Menthol Based Thixotropic Hydrogel and Its Application as a Universal Antibacterial Carrier. Soft Matter 2014, 10, 3077–3085. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Facile Fabrication of Novel pH-Sensitive Poly(Aspartic Acid) Hydrogel by Crosslinking Nanofibers. Mater. Lett. 2014, 132, 393–396. [Google Scholar] [CrossRef]
- Cao, H.; Zhu, X.; Liu, M. Self-Assembly of Racemic Alanine Derivatives: Unexpected Chiral Twist and Enhanced Capacity for the Discrimination of Chiral Species. Angew. Chem. Int. Ed. 2013, 52, 4122–4126. [Google Scholar] [CrossRef] [PubMed]
- Chen, S. Topological Structure Influences on the Gel Formation Process and Mechanical Properties of L-Lysine Based Supramolecular Gels. RSC Adv. 2015, 5, 101437–101443. [Google Scholar] [CrossRef]
- Brar, S.K.; Singh, P.; Bajaj, M.; Deep, A.; Wangoo, N.; Sharma, R.K. Self Assembly of a C-Amino Butyric Acid Based Derivative into Tunable Nano/Micro Structures. Mater. Chem. Front. 2017, 1, 449–454. [Google Scholar] [CrossRef]
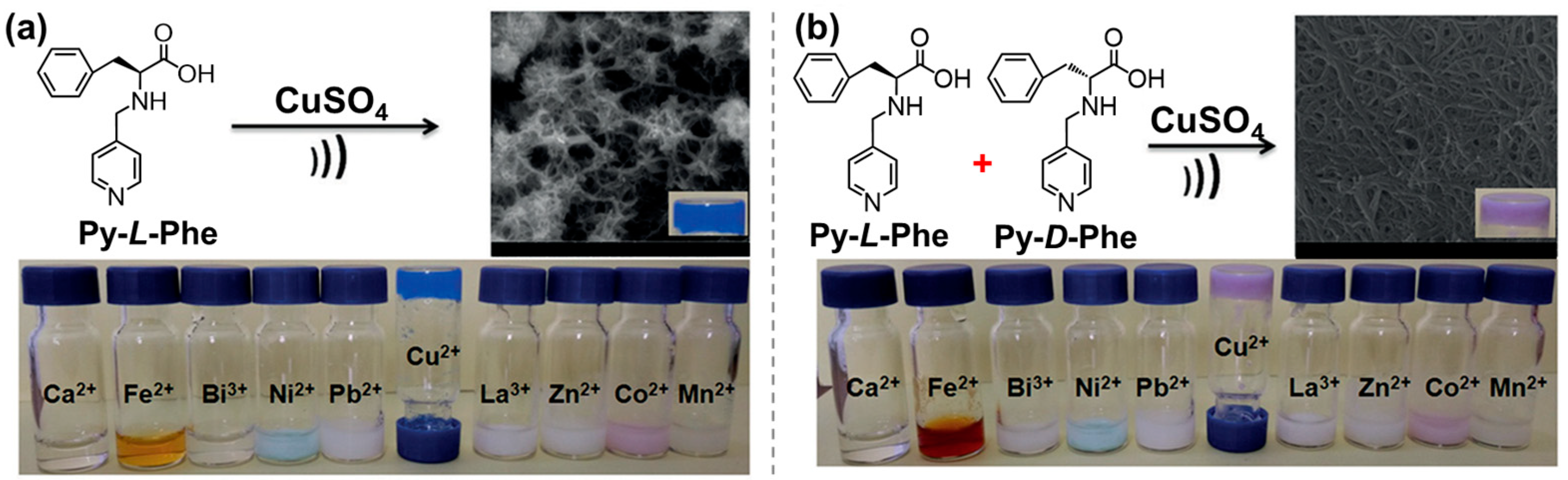
- Adhikari, B.; Nanda, J.; Banerjee, A. Multicomponent Hydrogels from Enantiomeric Amino Acid Derivatives: Helical Nanofibers, Handedness and Self-Sorting. Soft Matter 2011, 7, 8913–8922. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Qin, X.-T.; Feng, J.-Y.; Zhong, C.; Jia, S.-R. A Self-Assembled Amino Acid-Based Hydrogel with Broad-Spectrum Antibacterial Activity. J. Mater. Sci. 2021, 56, 7626–7636. [Google Scholar] [CrossRef]
- Wang, Q.; Li, L.; Xu, B. Bioinspired Supramolecular Confinement of Luminol and Heme Proteins to Enhance the Chemiluminescent Quantum Yield. Chem. Eur. J. 2009, 15, 3168–3172. [Google Scholar] [CrossRef]
- Ryan, D.M.; Doran, T.M.; Nilsson, B.L. Stabilizing Self-Assembled Fmoc–F5–Phe Hydrogels by Co-Assembly with PEG-Functionalized Monomerswz. Chem. Commun. 2011, 47, 475–477. [Google Scholar] [CrossRef]
- Misra, R.; Tang, Y.; Chen, Y.; Chakraborty, P.; Netti, F.; Vijayakanth, T.; Shimon, L.J.W.; Wei, G.; Adler-Abramovich, L. Exploiting Minimalistic Backbone Engineered γ-Phenylalanine for the Formation of Supramolecular Co-Polymer. Macromol. Rapid Commun. 2022, 43, e2200223. [Google Scholar] [CrossRef] [PubMed]
- Hsu, L.-H. Nanofibrous Hydrogels Self-Assembled from Naphthalene Diimide (NDI)/Amino Acid Conjugates. RSC Adv. 2015, 5, 20410–20413. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, M.; Wang, A.; Yu, C.; Bai, S.; Yin, J.; You, Q. Self-Assembly of Fmoc-Amino Acids in Capillary Confined Space Forming a Parallel Ordered Fiber Network for Application in Vascularization. Biomater. Sci. 2022, 10, 1470–1475. [Google Scholar] [CrossRef]
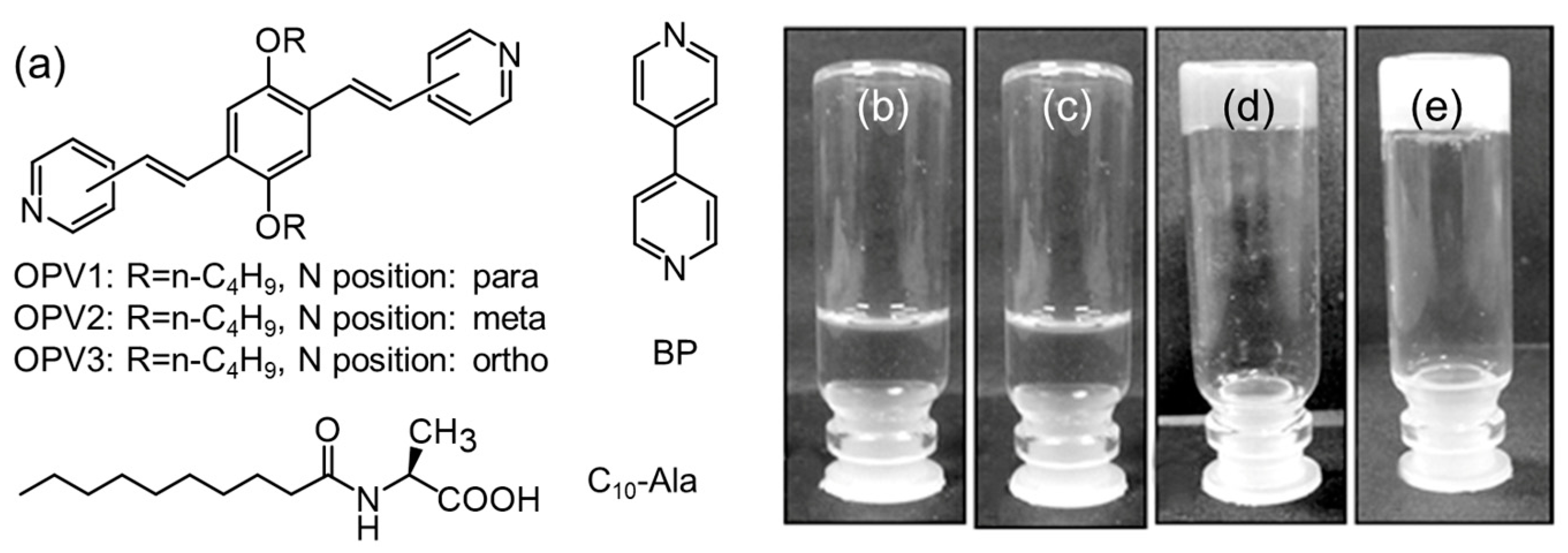
- Bhattacharjee, S.; Datta, S.; Bhattacharya, S. Remarkable Regioisomer Control in the Hydrogel Formation from a Two-Component Mixture of Pyridine-End Oligo( p -phenylenevinylene)s and N -Decanoyl- L -alanine. Chem. Eur. J. 2013, 19, 16672–16681. [Google Scholar] [CrossRef] [PubMed]
- Nie, B.; Zhan, T.; Zhou, T.; Xiao, Z.; Jiang, G.; Zhao, X. Self-Assembly of Chiral Propeller-like Supermolecules with Unusual “Sergeants-and-Soldiers” and “Majority-Rules” Effects. Chem. Asian J. 2014, 9, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Green, M.M.; Reidy, M.P.; Johnson, R.D.; Darling, G.; O’Leary, D.J.; Willson, G. Macromolecular Stereochemistry: The out-of-Proportion Influence of Optically Active Comonomers on the Conformational Characteristics of Polyisocyanates. The Sergeants and Soldiers Experiment. J. Am. Chem. Soc. 1989, 111, 6452–6454. [Google Scholar] [CrossRef]
- Xing, P.; Phua, S.Z.F.; Wei, X.; Zhao, Y. Programmable Multicomponent Self-Assembly Based on Aromatic Amino Acids. Adv. Mater. 2018, 30, 1805175. [Google Scholar] [CrossRef]
- Xing, P.; Li, P.; Chen, H.; Hao, A.; Zhao, Y. Understanding Pathway Complexity of Organic Micro/Nanofiber Growth in Hydrogen-Bonded Coassembly of Aromatic Amino Acids. ACS Nano 2017, 11, 4206–4216. [Google Scholar] [CrossRef]
- Xing, P.; Chen, H.; Xiang, H.; Zhao, Y. Selective Coassembly of Aromatic Amino Acids to Fabricate Hydrogels with Light Irradiation-Induced Emission for Fluorescent Imprint. Adv. Mater. 2018, 30, 1705633. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, L.; Liu, C.; Xie, Y.; Liu, W.; Fan, Y.; Li, X.; Fan, X. Hierarchical Self-Assembly of Amino Acid Derivatives into Stimuli-Responsive Luminescent Gels. Soft Matter 2014, 10, 8261–8266. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W.; Cheng, L.; Huang, P.; Peng, Y.; Wu, Y.; Li, X.; Li, X.; Fan, X. A Smart pH-Responsive Three Components Luminescent Hydrogel. J. Funct. Biomater. 2016, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Mulvee, M.; Vasiljevic, N.; Mann, S.; Patil, A.J. Stimuli-Responsive Nucleotide–Amino Acid Hybrid Supramolecular Hydrogels. Gels 2021, 7, 146. [Google Scholar] [CrossRef] [PubMed]
- Gahane, A.Y.; Singh, V.; Kumar, A.; Kumar Thakur, A. Development of Mechanism-Based Antibacterial Synergy between Fmoc-Phenylalanine Hydrogel and Aztreonam. Biomater. Sci. 2020, 8, 1996–2006. [Google Scholar] [CrossRef] [PubMed]
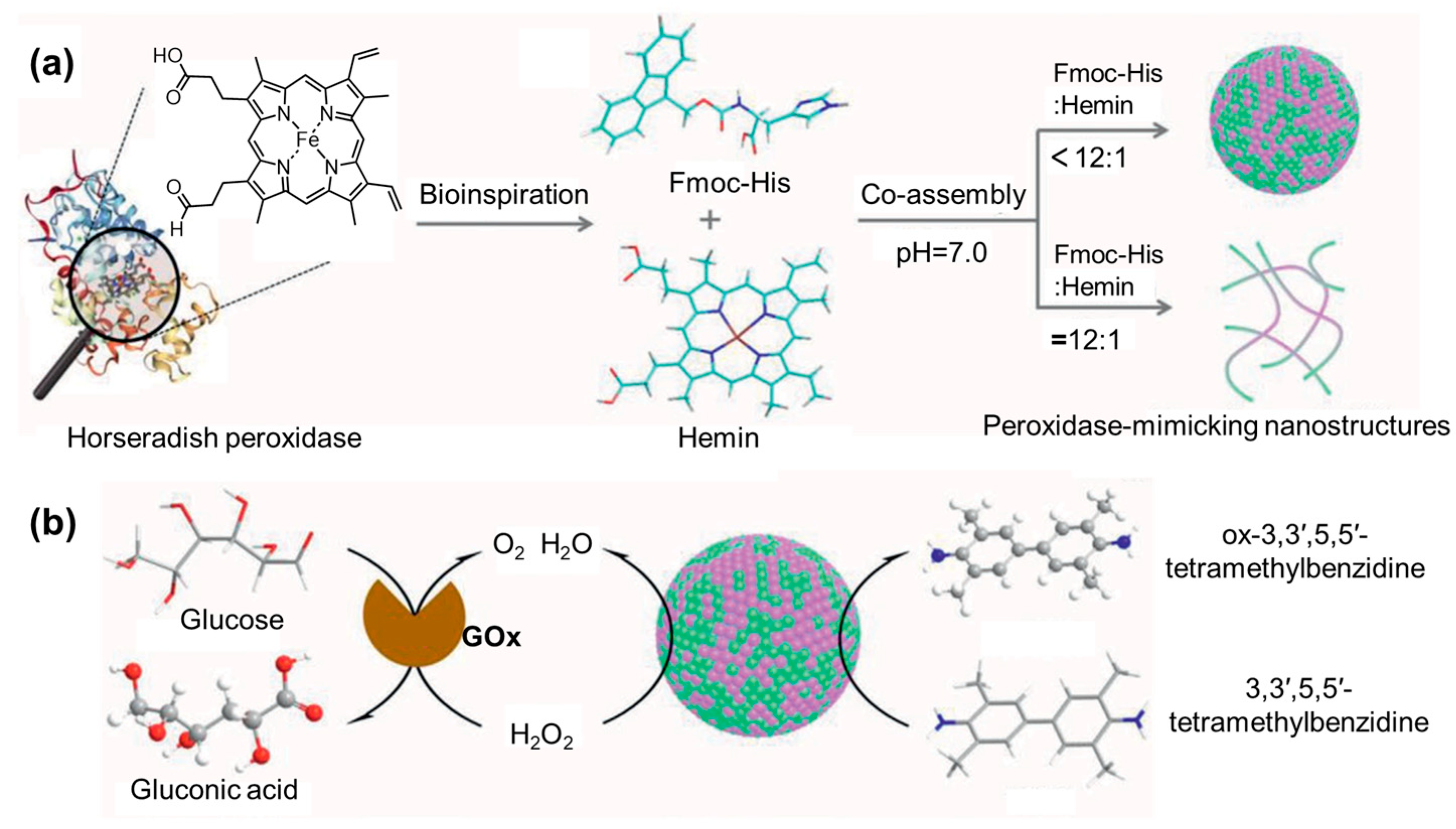
- Geng, R.; Chang, R.; Zou, Q.; Shen, G.; Jiao, T.; Yan, X. Biomimetic Nanozymes Based on Coassembly of Amino Acid and Hemin for Catalytic Oxidation and Sensing of Biomolecules. Small 2021, 17, 2008114. [Google Scholar] [CrossRef]
- Sitsanidis, E.D.; Dutra, L.A.L.; Schirmer, J.; Chevigny, R.; Lahtinen, M.; Johansson, A.; Piras, C.C.; Smith, D.K.; Tiirola, M.; Pettersson, M.; et al. Probing the Gelation Synergies and Anti-Escherichia Coli Activity of Fmoc-Phenylalanine/Graphene Oxide Hybrid Hydrogel. ACS Omega 2023, 8, 10225–10234. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, J.; Wang, Y.; Qi, W.; Su, R.; He, Z. Self-Assembly of Ferrocene-Phenylalanine@Graphene Oxide Hybrid Hydrogels for Dopamine Detection. ChemPlusChem 2020, 85, 2341–2348. [Google Scholar] [CrossRef]
- Chakraborty, P.; Bairi, P.; Mondal, S.; Nandi, A.K. Co-Assembled Conductive Hydrogel of N -Fluorenylmethoxycarbonyl Phenylalanine with Polyaniline. J. Phys. Chem. B 2014, 118, 13969–13980. [Google Scholar] [CrossRef]
- Aramaki, K.; Shiozaki, Y.; Kosono, S.; Ikeda, N. Coacervation in Cationic Polyelectrolyte Solutions with Anionic Amino Acid Surfactants. J. Oleo Sci. 2020, 69, 1411–1416. [Google Scholar] [CrossRef]
- Wang, F.; Feng, W.; Zhu, Z.; Zhang, J.; Wei, H.; Dang, L. Coacervating Behavior of Amino Acid Anionic and Amphoteric Mixed Micelle-Polymer. Soft Matter 2024, 20, 5733–5744. [Google Scholar] [CrossRef]
- Wang, X.; Wei, C.; Su, J.; He, B.; Wen, G.; Lin, Y.; Zhang, Y. A Chiral Ligand Assembly That Confers One-Electron O 2 Reduction Activity for a Cu 2+-Selective Metallohydrogel. Angew. Chem. Int. Ed. 2018, 57, 3504–3508. [Google Scholar] [CrossRef]
- Wang, X.; Wei, C.; Gao, S.; He, B.; Lin, Y. Assembly of ( l+d)-Tryptophan Derivatives Containing an Imidazole Group Selectively Forms a Rare Purple Ni 2+-Hydrogel. ChemistryOpen 2019, 8, 1172–1175. [Google Scholar] [CrossRef] [PubMed]
- Travaglini, L.; Di Gregorio, M.C.; Severoni, E.; D’Annibale, A.; Sennato, S.; Tardani, F.; Giustini, M.; Gubitosi, M.; Del Giudice, A.; Galantini, L. Deoxycholic Acid and L-Phenylalanine Enrich Their Hydrogel Properties When Combined in a Zwitterionic Derivative. J. Colloid Interface Sci. 2019, 554, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Wang, T.; Liu, R.; Jiang, W.; Niu, Z.; Bai, M.; Wu, W.; Hao, A.; Shang, W. A Novel Green Amino Acid Derivative Hydrogel with Multi-Stimulus Responsiveness. Colloid Polym. Sci. 2023, 301, 569–576. [Google Scholar] [CrossRef]
- Song, J.; Yuan, C.; Jiao, T.; Xing, R.; Yang, M.; Adams, D.J.; Yan, X. Multifunctional Antimicrobial Biometallohydrogels Based on Amino Acid Coordinated Self-Assembly. Small 2020, 16, 1907309. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Q. Fabrication of Amino Acid-Based Supramolecular Hydrogel with Silver Ions for Improved Antibacterial Properties. Mater. Lett. 2021, 300, 130161. [Google Scholar] [CrossRef]
- Li, W.; Hu, X.; Chen, J.; Wei, Z.; Song, C.; Huang, R. N-(9-Fluorenylmethoxycarbonyl)-L-Phenylalanine/Nano-Hydroxyapatite Hybrid Supramolecular Hydrogels as Drug Delivery Vehicles with Antibacterial Property and Cytocompatibility. J. Mater. Sci.-Mater. M. 2020, 31, 73. [Google Scholar] [CrossRef]
- Cao, S.; Fan, W.; Chang, R.; Yuan, C.; Yan, X. Metal Ion-Coordinated Biomolecular Noncovalent Glass with Ceramic-like Mechanics. CCS Chem. 2024. [Google Scholar] [CrossRef]
- Romanski, J.; Karbarz, M.; Pyrzynska, K.; Jurczak, J.; Stojek, Z. Polymeric Hydrogels Modified with Ornithine and Lysine: Sorption and Release of Metal Cations and Amino Acids. J. Polym. Sci. A Polym. Chem. 2012, 50, 542–550. [Google Scholar] [CrossRef]
- Gharakhloo, M.; Jagleniec, D.; Romanski, J.; Karbarz, M. A Novel Self-Healing Hydrogel Based on Derivatives of Natural α-Amino Acids with Potential Applications as a Strain Sensor. J. Mater. Chem. B 2022, 10, 4463–4472. [Google Scholar] [CrossRef]
- Zhang, H.; He, J.; Qu, J. Metal-Coordinated Amino Acid Hydrogels with Ultra-Stretchability, Adhesion, and Self-Healing Properties for Wound Healing. Eur. Polym. J. 2022, 179, 111548. [Google Scholar] [CrossRef]
- Jahier, C.; Nlate, S. L -Proline-Derived Dendritic Tetrakis(Diperoxotungsto)Phosphate: Synthesis and Enantioselective Oxidation Catalysis. Eur. J. Inorg. Chem. 2012, 2012, 833–840. [Google Scholar] [CrossRef]






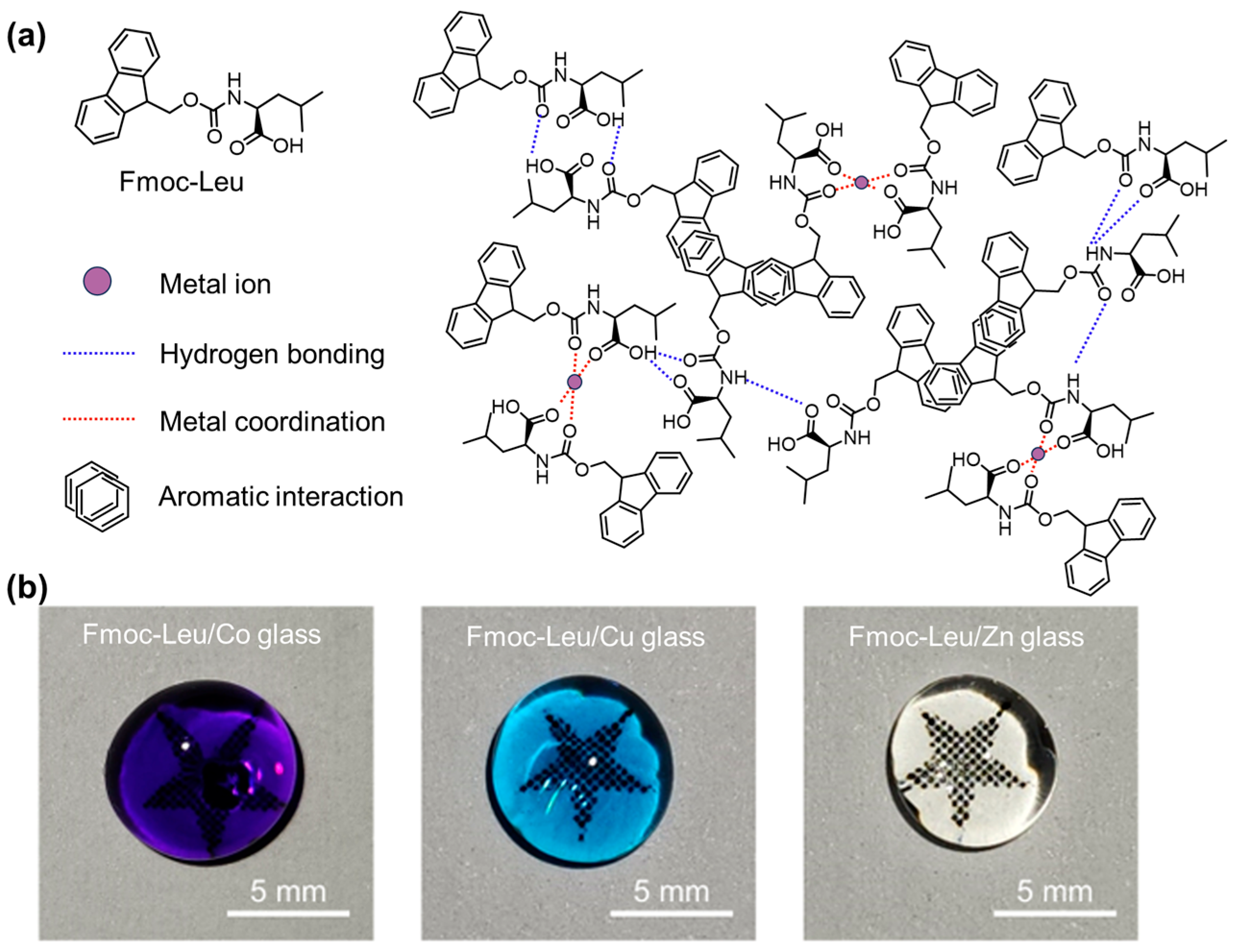
| Compounds | Assembled Structures |
|---|---|
| Phe/Phe@Au or Cit@Au [15] | nanofibers nanoribbons |
| His/Cit@Au [15] | |
| Phe/Zn2+ or Cd2+ or Hg2+ or Al3+ [48] | |
| Fmoc-His [97] | |
| Fmoc-Ala-C17 [114] | |
| Fmoc-Tyr or Fmoc-Thr or Fmoc-Ser or Fmoc-Leu/Leu-NH2[123] | |
| Phe or Trp [31] | |
| Phe/Tyr/Trp [33] | |
| Tyr [26] | |
| His/Phe@Au [15] | dendritic structures |
| Trp/Phe@Au or Cit@Au [15] | |
| Try or Trp [27] | nanotubes |
| (Ile)3PMo12O40 [72] | |
| (Ile)3PW12O40 [72] | |
| (Cys)3PMo12O40 [72] | |
| (Cys)3PW12O40 [72] | |
| L-Phe [28] | hydrogel with fibrillar structures hydrogel with twisted nanoribbons hydrogel with rod-like structures |
| Fmoc-Tyr [85,86,93] | |
| Fmoc-Met [93,94,95] | |
| Fmoc-Phe [17,88] | |
| Fmoc-Trp [93] | |
| Fmoc-β-Phe [92] | |
| N- and C-terminus of Asp were modified by Fmoc [96] | |
| Fmoc-Met or Fmoc-Gly or Fmoc-Ile [94] | |
| Cbz-Phe [99,100] | |
| Fc-Phe [105] | |
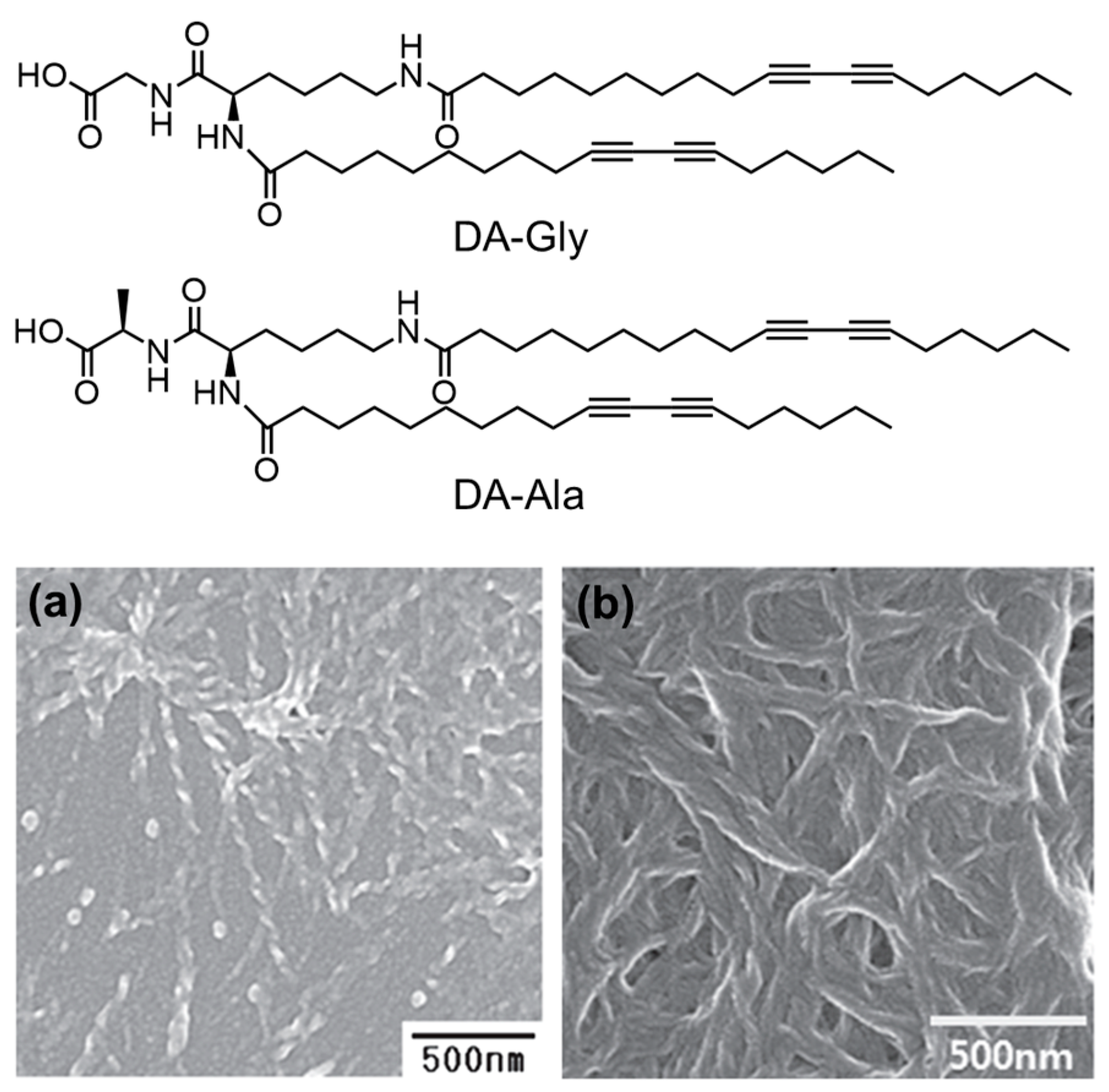
| DA-Gly or DA-Ala [108] | |
| Cn-His [109] | |
| β-L-PheDC/Ca2+ [142] | |
| menthol methyl ester group-modified Lys [112] | |
| Fmoc-Glu/Lys [117] | |
| Fmoc-F5-Phe/Fmoc-F5-Phe-PEG [120] | |
| Fmoc-Leu/Fmoc-Lys [118] | |
| L-Phe/Zn2+ [46] | |
| Phe [29] | hydrogels |
| Lys or Glu/chitosan/αβ-glycerophosphate [40] | |
| β-Ala/chitosan/poly-(γ-glutamic acid) [41] | |
| Cys/silver nitrate [14,42,43] | |
| Fmoc-F5-Phe [91] | |
| Cbz-Trp [101] | |
| N-(4-nitrobenzoyl)-Phe [102] | |
| naphthyl-Phe [103] | |
| naphthalenoxyl-Phe [103] | |
| cinnamoyl-Phe [103] | |
| Pyr-Phe [104] | |
| C12-Glu [107] | |
| POSS-Lys or C12-Lys [115] | |
| Fmoc-Phe/Fmoc-(Nε)-Lys [119] | |
| Fmoc-γ-Phe/Fmoc-(3-hydroxy)-γ-Phe [121] | |
| (NDI)-Ser/NDI-Lys [122] | |
| Fmoc-Phe/BPE [129] | |
| Fmoc-Tyr/GMP/Ag+ [132] | |
| Fmoc-Phe/AZT [133] | |
| Fmoc-Phe/GO [135] | |
| Fc-Phe/GO [136] | |
| Fmoc-Phe/PNAI [137] | |
| Fmoc-His or Fmoc-Pro or Fmoc-Ala or Fmoc-Leu/Ag+ [144] | |
| Fmoc-Phe/Ag+ [145] | |
| Fmoc-Phe/nHAP/CGA [146] | |
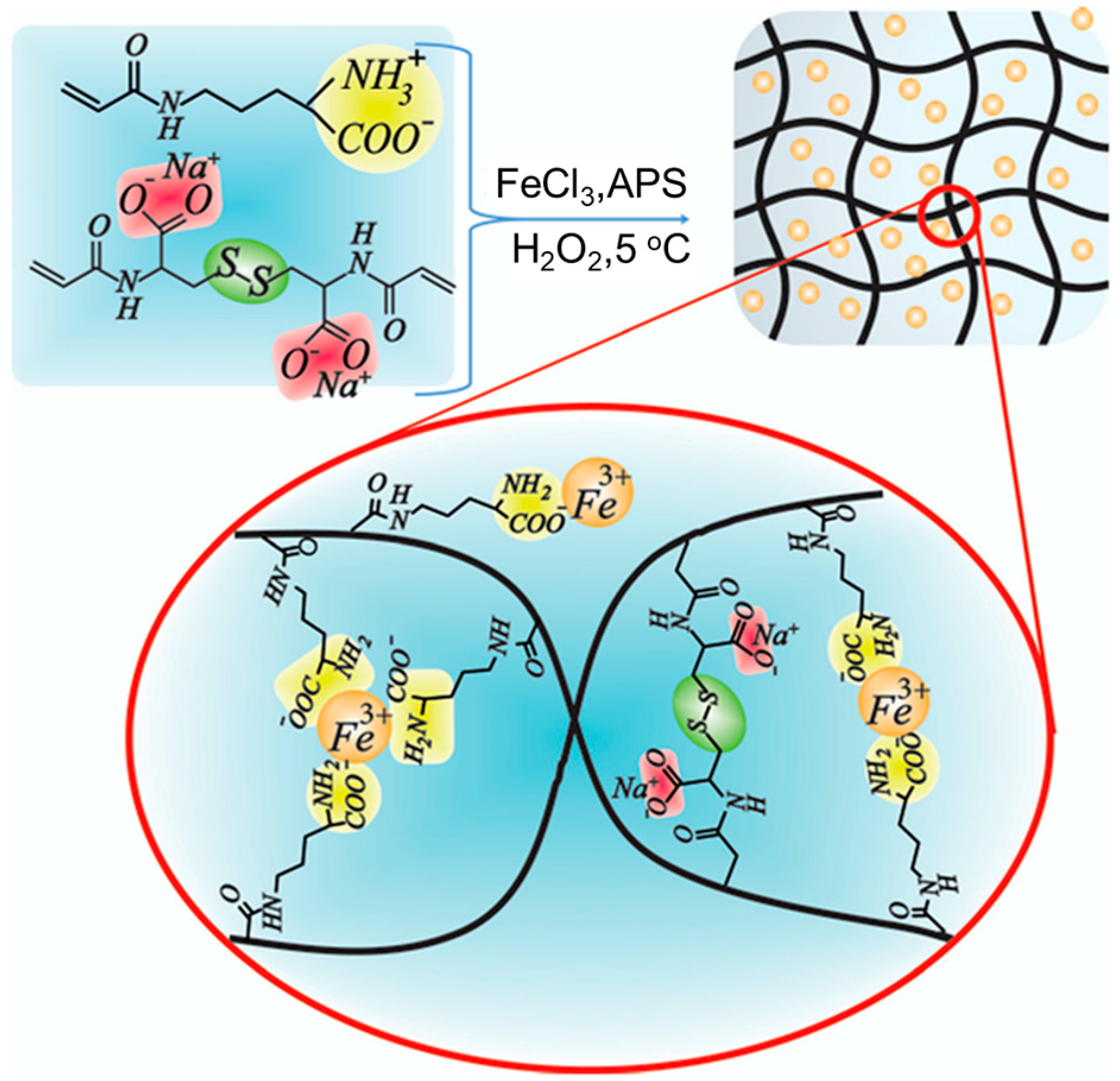
| Ac-Orn/N-N’-bisacryloylcystine/Fe3+ [149] | |
| DL-Phe [30] | 2D plate-like structures |
| DL-mixed Phe or Trp [31] | |
| Phe/Met [32] | |
| Phe/Co2+ and Pb2+ [49] | |
| Fmoc-Nphe [89] | |
| Phe/Ile [32] Carboxyl-protected (methyl ester) Phe/[PW11O39{Sn(C6H4)CuC(C6H4)COOH}]4- [60] C24-Cys or C24-Val [110] | spherical nanostructures |
| TA/Lys or Arg or His [12] | adhesive with crosslinked networks |
| His/Zn2+ [44] | metallo-hydrogel with nanofiber structures |
| Phe/Cu2+ [45] | |
| Py-Phe/Cu2+ [140] | metallo-hydrogel with sheet-like structures |
| Im-Trp/Ni2+ [141] | |
| Fmoc-Val/Zn2+ or Cu2+ [143] | metallo-hydrogel |
| Phe/Ga3+ or In3+ [48] | vesicular structures |
| Arg or Lys or His or Glu or Asp or Leu or Ala or Phe/[EuW10O36]9- [73] | |
| Fmoc-Phe/Ag+ [16] | |
| C12BZ-Val [106] | |
| Phe/Cd2+ or Zn2+ [49] | needle-like structures |
| Gly or Pro/Cu2+/[BW12O40]5- [61] | three-dimensional open frameworks |
| His/H4SiW12O40 (SiW) [79] | coacervate |
| C12BZ-Val [106] | tubules, straps, double helix ropes, and rod-like nanostructures |
| Fmoc-L-Ala-C17/Fmoc-D-Ala-C17 [114] | twisted ribbons |
| perylene-functionalized Phe/4,4’-bipyridine [130] | luminescent gels |
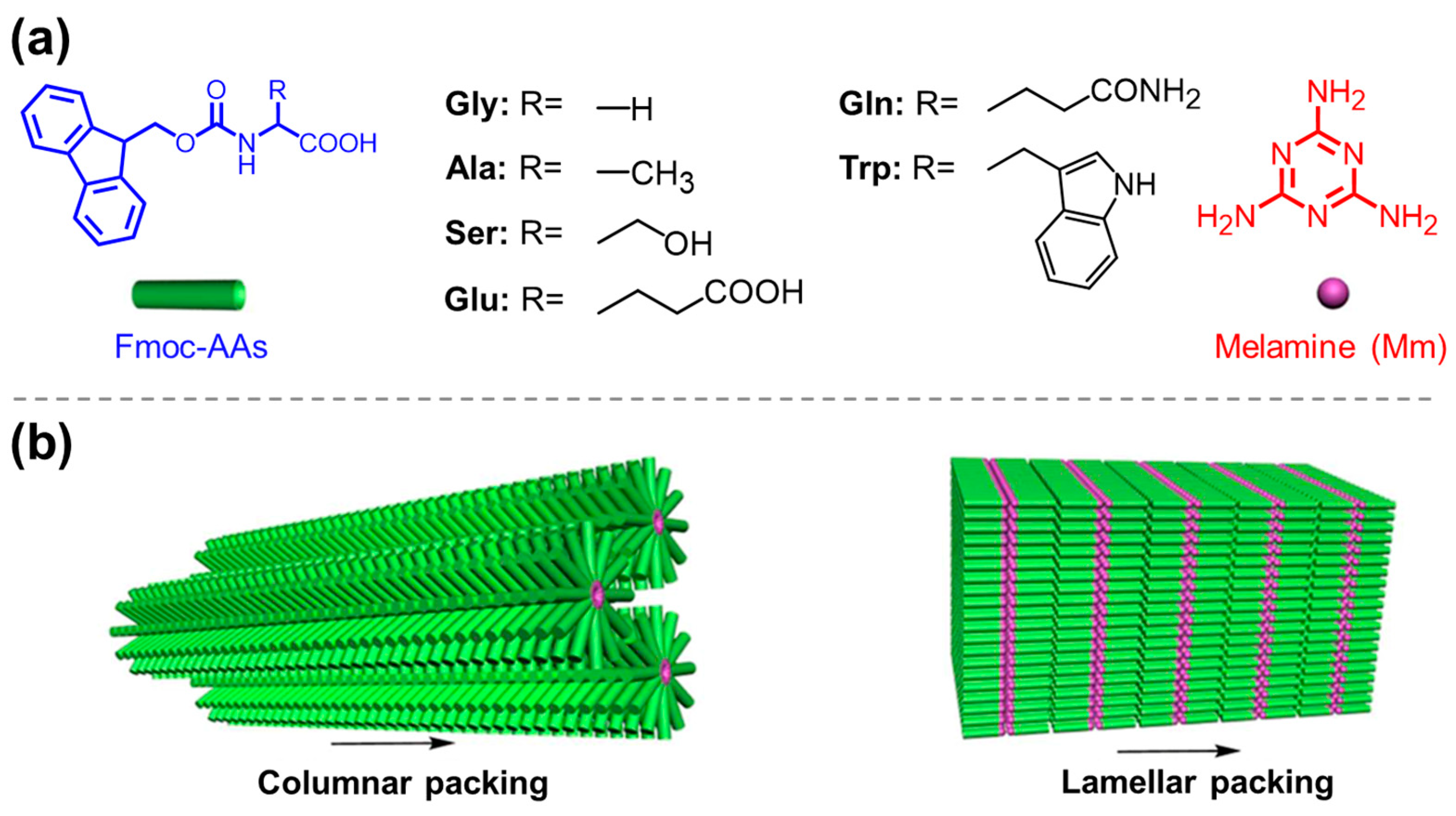
| N,N’-divaline-3,4,9,10-perylenetetracarboxylic acid/riboflavin/Mm [131] | |
| Fmoc-Leu/Co2+ or Cu2+ or Zn2+ [147] | bioglass with amorphous structures |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, S.; Zhao, H.; Sun, J.; Liu, Q.; Cui, Y.; Li, W. Amino Acid-Derived Supramolecular Assembly and Soft Materials. Molecules 2024, 29, 4705. https://doi.org/10.3390/molecules29194705
Nie S, Zhao H, Sun J, Liu Q, Cui Y, Li W. Amino Acid-Derived Supramolecular Assembly and Soft Materials. Molecules. 2024; 29(19):4705. https://doi.org/10.3390/molecules29194705
Chicago/Turabian StyleNie, Shuaishuai, He Zhao, Jiayi Sun, Qingtao Liu, Yongming Cui, and Wen Li. 2024. "Amino Acid-Derived Supramolecular Assembly and Soft Materials" Molecules 29, no. 19: 4705. https://doi.org/10.3390/molecules29194705
APA StyleNie, S., Zhao, H., Sun, J., Liu, Q., Cui, Y., & Li, W. (2024). Amino Acid-Derived Supramolecular Assembly and Soft Materials. Molecules, 29(19), 4705. https://doi.org/10.3390/molecules29194705






