Enhancing the Photocatalytic Performance for Norfloxacin Degradation by Fabricating S-Scheme ZnO/BiOCl Heterojunction
Abstract
1. Introduction
2. Experimental
2.1. Chemicals and Material Preparation
2.1.1. Chemicals
2.1.2. Preparation of ZnO
2.1.3. Preparation of BiOCl
2.1.4. Preparation of ZnO/BiOCl Composite
2.2. Characterization
2.3. The ZnO:BiOCl Ratio First Optimized
2.4. Photoelectrochemical Performance
3. Results and Discussion
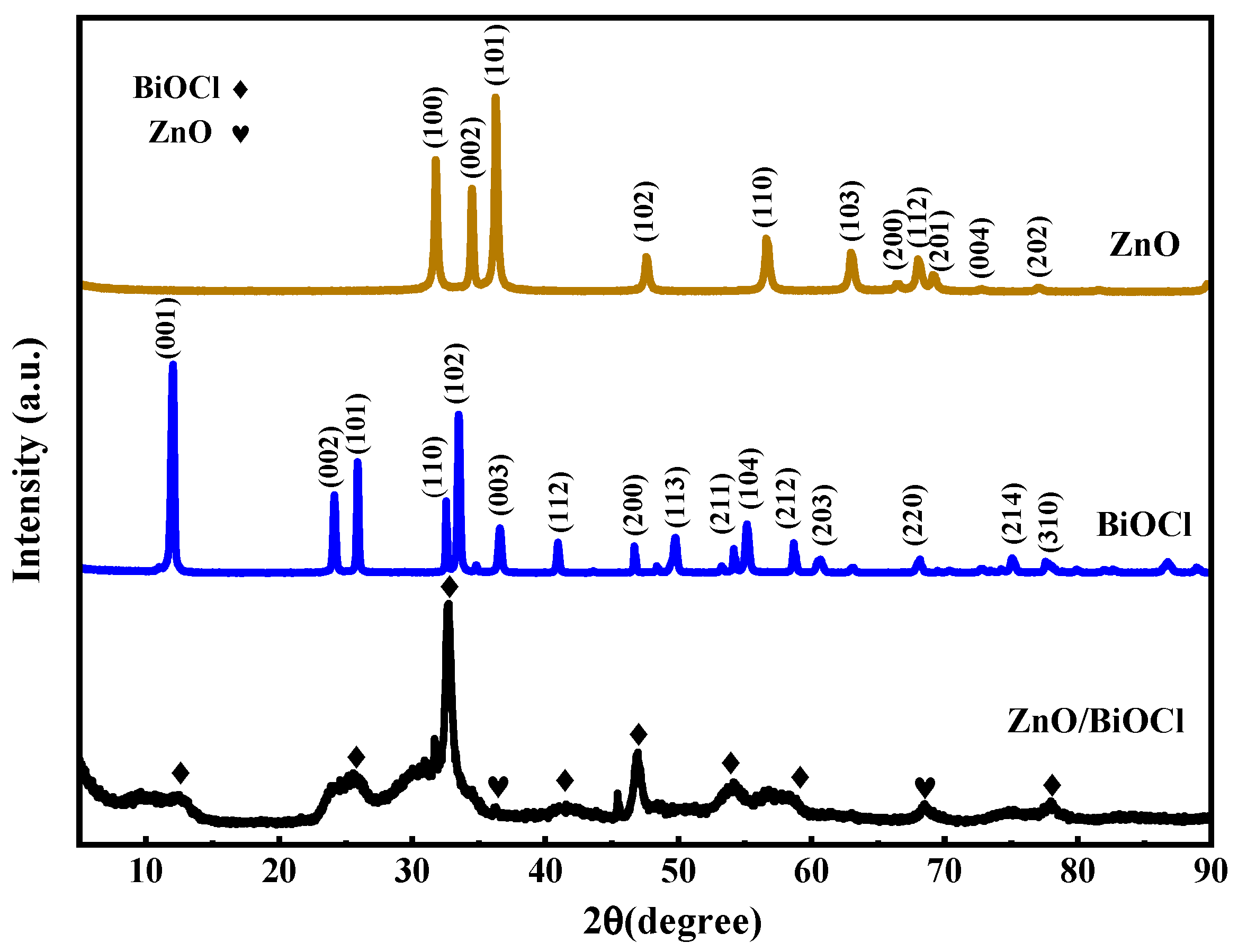
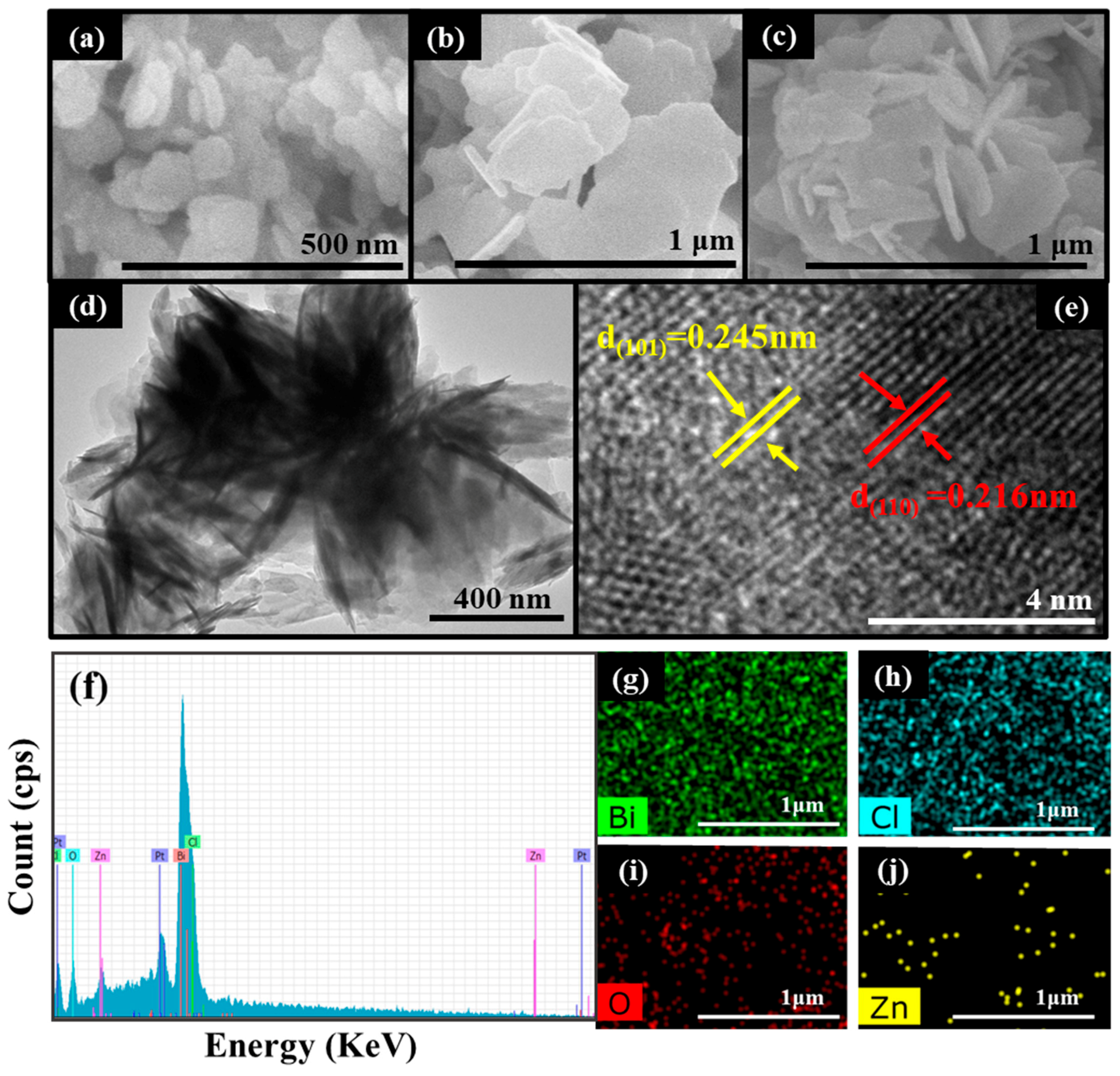
3.1. Structure, Composition and Morphology
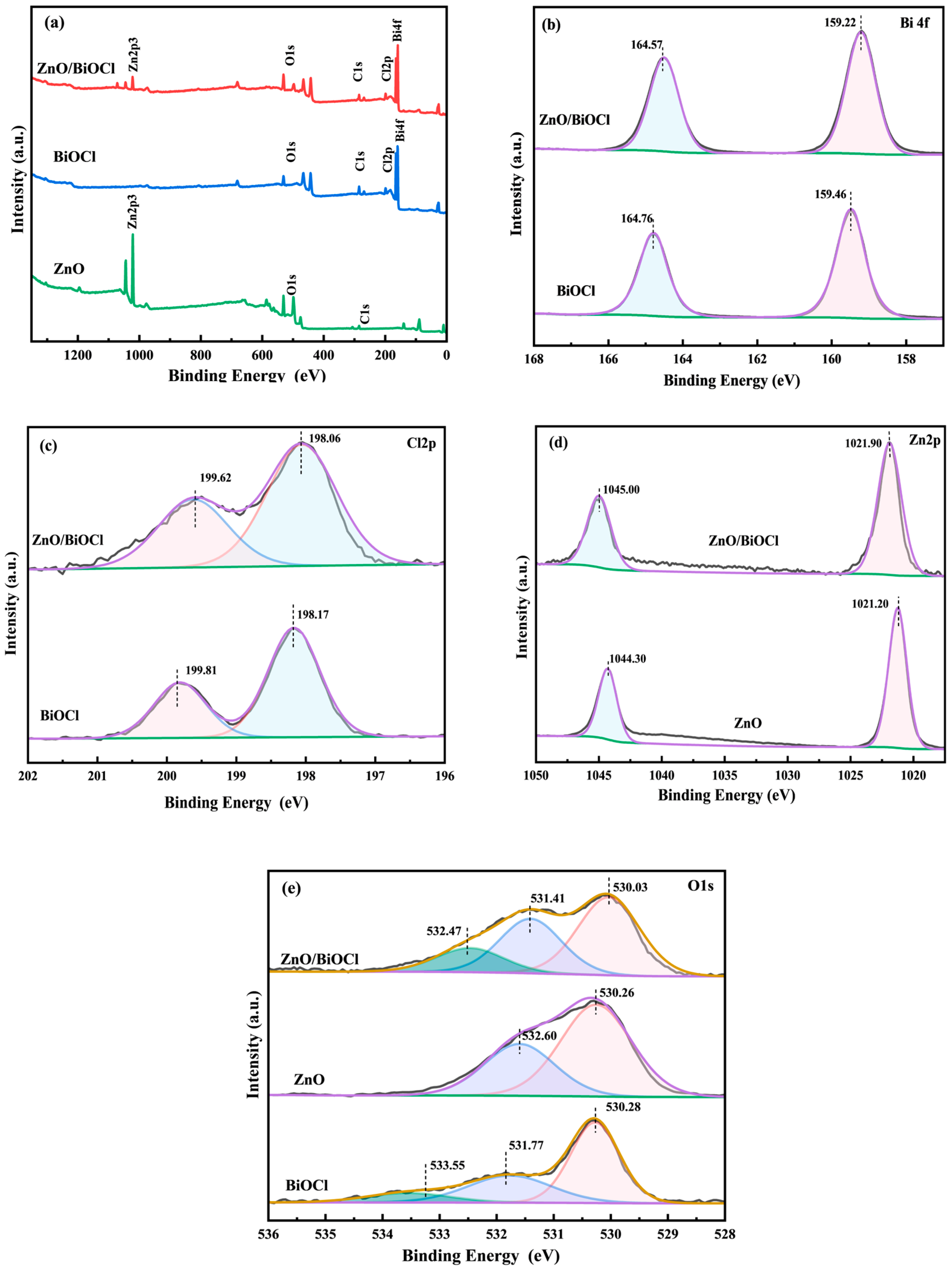
3.2. XPS Analysis
3.3. Optical Properties
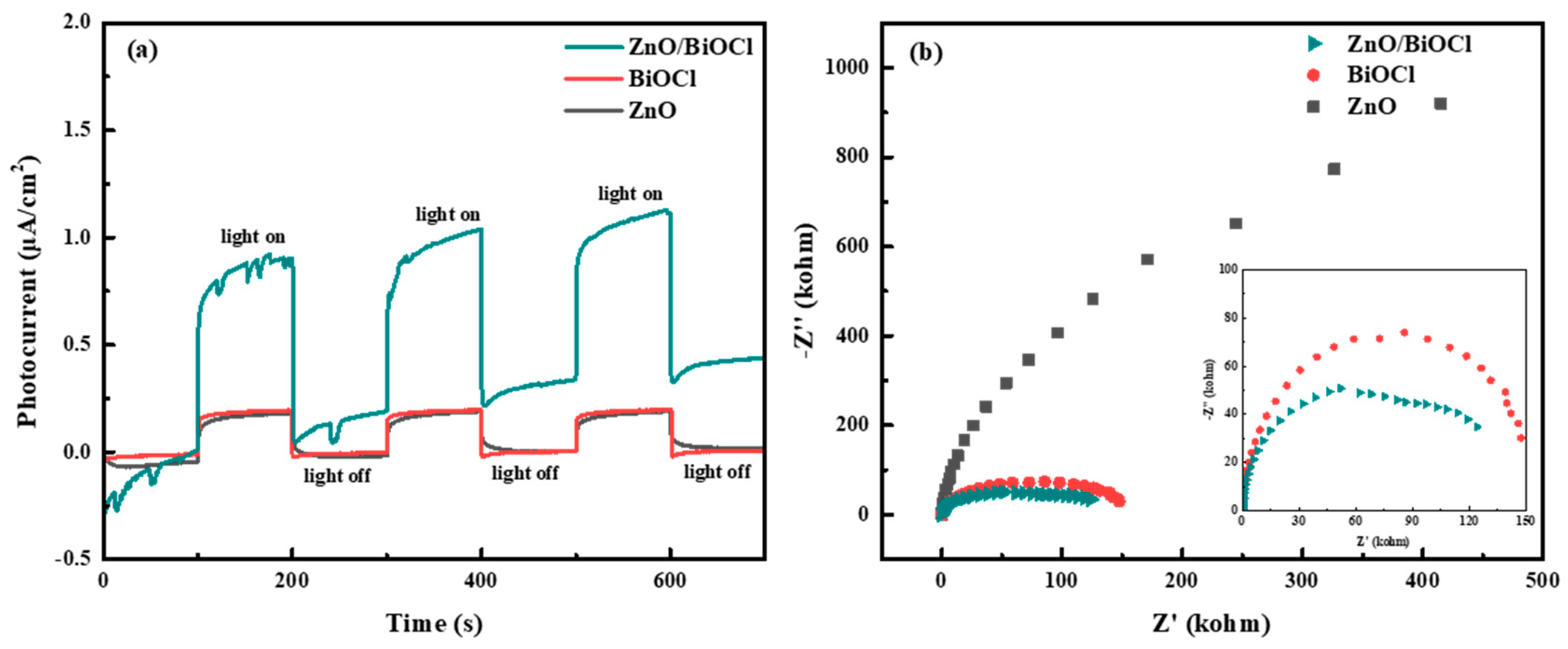
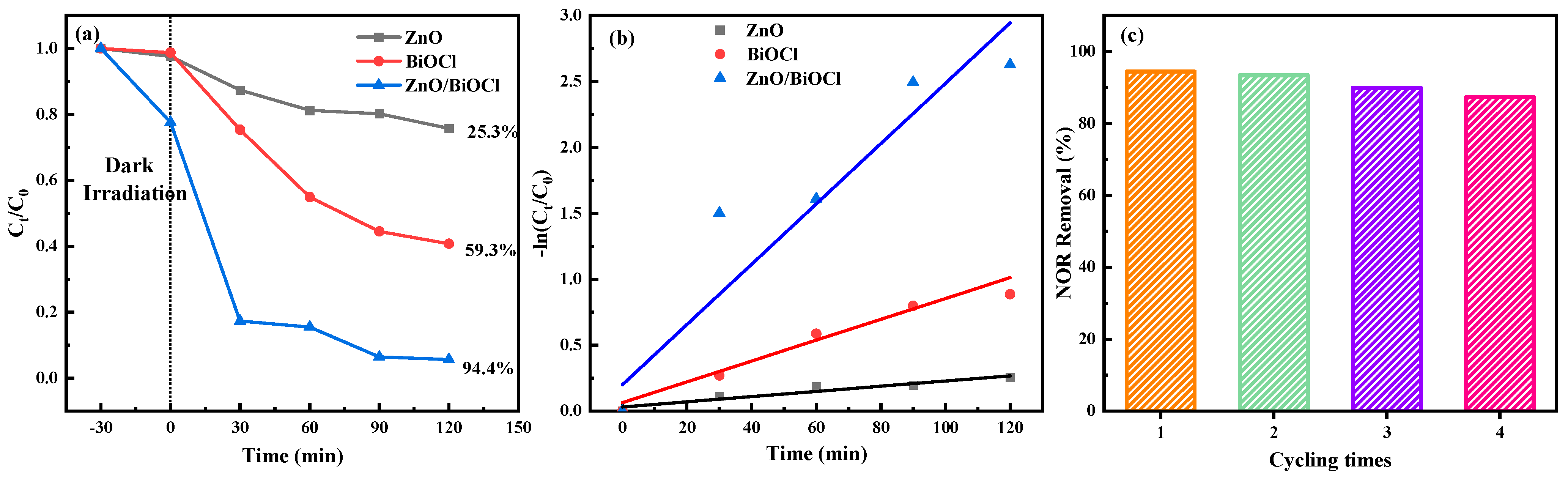
3.4. Photoelectric Performance and Photocatalytic Activity
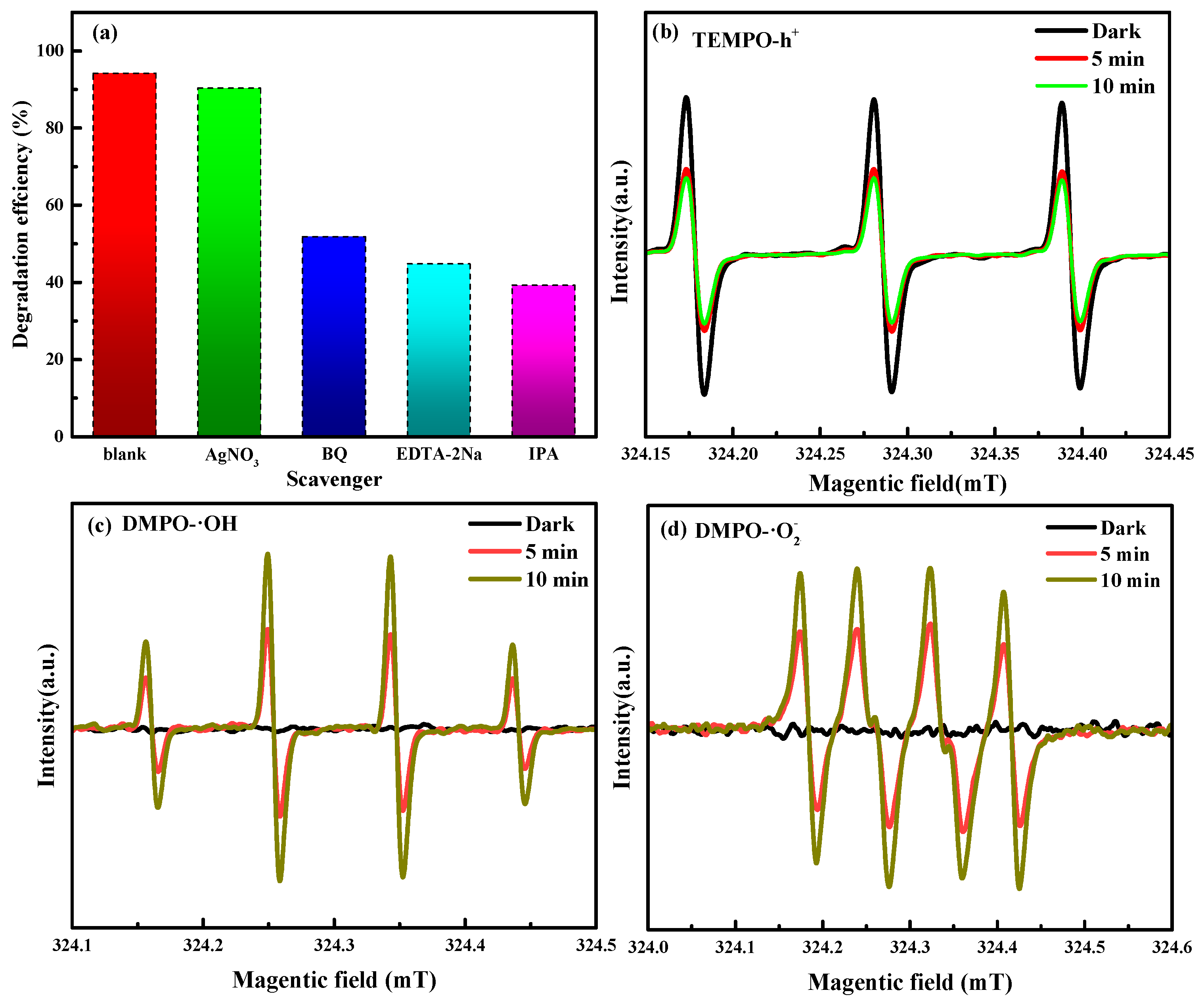
3.5. Photocatalytic Degradation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.; Gan, W.; Guo, J.; Lu, Y.; Ding, S.; Liu, R.; Zhang, M.; Sun, Z. Internal electric field and oxygen vacancies synergistically boost S-scheme VO/BiOCl-TiO2 heterojunction film for photocatalytic degradation of norfloxacin. Chem. Eng. J. 2024, 489, 151260. [Google Scholar] [CrossRef]
- Yang, Q.; Tan, G.; Yin, L.; Liu, W.; Zhang, B.; Feng, S.; Bi, Y.; Liu, Y.; Liu, T.; Wang, Z.; et al. Full-spectrum broad-spectrum degradation of antibiotics by BiVO4@BiOCl crystal plane S-type and Z-type heterojunctions. Chem. Eng. J. 2023, 467, 143450. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, H.; Jin, D.; Yang, H.; He, D.; Zhang, Z.; Zhang, Y.; Qu, J.; Zhang, Y. Effective degradation of norfloxacin on Ag3PO4/CNTs photoanode: Z-scheme mechanism, reaction pathway, and toxicity assessment. Chem. Eng. J. 2022, 429, 132092–132104. [Google Scholar] [CrossRef]
- Zhuang, Y.; Luan, J. Improved photocatalytic property of peony-like InOOH for degrading norfloxacin. Chem. Eng. J. 2020, 382, 122770. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J. Degradation of norfloxacin in aqueous solution by ionizing irradiation: Kinetics, pathway and biological toxicity. Chem. Eng. J. 2020, 395, 125095. [Google Scholar] [CrossRef]
- Amorim, C.L.; Moreira, I.; Maia, A.S.; Tiritan, M.E.; Castro, P.M.L. Biodegradation of ofloxacin, norfloxacin, and ciprofloxacin as single and mixed substrates by Labrys portucalensis F11. Appl. Microbiol. Biotechnol. 2014, 98, 3181–3190. [Google Scholar] [CrossRef]
- Santos, L.V.d.S.; Teixeira, D.C.; Jacob, R.S.; Amaral, M.C.S.D.; Lange, L.C. Evaluation of the aerobic and anaerobic biodegradability of the antibiotic norfloxacin. Water Sci. Technol. 2014, 70, 265–271. [Google Scholar] [CrossRef]
- Isari, A.A.; Mehregan, M.; Mehregan, S.; Hayati, F.; Kalantary, R.R.; Kakavandi, B. Sono-photocatalytic degradation of tetracycline and pharmaceutical wastewater using WO3/CNT heterojunction nanocomposite under US and visible light irradiations, A novel hybrid system. J. Hazard. Mater. 2020, 390, 122050–122063. [Google Scholar] [CrossRef]
- Dong, Y.P.; Ji, P.Z.; Xu, X.Y.; Li, R.; Wang, Y.; Homewood, K.P.; Xia, X.H.; Gao, Y.; Chen, X.X. Rational Design and Construction of a CdS QDs/InVO4 Atomic-Layer (110)/(110) Facet S-Scheme Heterojunction for Highly Efficient Photocatalytic Degradation of C2H4. Energy. Environ. Mater. 2023, 7, e12643. [Google Scholar] [CrossRef]
- Fan, J.; Bai, M.; Zhang, H.; Chu, Y. Synthesis and immobilization of HC/BiVO4 catalyst particles with PTFE for photocatalytic tetracycline degradation: Preparation, performance and mechanism. J. Water Process. Eng. 2023, 56, 104474. [Google Scholar] [CrossRef]
- Li, Q.H.; Wang, M.; He, J.Y.; Wang, Y.W. In situ synthesis of core-shell like BiVO4/BiOCl heterojunction with excellent visible-light photocatalytic activity. Opt. Mater. 2023, 144, 114266. [Google Scholar] [CrossRef]
- Wang, R.; Chu, C.; Wang, L.; Liu, Q. Investigation of the photocatalytic performance of Mg-doped modified BiOCl. Inorg. Chem. Commun. 2023, 157, 111286. [Google Scholar] [CrossRef]
- Repp, S.; Weber, S.; Erdem, E. Defect Evolution of Nonstoichiometric ZnO Quantum Dots. J. Phys. Chem. C 2016, 120, 25124–25130. [Google Scholar] [CrossRef]
- Nguyen, T.X.Q.; Chen, S.S.; Pasawan, M.; Chang, H.M. Enhanced photocatalytic activity of g-C3N4–n-p type flower-like ZnO/BiOBr heterojunction for hexavalent chromium and dye wastewater degradation. Environ. Technol. Innov. 2023, 31, 103154. [Google Scholar] [CrossRef]
- Jadhav, S.R.; Mohite, S.V.; Lee, C.; Bae, J.; Pedanekar, R.S.; Kim, Y.; Rajpure, K.Y. In-situ synthesized oxygen vacancy filled ZnS/Vo-ZnO heterojunction photocatalysts for efficient H2 production. Sustain. Mater. Technol. 2023, 38, e00731. [Google Scholar] [CrossRef]
- Silva, T.E.; Moreira, A.J.; Nobrega, E.T.; Alencar, R.G.; Rabello, P.T.; Blaskievicz, S.F.; Marques, G.N.; Mascaro, L.H.; Paris, E.C.; Lemos, S.G.; et al. Hierarchical structure of 3D ZnO experimentally designed to achieve high performance in the sertraline photocatalysis in natural waters. Chem. Eng. J. 2023, 475, 146235. [Google Scholar] [CrossRef]
- Liu, R.; Fu, X.; Guo, Y.; Zhang, J.; Tian, W. A study on Ag or Ce doped and co-doped ZnO for the photocatalytic degradation of RhB dye. Vacuum 2023, 215, 112337. [Google Scholar] [CrossRef]
- Wang, K.; Sun, T.; Ma, H.; Wang, Y.; He, Z.-H.; Wang, H.; Wang, W.; Yang, Y.; Wang, L.; Liu, Z.-T. Energy band modulating of NiO/BiOCl heterojunction with transition from type-II to S-scheme for enhancing photocatalytic CO2 reduction. Chem. Eng. J. 2024, 497, 154711. [Google Scholar] [CrossRef]
- Tang, H.; Hengchao, E.; Yao, C.; Wang, X.; Zhou, J.; Song, W.; Zhang, Z. Boosted antibiotic elimination over 2D/2D mesoporous CeO2/BiOCl S-scheme photocatalyst. Sep. Purif. Technol. 2025, 354, 128977. [Google Scholar] [CrossRef]
- Wang, Z.; Yue, X.; Xiang, Q. MOFs-based S-scheme heterojunction photocatalysts. Co-ord. Chem. Rev. 2024, 504, 215674. [Google Scholar] [CrossRef]
- Długosz, O.; Szostak, K.; Matysik, J.; Matyjasik, W.; Banach, M. Fabrication of ZnO/Ag and ZnO/Fe nanoparticles with immobilised peroxidase as a biocatalyst for photodegradation of ibuprofen. Sustain. Mater. Technol. 2023, 38, e00763. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, L.; Tu, X.; Hu, F. Hydrothermal synthesis of ZnO Crystals: Diverse morphologies and characterization of the photocatalytic properties. Polyhedron 2023, 246, 116668. [Google Scholar] [CrossRef]
- Yang, X.; Sun, S.; Cui, J.; Yang, M.; Yang, Q.; Xiao, P.; Liang, S. One-pot construction of robust BiOCl/ZnO p–n heterojunctions with semi-coherent interfaces toward improving charge separation for photodegradation enhancement. Nanoscale Adv. 2021, 3, 4851–48577. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Liu, Y.; Wang, M.; Wang, Y.; Long, F. Ionic liquid-hydrothermal synthesis of Z-scheme BiOBr/Bi2WO6 heterojunction with enhanced photocatalytic activity. J. Alloys Compd. 2021, 865, 158760. [Google Scholar] [CrossRef]
- Chen, M.X.; Dai, Y.Z.; Guo, J.; Yang, H.T.; Liu, D.N.; Zhai, Y.L. Solvothermal synthesis of biochar@ZnFe2O4/BiOBr Z-scheme heterojunction for efficient photocatalytic ciprofloxacin degradation under visible light. Appl. Surf. Sci. 2019, 493, 1361–1367. [Google Scholar] [CrossRef]
- Le Minh Tri, N.; Cam, N.T.D.; Pham, H.D.; Van Thuan, D.; Pham, T.-D.; Nguyen, V.T.; Trung, N.T.; Tung, M.H.T.; Phuong, T.T.T.; Nguyen, T.T.P.; et al. Development of g-C3N4/BiVO4 binary component heterojunction as an advanced visible light-responded photocatalyst for polluted antibiotics degradation. Top. Catal. 2020, 63, 1206–1214. [Google Scholar] [CrossRef]
- Huang, L.Y.; Liu, J.W.; Li, P.P.; Li, Y.P.; Wang, C.B.; Shu, S.X.; Song, Y.H. CQDs modulating Z-scheme g-C3N4/BiOBr heterostructure for photocatalytic removing RhB, BPA and TC and E. coli by LED light. J. Alloys Compd. 2022, 895, 162637. [Google Scholar] [CrossRef]
- Zhang, F.; Xiao, X.; Xiao, Y. In situ fabrication of type II 3D hierarchical flower-like BiOBr/Bi3O4Br heterojunction with improved photocatalytic activity. J. Alloys Compd. 2022, 923, 166417. [Google Scholar] [CrossRef]
- Zhang, J.W.; Shan, L.W.; Xu, H.Y.; Fang, Z.L.; Wu, H.T.; Li, D.; Dong, L.M.; Cheng, C.; Suriyaprakash, J.; Zhang, F.M. Multiscale understand the tuning photocatalytic hydrogen evolution performances of BiOCl stemmed from engineered crystal facet. Appl. Surf. Sci. 2024, 652, 159321. [Google Scholar] [CrossRef]
- Xu, H.; Yang, J.; Li, Y.; Fu, F.; Da, K.; Cao, S.; Chen, W.; Fan, X. Fabrication of Bi2O3 QDs decorated TiO2/BiOBr dual Z-scheme photocatalysts for efficient degradation of gaseous toluene under visible-light. J. Alloys Compd. 2023, 950, 169959. [Google Scholar] [CrossRef]
- Zhu, J.; Shen, Y.; Yu, X.; Guo, J.; Zhu, Y.; Zhang, Y. A facile two-step method to synthesize immobilized CdS/BiOCl film photocatalysts with enhanced photocatalytic activities. J. Alloys Compd. 2019, 771, 309–316. [Google Scholar] [CrossRef]
- Pinder, J.W.; Major, G.H.; Baer, D.R.; Terry, J.; Whitten, J.E.; Čechal, J.; Crossman, J.D.; Lizarbe, A.J.; Jafari, S.; Easton, C.D.; et al. Avoiding common errors in X-ray photoelectron spectroscopy data collection and analysis, and properly reporting instrument parameters. Appl. Surf. Sci. Adv. 2024, 19, 100534. [Google Scholar] [CrossRef]
- Lü, Z.; Cheng, Y.; Xue, L.; Wang, H.; Lin, H.; Sun, X.; Miao, Z.; Zhuo, S.; Zhou, J. MCr–LDHs/BiOBr heterojunction nanocomposites for efficient photocatalytic removal of organic pollutants under visible-light irradiation. J. Alloys Compd. 2022, 898, 162871. [Google Scholar] [CrossRef]
- Sharma, A.; Kanth, S.K.; Xu, S.; Han, N.; Zhu, L.; Fan, L.; Liu, C.; Zhang, Q. Visible light driven g-C3N4/Bi4NbO8X ([X] = Cl, Br) heterojunction photocatalyst for the degradation of organic pollutants. J. Alloys Compd. 2022, 895, 162576. [Google Scholar] [CrossRef]
- Hu, C.; Zhuang, J.; Zhong, L.; Zhong, Y.; Wang, D.; Zhou, H. Significantly enhanced photocatalytic activity of visible light responsive AgBr/Bi2Sn2O7 heterostructured composites. Appl. Surf. Sci. 2017, 426, 1173–1181. [Google Scholar] [CrossRef]
- Zhou, H.; Zhong, S.; Shen, M.; Hou, J.; Chen, W. Formamide-assisted one-pot synthesis of a Bi/Bi2O2CO3 heterojunction photocatalyst with enhanced photocatalytic activity. J. Alloys Compd. 2018, 769, 301–310. [Google Scholar] [CrossRef]
- Liang, C.; Guo, H.; Zhang, L.; Ruan, M.; Niu, C.G.; Feng, H.P.; Wen, X.J.; Tang, N.; Liu, H.Y.; Zeng, G.M. Boosting molecular oxygen activation ability in self-assembled plasmonic p-n semiconductor photocatalytic heterojunction of WO3/Ag@ Ag2O. Chem. Eng. J. 2019, 372, 12–25. [Google Scholar] [CrossRef]
- Huang, H.; Xiao, K.; Zhang, T.; Dong, F.; Zhang, Y. Rational design on 3D hierarchical bismuth oxyiodides via in situ self-template phase transformation and phase-junction construction for optimizing photocatalysis against diverse contaminants. Appl. Catal. B Environ. 2017, 203, 879–888. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, Z.; Peng, Y.; Yang, S.; Zhang, Y. Modulation of single-band red upconversion luminescence in Er3+/Yb3+ codoped bismuth oxyiodide nanoplates by bandgap engineering and their application in NIR photocatalysis. Ceram. Int. 2020, 46, 6351–6359. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, X.Y.; Chen, J.Y.; Zeng, X.Y. AgBr and GO co-decorated g-C3N4/Ag2WO4 composite for enhanced photocatalytic activity of contaminants degradation. J. Photoch. Photobio. A 2019, 382, 111957. [Google Scholar] [CrossRef]
- Zhang, J.W.; Xu, H.Y.; Zheng, Y.G.; Shen, Y.; Mu, C.Q.; Wang, Y.; Niyazi, A.; He, Z.X.; Zhang, Z.Q.; Zhang, L.; et al. Visible light photocatalytic degradation of oxytetracycline hydrochloride using chitosan-loaded Z-scheme heterostructured material BiOCOOH/O-gC3N4. Int. J. Biol. Macromol. 2024, 275, 133373. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, W.; Niu, Z.; Zhang, Y. Improvement of photocatalytic activity of BiOBr and BiOBr/ZnO under visible-light irradiation by short-time low temperature plasma treatment. J. Alloys Compd. 2022, 924, 166608. [Google Scholar] [CrossRef]
- Xu, Q.; Wageh, S.; Al-Ghamdi, A.A.; Li, X. Design principle of S-scheme heterojunction photocatalyst. J. Mater. Sci. Technol. 2022, 124, 171–173. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Wei, X.; Alothman, Z.A.; Xu, X. Direct Z-scheme CuInS2/Bi2MoO6 heterostructure for enhanced photocatalytic degradation of tetracycline under visible light. J. Hazard. Mater. 2021, 415, 125591. [Google Scholar] [CrossRef]
- Liu, C.; Mao, S.; Shi, M.; Wang, F.; Xia, M.; Chen, Q.; Ju, X. Peroxymonosulfate activation through 2D/2D Z-scheme CoAl-LDH/BiOBr photocatalyst under visible light for ciprofloxacin degradation. J. Hazard. Mater. 2021, 420, 126613. [Google Scholar] [CrossRef]
- Wang, J.; Fang, Y.; Zhang, W.; Yu, X.; Wang, L.; Zhang, Y. TiO2/BiOBr 2D–2D heterostructure via in-situ approach for enhanced visible-light photocatalytic N2 fixation. Appl. Surf. Sci. 2021, 567, 150623. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, P.; Li, H.; Huang, R.; Hu, B.; Liu, Y.; Li, T. Bimetallic-atom-hybridization-driven catalytic reaction kinetics and solar utilization to accelerate norfloxacin degradation. Appl. Catal. B Environ. 2021, 298, 120525. [Google Scholar] [CrossRef]
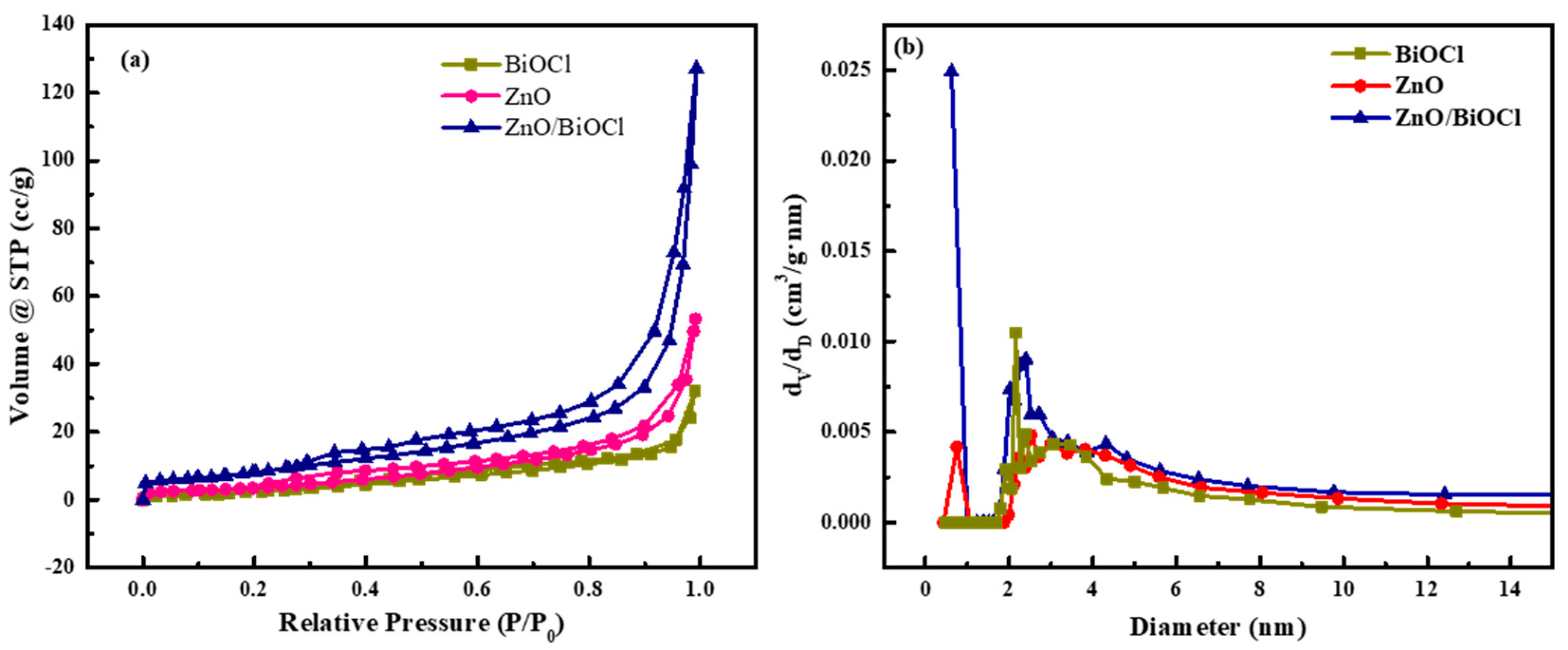


| Sample | Surface Area (m2/g) | Total Pore Volumes (cc/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| ZnO | 34.573 | 0.089 | 2.518 |
| BiOCl | 21.646 | 0.054 | 2.158 |
| ZnO/BiOCl | 115.912 | 0.212 | 0.619 |
| Sample | Eg (eV) | ECB vs. NHE (eV) | EVB vs. NHE (eV) | Ef vs. NHE (eV) |
|---|---|---|---|---|
| ZnO | 3.15 | −0.46 | 2.69 | −0.26 |
| BiOCl | 3.42 | −0.88 | 2.54 | −0.68 |
| Catalysts | Pollutants | Light Source | Reaction Time (min) | Removal Efficiency | References |
|---|---|---|---|---|---|
| BiVO4@BiOCl | TC | Vis/NIR | 180 | 90.32% | [2] |
| g-C3N4 ZnO/BiOBr | Cr(Ⅵ) | UV light | 180 | 96% | [14] |
| MO | UV light | 130 | 100% | ||
| ZnO/Ag ZnO/Fe | Ibuprofen | Vis | 120 | 86% | [21] |
| BiOCOOH/O-gC3N4 | NOR | Vis | 90 | 82% | [41] |
| BiOBr/ZnO | MO | Vis | 120 | 76% | [42] |
| ZnO/BiOCl | NOR | Sunlight | 120 | 94.40% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Lu, G.; Liang, H.; Li, Z.; Liang, J.; Chen, Z. Enhancing the Photocatalytic Performance for Norfloxacin Degradation by Fabricating S-Scheme ZnO/BiOCl Heterojunction. Molecules 2024, 29, 4738. https://doi.org/10.3390/molecules29194738
Yang R, Lu G, Liang H, Li Z, Liang J, Chen Z. Enhancing the Photocatalytic Performance for Norfloxacin Degradation by Fabricating S-Scheme ZnO/BiOCl Heterojunction. Molecules. 2024; 29(19):4738. https://doi.org/10.3390/molecules29194738
Chicago/Turabian StyleYang, Rongpeng, Guang Lu, Hongyu Liang, Zheng Li, Jiling Liang, and Zhen Chen. 2024. "Enhancing the Photocatalytic Performance for Norfloxacin Degradation by Fabricating S-Scheme ZnO/BiOCl Heterojunction" Molecules 29, no. 19: 4738. https://doi.org/10.3390/molecules29194738
APA StyleYang, R., Lu, G., Liang, H., Li, Z., Liang, J., & Chen, Z. (2024). Enhancing the Photocatalytic Performance for Norfloxacin Degradation by Fabricating S-Scheme ZnO/BiOCl Heterojunction. Molecules, 29(19), 4738. https://doi.org/10.3390/molecules29194738






