Chemical Constituents of Halophyte Suaeda glauca and Their Therapeutic Potential for Hair Loss
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of Compounds 1~8 from Suaeda glauca
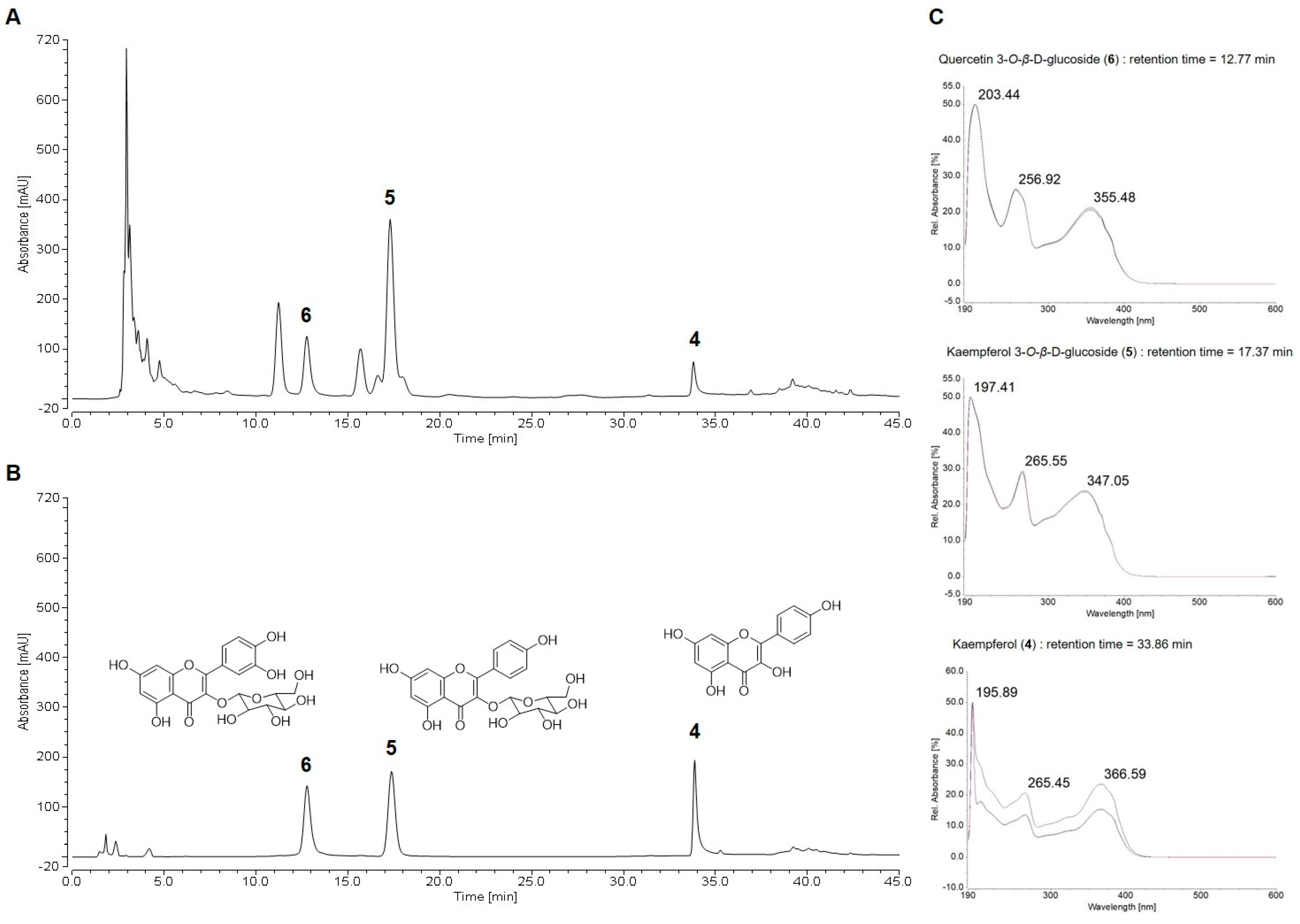
2.2. HPLC Chemical Profile of Suaeda glauca Extract
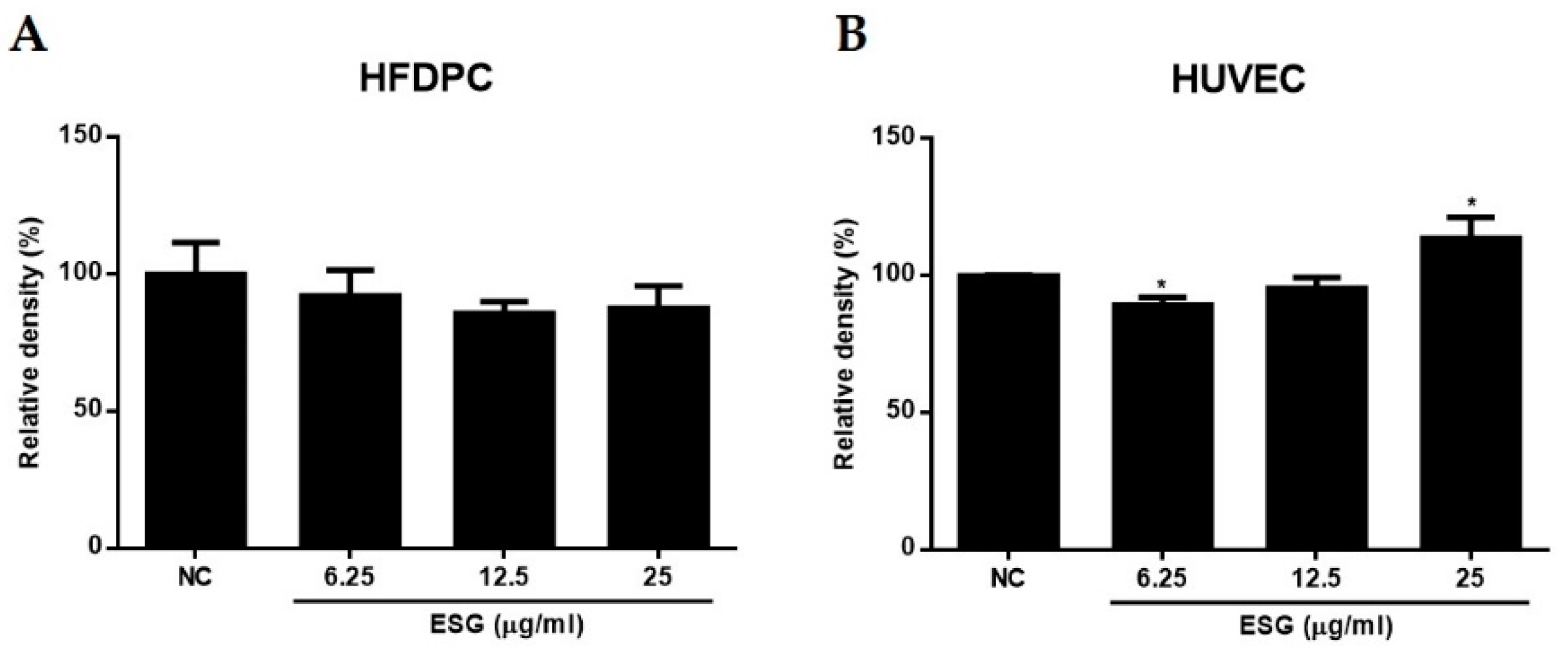
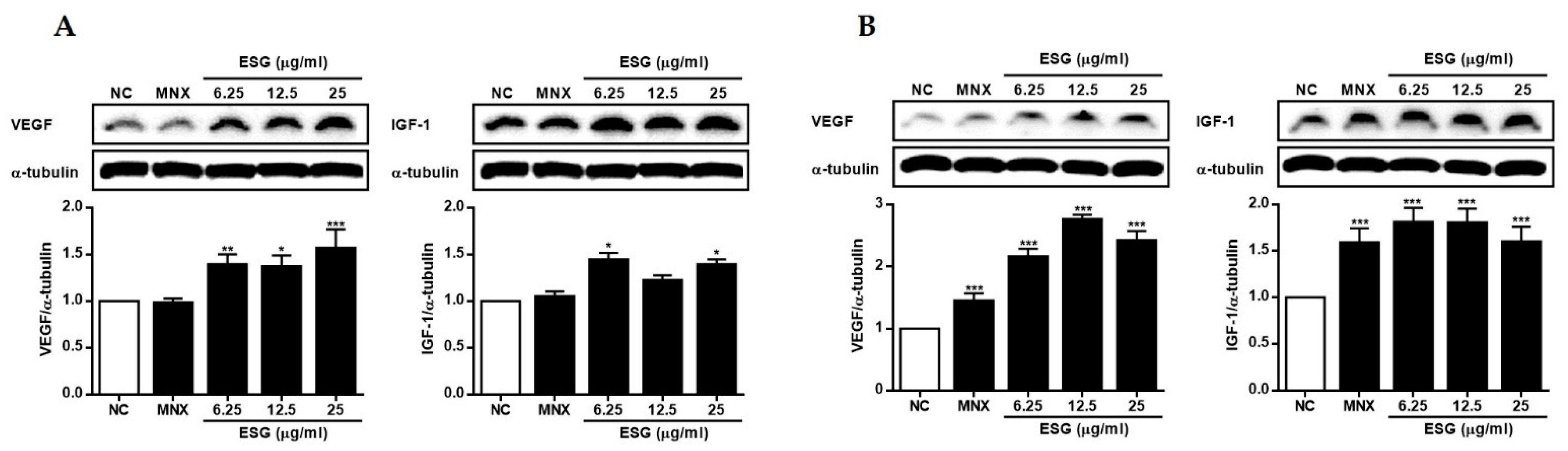
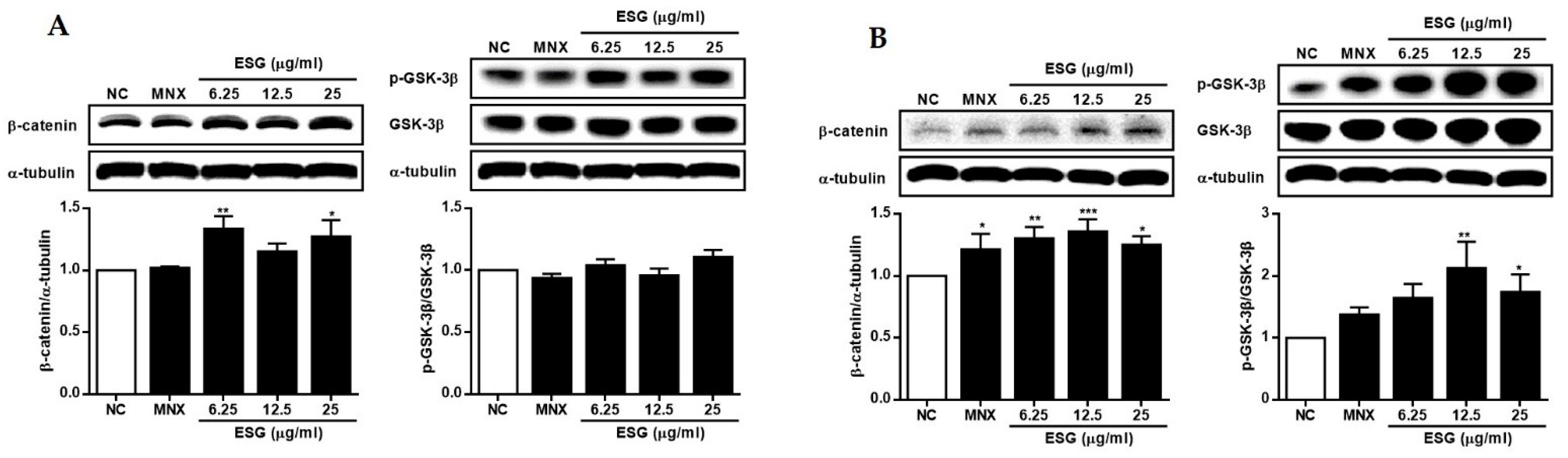
2.3. The Effects of Suaeda glauca Extract on Wnt/β-Catenin Signaling and the Expressions of VEGF, IGF-1, p-GSK-3β in Human Follicle Dermal Papilla Cells (HFDPCs) and Human Umbilical Vein Endothelial Cells (HUVECs)
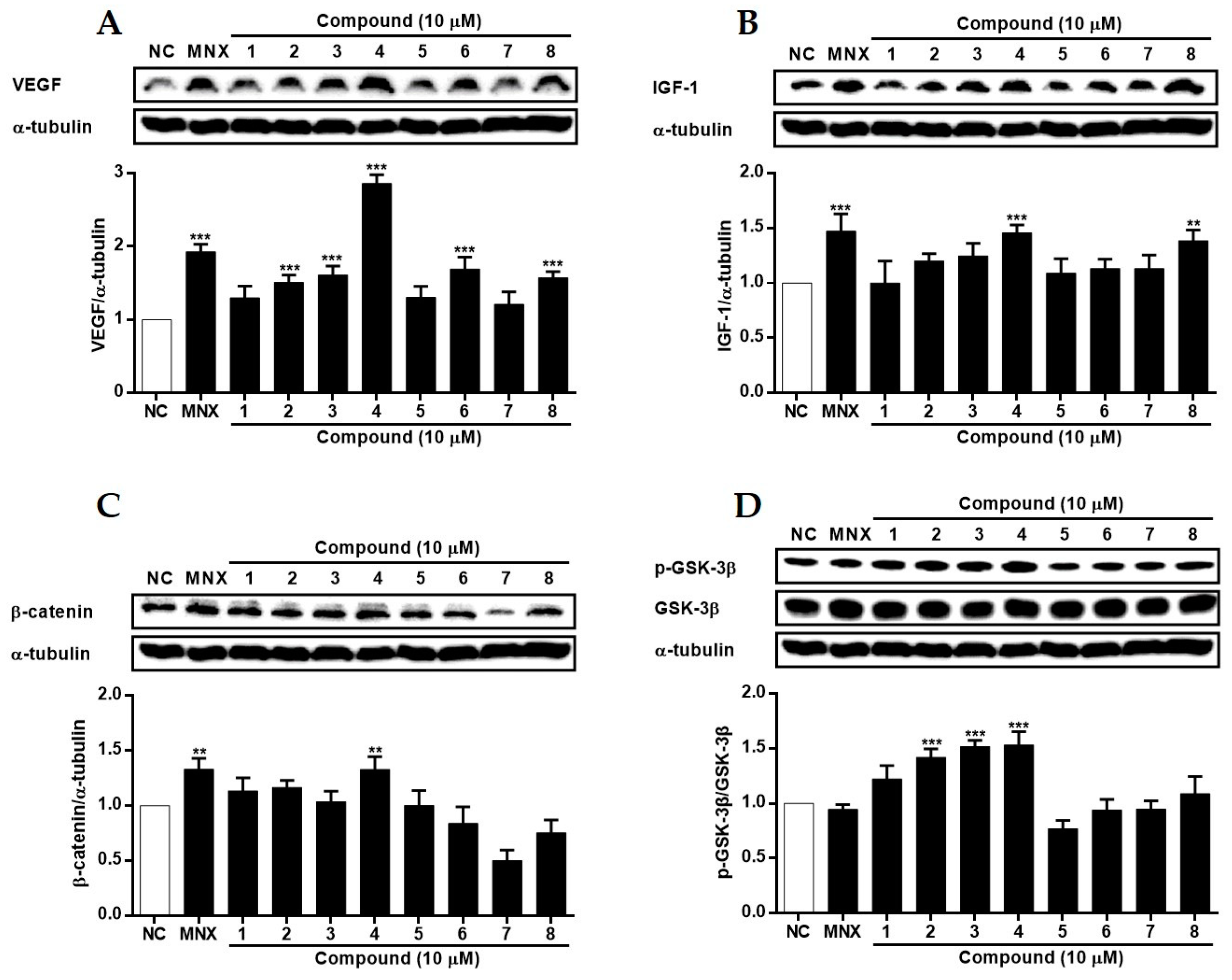
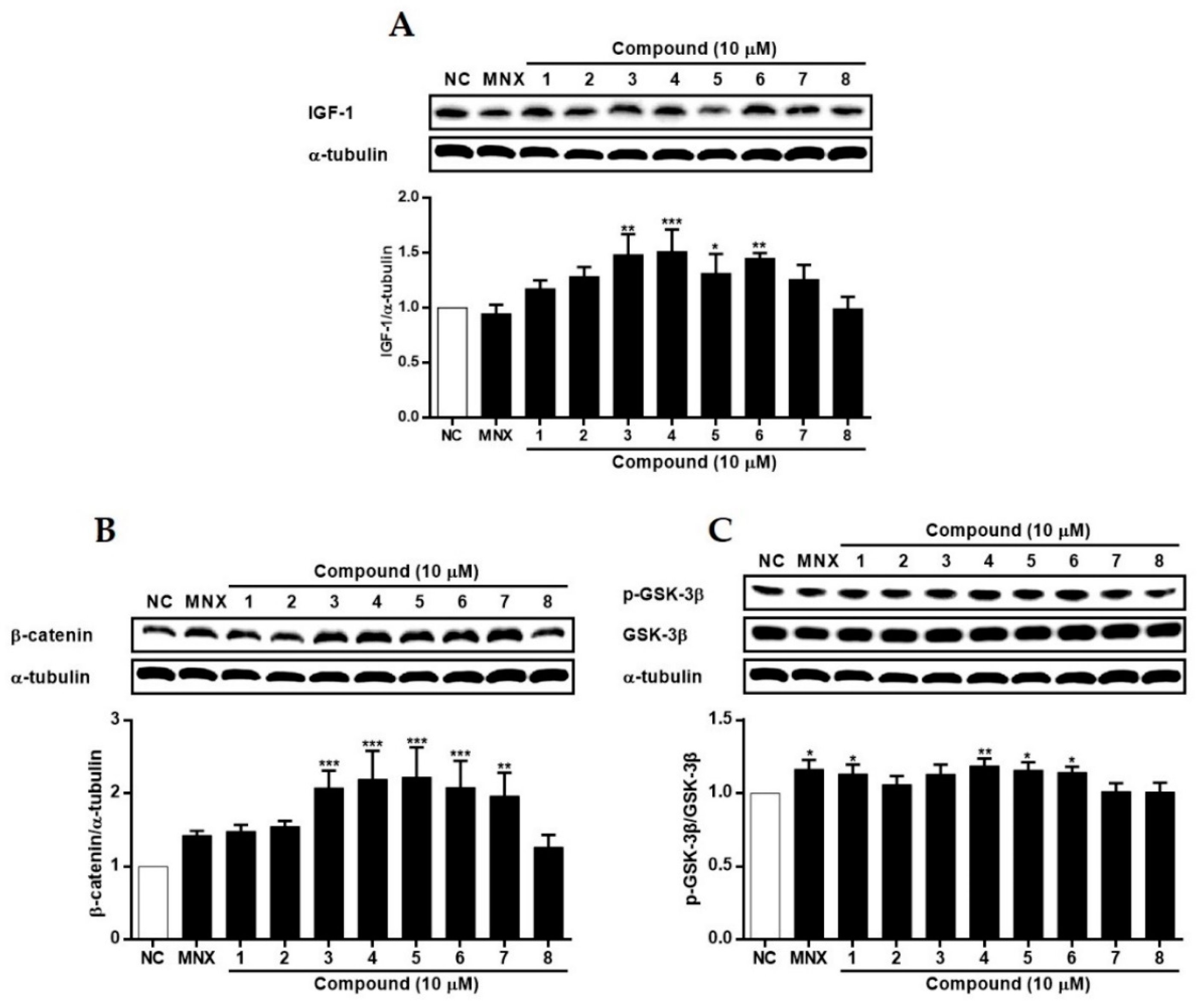
2.4. The Effects of 1~8 Isolated from S. glauca on Wnt/β-Catenin Signaling and the Expressions of VEGF, IGF-1, and p-GSK-3β in Human Follicle Dermal Papilla Cells (HFDPCs) and Human Umbilical Vein Endothelial Cells (HUVECs)
3. Materials and Methods
3.1. Plant Material
3.2. Instrumentation and Reagents
3.3. Extraction and Isolation
3.4. HPLC Chromatographic Conditions
3.5. Cell Cultures
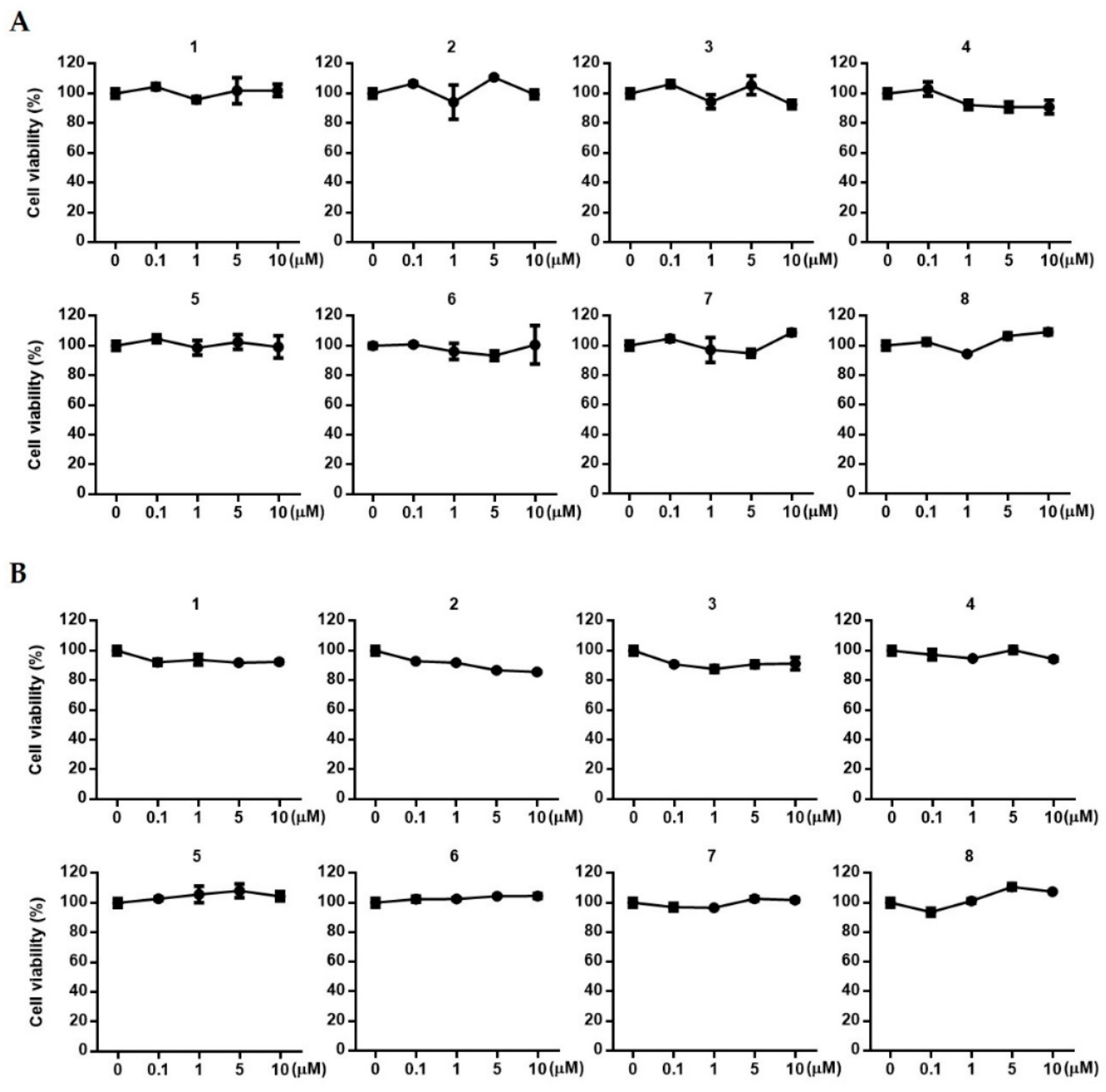
3.6. Estimation of Cell Viability
3.7. Western Blotting
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, R.B.; Sohn, D.H.; Jeong, G.S.; Kim, Y.C. In vitro hepatoprotective compounds from Suaeda glauca. Arch. Pharm. Res. 2008, 31, 594–597. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.X.; Zhong, R.Z.; Liu, H.W.; Wang, M.L.; Sun, J.Y.; Zhou, D.W. Meat quality, fatty acid composition of tissue and gastrointestinal content, and antioxidant status of lamb fed seed of a halophyte (Suaeda glauca). Meat Sci. 2015, 100, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shi, D.; Wang, D. Comparative effects of salt and alkali stresses on growth, osmotic adjustment and ionic balance of an alkali-resistant halophyte Suaeda glauca (Bge.). Plant Growth Reg. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Zhang, A.; Zang, W.; Wei, L.; Yan, X. Integrated proteomics and metabolomics for dissecting the mechanism of global responses to salt and alkali stress in Suaeda corniculata. Plant Soil 2016, 402, 379–394. [Google Scholar] [CrossRef]
- Zhao, K.F.; Fan, H.; Song, J.; Sun, M.X.; Wang, B.Z.; Zhang, S.Q.; Ungar, I.A. Two Na+ and Cl− Hyperaccumulators of the Chenopodiaceae. Integr. Plant Biol. 2005, 47, 311–318. [Google Scholar] [CrossRef]
- Kong, Y.; Zheng, Y. Variation of sodium uptake rate in Suaeda glauca (Bunge) and its relation to plant size and salt acclimation. Can. J. Plant Sci. 2016, 97, 466–472. [Google Scholar]
- Qiu, P.; Wang, Q.Z.; Yin, M.; Wang, M.; Zhao, Y.Y.; Shan, Y.; Feng, X. Chemical Constituents of Ethyl Acetate Fraction of Suaeda glauca. Zhong Yao Cai 2015, 38, 751–753. [Google Scholar]
- Wang, X.H.; Cai, C.; Li, X.M. Optimal extraction of gallic acid from Suaeda glauca Bge. leaves and enhanced efficiency by ionic liquids. Int. J. Chem. Eng. 2016, 2016, 5217802. [Google Scholar] [CrossRef]
- Hong, Y.J.; Kim, G.H.; Park, Y.; Jo, H.J.; Nam, M.W.; Kim, D.G.; Cho, H.; Shim, H.J.; Jin, J.S.; Rho, H.; et al. Suaeda glauca Attenuates Liver Fibrosis in Mice by Inhibiting TGFβ1-Smad2/3 Signaling in Hepatic Stellate Cells. Nutrients 2023, 15, 3740. [Google Scholar] [CrossRef]
- Norwood, O.T. Incidence of female androgenetic alopecia (female pattern alopecia). Dermatol. Surg. 2001, 27, 53–54. [Google Scholar] [PubMed]
- Cranwell, W.; Sinclair, R. Male Androgenetic Alopecia; MDText.com, Inc.: South Dartmouth, MA, USA, 2015. [Google Scholar]
- Sarker, S.D.; Bartholomew, B.; Nash, R.J. Alkaloids from Balanites aegyptiaca. Fitoterapia 2000, 71, 328–330. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Previtera, L.; Purcaro, R.; Zarrelli, A. Cinnamic acid amides and lignanamides from Aptenia cordifolia. Tetrahedron 2006, 62, 2877–2882. [Google Scholar] [CrossRef]
- Inuwa, H.M.; Ahmad, K. In silico physico-chemical evaluation, anti-inflammatory and mcf-7 breast cancer cell line growth inhibition effects of trolline isolated from Mirabilis jalapa. J. Med. Plants Res. 2016, 10, 783–789. [Google Scholar]
- Lee, D.Y.; Lyu, H.N.; Kwak, H.Y.; Jung, L.K.; Lee, Y.H.; Kim, D.K.; Chung, I.S.; Kim, S.H.; Baek, N.I. Isolation of flavonoids from the fruits of Cornus kousa Burg. J. Appl. Biol. Chem. 2007, 50, 144–147. [Google Scholar]
- Kim, H.Y.; Moon, B.H.; Lee, H.J.; Choi, D.H. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J. Ethnopharmacol. 2004, 93, 227–230. [Google Scholar] [CrossRef]
- Shim, J.S.; Kim, S.D.; Kim, T.S.; Kim, K.N. Biological activities of flavonoid glycosides isolated from Angelica keiskei. Korean J. Food Sci. Technol. 2005, 37, 78–83. [Google Scholar]
- Rovirosa, J.; Diaz-Marrero, A.N.A.; Darias, J.; Painemal, K.; San Martín, A. Secondary metabolites from marine Penicillium brevicompactum. J. Chil. Chem. Soc. 2006, 51, 775–778. [Google Scholar] [CrossRef]
- Romero, C.; Brenes, M.; García, P.; Garrido, A. Hydroxytyrosol 4-β-D-glucoside, an important phenolic compound in olive fruits and derived products. J. Agric. Food Chem. 2002, 50, 3835–3839. [Google Scholar] [CrossRef]
- Fan, P.; Terrier, L.; Hay, A.E.; Marston, A.; Hostettmann, K. Antioxidant and enzyme inhibition activities and chemical profiles of Polygonum sachalinensis F. Schmidt ex Maxim (Polygonaceae). Fitoterapia 2010, 81, 124–131. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Xiang, L.; Zhou, F.; Li, H.; Su, Y.; Xu, X.; Wang, Q. Metabolites identification of bioactive compounds daturataturin A, daturametelin I, n-trans-feruloyltyramine, and cannabisin F from the seeds of Datura metel in rats. Front. Pharmacol. 2018, 9, 731. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, C.; Chen, Z.; Chen, Y.; Santhanam, R.K.; Xue, Z.; Ma, Q.; Guo, Q.; Liu, W.; Zhang, M.; et al. Effects of N-trans-feruloyltyramine isolated from laba garlic on antioxidant, cytotoxic activities and H2O2-induced oxidative damage in HepG2 and L02 cells. Food Chem. Toxicol. 2019, 130, 130–141. [Google Scholar] [CrossRef]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, tyrosol and derivatives and their potential effects on human health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed]
- Madaan, A.; Verma, R.; Singh, A.T.; Jaggi, M. Review of hair follicle dermal papilla cells as in vitro screening model for hair growth. Int. J. Cosmet. Sci. 2018, 40, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Chun, Y.S.; Fukuda, J. Hair follicle germs containing vascular endothelial cells for hair regenerative medicine. Sci. Rep. 2021, 11, 624. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.; Liu, Z.; Qian, C.; Huang, J.; Zhou, Y.; Mao, X.; Qu, Q.; Liu, B.; Wang, J.; Hu, Z.; et al. 3D bioprinting of a gelatin-alginate hydrogel for tissue-engineered hair follicle regeneration. Acta Biomater. 2023, 165, 19–30. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of hair growth and follicle size by VEGF-mediated angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Kishimoto, J.; Burgeson, R.E.; Morgan, B.A. Wnt signaling maintains the hair-inducing activity of the dermal papilla. Genes Dev. 2000, 14, 1181–1185. [Google Scholar] [CrossRef]
- Andl, T.; Reddy, S.T.; Gaddapara, T.; Millar, S.E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2002, 2, 643–653. [Google Scholar] [CrossRef]
- Kitagawa, T.; Matsuda, K.; Inui, S.; Takenaka, H.; Katoh, N.; Itami, S.; Kishimoto, S.; Kawata, M. Keratinocyte growth inhibition through the modification of Wnt signaling by androgen in balding dermal papilla cells. J. Clin. Endocrinol. Metab. 2009, 94, 1288–1294. [Google Scholar] [CrossRef]
- Soma, T.; Fujiwara, S.; Shirakata, Y.; Hashimoto, K.; Kishimoto, J. Hair-inducing ability of human dermal papilla cells cultured under Wnt/β-catenin signalling activation. Exp. Dermatol. 2012, 21, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.Y.; Sennett, R.; Rezza, A.; Clavel, C.; Grisanti, L.; Zemla, R.; Najam, S.; Rendl, M. Wnt/β-catenin signaling in dermal condensates is required for hair follicle formation. Dev. Biol. 2014, 385, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Andl, T.; Bagasra, A.; Lu, M.M.; Epstein, D.J.; Morrisey, E.E.; Millar, S.E. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 2001, 107, 69–82. [Google Scholar] [CrossRef] [PubMed]
- Gat, U.; DasGupta, R.; Degenstein, L.; Fuchs, E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 1998, 95, 605–614. [Google Scholar] [CrossRef]
- Huelsken, J.; Vogel, R.; Erdmann, B.; Cotsarelis, G.; Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 2001, 105, 533–545. [Google Scholar] [CrossRef]
- Brock, R. The uptake of arginine-rich cell-penetrating peptides: Putting the puzzle together. Bioconjugate Chem. 2014, 25, 863–868. [Google Scholar] [CrossRef]
- Lv, S.; Wang, L.; Duan, Y.; Huang, D.; Yang, D. Prediction of the Mechanism of Shaoyao Gancao Decoction in the Treatment of Alopecia Areata by Network Pharmacology and Its Preliminary Verification Study. Evid.-Based Complement. Altern. Med. 2022, 2022, 5764107. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-N.; Park, M.-G.; Kim, Y.-J.; Lee, J.-S.; Kwon, B.-O.; Rho, J.-R.; Jeong, E.-J. Chemical Constituents of Halophyte Suaeda glauca and Their Therapeutic Potential for Hair Loss. Molecules 2024, 29, 298. https://doi.org/10.3390/molecules29020298
Kim Y-N, Park M-G, Kim Y-J, Lee J-S, Kwon B-O, Rho J-R, Jeong E-J. Chemical Constituents of Halophyte Suaeda glauca and Their Therapeutic Potential for Hair Loss. Molecules. 2024; 29(2):298. https://doi.org/10.3390/molecules29020298
Chicago/Turabian StyleKim, Yun-Na, Min-Gyu Park, Yu-Jung Kim, Jae-Sun Lee, Bong-Oh Kwon, Jung-Rae Rho, and Eun-Ju Jeong. 2024. "Chemical Constituents of Halophyte Suaeda glauca and Their Therapeutic Potential for Hair Loss" Molecules 29, no. 2: 298. https://doi.org/10.3390/molecules29020298
APA StyleKim, Y.-N., Park, M.-G., Kim, Y.-J., Lee, J.-S., Kwon, B.-O., Rho, J.-R., & Jeong, E.-J. (2024). Chemical Constituents of Halophyte Suaeda glauca and Their Therapeutic Potential for Hair Loss. Molecules, 29(2), 298. https://doi.org/10.3390/molecules29020298







