Design, Synthesis, and Evaluation of New Hybrid Derivatives of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one as Potential Dual Inhibitors of Blood Coagulation Factors Xa and XIa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Design of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one-Based Derivatives
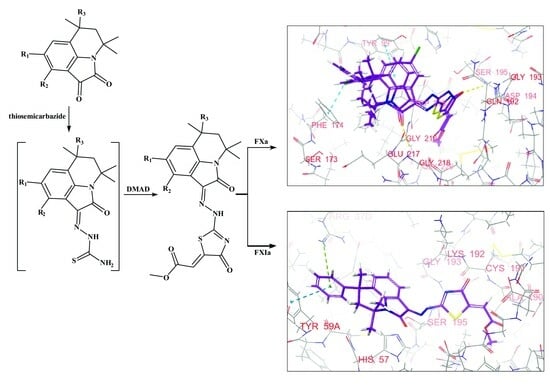
2.2. Synthesis
2.3. Anticoagulant Studies
2.4. Identifying Compound-Protein Interactions
3. Materials and Methods
3.1. Synthesis
- Methyl (Z)-2-(4-oxo-2-(2-((Z)-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)thiazol-5(4H)-ylidene)acetate (3a). Orange solid; 0.33 g; yield 80%; m.p. 285–287 °C; 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 1.30–1.34 (6H, m, C6-CH3+C4-CH3); 1.56 (1H, t, J = 12.9 Hz, C5-H); 1.70 (3H, s, C4-CH3); 1.86 (1H, dd, J = 13.7 Hz, J = 4.5 Hz, C5-H); 2.86–2.93 (1H, m, C6-H), 3.80 (3H, s, CH3O), 6.72 (1H, s, C=CH); 7.04 (1H, t, J = 7.6 Hz, CHarom); 7.37 (1H, d, J = 7.8 Hz, CHarom); 7.41 (1H, d, J = 7.4 Hz, CHarom); 13.35 (1H, br.s, NH). 13C NMR, δ (ppm): 18.0, 24.0, 25.3, 26.6, 45.2, 52.5, 53.7, 115.3, 117.9, 119.2, 122.3, 125.3, 129.4, 139.9, 140.8, 142.1, 147.2, 156.4, 165.8. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C20H20N4O4S + H+ 413.1279, found 413.1282.
- Methyl (Z)-2-(2-(2-((Z)-8-methoxy-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3b). Brown solid; 0.34 g; yield 77%; m.p. 232–234 °C; 1H NMR (500.13 MHz, DMSO-d6), δ (ppm): 1.29–1.32 (6H, m, C6-CH3+C4-CH3); 1.54 (1H, t, J = 12.9 Hz, C5-H); 1.68 (3H, s, C4-CH3); 1.82–1.87 (1H, m, C5-H); 2.82–2.91 (1H, m, C6-H), 3.79 (6H, s, 2-CH3O), 6.73 (1H, s, C=CH); 6.92–6.93 (1H, m, CHarom); 6.95–6.96 (1H, m, CHarom); 13.47 (1H, br.s, NH). 13C NMR, δ (ppm): 18.0, 23.8, 25.5, 26.5, 45.2, 52.5, 53.6, 55.7, 104.0, 112.0, 115.3, 115.6, 116.1, 118.3, 126.6, 134.9, 142.1, 147.5, 155.5, 156.3, 165.8. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C21H22N4O5S + H+ 443.1385, found 443.1389.
- Methyl (Z)-2-(2-(2-((Z)-8-chloro-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3c). Red solid; 0.31 g; yield 69%; m.p. 285–287 °C; 1H NMR (600.13 MHz, DMSO-d6), δ (ppm): 1.31–1.33 (6H, m, C6-CH3+C4-CH3); 1.56 (1H, t, J = 12.9 Hz, C5-H); 1.69 (3H, s, C4-CH3); 1.87 (1H, dd, J = 13.7 Hz, J = 4.5 Hz, C5-H); 2.82–2.93 (1H, m, C6-H), 3.80 (3H, s, CH3O), 6.75 (1H, s, C=CH); 7.36 (1H, s, CHarom); 7.42 (1H, s, CHarom); 13.45 (1H, br.s, NH). 13C NMR, δ (ppm): 17.8, 24.1, 25.5, 26.4, 44.9, 52.5, 53.9, 115.5, 118.5, 119.5, 126.6, 127.5, 128.8, 139.5, 141.9, 146.1, 155.9, 165.7, 166.2. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C20H19ClN4O4S + H+ 447.0889, found 447.0884.
- Methyl (Z)-2-(2-(2-((Z)-8-bromo-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3d). Orange solid; 0.35 g; yield 71%; m.p. 185–187 °C; 1H NMR (600.13 MHz, DMSO-d6), δ (ppm): 1.30–1.33 (6H, m, C6-CH3+C4-CH3); 1.55 (1H, t, J = 12.9 Hz, C5-H); 1.69 (3H, s, C4-CH3); 1.85 (1H, dd, J = 13.7 Hz, J = 4.6 Hz, C5-H); 2.88–2.93 (1H, m, C6-H), 3.80 (3H, s, CH3O), 6.73 (1H, s, C=CH); 7.46 (1H, s, CHarom); 7.52 (1H, s, CHarom); 13.45 (1H, br.s, NH). 13C NMR, δ (ppm): 17.8, 24.1, 25.5, 26.4, 44.9, 52.5, 53.9, 114.2, 115.5, 119.9, 121.2, 127.8, 131.4, 139.9, 141.8, 145.9, 155.8, 165.7, 166.1, 167.3. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C20H19BrN4O4S + H+ 491.0384, found 491.0381.
- Methyl 2-(2-(2-(8-iodo-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3e). Red solid; 0.41g; yield 76%; m.p. 175–177 °C; A mixture of isomers at the ratio of 11:1. 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 1.27–1.32 (6H, m, C6-CH3+C4-CH3); 1.33* (3H, s, C4-CH3); 1.53 (1H, t, J = 13.0 Hz, C5-H); 1.67, 1.70* (3H, s, C4-CH3); 1.82, 1.82–1.86* (1H, dd, J = 13.7 Hz, J = 4.6 Hz, C5-H); 2.84–2.91 (1H, m, C6-H), 3.79, 3.80* (3H, s, CH3O), 6.71, 6.73* (1H, s, C=CH); 7.59 (1H, s, CHarom); 7.64 (1H, s, CHarom); 13.45 (1H, br.s, NH). 13C NMR, δ (ppm): 17.8, 24.1, 24.3*, 25.4, 26.4, 26.6*, 44.8*, 44.9, 52.6, 53.9, 85.3, 115.5, 115.8*, 120.2, 126.8, 128.1, 133.7, 137.2, 137.6*, 140.3, 141.9, 145.8, 155.6, 165.7, 166.0. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C20H19IN4O4S + H+ 539.0246, found 539.0242.
- Methyl (Z)-2-(4-oxo-2-(2-((Z)-4,4,6,8,9-pentamethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)thiazol-5(4H)-ylidene)acetate (3f). Orange solid; 0.38 g; yield 86%; m.p. 273–275 °C; 1H NMR (500.13 MHz, DMSO-d6), δ (ppm): 1.28–1.32 (6H, m, C6-CH3+C4-CH3); 1.51 (1H, t, J = 12.9 Hz, C5-H); 1.69 (3H, s, C4-CH3); 1.80–1.85 (1H, m, C5-H); 2.24 (3H, s, C8-CH3); 2.47 (3H, s, C9-CH3); 2.78–2.86 (1H, m, C6-H), 3.79 (3H, s, 2-CH3O), 6.71 (1H, s, C=CH); 7.14 (1H, s, CHarom); 13.37 (1H, br.s, NH). 13C NMR, δ (ppm): 15.0, 18.2, 18.8, 23.9, 25.1, 26.7, 45.6, 52.4, 53.6, 115.5, 115.6, 122.2, 130.2, 130.8, 131.5, 132.8, 138.9, 142.1, 142.5, 156.7, 165.7. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C22H24N4O4S + H+ 441.1592, found 441.1590.
- Methyl (Z)-2-(2-(2-(8-chloro-4,4,6,9-tetramethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3g). Orange solid; 0.37 g; yield 80%; m.p. 264–266 °C; 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 1.28–1.33 (6H, m, C6-CH3+C4-CH3); 1.53 (1H, t, J = 12.9 Hz, C5-H); 1.69 (3H, s, C4-CH3); 1.85 (1H, dd, J = 13.7 Hz, J = 4.5 Hz, C5-H); 2.56 (3H, s, C9-CH3); 2.82–2.90 (1H, m, C6-H), 3.79 (3H, s, CH3O), 6.71 (1H, s, C=CH); 7.37 (1H, s, CHarom); 13.40 (1H, br.s, NH). 13C NMR, δ (ppm): 15.5, 18.0, 23.9, 25.2, 26.6, 45.0, 52.5, 53.8, 115.5, 116.9, 124.4, 127.7, 128.9, 131.1, 139.7, 142.1, 148.1, 155.9, 165.7, 166.5, 167.5. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C21H21ClN4O4S + H+ 461.1046, found 461.1041.
- Methyl (Z)-(2-(2-(2-(8-bromo-4,4,6,9-tetramethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3h). Orange solid; 0.42 g; yield 83%; m.p. 266–268 °C; 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 1.28–1.33 (6H, m, C6-CH3+C4-CH3); 1.49–1.57 (1H, m, C5-H); 1.69 (3H, s, C4-CH3); 1.84 (1H, dd, J = 13.7 Hz, J = 4.6 Hz, C5-H); 2.57 (3H, s, C9-CH3); 2.83–2.90 (1H, m, C6-H), 3.79 (3H, s, CH3O), 6.69 (1H, s, C=CH); 7.50 (1H, s, CHarom); 13.46 (1H, br.s, NH). 13C NMR, δ (ppm): 18.0, 18.5, 23.9, 25.2, 26.5, 45.0, 52.5, 53.8, 115.5, 116.9, 118.4, 124.9, 132.0, 132.8, 140.3, 142.1, 147.8, 155.8, 165.7, 166.7. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C21H21BrN4O4S + H+ 505.0541, found 505.0543.
- Methyl (Z)-2-(2-(2-((Z)-6-(4-chlorophenyl)-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3i). Orange solid; 0.40 g; yield 76%; m.p. 165–167 °C; 1H NMR (600.13 MHz, DMSO-d6), δ (ppm): 0.75 (3H, s, C6-CH3); 1.62 (3H, s, C4-CH3); 1.68 (3H, s, C4-CH3); 2.13 (1H, d, J = 14.4 Hz, C5-H); 2.47 (1H, d, J = 14.4 Hz, C5-H); 3.81 (3H, s, CH3O), 6.75 (1H, s, C=CH); 7.13 (2H, d, J = 8.4 Hz, CHarom); 7.17 (1H, t, J = 7.6 Hz, CHarom); 7.32 (2H, d, J = 8.5 Hz, CHarom); 7.45 (1H, d, J = 7.9 Hz, CHarom); 7.56 (1H, d, J = 7.4 Hz, CHarom); 13.43 (1H, br.s, NH). 13C NMR, δ (ppm): 24.7, 27.6, 30.2, 39.1, 50.7, 52.5, 53.7, 115.3, 118.7, 120.0, 122.5, 125.5, 128.1, 128.5, 130.8, 131.1, 140.6, 142.1, 146.9, 147.0, 156.1, 165.8, 166.2. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H23ClN4O4S + H+ 523.1203, found 523.1199.
- Methyl 2-(2-(2-(6-(4-chlorophenyl)-4,4,6,8-tetramethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3j). Orange solid; 0.46 g; yield 86%; m.p. 193–195 °C; a mixture of isomers at the ratio of 4:1. 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 0.75, 0.77* (3H, s, C6-CH3); 1.59, 1.62* (3H, s, C4-CH3); 1.67 (3H, s, C4-CH3); 2.10 (1H, d, J = 14.5 Hz, C5-H); 2.33*, 2.37 (3H, s, C8-CH3); 2.43, 2.44* (1H, d, J = 14.4 Hz, C5-H); 3.81 (3H, s, CH3O), 6.73 (1H, s, C=CH); 7.10–7.14*, 7.11–7.15 (2H, m, CHarom); 7.26 (1H, s, CHarom); 7.32 (2H, d, J = 8.7 Hz, CHarom); 7.37 (1H, s, CHarom); 13.33 (1H, br.s, NH). 13C NMR, δ (ppm): 20.7, 20.8, 24.7, 24.7, 27.6, 27.8*, 30.2, 51.0, 52.4, 52.6, 53.5*, 53.6, 115.1, 115.4*, 118.7, 120.4, 125.2*, 125.4, 127.6*, 128.1, 128.6, 130.7, 131.3*, 131.4, 131.7, 138.5, 139.1*, 142.4, 147.0, 156.2, 162.0, 165.9, 166.5. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C27H25ClN4O4S + H+ 537.1359, found 537.1360.
- Methyl (Z)-2-(2-(2-((Z)-8-chloro-6-(4-chlorophenyl)-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3k). Orange solid; 0.44 g; yield 79%; m.p. 210–212 °C; 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 0.75 (3H, s, C6-CH3); 1.60 (3H, s, C4-CH3); 1.69 (3H, s, C4-CH3); 2.11 (1H, d, J = 14.3 Hz, C5-H); 2.46 (1H, d, J = 14.4 Hz, C5-H); 3.81 (3H, s, CH3O), 6.75 (1H, s, C=CH); 7.14 (2H, d, J = 8.7 Hz, CHarom); 7.34 (2H, d, J = 8.8 Hz, CHarom); 7.48–7.51 (2H, m, CHarom); 13.34 (1H, br.s, NH). 13C NMR, δ (ppm): 24.7, 24.7, 27.5, 30.0, 50.7, 52.5, 52.6, 53.9, 115.6, 115.6, 119.4, 119.5, 120.4, 126.8, 127.6, 128.2, 128.5, 130.2, 130.2, 130.9, 139.5, 141.9, 146.0, 146.3, 155.7, 165.8, 166.2, 167.4. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H22Cl2N4O4S + H+ 557.0813, found 557.0810.
- Methyl (Z)-2-(2-(2-((Z)-8-bromo-6-(4-chlorophenyl)-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3l). Orange solid; 0.49 g; yield 81%; m.p. 208–210 °C; 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 0.75 (3H, s, C6-CH3); 1.60 (3H, s, C4-CH3); 1.69 (3H, s, C4-CH3); 2.11 (1H, d, J = 14.6 Hz, C5-H); 2.45 (1H, d, J = 14.4 Hz, C5-H); 3.81 (3H, s, CH3O), 6.75 (1H, s, C=CH); 7.14 (2H, d, J = 8.5 Hz, CHarom); 7.34 (2H, d, J = 8.5 Hz, CHarom); 7.59–7.62 (2H, m, CHarom); 13.49 (1H, br.s, NH). 13C NMR, δ (ppm): 24.7, 24.8, 27.5, 29.9, 50.7, 52.5, 52.6, 53.9, 114.4, 115.6, 115.6, 120.8, 122.2, 128.2, 128.5, 130.9, 132.9, 139.9, 141.9, 145.8, 146.4, 155.6, 165.8, 166.2. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H22BrClN4O4S + H+ 601.0308, found 601.0303.
- Methyl 2-(2-(2-(6-(4-chlorophenyl)-8-fluoro-4,4,6-trimethyl-2-oxo-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3m). Orange solid; 0.46 g; yield 85%; m.p. 181–183 °C; a mixture of isomers at the ratio of 3:1. 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 0.74, 0.76* (3H, s, C6-CH3); 1.60, 1.63* (3H, s, C4-CH3); 1.68 (3H, s, C4-CH3); 2.11, 2.12* (1H, d, J = 14.5 Hz, C5-H); 2.46, 2.47* (1H, d, J = 14.5 Hz, C5-H); 3.81 (3H, s, CH3O), 6.72, 6.74* (1H, s, C=CH); 7.13*, 7.14 (2H, d, J = 8.7 Hz, CHarom); 7.30–7.37 (4H, m, CHarom); 13.49 (1H, br.s, NH). 13C NMR, δ (ppm): 24.6, 24.9*, 27.5, 27.7*, 30.1, 50.5*, 50.7, 52.5, 52.6*, 53.7*, 53.8, 107.0, 107.3, 113.9*, 114.2*, 115.5, 115.8*, 117.3, 117.5, 117.7*, 117.9*, 119.8, 119.9, 127.0*, 127.2, 127.3, 128.1, 128.5, 130.9, 137.0, 137.7*, 142.0, 146.4, 155.9, 156.9*, 157.3, 159.3*, 159.7, 161.7. 165.7, 166.2, 167.2. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H22ClFN4O4S + H+ 541.1108, found 541.1111.
- Methyl (Z)-2-(4-oxo-2-(2-((Z)-4,4,6-trimethyl-2-oxo-6-phenyl-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)thiazol-5(4H)-ylidene)acetate (3n). Orange solid; 0.43 g; yield 88%; m.p. 232–234 °C; 1H NMR (500.13 MHz, DMSO-d6), δ (ppm): 0.72 (3H, s, C6-CH3); 1.61 (3H, s, C4-CH3); 1.70 (3H, s, C4-CH3); 2.13 (1H, d, J = 14.3 Hz, C5-H); 2.46–2.51 (m, C5-H+H2O); 3.81 (3H, s, CH3O), 6.75 (1H, s, C=CH); 7.10 (2H, d, J = 7.5 Hz, CHarom); 7.15–7.19 (2H, m, CHarom); 7.24–7.28 (2H, m, CHarom); 7.45 (1H, d, J = 7.7 Hz, CHarom); 7.56 (1H, d, J = 7.3 Hz, CHarom); 13.34 (1H, br.s, NH). 13C NMR, δ (ppm): 24.6, 27.7, 30.4, 51.0, 52.5, 53.8, 115.4, 119.0, 119.9, 122.4, 122.7, 126.1, 126.5, 128.1, 129.4, 131.3, 140.7, 142.1, 147.7, 147.8, 156.2, 160.2, 165.8. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H24N4O4S + H+ 489.1592, found 489.1590.
- Methyl (Z)-2-(2-(2-((Z)-8-chloro-4,4,6-trimethyl-2-oxo-6-phenyl-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3o). Orange solid; 0.44 g; yield 84%; m.p. 165–167 °C; 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 0.72 (3H, s, C6-CH3); 1.60 (3H, s, C4-CH3); 1.71 (3H, s, C4-CH3); 2.11 (1H, d, J = 14.4 Hz, C5-H); 2.45–2.51 (m, C5-H+H2O); 3.82 (3H, s, CH3O), 6.76 (1H, s, C=CH); 7.10 (2H, d, J = 7.6 Hz, CHarom); 7.17–7.21 (1H, m, CHarom); 7.26–7.31 (2H, m, CHarom); 7.48–7.51 (2H, m, CHarom); 13.35 (1H, br.s, NH). 13C NMR, δ (ppm): 24.6, 27/5, 30.0, 50.8, 52.5, 53.9, 115.5, 119.3, 120.3, 126.2, 126.5, 126.7, 128.2, 128.3, 130.3, 139.5, 147.3, 155.8, 165.8. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H23ClN4O4S + H+ 523.1203, found 523.1198.
- Methyl (Z)-2-(2-(2-((Z)-8-bromo-4,4,6-trimethyl-2-oxo-6-phenyl-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3p). Orange solid; 0.38 g; yield 67%; m.p. 208–210 °C; 1H NMR (500.13 MHz, DMSO-d6), δ (ppm): 0.72 (3H, s, C6-CH3); 1.60 (3H, s, C4-CH3); 1.71 (3H, s, C4-CH3); 2.11 (1H, d, J = 14.4 Hz, C5-H); 2.46 (1H, d, J = 14.4 Hz, C5-H); 3.82 (3H, s, CH3O), 6.76 (1H, s, C=CH); 7.10 (2H, d, J = 7.7 Hz, CHarom); 7.19 (1H, t, J = 7.3 Hz, CHarom); 7.28 (2H, d, J = 7.7 Hz, CHarom); 7.60–7.62 (2H, m, CHarom); 13.44 (1H, br.s, NH). 13C NMR, δ (ppm): 24.5, 24.6, 27.5, 30.0, 50.9, 52.5, 54.0, 114.3, 115.6, 115.6, 120.6, 122.0, 126.2, 126.5, 128.3, 128.7, 133.0, 139.9, 141.9, 145.9, 147.3, 155.6, 165.8. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H23BrN4O4S + H+ 567.0697, found 567.0699.
- Methyl (Z)-2-(2-(2-((Z)-8-fluoro-4,4,6-trimethyl-2-oxo-6-phenyl-5,6-dihydro-4H-pyrrolo[3,2,1-ij]quinolin-1(2H)-ylidene)hydrazineyl)-4-oxothiazol-5(4H)-ylidene)acetate (3q). Orange solid; 0.39 g; yield 77%; m.p. 171–173 °C; 1H NMR (400.16 MHz, DMSO-d6), δ (ppm): 0.69 (3H, s, C6-CH3); 1.60 (3H, s, C4-CH3); 1.68 (3H, s, C4-CH3); 2.10 (1H, d, J = 14.3 Hz, C5-H); 2.44–2.51 (m, C5-H+H2O); 3.80 (3H, s, CH3O), 6.72 (1H, s, C=CH); 7.11 (2H, d, J = 7.8 Hz, CHarom); 7.18 (1H, t, J = 7.3 Hz, CHarom); 7.24–7.36 (4H, m, CHarom); 13.43 (1H, br.s, NH). 13C NMR, δ (ppm): 24.5, 27.6, 30.2, 50.8, 52.5, 53.8, 106.9, 107.1, 115.5, 117.5, 117.7, 119.6, 119.7, 126.2, 126.4, 127.8, 127.9, 128.2, 137.1, 141.9, 146.6, 147.3, 155.9, 157.3, 159.7, 165.7, 166.1, 167.1. HPLC-HRMS-ESI, m/z ([M + H]+), calcd for C26H23FN4O4S + H+ 507.1498, found 507.1502.
3.2. In Vitro Assays
3.3. Molecular Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Sobieraj-Teague, M.; Eikelboom, J.W. Direct oral anticoagulants: Evidence and unresolved issues. Lancet 2020, 396, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Mesa, J.E.; Galindo-Coral, S.; Montes, M.C.; Martin, A.J.M. Thrombosis and Coagulopathy in COVID-19. Curr. Probl. Cardiol. 2021, 46, 100742. [Google Scholar] [CrossRef]
- Sutanto, H.; Soegiarto, G. Risk of Thrombosis during and after a SARS-CoV-2 Infection: Pathogenesis, Diagnostic Approach, and Management. Hematol. Rep. 2023, 15, 225–243. [Google Scholar] [CrossRef]
- Prezelj, A.; Stefanic Anderluh, P.; Peternel, L.; Urleb, U. Recent advances in serine protease inhibitors as anticoagulant agents. Curr. Pharm. Des. 2007, 13, 287–312. [Google Scholar] [CrossRef]
- Neves, A.R.; Correia-da-Silva, M.; Sousa, E.; Pinto, M. Structure–activity relationship studies for multitarget antithrombotic drugs. Future Med. Chem. 2016, 8, 2305–2355. [Google Scholar] [CrossRef] [PubMed]
- Gould, W.R.; McClanahan, T.B.; Welch, K.M.; Baxi, S.M.; Saiya-Cork, K.; Chi, L.; Johnson, T.R.; Leadley, R.J. Inhibitors of blood coagulation factors Xa and IIa synergize to reduce thrombus weight and thrombin generation in vivo and in vitro. J. Thromb. Haemost. 2006, 4, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Xin, H.; Olson, S.T. Kinetic Characterization of the Protein Z-dependent Protease Inhibitor Reaction with Blood Coagulation Factor Xa. J. Biol. Chem. 2008, 283, 29770–29783. [Google Scholar] [CrossRef]
- Gan, W.; Deng, L.; Yang, C.; He, Q.; Hu, J.; Yin, H.; Jin, X.; Lu, C.; Wu, Y.; Peng, L. An anticoagulant peptide from the human hookworm, Ancylostoma duodenale that inhibits coagulation factors Xa and XIa. FEBS Lett. 2009, 583, 1976–1980. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.K.; Tirloni, L.; Radulovic, Z.; Lewis, L.; Bakshi, M.; Hill, C.; da Silva Vaz, I., Jr.; Logullo, C.; Termignoni, C.; Mulenga, A. Conserved Amblyomma americanum tick Serpin19, an inhibitor of blood clotting factors Xa and XIa, trypsin and plasmin, has anti-haemostatic functions. Int. J. Parasitol. 2015, 45, 613–627. [Google Scholar] [CrossRef]
- Young, R.J.; Brown, D.; Burns-Kurtis, C.L.; Chan, C.; Convery, M.A.; Hubbard, J.A.; Kelly, H.A.; Pateman, A.J.; Patikis, A.; Senger, S.; et al. Selective and dual action orally active inhibitors of thrombin and factor Xa. Bioorg. Med. Chem. Lett. 2007, 17, 2927–2930. [Google Scholar] [CrossRef] [PubMed]
- Dönnecke, D.; Schweinitz, A.; Stürzebecher, A.; Steinmetzer, P.; Schuster, M.; Stürzebecher, U.; Nicklisch, S.; Stürzebecher, J.; Steinmetzer, T. From selective substrate analogue factor Xa inhibitors to dual inhibitors of thrombin and factor Xa. Part 3. Bioorg. Med. Chem. Lett. 2007, 17, 3322–3329. [Google Scholar] [CrossRef] [PubMed]
- Remko, M.; Remková, A.; Broer, R. Theoretical study of molecular structure and physicochemical properties of novel factor Xa inhibitors and dual factor Xa and factor IIa inhibitors. Molecules 2016, 21, 185. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, R.; Guan, H. Dual-target inhibitor screening against thrombin and factor Xa simultaneously by mass spectrometry. Anal. Chim. Acta 2017, 990, 1–10. [Google Scholar] [CrossRef]
- Olson, S.T.; Swanson, R.; Petitou, M. Specificity and selectivity profile of EP217609: A new neutralizable dual-action anticoagulant that targets thrombin and factor Xa. Blood 2012, 119, 2187–2195. [Google Scholar] [CrossRef]
- Philippides, G.J.; Loscalzo, J. Potential advantages of direct-acting thrombin inhibitors. Coron. Artery Dis. 1996, 7, 497–507. [Google Scholar] [CrossRef]
- Antman, E.M. Hirudin in acute myocardial infarction. Safety report from the Thrombolysis and Thrombin Inhibition in Myocardial Infarction (TIMI) 9A Trial. Circulation 1994, 90, 1624–1630. [Google Scholar] [CrossRef]
- Neuhaus, K.L.; Von Essen, R.; Tebbe, U.; Jessel, A.; Heinrichs, H.; Mäurer, W.; Döring, W.; Harmjanz, D.; Kötter, V.; Kalhammer, E. Safety observations from the pilot phase of the randomized r-Hirudin for Improvement of Thrombolysis (HIT-III) study. A study of the Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte (ALKK). Circulation 1994, 90, 1638–1642. [Google Scholar] [CrossRef]
- Wheeler, A.P.; Gailani, D. The Intrinsic pathway of coagulation as a target for antithrombotic therapy. Hematol. Oncol. Clin. N. Am. 2016, 30, 1099–1114. [Google Scholar] [CrossRef]
- Tillman, B.F.; Gruber, A.; McCarty, O.J.; Gailani, D. Plasma contact factors as therapeutic targets. Blood Rev. 2018, 32, 433–448. [Google Scholar] [CrossRef]
- Al-Horani, R.A.; Afosah, D.K. Recent advances in the discovery and development of factor XI/XIa inhibitors. Med. Res. Rev. 2018, 38, 1974–2023. [Google Scholar] [CrossRef]
- Gailani, D.; Bane, C.E.; Gruber, A. Factor XI and contact activation as targets for antithrombotic therapy. J. Thromb. Haemost. 2015, 13, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- D’Allesandro, N.; Cave, B.; Hough, A. Asundexian: An oral small molecule factor XIa inhibitor for the treatment of thrombotic disorders. Future Cardiol. 2023, 19, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Wichaiyo, S.; Parichatikanond, W.; Visansirikul, S.; Saengklub, N.; Rattanavipanon, W. Determination of the potential clinical benefits of small molecule factor XIa inhibitors in arterial thrombosis. ACS Pharmacol. Transl. Sci. 2023, 6, 970–981. [Google Scholar] [CrossRef]
- Gailani, D.; Lasky, N.M.; Broze, G.J., Jr. A murine model of factor XI deficiency. Blood Coagul. Fibrinolysis 1997, 8, 134–144. [Google Scholar] [CrossRef]
- Althagafi, I.; El-Metwaly, N.; Farghaly, T.A. New Series of Thiazole Derivatives: Synthesis, Structural Elucidation, Antimicrobial Activity, Molecular Modeling and MOE Docking. Molecule 2019, 24, 1741. [Google Scholar] [CrossRef]
- Demirci, S. Synthesis of Thiazole Derivatives as Antimicrobial Agents by Green Chemistry Techniques. J. Turk. Chem. Soc. Sect. A Chem. 2018, 5, 393–414. [Google Scholar] [CrossRef]
- Liaras, K.; Fesatidou, M.; Geronikaki, A. Thiazoles and Thiazolidinones asCOX/LOX Inhibitors. Molecule 2018, 23, 685. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, N.P. Synthesis, anti-inflammatory and analgesic evaluation of thiazole/oxazole substituted benzothiazole derivatives. Bioorg. Chem. 2021, 107, 104608. [Google Scholar] [CrossRef]
- Xie, W.; Wu, Y.; Zhang, J.; Mei, Q.; Zhang, Y.; Zhu, N.; Liu, R.; Zhang, H. Design, synthesis and biological evaluations of novel pyridonethiazole hybrid molecules as antitumor agents. Eur. J. Med. Chem. 2018, 145, 35–40. [Google Scholar] [CrossRef]
- de Santana, T.I.; Barbosa, M.O.; Gomes, P.A.T.M.; da Cruz, A.C.N.; da Silva, T.G.; Leite, A.C.L. Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem. 2018, 144, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Meleddu, R.; Distinto, S.; Corona, A.; Tramontano, E.; Bianco, G.; Melis, C.; Cottiglia, F.; Maccioni, E. Isatin thiazoline hybrids as dual inhibitors of HIV-1reverse transcriptase. J. Enzym. Inhib. Med. Chem. 2017, 32, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Khatik, G.L.; Datusalia, A.K.; Ahsan, W.; Kaur, P.; Vyas, M.; Mittal, A.; Nayak, S.K. A Retrospect Study on Thiazole Derivatives as the Potential Antidiabetic Agents in Drug Discovery and Developments. Curr. Drug Discov. Technol. 2018, 15, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Distinto, S.; Meleddu, R.; Ortuso, F.; Cottiglia, F.; Deplano, S.; Sequeira, L.; Melis, C.; Fois, B.; Angeli, A.; Capasso, C.; et al. Exploring new structural features of the 4-[(3-methyl-4-aryl-2,3-dihydro-1,3-thiazol-2-ylidene)amino]benzenesulphonamide scaffold for the inhibition of human carbonic anhydrases. J. Enzym. Inhib. Med. Chem. 2019, 34, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaidi, B.A.; Deb, P.K.; Telfah, S.T.; Dakkah, A.N.; Bataineh, Y.A.; Khames, A.Q.; Al-Dhoun, M.A.; Ahmad Al-Subeihi, A.A.; Odetallah, H.M.; Bardaweel, S.K.; et al. Synthesis and evaluation of 2,4,5-trisubstitutedthiazoles as carbonic anhydrase-III inhibitors. J. Enzym. Inhib. Med. Chem. 2020, 35, 1483–1490. [Google Scholar] [CrossRef]
- Karale, U.B.; Krishna, V.S.; Krishna, E.V.; Choudhari, A.S.; Shukla, M.; Gaikwad, V.R.; Mahizhaveni, B.; Chopra, S.; Misra, S.; Sarkar, D.; et al. Synthesis and biological evaluation of 2,4,5-trisubstituted thiazoles as antituberculosis agents effective against drug-resistant tuberculosis. Eur. J. Med. Chem. 2019, 178, 315–328. [Google Scholar] [CrossRef]
- Amr, A.E.-G.E.; Sabrry, N.M.; Abdalla, M.M.; Abdel-Wahab, B.F. Synthesis, antiarrhythmic and anticoagulant activities of novel thiazolo derivatives from methyl 2-(thiazol-2-ylcarbamoyl) acetate. Eur. J. Med. Chem. 2009, 44, 725–735. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, M.; Zhou, S.; Wu, S.; Zhang, X.; Ma, L.; Zhang, K.; Gong, P. Design, synthesis and structure–activity relationship of oxazolidinone derivatives containing novel S4 ligand as FXa inhibitors. Eur. J. Med. Chem. 2015, 96, 369–380. [Google Scholar] [CrossRef]
- Kumawat, M.K. Thiazole Containing Heterocycles with Antimalarial Activity. Curr. Drug Discov. Technol. 2018, 15, 196–200. [Google Scholar] [CrossRef]
- Mishchenko, M.; Shtrygol, S.; Kaminskyy, D.; Lesyk, R. Thiazole-Bearing 4-Thiazolidinones as NewAnticonvulsant Agents. Sci. Pharm. 2020, 88, 16. [Google Scholar] [CrossRef]
- Quan, M.; Wong, P.C.; Wang, C.; Woerner, F.; Smallheer, J.M.; Barbera, F.A.; Bozarth, J.M.; Brown, R.L.; Harpel, M.R.; Luettgen, J.M.; et al. Tetrahydroquinoline derivatives as potent and selective factor XIa inhibitors. J. Med. Chem. 2014, 57, 955–969. [Google Scholar] [CrossRef] [PubMed]
- Koul, A.; Vranckx, L.; Dhar, N.; Göhlmann, H.W.H.; Özdemir, E.; Neefs, J.-M.; Schulz, M.; Lu, P.; Mørtz, E.; McKinney, J.D.; et al. Delayed bactericidal response of Mycobacterium tuberculosis to bedaquiline involves remodelling of bacterial metabolism. Nat. Commun. 2014, 5, 3369. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.R.; Hu, J.H.; Yang, X.P.; Feng, X.; Li, X.S.; Huang, L.; Chan, A.S.C. Design, synthesis, and evaluation of orally bioavailable quinoline-indole derivatives as innovative multitarget-directed ligands: Promotion of cell proliferation in the adult murine hippocampus for the treatment of Alzheimer’s disease. J. Med. Chem. 2018, 61, 1871–1894. [Google Scholar] [CrossRef]
- Sharma, P.C.; Chaudhary, M.; Sharma, A.; Piplani, M.; Rajak, H.; Prakash, O. Insight View on Possible Role of Fluoroquinolones in Cancer Therapy. Curr. Top. Med. Chem. 2013, 13, 2076–2096. [Google Scholar] [CrossRef]
- Taha, M.; Sultan, S.; Imran, S.; Rahim, F.; Zaman, K.; Wadood, A.; Rehman, A.U.; Uddin, N.; Khan, K.M. Synthesis of quinoline derivatives as diabetic II inhibitors and molecular docking studies. Bioorg. Med. Chem. 2019, 27, 4081–4088. [Google Scholar] [CrossRef]
- Fan, Y.L.; Wu, J.B.; Cheng, X.W.; Zhang, F.Z.; Feng, L.S. Fluoroquinolone Derivatives and Their Anti-tubercular Activities. Eur. J. Med. Chem. 2018, 146, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, T.; Hashiguchi, Y.; Sakata, T.; Sakaguchi, T. Antioxidant activity of the fused heterocyclic compounds, 1,2,3,4-tetrahydroquinolines, and related compounds—Effect of ortho-substituents. Polym. Degrad. Stab. 2003, 79, 225–230. [Google Scholar] [CrossRef]
- Ökten, S.; Aydın, A.; Koçyiğit, Ü.M.; Çakmak, O.; Erkan, S.; Andac, C.A.; Taslimi, P.; Gülçin, İ. Quinoline-based promising anticancer and antibacterial agents, and some metabolic enzyme inhibitors. Arch. Pharm. 2020, 353, 2000086. [Google Scholar] [CrossRef]
- Idhayadhulla, A.; SurendraKumar, R.; Jamal Abdul Nasser, A.; Manilal, A. Synthesis of some pyrrole derivatives and their Anticoagulant Activity. Am. J. Drug Discov. Dev. 2012, 2, 40–49. [Google Scholar] [CrossRef]
- Mohamed, M.S.; El-Domany, R.A.; Abd El-Hameed, R.H. Synthesis of certain pyrrole derivatives as antimicrobial agents. Acta Pharm. 2009, 59, 145–158. [Google Scholar] [CrossRef]
- Said Fatahala, S.; Hasabelnaby, S.; Goudah, A.; Mahmoud, G.I.; Helmy Abd-El Hameed, R. Pyrrole and fused pyrrole compounds with bioactivity against inflammatory mediators. Molecules 2017, 22, 461. [Google Scholar] [CrossRef] [PubMed]
- Sarg, M.T.; Koraa, M.M.; Bayoumi, A.H.; Abd El Gilil, S.M. Synthesis of pyrroles and condensed pyrroles as anti-inflammatory agents with multiple activities and their molecular docking study. Open J. Med. Chem. 2015, 5, 49. [Google Scholar] [CrossRef]
- Mateev, E.; Georgieva, M.; Zlatkov, A. Pyrrole as an important scaffold of anticancer drugs: Recent advances. J. Pharm. Pharmaceut. Sci. 2022, 25, 24–40. [Google Scholar] [CrossRef] [PubMed]
- Bianco, M.D.C.A.D.; Marinho, D.I.L.F.; Hoelz, L.V.B.; Bastos, M.M.; Boechat, N. Pyrroles as privileged scaffolds in the search for new potential HIV inhibitors. Pharmaceuticals 2021, 14, 893. [Google Scholar] [CrossRef]
- Novichikhina, N.; Ilin, I.; Tashchilova, A.; Sulimov, A.; Kutov, D.; Ledenyova, I.; Krysin, M.; Shikhaliev, K.; Gantseva, A.; Gantseva, E.; et al. Synthesis, Docking, and In Vitro Anticoagulant Activity Assay of Hybrid Derivatives of Pyrrolo [3,2,1-ij]Quinolin-2(1H)-one as New Inhibitors of Factor Xa and Factor XIa. Molecules 2020, 25, 1889. [Google Scholar] [CrossRef] [PubMed]
- Novichikhina, N.P.; Shestakov, A.S.; Medvedeva, S.M.; Lagutina, A.M.; Krysin, M.Y.; Podoplelova, N.A.; Panteleev, M.A.; Ilin, I.S.; Sulimov, A.V.; Tashchilova, A.S.; et al. New Hybrid Tetrahydropyrrolo[3,2,1-ij]quinolin-1-ylidene-2-thioxothiazolidin-4-ones as New Inhibitors of Factor Xa and Factor XIa: Design, Synthesis, and In Silico and Experimental Evaluation. Molecules 2023, 28, 3851. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, S.M.; Potapov, A.Y.; Gribkova, I.V.; Katkova, E.V.; Sulimov, V.B.; Shikhaliev, K.S. Synthesis, docking, and anticoagulant activity of new factor-Xa inhibitors in a series of pyrrolo[3,2,1-ij]quinoline-1,2-diones. Pharm. Chem. J. 2018, 51, 975–979. [Google Scholar] [CrossRef]
- Novichikhina, N.P.; Ashrafova, Z.E.; Stolpovskaya, N.V.; Ledenyova, I.V.; Kholyavka, M.G.; Podoplelova, N.A.; Panteleev, M.A.; Shikhaliev, K.S. Synthesis and properties of novel hybrid molecules bearing 4H-pyrrolo[3,2,1-ij]quinolin-2-one and thiazole moieties. Russ. Chem. Bull. 2022, 71, 1969–1975. [Google Scholar] [CrossRef]
- Novichikhina, N.P.; Skoptsova, A.A.; Shestakov, A.S.; Potapov, A.Y.; Kosheleva, E.A.; Kozaderov, O.A.; Ledenyova, I.V.; Podoplelova, N.A.; Panteleev, M.A.; Shikhaliev, K.S. Synthesis and anticoagulant activity of new ethylidene and spiro derivatives of Pyrrolo[3,2,1-ij]quinolin-2-ones. Russ. J. Org. Chem. 2020, 56, 1550–1556. [Google Scholar] [CrossRef]
- Skoptsova, A.A.; Shestakov, A.S.; Ledenyova, I.V.; Stolpovskaya, N.V.; Podoplelova, N.A.; Panteleev, M.A.; Paponov, B.V.; Sidorenko, O.E.; Shikhaliev, K.S.; Novichikhina, N.P. Reaction of 1-Phenacylidene pyrrolo [3,2,1-ij] quinolin-2-ones with Cyclic/Acyclic Enaminones and the Anticoagulant Activity of Synthesized Pyrrole-Quinoline Derivatives. ChemistrySelect 2022, 7, e202200730. [Google Scholar] [CrossRef]
- Vilar, S.; Quezada, E.; Santana, L.; Uriarte, E.; Yánez, M.; Fraiz, N.; Alcaide, C.; Cano, E.; Orallo, F. Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin–resveratrol hybrids. Bioorg. Med. Chem. Lett. 2006, 16, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Kondengaden, S.M.; Zhang, Q.; Li, X.; Sigalapalli, D.K.; Kondengadan, S.M.; Huang, K.; Li, K.K.; Li, S.; Xiao, Z.; et al. Structure based design, synthesis and activity studies of small hybrid molecules as HDAC and G9a dual inhibitors. Oncotarget 2017, 8, 63187. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.A.; Naphade, S.S.; Bangalore, P.K.; Palkar, M.B.; Shaikh, M.S.; Karpoormath, R. Synthesis of novel 4-nitropyrrole-based semicarbazide and thiosemicarbazide hybrids with antimicrobial and anti-tubercular activity. Bioorg. Med. Chem. Lett. 2014, 24, 3079–3083. [Google Scholar] [CrossRef]
- Silva, D.; Mendes, E.; Summers, E.J.; Neca, A.; Jacinto, A.C.; Reis, T.; Agostinho, P.; Bolea, I.; Jimeno, M.L.; Mateus, M.L.; et al. Synthesis, biological evaluation, and molecular modeling of nitrile-containing compounds: Exploring multiple activities as anti-Alzheimer agents. Drug Dev. Res. 2020, 81, 215–231. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Pourshab, M.; Mohseni, M. Synthesis, characterization, and evaluation of antioxidant and antibacterial activities of novel indole-hydrazono thiazolidinones. Monatshefte Chem. 2018, 149, 2327–2336. [Google Scholar] [CrossRef]
- Matesic, L.; Locke, J.M.; Vine, K.L.; Ranson, M.; Bremner, J.B.; Skropeta, D. Synthesis and anti-leukaemic activity of pyrrolo[3,2,1-hi]indole-1,2-diones, pyrrolo[3,2,1-ij]quinoline-1,2-diones and other polycyclic isatin derivatives. Tetrahedron 2012, 68, 6810–6819. [Google Scholar] [CrossRef]
- Patel, N.R.; Patel, D.V.; Murumkar, P.R.; Yadav, M.R. Contemporary developments in the discovery of selective factor Xa inhibitors: A review. Eur. J. Med. Chem. 2016, 121, 671–698. [Google Scholar] [CrossRef] [PubMed]
- Sakami, S.; Kawai, K.; Maeda, M.; Aoki, T.; Fujii, H.; Ohno, H.; Ito, T.; Saitoh, A.; Nakao, K.; Izumimoto, N.; et al. Design and synthesis of a metabolically stable and potent antitussive agent, a novel δ opioid receptor antagonist, TRK-851. Bioorg. Med. Chem. 2008, 16, 7956–7967. [Google Scholar] [CrossRef]
- Yakaiah, S.; Kumar, P.S.V.; Rani, P.B.; Prasad, K.D.; Aparna, P. Design, synthesis and biological evaluation of novel pyrazolo-oxothiazolidine derivatives as antiproliferative agents against human lung cancer cell line A549. Bioorg. Med. Chem. Lett. 2018, 28, 630–636. [Google Scholar] [CrossRef]
- Gomha, S.M.; Salaheldin, T.A.; Hassaneen, H.M.; Abdel-Aziz, H.M.; Khedr, M.A. Synthesis, characterization and molecular docking of novel bioactive thiazolyl-thiazole derivatives as promising cytotoxic antitumor drug. Molecules 2015, 21, 3. [Google Scholar] [CrossRef]
- Abbas, E.M.H.; Gomha, S.M.; Farghaly, T.A.; Abdalla, M.M. Synthesis of new thiazole derivatives as antitumor agents. Curr. Org. Synth. 2016, 13, 456–465. [Google Scholar] [CrossRef]
- Channar, P.A.; Aziz, M.; Ejaz, S.A.; Chaudhry, G.E.S.; Saeed, A.; Ujan, R.; Hasan, A.; Ejaz, S.R.; Saeed, A. Structural and functional insight into thiazolidinone derivatives as novel candidates for anticancer drug design: In vitro biological and in-silico strategies. J. Biomol. Struct. Dyn. 2023, 41, 942–953. [Google Scholar] [CrossRef]
- Alzahrani, A.Y.; Ammar, Y.A.; Abu-Elghait, M.; Salem, M.A.; Assiri, M.A.; Ali, T.E.; Ragab, A. Development of novel indolin-2-one derivative incorporating thiazole moiety as DHFR and quorum sensing inhibitors: Synthesis, antimicrobial, and antibiofilm activities with molecular modelling study. Bioorg. Chem. 2022, 119, 105571. [Google Scholar] [CrossRef] [PubMed]
- Grybaitė, B.; Vaickelionienė, R.; Stasevych, M.; Komarovska-Porokhnyavets, O.; Kantminienė, K.; Novikov, V.; Mickevičius, V. Synthesis and antimicrobial activity of novel thiazoles with reactive functional groups. ChemistrySelect 2019, 4, 6965–6970. [Google Scholar] [CrossRef]
- Ahani, Z.; Nikbin, M.; Maghsoodlou, M.T.; Farhadi-Ghalati, F.; Valizadeh, J.; Beyzaei, H.; Moghaddam-Manesh, M. Semi-synthesis, antibacterial and antifungal activities of three novel thiazolidin-4-one by essential oil of Anethum graveolens seeds as starting material. J. Iran. Chem. Soc. 2018, 15, 2423–2430. [Google Scholar] [CrossRef]
- Vaarla, K.; Vishwapathi, V.; Vermeire, K.; Vedula, R.R.; Kulkarni, C.V. A facile one pot multi component synthesis of alkyl 4-oxo-coumarinyl ethylidene hydrazono-thiazolidin-5-ylidene acetates and their antiviral activity. J. Mol. Struct. 2022, 1249, 131662. [Google Scholar] [CrossRef]
- Saeed, A.; Al-Masoudi, N.A.; Latif, M. Synthesis and Antiviral Activity of New Substituted Methyl [2-(arylmethylene-hydrazino)-4-oxo-thiazolidin-5-ylidene] acetates. Arch. Pharm. 2013, 346, 618–625. [Google Scholar] [CrossRef]
- El-Helw, E.A.; Sallam, H.A.; Elgubbi, A.S. Antioxidant activity of some N-heterocycles derived from 2-(1-(2-oxo-2 H-chromen-3-yl) ethylidene) hydrazinecarbothioamide. Synth. Commun. 2019, 49, 2651–2661. [Google Scholar] [CrossRef]
- Aly, A.A.; Ibrahim, M.A.; El-Sheref, E.M.; Hassan, A.M.; Brown, A.B. Prospective new amidinothiazoles as leukotriene B4 inhibitors. J. Mol. Struct. 2019, 1175, 414–427. [Google Scholar] [CrossRef]
- Kato, M.; Ito, K.; Yamanaka, T. New Heterocyclic Derivative. Japanese Patent JPH07285952A, 31 October 1995. [Google Scholar]
- Leshcheva, Y.V.; Shikhaliev, K.S.; Shatalov, G.V.; Yermolova, G.I. New functional derivatives 4,4,6-trimethyl-4H-pyrrolo[3,2,1-ij]quinoline-1,2-diones. Izv. Vuzov. Khimiya Khim. Tekhnologia [ChemChemTech] 2003, 46, 105. (In Russian) [Google Scholar]
- Aly, A.A.; Mohamed, N.K.; Hassan, A.A.; El-Shaieb, K.M.; Makhlouf, M.M.; Bräse, S.; Nieger, M.; Brown, A.B. Functionalized 1,3-Thiazolidin-4-Ones from 2-Oxo-Acenaphthoquinylidene-and [2.2] Paracyclophanylidene-Thiosemicarbazones. Molecules 2019, 24, 3069. [Google Scholar] [CrossRef]
- Venkateswarlu, P.; Dubey, P.K. Synthesis of 3-Substituted Pyrido[1,2-a]pyrimidinethylidenehydrazinylthiozole Derivatives from Pyridine-2-amines. Asian J. Chem. 2017, 29, 2387–2390. [Google Scholar] [CrossRef]
- Hassan, A.A.; Ibrahim, Y.R.; Aly, A.A.; El-Sheref, E.M.; Yamato, T. Reactions of dimethyl ethynedicarboxylate with (substituted ethylidene) hydrazinecarbothioamides. J. Heterocycl. Chem. 2013, 50, 473–477. [Google Scholar] [CrossRef]
- Laamari, Y.; Bimoussa, A.; Fawzi, M.; Oubella, A.; Rohand, T.; Van Meervelt, L.; Itto, M.Y.A.; Morjani, H.; Auhmani, A. Synthesis, crystal structure and evaluation of anticancer activities of some novel heterocyclic compounds based on thymol. J. Mol. Struct. 2023, 1278, 134906. [Google Scholar] [CrossRef]
- Singla, R.; Gautam, D.; Gautam, P.; Chaudhary, R.P. Efficient “on water” green route heterocyclization of thiosemicarbazones with DMAD. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 740–745. [Google Scholar] [CrossRef]
- Danilkina, N.A.; Mikhailov, L.E.; Ivin, B.A. Condensations of thioamides with acetylenecarboxylic acid derivatives. Russ. J. Org. Chem. 2006, 42, 783–814. [Google Scholar] [CrossRef]
- Perzborn, E.; Kubitza, D.; Misselwitz, F. Rivaroxaban. Hämostaseologie 2007, 27, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Maignan, S.; Mikol, V. The use of 3D structural data in the design of specific factor Xa inhibitors. Curr. Top. Med. Chem. 2001, 1, 161–174. [Google Scholar] [CrossRef]
- Sulimov, A.V.; Kutov, D.C.; Oferkin, I.V.; Katkova, E.V.; Sulimov, V.B. Application of the Docking Program SOL for CSAR Benchmark. J. Chem. Inf. Model. 2013, 53, 1946–1956. [Google Scholar] [CrossRef]
- Halgren, T.A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput. Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Romanov, A.N.; Jabin, S.N.; Martynov, Y.B.; Sulimov, A.V.; Grigoriev, F.V.; Sulimov, V.B. Surface Generalized Born method: A simple, fast, and precise implicit solvent model beyond the Coulomb approximation. J. Phys. Chem. A 2004, 108, 9323–9327. [Google Scholar] [CrossRef]
- Ilin, I.; Lipets, E.; Sulimov, A.; Kutov, D.; Shikhaliev, K.; Potapov, A.; Krysin, M.; Zubkov, F.; Sapronova, L.; Ataullakhanov, F.; et al. New factor Xa inhibitors based on 1,2,3,4-tetrahydroquinoline developed by molecular modelling. J. Mol. Graph. Model. 2019, 89, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Novichikhina, N.P.; Shestakov, A.S.; Potapov, A.Y.; Kosheleva, E.A.; Shatalov, G.V.; Verezhnikov, V.N.; Vandyshev, D.Y.; Ledeneva, I.V.; Shikhaliev, K.S. Synthesis of 4H-pyrrolo[3,2,1-ij]quinoline-1,2-diones containing a piperazine fragment and study of their inhibitory properties against protein kinases. Russ. Chem. Bull. 2020, 69, 787–792. [Google Scholar] [CrossRef]
- Sulimov, A.; Ilin, I.; Kutov, D.; Shikhaliev, K.; Shcherbakov, D.; Pyankov, O.; Stolpovskaya, N.; Medvedeva, S.; Sulimov, V. New Chemicals Suppressing SARS-CoV-2 Replication in Cell Culture. Molecules 2022, 27, 5732. [Google Scholar] [CrossRef] [PubMed]
- Sulimov, A.V.; Ilin, I.S.; Kutov, D.C.; Stolpovskaya, N.V.; Shikhaliev, K.S.; Sulimov, V.B. Supercomputing, Docking and Quantum Mechanics in Quest for Inhibitors of Papain-like Protease of SARS-CoV-2. Lobachevskii J. Math. 2021, 42, 1571–1579. [Google Scholar] [CrossRef]
- ChemAxon. Marvin was Used for Drawing, Displaying and Characterizing Chemical Structures, Substructures and Reactions, Marvin 21.3.0. 2021. Available online: https://chemaxon.com/products/marvin (accessed on 10 February 2022).
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Voevodin, V.V.; Antonov, A.S.; Nikitenko, D.A.; Shvets, P.A.; Sobolev, S.I.; Sidorov, I.Y.; Stefanov, K.S.; Voevodin, V.V.; Zhumatiy, S.A. Supercomputer Lomonosov-2: Large scale, deep monitoring and fine analytics for the user community. Supercomput. Front. Innov. 2019, 6, 4–11. [Google Scholar] [CrossRef]





| № | Factor Xa, SOL Score, kcal/mol | Factor XIa, SOL Score, kcal/mol | Percent Inhibition at 30 μM | IC50, μM | ||
|---|---|---|---|---|---|---|
| Xa | XIa | Xa | XIa | |||
| 3a | −5.39 | −4.16 | −1 | −11 | – | – |
| 3b | −5.49 | −4.46 | 91 | 60 | 5.76 ± 1.08 | 8.10 ± 0.20 |
| 3c | −5.76 | −4.69 | 87 | 93 | 2.84 ± 0.04 | 12.20 ± 0.20 |
| 3d | −5.86 | −4.85 | 77 | 96 | – | – |
| 3e | −5.93 | −4.86 | 99 | 100 | 4.23 ± 0.31 | 8.03 ± 0.68 |
| 3f | −5.91 | −4.78 | 90 | 87 | 4.02 ± 0.60 | 7.97 ± 0.63 |
| 3g | −5.90 | −4.79 | 24 | 62 | – | – |
| 3h | −5.98 | −4.90 | −1 | −16 | – | – |
| 3i | −6.03 | −4.73 | 87 | 65 | – | – |
| 3j | −6.10 | −4.55 | 64 | 83 | – | – |
| 3k | −6.35 | −5.00 | 95 | 79 | 1.17 ± 0.26 | 4.59 ± 0.54 |
| 3l | −5.76 | −5.13 | 92 | 90 | 1.74 ± 0.21 | 3.61 ± 1.70 |
| 3m | −6.14 | −4.43 | 63 | 16 | – | – |
| 3n | −5.42 | −4.44 | 93 | 85 | 1.86 ± 0.07 | 11.46 ± 0.98 |
| 3o | −5.86 | −4.74 | 87 | 90 | 1.34 ± 0.16 | 7.21 ± 0.22 |
| 3p | −5.97 | −4.93 | 83 | 97 | 1.83 ± 0.05 | 3.87 ± 0.33 |
| 3q | −5.78 | −4.22 | 85 | 84 | – | – |
| Rivaroxaban | −6.89 | 94 | 8 | 0.007 ± 0.001 | ||
| № | Thrombin, SOL Score, kcal/mol | Percent Inhibition of Thrombin at 30 μM |
|---|---|---|
| 3a | −5.53 | 2 |
| 3b | −5.78 | 24 |
| 3c | −6.01 | 29 |
| 3d | −5.94 | 31 |
| 3e | −5.72 | 96 |
| 3f | −5.68 | 43 |
| 3g | −5.72 | 20 |
| 3h | −6.12 | −6 |
| 3i | −5.27 | 39 |
| 3j | −5.43 | 28 |
| 3k | −5.60 | 43 |
| 3l | −5.70 | 48 |
| 3m | −5.19 | 18 |
| 3n | −5.97 | 27 |
| 3o | −6.28 | 71 |
| 3p | −6.55 | 45 |
| 3q | −5.92 | 26 |
| Argatroban | - | 99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skoptsova, A.A.; Geronikaki, A.; Novichikhina, N.P.; Sulimov, A.V.; Ilin, I.S.; Sulimov, V.B.; Bykov, G.A.; Podoplelova, N.A.; Pyankov, O.V.; Shikhaliev, K.S. Design, Synthesis, and Evaluation of New Hybrid Derivatives of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one as Potential Dual Inhibitors of Blood Coagulation Factors Xa and XIa. Molecules 2024, 29, 373. https://doi.org/10.3390/molecules29020373
Skoptsova AA, Geronikaki A, Novichikhina NP, Sulimov AV, Ilin IS, Sulimov VB, Bykov GA, Podoplelova NA, Pyankov OV, Shikhaliev KS. Design, Synthesis, and Evaluation of New Hybrid Derivatives of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one as Potential Dual Inhibitors of Blood Coagulation Factors Xa and XIa. Molecules. 2024; 29(2):373. https://doi.org/10.3390/molecules29020373
Chicago/Turabian StyleSkoptsova, Anna A., Athina Geronikaki, Nadezhda P. Novichikhina, Alexey V. Sulimov, Ivan S. Ilin, Vladimir B. Sulimov, Georgii A. Bykov, Nadezhda A. Podoplelova, Oleg V. Pyankov, and Khidmet S. Shikhaliev. 2024. "Design, Synthesis, and Evaluation of New Hybrid Derivatives of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one as Potential Dual Inhibitors of Blood Coagulation Factors Xa and XIa" Molecules 29, no. 2: 373. https://doi.org/10.3390/molecules29020373
APA StyleSkoptsova, A. A., Geronikaki, A., Novichikhina, N. P., Sulimov, A. V., Ilin, I. S., Sulimov, V. B., Bykov, G. A., Podoplelova, N. A., Pyankov, O. V., & Shikhaliev, K. S. (2024). Design, Synthesis, and Evaluation of New Hybrid Derivatives of 5,6-Dihydro-4H-pyrrolo[3,2,1-ij]quinolin-2(1H)-one as Potential Dual Inhibitors of Blood Coagulation Factors Xa and XIa. Molecules, 29(2), 373. https://doi.org/10.3390/molecules29020373