Antimicrobial and Other Pharmacological Properties of Ocimum basilicum, Lamiaceae
Abstract
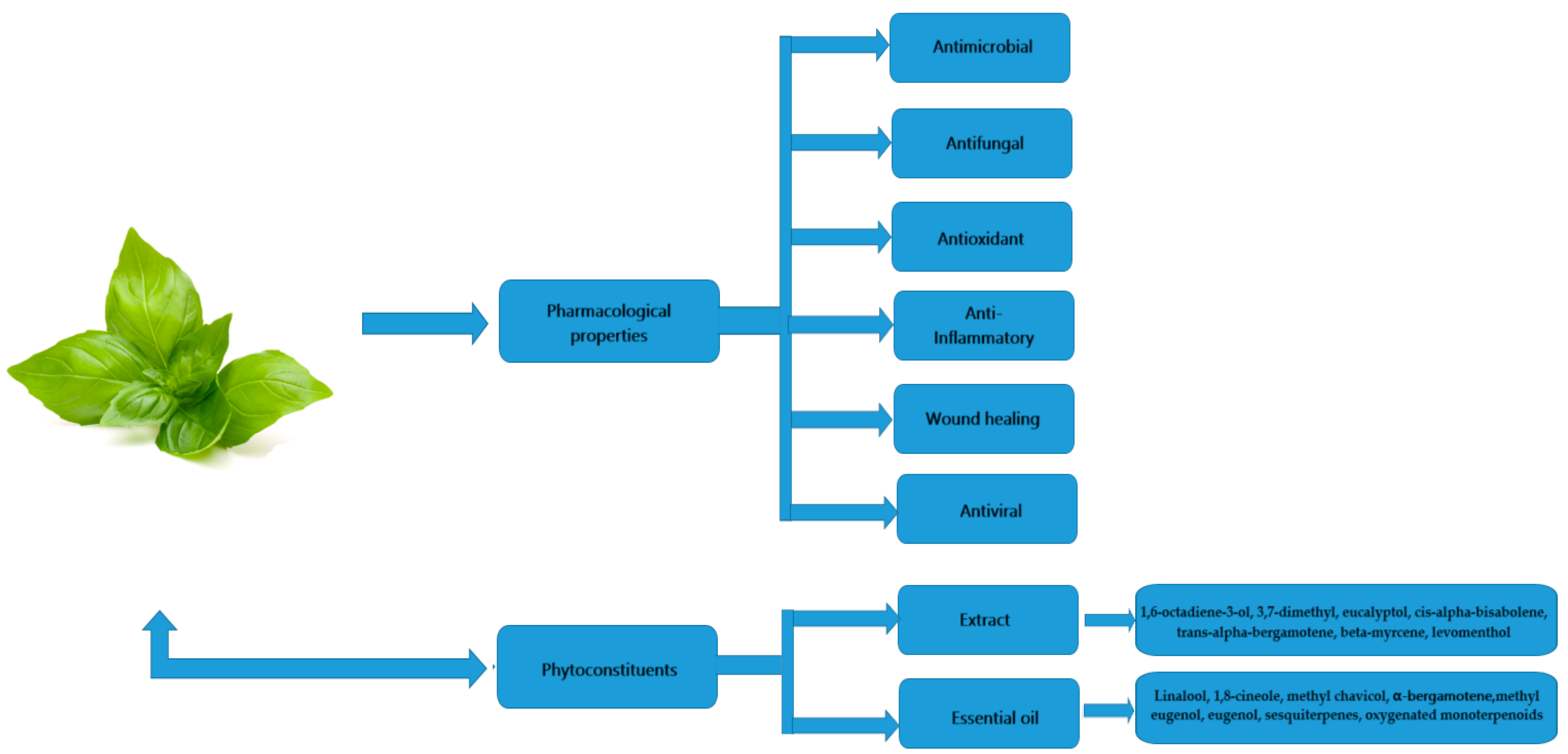
:1. Introduction
2. Methods
3. Phytoconstituents
4. Antibacterial Activity
5. Antifungal Activity
6. Antioxidant Activity
7. Anti-Inflammatory Activity
8. Wound Healing Effect
9. Antiviral Activity
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salehi, B.; Ata, A.; Anil Kumar, V.N.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef] [PubMed]
- Tesfahuneygn, G.; Gebreegziabher, G. Medicinal Plants Used in Traditional Medicine by Ethiopians: A Review Article. J. Resp. Med. Lung Dis. 2019, 4, 1040. [Google Scholar]
- Tiwari, R.; Latheef, S.K.; Ahmed, I.; Iqbal, H.M.N.; Bule, M.H.; Dhama, K.; Samad, H.A.; Karthik, K.; Alagawany, M.; El-Hack, M.E.A.; et al. Herbal Immunomodulators—A Remedial Panacea for Designing and Developing Effective Drugs and Medicines: Current Scenario and Future Prospects. Curr. Drug Metab. 2018, 19, 264–301. [Google Scholar] [CrossRef]
- Dhama, K.; Karthik, K.; Khandia, R.; Munjal, A.; Tiwari, R.; Rana, R.; Khurana, S.K.; Ullah, S.; Khan, R.U.; Alagawany, M.; et al. Medicinal and Therapeutic Potential of Herbs and Plant Metabolites/extracts Countering Viral Pathogens—Current Knowledge and Future Prospects. Curr. Drug Metab. 2018, 19, 236–263. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Tiwari, R.; Chakraborty, S.; Saminathan, M.; Kumar, A.; Karthik, K. Evidence Based Antibacterial Potentials of Medicinal Plants and Herbs Countering Bacterial Pathogens Especially in the Era of Emerging Drug Resistance: An Integrated Update. Int. J. Pharmacol. 2014, 10, 1–43. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; Jayaweera, L.D.; Dias, A.D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Mahima; Rahal, A.; Deb, R.; Latheef, S.K.; Samad, H.A.; Tiwari, R.; Verma, A.K.; Kumar, A.; Dhama, K. Immunomodulatory and Therapeutic Potentials of Herbal, Traditional/indigenous and Ethnoveterinary Medicines. Pak. J. Biol. Sci. 2012, 15, 754–774. [Google Scholar]
- Dhama, K.; Tiwari, R.; Khan, R.U.; Chakraborty, S.; Gopi, M.; Karthik, K.; Saminathan, M.; Desingu, P.A.; Sunkara, L.T. Growth Promoters and Novel Feed Additives Improving Poultry Production and Health, Bioactive Principles and Beneficial Applications: The Trends and Advances—A Review. Int. J. Pharmacol. 2014, 10, 129–159. [Google Scholar] [CrossRef]
- Thakur, P.; Chawla, R.; Chakotiya, A.S.; Tanwar, A.; Goel, R.; Narula, A.; Arora, R.; Sharma, R.K. Camellia Sinensis Ameliorates the Efficacy of Last Line Antibiotics against Carbapenem Resistant Escherichia Coli. Phytother. Res. 2016, 30, 314–322. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- Islam, M.T.; Bardaweel, S.K.; Mubarak, M.S.; Koch, W.; Gaweł-Beben, K.; Antosiewicz, B.; Sharifi-Rad, J. Immunomodulatory Effects of Diterpenes and Their Derivatives through Nlrp3 Inflammasome Pathway: A Review. Front. Immunol. 2020, 11, 572136. [Google Scholar] [CrossRef] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants and Antioxidants: The Interplay. Biomed. Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef]
- Yatoo, M.I.; Gopalakrishnan, A.; Saxena, A.; Parray, O.R.; Tufani, N.A.; Chakraborty, S.; Tiwari, R.; Dhama, K.; Iqbal, H.M.N. Anti-inflammatory Drugs and Herbs with Special Emphasis on Herbal Medicines for Countering Inflammatory Diseases and Disorders—A Review. Recent. Pat. Inflamm. Allergy Drug Discov. 2018, 12, 39–58. [Google Scholar] [CrossRef]
- Abdoly, M.; Farnam, A.; Fathiazad, F.; Khaki, A.; Ibrahimi, A.; Khaki, A.A.; Ibrahimi, O.A. Antidepressant-like Activities of Ocimum Basilicum (Sweet Basil) in the Forced Swimming Test of Rats Exposed to Electromagnetic Field (EMF). Afr. J. Pharm. Pharmacol. 2012, 6, 211–215. [Google Scholar] [CrossRef]
- Hozayen, W.G.; El-Desouky, M.A.; Soliman, H.A.; Ahmed, R.R.; Khaliefa, A.K. Antiosteoporotic Effect of Petroselinumcrispum, Ocimum Basilicum and Cichoriumintybus L. In Glucocorticoid Induced Osteoporosis in Rats. BMC Complement. Altern. Med. 2016, 16, 165. [Google Scholar] [CrossRef]
- Rubab, S.; Hussain, I.; Khan, B.A.; Unar, A.A.; Abbas, K.A.; Khich, Z.H.; Khan, M.; Khanum, S.; Khan, K.U.H. Biomedical Description of Ocimum basilicum L. J. Islam. Int. Med. Colleg. 2017, 12, 59–67. [Google Scholar]
- Shehata, A.M.; Nosir, W. Response of Sweet Basil Plants (Ocimum Basilicum, L.) Grown under Salinity Stress to Spraying Seaweed Extract. Future J. Biol. 2019, 2, 16–28. [Google Scholar]
- Brar, B.; Duhan, J.S.; Rakha, P. Antidepressant Activity of Various Extract from Seed of Ocimum Basilicum Linn. Int. J. Sci. Res. 2015, 4, 41–43. [Google Scholar]
- Ahmad, C.M.; Naz, S.B.; Sharif, A.; Akram, M.; Saeed, M.A. Biological and Pharmacological Properties of the Sweet Basil (Ocimum Basilicum). Br. J. Pharm. Res. 2015, 7, 330–339. [Google Scholar]
- Sestili, P.; Ismail, T.; Calcabrini, C.; Guescini, M.; Catanzaro, E.; Turrini, E.; Layla, A.; Akhtar, S.; Fimognari, C. The Potential Effects of Ocimum Basilicum on Health: A Review of Pharmacological and Toxicological Studies. Expert. Opin. Drug Metab. Toxicol. 2018, 14, 679–692. [Google Scholar] [CrossRef]
- Dhama, K.; Sharun, K.; Gugjoo, M.B.; Tiwari, R.; Alagawany, M.; Iqbal Yatoo, M.; Thakur, P.; Iqbal, H.M.N.; Chaicumpa, W.; Michalak, I.; et al. A comprehensive review on chemical profile and pharmacological activities of Ocimum basilicum. Food Rev. Int. 2023, 39, 119–147. [Google Scholar] [CrossRef]
- Da-Silva, F.; Santos, R.H.S.; Diniz, E.R.; Barbosa, L.C.A.; Casali, V.W.D.; De-Lima, R.R. Content and composition of basil essential oil at two different hours in the day and two seasons. Rev. Bras. De Plants Med. 2003, 6, 33–38. [Google Scholar]
- Sajjadi, S. Analysis of the essential oils of two cultivated basil (Ocimum basilicum L.) from Iran. Daru 2006, 14, 128–130. [Google Scholar]
- Hussain, A.I.; Anwar, F.; Sherazi, S.T.H.; Przybylskmi, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Hanganu, D.; Oniga, I. Antioxidant potential and polyphenolic content of Romanian Ocimum basilicum. Dig. J. Nanomater. Biostructures 2012, 7, 1263–1270. [Google Scholar]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Kwee, E.; Niemeyer, E. Variations in phenolic compositions and antioxidant properties among 15 basil (Ocimum basilicum L.) cultivars. Food Chem. 2011, 128, 1044–1050. [Google Scholar] [CrossRef]
- Güez, C.; de Souza, R.; Fischer, P.; de Moura Leão, M.; Duarte, J.; Boligon, A.; Athayde, M.; Zuravski, L.; de Oliveira, L.; Machado, M. Evaluation of basil extract (Ocimum basilicum L.) on oxidative, anti-genotoxic and antiinflammatory effects in human leukocytes cell cultures exposed to challenging agents. Braz. J. Pharm. Sci. 2017, 53, e15098. [Google Scholar] [CrossRef]
- Marzouk, A. Hepatoprotective triterpenes from hairy root cultures of Ocimum basilicum L. Z. Fur Naturforschung C 2009, 64, 201–209. [Google Scholar] [CrossRef]
- Zhan, Y.; An, X.; Wang, S.; Sun, M.; Zhou, H. Basil polysaccharides: A review on extraction, bioactivities and pharmacological applications. Bioorg Med. Chem. 2020, 28, 115179. [Google Scholar] [CrossRef]
- Al-Snafi, A.E. Chemical constituents and pharmacological effects of Ocimum basilicum—A review. Int. J. Pharm. Res. 2021, 13, 2997–3013. [Google Scholar]
- Hassanpouraghdam, B.; Hassani, A.; Shalamzari, S. Menthone and estragole-rich essential oil of cultivated Ocimum basilicum L. from northwest Iran. Chemija 2010, 21, 59–62. [Google Scholar]
- Wesolowska, A.; Kosecka, D.; Jadczak, D. Essential oil composition of three sweet basil (Ocimum basilicum L.) cultivars. Herba Pol. 2012, 58, 5–16. [Google Scholar]
- Falowo, A.; Mukumbo, F.; Idamokoro, E.; Afolayan, A.; Muchenje, V. Phytochemical constituents and antioxidant activity of sweet basil (Ocimum basilicum L.) essential oil on ground beef from Boran and Nguni cattle. Hindawi Intern. J. Food Sci. 2019, 2019, 2628747. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, E.; El-Khateeb, A.; Azzaz, N. Chemical composition and fungicidal effects of Ocimum basilicum essential oil on Bipolaris and Cochliobolus species. J. Agr. Sci. Tech. 2016, 18, 1143–1152. [Google Scholar]
- Rezzoug, M.; Bakchiche, B.; Gherib, A.; Roberta, A.; Flamini, G.; Kilinçarslan, Ö.; Mammadov, R.; Bardaweel, S. Chemical composition and bioactivity of essential oils and ethanolic extracts of Ocimum basilicum L. and Thymus algeriensis Boiss. &Reut. from the Algerian Saharan Atlas. BMC Compl. Altern. Med. 2019, 19, 146. [Google Scholar]
- Loughrin, J.; Kasperbauer, M. Aroma content of fresh basil (Ocimum basilicum L.) leaves is affected by light reflected from colored mulches. J. Agric. Food Chem. 2003, 51, 2272–2276. [Google Scholar] [CrossRef]
- Beatovi, D.; Krstic-Miloševi, D.; Trifunovic, S.; Šiljegovic, G.; Glamoclija, J.; Ristic, M.; Jelacic, S. Chemical composition, antioxidant and antimicrobial activities of the essential oils of twelve Ocimum basilicum L. cultivars grown in Serbia. Rec. Nat. Prod. 2015, 9, 62–75. [Google Scholar]
- Belong, P.; Ntonga, P.; Fils, E.; Dadji, G.; Tamesse, J. Chemical composition and residue activities of Ocimum canum Sims and Ocimum basilicum L. essential oils on adult female Anopheles funestus ss. J. Anim. Plant Sci. 2013, 19, 2854–2863. [Google Scholar]
- Saaban, K.F.; Ang, C.H.; Khor, S.M.; Chuah, C.H. Chemical constituents and antioxidant capacity of Ocimum basilicum and Ocimum sanctum. Iran J. Chem. Chem. Eng. 2019, 38, 139–152. [Google Scholar]
- Dev, N.; Das, A.; Hossain, M.; Rahman, S. Chemical compositions of different extracts of Ocimum basilicum leaves. J. Sci. Res. 2011, 3, 197–206. [Google Scholar] [CrossRef]
- Ololade, Z.; Fakankun, O.; Alao, F.; Udi, O. Ocimum basilicum var purpureum floral essential oil: Phytochemicals phenolic content antioxidant free radical scavenging and antimicrobial potentials. Glob. J. Sci. Front. Res. B 2014, 14, 31–38. [Google Scholar]
- Adigüzel, A.; Güllüce, M.; Şengül, M.; Öğütcü, H.; Şahin, F.; Karaman, I. Antimicrobial effects of Ocimum basilicum (Labiatae) extract. Turk. J. Biol. 2005, 29, 155–160. [Google Scholar]
- Ababutain, I.M. Antimicrobial Activity and Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Saudi Arabian Ocimum basilicum Leaves Extracts. J. Pure Appl. Microbiol. 2019, 13, 61. [Google Scholar] [CrossRef]
- Backiam, A.D.S.; Duraisamy, S.; Karuppaiya, P.; Balakrishnan, S.; Sathyan, A.; Kumarasamy, A.; Raju, A. Analysis of the main bioactive compounds from Ocimum basilicum for their antimicrobial and antioxidant activity. Biotechnol. Appl. Biochem. 2023, 70, 2038–2051. [Google Scholar] [CrossRef]
- Yibeltal, G.; Yusuf, Z.; Desta, M. Physicochemical properties, antioxidant and antimicrobial activities of Ethiopian sweet basil (Ocimum basilicum L.) leaf and flower oil extracts. Recent. Adv. Anti-Infect. Drug Discov. Former. Recent. Pat. Anti-Infect. Drug Discov. 2022, 17, 131–138. [Google Scholar] [CrossRef]
- Stanojevic, L.P.; Marjanovic-Balaban, Z.R.; Kalaba, V.D.; Stanojevic, J.S.; Cvetkovic, D.J.; Cakic, M.D. Chemical composition, antioxidant and antimicrobial activity of basil (Ocimum basilicum L.) essential oil. J. Essent. Oil Bear. Plants 2017, 20, 1557–1569. [Google Scholar] [CrossRef]
- Shafique, M.; Khan, S.J.; Khan, N.H. Study of antioxidant and antimicrobial activity of sweet basil (Ocimum basilicum) essential oil. Pharmacologyonline 2011, 1, 105–111. [Google Scholar]
- Joshi, R.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum basilicum L. (sweet basil) from Western Ghats of North West Karnataka, India. Anc. Sci. Life 2014, 33, 151. [Google Scholar] [CrossRef]
- Sahu, A.; Nayak, G.; Bhuyan, S.K.; Bhuyan, R.; Kar, D.; Kuanar, A. Antioxidant and antimicrobial activities of Ocimum basilicum var. thyrsiflora against some oral microbes. Multidiscip. Sci. J. 2024, 6, 2024026. [Google Scholar] [CrossRef]
- Tarayrah, H.; Akkawi, M.; Yaghmour, R. Investigations of the Palestinian medicinal plant basil (Ocimum basilicum): Antioxidant, antimicrobial activities, and their phase behavior. Pharm. Pharmacol. Int. J. 2022, 10, 97–104. [Google Scholar]
- Abbasi, F.; Pournaghi, P. Investigating the Anti-Depressant and Anxiolytic Effects of the Rosa canina L. Fruit in Syrian Rats Treated with Bisphenol A. J. Ardabil Univ. Med. Sci. 2022, 22, 154–167. [Google Scholar] [CrossRef]
- Kocić-Tanackov, S.; Dimić, G.; Lević, J.; Tanackov, I.; Tuco, D. Antifungal activities of basil (Ocimum basilicum L.) extract on Fusarium species. Afr. J. Biotechnol. 2011, 10, 10188–10195. [Google Scholar]
- Ahmad, K.; talha Khalil, A.; Somayya, R. Antifungal, phytotoxic and hemagglutination activity of methanolic extracts of Ocimum basilicum. J. Tradit. Chin. Med. 2016, 36, 794–798. [Google Scholar] [CrossRef]
- Jacob, J.K.S.; Carlos, R.C.A.; Divina, C.C. Phytochemical composition, antibacterial and antifungal activities of sweet basil (Ocimum basilicum). Adv. Environ. Biol. 2016, 7, 84–90. [Google Scholar]
- Piyo, A.; Udomsilp, J.; Khang-Khun, P.; Thobunluepop, P. Antifungal activity of essential oils from basil (Ocimum basilicum Linn.) and sweet fennel (Ocimum gratissimum Linn.): Alternative strategies to control pathogenic fungi in organic rice. Asian J. Food Agro-Ind. 2009, S2–S9. Available online: https://www.thaiscience.info/Journals/Article/AFAI/10850172.pdf (accessed on 8 January 2024).
- Gucwa, K.; Milewski, S.; Dymerski, T.; Szweda, P. Investigation of the antifungal activity and mode of action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus essential oils. Molecules 2018, 23, 1116. [Google Scholar] [CrossRef]
- Gülçin, I.; Elmastaş, M.; Aboul-Enein, H.Y. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Product. Deriv. 2007, 21, 354–361. [Google Scholar] [CrossRef]
- Nadeem, H.R.; Akhtar, S.; Sestili, P.; Ismail, T.; Neugart, S.; Qamar, M.; Esatbeyoglu, T. Toxicity, antioxidant activity, and phytochemicals of basil (Ocimum basilicum L.) leaves cultivated in Southern Punjab, Pakistan. Foods 2022, 11, 1239. [Google Scholar] [CrossRef]
- Bravo, E.; Amrani, S.; Aziz, M.; Harnafi, H.; Napolitano, M. Ocimum basilicum ethanolic extract decreases cholesterol synthesis and lipid accumulation in human macrophages. Fitoterapia 2008, 79, 515–523. [Google Scholar] [CrossRef]
- Khaki, A.; Khaki, A.A.; Ezzatzadeh, A.; Hamidreza, A. Effect of Ocimum basilicum on ovary tissue histopathology after exposure to electromagnetic fields (EMF) in rats. Afr. J. Pharm. Pharmacol. 2013, 7, 1703–1706. [Google Scholar] [CrossRef]
- Kaurinovic, B.; Popovic, M.; Vlaisavljevic, S.; Trivic, S. Antioxidant capacity of Ocimum basilicum and Origanum vulgare L. extracts. Molecules 2011, 16, 7401–7414. [Google Scholar] [CrossRef] [PubMed]
- Fokou, J.B.H.; Pierre, N.J.; Gisele, E.L.; Christian, N.C.; Michel, J.D.P.; Laza, I.M.; Boyom, F.F.; Emmanuel, B. In vitro antioxidant and anti-inflammatory potential of the optimized combinations of essential oils from three cameroon grew Ocimum L. J. Pharm. Pharmacol. 2020, 8, 207–219. [Google Scholar]
- Ahmed, A.S.; Fanokh, A.K.M.; Mahdi, M.A. Phytochemical identification and antioxidant study of essential oil constituents of Ocimum basilicum L. Growing in Iraq. Pharmacog J. 2019, 11, 724–729. [Google Scholar] [CrossRef]
- Teofilović, B.; Tomas, A.; Martić, N.; Stilinović, N.; Popović, M.; Čapo, I.; Grujić, N.; Ilinčić, B.; Rašković, A. Antioxidant and hepatoprotective potential of sweet basil (Ocimum basilicum L.) extract in acetaminophen-induced hepatotoxicity in rats. J. Funct. Foods. 2021, 87, 104783. [Google Scholar] [CrossRef]
- Soliman, A.; Rizk, M.; Shalaby, M.; Elkomy, A. Mechanisms of hepato-renal protective activity of Ocimum basilicum leaf extract against paracetamol toxicity in rat model. Adv. Anim. Vet. Sci. 2020, 8, 385–391. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Human. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Politeo, O.; Jukic, M.; Milos, M. Chemical composition and antioxidant capacity of free volatile aglycones from basil (Ocimum basilicum L.) compared with its essential oil. Food Chem. 2007, 101, 379–385. [Google Scholar] [CrossRef]
- Zhakipbekov, K.; Turgumbayeva, A.; Issayeva, R.; Kipchakbayeva, A.; Kadyrbayeva, G.; Tleubayeva, M.; Akhayeva, T.; Tastambek, K.; Sainova, G.; Serikbayeva, E.; et al. Antimicrobial and Other Biomedical Properties of Extracts from Plantago major, Plantaginaceae. Pharmaceuticals 2023, 16, 1092. [Google Scholar] [CrossRef]
- Shegebayev, Z.; Turgumbayeva, A.; Datkhayev, U.; Zhakipbekov, K.; Kalykova, A.; Kartbayeva, E.; Beyatli, A.; Tastambek, K.; Altynbayeva, G.; Dilbarkhanov, B.; et al. Pharmacological Properties of Four Plant Species of the Genus Anabasis, Amaranthaceae. Molecules 2023, 28, 4454. [Google Scholar] [CrossRef]
- Pandey, A.K.; Tiwari, S.P.; Biswas, D.; Patel, Y.; Jajda, H.M.; Dave, G.S. Evaluation of Phytochemicals, Antioxidant and Anti-inflammatory properties of leaves of Ocimum basilicum L. Res. J. Pharm. Technol. 2023, 16, 1981–1986. [Google Scholar] [CrossRef]
- Raina, P.; Deepak, M.; Chandrasekaran, C.V.; Agarwal, A.; Wagh, N.; Kaul-Ghanekar, R. Comparative analysis of anti-inflammatory activity of aqueous and methanolic extracts of Ocimum basilicum (basil) in RAW264. 7, SW1353 and human primary chondrocytes in respect of the management of osteoarthritis. J. Herbal. Med. 2016, 6, 28–36. [Google Scholar] [CrossRef]
- Yadav, N.P.; Khatri, R.; Bawankule, D.U.; Pal, A.; Shanker, K.; Srivastava, P.; Gupta, A.K.; Chanda, D. Topical anti-inflammatory effects of Ocimum basilicum leaf extract in the phorbol-12, 13-dibutyrate model of mouse ear inflammation. Planta Medica 2009, 75, PA72. [Google Scholar] [CrossRef]
- Eftekhar, N.; Moghimi, A.; Mohammadian Roshan, N.; Saadat, S.; Boskabady, M.H. Immunomodulatory and anti-inflammatory effects of hydro-ethanolic extract of Ocimum basilicum leaves and its effect on lung pathological changes in an ovalbumin-induced rat model of asthma. BMC Complement. Altern. Med. 2019, 19, 349. [Google Scholar] [CrossRef]
- Osei Akoto, C.; Acheampong, A.; Boakye, Y.D.; Naazo, A.A.; Adomah, D.H. Anti-inflammatory, antioxidant, and anthelmintic activities of Ocimum basilicum (Sweet Basil) fruits. J. Chem. 2020, 2020, 2153534. [Google Scholar] [CrossRef]
- Złotek, U.; Szymanowska, U.; Karaś, M.; Świeca, M. Antioxidative and anti-inflammatory potential of phenolics from purple basil (Ocimum basilicum L.) leaves induced by jasmonic, arachidonic and β-aminobutyric acid elicitation. Int. J. Food Sci. Technol. 2016, 51, 163–170. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Cesário, F.R.A.S.; e Castro, F.F.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Júnior, L.J.Q.; da Costa, J.G.M.; Coutinho, H.D.M.; et al. Anti-inflammatory and antiedematogenic activity of the Ocimum basilicum essential oil and its main compound estragole: In vivo mouse models. Chem.-Biol. Interact. 2016, 257, 14–25. [Google Scholar] [CrossRef]
- Okoye-Festus, B.C.; Willfred, O.O.; Felix, A.O.; Ogheneogaga, I.O.; Peace, O.; Ngozi, I.N.; Jane, A.; Okechukwu, O.N. Chemical composition and anti-inflammatory activity of essential oils from the leaves of Ocimum basilicum L. and Ocimum gratissimum L. (Lamiaceae). Int. J. Pharmacogn. 2014, 1, 59–65. [Google Scholar]
- Takeuchi, H.; Takahashi-Muto, C.; Nagase, M.; Kassai, M.; Tanaka-Yachi, R.; Kiyose, C. Anti-inflammatory effects of extracts of sweet basil (Ocimum basilicum L.) on a co-culture of 3T3-L1 adipocytes and RAW264. 7 macrophages. J. Oleo Sci. 2020, 69, 487–493. [Google Scholar] [CrossRef]
- Rodrigues, L.B.; Martins, A.O.B.P.B.; Ribeiro-Filho, J.; Cesário, F.R.A.S.; e Castro, F.F.; de Albuquerque, T.R.; Fernandes, M.N.M.; da Silva, B.A.F.; Júnior, L.J.Q.; de Sousa Araújo, A.A.; et al. Anti-inflammatory activity of the essential oil obtained from Ocimum basilicum complexed with β-cyclodextrin (β-CD) in mice. Food Chem. Toxicol. 2017, 109, 836–846. [Google Scholar] [CrossRef]
- Khan, B.A.; Ullah, S.; Khan, M.K.; Alshahrani, S.M.; Braga, V.A. Formulation and evaluation of Ocimum basilicum-based emulgel for wound healing using animal model. Saudi Pharm. J. 2020, 28, 1842–1850. [Google Scholar] [CrossRef] [PubMed]
- Antonescu, I.A.; Antonescu, A.; Miere, F.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; et al. Evaluation of wound healing potential of novel hydrogel based on Ocimum basilicum and Trifolium pratense extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Zangeneh, M.M.; Zangeneh, A.; Seydi, N.; Moradi, R. Evaluation of cutaneous wound healing activity of Ocimum basilicum aqueous extract ointment in rats. Comp. Clin. Pathol. 2019, 28, 1447–1454. [Google Scholar] [CrossRef]
- Dubey, G.D.G.; Pathak, A.K.P.A.K. Wound healing activity of hydro-alcoholic extract of Ocimum basilicum Linn. aerial parts in wistar rats. Int. J. Indig. Herbs Drugs 2017, 11–13, 76. [Google Scholar]
- Chiang, L.C.; Ng, L.T.; Cheng, P.W.; Chiang, W.; Lin, C.C. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin. Exp. Pharmacol. Physiol. 2005, 32, 811–816. [Google Scholar] [CrossRef]
- Kurnia, D.; Putri, S.A.; Tumilaar, S.G.; Zainuddin, A.; Dharsono, H.D.A.; Amin, M.F. In silico Study of Antiviral Activity of Polyphenol Compounds from Ocimum basilicum by Molecular Docking, ADMET, and Drug-Likeness Analysis. Adv. Appl. Bioinform. Chem. 2023, 16, 37–47. [Google Scholar] [CrossRef]
- Behbahani, M.; Mohabatkar, H.; Soltani, M. Anti-HIV-1 activities of aerial parts of Ocimum basilicum and its parasite Cuscuta campestris. J. Antivir. Antiretrovir. 2013, 5, 57–61. [Google Scholar] [CrossRef]
- Al-Amri, S.A. In vitro antiviral potential of Ocimum basilicum and Olea europaea leaves extract against Newcastle Disease Virus of poultry: Sarah AH Al-Amri1@, Shony M. Odisho1 and Orooba MS Ibrahem2. Iraqi J. Vet. Med. 2015, 39, 94–99. [Google Scholar] [CrossRef]
- Romeilah, R.M.; Fayed, S.A.; Mahmoud, G.I. Chemical compositions, antiviral and antioxidant activities of seven essential oils. J. Appl. Sci. Res. 2010, 6, 50–62. [Google Scholar]
- Yucharoen, R.; Anuchapreeda, S.; Tragoolpua, Y. Anti-herpes simplex virus activity of extracts from the culinary herbs Ocimum sanctum L.; Ocimum basilicum L. and Ocimum americanum L. Afr. J. Biotechnol. 2011, 10, 860–866. [Google Scholar]
- Kubiça, T.F.; Alves, S.H.; Weiblen, R.; Lovato, L.T. In vitro inhibition of the bovine viral diarrhoea virus by the essential oil of Ocimum basilicum (basil) and monoterpenes. Braz. J. Microbiol. 2014, 45, 209–214. [Google Scholar] [CrossRef] [PubMed]

| Extracts | Plant Part | Method | Biological Active Compounds | Pharmacology Activity | Country | Ref. |
|---|---|---|---|---|---|---|
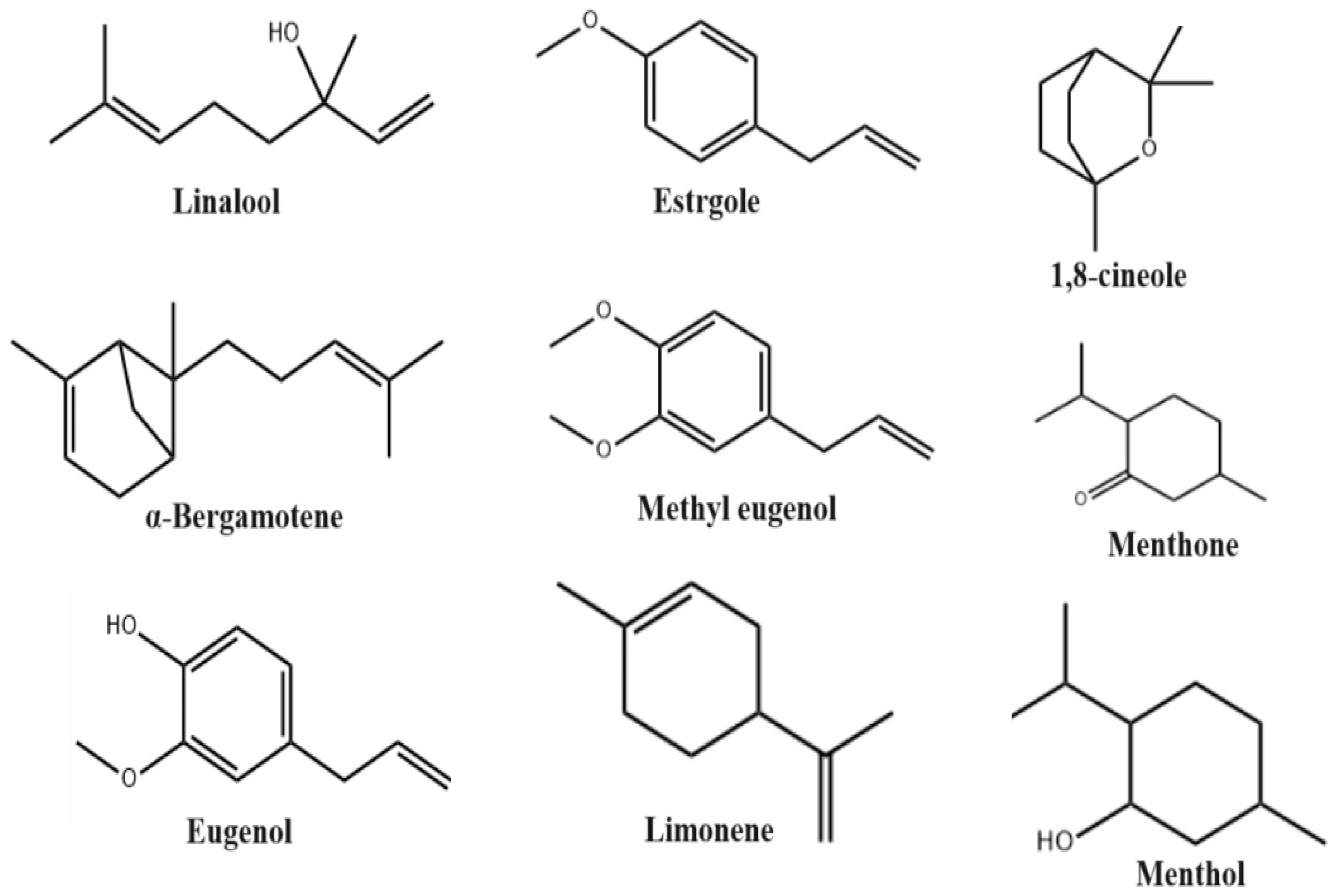
| Essential oil | Leaves, seed, root | GC/MS | Menthone (33.1%), oxygenated monoterpenoids (77.8%), estragole (21.5%), oxygenated monoterpenes (75.3%), isoneomenthol (7.5%), transcaryophyllene (2.2%), menthol (6.1%), limonene (1.5%), pulegone (3.7%), sesquiterpene hydrocarbons (8.8%), trans-β-farnesene (1.1%), germacrene D (1.4%), α-amorphene (1.1%), menthyl acetate (5.6%), α-cadinol (2.9%), methyl eugenol (1%), sesquiterpenoids (12.8%). | Antioxidant, Antimicrobial, Ani-Inflammatory | Iran | [32] |
| Essential oil | Leaves, seed | GC/MS | Bolloso Napoletano: linalool (47.75%), 1,8-cineole (10.23%), methyl chavicol (20.21%); Foglie di Lattuga: linalool (48.65%), 1,8-cineole (12.59%), methyl chavicol (18.55%). Thai Siam: linalool (36.60%), methyl chavicol (7.50%), (E)-methyl cinnamate (21.90%). | Antioxidant, Anti-Inflammatory, Antiviral | Poland | [33] |
| Etanolic, Metanolic | Stem, seed | HPLC, GC/MS | 1,6-octadiene-3-ol, 3,7-dimethyl (29.49%), eucalyptol (3.31%), cis-alpha-bisabolene (1.92%), trans-alpha-bergamotene (5.32%), beta-myrcene (1.11%), levomenthol (1.81%). | Antimicrobial, Antioxidant | South Africa | [34] |
| Etanolic, Metanolic | Leaves, seed, root | HPLC, GC/MS | 1,8-cineole (10.56%), linalool 48.4%, methyl chavicol 14.3%, α-bergamotene 27%, oxygen monoterpenes (57.42%), β-bisabolol 4.1%, methyl eugenol (10.09%), stragol (55.95%), sesquiterpene hydrocarbons (6.9%). | Antioxidant, Anti-Inflammatory, Antifungal | Egypt | [35] |
| Essential oil | Leaves | GC/MS | Linalyl acetate (19.1%), linalool (52.1%). Aliphatic compounds (9980–17,929 nanograms per gram fresh weight), including (E)-2-hexenal: 1519–1991, (Z)-3-hexenal (4991–10731), (E)-2-hexen-1-ol: 75–144, (Z)-3-hexen-1-ol: 1436–2219, n-hexanol: 73 –175, 1-octen-3-ol: 1610–2689, (Z)-3-hexenyl acetate: 54–99; eugenol (66,142–131,926). α-pinene: 875–1198, camphene: 153–295, β-pinene: 1780–2771, 2-carene: 42–142, myrcene: 2770–3030, limonene: 712–870, 1,8-cineole: 26,640–52,799, 3-carene: 41–48, linalool: 42,726–65,033, bornyl acetate: 332–1163, camphor: 164–463, tepinen-4-ol: 185–364, eugenol: 945 –1948, α-terpineol: 159–310, α-bergamotene: 202–406 and (E,E)-α farnesene: 32–65), α-humulene: 141–538, caryophyllene: 641–1432. | Wound Healing, Antiviral, Antimicrobial | Algeria | [36,37] |
| Essential oil | Leaves | HPLC, GC/MS | Linalool and 1,8-cineole. | Antimicrobial and Antioxidant | Serbia | [38] |
| Essential oil | Leaves, stem | HPLC, GC/MS | Limonene (30.9%), p-cymene (2.6%), linalool (18.9%), thymol (6.5%), B-phellandrene (15.3%), O-cardinol (2.6%). | Antimicrobial and Antioxidant | Cameroon | [39] |
| Etanolic, n-hexane | Leaves, stem | TLC, HPLC | Estragole (>35.71%), trans-α-bergamotene (>0.83%), (E)-β-ocimene (>1.47%), eucalyptol (>0.25%), τ-cadinol (>0.41%). | Antimicrobial and Antioxidant | Malaysia | [40] |
| Essential oil | Leaves | FT-IR, GC/MS | Eugenol (61.76%), [2-methyl-4-(1))-propyl)phenoxy]silane (2.01%), 2,3-dihydroxypropyl elaidate (5.10%), isopropyl palpitate (11.36%), 2-methoxy-4-(1-propyl)phenol (2.65%), α-cubene (3.85%), vanillin (1.27%), 1-methyl-3-(1-methyl)benzene (1.73%), 1,4-diethylbenzene (1.03%), hexadecanoic acid methyl ester (2.51%). | Wound Healing | Bangladesh | [41] |
| Essential oil | Leaves | HPLC | Methyleugenol (15.5%), patchoulan (6.7%), 2-phenyl-1-hexanol (14.0%), o-nitrocumene (14.0%), 2-methyl- 3,5-dodecadiine (14.0%), 1-(4,5-dimethyl-2-nitrophenyl)-1H-tetraazole (14.0%). | Antimicrobial and Antioxidant | Nigeria | [42] |
| Tested Microorganism | Ethanolic Extract | Methanolic Extract | Aqueous Extract | Acetone Extract | Linalool | Ref. |
|---|---|---|---|---|---|---|
| Diameter of inhibition zone (mm) | ||||||
| S. aureus | 20.4 ± 1.0 | 26.9 ± 1.2 | 24.1 ± 1.2 | 21.2 ± 1.2 | 26.1 ± 1.1 | [44,45,46] |
| P. multocida | 24.4 ± 1.1 | 25.3 ± 1.1 | 23.2 ± 1.4 | 22.2 ± 1.3 | 24.0 ± 1.0 | [43] |
| B. subtilis | 13.2 ± 0.8 | 19.5 ± 1.1 | 13.5 ± 0.8 | 11.4 ± 0.6 | 16.2 ± 1.0 | [44] |
| E. coli | 13.6 ± 0.8 | 22.3 ± 1.0 | 18.4 ± 1.0 | 16.1 ± 1.0 | 18.0 ± 0.9 | [44] |
| M. mucedo | 19.4 ± 1.1 | 21.4 ± 1.0 | 17.7 ± 1.3 | 15.2 ± 0.7 | 11.7 ± 0.7 | [43] |
| A. niger | 21.6 ± 1.2 | 23.3 ± 0.8 | 20.4 ± 1.2 | 18.4 ± 1.2 | 18.7 ± 0.7 | [43] |
| F. solani | 13.6 ± 0.8 | 11.2 ± 0.6 | 9.7 ± 0.6 | 11.1 ± 0.9 | 9.7 ± 0.6 | [43] |
| R. solani | 17.2 ± 1.0 | 17.6 ± 1.0 | 16.6 ± 1.0 | 14.3 ± 1.1 | 13.6 ± 0.8 | [43] |
| B. theobromae | 13.5 ± 0.8 | 17.3 ± 0.8 | 14.3 ± 0.8 | 12.3 ± 0.7 | 10.3 ± 0.6 | [43] |
| Minimum inhibitory concentration (mg/mL) | ||||||
| S. aureus | 1.2 ± 0.0 | 0.8 ± 0.0 | 0.8 ± 0.0 | 1.4 ± 0.0 | 0.3 ± 0.0 | [44,45,46] |
| P. multocida | 1.5 ± 0.0 | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.3 ± 0.0 | 0.4 ± 0.0 | [43] |
| B. subtilis | 0.06 ± 0.1 | 0.03 ± 0.1 | 2.0 ± 0.1 | 2.6 ± 0.1 | 0.9 ± 0.0 | [44] |
| E. coli | 2.2 ± 0.1 | 4.5 ± 0.2 | 2.7 ± 0.1 | 3.2 ± 0.2 | 1.0 ± 0.1 | [44] |
| M. mucedo | 2.0 ± 0.1 | 1.7 ± 0.1 | 2.3 ± 0.1 | 1.9 ± 0.1 | 0.9 ± 0.0 | [43] |
| A. niger | 3.0 ± 0.2 | 5.0 ± 0.3 | 2.9 ± 0.2 | 4.3 ± 0.2 | 1.5 ± 0.1 | [43] |
| F. solani | 2.7 ± 0.1 | 4.9 ± 0.2 | 3.2 ± 0.2 | 3.6 ± 0.2 | 1.6 ± 0.1 | [43] |
| R. solani | 2.3 ± 0.1 | 4.6 ± 0.2 | 2.9 ± 0.2 | 4.1 ± 0.2 | 1.1 ± 0.0 | [43] |
| B. theobromae | 3.8 ± 0.2 | 5.1 ± 0.3 | 4.6 ± 0.2 | 4.9 ± 0.3 | 1.9 ± 0.1 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhakipbekov, K.; Turgumbayeva, A.; Akhelova, S.; Bekmuratova, K.; Blinova, O.; Utegenova, G.; Shertaeva, K.; Sadykov, N.; Tastambek, K.; Saginbazarova, A.; et al. Antimicrobial and Other Pharmacological Properties of Ocimum basilicum, Lamiaceae. Molecules 2024, 29, 388. https://doi.org/10.3390/molecules29020388
Zhakipbekov K, Turgumbayeva A, Akhelova S, Bekmuratova K, Blinova O, Utegenova G, Shertaeva K, Sadykov N, Tastambek K, Saginbazarova A, et al. Antimicrobial and Other Pharmacological Properties of Ocimum basilicum, Lamiaceae. Molecules. 2024; 29(2):388. https://doi.org/10.3390/molecules29020388
Chicago/Turabian StyleZhakipbekov, Kairat, Aknur Turgumbayeva, Sholpan Akhelova, Kymbat Bekmuratova, Olga Blinova, Gulnara Utegenova, Klara Shertaeva, Nurlan Sadykov, Kuanysh Tastambek, Akzharkyn Saginbazarova, and et al. 2024. "Antimicrobial and Other Pharmacological Properties of Ocimum basilicum, Lamiaceae" Molecules 29, no. 2: 388. https://doi.org/10.3390/molecules29020388
APA StyleZhakipbekov, K., Turgumbayeva, A., Akhelova, S., Bekmuratova, K., Blinova, O., Utegenova, G., Shertaeva, K., Sadykov, N., Tastambek, K., Saginbazarova, A., Urazgaliyev, K., Tulegenova, G., Zhalimova, Z., & Karasova, Z. (2024). Antimicrobial and Other Pharmacological Properties of Ocimum basilicum, Lamiaceae. Molecules, 29(2), 388. https://doi.org/10.3390/molecules29020388






