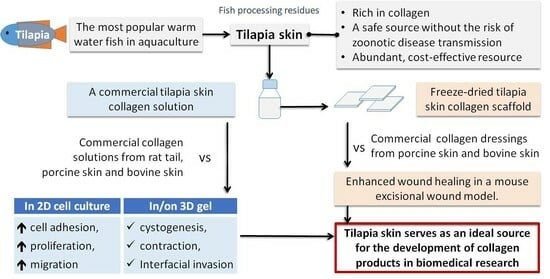
Assessment of Tilapia Skin Collagen for Biomedical Research Applications in Comparison with Mammalian Collagen
Abstract
1. Introduction
2. Results
2.1. The Composition and Fibril Formation of the Tilapia Skin Collagen
2.2. The Effects of Tilapia Skin Collagen on Cell Adhesion, Proliferation, and Migration
2.3. Application of Tilapia Skin Collagen in Three-Dimensional (3D) Cell Culture
2.3.1. The MDCK 3D Culture Model
2.3.2. The Fibroblast-Induced Gel Contraction Model
2.3.3. The Tumor Spheroid Interfacial Invasiveness Model
2.4. The Effects of the Freeze-Dried FC Collagen Scaffold on Wound Healing
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Collagen Solutions
4.3. SDS-PAGE
4.4. Collagen Fibril Formation
4.5. Cell Adhesion Assay
4.6. Cell Proliferation Assay
4.7. Cell Migration Assay
4.8. MDCK Cells in 3D Culture
4.9. Contractility Assay
4.10. Interfacial Invasiveness of Tumor Spheroid Assay
4.11. Fabrication of Collagen Scaffold and Scanning Electron Microscopy Observation
4.12. The Treatment of the Collagen Biomaterials on a Mouse Excisional Wound Model
4.13. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed]
- Felician, F.F.; Xia, C.; Qi, W.; Xu, H. Collagen from Marine Biological Sources and Medical Applications. Chem. Biodivers. 2018, 15, e1700557. [Google Scholar] [CrossRef] [PubMed]
- Coppola, D.; Lauritano, C.; Palma Esposito, F.; Riccio, G.; Rizzo, C.; de Pascale, D. Fish Waste: From Problem to Valuable Resource. Mar. Drugs 2021, 19, 116. [Google Scholar] [CrossRef] [PubMed]
- Akita, M.; Nishikawa, Y.; Shigenobu, Y.; Ambe, D.; Morita, T.; Morioka, K.; Adachi, K. Correlation of proline, hydroxyproline and serine content, denaturation temperature and circular dichroism analysis of type I collagen with the physiological temperature of marine teleosts. Food Chem. 2020, 329, 126775. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.S.; Ok, Y.J.; Hwang, S.Y.; Kwak, J.Y.; Yoon, S. Marine Collagen as A Promising Biomaterial for Biomedical Applications. Mar. Drugs 2019, 17, 467. [Google Scholar] [CrossRef]
- Karim, A.A.; Bhat, R. Fish gelatin: Properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll. 2009, 23, 563–576. [Google Scholar] [CrossRef]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll. 2004, 18, 581–592. [Google Scholar] [CrossRef]
- Renuhadevi, M.; Jeevagan, I.J.M.A.; Ahilan, B.; Rajagopalsamy, C.B.T.; Prabu, E. Tilapia—An Excellent Candidate Species for World Aquaculture: A Review. Annu. Res. Rev. Biol. 2019, 31, 1–14. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.; Xue, Y.; Ding, T.; Sun, J. Hydrolyzed tilapia fish collagen modulates the biological behavior of macrophages under inflammatory conditions. RSC Adv. 2015, 5, 30727–30736. [Google Scholar] [CrossRef]
- Liu, C.; Sun, J. Potential application of hydrolyzed fish collagen for inducing the multidirectional differentiation of rat bone marrow mesenchymal stem cells. Biomacromolecules 2014, 15, 436–443. [Google Scholar] [CrossRef]
- Tang, J.; Saito, T. Biocompatibility of Novel Type I Collagen Purified from Tilapia Fish Scale: An In Vitro Comparative Study. BioMed Res. Int. 2015, 2015, 139476. [Google Scholar] [CrossRef]
- Elbialy, Z.I.; Atiba, A.; Abdelnaby, A.; Al, H., II; Elsheshtawy, A.; El-Serehy, H.A.; Abdel-Daim, M.M.; Fadl, S.E.; Assar, D.H. Collagen extract obtained from Nile tilapia (Oreochromis niloticus L.) skin accelerates wound healing in rat model via up regulating VEGF, bFGF, and alpha-SMA genes expression. BMC Vet. Res. 2020, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Ge, B.; Wang, H.; Li, J.; Liu, H.; Yin, Y.; Zhang, N.; Qin, S. Comprehensive Assessment of Nile Tilapia Skin (Oreochromis niloticus) Collagen Hydrogels for Wound Dressings. Mar. Drugs 2020, 18, 178. [Google Scholar] [CrossRef] [PubMed]
- Darvish, D.M. Collagen fibril formation in vitro: From origin to opportunities. Mater. Today Bio 2022, 15, 100322. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Yang, T.P.; Jiang, S.T.; Yang, H.Y.; Tang, M.J. Bcl-2 overexpression prevents apoptosis-induced Madin-Darby canine kidney simple epithelial cyst formation. Kidney Int. 1999, 55, 168–178. [Google Scholar] [CrossRef]
- Shen, Y.; Guan, D.; Serien, D.; Takeuchi, S.; Tong, P.; Yobas, L.; Huang, P. Mechanical Characterization of Microengineered Epithelial Cysts by Using Atomic Force Microscopy. Biophys. J. 2017, 112, 398–409. [Google Scholar] [CrossRef]
- Engelberg, J.A.; Datta, A.; Mostov, K.E.; Hunt, C.A. MDCK cystogenesis driven by cell stabilization within computational analogues. PLoS Comput. Biol. 2011, 7, e1002030. [Google Scholar] [CrossRef]
- Ngo, P.; Ramalingam, P.; Phillips, J.A.; Furuta, G.T. Collagen gel contraction assay. Methods Mol. Biol. 2006, 341, 103–109. [Google Scholar] [CrossRef]
- Zhang, T.; Day, J.H.; Su, X.; Guadarrama, A.G.; Sandbo, N.K.; Esnault, S.; Denlinger, L.C.; Berthier, E.; Theberge, A.B. Investigating Fibroblast-Induced Collagen Gel Contraction Using a Dynamic Microscale Platform. Front. Bioeng. Biotechnol. 2019, 7, 196. [Google Scholar] [CrossRef]
- Hsu, C.K.; Lin, H.H.; Harn, H.I.; Ogawa, R.; Wang, Y.K.; Ho, Y.T.; Chen, W.R.; Lee, Y.C.; Lee, J.Y.; Shieh, S.J.; et al. Caveolin-1 Controls Hyperresponsiveness to Mechanical Stimuli and Fibrogenesis-Associated RUNX2 Activation in Keloid Fibroblasts. J. Investig. Dermatol. 2018, 138, 208–218. [Google Scholar] [CrossRef]
- Mao, B.H.; Nguyen Thi, K.M.; Tang, M.J.; Kamm, R.D.; Tu, T.Y. The interface stiffness and topographic feature dictate interfacial invasiveness of cancer spheroids. Biofabrication 2023, 15, 015023. [Google Scholar] [CrossRef]
- Shoval, H.; Karsch-Bluman, A.; Brill-Karniely, Y.; Stern, T.; Zamir, G.; Hubert, A.; Benny, O. Tumor cells and their crosstalk with endothelial cells in 3D spheroids. Sci. Rep. 2017, 7, 10428. [Google Scholar] [CrossRef] [PubMed]
- Berens, E.B.; Holy, J.M.; Riegel, A.T.; Wellstein, A. A Cancer Cell Spheroid Assay to Assess Invasion in a 3D Setting. J. Vis. Exp. JoVE 2015, 105, e53409. [Google Scholar] [CrossRef]
- Frangogiannis, N. Transforming growth factor-beta in tissue fibrosis. J. Exp. Med. 2020, 217, e20190103. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Gong, D.J.; Lee, H.H.; Seo, J.Y.; Song, K.M.; Eom, S.J.; Yeo, S.Y. Mechanical Properties of Porcine and Fish Skin-Based Collagen and Conjugated Collagen Fibers. Polymers 2021, 13, 2151. [Google Scholar] [CrossRef] [PubMed]
- Gallo, N.; Natali, M.L.; Sannino, A.; Salvatore, L. An Overview of the Use of Equine Collagen as Emerging Material for Biomedical Applications. J. Funct. Biomater. 2020, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Gistelinck, C.; Gioia, R.; Gagliardi, A.; Tonelli, F.; Marchese, L.; Bianchi, L.; Landi, C.; Bini, L.; Huysseune, A.; Witten, P.E.; et al. Zebrafish Collagen Type I: Molecular and Biochemical Characterization of the Major Structural Protein in Bone and Skin. Sci. Rep. 2016, 6, 21540. [Google Scholar] [CrossRef]
- Ohara, H.; Matsumoto, H.; Ito, K.; Iwai, K.; Sato, K. Comparison of quantity and structures of hydroxyproline-containing peptides in human blood after oral ingestion of gelatin hydrolysates from different sources. J. Agric. Food Chem. 2007, 55, 1532–1535. [Google Scholar] [CrossRef]
- Michael, S.; Winters, C.; Khan, M. Acellular Fish Skin Graft Use for Diabetic Lower Extremity Wound Healing: A Retrospective Study of 58 Ulcerations and a Literature Review. Wounds A Compend. Clin. Res. Pract. 2019, 31, 262–268. [Google Scholar]
- Wallner, C.; Holtermann, J.; Drysch, M.; Schmidt, S.; Reinkemeier, F.; Wagner, J.M.; Dadras, M.; Sogorski, A.; Houschyar, K.S.; Becerikli, M.; et al. The Use of Intact Fish Skin as a Novel Treatment Method for Deep Dermal Burns Following Enzymatic Debridement: A Retrospective Case-Control Study. Eur. Burn J. 2022, 3, 43–55. [Google Scholar] [CrossRef]
- Li, D.; Sun, W.Q.; Wang, T.; Gao, Y.; Wu, J.; Xie, Z.; Zhao, J.; He, C.; Zhu, M.; Zhang, S.; et al. Evaluation of a novel tilapia-skin acellular dermis matrix rationally processed for enhanced wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 127, 112202. [Google Scholar] [CrossRef] [PubMed]
- Lima Junior, E.M.; De Moraes Filho, M.O.; Costa, B.A.; Rohleder, A.V.P.; Sales Rocha, M.B.; Fechine, F.V.; Forte, A.J.; Alves, A.; Silva Junior, F.R.; Martins, C.B.; et al. Innovative Burn Treatment Using Tilapia Skin as a Xenograft: A Phase II Randomized Controlled Trial. J. Burn Care Res. Off. Publ. Am. Burn Assoc. 2020, 41, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Lima Junior, E.M.; de Moraes Filho, M.O.; Costa, B.A.; Fechine, F.V.; Vale, M.L.; Diogenes, A.K.L.; Neves, K.R.T.; Uchoa, A.; Soares, M.; de Moraes, M.E.A. Nile Tilapia Fish Skin-Based Wound Dressing Improves Pain and Treatment-Related Costs of Superficial Partial-Thickness Burns: A Phase III Randomized Controlled Trial. Plast. Reconstr. Surg. 2021, 147, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.; de Almeida Cruz, M.; de Araujo, T.A.T.; Parisi, J.R.; do Vale, G.C.A.; Dos Santos Jorge Sousa, K.; Ribeiro, D.A.; Granito, R.N.; Renno, A.C.M. Fish collagen for skin wound healing: A systematic review in experimental animal studies. Cell Tissue Res. 2022, 388, 489–502. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Postlethwaite, A.E.; Seyer, J.M.; Kang, A.H. Chemotactic attraction of human fibroblasts to type I, II, and III collagens and collagen-derived peptides. Proc. Natl. Acad. Sci. USA 1978, 75, 871–875. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, P.; Zhou, C.; Li, S.; Hong, P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar. Drugs 2017, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.R.; Gelman, R.A.; Poppke, D.C.; Piez, K.A. Collagen fibril formation. Optimal in vitro conditions and preliminary kinetic results. J. Biol. Chem. 1978, 253, 6578–6585. [Google Scholar] [CrossRef]
- Humphries, M.J. Cell adhesion assays. Methods Mol. Biol. 2009, 522, 203–210. [Google Scholar] [CrossRef]
- Reed, M.J.; Vernon, R.B.; Abrass, I.B.; Sage, E.H. TGF-beta 1 induces the expression of type I collagen and SPARC, and enhances contraction of collagen gels, by fibroblasts from young and aged donors. J. Cell. Physiol. 1994, 158, 169–179. [Google Scholar] [CrossRef]
- Rojkind, M.; Giambrone, M.A.; Biempica, L. Collagen types in normal and cirrhotic liver. Gastroenterology 1979, 76, 710–719. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-Y.; Wong, T.-Y.; Tu, T.-Y.; Tang, M.-J.; Lin, H.-H.; Hsueh, Y.-Y. Assessment of Tilapia Skin Collagen for Biomedical Research Applications in Comparison with Mammalian Collagen. Molecules 2024, 29, 402. https://doi.org/10.3390/molecules29020402
Huang J-Y, Wong T-Y, Tu T-Y, Tang M-J, Lin H-H, Hsueh Y-Y. Assessment of Tilapia Skin Collagen for Biomedical Research Applications in Comparison with Mammalian Collagen. Molecules. 2024; 29(2):402. https://doi.org/10.3390/molecules29020402
Chicago/Turabian StyleHuang, Jyun-Yuan, Tzyy-Yue Wong, Ting-Yuan Tu, Ming-Jer Tang, Hsi-Hui Lin, and Yuan-Yu Hsueh. 2024. "Assessment of Tilapia Skin Collagen for Biomedical Research Applications in Comparison with Mammalian Collagen" Molecules 29, no. 2: 402. https://doi.org/10.3390/molecules29020402
APA StyleHuang, J.-Y., Wong, T.-Y., Tu, T.-Y., Tang, M.-J., Lin, H.-H., & Hsueh, Y.-Y. (2024). Assessment of Tilapia Skin Collagen for Biomedical Research Applications in Comparison with Mammalian Collagen. Molecules, 29(2), 402. https://doi.org/10.3390/molecules29020402