Porphyrin-Based Metal-Organic Framework Materials: Design, Construction, and Application in the Field of Photocatalysis
Abstract
:1. Introduction
2. Photocatalytic Mechanism
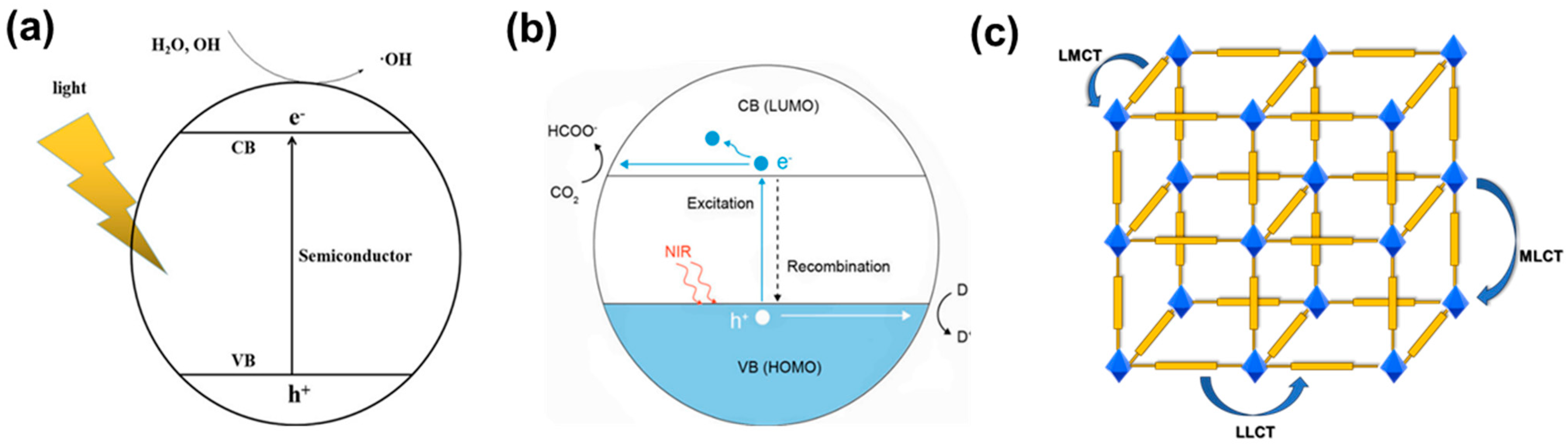
2.1. Light Absorption
2.2. Charge Transfer Pathway
3. Design and Construction of Porphyrin-Based MOFs
3.1. Design of Porphyrin-Based MOFs
3.2. Construction of Porphyrin-Based MOFs
3.2.1. Porphyrin-Based MOFs with Carboxylic Acid Linkers
3.2.2. Porphyrin-Based MOFs with Nitrogen-Containing Heterocyclic Linkers
4. Improvement in Photocatalytic Performance of Porphyrin-Based MOFs
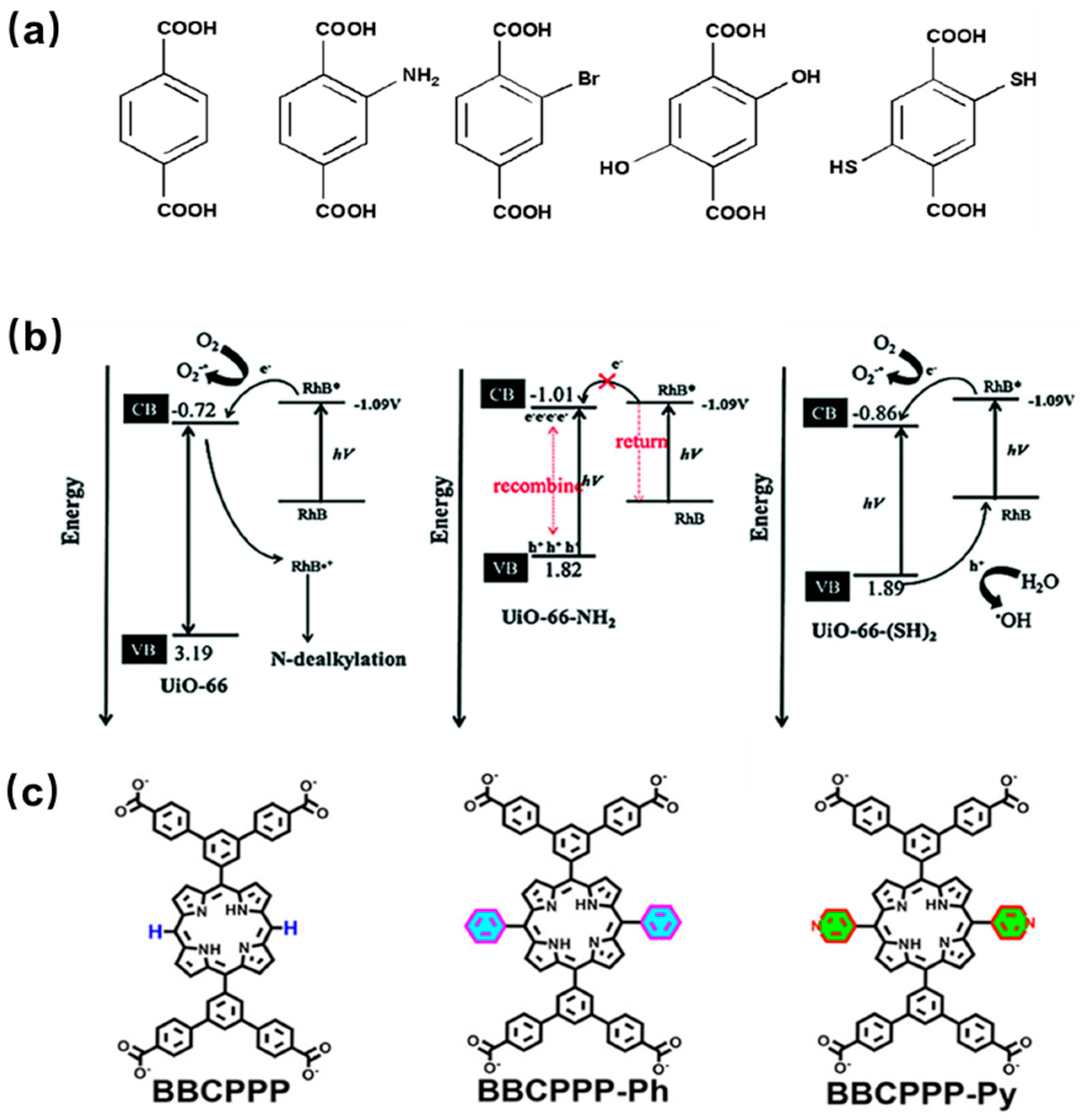
4.1. Modification of Porphyrin Ligands
4.1.1. The Introduction of Metal Coordination Center
4.1.2. Incorporation of Functional Groups
4.2. Construction of Donor-Acceptor System
4.2.1. Effect of D-A System in Promoting Light Harvesting
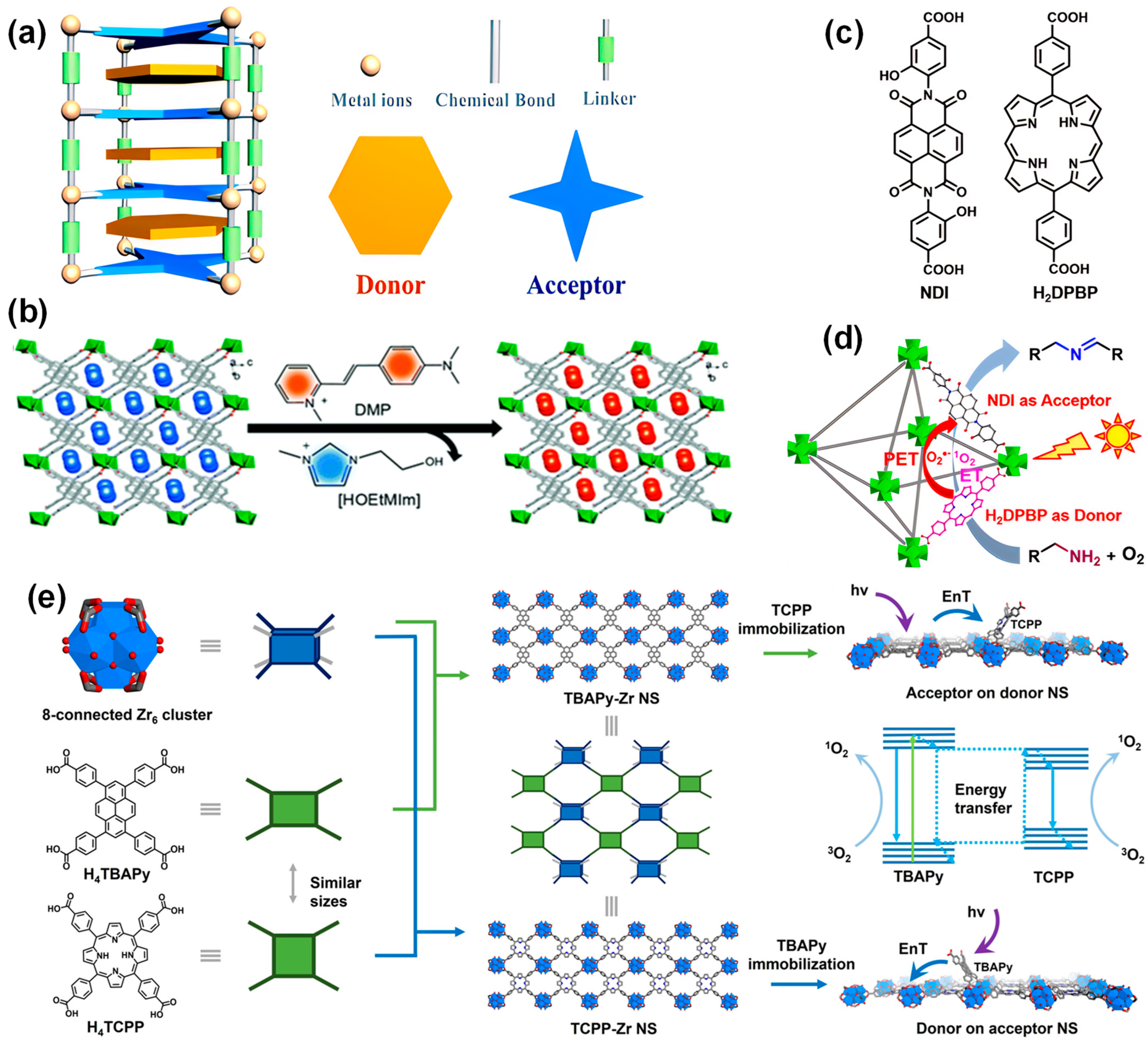
4.2.2. Effect of D-A System in Facilitating Electron Separation
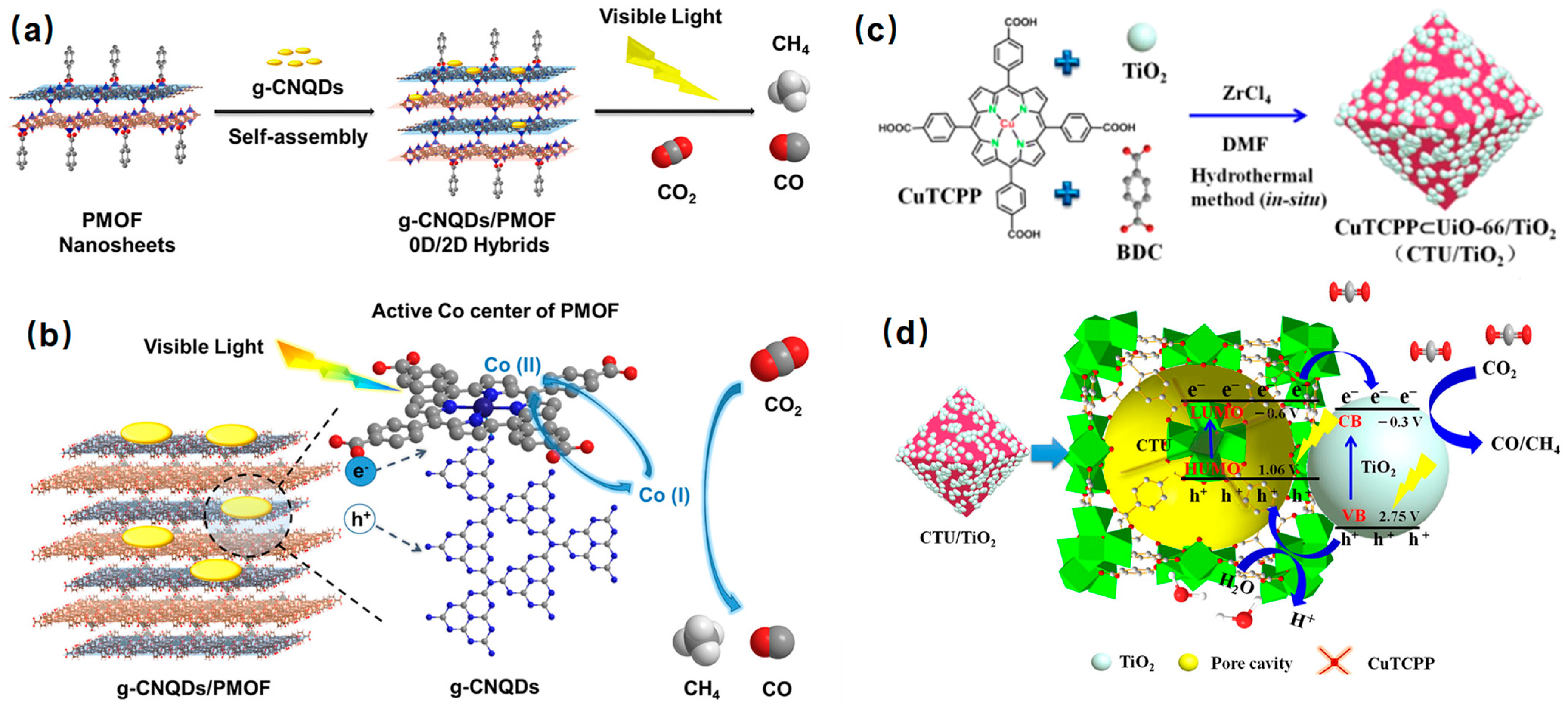
4.3. The Introduction of Co-Catalysts
5. Application of Porphyrin-Based MOFs in Photocatalysis
5.1. Photocatalytic Hydrogen Evolution
5.2. Photocatalytic CO2 Reduction
5.3. Photocatalytic Synthesis of Organic Compounds
5.4. Photocatalytic Removal of Pollutants
5.5. Photocatalytic Nitrogen Fixation
6. Conclusions and Foresight Perspective Outlooks
Funding
Conflicts of Interest
References
- McGlade, C.; Ekins, P. The geographical distribution of fossil fuels unused when limiting global warming to 2 degrees C. Nature 2015, 517, 187–190. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, Y.; Zhang, W.; Zhong, Q.; Ma, X. In situ self-assembly of 3D hierarchical 2D/2D CdS/g-C3N4 hereojunction with excellent photocatalytic performance. Mater. Sci. Semicond. Proc. 2020, 105, 104734. [Google Scholar] [CrossRef]
- Wang, S.; Li, M.; Patil, A.J.; Sun, S.; Tian, L.; Zhang, D.; Cao, M.; Mann, S. Design and construction of artificial photoresponsive protocells capable of converting day light to chemical energy. J. Mater. Chem. A 2017, 5, 24612–24616. [Google Scholar] [CrossRef]
- Liu, F.; Cao, H.; Xu, L.; Fu, H.; Sun, S.; Xiao, Z.; Sun, C.; Long, X.; Xia, Y.; Wang, S. Design and preparation of highly active TiO2 photocatalysts by modulating their band structure. J. Colloid Interface Sci. 2023, 629, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, K.; Wang, L.; Wang, L.; Fan, Z. Porphyrin-based heterogeneous photocatalysts for solar energy conversion. Chin. Chem. Lett. 2022, 33, 33–60. [Google Scholar] [CrossRef]
- Ni, Y.; Yan, K.; Xu, F.; Zhong, W.; Zhao, Q.; Liu, K.; Yan, K.; Wang, D. Synergistic effect on TiO2 doped poly (vinyl alcohol-co-ethylene) nanofibrous film for filtration and photocatalytic degradation of methylene blue. Compos. Commun. 2019, 12, 112–116. [Google Scholar] [CrossRef]
- Qian, Y.T.; Zhang, F.F.; Pang, H. A Review of MOFs and Their Composites-Based Photocatalysts: Synthesis and Applications. Adv. Funct. Mater. 2021, 31, 2104231. [Google Scholar] [CrossRef]
- Foo, C.; Li, Y.; Lebedev, K.; Chen, T.; Day, S.; Tang, C.; Tsang, S.C.E. Characterisation of oxygen defects and nitrogen impurities in TiO(2) photocatalysts using variable-temperature X-ray powder diffraction. Nat. Commun. 2021, 12, 661. [Google Scholar] [CrossRef]
- Nguyen, C.T.K.; Quang Tran, N.; Seo, S.; Hwang, H.; Oh, S.; Yu, J.; Lee, J.; Anh Le, T.; Hwang, J.; Kim, M.; et al. Highly efficient nanostructured metal-decorated hybrid semiconductors for solar conversion of CO2 with almost complete CO selectivity. Mater. Today 2020, 35, 25–33. [Google Scholar]
- Cao, S.; Chen, Y.; Wang, H.; Chen, J.; Shi, X.; Li, H.; Cheng, P.; Liu, X.; Liu, M.; Piao, L. Ultrasmall CoP Nanoparticles as Efficient Cocatalysts for Photocatalytic Formic Acid Dehydrogenation. Joule 2018, 2, 549–557. [Google Scholar]
- Dong, H.; Xiao, M.; Yu, S.; Wu, H.; Wang, Y.; Sun, J.; Chen, G.; Li, C. Insight into the Activity and Stability of RhxP Nano-Species Supported on g-C3N4 for Photocatalytic H2 Production. ACS Catal. 2019, 10, 458–462. [Google Scholar] [CrossRef]
- Yu, J.; Seo, S.; Luo, Y.; Sun, Y.; Oh, S.; Nguyen, C.T.K.; Seo, C.; Kim, J.H.; Kim, J.; Lee, H. Efficient and Stable Solar Hydrogen Generation of Hydrophilic Rhenium-Disulfide-Based Photocatalysts via Chemically Controlled Charge Transfer Paths. ACS Nano 2020, 14, 1715–1726. [Google Scholar]
- Sun, Q.; Wang, N.; Yu, J.; Yu, J.C. A Hollow Porous CdS Photocatalyst. Adv. Mater. 2018, 30, e1804368. [Google Scholar] [CrossRef]
- Meng, X.-B.; Sheng, J.-L.; Tang, H.-L.; Sun, X.-J.; Dong, H.; Zhang, F.-M. Metal-organic framework as nanoreactors to co-incorporate carbon nanodots and CdS quantum dots into the pores for improved H2 evolution without noble-metal cocatalyst. Appl. Catal. B Environ. 2019, 244, 340–346. [Google Scholar] [CrossRef]
- Velumani, A.; Sengodan, P.; Arumugam, P.; Rajendran, R.; Santhanam, S.; Palanisamy, M. Carbon quantum dots supported ZnO sphere based photocatalyst for dye degradation application. Curr. Appl. Phys. 2020, 20, 1176–1184. [Google Scholar]
- Syed, N.; Huang, J.; Feng, Y.; Wang, X.; Cao, L. Carbon-Based Nanomaterials via Heterojunction Serving as Photocatalyst. Front. Chem. 2019, 7, 713. [Google Scholar] [CrossRef]
- Kundu, S.; Bramhaiah, K.; Bhattacharyya, S. Carbon-based nanomaterials: In the quest of alternative metal-free photocatalysts for solar water splitting. Nanoscale Adv. 2020, 2, 5130–5151. [Google Scholar] [CrossRef]
- Srisasiwimon, N.; Chuangchote, S.; Laosiripojana, N.; Sagawa, T. TiO2/Lignin-Based Carbon Composited Photocatalysts for Enhanced Photocatalytic Conversion of Lignin to High Value Chemicals. ACS Sustain. Chem. Eng. 2018, 6, 13968–13976. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, R.; Xiao, Y.; Huang, H.; Sun, Y.; Chen, Y.; Ma, T.; Sun, X. Trace to the Source: Self-Tuning of MOF Photocatalysts. Adv. Energy Mater. 2023, 13, 2300086. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; O’Keeffe, M.; Yaghi, O.M. Modular chemistry: Secondary building units as a basis for the design of highly porous and robust metal-organic carboxylate frameworks. Accounts. Chem. Res. 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Wang, K.-Y.; Joseph, E.; Zhou, H.-C. Catalytic Porphyrin Framework Compounds. Trends Chem. 2020, 2, 555–568. [Google Scholar] [CrossRef]
- Qin, Y.; Hao, M.; Li, Z. Metal-organic frameworks for photocatalysis. In Surface Science of Photocatalysis; Elsevier: London, UK, 2020; Volume 31, pp. 541–579. [Google Scholar]
- Chen, Z.; Li, P.; Anderson, R.; Wang, X.; Zhang, X.; Robison, L.; Redfern, L.R.; Moribe, S.; Islamoglu, T.; Gomez-Gualdron, D.A.; et al. Balancing volumetric and gravimetric uptake in highly porous materials for clean energy. Science 2020, 368, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhao, Z.; Chen, Y.; Tan, J.; Shi, Y.; Ren, H.; Yang, D.; Jiang, Z. Dual-Ligand Ti-MOFs with Push-Pull Effect for Photocatalytic H(2) Production. ACS Appl. Mater. Interfaces 2023, 15, 1053–1062. [Google Scholar] [CrossRef]
- Hussain, M.B.; Kang, B.; Cheng, X.; Ma, C.; Wang, X.; Mehmood, R.; Iqbal, S. Oxygen vacancy induced Pt-decorated MOF photocatalyst for hydrogen production. Int. J. Hydrogen Energy 2023, 48, 13780–13790. [Google Scholar]
- Ko, M.; Mendecki, L.; Eagleton, A.M.; Durbin, C.G.; Stolz, R.M.; Meng, Z.; Mirica, K.A. Employing Conductive Metal-Organic Frameworks for Voltammetric Detection of Neurochemicals. J. Am. Chem. Soc. 2020, 142, 11717–11733. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar]
- Rojas, S.; Garcia-Gonzalez, J.; Salcedo-Abraira, P.; Rincon, I.; Castells-Gil, J.; Padial, N.M.; Marti-Gastaldo, C.; Horcajada, P. Ti-based robust MOFs in the combined photocatalytic degradation of emerging organic contaminants. Sci. Rep. 2022, 12, 14513. [Google Scholar]
- Xie, W.; Yuan, Y.; Jiang, W.; Zhang, S.-R.; Xu, G.-J.; Xu, Y.-H.; Su, Z.-M. Heterogeneous activation of peroxymonosulfate by stable Co-MOF for the efficient degradation of organic dye pollutants. CrystEngComm 2022, 24, 6786–6792. [Google Scholar] [CrossRef]
- Zhang, X.; Wasson, M.C.; Shayan, M.; Berdichevsky, E.K.; Ricardo-Noordberg, J.; Singh, Z.; Papazyan, E.K.; Castro, A.J.; Marino, P.; Ajoyan, Z.; et al. A historical perspective on porphyrin-based metal-organic frameworks and their applications. Coord. Chem. Rev. 2021, 429, 213615. [Google Scholar]
- Tasaki, M.; Okabe, Y.; Iwami, H.; Akatsuka, C.; Kosugi, K.; Negita, K.; Kusaka, S.; Matsuda, R.; Kondo, M.; Masaoka, S. Modulation of Self-Assembly Enhances the Catalytic Activity of Iron Porphyrin for CO2 Reduction. Small 2021, 17, 2006150. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Du, P.; Zhang, Z.; Ning, X.; Deng, Y.; Yin, D.; Chen, J.; Han, Z.; Lu, X. Cross-Linked Surface Engineering to Improve Iron Porphyrin Catalytic Activity. Small 2020, 16, 1905889. [Google Scholar] [CrossRef]
- Liu, J.; Fan, Y.Z.; Li, X.; Xu, Y.W.; Zhang, L.; Su, C.Y. Catalytic Space Engineering of Porphyrin Metal-Organic Frameworks for Combined CO2 Capture and Conversion at a Low Concentration. ChemSusChem 2018, 11, 2340–2347. [Google Scholar]
- Xiu, Y.; Xu, L.; Zhang, X.; Wang, X.; Liu, F.; Xia, Y.; Cao, M.; Wang, S. Mechanistic Process Understanding of the Biomimetic Construction of Porphyrin-Based Light-Capturing Antennas from Self-Assembled Fmoc-Blocked Peptide Templates. ACS Sustain. Chem. Eng. 2020, 8, 15761–15771. [Google Scholar]
- Xiu, Y.; Zhang, X.; Feng, Y.; Wei, R.; Wang, S.; Xia, Y.; Cao, M.; Wang, S. Peptide-mediated porphyrin based hierarchical complexes for light-to-chemical conversion. Nanoscale 2020, 12, 15201–15208. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L.; Masoomi, M.Y.; Morsali, A. Template strategies with MOFs. Coord. Chem. Rev. 2019, 387, 415–435. [Google Scholar]
- Huang, Z.W.; Hu, K.Q.; Mei, L.; Kong, X.H.; Yu, J.P.; Liu, K.; Zeng, L.W.; Chai, Z.F.; Shi, W.Q. A mixed-ligand strategy regulates thorium-based MOFs. Dalton Trans. 2020, 49, 983–987. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, W.; Liu, J.; Fujimori, Y.; Higashino, T.; Imahori, H.; Jiang, X.; Zhao, J.; Sakurai, T.; Hattori, Y.; et al. A new class of epitaxial porphyrin metal-organic framework thin films with extremely high photocarrier generation efficiency: Promising materials for all-solid-state solar cells. J. Mater. Chem. A 2016, 4, 12739–12747. [Google Scholar]
- He, Y.; Jiang, G.; Zhang, C.; Tu, Q.; Jiang, Y.; Gu, L.; Wang, S.; Tan, J.; Jiang, M.; Liu, X. In situ grown CdS on 2D Cd-based porphyrin MOFs enhances the significant separation and transfer of charge carriers with an appropriate heterojunction during photocatalytic hydrogen evolution. Catal. Sci. Technol. 2022, 12, 5077–5085. [Google Scholar]
- Johnson, J.A.; Luo, J.; Zhang, X.; Chen, Y.-S.; Morton, M.D.; Echeverría, E.; Torres, F.E.; Zhang, J. Porphyrin-Metalation-Mediated Tuning of Photoredox Catalytic Properties in Metal-Organic Frameworks. ACS Catal. 2015, 5, 5283–5291. [Google Scholar] [CrossRef]
- Li, S.; Mei, H.M.; Yao, S.L.; Chen, Z.Y.; Lu, Y.L.; Zhang, L.; Su, C.Y. Well-distributed Pt-nanoparticles within confined coordination interspaces of self-sensitized porphyrin metal-organic frameworks: Synergistic effect boosting highly efficient photocatalytic hydrogen evolution reaction. Chem. Sci. 2019, 10, 10577–10585. [Google Scholar] [CrossRef]
- Xu, D.; Duan, Q.; Yu, H.; Dong, W. Photodynamic therapy based on porphyrin-based metal-organic frameworks. J. Mater. Chem. B 2023, 11, 5976–5989. [Google Scholar]
- Wang, S.; Chen, Y.; Wang, S.; Li, P.; Mirkin, C.A.; Farha, O.K. DNA-Functionalized Metal-Organic Framework Nanoparticles for Intracellular Delivery of Proteins. J. Am. Chem. Soc. 2019, 141, 2215–2219. [Google Scholar]
- Zhao, X.; Zhang, Z.; Cai, X.; Ding, B.; Sun, C.; Liu, G.; Hu, C.; Shao, S.; Pang, M. Postsynthetic Ligand Exchange of Metal-Organic Framework for Photodynamic Therapy. ACS Appl. Mater. Interfaces 2019, 11, 7884–7892. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.-Y.; Wang, X.-S.; Zhang, M.-K.; Li, Z.-H.; Gong, D.; Pan, P.; Huang, L.; Cheng, S.-X.; Cheng, H.; Zhang, X.-Z. Universal Porphyrinic Metal-Organic Framework Coating to Various Nanostructures for Functional Integration. ACS Appl. Mater. Interfaces 2017, 9, 43143–43153. [Google Scholar] [PubMed]
- Liang, Z.; Guo, H.; Zhou, G.; Guo, K.; Wang, B.; Lei, H.; Zhang, W.; Zheng, H.; Apfel, U.P.; Cao, R. Metal-Organic-Framework-Supported Molecular Electrocatalysis for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2021, 60, 8472–8476. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Jaramillo, T.F. Addressing the terawatt challenge: Scalability in the supply of chemical elements for renewable energy. RSC Adv. 2012, 2, 7933–7947. [Google Scholar]
- Dong, B.-X.; Qian, S.-L.; Bu, F.-Y.; Wu, Y.-C.; Feng, L.-G.; Teng, Y.-L.; Liu, W.-L.; Li, Z.-W. Electrochemical Reduction of CO2 to CO by a Heterogeneous Catalyst of Fe-Porphyrin-Based Metal-Organic Framework. ACS Appl. Energy Mater. 2018, 1, 4662–4669. [Google Scholar] [CrossRef]
- Vesborg, P.C.; Seger, B.; Chorkendorff, I. Recent Development in Hydrogen Evolution Reaction Catalysts and Their Practical Implementation. J. Phys. Chem. Lett. 2015, 6, 951–957. [Google Scholar]
- Zou, C.; Wu, C.D. Functional porphyrinic metal-organic frameworks: Crystal engineering and applications. Dalton Trans. 2012, 41, 3879–3888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, Q.; Al-Enizi, A.M.; Nafady, A.; Ma, S. Recent advances in MOF-based photocatalysis: Environmental remediation under visible light. Inorg. Chem. Front. 2020, 7, 300–339. [Google Scholar] [CrossRef]
- Li, X.; Tang, C.; Zhang, L.; Song, M.; Zhang, Y.; Wang, S. Porphyrin-Based Covalent Organic Frameworks: Design, Synthesis, Photoelectric Conversion Mechanism, and Applications. Biomimetics 2023, 8, 171. [Google Scholar] [CrossRef]
- Xu, C.; Pan, Y.; Wan, G.; Liu, H.; Wang, L.; Zhou, H.; Yu, S.-H.; Jiang, H.-L. Turning on Visible-Light Photocatalytic C-H Oxidation over Metal-Organic Frameworks by Introducing Metal-to-Cluster Charge Transfer. J. Am. Chem. Soc. 2019, 141, 19110–19117. [Google Scholar] [CrossRef]
- Li, S.; Luo, P.; Wu, H.; Wei, C.; Hu, Y.; Qiu, G. Strategies for Improving the Performance and Application of MOFs Photocatalysts. ChemCatChem 2019, 11, 2978–2993. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Wang, X.S.; Xie, B.R.; Li, Q.R.; Zhang, X.Z. Large pi-Conjugated Metal-Organic Frameworks for Infrared-Light-Driven CO(2) Reduction. J. Am. Chem. Soc. 2022, 144, 1218–1231. [Google Scholar] [CrossRef]
- Xiu, Y.; Zhang, D.; Xu, L.; Li, J.; Chen, Y.; Xia, Y.; Cao, M.; Wang, S. Bioinspired construction of light-harvesting antenna via hierarchically co-assembling approach. J. Colloid. Interface Sci. 2021, 587, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Tian, X.; Xu, X.; He, J. Enhancing visible-light-activity of Ti-based MOFs based on extending the conjugated degree of organic ligands and photocatalytic degradation process and mechanism in real industrial textile wastewaters. J. Environ. Chem. Eng. 2021, 9, 106428. [Google Scholar]
- Xiao, J.D.; Jiang, H.L. Metal-Organic Frameworks for Photocatalysis and Photothermal Catalysis. Account. Chem. Res. 2019, 52, 356–366. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Alvaro, M.; Carbonell, E.; Ferrer, B.; Llabrés i Xamena, F.X.; Garcia, H. Semiconductor Behavior of a Metal-Organic Framework (MOF). Chem.-Eur. J. 2007, 13, 5106–5112. [Google Scholar] [PubMed]
- Wu, T.; Liu, X.; Liu, Y.; Cheng, M.; Liu, Z.; Zeng, G.; Shao, B.; Liang, Q.; Zhang, W.; He, Q.; et al. Application of QD-MOF composites for photocatalysis: Energy production and environmental remediation. Coord. Chem. Rev. 2020, 403, 213097. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhou, W.; Liu, L.; Ye, J.; Wang, D. An ultrathin porphyrin-based metal-organic framework for efficient photocatalytic hydrogen evolution under visible light. Nano Energy 2019, 62, 250–258. [Google Scholar]
- Wu, X.P.; Choudhuri, I.; Truhlar, D.G. Computational Studies of Photocatalysis with Metal-Organic Frameworks. Energy Environ. Mater. 2019, 2, 251–263. [Google Scholar]
- Wen, M.; Li, G.; Liu, H.; Chen, J.; An, T.; Yamashita, H. Metal-organic framework-based nanomaterials for adsorption and photocatalytic degradation of gaseous pollutants: Recent progress and challenges. Environ. Sci. Nano 2019, 6, 1006–1025. [Google Scholar] [CrossRef]
- Pan, Y.; Abazari, R.; Yao, J.; Gao, J. Recent progress in 2D metal-organic framework photocatalysts: Synthesis, photocatalytic mechanism and applications. J. Phys.-Energy 2021, 3, 032010. [Google Scholar] [CrossRef]
- Fan, L.; Yu, Q.; Chen, J.; Khan, U.; Wang, X.; Gao, J. Achievements and Perspectives in Metal-Organic Framework-Based Materials for Photocatalytic Nitrogen Reduction. Catalysts 2022, 12, 1005. [Google Scholar] [CrossRef]
- Li, L.-L.; Yang, C.-J.; Chen, W.-H.; Lin, K.-J. Towards the Development of Electrical Conduction and Lithium-Ion Transport in a Tetragonal Porphyrin Wire. Angew. Chem. 2003, 115, 1543–1546. [Google Scholar] [CrossRef]
- Medforth, C.J.; Wang, Z.; Martin, K.E.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 47, 7261–7277. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar] [CrossRef]
- Tian, Y.-Q.; Zhao, Y.-M.; Chen, Z.-X.; Zhang, G.-N.; Weng, L.-H.; Zhao, D.-Y. Design and generation of extended zeolitic metal-organic frameworks (ZMOFs): Synthesis and crystal structures of zinc(II) imidazolate polymers with zeolitic topologies. Chem.-Eur. J. 2007, 13, 4146–4154. [Google Scholar] [CrossRef] [PubMed]
- Wong-Foy, A.G.; Matzger, A.J.; Yaghi, O.M. Exceptional H2 saturation uptake in microporous metal-organic frameworks. J. Am. Chem. Soc. 2006, 128, 3494–3495. [Google Scholar] [CrossRef]
- Dybtsev, D.N.; Chun, H.; Kim, K. Three-dimensional metal-organic framework with (3,4)-connected net, synthesized from an ionic liquid medium. Chem. Commun. 2004, 14, 1594–1595. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Sun, Q.; Liu, B.; Kuang, Y.; Gulzar, A.; He, F.; Gai, S.; Yang, P.; Lin, J. Recent advances in porphyrin-based MOFs for cancer therapy and diagnosis therapy. Coord. Chem. Rev. 2021, 439, 213945. [Google Scholar] [CrossRef]
- Kubovics, M.; Careta, O.; Vallcorba, O.; Romo-Islas, G.; Rodríguez, L.; Ayllón, J.A.; Domingo, C.; Nogués, C.; López-Periago, A.M. Supercritical CO2 Synthesis of Porous Metalloporphyrin Frameworks: Application in Photodynamic Therapy. Chem. Mater. 2023, 35, 1080–1093. [Google Scholar] [CrossRef]
- Zhao, Y.; Qi, S.; Niu, Z.; Peng, Y.; Shan, C.; Verma, G.; Wojtas, L.; Zhang, Z.; Zhang, B.; Feng, Y.; et al. Robust Corrole-Based Metal-Organic Frameworks with Rare 9-Connected Zr/Hf-Oxo Clusters. J. Am. Chem. Soc. 2019, 141, 14443–14450. [Google Scholar] [CrossRef]
- Wu, H.; Yang, F.; Lv, X.-L.; Wang, B.; Zhang, Y.-Z.; Zhao, M.-J.; Li, J.-R. A stable porphyrinic metal-organic framework pore-functionalized by high-density carboxylic groups for proton conduction. J. Mater. Chem. A 2017, 5, 14525–14529. [Google Scholar]
- Yang, X.-L.; Xie, M.-H.; Zou, C.; He, Y.; Chen, B.; O’Keeffe, M.; Wu, C.-D. Porous Metalloporphyrinic Frameworks Constructed from Metal 5,10,15,20-Tetrakis(3,5-biscarboxylphenyl)porphyrin for Highly Efficient and Selective Catalytic Oxidation of Alkylbenzenes. J. Am. Chem. Soc. 2012, 134, 10638–10645. [Google Scholar] [CrossRef]
- Johnson, J.A.; Zhang, X.; Reeson, T.C.; Chen, Y.-S.; Zhang, J. Facile Control of the Charge Density and Photocatalytic Activity of an Anionic Indium Porphyrin Framework via in Situ Metalation. J. Am. Chem. Soc. 2014, 136, 15881–15884. [Google Scholar]
- Shultz, A.M.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. A Catalytically Active, Permanently Microporous MOF with Metalloporphyrin Struts. J. Am. Chem. Soc. 2009, 131, 4204–4205. [Google Scholar]
- Liu, D.; Liu, T.-F.; Chen, Y.-P.; Zou, L.; Feng, D.; Wang, K.; Zhang, Q.; Yuan, S.; Zhong, C.; Zhou, H.-C. A Reversible Crystallinity-Preserving Phase Transition in Metal-Organic Frameworks: Discovery, Mechanistic Studies, and Potential Applications. J. Am. Chem. Soc. 2015, 137, 7740–7746. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Shang, Q.; Wang, Y.; Jiao, L.; Yao, T.; Li, Y.; Zhang, Q.; Luo, Y.; Jiang, H.L. Single Pt Atoms Confined into a Metal-Organic Framework for Efficient Photocatalysis. Adv. Mater. 2018, 30, 1705112. [Google Scholar]
- Hou, Y.; Liu, L.; Zhang, Z.; Sun, J.; Zhang, Y.; Jiang, J. Synthesis, crystal structures, and fluorescence properties of porphyrin alkaline earth MOFs. Inorg. Chem. Commun. 2018, 95, 36–39. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, J.; Zhang, D.; Qi, D.; Jiang, J. Porphyrin-Alkaline Earth MOFs with the Highest Adsorption Capacity for Methylene Blue. Chemistry 2016, 22, 6345–6352. [Google Scholar] [CrossRef]
- Jin, J. Porphyrin-based metal-organic framework catalysts for photoreduction of CO2: Understanding the effect of node connectivity and linker metalation on activity. New J. Chem. 2020, 44, 15362–15368. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Hoskins, B.F.; Michail, D.M.; Robson, R. Assembly of porphyrin building blocks into network structures with large channels. Nature 1994, 369, 727–729. [Google Scholar]
- Masih, D.; Chernikova, V.; Shekhah, O.; Eddaoudi, M.; Mohammed, O.F. Zeolite-like Metal-Organic Framework(MOF) Encaged Pt(II)-Porphyrin for Anion-Selective Sensing. ACS Appl. Mater. Interfaces 2018, 10, 11399–11405. [Google Scholar]
- Zha, Q.; Ding, C.; Rui, X.; Xie, Y. A Novel Porphyrin-Based Ligand Containing Four 4,4′-Dipyridylamine Moieties: Syntheses, Structures, and Luminescent Properties of Mn(II), Cu(II), Zn(II), and Cd(II) Coordination Polymers. Cryst. Growth Des. 2013, 13, 4583–4590. [Google Scholar] [CrossRef]
- Lv, X.L.; Wang, K.; Wang, B.; Su, J.; Zou, X.; Xie, Y.; Li, J.R.; Zhou, H.C. A Base-Resistant Metalloporphyrin Metal-Organic Framework for C-H Bond Halogenation. J. Am. Chem. Soc. 2017, 139, 211–217. [Google Scholar]
- Wang, K.; Lv, X.L.; Feng, D.; Li, J.; Chen, S.; Sun, J.; Song, L.; Xie, Y.; Li, J.R.; Zhou, H.C. Pyrazolate-Based Porphyrinic Metal-Organic Framework with Extraordinary Base-Resistance. J. Am. Chem. Soc. 2016, 138, 914–919. [Google Scholar]
- Feng, L.; Day, G.S.; Wang, K.-Y.; Yuan, S.; Zhou, H.-C. Strategies for Pore Engineering in Zirconium Metal-Organic Frameworks. Chem 2020, 6, 2902–2923. [Google Scholar] [CrossRef]
- Zhang, X.; Frey, B.L.; Chen, Y.S.; Zhang, J. Topology-Guided Stepwise Insertion of Three Secondary Linkers in Zirconium Metal-Organic Frameworks. J. Am. Chem. Soc. 2018, 140, 7710–7715. [Google Scholar] [PubMed]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’Arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal-organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef]
- Pan, S.; Kong, X.; Zhang, Q.; Xu, Q.; Wang, M.; Wei, C.; Zhao, Y.; Zhang, X. Rational modulating electronegativity of substituents in amorphous metal-organic frameworks for water oxidation catalysis. Int. J. Hydrogen Energy 2020, 45, 9723–9732. [Google Scholar]
- De, S.; Devic, T.; Fateeva, A. Porphyrin and phthalocyanine-based metal-organic frameworks beyond metal-carboxylates. Dalton Trans. 2021, 50, 1166–1188. [Google Scholar] [CrossRef]
- Shi, L.; Yang, L.; Zhang, H.; Chang, K.; Zhao, G.; Kako, T.; Ye, J. Implantation of Iron(III) in porphyrinic metal-organic frameworks for highly improved photocatalytic performance. Appl. Catal. B Environ. 2018, 224, 60–68. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, H.; Wang, H. Synthesis of iron(iii)-based metal-organic framework/graphene oxide composites with increased photocatalytic performance for dye degradation. RSC Adv. 2014, 4, 40435–40438. [Google Scholar]
- Wang, M.; Ma, Y.; Lv, B.; Hua, F.; Meng, S.; Lei, X.; Wang, Q.; Su, B.; Lei, Z.; Yang, Z. Fe Doped MIL-101/Graphene Nanohybrid for Photocatalytic Oxidation of Alcohols Under Visible-Light Irradiation. Catal. Lett. 2021, 151, 2384–2395. [Google Scholar] [CrossRef]
- Choi, S.; Jung, W.-J.; Park, K.; Kim, S.-Y.; Baeg, J.-O.; Kim, C.H.; Son, H.-J.; Pac, C.; Kang, S.O. Rapid Exciton Migration and Amplified Funneling Effects of Multi-Porphyrin Arrays in a Re(I)/Porphyrinic MOF Hybrid for Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2021, 13, 2710–2722. [Google Scholar] [CrossRef]
- Kong, X.J.; He, T.; Zhou, J.; Zhao, C.; Li, T.C.; Wu, X.Q.; Wang, K.; Li, J.R. In Situ Porphyrin Substitution in a Zr(IV)-MOF for Stability Enhancement and Photocatalytic CO2 Reduction. Small 2021, 17, 2005357. [Google Scholar] [CrossRef]
- Fang, Z.-B.; Liu, T.-T.; Liu, J.; Jin, S.; Wu, X.-P.; Gong, X.-Q.; Wang, K.; Yin, Q.; Liu, T.-F.; Cao, R.; et al. Boosting Interfacial Charge-Transfer Kinetics for Efficient Overall CO2 Photoreduction via Rational Design of Coordination Spheres on Metal-Organic Frameworks. J. Am. Chem. Soc. 2020, 142, 12515–12523. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.-H.; Xu, P.; Huang, Y.-D.; Xiao, L.-Y.; Lu, W.; Yang, X.-G.; Ma, L.-F.; Zang, S.-Q. High loading of Mn(ii)-metallated porphyrin in a MOF for photocatalytic CO2 reduction in gas-solid conditions. Chem. Commun. 2021, 57, 8468–8471. [Google Scholar]
- Chen, M.; Zhang, C.; Tang, Y.; Cai, Q.; Yu, J.; Chen, Y.; He, Y.; Bai, J.; Fu, M.; Chen, S.; et al. Cu ions anchored in the porphyrin center act as transient metal centers of 2D-MOFs to enhance photocatalytic hydrogen production. Catal. Sci. Technol. 2023, 13, 581–586. [Google Scholar]
- Pan, Y.; Abazari, R.; Tahir, B.; Sanati, S.; Zheng, Y.; Tahir, M.; Gao, J. Iron-based metal-organic frameworks and their derived materials for photocatalytic and photoelectrocatalytic reactions. Coord. Chem. Rev. 2024, 499, 215538. [Google Scholar]
- Mu, X.; Jiang, J.; Chao, F.; Lou, Y.; Chen, J. Ligand modification of UiO-66 with an unusual visible light photocatalytic behavior for RhB degradation. Dalton Trans. 2018, 47, 1895–1902. [Google Scholar]
- Castellanos, N.J.; Martinez Rojas, Z.; Camargo, H.A.; Biswas, S.; Granados-Oliveros, G. Congo red decomposition by photocatalytic formation of hydroxyl radicals (·OH) using titanium metal-organic frameworks. Transit. Metal Chem. 2018, 44, 77–87. [Google Scholar]
- Wu, W.; Xie, Y.; Lv, X.-L.; Xie, L.-H.; Zhang, X.; He, T.; Si, G.-R.; Wang, K.; Li, J.-R. Expanding the Structural Topologies of Rare-Earth Porphyrinic Metal-Organic Frameworks through Ligand Modulation. ACS Appl. Mater. Interfaces 2023, 15, 5357–5364. [Google Scholar] [CrossRef]
- Jin, S.; Supur, M.; Addicoat, M.; Furukawa, K.; Chen, L.; Nakamura, T.; Fukuzumi, S.; Irle, S.; Jiang, D. Creation of Superheterojunction Polymers via Direct Polycondensation: Segregated and Bicontinuous Donor-Acceptor pi-Columnar Arrays in Covalent Organic Frameworks for Long-Lived Charge Separation. J. Am. Chem. Soc. 2015, 137, 7817–7827. [Google Scholar]
- Jin, S.; Furukawa, K.; Addicoat, M.; Chen, L.; Takahashi, S.; Irle, S.; Nakamura, T.; Jiang, D. Large pore donor-acceptor covalent organic frameworks. Chem. Sci. 2013, 4, 4505–4511. [Google Scholar] [CrossRef]
- Zeng, Y.; Fu, Z.; Chen, H.; Liu, C.; Liao, S.; Dai, J. Photo- and thermally induced coloration of a crystalline MOF accompanying electron transfer and long-lived charge separation in a stable host-guest system. Chem. Commun. 2012, 48, 8114–8116. [Google Scholar] [CrossRef]
- Zhang, B.; Qian, B.-B.; Li, C.-T.; Li, X.-W.; Nie, H.-X.; Yu, M.-H.; Chang, Z. Donor-acceptor systems in metal-organic frameworks: Design, construction, and properties. CrystEngComm 2022, 24, 5538–5551. [Google Scholar] [CrossRef]
- Antonietta Loi, M.; Denk, P.; Hoppe, H.; Neugebauer, H.; Winder, C.; Meissner, D.; Brabec, C.; Serdar Sariciftci, N.; Gouloumis, A.; Vázquez, P.; et al. Long-lived photoinduced charge separation for solar cell applications in phthalocyanine-fulleropyrrolidine dyad thin filmsElectronic supplementary information (ESI) available: Plots of the refractive index, extinction coefficient and dielectric function of Pc-C60. J. Mater. Chem. 2003, 13, 700–704. [Google Scholar]
- Yang, X.-G.; Qin, J.-H.; Huang, Y.-D.; Zhai, Z.-M.; Ma, L.-F.; Yan, D. Highly enhanced UV-vis-NIR light harvesting and photoelectric conversion of a pyrene MOF by encapsulation of the D-π-A cyanine dye. J. Mater. Chem. C 2020, 8, 17169–17175. [Google Scholar] [CrossRef]
- Lee, C.Y.; Farha, O.K.; Hong, B.J.; Sarjeant, A.A.; Nguyen, S.T.; Hupp, J.T. Light-harvesting metal-organic frameworks (MOFs): Efficient strut-to-strut energy transfer in bodipy and porphyrin-based MOFs. J. Am. Chem. Soc. 2011, 133, 15858–15861. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.-J.; Huang, B.-X.; Li, G.; Yang, F.; Lin, W.; Gu, J.-X.; Deng, H.-G.; Gu, Z.-G.; Jin, H.-G. Donor-Acceptor Mixed-Naphthalene Diimide-Porphyrin MOF for Boosting Photocatalytic Oxidative Coupling of Amines. ACS Catal. 2023, 13, 5723–5732. [Google Scholar] [CrossRef]
- Cai, P.; Xu, M.; Meng, S.S.; Lin, Z.; Yan, T.; Drake, H.F.; Zhang, P.; Pang, J.; Gu, Z.Y.; Zhou, H.C. Precise Spatial-Designed Metal-Organic-Framework Nanosheets for Efficient Energy Transfer and Photocatalysis. Angew. Chem. Int. Ed. 2021, 60, 27258–27263. [Google Scholar]
- Cui, G.W.; Wang, W.L.; Ma, M.Y.; Zhang, M.; Xia, X.Y.; Han, F.Y.; Shi, X.F.; Zhao, Y.Q.; Dong, Y.B.; Tang, B. Rational design of carbon and TiO2 assembly materials: Covered or strewn, which is better for photocatalysis? Chem. Commun. 2013, 49, 6415–6417. [Google Scholar] [CrossRef]
- Han, M.; Zhu, S.; Lu, S.; Song, Y.; Feng, T.; Tao, S.; Liu, J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218. [Google Scholar] [CrossRef]
- Liu, X.; Fan, P.; Xiao, L.; Weng, J.; Xu, Q.; Xu, J. Reduced Ti-MOFs encapsulated black phosphorus with high stability and enhanced photocatalytic activity. J. Energy Chem. 2021, 53, 185–191. [Google Scholar] [CrossRef]
- Jin, S.; Son, H.J.; Farha, O.K.; Wiederrecht, G.P.; Hupp, J.T. Energy transfer from quantum dots to metal-organic frameworks for enhanced light harvesting. J. Am. Chem. Soc. 2013, 135, 955–958. [Google Scholar] [CrossRef]
- Yu, Q.; Wang, X.; Wu, W.; Feng, X.; Kong, D.; Khan, U.; Ren, X.; Li, L. In Situ Encapsulation of Graphene Quantum Dots in Highly Stable Porphyrin Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Molecules 2023, 28, 4703. [Google Scholar] [PubMed]
- Wang, Q.; Xu, R.; Wang, X.-S.; Liu, S.-D.; Huang, Y.-B.; Cao, R. Platinum Nanoparticle-Decorated Porous Porphyrin-Based Metal-Organic Framework for Photocatalytic Hydrogen Production. Chin. J. Inorg. Chem. 2017, 33, 2038–2044. [Google Scholar]
- Zuo, Q.; Liu, T.; Chen, C.; Ji, Y.; Gong, X.; Mai, Y.; Zhou, Y. Ultrathin Metal-Organic Framework Nanosheets with Ultrahigh Loading of Single Pt Atoms for Efficient Visible-Light-Driven Photocatalytic H(2) Evolution. Angew. Chem. Int. Ed. 2019, 58, 10198–10203. [Google Scholar]
- He, T.; Chen, S.; Ni, B.; Gong, Y.; Wu, Z.; Song, L.; Gu, L.; Hu, W.; Wang, X. Zirconium-Porphyrin-Based Metal-Organic Framework Hollow Nanotubes for Immobilization of Noble-Metal Single Atoms. Angew. Chem. Int. Ed. 2018, 57, 3493–3498. [Google Scholar] [CrossRef]
- Sasan, K.; Lin, Q.; Mao, C.; Feng, P. Incorporation of iron hydrogenase active sites into a highly stable metal-organic framework for photocatalytic hydrogen generation. Chem. Commun. 2014, 50, 10390–10393. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Guo, H.; Lei, H.; Cao, R. Co porphyrin-based metal-organic framework for hydrogen evolution reaction and oxygen reduction reaction. Chin. Chem. Lett. 2022, 33, 3999–4002. [Google Scholar] [CrossRef]
- Fateeva, A.; Chater, P.A.; Ireland, C.P.; Tahir, A.A.; Khimyak, Y.Z.; Wiper, P.V.; Darwent, J.R.; Rosseinsky, M.J. A water-stable porphyrin-based metal-organic framework active for visible-light photocatalysis. Angew. Chem. Int. Ed. 2012, 51, 7440–7444. [Google Scholar]
- Rahman, F.A.; Aziz, M.M.A.; Saidur, R.; Bakar, W.A.; Hainin, M.R.; Putrajaya, R.; Hassan, N.A. Pollution to solution: Capture and sequestration of carbon dioxide (CO2) and its utilization as a renewable energy source for a sustainable future. Renew. Sustain. Energy Rev. 2017, 71, 112–126. [Google Scholar] [CrossRef]
- Afroz, R.; Hassan, M.N.; Ibrahim, N.A. Review of air pollution and health impacts in Malaysia. Environ. Res. 2003, 92, 71–77. [Google Scholar]
- Choe, J.H.; Kim, H.; Hong, C.S. MOF-74 type variants for CO2 capture. Mater. Chem. Front. 2021, 5, 5172–5185. [Google Scholar] [CrossRef]
- Liang, J.; Yu, H.; Shi, J.; Li, B.; Wu, L.; Wang, M. Dislocated Bilayer MOF Enables High-Selectivity Photocatalytic Reduction of CO2 to CO. Adv. Mater. 2023, 35, 2209814. [Google Scholar]
- Xu, L.; Xiu, Y.; Liu, F.; Liang, Y.; Wang, S. Research Progress in Conversion of CO2 to Valuable Fuels. Molecules 2020, 25, 3653. [Google Scholar] [CrossRef] [PubMed]
- Schneider, J.; Jia, H.; Muckerman, J.T.; Fujita, E. Thermodynamics and kinetics of CO2, CO, and H+ binding to the metal centre of CO2 reduction catalysts. Chem. Soc. Rev. 2012, 41, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Peter, S.C. Reduction of CO2 to Chemicals and Fuels: A Solution to Global Warming and Energy Crisis. ACS Energy Lett. 2018, 3, 1557–1561. [Google Scholar]
- Chen, X.; Cong, M.; Tang, M.; Liu, J.; Chen, S.; Gao, Y. Tandem ZnCo-porphyrin metal-organic frameworks for enhanced photoreduction of CO2. Inorg. Chem. Front. 2022, 9, 4369–4375. [Google Scholar] [CrossRef]
- Sadeghi, N.; Sharifnia, S.; Sheikh Arabi, M. A porphyrin-based metal-organic framework for high rate photoreduction of CO2 to CH4 in gas phase. J. CO2 Util. 2016, 16, 450–457. [Google Scholar] [CrossRef]
- Chen, E.X.; Qiu, M.; Zhang, Y.F.; Zhu, Y.S.; Liu, L.Y.; Sun, Y.Y.; Bu, X.; Zhang, J.; Lin, Q. Acid and Base Resistant Zirconium Polyphenolate-Metalloporphyrin Scaffolds for Efficient CO(2) Photoreduction. Adv. Mater. 2018, 30, 1704388. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.; Yu, F.; Shen, X.; Duan, C. A simple strategy for engineering heterostructures of Au nanoparticle-loaded metal-organic framework nanosheets to achieve plasmon-enhanced photocatalytic CO2 conversion under visible light. J. Mater. Chem. A 2019, 7, 11355–11361. [Google Scholar]
- Sun, D.; Fu, Y.; Liu, W.; Ye, L.; Wang, D.; Yang, L.; Fu, X.; Li, Z. Studies on photocatalytic CO(2) reduction over NH2-Uio-66(Zr) and its derivatives: Towards a better understanding of photocatalysis on metal-organic frameworks. Chemistry 2013, 19, 14279–14285. [Google Scholar] [CrossRef]
- Xu, H.Q.; Hu, J.; Wang, D.; Li, Z.; Zhang, Q.; Luo, Y.; Yu, S.H.; Jiang, H.L. Visible-Light Photoreduction of CO2 in a Metal-Organic Framework: Boosting Electron-Hole Separation via Electron Trap States. J. Am. Chem. Soc. 2015, 137, 13440–13443. [Google Scholar] [CrossRef]
- Ye, L.; Gao, Y.; Cao, S.; Chen, H.; Yao, Y.; Hou, J.; Sun, L. Assembly of highly efficient photocatalytic CO2 conversion systems with ultrathin two-dimensional metal-organic framework nanosheets. Appl. Catal. B Environ. 2018, 227, 54–60. [Google Scholar]
- Zheng, C.; Qiu, X.; Han, J.; Wu, Y.; Liu, S. Zero-Dimensional-g-CNQD-Coordinated Two-Dimensional Porphyrin MOF Hybrids for Boosting Photocatalytic CO2 Reduction. ACS Appl. Mater. Interfaces 2019, 11, 42243–42249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, P.; Duan, S.; She, H.; Huang, J.; Wang, Q. In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO(2) for efficient photocatalytic CO(2) reduction. Sci. Bull. 2019, 64, 926–933. [Google Scholar]
- Ogilby, P.R. Singlet oxygen: There is indeed something new under the sun. Chem. Soc. Rev. 2010, 39, 3181–3209. [Google Scholar] [CrossRef]
- Jiang, Z.W.; Zou, Y.C.; Zhao, T.T.; Zhen, S.J.; Li, Y.F.; Huang, C.Z. Controllable Synthesis of Porphyrin-Based 2D Lanthanide Metal-Organic Frameworks with Thickness- and Metal-Node-Dependent Photocatalytic Performance. Angew. Chem. Int. Ed. 2020, 59, 3300–3306. [Google Scholar] [CrossRef]
- Liu, Y.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Selective Photooxidation of a Mustard-Gas Simulant Catalyzed by a Porphyrinic Metal-Organic Framework. Angew. Chem. Int. Ed. 2015, 54, 9001–9005. [Google Scholar]
- Buru, C.T.; Majewski, M.B.; Howarth, A.J.; Lavroff, R.H.; Kung, C.W.; Peters, A.W.; Goswami, S.; Farha, O.K. Improving the Efficiency of Mustard Gas Simulant Detoxification by Tuning the Singlet Oxygen Quantum Yield in Metal-Organic Frameworks and Their Corresponding Thin Films. ACS Appl. Mater. Interfaces 2018, 10, 23802–23806. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, X.; Xu, L.; Zhao, Y. Fabrication of a novel type visible-light-driven heterojunction photocatalyst: Metal-porphyrinic metal-organic framework coupled with PW12/TiO2. Chem. Eng. J. 2020, 386, 123955. [Google Scholar] [CrossRef]
- Padwa, A.; Bullock, W.H.; Dyszlewski, A.D. Studies dealing with the alkylation-[1,3]-rearrangement reaction of some phenylthio-substituted allylic sulfones. J. Org. Chem. 2002, 55, 955–964. [Google Scholar] [CrossRef]
- Fernandez, I.; Khiar, N. Recent developments in the synthesis and utilization of chiral sulfoxides. Chem. Rev. 2003, 103, 3651–3705. [Google Scholar] [CrossRef]
- Ren, C.; Fang, R.; Yu, X.; Wang, S. A highly efficient reusable homogeneous copper catalyst for the selective aerobic oxygenation sulfides to sulfoxides. Tetrahedron Lett. 2018, 59, 982–986. [Google Scholar]
- Wang, J.; Zhang, X.; Liu, Y.; Wang, Z.; Wang, P.; Zheng, Z.; Cheng, H.; Dai, Y.; Huang, B. Enhanced singlet oxygen production over a photocatalytic stable metal-organic framework composed of porphyrin and Ag. J. Colloid. Interface Sci. 2021, 602, 300–306. [Google Scholar] [PubMed]
- Sheng, W.; Huang, F.; Dong, X.; Lang, X. Solvent-controlled synthesis of Ti-based porphyrinic metal-organic frameworks for the selective photocatalytic oxidation of amines. J. Colloid. Interface Sci. 2022, 628 Pt A, 784–793. [Google Scholar]
- Demel, J.; Kubat, P.; Millange, F.; Marrot, J.; Cisarova, I.; Lang, K. Lanthanide-porphyrin hybrids: From layered structures to metal-organic frameworks with photophysical properties. Inorg. Chem. 2013, 52, 2779–2786. [Google Scholar] [PubMed]
- Wei, F.; Zhang, H.; Ren, Q.; Chen, H.; Yang, L.; Ding, B.; Yu, M.; Liang, Z. Removal of organic contaminants from wastewater with GO/MOFs composites. PLoS ONE 2021, 16, e0253500. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yang, L.; Johansson, E.M.J.; Wang, Y.; Jin, P. Photocatalytic activity and mechanism of bisphenol a removal over TiO2-x/rGO nanocomposite driven by visible light. Chem. Eng. J. 2018, 350, 1043–1055. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Johnson, M.S.; Yoshimura, C. Photodegradation of pharmaceuticals and personal care products in water treatment using carbonaceous-TiO(2) composites: A critical review of recent literature. Water Res. 2018, 142, 26–45. [Google Scholar] [CrossRef]
- Fang, H.; Zhang, H.; Han, L.; Mei, J.; Ge, Q.; Long, Z.; Yu, Y. Exploring bacterial communities and biodegradation genes in activated sludge from pesticide wastewater treatment plants via metagenomic analysis. Environ. Pollut. 2018, 243 Pt B, 1206–1216. [Google Scholar] [CrossRef]
- Ramasubbu, V.; Kumar, P.R.; Chellapandi, T.; Madhumitha, G.; Mothi, E.M.; Shajan, X.S. Zn(II) porphyrin sensitized (TiO2@Cd-MOF) nanocomposite aerogel as novel photocatalyst for the effective degradation of methyl orange (MO) dye. Opt. Mater. 2022, 132, 112558. [Google Scholar] [CrossRef]
- Akpinar, I.; Yazaydin, A.O. Rapid and Efficient Removal of Carbamazepine from Water by UiO-67. Ind. Eng. Chem. Res. 2017, 56, 15122–15130. [Google Scholar] [CrossRef]
- Trapido, M.; Tenno, T.; Goi, A.; Dulova, N.; Kattel, E.; Klauson, D.; Klein, K.; Tenno, T.; Viisimaa, M. Bio-recalcitrant pollutants removal from wastewater with combination of the Fenton treatment and biological oxidation. J. Water Process Eng. 2017, 16, 277–282. [Google Scholar] [CrossRef]
- Zhang, S.; Li, B.; Wang, X.; Zhao, G.; Hu, B.; Lu, Z.; Wen, T.; Chen, J.; Wang, X. Recent developments of two-dimensional graphene-based composites in visible-light photocatalysis for eliminating persistent organic pollutants from wastewater. Chem. Eng. J. 2020, 390, 14642. [Google Scholar] [CrossRef]
- Cao, H.; Liu, F.; Tai, Y.; Wang, W.; Li, X.; Li, P.; Zhao, H.; Xia, Y.; Wang, S. Promoting photocatalytic performance of TiO2 nanomaterials by structural and electronic modulation. Chem. Eng. J. 2023, 466, 143219. [Google Scholar]
- Al-Nuaim, M.A.; Alwasiti, A.A.; Shnain, Z.Y. The photocatalytic process in the treatment of polluted water. Chem. Zvest. 2022, 77, 677–701. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Luo, C.J.; Chen, X.S.; Gu, J.C.; Zhang, Y.M.; Chao, M.; Li, M.R.; Chen, T.; Chen, X.; Wang, X.; et al. Shell inspired heterogeneous membrane with smaller bandgap toward sunlight-activated sustainable water purification. Chem. Eng. J. 2022, 440, 135910. [Google Scholar]
- Li, L.X.; Luo, C.J.; Chen, X.S.; Chu, N.; Li, L.; Chao, M.; Yan, L.K. A Novel Multifunctional Photocatalytic Separation Membrane Based on Single-Component Seaweed-Like g-C3N4. Adv. Funct. Mater. 2023, 33, 2213974. [Google Scholar] [CrossRef]
- Yu, K.; Ahmed, I.; Won, D.I.; Lee, W.I.; Ahn, W.S. Highly efficient adsorptive removal of sulfamethoxazole from aqueous solutions by porphyrinic MOF-525 and MOF-545. Chemosphere 2020, 250, 126133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhu, X.; Zhang, C.; Huo, T.; Hou, X.; Guo, D.; Zhang, H.; Xia, D. Enhanced visible-light catalytic degradation of methylene blue by improving adsorption of porous zirconium-based porphyrin MOFs sensitized TiO2 photocatalyst. J. Mater. Res. 2021, 36, 2961–2972. [Google Scholar]
- He, J.; Zhang, Y.; He, J.; Zeng, X.; Hou, X.; Long, Z. Enhancement of photoredox catalytic properties of porphyrinic metal-organic frameworks based on titanium incorporation via post-synthetic modification. Chem. Commun. 2018, 54, 8610–8613. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Duan, S.; Huang, J.; She, H.; Wang, Q.; An, T. Accelerated Fenton-like kinetics by visible-light-driven catalysis over iron(iii) porphyrin functionalized zirconium MOF: Effective promotion on the degradation of organic contaminants. Environ. Sci. Nano 2019, 6, 2652–2661. [Google Scholar] [CrossRef]
- Shi, M.; Lin, D.; Huang, R.; Qi, W.; Su, R.; He, Z. Construction of a Mercapto-Functionalized Zr-MOF/Melamine Sponge Composite for the Efficient Removal of Oils and Heavy Metal Ions from Water. Ind. Eng. Chem. Res. 2020, 59, 13220–13227. [Google Scholar] [CrossRef]
- Li, L.; Lv, X.; Jin, L.; Du, K.; Jiang, J.; Zhao, X.; Liang, H.; Guo, Y.; Wang, X. Facile synthesis of Sn-doped MOF-5 catalysts for efficient photocatalytic nitrogen fixation. Appl. Catal. B Environ. 2024, 344, 123586. [Google Scholar]
- Huang, H.; Wang, X.-S.; Philo, D.; Ichihara, F.; Song, H.; Li, Y.; Li, D.; Qiu, T.; Wang, S.; Ye, J. Toward visible-light-assisted photocatalytic nitrogen fixation: A titanium metal organic framework with functionalized ligands. Appl. Catal. B Environ. 2020, 267, 118686. [Google Scholar] [CrossRef]
- Shang, S.; Xiong, W.; Yang, C.; Johannessen, B.; Liu, R.; Hsu, H.-Y.; Gu, Q.; Leung, M.K.H.; Shang, J. Atomically Dispersed Iron Metal Site in a Porphyrin-Based Metal-Organic Framework for Photocatalytic Nitrogen Fixation. ACS Nano 2021, 15, 9670–9678. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-J.; Jin, S.; Patwardhan, S.; Wezenberg, S.J.; Jeong, N.C.; So, M.; Wilmer, C.E.; Sarjeant, A.A.; Schatz, G.C.; Snurr, R.Q.; et al. Light-Harvesting and Ultrafast Energy Migration in Porphyrin-Based Metal-Organic Frameworks. J. Am. Chem. Soc. 2013, 135, 862–869. [Google Scholar] [CrossRef]
- Feng, L.; Wang, Y.; Yuan, S.; Wang, K.-Y.; Li, J.-L.; Day, G.S.; Qiu, D.; Cheng, L.; Chen, W.-M.; Madrahimov, S.T.; et al. Porphyrinic Metal-Organic Frameworks Installed with Bronsted Acid Sites for Efficient Tandem Semisynthesis of Artemisinin. ACS Catal. 2019, 9, 5111–5118. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Li, X.; Hu, Y.; Du, X.; Wang, S.; Chen, B.; Wang, S. Porphyrin-Based Metal-Organic Framework Materials: Design, Construction, and Application in the Field of Photocatalysis. Molecules 2024, 29, 467. https://doi.org/10.3390/molecules29020467
Tang C, Li X, Hu Y, Du X, Wang S, Chen B, Wang S. Porphyrin-Based Metal-Organic Framework Materials: Design, Construction, and Application in the Field of Photocatalysis. Molecules. 2024; 29(2):467. https://doi.org/10.3390/molecules29020467
Chicago/Turabian StyleTang, Chuanyin, Xiaoyu Li, Yingxu Hu, Xin Du, Shuo Wang, Bo Chen, and Shengjie Wang. 2024. "Porphyrin-Based Metal-Organic Framework Materials: Design, Construction, and Application in the Field of Photocatalysis" Molecules 29, no. 2: 467. https://doi.org/10.3390/molecules29020467
APA StyleTang, C., Li, X., Hu, Y., Du, X., Wang, S., Chen, B., & Wang, S. (2024). Porphyrin-Based Metal-Organic Framework Materials: Design, Construction, and Application in the Field of Photocatalysis. Molecules, 29(2), 467. https://doi.org/10.3390/molecules29020467








