Solid-Phase Microextraction/Gas Chromatography–Time-of-Flight Mass Spectrometry Approach Combined with Network Pharmacology Analysis to Evaluate the Quality of Agarwood from Different Regions against Anxiety Disorder
Abstract
:1. Introduction
2. Results
2.1. Identification of Volatile and Semi-Volatile Compounds of Agarwood
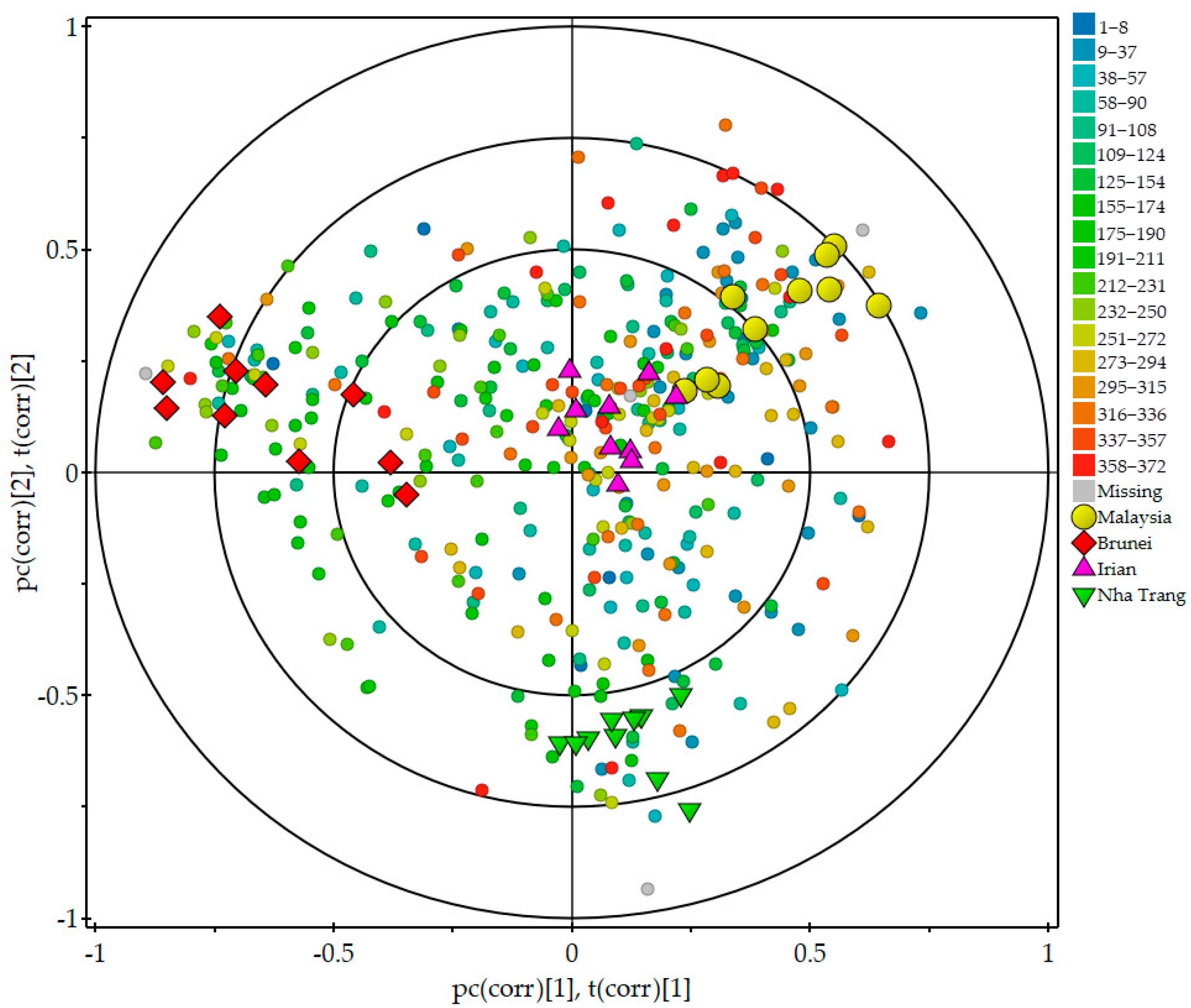
2.2. Relationships among Agarwood Groups and Compounds as Indicated by PLS-DA Biplot of Scores and Loadings
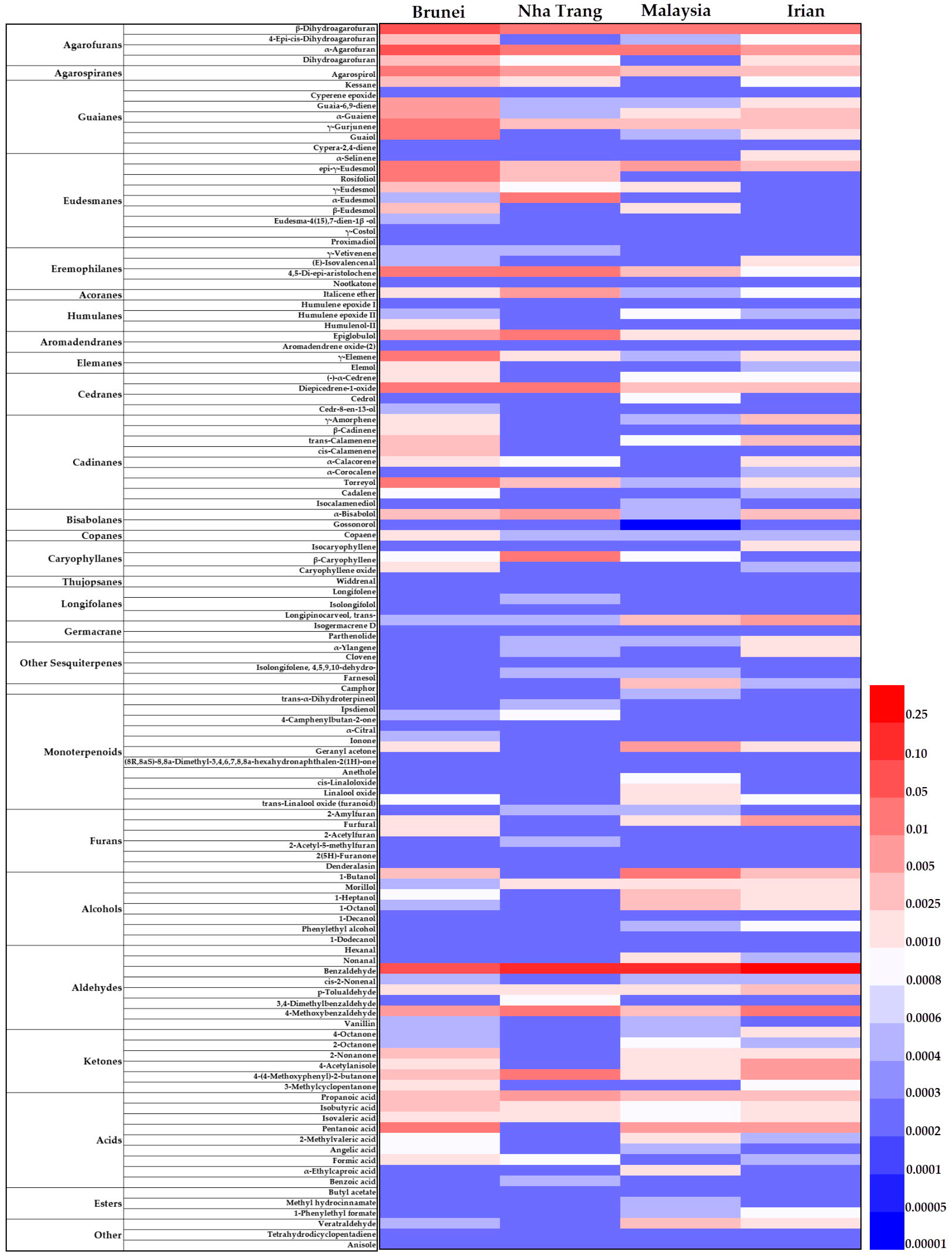
2.3. Differential Compounds across Four Groups of Agarwood
2.4. Network Pharmacology Analysis
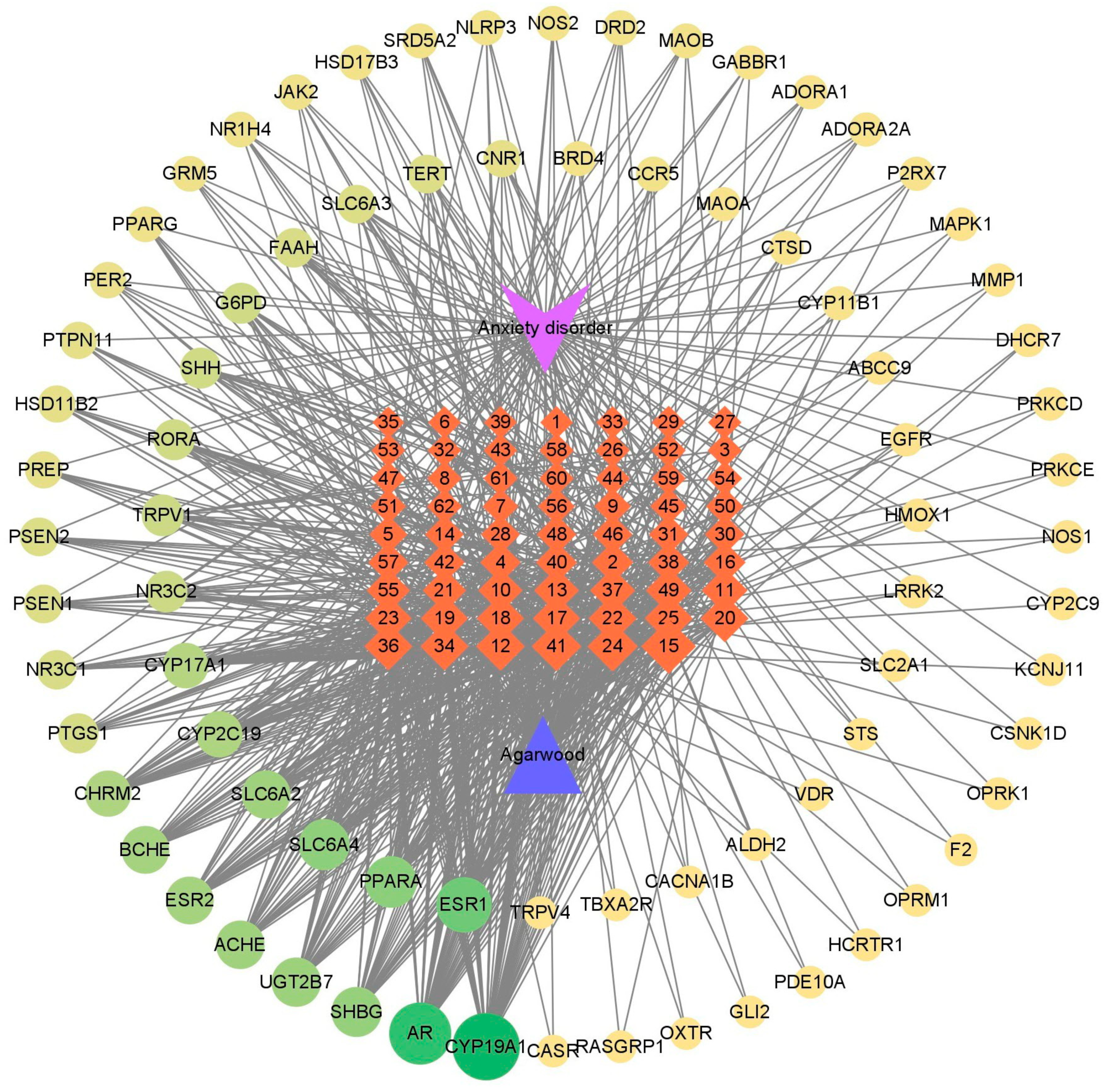
2.4.1. Compound–Target–Disease Network Construction
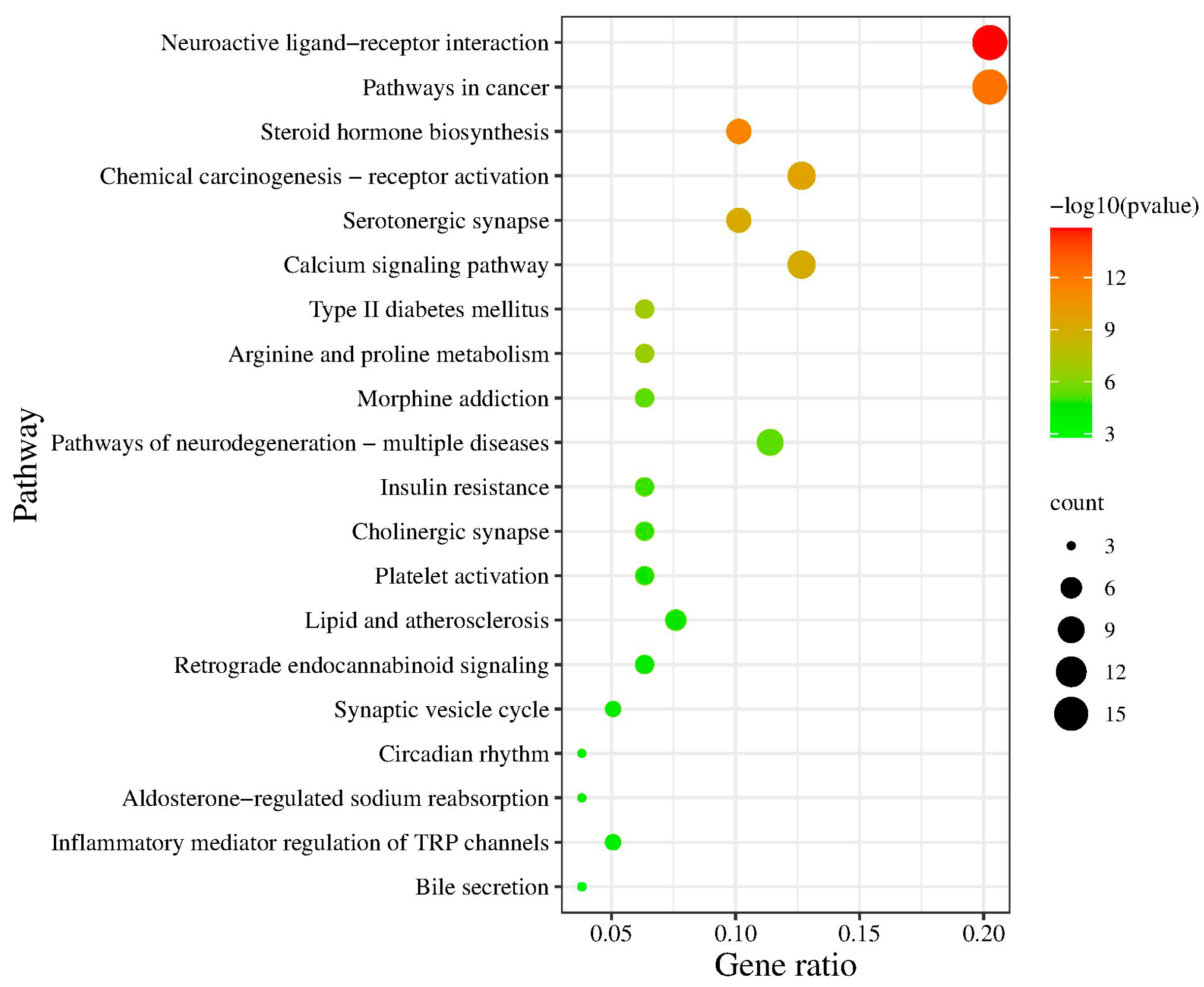
2.4.2. Pathway Enrichment Analysis
3. Discussion
3.1. Unique Sesquiterpenoids with Pharmacological Activities in Four Agarwood Groups
3.2. Potential Therapeutic Targets for Anxiety Treatment as Indicated by Compound–Target–Disease Network
4. Materials and Methods
4.1. Sample Preparation
4.2. GC-TOFMS Analysis
4.3. Compound Identification and Quantification
4.4. Statistical Analysis
4.5. Network Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takemoto, H.; Ito, M.; Shiraki, T.; Yagura, T.; Honda, G. Sedative Effects of Vapor Inhalation of Agarwood Oil and Spikenard Extract and Identification of Their Active Components. J. Nat. Med. 2007, 62, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Mujahid, M.; Badr, B.; Rahman, M.; Akhtar, J.; Khalid, M.; Jahan, Y.; Basit, A.; Khan, A.; Shawwal, M.; et al. An Insight of Pharmacognostic Study and Phytopharmacology of Aquilaria agallocha. J. Appl. Pharm. Sci. 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Yu, Z.; Wu, C.; Peng, D.; Liu, X.; Liu, Y.; Yang, Y.; Guo, P.; Wei, J. Agarwood Essential Oil Ameliorates Restrain Stress-Induced Anxiety and Depression by Inhibiting HPA Axis Hyperactivity. Int. J. Mol. Sci. 2018, 19, 3468. [Google Scholar] [CrossRef]
- Dong, M.; Du, H.; Li, X.; Zhang, L.; Wang, X.; Wang, Z.; Jiang, H. Discovery of Biomarkers and Potential Mechanisms of Agarwood Incense Smoke Intervention by Untargeted Metabolomics and Network Pharmacology. Drug Des. Dev. Ther. 2022, 16, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, R.; Lee, S.Y. Keeping Up Appearances: Agarwood Grades and Quality. In Agarwood; Mohamed, R., Ed.; Tropical Forestry; Springer: Singapore, 2016; pp. 149–167. ISBN 978-981-10-0832-0. [Google Scholar]
- Naef, R. The Volatile and Semi-Volatile Constituents of Agarwood, the Infected Heartwood of Aquilaria Species: A Review. Flavour. Fragr. J. 2011, 26, 73–87. [Google Scholar] [CrossRef]
- Kao, W.-Y.; Hsiang, C.-Y.; Ho, S.-C.; Ho, T.-Y.; Lee, K.-T. Chemical Profiles of Incense Smoke Ingredients from Agarwood by Headspace Gas Chromatography-Tandem Mass Spectrometry. Molecules 2018, 23, 2969. [Google Scholar] [CrossRef]
- Gao, M.; Han, X.; Sun, Y.; Chen, H.; Yang, Y.; Liu, Y.; Meng, H.; Gao, Z.; Xu, Y.; Zhang, Z.; et al. Overview of Sesquiterpenes and Chromones of Agarwood Originating from Four Main Species of the Genus Aquilaria. RSC Adv. 2019, 9, 4113–4130. [Google Scholar] [CrossRef]
- Hashim, Y.Z.H.-Y.; Kerr, P.G.; Abbas, P.; Mohd Salleh, H. Aquilaria Spp. (Agarwood) as Source of Health Beneficial Compounds: A Review of Traditional Use, Phytochemistry and Pharmacology. J. Ethnopharmacol. 2016, 189, 331–360. [Google Scholar] [CrossRef]
- Yang, L.; Qiao, L.; Ji, C.; Xie, D.; Gong, N.-B.; Lu, Y.; Zhang, J.; Dai, J.; Guo, S. Antidepressant Abietane Diterpenoids from Chinese Eaglewood. J. Nat. Prod. 2013, 76, 216–222. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Wang, C.; Wu, C.; Guo, P.; Wei, J. Chemical Constituents and Pharmacological Activity of Agarwood and Aquilaria Plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef]
- Tan, C.S.; Isa, N.M.; Ismail, I.; Zainal, Z. Agarwood Induction: Current Developments and Future Perspectives. Front. Plant Sci. 2019, 10, 122. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Maulidiani, M.; Akhtar, M.; Abas, F.; Ismail, I.; Khatib, A.; Ali, N.; Shaari, K. Discriminative Analysis of Different Grades of Gaharu (Aquilaria malaccensis Lamk.) via 1H-NMR-Based Metabolomics Using PLS-DA and Random Forests Classification Models. Molecules 2017, 22, 1612. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, K.; Sekar, I.; Parthiban, K.T.; Amirtham, D.; Suresh, K.K. Analysis of Different Grades of Agarwood (Aquilaria malaccensis Lamk.) Oil through GC-MS. Indian. J. Nat. Prod. Resour. 2014, 5, 44–47. [Google Scholar]
- Hung, C.-H.; Lee, C.-Y.; Yang, C.-L.; Lee, M.-R. Classification and Differentiation of Agarwoods by Using Non-Targeted HS-SPME-GC/MS and Multivariate Analysis. Anal. Methods 2014, 6, 7449. [Google Scholar] [CrossRef]
- Pripdeevech, P.; Khummueng, W.; Park, S.-K. Identification of Odor-Active Components of Agarwood Essential Oils from Thailand by Solid Phase Microextraction-GC/MS and GC-O. J. Essen. Oil Res. 2011, 23, 46–53. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Q.; Chen, Q.; Su, S. Network Pharmacology: A New Approach for Chinese Herbal Medicine Research. Evid. Based Compl. Alt. 2013, 2013, 621423. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, B. Traditional Chinese Medicine Network Pharmacology: Theory, Methodology and Application. Chin. J. Nat. Med. 2013, 11, 110–120. [Google Scholar] [CrossRef]
- Wang, C.H.; Wang, S.; Peng, D.Q.; Yu, Z.X.; Liu, Y.Y.; Yang, Y.; Guo, P.; Wei, J.H. Prediction and analysis of the components and therapeutic targets of agarwood essential oil. Chin. Pharm. J. 2019, 54, 1958–1964. [Google Scholar]
- Rasool, S.; Mohamed, R. Understanding Agarwood Formation and Its Challenges. In Agarwood; Mohamed, R., Ed.; Tropical Forestry; Springer: Singapore, 2016; pp. 39–56. ISBN 978-981-10-0832-0. [Google Scholar]
- Rabgay, T.; Gurung, D.; Jambay, J.; Wangchuk, K.; Thinley, P.; Tshering, B.; Penjor, P.; Dorji, T.; Tshering, K.; Sitaula, B.; et al. Environmental Factors Affecting Growth of Agarwood (Aquilaria malaccensis Lamarck, 1783) Forests of Bhutan. BJNRD 2020, 7, 12–25. [Google Scholar] [CrossRef]
- Okugawa, H.; Ueda, R.; Matsumoto, K.; Kawanishi, K.; Kato, A. Effect of Jinkoh-Eremol and Agarospirol from Agarwood on the Central Nervous System in Mice. Planta Med. 1996, 62, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Mudgal, V.; Agrawal, J.; Maurya, A.; Bawankule, D.; Chanotiya, C.; Khan, F.; Thul, S. Molecular Docking and ADME Studies of Natural Compounds of Agarwood Oil for Topical Anti-Inflammatory Activity. Curr. Comput. Aided Drug Des. 2013, 9, 360–370. [Google Scholar] [CrossRef]
- Martínez-Hernández, G.B.; Jiménez-Ferrer, E.; González-Cortazar, M.; Román-Ramos, R.; Tortoriello, J.; Vargas-Villa, G.; Herrera-Ruiz, M. Antidepressant and Anxiolytic Compounds Isolated from Salvia elegans Interact with Serotonergic Drugs. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, W.D.; Buigues, N.M. Sesquiterpenes. I. Nootkatone, A New Grapefruit Flavor Constituent. J. Food Sci. 1964, 29, 565–568. [Google Scholar] [CrossRef]
- Fan, J.; Liu, Z.; Xu, S.; Yan, X.; Cheng, W.; Yang, R.; Guo, Y. Non-Food Bioactive Product (+)-Nootkatone: Chemistry and Biological Activities. Ind. Crop Prod. 2022, 177, 114490. [Google Scholar] [CrossRef]
- Yan, T.; Li, F.; Xiong, W.; Wu, B.; Xiao, F.; He, B.; Jia, Y. Nootkatone Improves Anxiety- and Depression-like Behavior by Targeting Hyperammonemia-induced Oxidative Stress in D-galactosamine Model of Liver Injury. Environ. Toxicol. 2021, 36, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Ishola, I.O.; Olubodun-Obadun, T.G.; Ochieng, C.O.; Nwajuwe, M.C.; Mukoro, R.U.; Adeyemi, O.O. Monoaminergic System Involvement in the Antidepressant-like and Anxiolytic-like Properties of Novel β-Dihydroagarofuran Sesquiterpene Alkaloid and Triterpenes Isolated from Gymnosporia heterophylla Aerial Parts in Mice. Neurochem. Int. 2022, 158, 105379. [Google Scholar] [CrossRef] [PubMed]
- Eldeen, I.M.S.; Mohamed, H.; Tan, W.-N.; Siong, J.Y.F.; Andriani, Y.; Tengku-Muh, T.S. Cyclooxygenase, 5-Lipoxygenase and Acetylcholinesterase Inhibitory Effects of Fractions Containing, α-Guaiene and Oil Isolated from the Root of Xylocarpus moluccensis. Res. J. Med. Plants 2016, 10, 286–294. [Google Scholar] [CrossRef]
- Asakura, K.; Matsuo, Y.; Oshima, T.; Kihara, T.; Minagawa, K.; Araki, Y.; Kagawa, K.; Kanemasa, T.; Ninomiya, M. ω-Agatoxin IVA-Sensitive Ca2+ Channel Blocker, α-Eudesmol, Protects against Brain Injury after Focal Ischemia in Rats. Eur. J. Pharmacol. 2000, 394, 57–65. [Google Scholar] [CrossRef]
- Asakura, K.; Kanemasa, T.; Minagawa, K.; Kagawa, K.; Ninomiya, M. The Nonpeptide α-Eudesmol from Juniperus virginiana Linn. (Cupressaceae) Inhibits ω-Agatoxin IVA-Sensitive Ca2+ Currents and Synaptosomal 45Ca2+ Uptake. Brain Res. 1999, 823, 169–176. [Google Scholar] [CrossRef]
- Asakura, K.; Kanemasa, T.; Minagawa, K.; Kagawa, K.; Yagami, T.; Nakajima, M.; Ninomiya, M. α-Eudesmol, a P/Q-Type Ca2+ Channel Blocker, Inhibits Neurogenic Vasodilation and Extravasation Following Electrical Stimulation of Trigeminal Ganglion. Brain Res. 2000, 873, 94–101. [Google Scholar] [CrossRef]
- Alves, A.D.M.H.; Gonçalves, J.C.R.; Cruz, J.S.; Araújo, D.A.M. Evaluation of the Sesquiterpene (−)-α-Bisabolol as a Novel Peripheral Nervous Blocker. Neurosci. Lett. 2010, 472, 11–15. [Google Scholar] [CrossRef]
- Rocha, N.F.M.; Oliveira, G.V.D.; Araújo, F.Y.R.D.; Rios, E.R.V.; Carvalho, A.M.R.; Vasconcelos, L.F.; Macêdo, D.S.; Soares, P.M.G.; Sousa, D.P.D.; Sousa, F.C.F.D. (−)-α-Bisabolol-Induced Gastroprotection Is Associated with Reduction in Lipid Peroxidation, Superoxide Dismutase Activity and Neutrophil Migration. Eur. J. Pharm. Sci. 2011, 44, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Tabari, M.A.; Tehrani, M.A.B. Evidence for the Involvement of the GABAergic, but Not Serotonergic Transmission in the Anxiolytic-like Effect of Bisabolol in the Mouse Elevated plus Maze. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Machado, K.D.C.; Islam, M.T.; Ali, E.S.; Rouf, R.; Uddin, S.J.; Dev, S.; Shilpi, J.A.; Shill, M.C.; Reza, H.M.; Das, A.K.; et al. A Systematic Review on the Neuroprotective Perspectives of Beta-caryophyllene. Phytother. Res. 2018, 32, 2376–2388. [Google Scholar] [CrossRef]
- Anselmi, C.; Centini, M.; Mariani, M.; Sega, A.; Pelosi, P. Odor Properties of Some Tetrahydropyranyl Ethers. J. Agric. Food Chem. 1992, 40, 853–856. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Ngassoum, M.B.; Geissler, M. Aroma Compound Analysis of Piper nigrum and Piper guineense Essential Oils from Cameroon Using Solid-Phase Microextraction–Gas Chromatography, Solid-Phase Microextraction–Gas Chromatography–Mass Spectrometry and Olfactometry. J. Chromatogr. A 2002, 976, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Han, Y.P. Effects of volatile rose aroma in grape wine on People’s psychological regulation. Liquor. Mak. Sci. Technol. 2020, 4, 53–56+69. [Google Scholar] [CrossRef]
- Shuai, S.Y.; Zheng, Q.; Yue, P.F.; Hu, P.Y.; Huang, X.Y.; Liu, S.S.; Liu, X.J.; Yang, M. Research progress on mechanism of aromatic Chinese medicine and their active ingredients in refreshing the brain. Herbal. Drugs. 2021, 52, 6403–6412. [Google Scholar] [CrossRef]
- Barden, A.; Anak, N.A.; Mulliken, T.; Song, M. Heart of the Matter: Agarwood Use and Trade and Cites Implementation for Aquilaria malaccensis; TRAFFIC International: Cambridge, UK, 2000; Volume 46. [Google Scholar]
- Api, A.M.; Belsito, D.; Biserta, S.; Botelho, D.; Bruze, M.; Burton, G.A.; Buschmann, J.; Cancellieri, M.A.; Dagli, M.L.; Date, M.; et al. RIFM Fragrance Ingredient Safety Assessment, p-Tolualdehyde, CAS Registry Number 104-87-0. Food Chem. Toxicol. 2021, 149, 111982. [Google Scholar] [CrossRef] [PubMed]
- Ancelin, M.; Norton, J.; Canonico, M.; Scarabin, P.; Ritchie, K.; Ryan, J. Aromatase (CYP19A1) Gene Variants, Sex Steroid Levels, and Late-life Depression. Depress. Anxiety 2020, 37, 146–155. [Google Scholar] [CrossRef]
- Frye, C.; Koonce, C.; Edinger, K.; Osborne, D.; Walf, A. Androgens with Activity at Estrogen Receptor Beta Have Anxiolytic and Cognitive-Enhancing Effects in Male Rats and Mice. Horm. Behav. 2008, 54, 726–734. [Google Scholar] [CrossRef]
- Pugeat, M.; Nader, N.; Hogeveen, K.; Raverot, G.; Déchaud, H.; Grenot, C. Sex Hormone-Binding Globulin Gene Expression in the Liver: Drugs and the Metabolic Syndrome. Mol. Cell Endocrinol. 2010, 316, 53–59. [Google Scholar] [CrossRef]
- Gestl, S.A.; Green, M.D.; Shearer, D.A.; Frauenhoffer, E.; Tephly, T.R.; Weisz, J. Expression of UGT2B7, a UDP-Glucuronosyltransferase Implicated in the Metabolism of 4-Hydroxyestrone and All-Trans Retinoic Acid, in Normal Human Breast Parenchyma and in Invasive and in Situ Breast Cancers. Am. J. Pathol. 2002, 160, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Scariot, R.; Tomaz, C.O.; Calixto, R.D.; Gerber, J.T.; Pivetta Petinati, M.F.; Cavalcante, R.C.; Küchler, E.C.; João Da Costa, D. Association between Gender, Estrogen Receptors Genes and Anxiety Levels in Patients Undergoing Orthognathic Surgery. J. Cranio Maxill Surg. 2019, 47, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Sundermann, E.E.; Maki, P.M.; Bishop, J.R. A Review of Estrogen Receptor α Gene (ESR1) Polymorphisms, Mood, and Cognition. Menopause 2010, 17, 874–886. [Google Scholar] [CrossRef] [PubMed]
- Borrow, A.P.; Handa, R.J. Estrogen Receptors Modulation of Anxiety-Like Behavior. In Vitamins and Hormones; Elsevier: Amsterdam, The Netherlands, 2017; Volume 103, pp. 27–52. ISBN 978-0-12-811914-3. [Google Scholar]
- Spiteri, T.; Musatov, S.; Ogawa, S.; Ribeiro, A.; Pfaff, D.W.; Ågmo, A. The Role of the Estrogen Receptor α in the Medial Amygdala and Ventromedial Nucleus of the Hypothalamus in Social Recognition, Anxiety and Aggression. Behav. Brain Res. 2010, 210, 211–220. [Google Scholar] [CrossRef]
- Spiteri, T.; Ogawa, S.; Musatov, S.; Pfaff, D.W.; Ågmo, A. The Role of the Estrogen Receptor α in the Medial Preoptic Area in Sexual Incentive Motivation, Proceptivity and Receptivity, Anxiety, and Wheel Running in Female Rats. Behav. Brain Res. 2012, 230, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bethea, C.L.; Mirkes, S.J.; Shively, C.A.; Adams, M.R. Steroid Regulation of Tryptophan Hydroxylase Protein in the Dorsal Raphe of Macaques. Biol. Psychiat 2000, 47, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Lawther, A.J.; Hale, M.W.; Lowry, C.A. Serotonin and the Neurobiology of Anxious States. In Handbook of Behavioral Neuroscience; Elsevier: Amsterdam, The Netherlands, 2020; Volume 31, pp. 505–520. ISBN 978-0-444-64125-0. [Google Scholar]
- Yamashita, P.S.; Rosa, D.S.; Lowry, C.A.; Zangrossi, H. Serotonin Actions within the Prelimbic Cortex Induce Anxiolysis Mediated by Serotonin 1a Receptors. J. Psychopharmacol. 2019, 33, 3–11. [Google Scholar] [CrossRef]
- Brimijoin, S.; Gao, Y.; Geng, L.; Chen, V.P. Treating Cocaine Addiction, Obesity, and Emotional Disorders by Viral Gene Transfer of Butyrylcholinesterase. Front. Pharmacol. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Kao, W.-Y.; Hsiang, C.-Y.; Ho, S.-C.; Ho, T.-Y.; Lee, K.-T. Novel Serotonin-Boosting Effect of Incense Smoke from Kynam Agarwood in Mice: The Involvement of Multiple Neuroactive Pathways. J. Ethnopharmacol. 2021, 275, 114069. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Guo, Q.; Li, H.; Wang, Z.; Li, J.; Li, T.; Tang, T.; Wang, Y.; Jia, Y.; et al. Valerian Essential Oil for Treating Insomnia via the Serotonergic Synapse Pathway. Front. Nutr. 2022, 9, 927434. [Google Scholar] [CrossRef]
- Mazzanti, C.M.; Lappalainen, J.; Long, J.C.; Bengel, D.; Naukkarinen, H.; Eggert, M.; Virkkunen, M.; Linnoila, M.; Goldman, D. Role of the Serotonin Transporter Promoter Polymorphism in Anxiety-Related Traits. Arch. Gen. Psychiatry 1998, 55, 936. [Google Scholar] [CrossRef] [PubMed]
- Ponder, K.L.; Salisbury, A.; McGonnigal, B.; Laliberte, A.; Lester, B.; Padbury, J.F. Maternal Depression and Anxiety Are Associated with Altered Gene Expression in the Human Placenta without Modification by Antidepressant Use: Implications for Fetal Programming. Dev. Psychobiol. 2011, 53, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.Q.; Hou, S.R.; Huang, X.L.; Lin, X.S.; Zhu, Z.X.; Huang, F.; Ma, Y.F. Research on authenticity identification of agarwood by SPME/GC-MS. J. Instrum. Anal. 2016, 35, 1369–1375. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A Web Server for Target Prediction of Bioactive Small Molecules. NAR 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Yu, S.; Guo, Q.; Jia, T.; Zhang, X.; Guo, D.; Jia, Y.; Li, J.; Sun, J. Mechanism of Action of Nicotiflorin from Tricyrtis maculata in the Treatment of Acute Myocardial Infarction: From Network Pharmacology to Experimental Pharmacology. Drug Des. Dev. Ther. 2021, 15, 2179–2191. [Google Scholar] [CrossRef]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. DrugBank: A Knowledgebase for Drugs, Drug Actions and Drug Targets. Nucleic Acids Res. 2008, 36, D901–D906. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape Provides a Biologist-Oriented Resource for the Analysis of Systems-Level Datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Brouwer, C. Pathview: An R/Bioconductor Package for Pathway-Based Data Integration and Visualization. Bioinformatics 2013, 29, 1830–1831. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]

| Name | CAS | Degree |
|---|---|---|
| Nootkatone | 4674-50-4 | 31 |
| Proximadiol | 4666-84-6 | 25 |
| α-Bisabolol | 515-69-5 | 24 |
| β-Vatirenene | 27840-40-0 | 23 |
| α-Eudesmol | 473-16-5 | 22 |
| Eudesma-4(15),7-dien-1β-ol | 119120-23-9 | 22 |
| Agarospirol | 1460-73-7 | 22 |
| Torreyol | 19435-97-3 | 22 |
| Isocalamenediol | 25330-21-6 | 22 |
| epi-γ-Eudesmol | 117066-77-0 | 21 |
| Rosifoliol | 63891-61-2 | 21 |
| γ-Eudesmol | 1209-71-8 | 21 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Y.; Yu, W.; Liang, W.; Gao, Y.; Yang, F.; Zhu, Y.; Feng, L.; Yin, H.; Liu, Y. Solid-Phase Microextraction/Gas Chromatography–Time-of-Flight Mass Spectrometry Approach Combined with Network Pharmacology Analysis to Evaluate the Quality of Agarwood from Different Regions against Anxiety Disorder. Molecules 2024, 29, 468. https://doi.org/10.3390/molecules29020468
Pang Y, Yu W, Liang W, Gao Y, Yang F, Zhu Y, Feng L, Yin H, Liu Y. Solid-Phase Microextraction/Gas Chromatography–Time-of-Flight Mass Spectrometry Approach Combined with Network Pharmacology Analysis to Evaluate the Quality of Agarwood from Different Regions against Anxiety Disorder. Molecules. 2024; 29(2):468. https://doi.org/10.3390/molecules29020468
Chicago/Turabian StylePang, Yue, Wenjuan Yu, Wenyi Liang, Yu Gao, Fan Yang, Yuanyuan Zhu, Lei Feng, Hongmei Yin, and Yumin Liu. 2024. "Solid-Phase Microextraction/Gas Chromatography–Time-of-Flight Mass Spectrometry Approach Combined with Network Pharmacology Analysis to Evaluate the Quality of Agarwood from Different Regions against Anxiety Disorder" Molecules 29, no. 2: 468. https://doi.org/10.3390/molecules29020468
APA StylePang, Y., Yu, W., Liang, W., Gao, Y., Yang, F., Zhu, Y., Feng, L., Yin, H., & Liu, Y. (2024). Solid-Phase Microextraction/Gas Chromatography–Time-of-Flight Mass Spectrometry Approach Combined with Network Pharmacology Analysis to Evaluate the Quality of Agarwood from Different Regions against Anxiety Disorder. Molecules, 29(2), 468. https://doi.org/10.3390/molecules29020468







