Development of Fluorescent Chemosensors for Calcium and Lead Detection
Abstract
1. Introduction

2. Results and Discussion
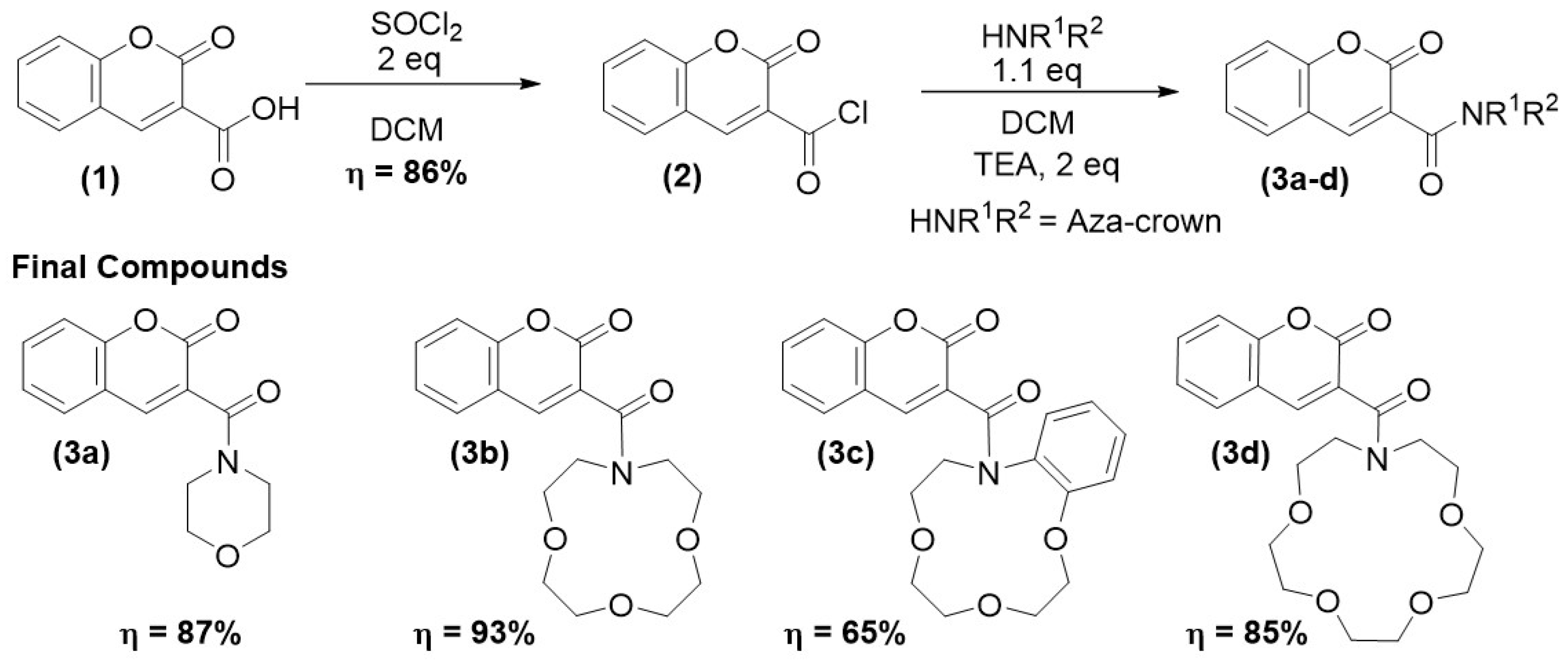
2.1. Synthesis
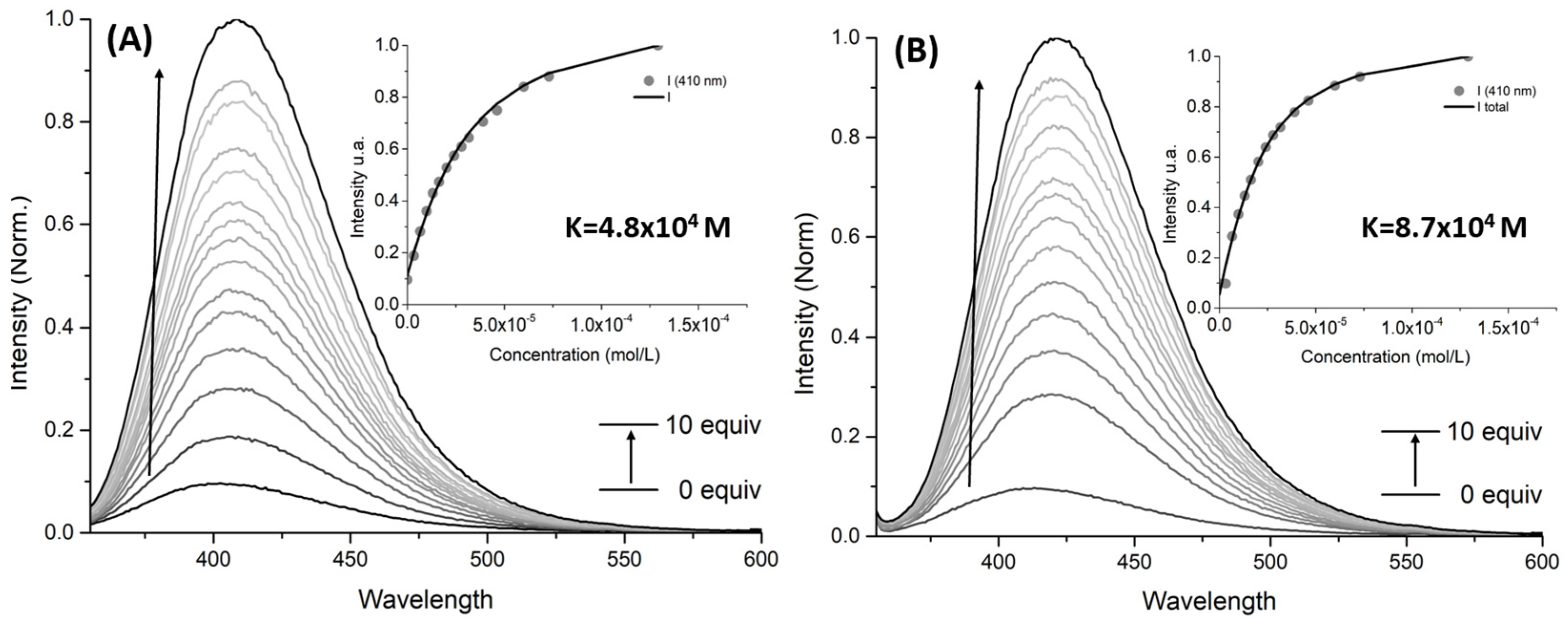
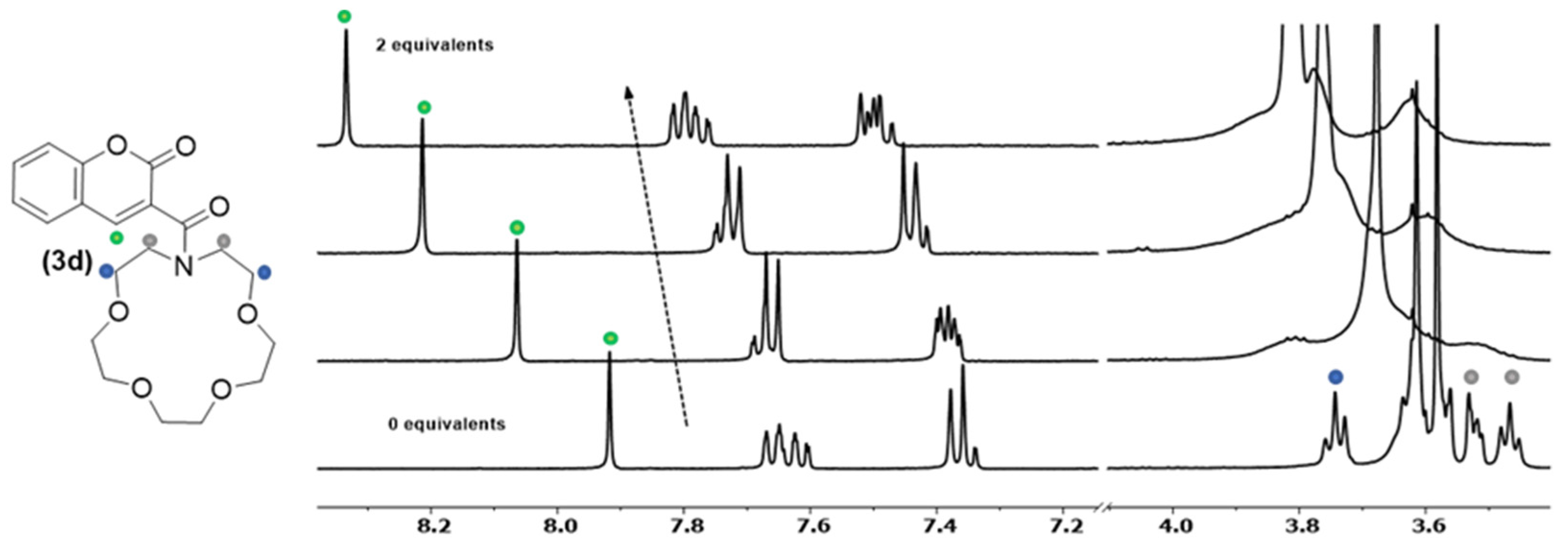
2.2. UV–Vis and Fluorescence Studies
2.3. Computational Studies
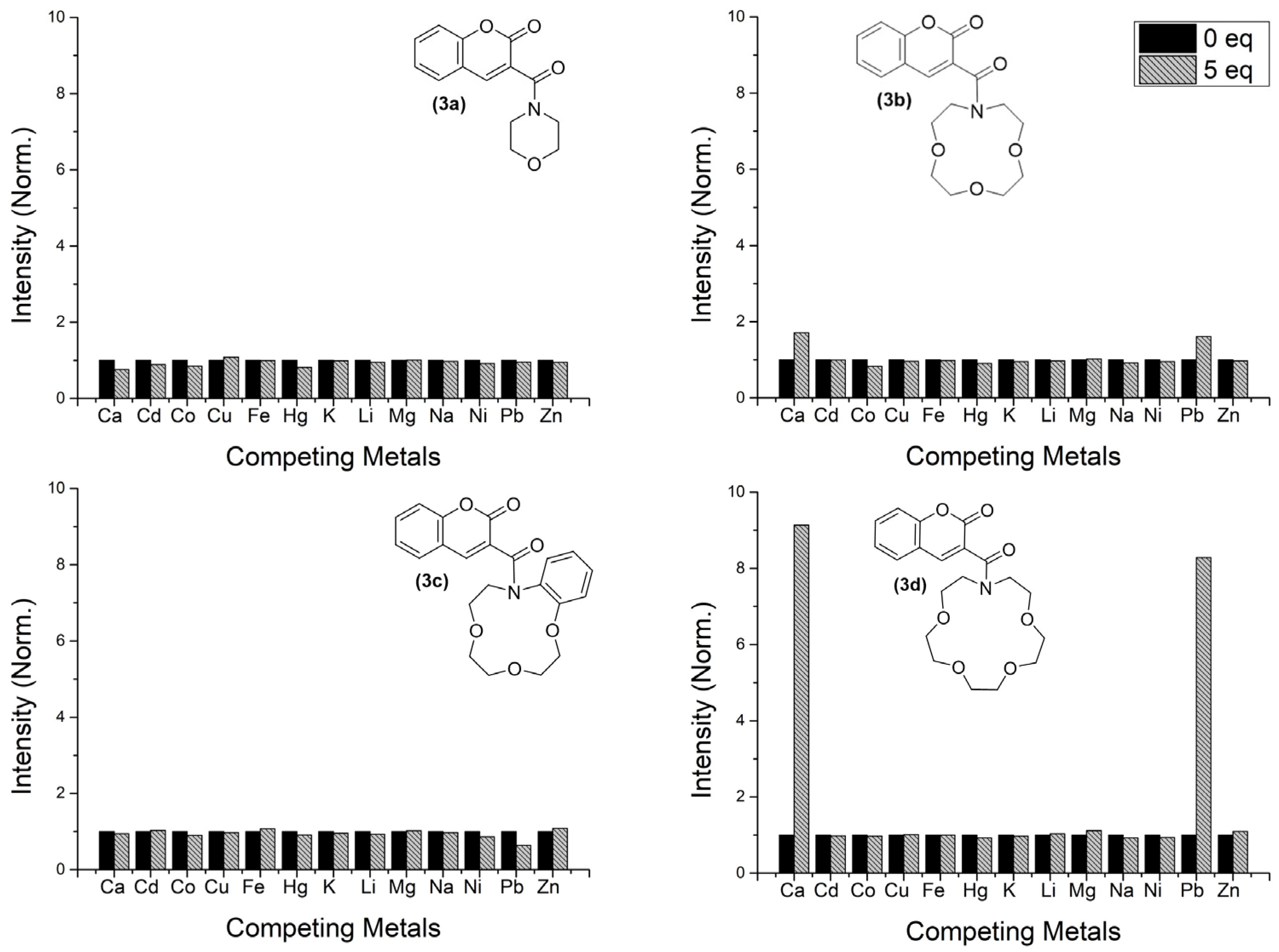
2.4. Metal Competition
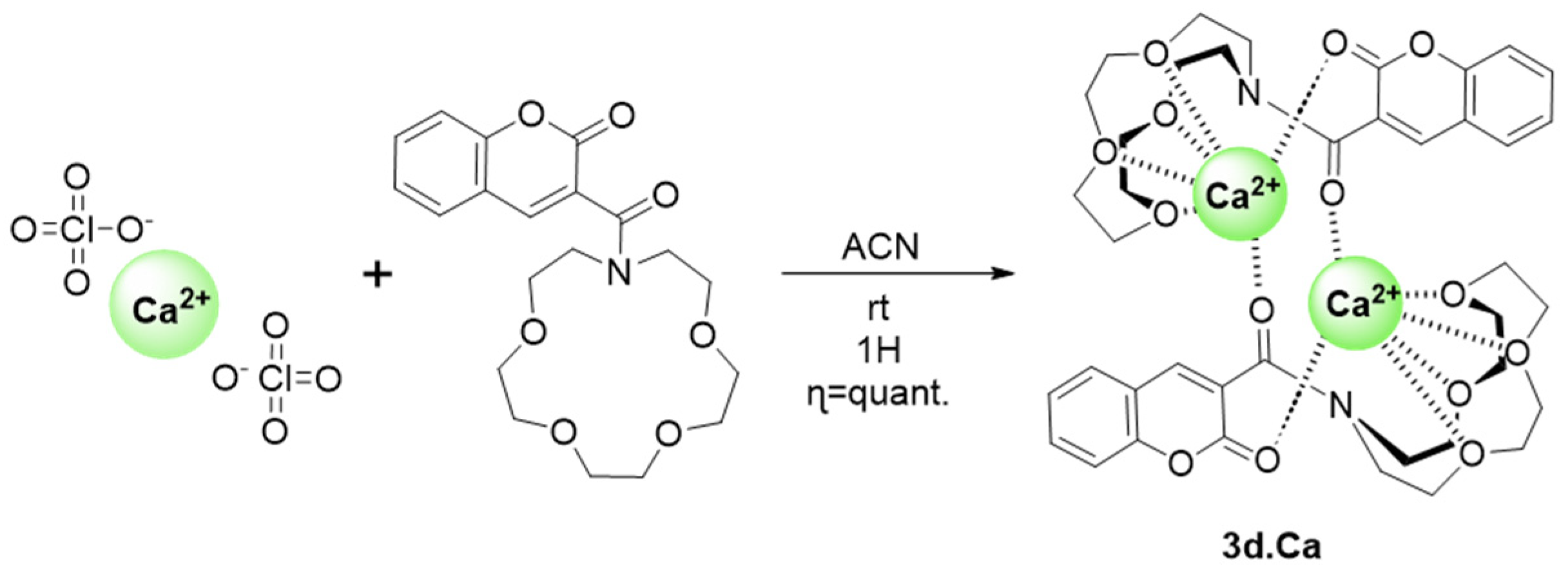
2.5. Complex Synthesis and X-ray Crystallography Studies
3. Experimental Section
3.1. General Information and Instruments
3.2. Synthesis
3.2.1. Synthesis of 2-oxo-2H-Chromene-3-Carbonyl Chloride (2)
3.2.2. Synthesis of Coumarin-3-Carboxamide Derivatives (3a-d): General Procedure
- 3-(Morpholine-4-carbonyl)-2H-chromen-2-one (3a)
- 3-(1,4,7-Trioxa-10-azacyclododecane-10-carbonyl)-2H-chromen-2-one (3b)
- 3-(2,3,5,6,9,10-hexahydro-8H-benzo[h][1,4,7]trioxa[10]azacyclododecine-10-carbonyl)-2H-chromen-2-one (3c)
- 3-(1,4,7,10-Tetraoxa-13-azacyclopentadecane-13-carbonyl)-2H-chromen-2-one (3d)
- Complex 3-(1,4,7,10-Tetraoxa-13-azacyclopentadecane-13-carbonyl)-2H-chromen-2-one with calcium (3d.Ca)
3.3. UV–Vis and Fluorescence Measurements
3.4. DFT Calculations
3.5. X-ray Diffraction Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Kumar, N.; Mukhopadhyay, S.K.; Banerjee, P. Sensitive and Fluorescent Schiff Base Chemosensor for Pico Molar Level Fluoride Detection: In Vitro Study and Mimic of Logic Gate Function. Sens. Actuators B Chem. 2016, 224, 899–906. [Google Scholar] [CrossRef]
- Pooja; Pandey, H.; Aggarwal, S.; Vats, M.; Rawat, V.; Pathak, S.R. Coumarin-based Chemosensors for Metal Ions Detection. Asian J. Org. Chem. 2022, 11, e202200455. [Google Scholar] [CrossRef]
- Jomova, K.; Makova, M.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Rhodes, C.J.; Valko, M. Essential Metals in Health and Disease. Chem. Biol. Interact. 2022, 367, 110173. [Google Scholar] [CrossRef]
- Godt, J.; Scheidig, F.; Grosse-Siestrup, C.; Esche, V.; Brandenburg, P.; Reich, A.; Groneberg, D.A. The Toxicity of Cadmium and Resulting Hazards for Human Health. J. Occup. Med. Toxicol. 2006, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, J.S.; Krakowiak, K.E.; Izatt, R.M. Aza-Crown Macrocycles: An Overview; Jonh Wiley & Sons, Inc.: Ogden, UT, USA, 1993; Volume 51, pp. 1–29. [Google Scholar] [CrossRef]
- Mazor, M.H.; McCammon, J.A.; Lybrand, T.P. Molecular Recognition in Nonaqueous Solvents: Sodium Ion, Potassium Ion, and 18-Crown-6 in Methanol. J. Am. Chem. Soc. 1989, 111, 55–56. [Google Scholar] [CrossRef]
- Citterio, D.; Omagari, M.; Kawada, T.; Sasaki, S.; Suzuki, Y.; Suzuki, K. Chromogenic betaine lariates for highly selective calcium ion sensing in aqueous environment. Anal. Chim. Acta 2004, 504, 227–234. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yin, Y.; Luo, J.; Kong, L. Coumarin-Naphthol Conjugated Schiff Base as a “Turn-on” Fluorescent Probe for Cu2+ via Selective Hydrolysis of Imine and Its Application in Live Cell Imaging. J. Photochem. Photobiol. A Chem. 2017, 333, 213–219. [Google Scholar] [CrossRef]
- Hatai, J.; Pal, S.; Jose, G.P.; Sengupta, T.; Bandyopadhyay, S. A Single Molecule Multi Analyte Chemosensor Differentiates among Zn2+, Pb2+ and Hg2+: Modulation of Selectivity by Tuning of Solvents. RSC Adv. 2012, 2, 7033. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Matsunaga, Y.; Hongpitakpong, P.; Hirai, T. A phenylbenzoxazole–amide–azacrown linkage as a selective fluorescent receptor for ratiometric sensing of Pb(II) in aqueous media. Chem. Commun. 2013, 49, 3434. [Google Scholar] [CrossRef]
- Azadbakht, R.; Hakimi, M.; Khanabadia, J.; Rudbarib, H.A. A new macrocyclic ligand as a turn-on fluorescent chemosensor for the recognition of Pb2+ ions. New J. Chem. 2017, 41, 12198–12204. [Google Scholar] [CrossRef]
- Chen, C.-T.; Huang, W.-P. A Highly Selective Fluorescent Chemosensor for Lead Ions. J. Am. Chem. Soc. 2002, 124, 6246–6247. [Google Scholar] [CrossRef] [PubMed]
- Orrego-Hernández, J.; Nuñez-Dallos, N.; Portilla, J. Recognition of Mg2+ by a New Fluorescent “Turn-on” Chemosensor Based on Pyridyl-Hydrazono-Coumarin. Talanta 2016, 152, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Khairy, G.M.; Amin, A.S.; Moalla, S.M.N.; Medhat, A.; Hassan, N. Fluorescence Determination of Fe (iii) in Drinking Water Using a New Fluorescence Chemosensor. RSC Adv. 2022, 12, 27679–27686. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Liu, X.; Pan, J.; Spring, D.R. Coumarin-Derived Transformable Fluorescent Sensor for Zn2+. Chem. Commun. 2012, 48, 4764. [Google Scholar] [CrossRef]
- Shaily, S.; Kumar, A.; Ahmed, N. A Coumarin–Chalcone Hybrid Used as a Selective and Sensitive Colorimetric and Turn-on Fluorometric Sensor for Cd2+ Detection. New J. Chem. 2017, 41, 14746–14753. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S. Database of Absorption and Fluorescence Spectra of >300 Common Compounds for Use in Photochem CAD. Photochem. Photobiol. 2018, 94, 290–327. [Google Scholar] [CrossRef]
- Reichenbach-Klinke, R.; König, B. Metal Complexes of Azacrown Ethers in Molecular Recognition and Catalysis. J. Chem.Soc. Dalton Trans. 2002, 2, 121–130. [Google Scholar] [CrossRef]
- Lide, D.R.; Baysinger, G.; Berger, L.I.; Goldberg, R.N.; Kehiaian, H.V.; Kuchitsu, K.; Roth, D.L.; Zwillinger, D. CRC Handbook of Chemistry and Physics, 2009−2010, 90th ed. J. Am. Chem. Soc. 2009, 131, 12862. [Google Scholar] [CrossRef]
- Escudero, D. Revising Intramolecular Photoinduced Electron Transfer (PET) from First-Principles. Acc. Chem. Res. 2016, 49, 1816–1824. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B Struct. Sci. Cryst. Eng. Mater. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Still, W.C.; Kahn, M.; Mitra, A. Rapid Chromatographic Technique for Preparative Separations with Moderate Resolution. J. Org. Chem. 1978, 43, 2923–2925. [Google Scholar] [CrossRef]
- Chalk, S.; McEwen, L. The IUPAC Gold Book. Chem. Int. 2017, 39, 25–30. [Google Scholar] [CrossRef]
- Thordarson, P. Determining Association Constants from Titration Experiments in Supramolecular Chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-Ray Sources for Single-Crystal Structure Determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef]





| Compound | λabs (nm) | λem (nm) | ε b (cm−1M−1) | Φ c (×10−4) |
|---|---|---|---|---|
| 3a | 285 a, 310 | 408 | 10,908 | 1.62 |
| 3b | 285 a, 315 | 403 | 7501 | 2.05 |
| 3c | 280 a, 320 | 400 | 7973 | 0.361 |
| 3d | 285 a, 315 | 405 | 10,414 | 3.51 |
| Compound | Kcalcium (M−1) | Klead (M−1) |
|---|---|---|
| 3b | 3.4 × 103 | 5.3 × 103 |
| 3c | - | 2.2 × 104 |
| 3d | 4.8 × 104 | 8.7 × 104 |
| Atoms | Distance (Å) | Moiety |
|---|---|---|
| O(2)-Ca(1) | 2.475(7) | Azacrown |
| O(3)-Ca(1) | 2.727(7) | Azacrown |
| O(4)-Ca(1) | 2.505(6) | Azacrown |
| O(5)-Ca(1) | 2.418(7) | Azacrown |
| O(6)-Ca(1) | 2.369(7) | Coumarin |
| O(8)-Ca(1) | 2.410(2) | - |
| O(13)-Ca(1) | 2.488(2) | - |
| O(1) #-Ca(1) * | 2.376(7) | Coumarin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.J.; Outis, M.; Gomes, C.S.B.; Tomé, A.C.; Moro, A.J. Development of Fluorescent Chemosensors for Calcium and Lead Detection. Molecules 2024, 29, 527. https://doi.org/10.3390/molecules29020527
Gomes LJ, Outis M, Gomes CSB, Tomé AC, Moro AJ. Development of Fluorescent Chemosensors for Calcium and Lead Detection. Molecules. 2024; 29(2):527. https://doi.org/10.3390/molecules29020527
Chicago/Turabian StyleGomes, Liliana J., Mani Outis, Clara S. B. Gomes, Augusto C. Tomé, and Artur J. Moro. 2024. "Development of Fluorescent Chemosensors for Calcium and Lead Detection" Molecules 29, no. 2: 527. https://doi.org/10.3390/molecules29020527
APA StyleGomes, L. J., Outis, M., Gomes, C. S. B., Tomé, A. C., & Moro, A. J. (2024). Development of Fluorescent Chemosensors for Calcium and Lead Detection. Molecules, 29(2), 527. https://doi.org/10.3390/molecules29020527









