High-Pressure Limit and Pressure-Dependent Rate Rules for β-Scission Reaction Class of Hydroperoxyl Alkyl Hydroperoxyl Radicals (•P(OOH)2) in Normal-Alkyl Cyclohexanes Combustion
Abstract
:1. Introduction
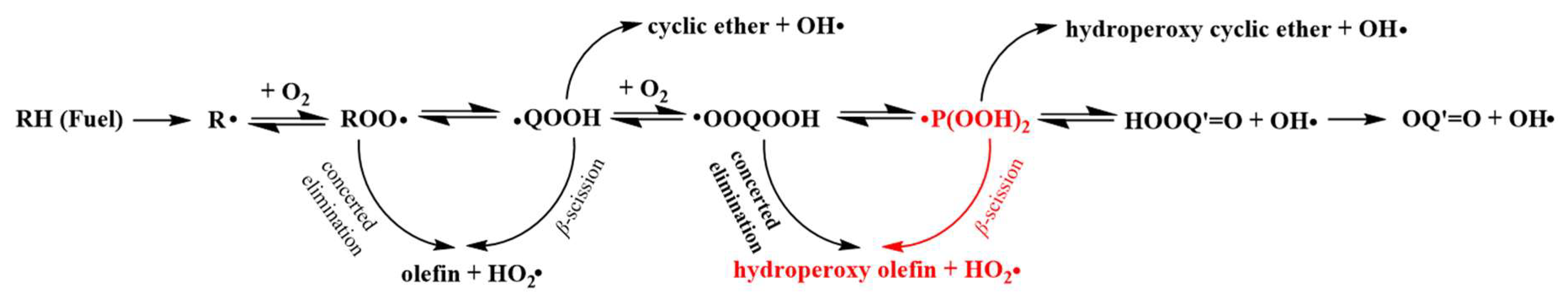
2. Results and Discussion
2.1. Conformational Analysis
2.2. Energy Barrier
2.3. Comparison of Energy Barriers for β-Scission Class
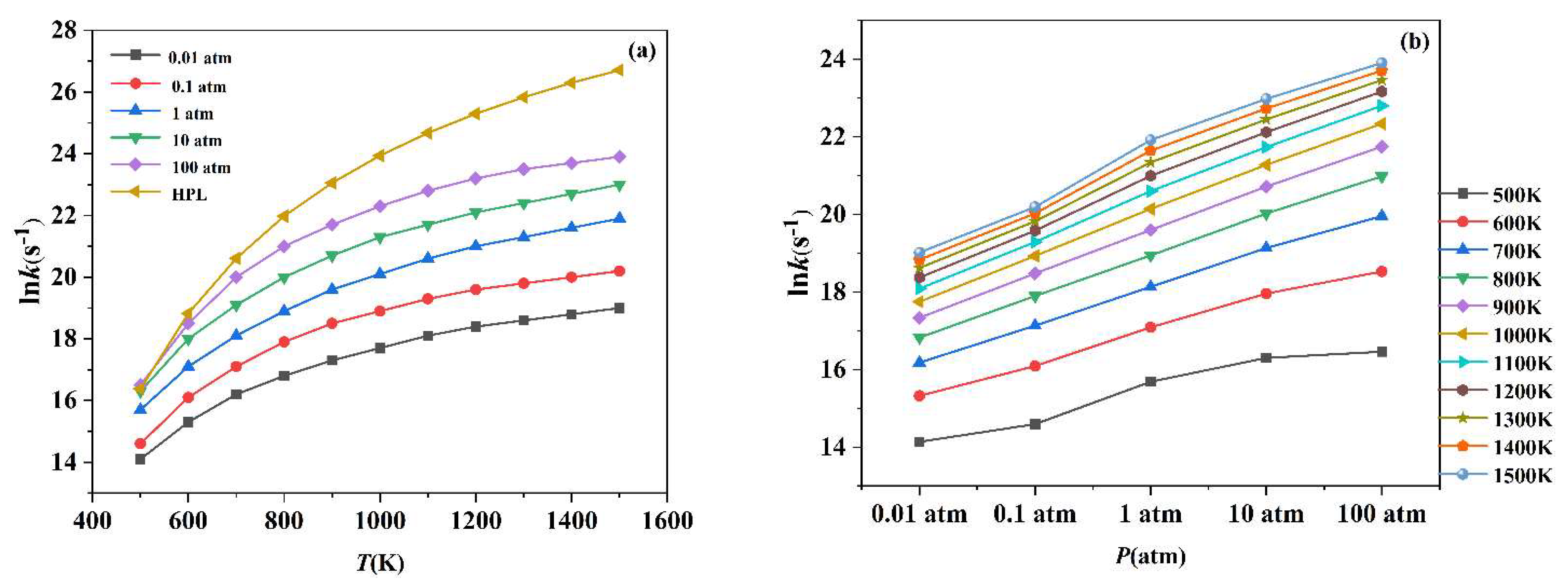
2.4. Rate Constants and Rate Rules
2.4.1. High-Pressure Limit Rate Constants and Rate Rules
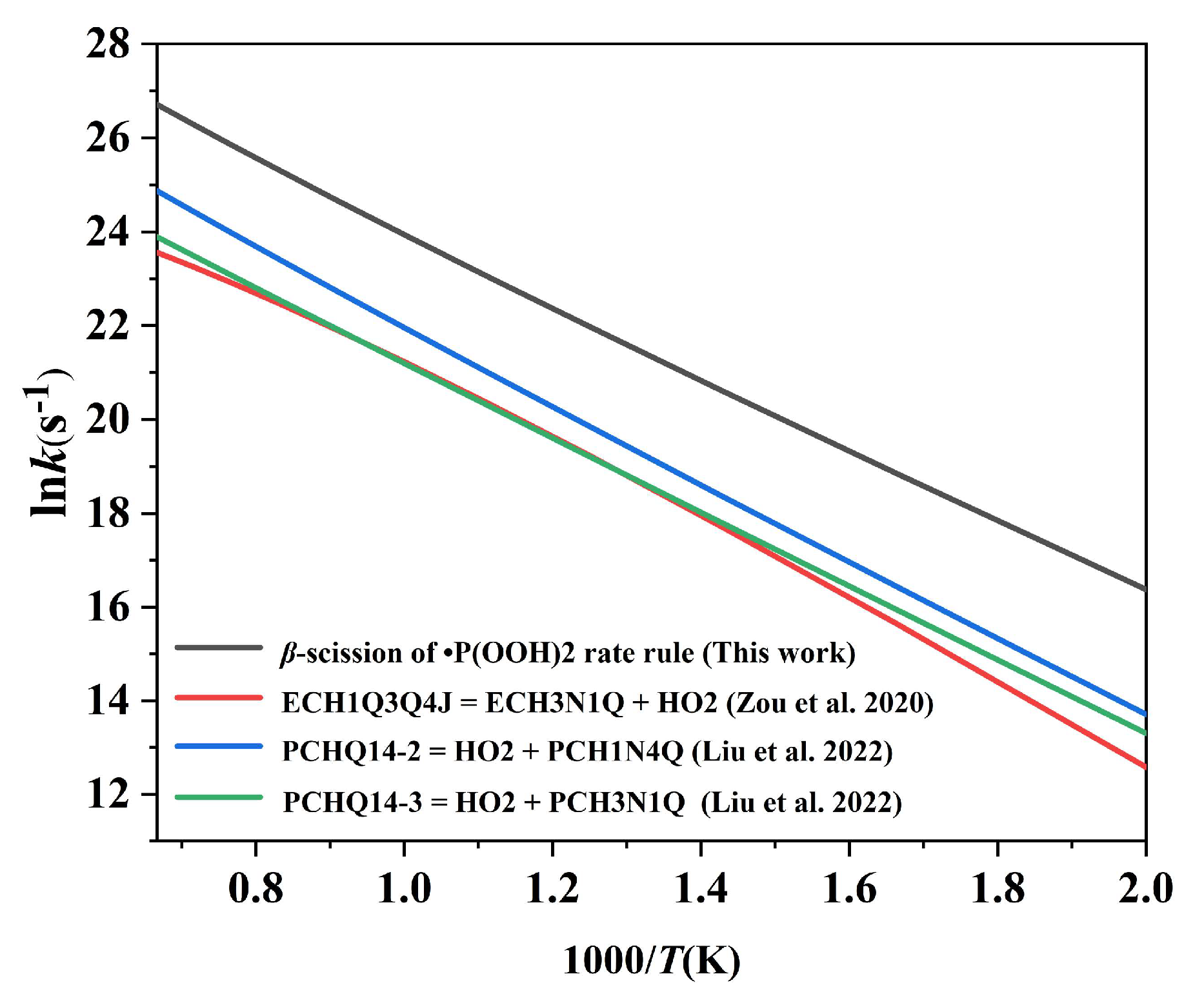
2.4.2. Comparison of the High-Pressure Limit Rate Constants with the Kinetic Data for the Corresponding Reactions in Published Mechanisms
2.4.3. Comparison of the High-Pressure Limit Rate Rule with the Kinetic Data for the β-Scission Class in Non-Cyclic Systems
2.5. Pressure-Dependent Rate Constants and Rate Rules
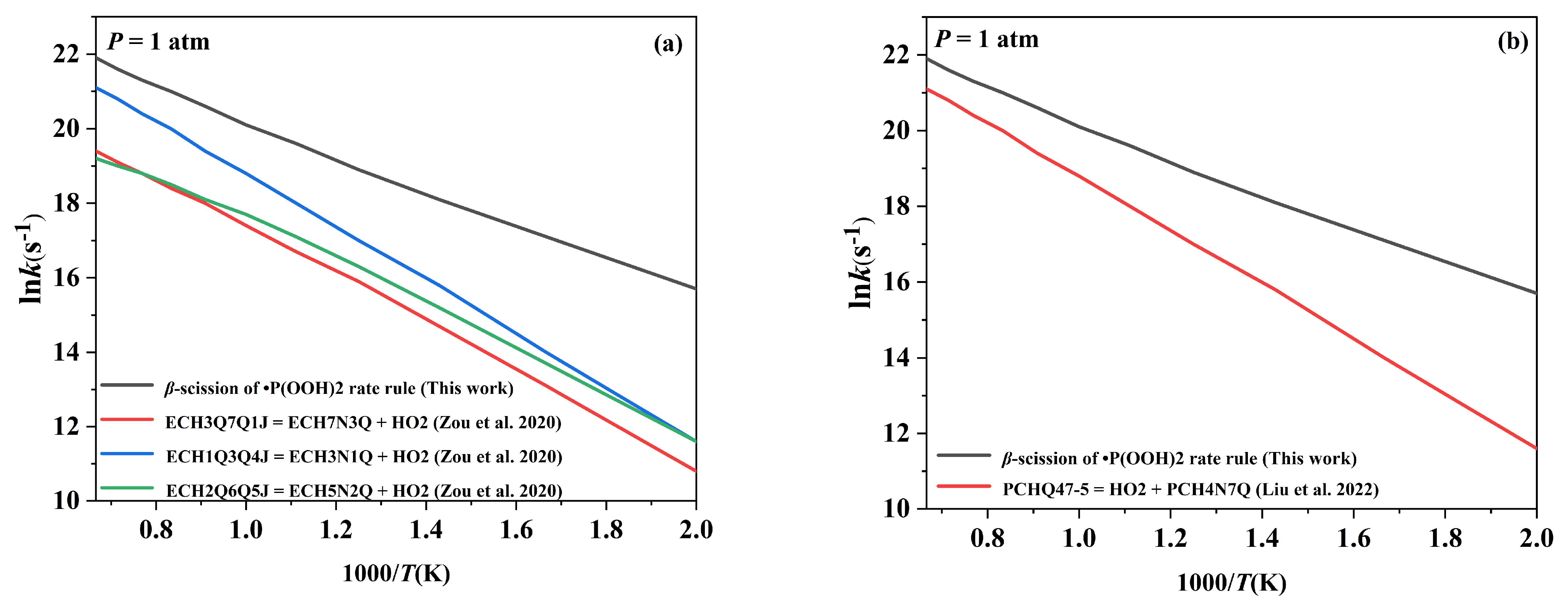
2.5.1. Comparison of the Pressure-Dependent Rate Constants with the Kinetic Data for the Corresponding Reactions in Published Mechanisms
2.5.2. Comparison of the Pressure-Dependent Rate Rules at Different Pressures
3. Methods
3.1. Electronic Structure Calculation
3.2. Calculation of Rate Constant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Violi, A.; Yan, S.; Eddings, E.G.; Sarofim, A.F.; Granata, S.; Faravelli, T.; Ranzi, E. Experimental formulation and kinetic model for JP-8 surrogate mixtures. Combust. Sci. Technol. 2002, 174, 399–417. [Google Scholar] [CrossRef]
- Walker, R.W.; Morley, C. Oxidation Kinetics and Autoignition of Hydrocarbons; Elsevier: London, UK, 1997; Volume 35. [Google Scholar]
- Yang, M.; Wang, J. Multi-structural variational kinetics study on hydrogen abstraction reactions of cyclopentanol and cyclopentane by hydroperoxyl radical with anharmonicity, recrossing and tunneling effects. Phys. Chem. Chem. Phys. 2023, 25, 12943. [Google Scholar] [CrossRef] [PubMed]
- Curran, H.; Gaffuri, P.; Pitz, W.; Westbrook, C. A comprehensive modeling study of iso-octane oxidation. Combust. Flame 2002, 129, 253–280. [Google Scholar] [CrossRef]
- Dorofeeva, O.V.; Ryzhova, O.N. Enthalpy of formation and O–H bond dissociation enthalpy of phenol: Inconsistency between theory and experiment. J. Phys. Chem. A 2016, 120, 2471–2479. [Google Scholar] [CrossRef] [PubMed]
- Pitz, W.J.; Mueller, C.J. Recent progress in the development of diesel surrogate fuels. Prog. Energy Combust. Sci. 2011, 37, 330–350. [Google Scholar] [CrossRef]
- Zheng, J.; Oyedepo, G.A.; Truhlar, D.G. Kinetics of the hydrogen abstraction re- action from 2-butanol by OH radical. J. Phys. Chem. A 2015, 119, 12182–12192. [Google Scholar] [CrossRef] [PubMed]
- Holley, A.; Dong, Y.; Andac, M.; Egolfopoulos, F.; Edwards, T. Ignition and extinction of non-premixed flames of single-component liquid hydrocarbons, jet fuels, and their surrogates. Proc. Combust. Inst. 2007, 31, 1205–1213. [Google Scholar] [CrossRef]
- Simmie, J.M. Detailed chemical kinetic models for the combustion of hydrocarbon fuels. Prog. Energy Combust. Sci. 2003, 29, 599–634. [Google Scholar] [CrossRef]
- Pitz, W.J.; Cernansky, N.P.; Dryer, F.L.; Egolfopoulos, F.N.; Farrell, J.T.; Friend, D.G.; Pitsch, H. Development of an experimental database and chemical kinetic models for surrogate gasoline fuels. SAE Int. 2007, 116, 195–216. [Google Scholar]
- Battin-Leclerc, F. Detailed chemical kinetic models for the low-temperature combustion of hydrocarbons with application to gasoline and diesel fuel surrogates. Prog. Energy Combust. Sci. 2008, 34, 440–498. [Google Scholar] [CrossRef]
- Westbrook, C.K. Chemical kinetics of hydrocarbon ignition in practical combustion systems. Proc. Combust. Inst. 2000, 28, 1563–1577. [Google Scholar] [CrossRef]
- Dec, J.E. Advanced compression-ignition engines—Understanding the in-cylinder processes. Proc. Combust. Inst. 2009, 32, 2727–2742. [Google Scholar] [CrossRef]
- Mati, K.; Ristori, A.; Gaïl, S.; Pengloan, G.; Dagaut, P. The oxidation of a diesel fuel at 1–10atm: Experimental study in a JSR and detailed chemical kinetic modeling. Proc. Combust. Inst. 2007, 31, 2939–2946. [Google Scholar] [CrossRef]
- Gauthier, B.M.; Davidson, D.F.; Hanson, R.K. Shock tube determination of ignition delay times in full-blend and surrogate fuel mixtures. Combust. Flame 2004, 139, 300–311. [Google Scholar] [CrossRef]
- Tanaka, S.; Ayala, F.; Keck, J.C.; Heywood, J.B. Two-stage ignition in HCCI combustion and HCCI control by fuels and additives. Combust. Flame 2003, 132, 219–239. [Google Scholar] [CrossRef]
- Tanaka, S.; Ayala, F.; Keck, J.C. A reduced chemical kinetic model for HCCI combustion of primary reference fuels in a rapid compression machine. Combust. Flame 2003, 133, 467–481. [Google Scholar] [CrossRef]
- Knepp, A.M.; Meloni, G.; Jusinski, L.E.; Taatjes, C.A.; Cavallotti, C.; Klippenstein, S.J. Theory, measurements, and modeling of OH and HO2 formation in the reaction of cyclohexyl radicals with O2. Phys. Chem. Chem. Phys. 2007, 9, 4315–4331. [Google Scholar] [CrossRef]
- Sirjean, B.; Glaude, P.A.; Ruiz-Lopez, M.F.; Fournet, R. Theoretical kinetic study of the reactions of cycloalkylperoxy radicals. J. Phys. Chem. A 2009, 113, 6924–6935. [Google Scholar] [CrossRef] [PubMed]
- Cavallotti, C.; Rota, R.; Faravelli, T.; Ranzi, E. Ab initio evaluation of primary cyclo-hexane oxidation reaction rates. Proc. Combust. Inst. 2007, 31, 201–209. [Google Scholar] [CrossRef]
- Fernandes, R.X.; Zádor, J.; Jusinski, L.E.; Miller, J.A.; Taatjes, C.A. Formally direct pathways and low-temperature chain branching in hydrocarbon autoignition: The cyclohexyl + O2 reaction at high pressure. Phys. Chem. Chem. Phys. 2009, 11, 1320. [Google Scholar] [CrossRef]
- Yang, Y.; Boehman, A.L.; Simmie, J.M. Uniqueness in the low temperature oxidation of cycloalkanes. Combust. Flame 2010, 157, 2357–2368. [Google Scholar] [CrossRef]
- Weber, B.W.; Pitz, W.J.; Mehl, M.; Silke, E.J.; Davis, A.C.; Sung, C.J. Experiments and modeling of the autoignition of methylcyclohexane at high pressure. Combust. Flame 2014, 161, 1972–1983. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, L.; Zhang, F.; Jiang, J. Theoretical kinetic studies for low temperature oxidation of two typical methylcyclohexyl radicals. Combust. Flame 2017, 182, 216–224. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, F.; Zhang, L. Theoretical studies for reaction kinetics of cyC6H11CH2 radical with O2. Proc. Combust. Inst. 2015, 36, 179–186. [Google Scholar] [CrossRef]
- Ning, H.; Gong, C.; Tan, N.; Li, Z.; Li, X. Low- and intermediate-temperature oxidation of ethylcyclohexane: A theoretical study. Combust. Flame 2015, 162, 4167–4182. [Google Scholar] [CrossRef]
- Liu, M.; Fang, R.; Sung, C.-J.; Aljohani, K.; Farooq, A.; Almarzooq, Y.; Mathieu, O.; Petersen, E.L.; Dagaut, P.; Zhao, J.; et al. A comprehensive experimental and modeling study of n-propylcyclohexane oxidation. Combust. Flame 2022, 238, 111944. [Google Scholar] [CrossRef]
- Ali, M.A.; Dillstrom, V.T.; Lai, J.Y.W.; Violi, A. Ab initio investigation of the thermal decomposition of n-butylcyclohexane. J. Phys. Chem. A 2014, 118, 1067–1076. [Google Scholar]
- Wang, Z.; Zhang, L.; Moshammer, K.; Popolan-Vaida, D.M.; Shankar, V.S.B.; Lucassen, A.; Hemken, C.; Taatjes, C.A.; Leone, S.R.; Kohse-Höinghaus, K.; et al. Additional chain-branching pathways in the low-temperature oxidation of branched alkanes. Combust. Flame 2016, 164, 386–396. [Google Scholar] [CrossRef]
- Yao, X.X.; Zhang, J.L.; Zhu, Y.F. A Theoretical Kinetic Study on Concerted Elimination Reaction Class of Peroxyl-hydroperoxyl-alkyl Radicals (•OOQOOH) in Normal-alkyl Cyclohexanes. Molecules 2023, 28, 6612. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.; Yao, X.; Sun, X.; Li, Z.; Wang, J.; Li, X. Automatic construction of transition states and on-the-fly accurate kinetic calculations for reaction classes in automated mechanism generators. Comput. Theor. Chem. 2020, 1184, 112852. [Google Scholar] [CrossRef]
- Zou, J.; Zhang, X.; Li, Y.; Ye, L.; Xing, L.; Li, W.; Cao, C.; Zhai, Y.; Qi, F.; Yang, J. Experimental and kinetic modeling investigation on ethylcyclohexane low-temperature oxidation in a jet-stirred reactor. Combust. Flame 2020, 214, 211–223. [Google Scholar] [CrossRef]
- Villano, S.M.; Huynh, L.K.; Carstensen, H.H.; Dean, A.M. High-pressure rate rules for alkyl + O2 reactions. 1. The dissociation, concerted elimination, and isomerization channels of the alkyl peroxy radical. J. Phys. Chem. A 2011, 115, 13425–13442. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, S.; Wu, Z.; Qiu, Y.; Yu, L.; Ruan, C.; Chen, F.; Zhu, L.; Lu, X. An experimental and kinetic modeling study of n-butylcyclohexane over low-to-high temperature ranges. Combust. Flame 2019, 206, 83–97. [Google Scholar] [CrossRef]
- Mao, Y.; Li, A.; Zhu, L.; Wu, Z.; Yu, L.; Wang, S.; Raza, M.; Lu, X. A detailed chemical mechanism for low to high temperature oxidation of n-butylcyclohexane and its validation. Combust. Flame 2019, 210, 360–373. [Google Scholar] [CrossRef]
- Miyoshi, A. Systematic computational study on the unimolecular reactions of alkylperoxy (RO2), hydroperoxyalkyl (QOOH), and hydroperoxyalkylperoxy (O2QOOH) radicals. J. Phys. Chem. A 2011, 115, 3301–3325. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, J.A.; Frisch, M.J.; Ochterski, J.W.; Petersson, G.A. A complete basis set model chemistry. VI. Use of density functional geometries and frequencies. J. Chem. Phys. 1999, 110, 2822–2827. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C. 01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Gonzalez, C.; Schlegel, H.B. An improved algorithm for reaction path following. J. Chem. Phys. 1989, 90, 2154–2161. [Google Scholar] [CrossRef]
- Mokrushin, V.; Tsang, W. Chemrate v.1.5.8; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2009. [Google Scholar]
- Laidler, K.J.; King, M.C. Development of transition-state theory. J. Phys. Chem. 1983, 87, 2657–2664. [Google Scholar] [CrossRef]
- Truhlar, D.G.; Garrett, B.C.; Klippenstein, S.J. Current Status of Transition-State Theory. J. Phys. Chem. 1996, 100, 12771–12800. [Google Scholar] [CrossRef]
- Gilbert, R.G.; Smith, S.C. Theory of Unimolecular and Recombination Reactions; Blackwell: Oxford, UK, 1990. [Google Scholar]
- Wang, H.; Frenklach, M. Transport properties of polycyclic aromatic hydrocarbons for flame modeling. Combust. Flame 1994, 96, 163–170. [Google Scholar] [CrossRef]
- Eckart, C. The penetration of a potential barrier by electrons. Phys. Rev. B 1930, 35, 1303–1309. [Google Scholar] [CrossRef]
- Johnston, H.S.; Heicklen, J. Tunnelling corrections for unsymmetrical Eckart potential energy barriers. J. Phys. Chem. 1962, 66, 532–533. [Google Scholar] [CrossRef]
- Klippenstein, S.J.; Pande, V.S.; Truhlar, D.G. Chemical kinetics and mechanisms of complex systems: A perspective on recent theoretical advances. J. Am. Chem. Soc. 2014, 136, 528–546. [Google Scholar] [CrossRef]
- Pitzer, K.S.; Gwinn, W.D. Energy levels and thermodynamic functions for molecules with internal rotation I. Rigid frame with attached tops. J. Chem. Phys. 1942, 10, 428–440. [Google Scholar] [CrossRef]
- Mammen, M.; Shakhnovich, E.I.; Whitesides, G.M. Using a convenient, quantitative model for torsional entropy to establish qualitative trends for molecular processes that restrict conformational freedom. J. Org. Chem. 1998, 63, 3168–3175. [Google Scholar] [CrossRef]
- CHEMKIN-PRO 15092; Reaction Design: San Diego, CA, USA, 2009.

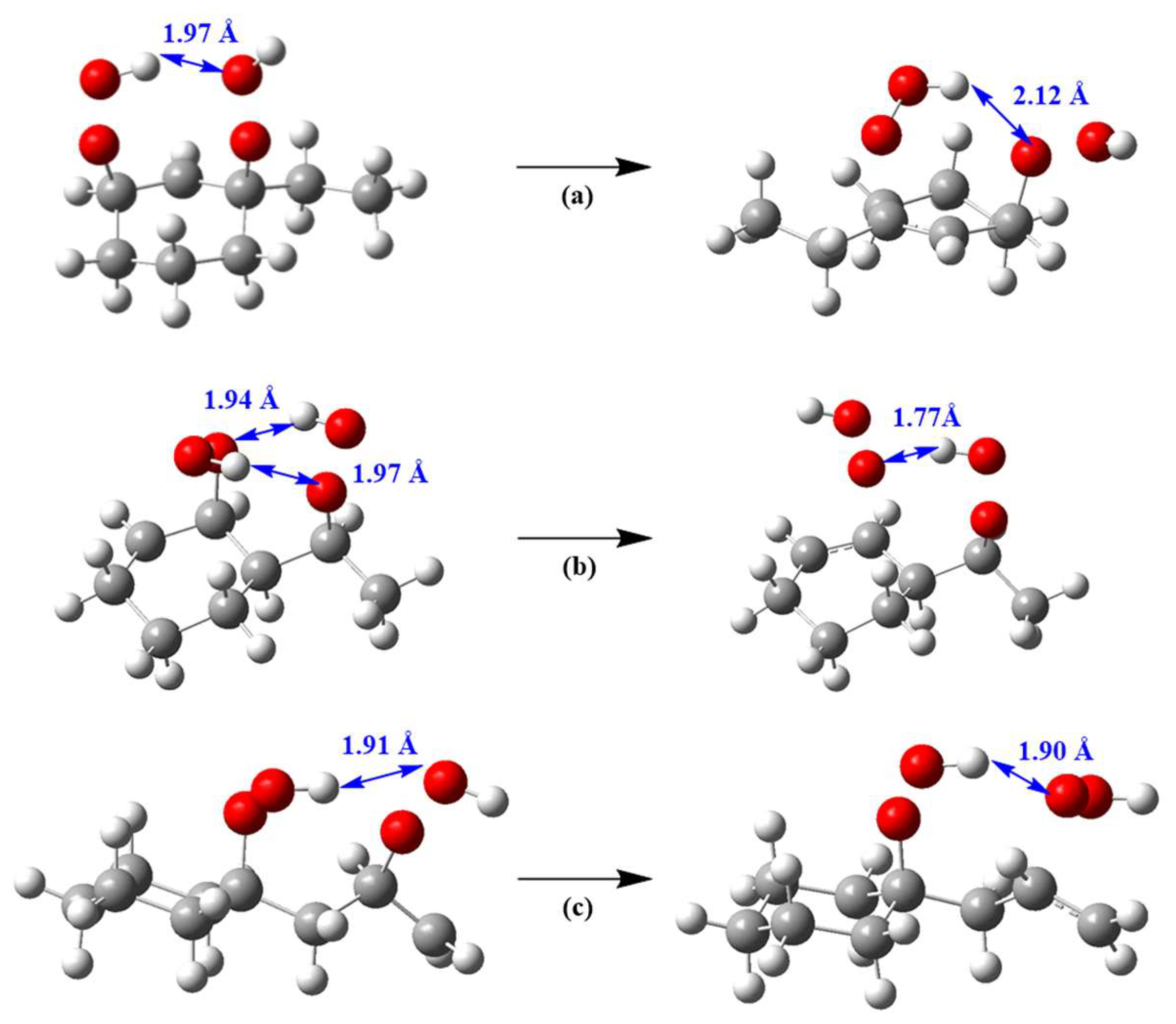

| Reactants | Transition States | Energy Barriers | |||
|---|---|---|---|---|---|
| (1) | R-no HB |  | TS-no HB |  | 16.4 |
| (2) | R-HB | 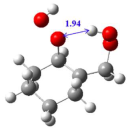 | TS-no HB | 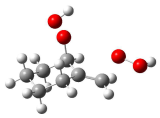 | 19.9 |
| (3) | R-no HB | 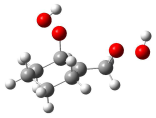 | TS-HB |  | 12.7 |
| (4) | R-HB | 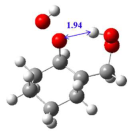 | TS-HB |  | 16.2 |
| Reaction | Energy Barrier | |
|---|---|---|
| R1 | 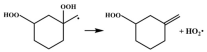 | 16.3 |
| R2 | 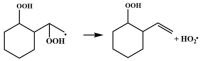 | 15.1 |
| R3 |  | 14.8 |
| R4 | 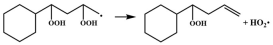 | 15.4 |
| R5 | 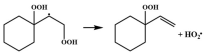 | 14.5 |
| R6 | 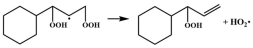 | 14.6 |
| R7 | 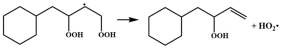 | 15.7 |
| R8 | 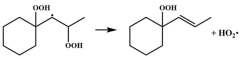 | 15.3 |
| R9 | 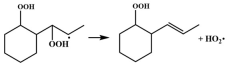 | 14.7 |
| R10 |  | 14.6 |
| R11 |  | 14.0 |
| R12 | 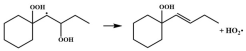 | 15.9 |
| R13 | 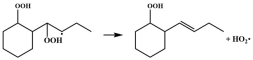 | 14.5 |
| R14 | 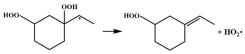 | 16.5 |
| R15 | 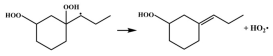 | 16.4 |
| R16 | 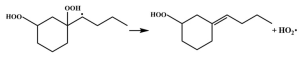 | 16.3 |
| R17 | 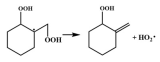 | 16.2 |
| R18 | 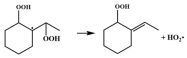 | 15.6 |
| R19 | 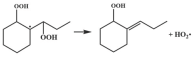 | 15.8 |
| R20 |  | 15.6 |
| R21 | 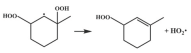 | 14.7 |
| R22 |  | 15.9 |
| R23 | 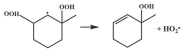 | 14.8 |
| R24 | 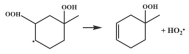 | 15.3 |
| R25 |  | 14.8 |
| R26 |  | 16.4 |
| R27 |  | 15.0 |
| R28 |  | 16.6 |
| R29 | 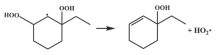 | 15.1 |
| R30 |  | 15.2 |
| R31 | 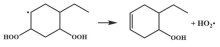 | 14.8 |
| R32 |  | 16.4 |
| R33 |  | 14.9 |
| R34 | 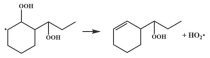 | 16.8 |
| R35 | 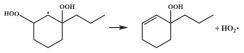 | 15.1 |
| R36 | 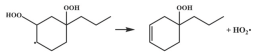 | 15.2 |
| R37 | 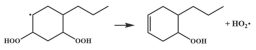 | 14.8 |
| R38 | 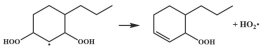 | 16.4 |
| R39 | 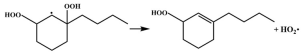 | 14.9 |
| R40 | 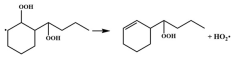 | 16.8 |
| R41 | 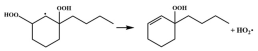 | 15.2 |
| R42 | 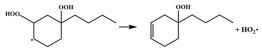 | 15.3 |
| R43 |  | 14.8 |
| R44 | 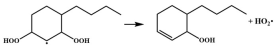 | 16.5 |
| β-scission class | (15.5 a, 2.8 b) |
| β-Scission of •P(OOH)2 This Work | Energy Barrier | β-Scission of •QOOH Previous Work | Energy Barrier |
|---|---|---|---|
 | 16.3 | 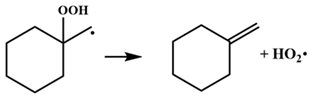 | 16.0 |
 | 15.1 |  | 16.0 |
 | 14.8 | 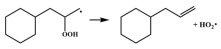 | 15.9 |
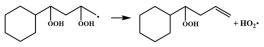 | 15.4 | 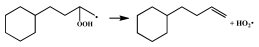 | 16.2 |
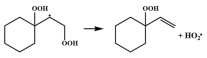 | 14.5 |  | 15.6 |
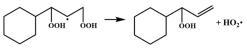 | 14.6 | 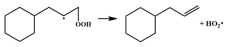 | 16.1 |
 | 15.7 |  | 16.0 |
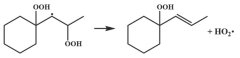 | 15.3 |  | 15.3 |
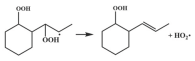 | 14.7 | 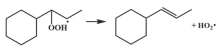 | 15.3 |
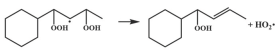 | 14.6 | 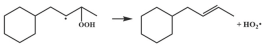 | 16.3 |
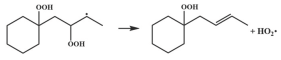 | 14.0 |  | 15.6 |
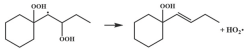 | 15.9 | 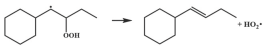 | 15.7 |
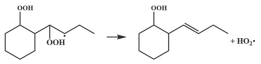 | 14.5 | 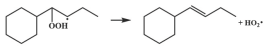 | 15.2 |
 | 16.5 |  | 16.6 |
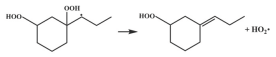 | 16.4 | 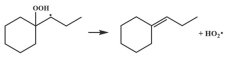 | 16.5 |
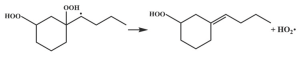 | 16.3 | 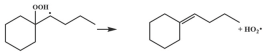 | 16.4 |
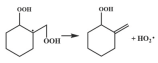 | 16.2 | 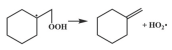 | 14.4 |
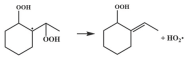 | 15.6 | 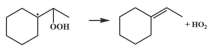 | 14.1 |
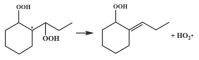 | 15.8 | 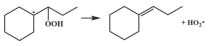 | 14.0 |
 | 15.6 | 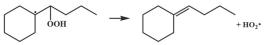 | 13.9 |
 | 14.7 |  | 15.3 |
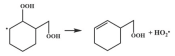 | 15.9 | 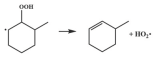 | 15.6 |
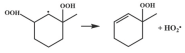 | 14.8 |  | 15.2 |
 | 15.3 | 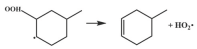 | 15.0 |
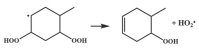 | 14.8 | 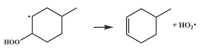 | 15.1 |
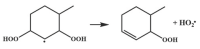 | 16.4 |  | 15.1 |
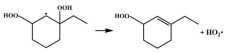 | 15.0 | 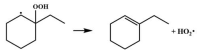 | 15.2 |
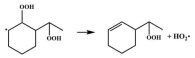 | 16.6 | 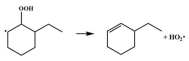 | 15.6 |
 | 15.1 | 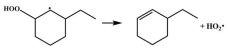 | 15.1 |
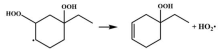 | 15.2 | 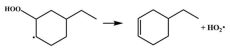 | 14.9 |
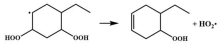 | 14.8 | 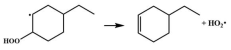 | 15.1 |
 | 16.4 | 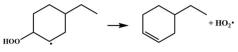 | 15.1 |
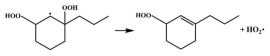 | 14.9 | 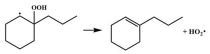 | 15.1 |
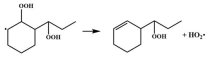 | 16.8 | 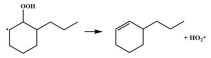 | 15.7 |
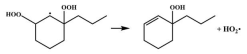 | 15.1 |  | 15.1 |
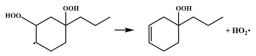 | 15.2 |  | 15.0 |
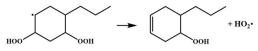 | 14.8 | 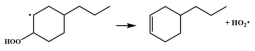 | 15.1 |
 | 16.4 | 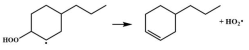 | 15.1 |
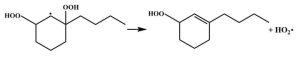 | 14.9 | 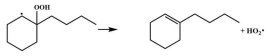 | 15.2 |
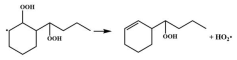 | 16.8 | 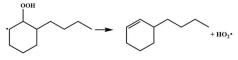 | 15.6 |
 | 15.2 |  | 15.0 |
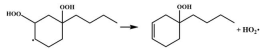 | 15.3 | 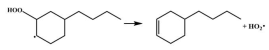 | 15.0 |
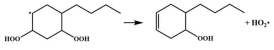 | 14.8 | 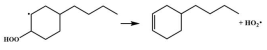 | 15.1 |
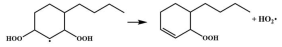 | 16.5 |  | 15.1 |
| Modified Arrhenius Parameters | T = 800 K | ||||
|---|---|---|---|---|---|
| Reaction | A (s−1) | n | E (cal mol−1) | k (s−1) | k/kave e |
| R1 | 7.89 × 1010 | 0.68 | 14,825 | 6.53 × 108 | 0.2 |
| R2 | 3.30 × 1010 | 0.72 | 12,917.3 | 1.21 × 109 | 0.4 |
| R3 | 1.76 × 1014 | −0.47 | 13,467.6 | 1.57 × 109 | 0.6 |
| R4 | 1.69 × 1021 | −2.79 | 15,678.3 | 6.97 × 108 | 0.3 |
| R5 | 8.10 × 1010 | 0.57 | 12,586.8 | 1.29 × 109 | 0.5 |
| R6 | 1.62 × 1011 | 0.57 | 12,591 | 2.58 × 109 | 0.9 |
| R7 | 3.42 × 1012 | 0.33 | 14,195.2 | 4.24 × 109 | 1.5 |
| R8 | 1.30 × 1015 | −0.53 | 15,412.3 | 2.35 × 109 | 0.8 |
| R9 | 1.54 × 1014 | −0.46 | 14,286.6 | 9.02 × 108 | 0.3 |
| R10 | 6.39 × 1014 | −0.61 | 14,562.3 | 1.11 × 109 | 0.4 |
| R11 | 5.28 × 1010 | 0.56 | 12,988.8 | 6.37 × 108 | 0.2 |
| R12 | 1.88 × 1014 | −0.20 | 16,039.2 | 2.10 × 109 | 0.8 |
| R13 | 6.92 × 1013 | −0.14 | 14,058.2 | 3.80 × 109 | 1.4 |
| R14 | 8.06 × 1010 | 0.75 | 15,413.8 | 7.54 × 108 | 0.3 |
| R15 | 8.91 × 1015 | −0.65 | 16,530.5 | 3.58 × 109 | 1.3 |
| R16 | 3.21 × 1014 | −0.21 | 16,328.9 | 2.75 × 109 | 1.0 |
| R17 | 3.37 × 1012 | 0.29 | 14,444.9 | 2.62 × 109 | 0.9 |
| R18 | 2.11 × 1012 | 0.39 | 14,762.5 | 2.70 × 109 | 1.0 |
| R19 | 1.21 × 1015 | −0.41 | 15,684.1 | 4.14 × 109 | 1.5 |
| R20 | 2.00 × 1010 | 1.00 | 14,149.3 | 2.25 × 109 | 0.8 |
| R21 | 1.11 × 1011 | 0.81 | 13,327.4 | 5.60 × 109 | 2.0 |
| R22 | 7.51 × 1012 | 0.45 | 15,685.9 | 7.67 × 109 | 2.8 |
| R23 | 4.22 × 1011 | 0.43 | 13,543.5 | 1.46 × 109 | 0.5 |
| R24 | 4.67 × 1011 | 0.37 | 14,010.2 | 8.15 × 108 | 0.3 |
| R25 | 5.82 × 1011 | 0.39 | 13,522.5 | 1.65 × 109 | 0.6 |
| R26 | 4.46 × 1011 | 0.49 | 15,149.1 | 8.33 × 108 | 0.3 |
| R27 | 1.31 × 1014 | −0.14 | 15,881.9 | 2.40 × 109 | 0.9 |
| R28 | 4.37 × 1014 | 0.01 | 17,887.1 | 6.07 × 109 | 2.2 |
| R29 | 7.82 × 1013 | −0.17 | 15,816.1 | 1.23 × 109 | 0.4 |
| R30 | 5.75 × 1013 | −0.17 | 15,859.5 | 8.64 × 108 | 0.3 |
| R31 | 1.06 × 1012 | 0.43 | 13,397.7 | 3.97 × 109 | 1.4 |
| R32 | 9.02 × 1011 | 0.48 | 15,188.5 | 1.62 × 109 | 0.6 |
| R33 | 1.68 × 1012 | 0.44 | 13,802.2 | 5.40 × 109 | 1.9 |
| R34 | 1.20 × 1011 | 0.99 | 16,357 | 3.11 × 109 | 1.1 |
| R35 | 2.14 × 1012 | 0.42 | 13,842.7 | 5.82 × 109 | 2.1 |
| R36 | 2.43 × 1012 | 0.31 | 14,131.4 | 2.61 × 109 | 0.9 |
| R37 | 1.98 × 1012 | 0.39 | 13,496.6 | 5.50 × 109 | 2.0 |
| R38 | 9.25 × 1012 | 0.44 | 16,624 | 4.96 × 109 | 1.8 |
| R39 | 6.00 × 1011 | 0.43 | 13,791.4 | 1.85 × 109 | 0.7 |
| R40 | 1.33 × 1010 | 1.43 | 15,998.7 | 8.14 × 109 | 2.9 |
| R41 | 1.73 × 1011 | 0.53 | 13,585.9 | 1.15 × 109 | 0.4 |
| R42 | 1.86 × 1014 | −0.17 | 15,912.6 | 2.75 × 109 | 1.0 |
| R43 | 1.09 × 1012 | 0.40 | 13,427.9 | 3.32 × 109 | 1.2 |
| R44 | 1.04 × 1012 | 0.47 | 15,255.5 | 1.63 × 109 | 0.6 |
| β scission class rate rule | 8.47 × 109 | 1.11 | 13,585.5 | 2.78 × 109 | 12.8 # |
| Modified Arrhenius Parameters | T = 800 K | |||||
|---|---|---|---|---|---|---|
| Reaction | Pressure (atm) | A (s−1) | n | E (cal mol−1) | k (s−1) | k/kave e |
| R1 | 0.01 | 1.99 × 1012 | −1.10 | 8262.1 | 6.9 × 106 | 0.5 |
| 0.1 | 2.05 × 1012 | −0.89 | 9158.4 | 1.7 × 107 | 0.4 | |
| 1 | 6.57 × 1015 | −1.66 | 11,742.7 | 6.2 × 107 | 0.4 | |
| 10 | 2.81 × 1024 | −3.94 | 17,150.0 | 2.1 × 108 | 0.4 | |
| 100 | 1.18 × 1028 | −4.75 | 20,526.9 | 4.7 × 108 | 0.4 | |
| R2 | 0.01 | 5.96 × 1010 | −0.66 | 6625.0 | 1.1 × 107 | 0.8 |
| 0.1 | 1.25 × 1029 | −5.87 | 16,057.4 | 4.6 × 107 | 1.2 | |
| 1 | 1.48 × 1013 | −0.77 | 10,088.1 | 1.5 × 108 | 1.0 | |
| 10 | 1.00 × 1020 | −2.54 | 14,480.4 | 4.7 × 108 | 1.0 | |
| 100 | 8.13 × 1021 | −2.88 | 16,729.3 | 9.2 × 108 | 0.7 | |
| R3 | 0.01 | 1.11 × 1012 | −1.03 | 6829.8 | 1.5 × 107 | 1.1 |
| 0.1 | 6.54 × 1037 | −8.51 | 19,359.7 | 6.8 × 107 | 1.8 | |
| 1 | 1.74 × 1044 | −10.13 | 23,253.2 | 3.0 × 108 | 2.1 | |
| 10 | 6.07 × 1018 | −2.19 | 13,287.4 | 6.2 × 108 | 1.3 | |
| 100 | 5.04 × 1021 | −2.86 | 15,842.2 | 1.2 × 109 | 1.0 | |
| R4 | 0.01 | 1.05 × 1012 | −1.01 | 7003.7 | 1.5 × 107 | 1.1 |
| 0.1 | 2.60 × 1030 | −6.26 | 16,431.0 | 5.5 × 107 | 1.4 | |
| 1 | 2.81 × 1013 | −0.87 | 9953.5 | 1.6 × 108 | 1.1 | |
| 10 | 3.57 × 1019 | −2.47 | 13,813.7 | 4.1 × 108 | 0.9 | |
| 100 | 1.66 × 1022 | −3.13 | 15,871.5 | 6.4 × 108 | 0.5 | |
| R5 | 0.01 | 1.65 × 1011 | −0.79 | 6515.2 | 1.4 × 107 | 1.0 |
| 0.1 | 3.02 × 1034 | −7.50 | 18,035.6 | 6.0 × 107 | 1.5 | |
| 1 | 5.24 × 1021 | −3.35 | 13,502.0 | 2.0 × 108 | 1.4 | |
| 10 | 6.29 × 1018 | −2.19 | 13,553.5 | 5.5 × 108 | 1.2 | |
| 100 | 6.48 × 1020 | −2.57 | 15,823.8 | 1.1 × 109 | 0.9 | |
| R6 | 0.01 | 1.18 × 1012 | −1.05 | 6650.9 | 1.6 × 107 | 1.2 |
| 0.1 | 3.54 × 1042 | −9.96 | 21,053.6 | 7.8 × 107 | 2.0 | |
| 1 | 2.01 × 1047 | −11.05 | 24,331.0 | 3.8 × 108 | 2.6 | |
| 10 | 8.61 × 1018 | −2.22 | 13,030.2 | 8.3 × 108 | 1.8 | |
| 100 | 2.49 × 1023 | −3.32 | 16,399.4 | 1.9 × 109 | 1.5 | |
| R7 | 0.01 | 4.21 × 1012 | −1.21 | 7051.3 | 1.6 × 107 | 1.1 |
| 0.1 | 3.44 × 107 | 0.63 | 5985.7 | 5.3 × 107 | 1.4 | |
| 1 | 4.77 × 10−67 | 23.12 | −25,993.3 | 7.7 × 107 | 0.5 | |
| 10 | 4.41 × 10−43 | 16.20 | −14,049.8 | 3.3 × 108 | 0.7 | |
| 100 | 4.84 × 10−16 | 8.37 | −404.5 | 1.2 × 109 | 1.0 | |
| R8 | 0.01 | 3.33 × 1011 | −0.87 | 7033.6 | 1.2 × 107 | 0.9 |
| 0.1 | 7.94 × 1038 | −8.81 | 20,325.8 | 5.9 × 107 | 1.5 | |
| 1 | 5.62 × 1048 | −11.45 | 25,781.3 | 3.0 × 108 | 2.0 | |
| 10 | 7.09 × 1022 | −3.35 | 15,688.8 | 6.9 × 108 | 1.5 | |
| 100 | 4.55 × 1028 | −4.84 | 19,707.2 | 1.6 × 109 | 1.3 | |
| R9 | 0.01 | 2.64 × 1011 | −0.84 | 6999.6 | 1.2 × 107 | 0.9 |
| 0.1 | 2.84 × 1028 | −5.66 | 15,908.9 | 4.6 × 107 | 1.2 | |
| 1 | 6.71 × 1013 | −0.96 | 10,556.9 | 1.4 × 108 | 1.0 | |
| 10 | 3.60 × 1019 | −2.40 | 14,479.2 | 4.2 × 108 | 0.9 | |
| 100 | 9.10 × 1021 | −2.92 | 16,844.3 | 7.7 × 108 | 0.6 | |
| R10 | 0.01 | 1.36 × 1012 | −1.04 | 7226.5 | 1.4 × 107 | 1.0 |
| 0.1 | 5.46 × 1033 | −7.25 | 18,099.7 | 5.5 × 107 | 1.4 | |
| 1 | 3.54 × 1013 | −0.86 | 10,347.0 | 1.7 × 108 | 1.1 | |
| 10 | 7.96 × 1020 | −2.79 | 14,975.2 | 5.0 × 108 | 1.1 | |
| 100 | 1.13 × 1023 | −3.22 | 17,261.3 | 9.5 × 108 | 0.8 | |
| R11 | 0.01 | 9.06 × 1010 | −0.70 | 6777.2 | 1.2 × 107 | 0.9 |
| 0.1 | 5.82 × 1010 | −0.47 | 7375.1 | 2.4 × 107 | 0.6 | |
| 1 | 3.62 × 1014 | −1.34 | 10,068.0 | 8.1 × 107 | 0.6 | |
| 10 | 7.72 × 1021 | −3.26 | 14,810.5 | 2.4 × 108 | 0.5 | |
| 100 | 4.46 × 1024 | −3.83 | 17,659.8 | 5.0 × 108 | 0.4 | |
| R12 | 0.01 | 1.07 × 1010 | −0.41 | 6594.7 | 1.1 × 107 | 0.8 |
| 0.1 | 6.71 × 1013 | −1.35 | 9102.0 | 2.6 × 107 | 0.7 | |
| 1 | 4.32 × 1016 | −1.91 | 11,204.9 | 1.0 × 108 | 0.7 | |
| 10 | 7.66 × 1022 | −3.49 | 15,299.8 | 3.8 × 108 | 0.8 | |
| 100 | 4.45 × 1029 | −5.21 | 20,083.2 | 1.1 × 109 | 0.8 | |
| R13 | 0.01 | 2.47 × 100 | 2.45 | 1496.2 | 1.3 × 107 | 0.9 |
| 0.1 | 1.13 × 108 | 0.37 | 5701.2 | 3.8 × 107 | 1.0 | |
| 1 | 9.51 × 1013 | −1.15 | 8877.3 | 1.6 × 108 | 1.1 | |
| 10 | 1.85 × 1018 | −2.17 | 11,771.2 | 5.8 × 108 | 1.2 | |
| 100 | 8.37 × 1024 | −3.88 | 16,277.2 | 1.6 × 109 | 1.3 | |
| R14 | 0.01 | 2.60 × 1013 | −1.42 | 8856.5 | 7.2 × 106 | 0.5 |
| 0.1 | 7.88 × 1017 | −2.56 | 11,783.8 | 1.8 × 107 | 0.5 | |
| 1 | 3.58 × 1017 | −2.18 | 12,665.9 | 6.0 × 107 | 0.4 | |
| 10 | 4.24 × 1025 | −4.29 | 17,845.4 | 2.0 × 108 | 0.4 | |
| 100 | 6.83 × 1029 | −5.25 | 21,550.1 | 4.9 × 108 | 0.4 | |
| R15 | 0.01 | 5.39 × 107 | 0.26 | 5428.8 | 1.0 × 107 | 0.7 |
| 0.1 | 1.90 × 1012 | −0.88 | 8313.7 | 2.9 × 107 | 0.8 | |
| 1 | 4.87 × 1015 | −1.62 | 10,489.8 | 1.3 × 108 | 0.9 | |
| 10 | 7.14 × 1020 | −2.88 | 13,890.4 | 5.0 × 108 | 1.1 | |
| 100 | 2.95 × 1028 | −4.86 | 18,946.0 | 1.5 × 109 | 1.2 | |
| R16 | 0.01 | 1.84 × 109 | −0.18 | 6215.5 | 1.1 × 107 | 0.8 |
| 0.1 | 1.45 × 1014 | −1.45 | 9211.8 | 2.8 × 107 | 0.7 | |
| 1 | 1.29 × 1017 | −2.05 | 11,335.5 | 1.1 × 108 | 0.8 | |
| 10 | 5.61 × 1022 | −3.44 | 15,100.8 | 4.3 × 108 | 0.9 | |
| 100 | 6.95 × 1029 | −5.26 | 20,023.1 | 1.3 × 109 | 1.0 | |
| R17 | 0.01 | 2.25 × 106 | 0.64 | 4682.3 | 8.6 × 106 | 0.6 |
| 0.1 | 5.97 × 108 | 0.16 | 6662.7 | 2.6 × 107 | 0.7 | |
| 1 | 2.31 × 1011 | −0.35 | 8454.8 | 1.1 × 108 | 0.8 | |
| 10 | 8.94 × 1017 | −2.04 | 12,490.5 | 4.2 × 108 | 0.9 | |
| 100 | 3.25 × 1025 | −4.01 | 17,488.3 | 1.2 × 109 | 1.0 | |
| R18 | 0.01 | 1.87 × 108 | 0.08 | 5649.0 | 9.4 × 106 | 0.7 |
| 0.1 | 5.99 × 1010 | −0.44 | 7649.2 | 2.6 × 107 | 0.7 | |
| 1 | 2.50 × 1013 | −0.95 | 9495.1 | 1.1 × 108 | 0.8 | |
| 10 | 3.68 × 1019 | −2.51 | 13,392.2 | 4.2 × 108 | 0.9 | |
| 100 | 8.87 × 1026 | −4.42 | 18,359.7 | 1.2 × 109 | 1.0 | |
| R19 | 0.01 | 1.63 × 105 | 1.01 | 4078.0 | 1.1 × 107 | 0.8 |
| 0.1 | 1.17 × 1010 | −0.21 | 7092.7 | 3.2 × 107 | 0.8 | |
| 1 | 2.14 × 1014 | −1.23 | 9561.2 | 1.4 × 108 | 1.0 | |
| 10 | 1.27 × 1019 | −2.37 | 12,701.4 | 5.6 × 108 | 1.2 | |
| 100 | 5.15 × 1026 | −4.36 | 17,686.6 | 1.7 × 109 | 1.4 | |
| R20 | 0.01 | 3.33 × 108 | 0.03 | 5695.6 | 1.1 × 107 | 0.8 |
| 0.1 | 2.80 × 1012 | −0.95 | 8256.4 | 2.8 × 107 | 0.7 | |
| 1 | 5.24 × 1015 | −1.65 | 10,516.9 | 1.1 × 108 | 0.8 | |
| 10 | 3.03 × 1021 | −3.08 | 14,356.4 | 4.0 × 108 | 0.9 | |
| 100 | 1.07 × 1028 | −4.74 | 19,031.9 | 1.1 × 109 | 0.9 | |
| R21 | 0.01 | 9.08 × 101 | 1.95 | 2409.0 | 9.3 × 106 | 0.7 |
| 0.1 | 5.65 × 107 | 0.47 | 5813.9 | 3.5 × 107 | 0.9 | |
| 1 | 4.28 × 1010 | −0.13 | 7557.5 | 1.6 × 108 | 1.1 | |
| 10 | 1.19 × 1015 | −1.19 | 10,382.0 | 6.3 × 108 | 1.3 | |
| 100 | 7.69 × 1022 | −3.24 | 15,314.7 | 2.0 × 109 | 1.6 | |
| R22 | 0.01 | 5.41 × 104 | 1.14 | 4218.3 | 7.8 × 106 | 0.6 |
| 0.1 | 1.18 × 109 | 0.10 | 7015.4 | 2.8 × 107 | 0.7 | |
| 1 | 3.71 × 1011 | −0.37 | 8628.7 | 1.4 × 108 | 1.0 | |
| 10 | 4.60 × 1016 | −1.61 | 11,796.9 | 5.9 × 108 | 1.3 | |
| 100 | 2.63 × 1025 | −3.93 | 17,215.9 | 2.1 × 109 | 1.7 | |
| R23 | 0.01 | 6.70 × 108 | −0.10 | 5765.5 | 9.2 × 106 | 0.7 |
| 0.1 | 1.24 × 1010 | −0.26 | 7201.3 | 2.4 × 107 | 0.6 | |
| 1 | 2.19 × 1012 | −0.66 | 8905.8 | 9.6 × 107 | 0.7 | |
| 10 | 1.45 × 1019 | −2.43 | 13,136.9 | 3.4 × 108 | 0.7 | |
| 100 | 9.74 × 1024 | −3.90 | 17,358.5 | 8.6 × 108 | 0.7 | |
| R24 | 0.01 | 1.66 × 1011 | −0.80 | 7216.6 | 8.2 × 106 | 0.6 |
| 0.1 | 3.62 × 1012 | −1.00 | 8681.2 | 2.0 × 107 | 0.5 | |
| 1 | 1.35 × 1014 | −1.20 | 10,249.4 | 7.2 × 107 | 0.5 | |
| 10 | 4.85 × 1021 | −3.17 | 14,987.9 | 2.4 × 108 | 0.5 | |
| 100 | 8.59 × 1025 | −4.17 | 18,547.1 | 5.6 × 108 | 0.5 | |
| R25 | 0.01 | 2.34 × 108 | 0.04 | 5540.1 | 9.2 × 106 | 0.7 |
| 0.1 | 9.09 × 109 | −0.21 | 7108.2 | 2.5 × 107 | 0.6 | |
| 1 | 1.55 × 1012 | −0.62 | 8769.5 | 1.0 × 108 | 0.7 | |
| 10 | 6.96 × 1018 | −2.33 | 12,888.1 | 3.5 × 108 | 0.8 | |
| 100 | 7.32 × 1024 | −3.86 | 17,180.7 | 9.1 × 108 | 0.7 | |
| R26 | 0.01 | 2.35 × 1012 | −1.13 | 8199.2 | 6.9 × 106 | 0.5 |
| 0.1 | 2.13 × 1014 | −1.50 | 10,006.9 | 1.7 × 107 | 0.4 | |
| 1 | 4.64 × 1015 | −1.63 | 11,580.4 | 6.1 × 107 | 0.4 | |
| 10 | 8.18 × 1023 | −3.79 | 16,759.2 | 2.1 × 108 | 0.4 | |
| 100 | 1.55 × 1028 | −4.79 | 20,430.8 | 5.0 × 108 | 0.4 | |
| R27 | 0.01 | 6.87 × 1010 | −0.67 | 7097.7 | 8.8 × 106 | 0.6 |
| 0.1 | 2.88 × 1013 | −1.23 | 9126.3 | 2.4 × 107 | 0.6 | |
| 1 | 1.98 × 1015 | −1.51 | 10,662.6 | 1.0 × 108 | 0.7 | |
| 10 | 4.02 × 1021 | −3.10 | 14,657.0 | 3.9 × 108 | 0.8 | |
| 100 | 6.16 × 1028 | −4.96 | 19,560.0 | 1.1 × 109 | 0.9 | |
| R28 | 0.01 | 2.03 × 1011 | −0.80 | 7747.2 | 7.4 × 106 | 0.5 |
| 0.1 | 8.46 × 1013 | −1.34 | 9817.3 | 2.3 × 107 | 0.6 | |
| 1 | 9.23 × 1015 | −1.66 | 11,416.7 | 1.1 × 108 | 0.7 | |
| 10 | 1.16 × 1022 | −3.18 | 15,260.7 | 4.6 × 108 | 1.0 | |
| 100 | 7.73 × 1030 | −5.50 | 20,920.8 | 1.6 × 109 | 1.3 | |
| R29 | 0.01 | 2.64 × 1012 | −1.14 | 8058.9 | 8.3 × 106 | 0.6 |
| 0.1 | 7.61 × 1015 | −1.97 | 10,446.3 | 2.1 × 107 | 0.5 | |
| 1 | 3.06 × 1016 | −1.87 | 11,604.9 | 7.7 × 107 | 0.5 | |
| 10 | 7.65 × 1023 | −3.79 | 16,287.9 | 2.7 × 108 | 0.6 | |
| 100 | 1.78 × 1029 | −5.11 | 20,420.3 | 6.8 × 108 | 0.5 | |
| R30 | 0.01 | 1.12 × 1013 | −1.32 | 8531.6 | 7.9 × 106 | 0.6 |
| 0.1 | 2.44 × 1017 | −2.41 | 11,325.2 | 1.9 × 107 | 0.5 | |
| 1 | 1.90 × 1017 | −2.11 | 12,232.9 | 6.6 × 107 | 0.5 | |
| 10 | 1.07 × 1025 | −4.13 | 17,166.4 | 2.3 × 108 | 0.5 | |
| 100 | 2.51 × 1029 | −5.16 | 20,859.7 | 5.3 × 108 | 0.4 | |
| R31 | 0.01 | 5.81 × 102 | 1.73 | 2660.4 | 1.1 × 107 | 0.8 |
| 0.1 | 5.18 × 108 | 0.18 | 6095.2 | 3.8 × 107 | 1.0 | |
| 1 | 3.37 × 1012 | −0.70 | 8305.1 | 1.7 × 108 | 1.1 | |
| 10 | 1.56 × 1017 | −1.83 | 11,349.6 | 6.1 × 108 | 1.3 | |
| 100 | 1.94 × 1024 | −3.67 | 16,046.1 | 1.8 × 109 | 1.4 | |
| R32 | 0.01 | 5.16 × 1011 | −0.93 | 7533.6 | 9.1 × 106 | 0.7 |
| 0.1 | 5.34 × 1013 | −1.32 | 9278.3 | 2.3 × 107 | 0.6 | |
| 1 | 5.11 × 1015 | −1.63 | 11,035.1 | 9.3 × 107 | 0.6 | |
| 10 | 8.96 × 1022 | −3.50 | 15,647.4 | 3.4 × 108 | 0.7 | |
| 100 | 1.26 × 1029 | −5.04 | 20,177.3 | 9.1 × 108 | 0.7 | |
| R33 | 0.01 | 6.72 × 100 | 2.32 | 1775.7 | 1.2 × 107 | 0.9 |
| 0.1 | 3.75 × 108 | 0.23 | 5997.9 | 4.1 × 107 | 1.1 | |
| 1 | 6.42 × 1013 | −1.08 | 8835.2 | 1.8 × 108 | 1.3 | |
| 10 | 9.82 × 1017 | −2.06 | 11,661.9 | 6.8 × 108 | 1.5 | |
| 100 | 1.01 × 1025 | −3.87 | 16,334.4 | 2.1 × 109 | 1.7 | |
| R34 | 0.01 | 1.83 × 1012 | −1.07 | 8069.1 | 9.0 × 106 | 0.7 |
| 0.1 | 4.35 × 1034 | −7.48 | 19,352.7 | 4.2 × 107 | 1.1 | |
| 1 | 1.55 × 1050 | −11.83 | 27,391.5 | 2.3 × 108 | 1.6 | |
| 10 | 2.15 × 1027 | −4.61 | 18,838.3 | 6.2 × 108 | 1.3 | |
| 100 | 1.40 × 1037 | −7.28 | 24,814.8 | 1.7 × 109 | 1.4 | |
| R35 | 0.01 | 1.56 × 100 | 2.51 | 1477.3 | 1.2 × 107 | 0.9 |
| 0.1 | 2.45 × 108 | 0.29 | 5897.7 | 4.2 × 107 | 1.1 | |
| 1 | 6.29 × 1013 | −1.07 | 8803.5 | 1.9 × 108 | 1.3 | |
| 10 | 7.40 × 1017 | −2.02 | 11,560.8 | 7.1 × 108 | 1.5 | |
| 100 | 7.65 × 1024 | −3.83 | 16,221.7 | 2.2 × 109 | 1.7 | |
| R36 | 0.01 | 1.38 × 106 | 0.74 | 4434.7 | 1.2 × 107 | 0.9 |
| 0.1 | 1.44 × 1011 | −0.55 | 7472.1 | 3.2 × 107 | 0.8 | |
| 1 | 5.96 × 1014 | −1.37 | 9734.3 | 1.4 × 108 | 0.9 | |
| 10 | 1.62 × 1020 | −2.72 | 13,314.0 | 4.9 × 108 | 1.0 | |
| 100 | 5.73 × 1026 | −4.39 | 17,893.3 | 1.3 × 109 | 1.1 | |
| R37 | 0.01 | 3.19 × 10−2 | 3.02 | 650.4 | 1.2 × 107 | 0.9 |
| 0.1 | 6.47 × 107 | 0.46 | 5539.3 | 4.4 × 107 | 1.1 | |
| 1 | 3.00 × 1013 | −0.98 | 8534.6 | 2.0 × 108 | 1.3 | |
| 10 | 2.62 × 1017 | −1.89 | 11,200.8 | 7.3 × 108 | 1.6 | |
| 100 | 1.98 × 1024 | −3.67 | 15,768.8 | 2.2 × 109 | 1.8 | |
| R38 | 0.01 | 2.98 × 1011 | −0.84 | 7443.0 | 1.0 × 107 | 0.7 |
| 0.1 | 1.13 × 1040 | −9.13 | 21,293.2 | 5.3 × 107 | 1.4 | |
| 1 | 1.08 × 1053 | −12.70 | 28,151.8 | 3.1 × 108 | 2.1 | |
| 10 | 1.04 × 1028 | −4.84 | 18,452.6 | 8.3 × 108 | 1.8 | |
| 100 | 1.36 × 1039 | −7.91 | 24,879.1 | 2.4 × 109 | 1.9 | |
| R39 | 0.01 | 9.00 × 107 | 0.20 | 5231.3 | 1.3 × 107 | 0.9 |
| 0.1 | 5.74 × 1011 | −0.75 | 7668.5 | 3.1 × 107 | 0.8 | |
| 1 | 3.11 × 1015 | −1.60 | 10,090.1 | 1.2 × 108 | 0.9 | |
| 10 | 1.52 × 1021 | −3.02 | 13,889.6 | 4.2 × 108 | 0.9 | |
| 100 | 7.59 × 1026 | −4.44 | 18,121.7 | 1.1 × 109 | 0.9 | |
| R40 | 0.01 | 2.76 × 108 | 0.08 | 6012.2 | 1.1 × 107 | 0.8 |
| 0.1 | 3.06 × 1012 | −0.89 | 8635.5 | 3.4 × 107 | 0.9 | |
| 1 | 1.37 × 1017 | −2.00 | 11,403.5 | 1.6 × 108 | 1.1 | |
| 10 | 5.27 × 1022 | −3.36 | 15,108.0 | 6.8 × 108 | 1.5 | |
| 100 | 1.21 × 1031 | −5.54 | 20,636.8 | 2.3 × 109 | 1.9 | |
| R41 | 0.01 | 2.69 × 1010 | −0.53 | 6504.2 | 1.3 × 107 | 0.9 |
| 0.1 | 5.85 × 1013 | −1.36 | 8680.1 | 2.8 × 107 | 0.7 | |
| 1 | 1.14 × 1016 | −1.78 | 10,614.9 | 9.9 × 107 | 0.7 | |
| 10 | 2.25 × 1022 | −3.37 | 14,822.9 | 3.2 × 108 | 0.7 | |
| 100 | 4.55 × 1026 | −4.39 | 18,443.6 | 7.5 × 108 | 0.6 | |
| R42 | 0.01 | 9.98 × 1028 | −5.16 | 21,464.5 | 1.4 × 108 | 10.1 |
| 0.1 | 1.01 × 1022 | −2.90 | 19,477.2 | 1.8 × 108 | 4.7 | |
| 1 | 1.21 × 1015 | −0.77 | 16,789.5 | 1.8 × 108 | 1.3 | |
| 10 | 1.96 × 1013 | −0.22 | 16,057.5 | 1.8 × 108 | 0.4 | |
| 100 | 1.19 × 1013 | −0.15 | 15,968.0 | 1.8 × 108 | 0.1 | |
| R43 | 0.01 | 1.92 × 101 | 2.19 | 1916.8 | 1.3 × 107 | 0.3 |
| 0.1 | 4.53 × 108 | 0.20 | 5964.1 | 4.0 × 107 | 1.3 | |
| 1 | 1.80 × 1014 | −1.23 | 9024.6 | 1.7 × 108 | 2.3 | |
| 10 | 7.77 × 1018 | −2.34 | 12,143.8 | 5.9 × 108 | 3.3 | |
| 100 | 2.31 × 1025 | −4.00 | 16,615.7 | 1.6 × 109 | 4.3 | |
| R44 | 0.01 | 3.11 × 1011 | −0.84 | 7368.0 | 1.1 × 107 | 0.8 |
| 0.1 | 3.17 × 1015 | −1.85 | 9912.4 | 2.6 × 107 | 0.7 | |
| 1 | 3.94 × 1017 | −2.20 | 11,797.4 | 1.0 × 108 | 0.7 | |
| 10 | 1.90 × 1024 | −3.89 | 16,222.3 | 3.5 × 108 | 0.8 | |
| 100 | 1.19 × 1030 | −5.33 | 20,629.3 | 9.3 × 108 | 0.7 | |
| β-scission class rate rule | 0.01 | 3.02 × 10−10 | 5.70 | −579.1 | 1.4 × 107 | 20.0 # |
| 0.1 | 4.78 × 109 | 0.005 | 7720.47 | 3.9 × 107 | 10.9 # | |
| 1 | 7.93 × 103 | 1.98 | 5569.14 | 1.4 × 108 | 6.1 # | |
| 10 | 3.09 × 1012 | −0.35 | 10,382.5 | 4.6 × 108 | 4.5 # | |
| 100 | 3.04 × 1022 | −3.08 | 16,288.8 | 1.3 × 109 | 13.2 # | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, X.; Sun, X.; Zhu, Y. High-Pressure Limit and Pressure-Dependent Rate Rules for β-Scission Reaction Class of Hydroperoxyl Alkyl Hydroperoxyl Radicals (•P(OOH)2) in Normal-Alkyl Cyclohexanes Combustion. Molecules 2024, 29, 544. https://doi.org/10.3390/molecules29020544
Yao X, Sun X, Zhu Y. High-Pressure Limit and Pressure-Dependent Rate Rules for β-Scission Reaction Class of Hydroperoxyl Alkyl Hydroperoxyl Radicals (•P(OOH)2) in Normal-Alkyl Cyclohexanes Combustion. Molecules. 2024; 29(2):544. https://doi.org/10.3390/molecules29020544
Chicago/Turabian StyleYao, Xiaoxia, Xiaoli Sun, and Yifei Zhu. 2024. "High-Pressure Limit and Pressure-Dependent Rate Rules for β-Scission Reaction Class of Hydroperoxyl Alkyl Hydroperoxyl Radicals (•P(OOH)2) in Normal-Alkyl Cyclohexanes Combustion" Molecules 29, no. 2: 544. https://doi.org/10.3390/molecules29020544
APA StyleYao, X., Sun, X., & Zhu, Y. (2024). High-Pressure Limit and Pressure-Dependent Rate Rules for β-Scission Reaction Class of Hydroperoxyl Alkyl Hydroperoxyl Radicals (•P(OOH)2) in Normal-Alkyl Cyclohexanes Combustion. Molecules, 29(2), 544. https://doi.org/10.3390/molecules29020544







