SARS-CoV-2 Nucleocapsid Protein Antagonizes GADD34-Mediated Innate Immune Pathway through Atypical Foci
Abstract
:1. Introduction
2. Results
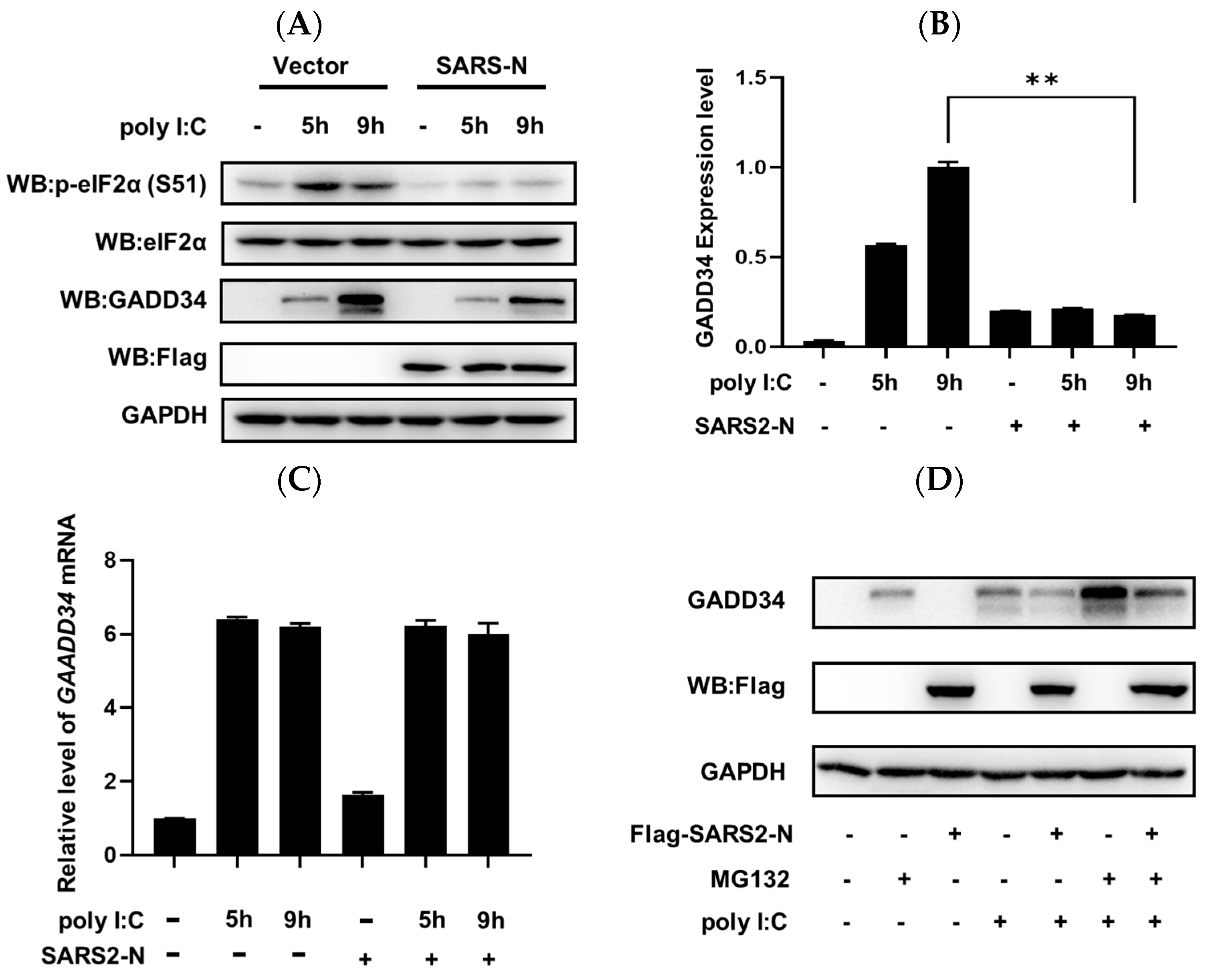
2.1. SARS-CoV-2 N Protein Inhibits dsRNA-Induced GADD34 Translation
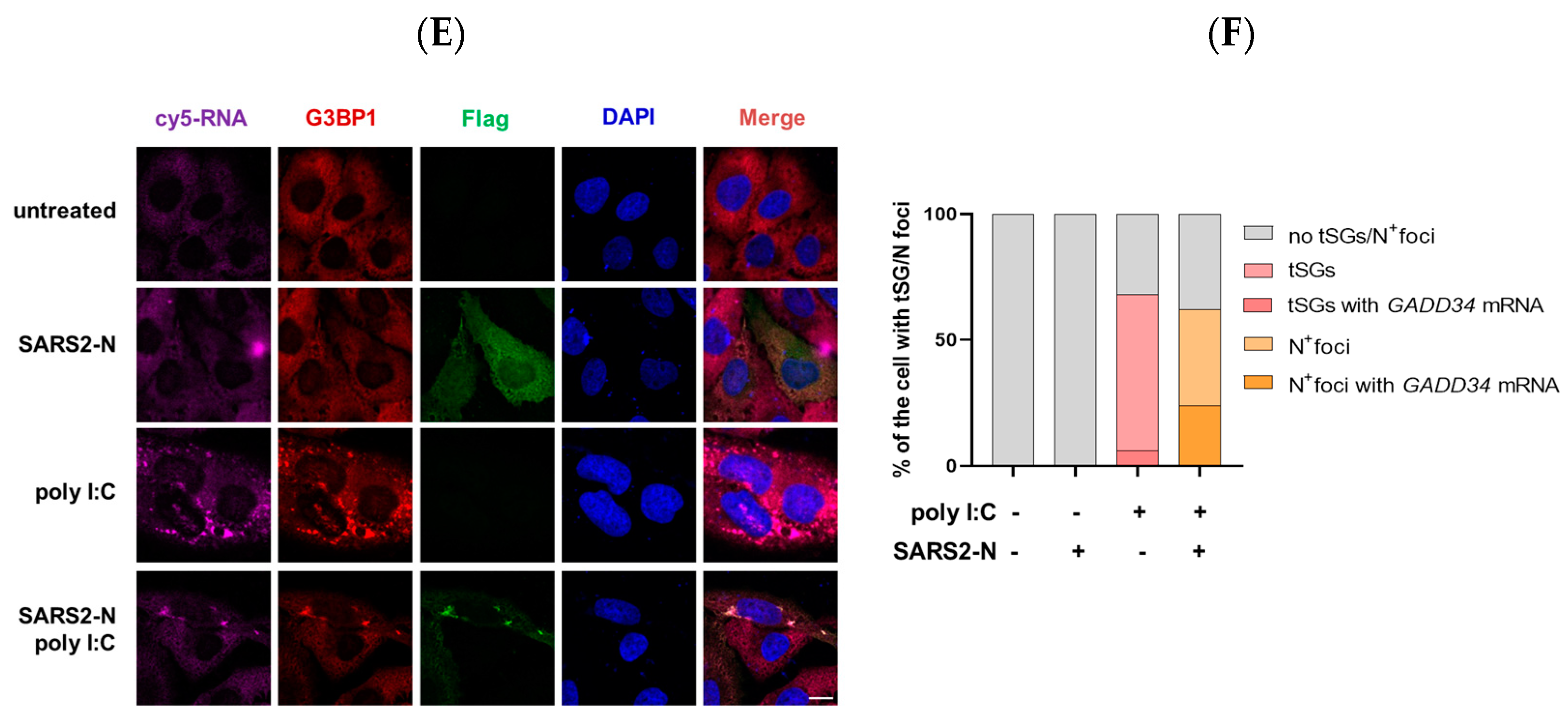
2.2. SARS2-N Protein Sequesters GADD34 mRNA into N+foci
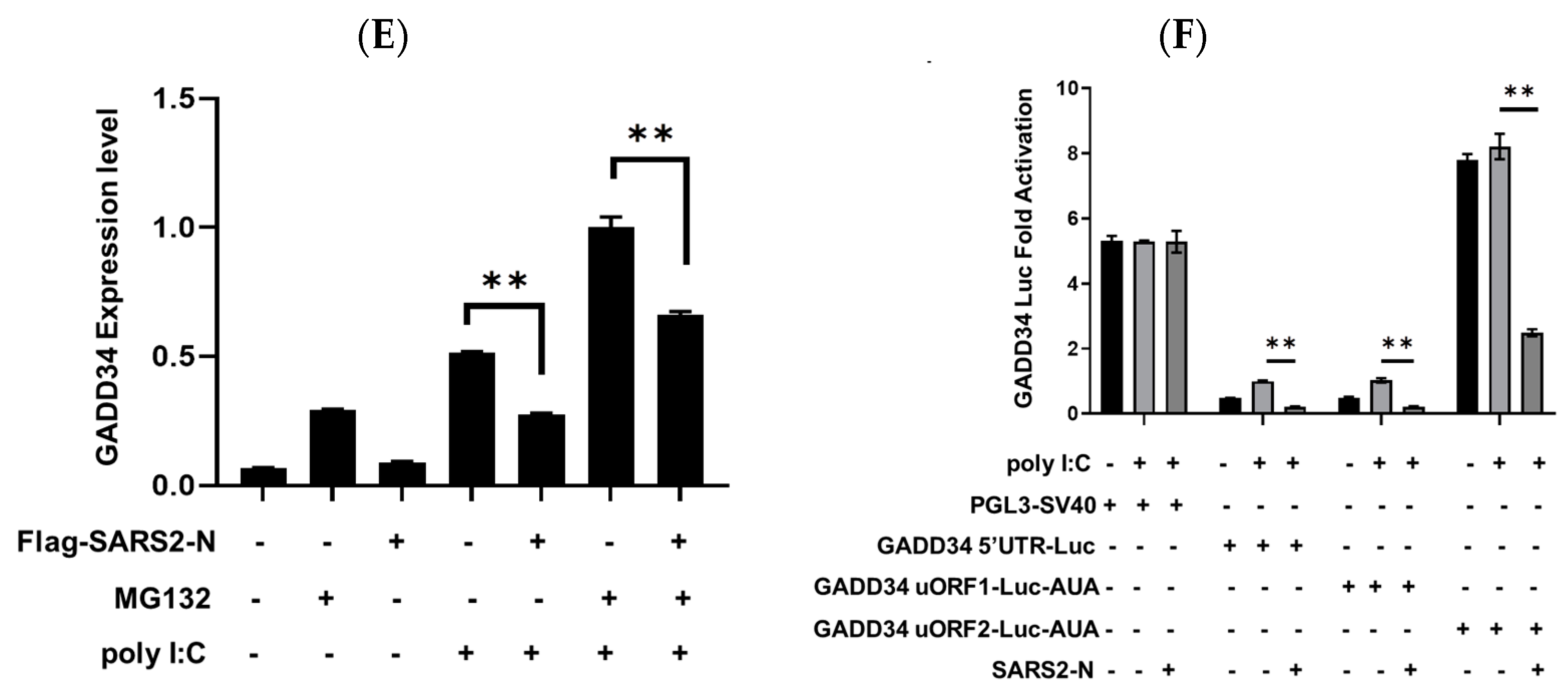
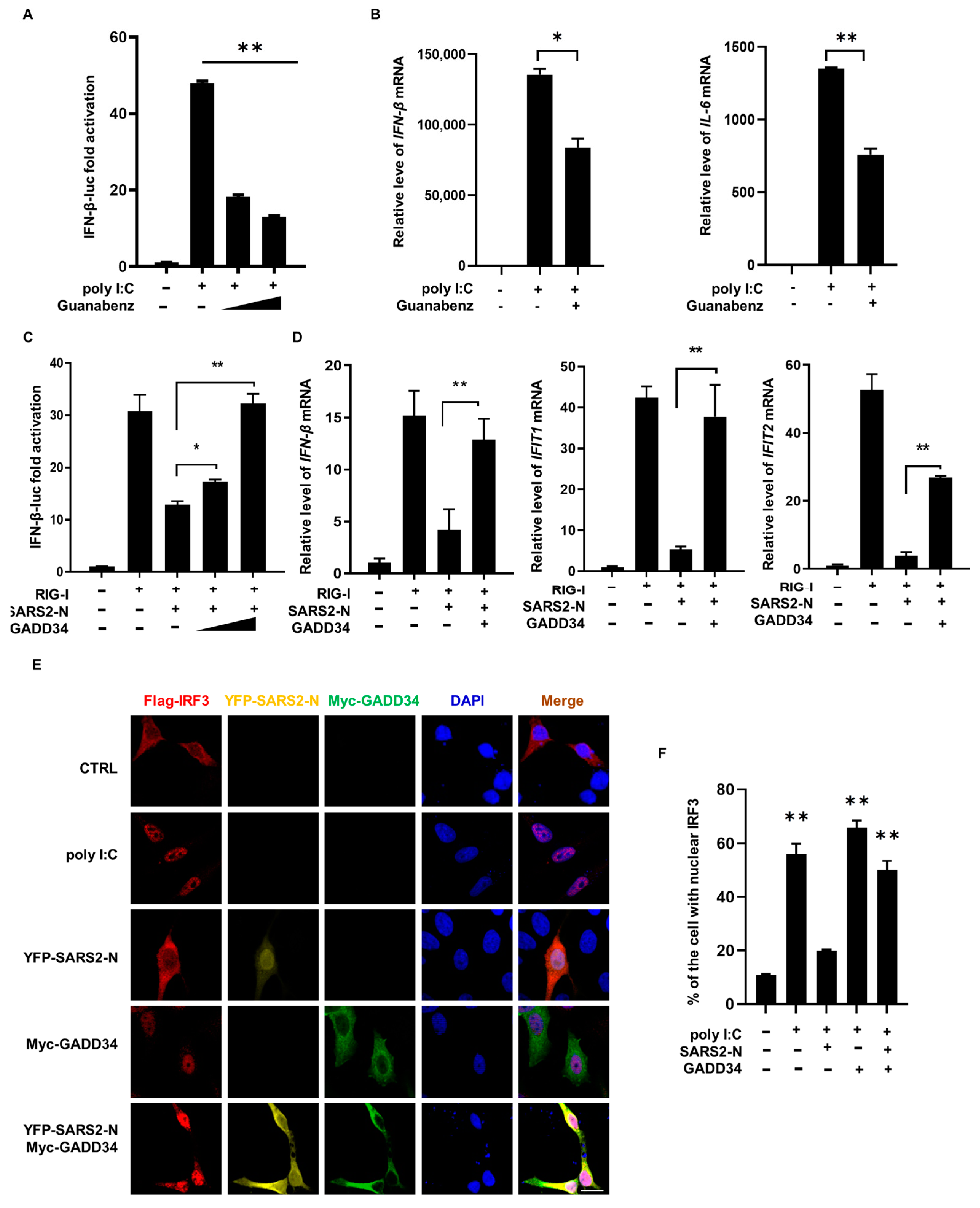
2.3. SARS2-N Protein Inhibits GADD34 Expression to Attenuate Innate Immune Response
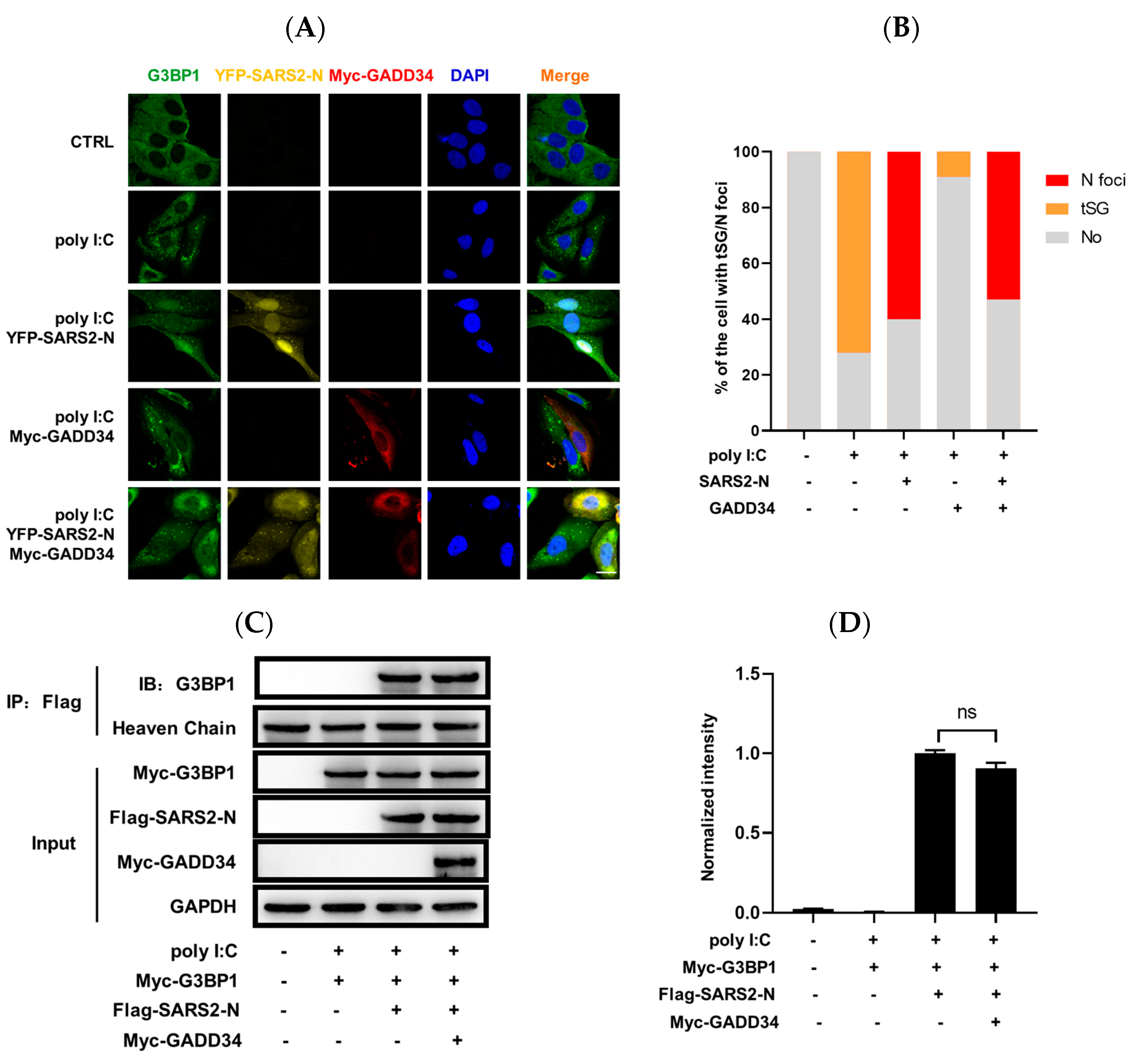
2.4. GADD34 Barely Affects SARS2-N Protein-Mediated Induction of N+foci
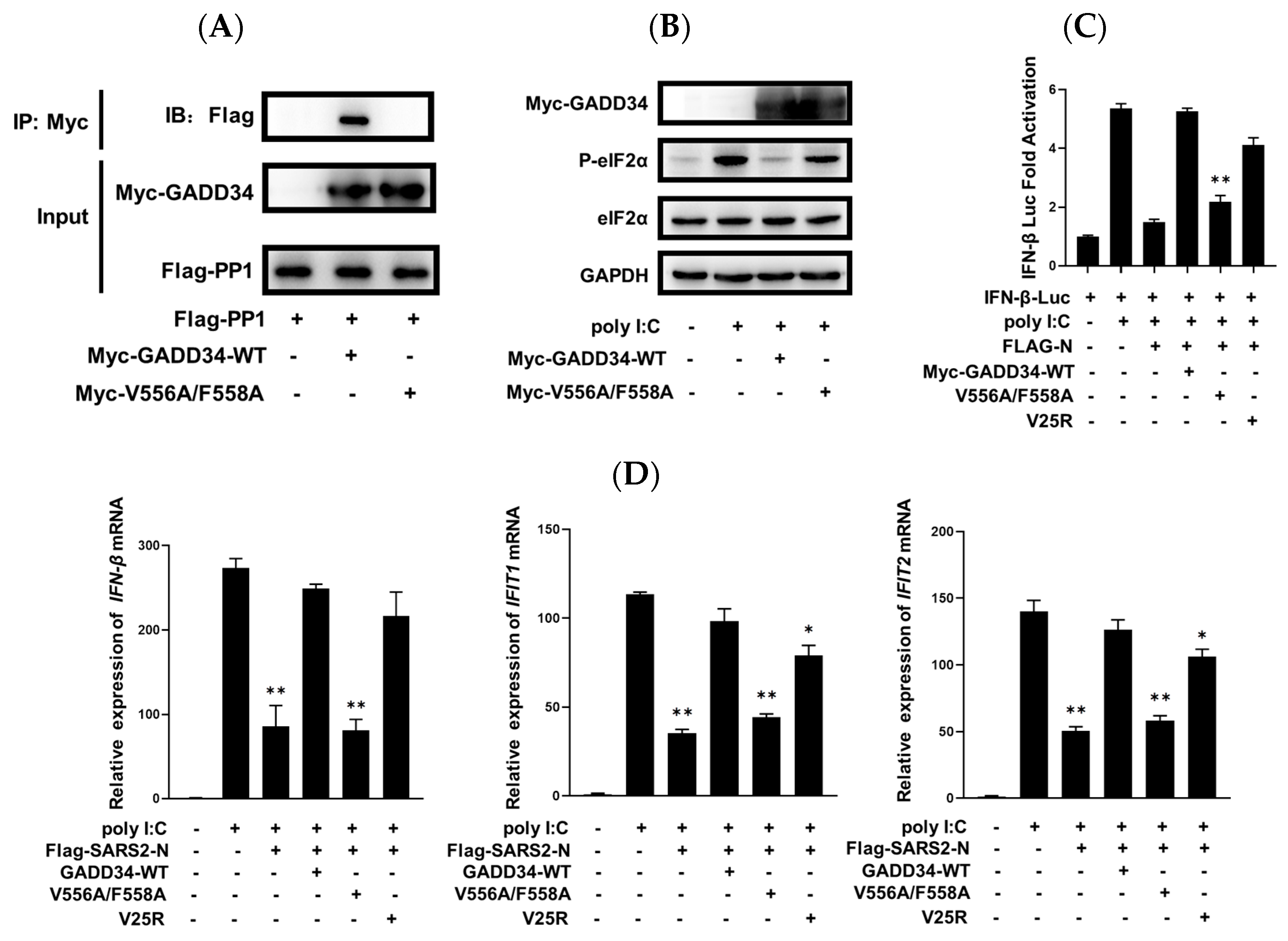
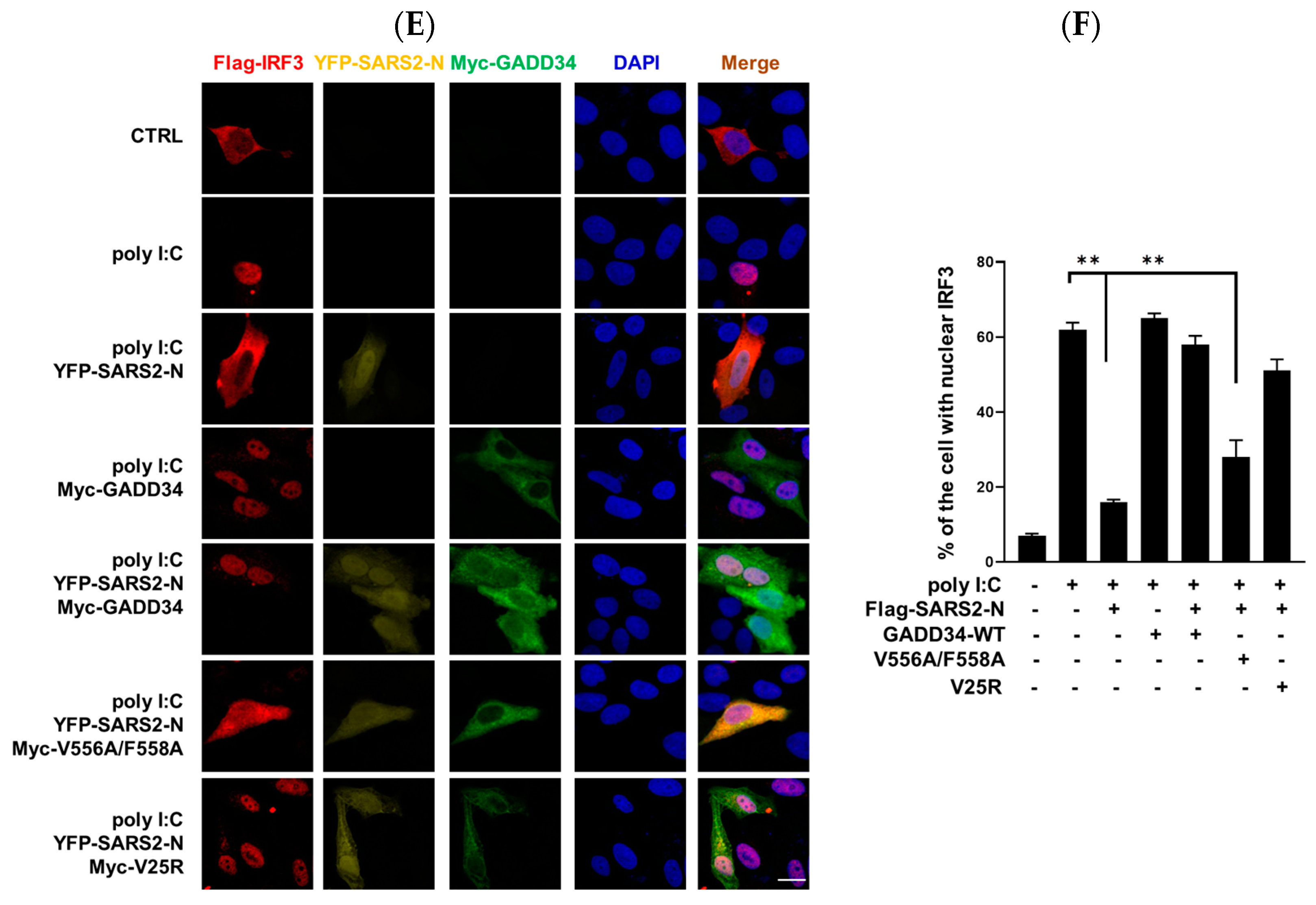
2.5. GADD34 Counteracts SARS2-N Protein in Regulation of Innate Immunity Dependent on KVRF Motif
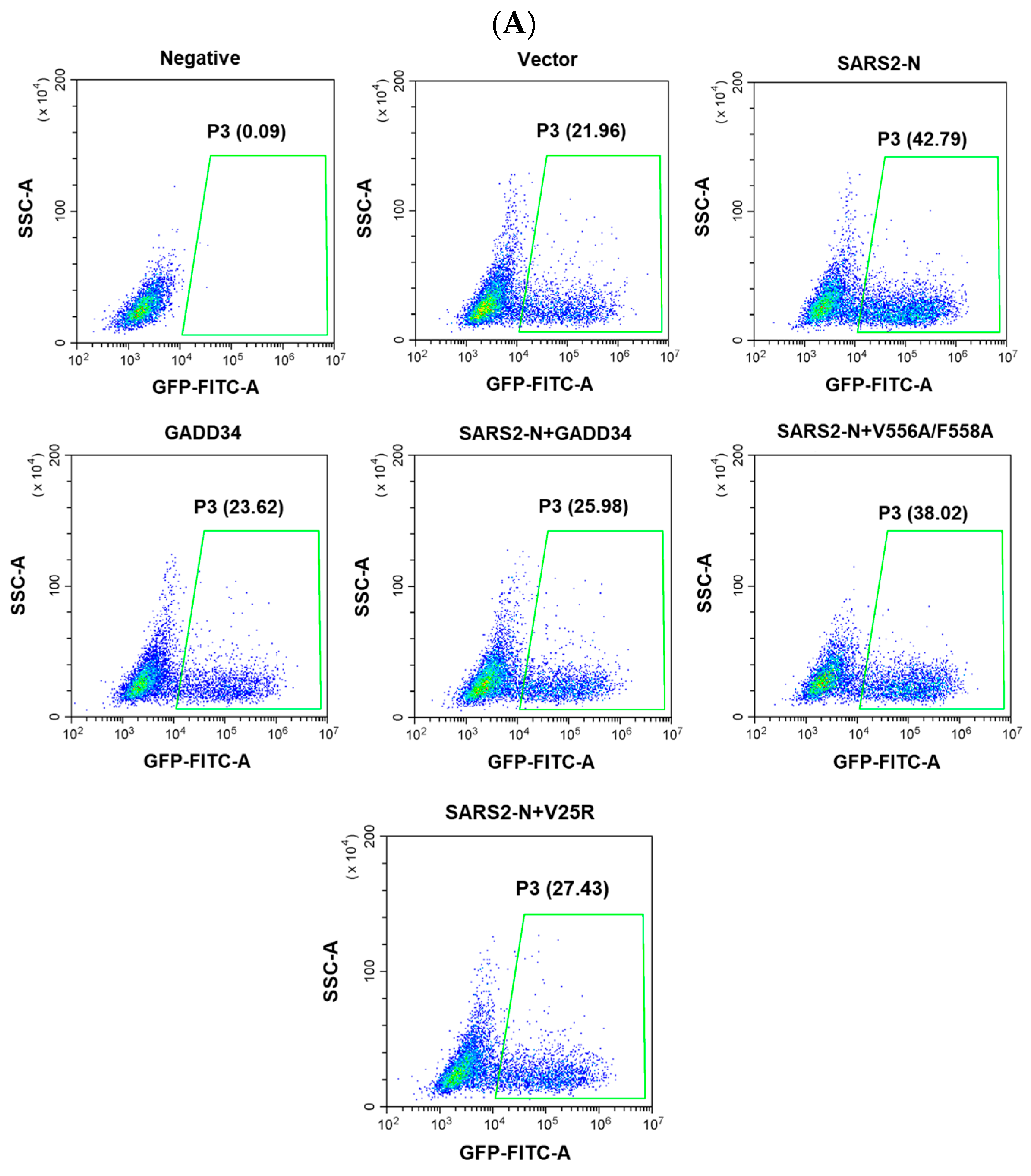
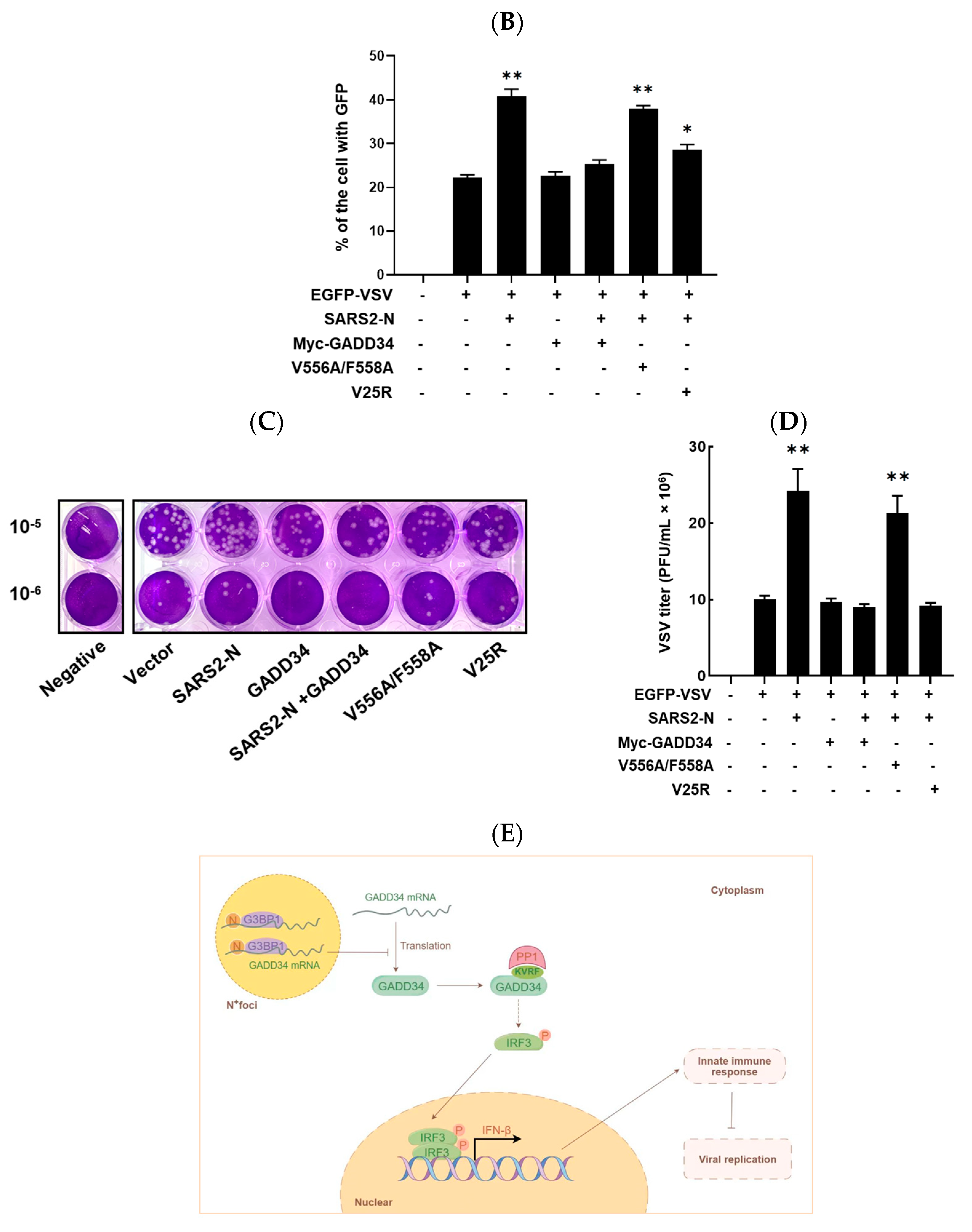
2.6. GADD34 Suppresses Viral Replication Facilitated by SARS2-N Protein
3. Materials and Methods
3.1. Cell Culture
3.2. Plasmids and Reagents
3.3. Stress Treatment
3.4. Immunofluorescence
3.5. Luciferase Assay
3.6. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
3.7. Immunoprecipitation and Immunoblotting
3.8. RNA Immunoprecipitation (RIP)
3.9. TurboID Proximity Labeling Assay
3.10. Viral Infection and Flow Cytometry Analysis
3.11. Viral Plaque Assays
3.12. In Vitro Transcription
3.13. Statistical Analyses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kawai, T.; Akira, S. Innate immune recognition of viral infection. Nat. Immunol. 2006, 7, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Reis e Sousa, C. Innate Recognition of Viruses. Immunity 2007, 27, 370–383. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takahashi, K.; Sato, S.; Coban, C.; Kumar, H.; Kato, H.; Ishii, K.J.; Takeuchi, O.; Akira, S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat. Immunol. 2005, 6, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.-M.; Maniatis, T. IKKε and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef]
- Yan, N.; Chen, Z.J. Intrinsic antiviral immunity. Nat. Immunol. 2012, 13, 214–222. [Google Scholar] [CrossRef]
- Zhao, Y.; Sui, L.; Wu, P.; Wang, W.; Wang, Z.; Yu, Y.; Hou, Z.; Tan, G.; Liu, Q.; Wang, G. A dual-role of SARS-CoV-2 nucleocapsid protein in regulating innate immune response. Signal Transduct. Target. Ther. 2021, 6, 331. [Google Scholar] [CrossRef]
- Freitas, R.S.; Crum, T.F.; Parvatiyar, K. SARS-CoV-2 Spike Antagonizes Innate Antiviral Immunity by Targeting Interferon Regulatory Factor 3. Front. Cell. Infect. Microbiol. 2022, 11, 789462. [Google Scholar] [CrossRef]
- Sui, L.; Zhao, Y.; Wang, W.; Wu, P.; Wang, Z.; Yu, Y.; Hou, Z.; Tan, G.; Liu, Q. SARS-CoV-2 Membrane Protein Inhibits Type I Interferon Production through Ubiquitin-Mediated Degradation of TBK1. Front. Immunol. 2021, 12, 662989. [Google Scholar] [CrossRef]
- Kumar, A.; Ishida, R.; Strilets, T.; Cole, J.; Lopez-Orozco, J.; Fayad, N.; Felix-Lopez, A.; Elaish, M.; Evseev, D.; Magor, K.E.; et al. SARS-CoV-2 Nonstructural Protein 1 Inhibits the Interferon Response by Causing Depletion of Key Host Signaling Factors. J. Virol. 2021, 95, e0026621. [Google Scholar] [CrossRef]
- Lei, X.; Dong, X.; Ma, R.; Wang, W.; Xiao, X.; Tian, Z.; Wang, C.; Wang, Y.; Li, L.; Ren, L.; et al. Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 2020, 11, 3810. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.-Y.; Siu, K.-L.; Lin, H.; Yeung, M.L.; Jin, D.-Y. SARS-CoV-2 main protease suppresses type I interferon production by preventing nuclear translocation of phosphorylated IRF3. Int. J. Biol. Sci. 2021, 17, 1547–1554. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Cao, Z.; Xie, X.; Zhang, X.; Chen, J.Y.-C.; Wang, H.; Menachery, V.D.; Rajsbaum, R.; Shi, P.-Y. Evasion of Type I Interferon by SARS-CoV-2. Cell Rep. 2020, 33, 108234. [Google Scholar] [CrossRef] [PubMed]
- Jin, D.-Y.; Vazquez, C.; Swanson, S.E.; Negatu, S.G.; Dittmar, M.; Miller, J.; Ramage, H.R.; Cherry, S.; Jurado, K.A. SARS-CoV-2 viral proteins NSP1 and NSP13 inhibit interferon activation through distinct mechanisms. PLoS ONE 2021, 16, e0253089. [Google Scholar] [CrossRef]
- Han, L.; Zhuang, M.W.; Deng, J.; Zheng, Y.; Zhang, J.; Nan, M.L.; Zhang, X.J.; Gao, C.; Wang, P.H. SARS-CoV-2 ORF9b antagonizes type I and III interferons by targeting multiple components of the RIG-I/MDA-5–MAVS, TLR3–TRIF, and cGAS–STING signaling pathways. J. Med. Virol. 2021, 93, 5376–5389. [Google Scholar] [CrossRef]
- Zhang, Q.; Sharma, N.R.; Zheng, Z.-M.; Chen, M. Viral Regulation of RNA Granules in Infected Cells. Virol. Sin. 2019, 34, 175–191. [Google Scholar] [CrossRef]
- Thomas, M.G.; Loschi, M.; Desbats, M.A.; Boccaccio, G.L. RNA granules: The good, the bad and the ugly. Cell. Signal. 2011, 23, 324–334. [Google Scholar] [CrossRef]
- McCormick, C.; Khaperskyy, D.A. Translation inhibition and stress granules in the antiviral immune response. Nat. Rev. Immunol. 2017, 17, 647–660. [Google Scholar] [CrossRef]
- Jackson, R.J.; Hellen, C.U.T.; Pestova, T.V. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010, 11, 113–127. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Fan, S.; Jin, Z.; Huang, C. Targeting stress granules: A novel therapeutic strategy for human diseases. Pharmacol. Res. 2020, 161, 105143. [Google Scholar] [CrossRef]
- Brownsword, M.J.; Locker, N. A little less aggregation a little more replication: Viral manipulation of stress granules. WIREs RNA 2022, 14, e1821. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Narayanan, K.; Wada, M.; Makino, S.; Gallagher, T. Inhibition of Stress Granule Formation by Middle East Respiratory Syndrome Coronavirus 4a Accessory Protein Facilitates Viral Translation, Leading to Efficient Virus Replication. J. Virol. 2018, 92, e00902-18. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W. Interferon-stimulated genes: Roles in viral pathogenesis. Curr. Opin. Virol. 2014, 6, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Racaniello, V.; Lloyd, R.E. How Do Viruses Interact with Stress-Associated RNA Granules? PLoS Pathog. 2012, 8, e1002741. [Google Scholar] [CrossRef]
- White, J.P.; Lloyd, R.E. Regulation of stress granules in virus systems. Trends Microbiol. 2012, 20, 175–183. [Google Scholar] [CrossRef]
- Poblete-Durán, N.; Prades-Pérez, Y.; Vera-Otarola, J.; Soto-Rifo, R.; Valiente-Echeverría, F. Who Regulates Whom? An Overview of RNA Granules and Viral Infections. Viruses 2016, 8, 180. [Google Scholar] [CrossRef]
- Sarnow, P.; Yang, X.; Hu, Z.; Fan, S.; Zhang, Q.; Zhong, Y.; Guo, D.; Qin, Y.; Chen, M. Picornavirus 2A protease regulates stress granule formation to facilitate viral translation. PLoS Pathog. 2018, 14, e1006901. [Google Scholar] [CrossRef]
- Heise, M.T.; Götte, B.; Panas, M.D.; Hellström, K.; Liu, L.; Samreen, B.; Larsson, O.; Ahola, T.; McInerney, G.M. Separate domains of G3BP promote efficient clustering of alphavirus replication complexes and recruitment of the translation initiation machinery. PLoS Pathog. 2019, 15, e1007842. [Google Scholar] [CrossRef]
- Scholte, F.E.M.; Tas, A.; Albulescu, I.C.; Žusinaite, E.; Merits, A.; Snijder, E.J.; van Hemert, M.J.; Diamond, M.S. Stress Granule Components G3BP1 and G3BP2 Play a Proviral Role Early in Chikungunya Virus Replication. J. Virol. 2015, 89, 4457–4469. [Google Scholar] [CrossRef]
- Heise, M.T.; Kim, D.Y.; Reynaud, J.M.; Rasalouskaya, A.; Akhrymuk, I.; Mobley, J.A.; Frolov, I.; Frolova, E.I. New World and Old World Alphaviruses Have Evolved to Exploit Different Components of Stress Granules, FXR and G3BP Proteins, for Assembly of Viral Replication Complexes. PLoS Pathog. 2016, 12, e1005810. [Google Scholar] [CrossRef]
- Dhillon, P.; Rao, C.D.; Dermody, T.S. Rotavirus Induces Formation of Remodeled Stress Granules and P Bodies and Their Sequestration in Viroplasms to Promote Progeny Virus Production. J. Virol. 2018, 92, e01363-18. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Li, Z.; Zhao, T.; Ju, X.; Ma, P.; Jin, B.; Zhou, Y.; He, S.; Huang, J.; Xu, X.; et al. SARS-CoV-2 nucleocapsid protein phase separates with G3BPs to disassemble stress granules and facilitate viral production. Sci. Bull. 2021, 66, 1194–1204. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Gou, H.; Zhou, Y.; Wu, C.; Ren, X.; Wu, X.; Guan, G.; Jin, B.; Huang, J.; Jin, Z.; et al. The SARS-CoV-2 nucleocapsid protein suppresses innate immunity by remodeling stress granules to atypical foci. FASEB J. 2023, 37, e23269. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Jiang, X.; Xin, H.; Zheng, X.; Xue, X.; Chen, J.-L.; Qi, B. GADD34-mediated dephosphorylation of eIF2α facilitates pseudorabies virus replication by maintaining de novo protein synthesis. Vet. Res. 2021, 52, 148. [Google Scholar] [CrossRef]
- Magg, V.; Manetto, A.; Kopp, K.; Wu, C.C.; Naghizadeh, M.; Lindner, D.; Eke, L.; Welsch, J.; Kallenberger, S.M.; Schott, J.; et al. Turnover of PPP1R15A mRNA encoding GADD34 controls responsiveness and adaptation to cellular stress. Cell Rep. 2024, 43, 114069. [Google Scholar] [CrossRef]
- Choy, M.S.; Yusoff, P.; Lee, I.C.; Newton, J.C.; Goh, C.W.; Page, R.; Shenolikar, S.; Peti, W. Structural and Functional Analysis of the GADD34:PP1 eIF2α Phosphatase. Cell Rep. 2015, 11, 1885–1891. [Google Scholar] [CrossRef]
- Heise, M.T.; Clavarino, G.; Cláudio, N.; Couderc, T.; Dalet, A.; Judith, D.; Camosseto, V.; Schmidt, E.K.; Wenger, T.; Lecuit, M.; et al. Induction of GADD34 Is Necessary for dsRNA-Dependent Interferon-β Production and Participates in the Control of Chikungunya Virus Infection. PLoS Pathog. 2012, 8, e1002708. [Google Scholar] [CrossRef]
- Dalet, A.; Argüello, R.J.; Combes, A.; Spinelli, L.; Jaeger, S.; Fallet, M.; Vu Manh, T.P.; Mendes, A.; Perego, J.; Reverendo, M.; et al. Protein synthesis inhibition and GADD34 control IFN-β heterogeneous expression in response to dsRNA. EMBO J. 2017, 36, 761–782. [Google Scholar] [CrossRef]
- Perego, J.; Mendes, A.; Bourbon, C.; Camosseto, V.; Combes, A.; Liu, H.; Manh, T.-P.V.; Dalet, A.; Chasson, L.; Spinelli, L.; et al. Guanabenz inhibits TLR9 signaling through a pathway that is independent of eIF2α dephosphorylation by the GADD34/PP1c complex. Sci. Signal. 2018, 11, eaam8104. [Google Scholar] [CrossRef]
- Clavarino, G.; Cláudio, N.; Dalet, A.; Terawaki, S.; Couderc, T.; Chasson, L.; Ceppi, M.; Schmidt, E.K.; Wenger, T.; Lecuit, M.; et al. Protein phosphatase 1 subunit Ppp1r15a/GADD34 regulates cytokine production in polyinosinic: Polycytidylic acid-stimulated dendritic cells. Proc. Natl. Acad. Sci. USA 2012, 109, 3006–3011. [Google Scholar] [CrossRef]
- Mukai, R.; Ohshima, T. HTLV-1 HBZ positively regulates the mTOR signaling pathway via inhibition of GADD34 activity in the cytoplasm. Oncogene 2013, 33, 2317–2328. [Google Scholar] [CrossRef]
- Mesman, A.W.; Zijlstra-Willems, E.M.; Kaptein, T.M.; de Swart, R.L.; Davis, M.E.; Ludlow, M.; Duprex, W.P.; Gack, M.U.; Gringhuis, S.I.; Geijtenbeek, T.B.H. Measles Virus Suppresses RIG-I-like Receptor Activation in Dendritic Cells via DC-SIGN-Mediated Inhibition of PP1 Phosphatases. Cell Host Microbe 2014, 16, 31–42. [Google Scholar] [CrossRef]
- Jasenosky, L.D.; Cadena, C.; Mire, C.E.; Borisevich, V.; Haridas, V.; Ranjbar, S.; Nambu, A.; Bavari, S.; Soloveva, V.; Sadukhan, S.; et al. The FDA-Approved Oral Drug Nitazoxanide Amplifies Host Antiviral Responses and Inhibits Ebola Virus. iScience 2019, 19, 1279–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, W.; Zhang, S.; Qiu, M.; Li, Z.; Lin, Y.; Tan, J.; Qiao, W.; Kolawole, A.O. Enterovirus 71 Activates GADD34 via Precursor 3CD to Promote IRES-Mediated Viral Translation. Microbiol. Spectr. 2022, 10, e0138821. [Google Scholar] [CrossRef] [PubMed]
- Brush, M.H.; Weiser, D.C.; Shenolikar, S. Growth Arrest and DNA Damage-Inducible Protein GADD34 Targets Protein Phosphatase 1α to the Endoplasmic Reticulum and Promotes Dephosphorylation of the α Subunit of Eukaryotic Translation Initiation Factor 2. Mol. Cell. Biol. 2023, 23, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Brush, M.H.; Choy, M.S.; Shenolikar, S. Association with Endoplasmic Reticulum Promotes Proteasomal Degradation of GADD34 Protein. J. Biol. Chem. 2011, 286, 21687–21696. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wu, W.; Chen, M.; Cheng, W.; Yu, J.; Fang, J.; Xu, L.; Yasunaga, J.-I.; Matsuoka, M.; Zhao, T.; et al. Long Noncoding RNA ANRIL Supports Proliferation of Adult T-Cell Leukemia Cells through Cooperation with EZH2. J. Virol. 2018, 92, e00909-18. [Google Scholar] [CrossRef]
- Zhao, T.; Yasunaga, J.-I.; Satou, Y.; Nakao, M.; Takahashi, M.; Fujii, M.; Matsuoka, M. Human T-cell leukemia virus type 1 bZIP factor selectively suppresses the classical pathway of NF-κB. Blood 2009, 113, 2755–2764. [Google Scholar] [CrossRef]
- Aguero, T.; Jin, Z.; Chorghade, S.; Kalsotra, A.; King, M.L.; Yang, J. Maternal Dead-end 1 promotes translation of nanos1 by binding the eIF3 complex. Development 2017, 144, 3755–3765. [Google Scholar] [CrossRef]
- Lloyd, R.E. Regulation of stress granules and P-bodies during RNA virus infection. WIREs RNA 2013, 4, 317–331. [Google Scholar] [CrossRef]
- Eiermann, N.; Haneke, K.; Sun, Z.; Stoecklin, G.; Ruggieri, A. Dance with the Devil: Stress Granules and Signaling in Antiviral Responses. Viruses 2020, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Heise, M.T.; Panas, M.D.; Schulte, T.; Thaa, B.; Sandalova, T.; Kedersha, N.; Achour, A.; McInerney, G.M. Viral and Cellular Proteins Containing FGDF Motifs Bind G3BP to Block Stress Granule Formation. PLoS Pathog. 2015, 11, e1004659. [Google Scholar] [CrossRef]
- White, J.P.; Cardenas, A.M.; Marissen, W.E.; Lloyd, R.E. Inhibition of Cytoplasmic mRNA Stress Granule Formation by a Viral Proteinase. Cell Host Microbe 2007, 2, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Shetty, M.; Harding, H.P.; George, G.; Zyryanova, A.; Labbé, K.; Mafi, A.; Hao, Q.; Sidrauski, C.; Ron, D. Substrate recruitment via eIF2γ enhances catalytic efficiency of a holophosphatase that terminates the integrated stress response. Proc. Natl. Acad. Sci. USA 2024, 121, e2320013121. [Google Scholar] [CrossRef]
- Wu, D.Y.; Tkachuck, D.C.; Roberson, R.S.; Schubach, W.H. The Human SNF5/INI1 Protein Facilitates the Function of the Growth Arrest and DNA Damage-Inducible Protein (GADD34) and Modulates GADD34-Bound Protein Phosphatase-1 Activity. J. Biol. Chem. 2002, 277, 27706–27715. [Google Scholar] [CrossRef]
- Minami, K.; Tambe, Y.; Watanabe, R.; Isono, T.; Haneda, M.; Isobe, K.-I.; Kobayashi, T.; Hino, O.; Okabe, H.; Chano, T.; et al. Suppression of Viral Replication by Stress-Inducible GADD34 Protein via the Mammalian Serine/Threonine Protein Kinase mTOR Pathway. J. Virol. 2007, 81, 11106–11115. [Google Scholar] [CrossRef]
- Watanabe, R.; Tambe, Y.; Inoue, H.; Isono, T.; Haneda, M.; Isobe, K.-I.; Kobayashi, T.; Hino, O.; Okabe, H.; Chano, T. GADD34 inhibits mammalian target of rapamycin signaling via tuberous sclerosis complex and controls cell survival under bioenergetic stress. Int. J. Mol. Med. 2007, 19, 475–483. [Google Scholar] [CrossRef]
- Zhou, W.; Jeyaraman, K.; Yusoff, P.; Shenolikar, S. Phosphorylation At Tyrosine 262 Promotes GADD34 Protein Turnover. J. Biol. Chem. 2013, 288, 33146–33155. [Google Scholar] [CrossRef]
- Klein, P.; Kallenberger, S.M.; Roth, H.; Roth, K.; Ly-Hartig, T.B.N.; Magg, V.; Ales, J.; Talemi, S.R.; Qiang, Y.; Wolf, S.; et al. Temporal control of the integrated stress response by a stochastic molecular switch. Sci. Adv. 2022, 8, eabk2022. [Google Scholar] [CrossRef]
- Arimoto, K.-I.; Takahashi, H.; Hishiki, T.; Konishi, H.; Fujita, T.; Shimotohno, K. Negative regulation of the RIG-I signaling by the ubiquitin ligase RNF125. Proc. Natl. Acad. Sci. USA 2007, 104, 7500–7505. [Google Scholar] [CrossRef]
- Gack, M.U.; Shin, Y.C.; Joo, C.-H.; Urano, T.; Liang, C.; Sun, L.; Takeuchi, O.; Akira, S.; Chen, Z.; Inoue, S.; et al. TRIM25 RING-finger E3 ubiquitin ligase is essential for RIG-I-mediated antiviral activity. Nature 2007, 446, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Gack, M.U.; Nistal-Villán, E.; Inn, K.-S.; García-Sastre, A.; Jung, J.U. Phosphorylation-Mediated Negative Regulation of RIG-I Antiviral Activity. J. Virol. 2010, 84, 3220–3229. [Google Scholar] [CrossRef] [PubMed]
- Maharaj, N.P.; Wies, E.; Stoll, A.; Gack, M.U. Conventional Protein Kinase C-α (PKC-α) and PKC-β Negatively Regulate RIG-I Antiviral Signal Transduction. J. Virol. 2012, 86, 1358–1371. [Google Scholar] [CrossRef] [PubMed]
- Wies, E.; Wang, M.K.; Maharaj, N.P.; Chen, K.; Zhou, S.; Finberg, R.W.; Gack, M.U. Dephosphorylation of the RNA Sensors RIG-I and MDA5 by the Phosphatase PP1 Is Essential for Innate Immune Signaling. Immunity 2013, 38, 437–449. [Google Scholar] [CrossRef]
- Shin, D.; Mukherjee, R.; Grewe, D.; Bojkova, D.; Baek, K.; Bhattacharya, A.; Schulz, L.; Widera, M.; Mehdipour, A.R.; Tascher, G.; et al. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature 2020, 587, 657–662. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Guan, G.; Wu, C.; Wang, B.; Chu, K.; Zhang, X.; He, S.; Zhang, N.; Yang, G.; Jin, Z.; et al. SARS-CoV-2 Nucleocapsid Protein Antagonizes GADD34-Mediated Innate Immune Pathway through Atypical Foci. Molecules 2024, 29, 4792. https://doi.org/10.3390/molecules29204792
Liu J, Guan G, Wu C, Wang B, Chu K, Zhang X, He S, Zhang N, Yang G, Jin Z, et al. SARS-CoV-2 Nucleocapsid Protein Antagonizes GADD34-Mediated Innate Immune Pathway through Atypical Foci. Molecules. 2024; 29(20):4792. https://doi.org/10.3390/molecules29204792
Chicago/Turabian StyleLiu, Jie, Guanwen Guan, Chunxiu Wu, Bingbing Wang, Kaifei Chu, Xu Zhang, Su He, Naru Zhang, Geng Yang, Zhigang Jin, and et al. 2024. "SARS-CoV-2 Nucleocapsid Protein Antagonizes GADD34-Mediated Innate Immune Pathway through Atypical Foci" Molecules 29, no. 20: 4792. https://doi.org/10.3390/molecules29204792
APA StyleLiu, J., Guan, G., Wu, C., Wang, B., Chu, K., Zhang, X., He, S., Zhang, N., Yang, G., Jin, Z., & Zhao, T. (2024). SARS-CoV-2 Nucleocapsid Protein Antagonizes GADD34-Mediated Innate Immune Pathway through Atypical Foci. Molecules, 29(20), 4792. https://doi.org/10.3390/molecules29204792




