Protective Role of Eicosapentaenoic and Docosahexaenoic and Their N-Ethanolamide Derivatives in Olfactory Glial Cells Affected by Lipopolysaccharide-Induced Neuroinflammation
Abstract
1. Introduction
2. Results
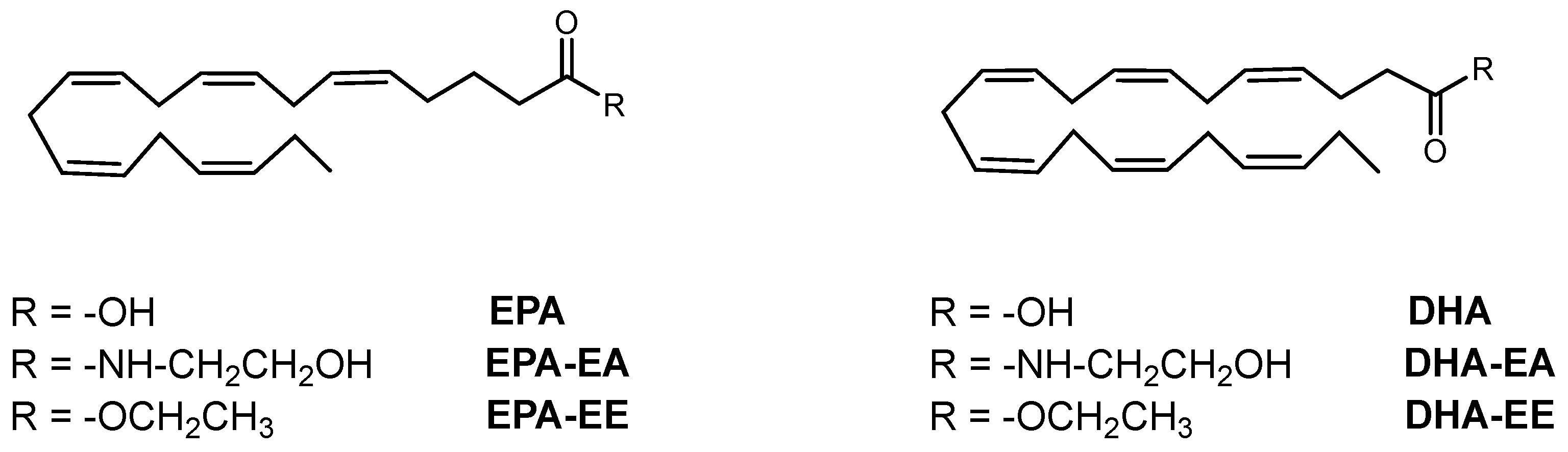
2.1. Chemical Compounds
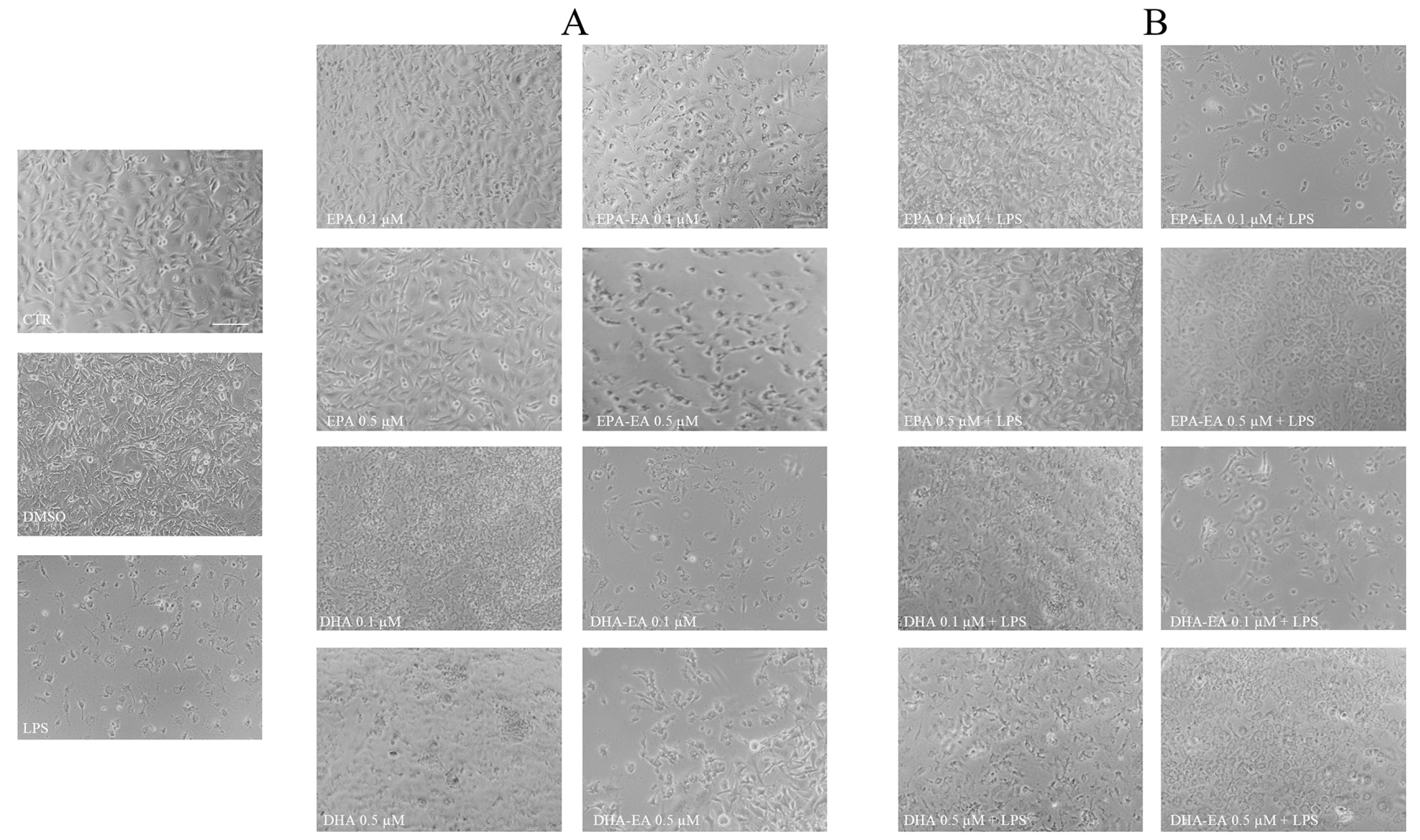
2.2. Morphological Features of OECs after Treatments
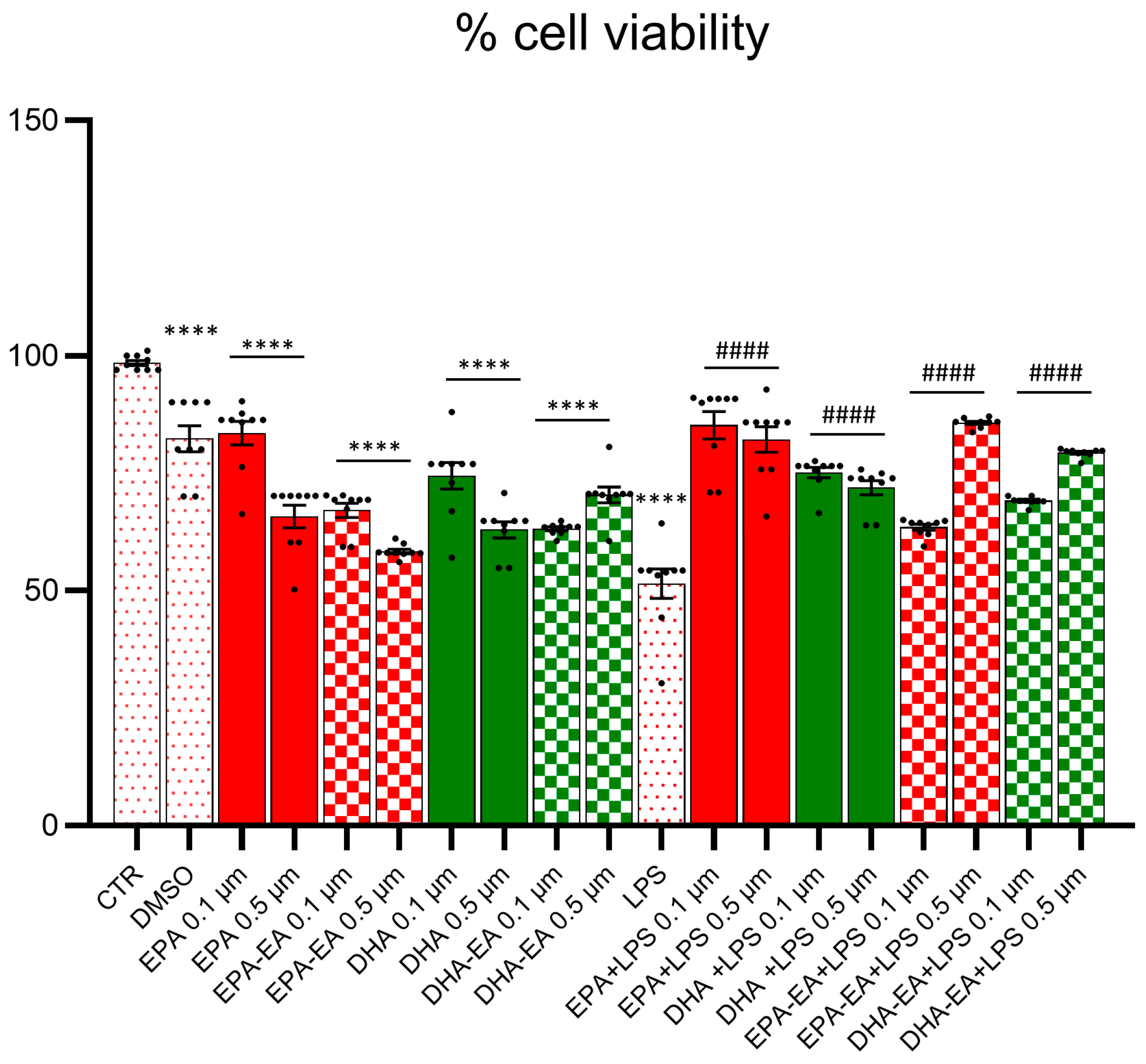
2.3. Cell Viability (MTT Test)
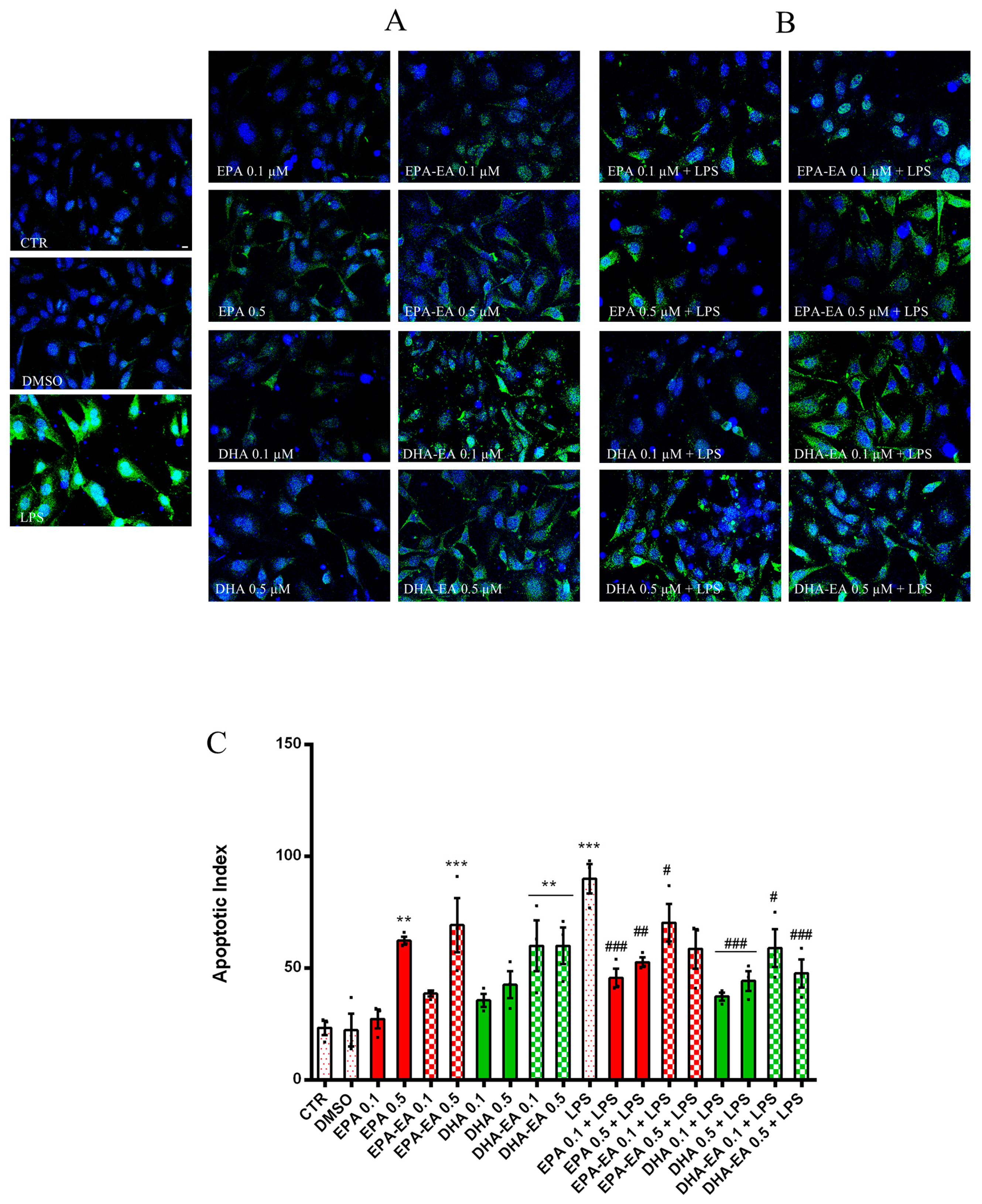
2.4. Influence on OEC Apoptosis (TUNEL Assay)
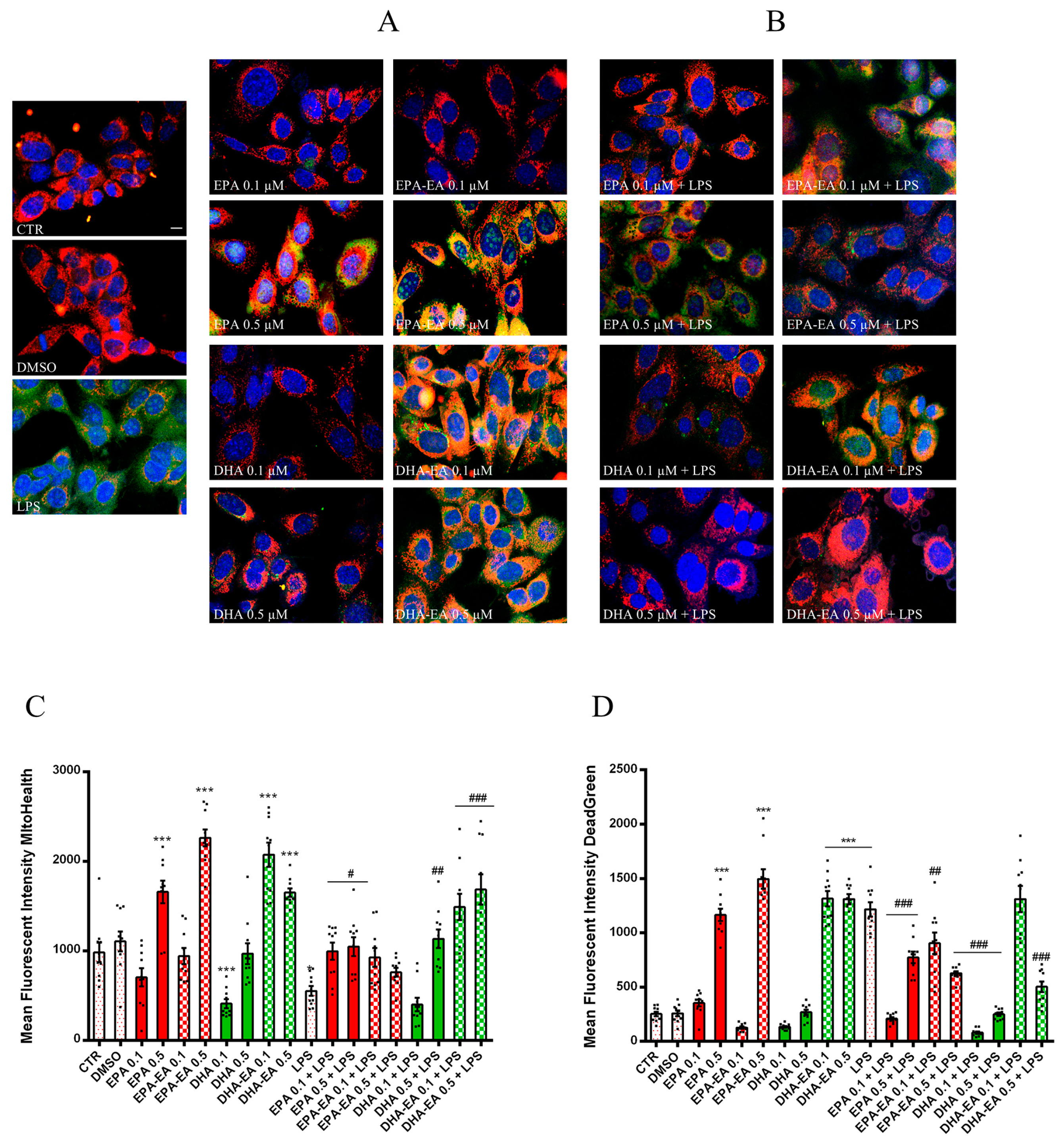
2.5. Mitotoxicity and Cytotoxicity of LPS in OECs (MitoHealth Assay)
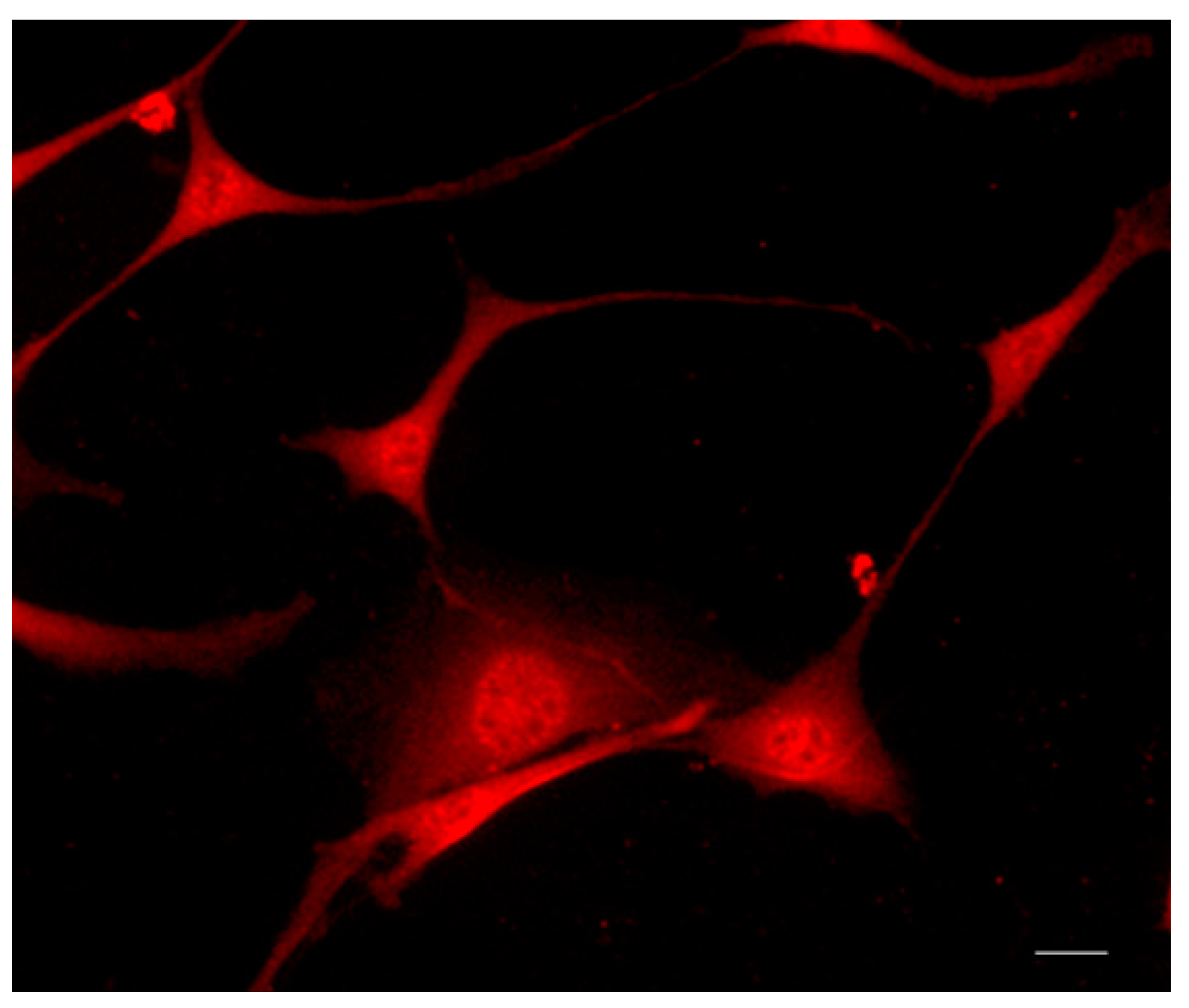
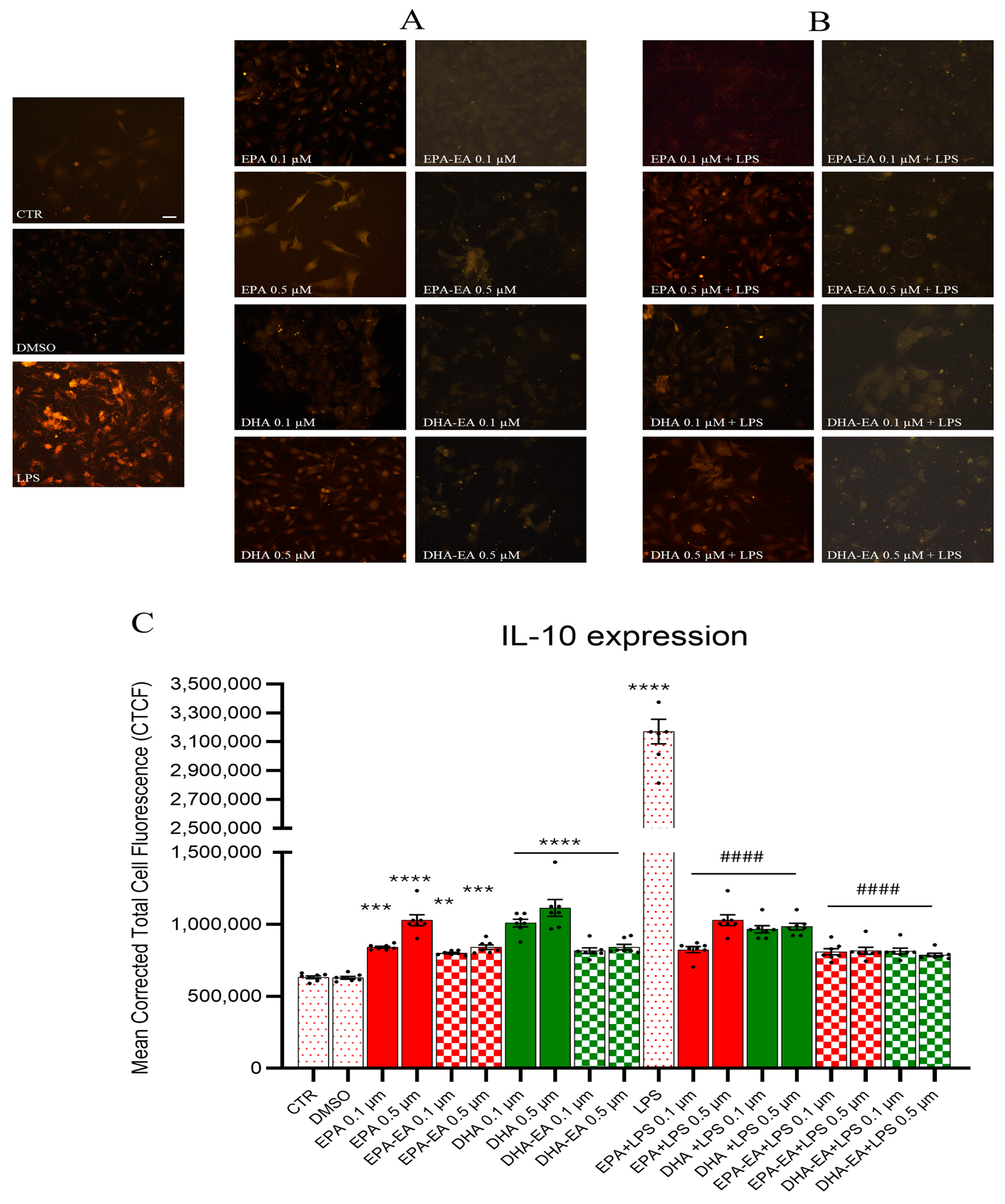
2.6. IL-10 Expression by Immunofluorescence
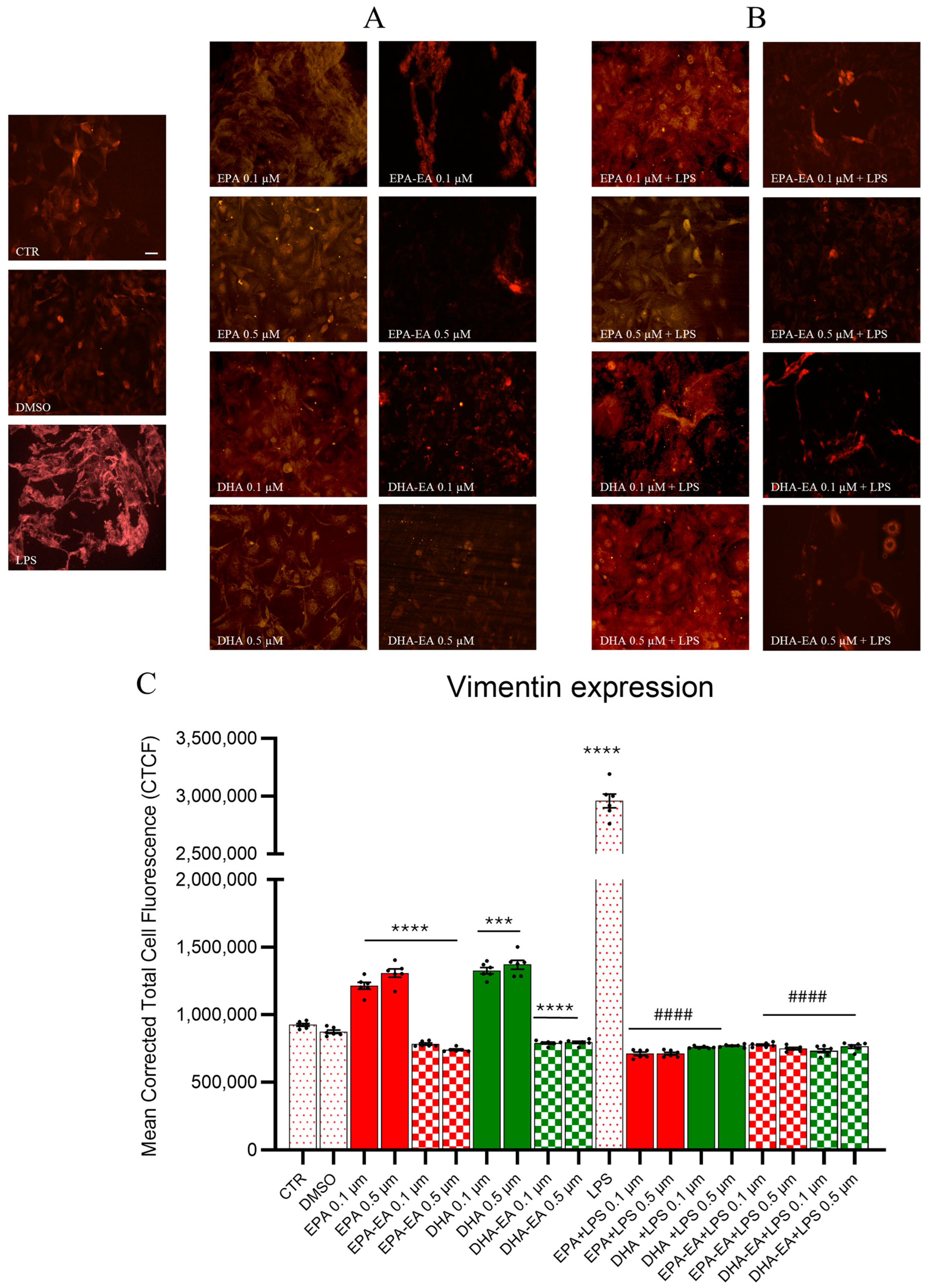
2.7. GFAP and Vimentin Expression by Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Synthesis of EPA-EA and DHA-EA
4.3. OEC Cultures
4.4. Treatment of Cells
4.5. MTT Assay
4.6. TUNEL (Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling) Test
4.7. Mitochondrial Health Assay
4.8. Immunocytochemistry
4.9. Immunostaining Quantification
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, Y.; Chen, X.; Liu, Z.; Peng, Y.P.; Qiu, Y.H. Interleukin-10 protection against lipopolysaccharide-induced neuro-inflammation and neurotoxicity in ventral mesencephalic cultures. Int. J. Mol. Sci. 2016, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron 2009, 64, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Bartels, T.; De Schepper, S.; Hong, S. Microglia modulate neurodegeneration in Alzheimer’s and Parkinson’s diseases. Science 2020, 370, 66–69. [Google Scholar] [CrossRef]
- Shao, F.; Wang, X.; Wu, H.; Wu, Q.; Zhang, J. Microglia and neuroinflammation: Crucial pathological mechanisms in traumatic brain injury-induced neurodegeneration. Front. Aging Neurosci. 2022, 14, 825086. [Google Scholar] [CrossRef]
- Paterniti, I.; Impellizzeri, D.; Di Paola, R.; Esposito, E.; Gladman, S.; Yip, P.; Priestley, J.V.; Michael-Titus, A.T.; Cuzzocrea, S. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: In-vivo and in-vitro studies. J. Neuroinflamm. 2014, 11, 6. [Google Scholar] [CrossRef]
- Guo, S.; Wang, H.; Yin, Y. Microglia polarization from M1 to M2 in neurodegenerative diseases. Front. Aging Neurosci. 2022, 14, 815347. [Google Scholar] [CrossRef] [PubMed]
- Aldana, B.I. Microglia-specific metabolic changes in neurodegeneration. J. Mol. Biol. 2019, 431, 1830–1842. [Google Scholar] [CrossRef]
- Yang, C.; Pan, R.Y.; Guan, F.; Yuan, Z. Lactate metabolism in neurodegenerative diseases. Regen. Res. 2024, 19, 69–74. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: Neuroinfammation; homeostasis; and stress. J. Neuroinflamm. 2021, 18, 258. [Google Scholar] [CrossRef]
- Stillwell, W.; Wassall, S.R. Docosahexaenoic acid: Membrane properties of a unique fatty acid. Chem. Phys. Lipids 2003, 126, 1–27. [Google Scholar] [CrossRef]
- Petermann, A.B.; Reyna-Jeldes, M.; Ortega, L.; Coddou, C.; Yévenes, G.E. Roles of the unsaturated fatty acid docosahexaenoic acid in the central nervous system: Molecular and cellular insights. Int. J. Mol. Sci. 2022, 23, 5390. [Google Scholar] [CrossRef] [PubMed]
- Bourre, J. Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J. Nutr. Health Aging 2004, 8, 163–174. [Google Scholar] [PubMed]
- Dyall, S.C. Long-chain omega-3 fatty acids and the brain: A review of the independent and shared effects of EPA; DPA and DHA. Front. Aging Neurosci. 2015, 7, 52. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Ceccarelli, V.; Codini, M.; Fettucciari, K.; Calvitti, M.; Cataldi, S.; Albi, E.; Vecchini, A.; Beccari, T. The polyunsaturated fatty acid EPA; but not DHA; enhances neurotrophic factor expression through epigenetic mechanisms and protects against parkinsonian neuronal cell death. Int. J. Mol. Sci. 2022, 23, 16176. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.R.; Supti, F.A.; Dhar, P.S.; Shohag, S.; Ferdous, J.; Shuvo, S.K.; Akter, A.; Hossain, M.S.; Sharma, R. Exploring the therapeutic effect of neurotrophins and neuropeptides in neurodegenerative diseases: At a glance. Mol. Neurobiol. 2023, 60, 4206–4231. [Google Scholar] [CrossRef]
- Kawakita, E.; Hashimoto, M.; Shido, O. Docosahexaenoic acid promotes neurogenesis in vitro and in vivo. Neuroscience 2006, 139, 991–997. [Google Scholar] [CrossRef]
- Chapkin, R.S.; Kim, W.; Lupton, J.R.; McMurray, D.N. Dietary docosahexaenoic and eicosapentaenoic acid; Emerging mediators of inflammation. Prostaglandins Leukot. Essent. Fatty Acids 2009, 81, 187–191. [Google Scholar] [CrossRef]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-inflammatory effects of omega-3 fatty acids in the brain: Physiological mechanisms and relevance to pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- Moon, D.O.; Kim, K.C.; Jin, C.Y.; Han, M.H.; Park, C.; Lee, K.J.; Park, Y.M.; Choi, Y.H.; Kim, G.Y. Inhibitory effects of eicosapentaenoic acid on lipopolysaccharide-induced activation in BV2 microglia. Int. Immunopharmacol. 2007, 7, 222–229. [Google Scholar] [CrossRef]
- Liuzzi, G.M.; Latronico, T.; Rossano, R.; Viggiani, S.; Fasano, A.; Riccio, P. Inhibitory effect of polyunsaturated fatty acids on MMP-9 release from microglial cells–implications for complementary multiple sclerosis treatment. Neurochem. Res. 2007, 32, 2184–2193. [Google Scholar] [CrossRef] [PubMed]
- De Smedt-Peyrusse, V.; Sargueil, F.; Moranis, A.; Harizi, H.; Mongrand, S.; Layé, S. Docosahexaenoic acid prevents lipopolysaccharide-induced cytokine production in microglial cells by inhibiting lipopolysaccharide receptor presentation but not its membrane subdomain localization. J. Neurochem. 2008, 105, 296–307. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Meijerink, J.; Balvers, M.; Witkamp, R. N-Acyl amines of docosahexaenoic acid and other n-3 polyunsatured fatty acids: From fishy endocannabinoids to potential leads. Br. J. Pharmacol. 2013, 169, 772–783. [Google Scholar] [CrossRef]
- Park, T.; Chen, H.; Kevala, K.; Lee, J.W.; Kim, H.Y. N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. J. Neuroinflamm. 2016, 13, 284. [Google Scholar] [CrossRef] [PubMed]
- Fairless, R.; Barnett, S. Olfactory ensheathing cells: Their role in central nervous system repair. Int. J. Bioch. Cell. Biol. 2005, 37, 693–699. [Google Scholar] [CrossRef]
- Graziadei, P.P.C.; Monti-Graziadei, G.A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J. Neurocytol. 1979, 8, 1–18. [Google Scholar] [CrossRef]
- Pellitteri, R.; Spatuzza, M.; Stanzani, S.; Zaccheo, D. Biomarkers expression in rat olfactory ensheathing cells. Front. Biosci. 2010, 2, 289–298. [Google Scholar] [CrossRef]
- Lipson, A.C.; Widenfalk, J.; Lindqvist, E.; Ebendal, T.; Olson, L. Neurotrophic properties of olfactory ensheathing glia. Exp. Neurol. 2003, 180, 167–171. [Google Scholar] [CrossRef]
- Pastrana, E.; Moreno-Flores, M.T.; Avila, J.; Wandosel, F.; Minichiello, L.; Diaz-Nido, J. BDNF production by olfactory ensheathing cells contributes to axonal regeneration of cultured adult CNS neurons. Neurochem. Int. 2007, 50, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Doucette, R. Immunohistochemical localization of laminin; fibronectin and collagen type IV in the nerve fiber layer of the olfactory bulb. Int. J. Dev. Neurosci. 1996, 14, 945–959. [Google Scholar] [CrossRef] [PubMed]
- Ramon-Cueto, A.; Avila, J. Olfactory ensheathing cells: Properties and function. Brain Res. Bull. 1998, 46, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Franssen, E.H.; de Bree, F.M.; Verhaagen, J. Olfactory ensheathing glia: Their contribution to primary olfactory nervous system regeneration and their regenerative potential following transplantation into the injured spinal cord. Brain Res. Rev. 2007, 56, 236–258. [Google Scholar] [CrossRef] [PubMed]
- Pellitteri, R.; Cova, L.; Zaccheo, D.; Silani, V.; Bossolasco, P. Phenotypic modulation and neuroprotective effects of olfactory ensheathing cells: A promising tool for cell therapy. Stem Cell Rev. Rep. 2016, 12, 224–234. [Google Scholar] [CrossRef]
- Delarue, Q.; Guérout, N. Transplantation of Olfactory Ensheathing Cells: Properties and therapeutic effects after transplantation into the lesioned nervous system. Neuroglia 2022, 3, 1–22. [Google Scholar] [CrossRef]
- Nazareth, L.; Lineburg, K.E.; Chuah, M.I.; Velasquez, J.T.; Chehrehasa, F.; John, J.S.; Ekberg, J.A. Olfactory ensheathing cells are the main phagocytic cells that remove axon debris during early development of the olfactory system. J. Comp. Neurol. 2015, 523, 479–494. [Google Scholar] [CrossRef]
- Nazareth, L.; Shelper, T.B.; Chacko, A.; Basu, S.; Delbaz, A.; Lee, J.Y.P.; Chen, M.; John, J.A.S.; Ekberg, J.A.K. Key differences between olfactory ensheathing cells and Schwann cells regarding phagocytosis of necrotic cells: Implications for transplantation therapies. Sci. Rep. 2020, 10, 18936. [Google Scholar] [CrossRef]
- Russo, C.; Patanè, M.; Vicario, N.; Di Bella, V.; Cosentini, I.; Barresi, V.; Gulino, R.; Pellitteri, R.; Russo, A.; Stanzani, S. Olfactory ensheathing cells express both ghrelin and ghrelin receptor in vitro: A new hypothesis in favor of a neurotrophic effect. Neuropeptides 2020, 79, 101997. [Google Scholar] [CrossRef]
- Erekat, N.S. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin. Anat. 2022, 35, 65–78. [Google Scholar] [CrossRef]
- Qin, L.; Wu, X.; Block, M.L.; Liu, Y.; Breese, G.R.; Hong, J.S.; Knapp, D.; Crews, F.T. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007, 55, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, C.; Patti, A. Biocatalytic regio- and stereoselective access to ω-3 endocannabinoid epoxides with peroxygenase from oat flour. Bioorg. Chem. 2021, 113, 105014. [Google Scholar] [CrossRef]
- Katakura, M.; Hashimoto, M.; Okui, T.; Shahdat, H.M.; Matsuzaki, K.; Shido, O. Omega-3 polyunsaturated-fatty acids enhance neuronal differentiation in cultured rat neural stem cells. Stem Cells Int. 2013, 2013, 490476. [Google Scholar] [CrossRef] [PubMed]
- Mazza, M.; Pomponi, M.; Janiri, L.; Bria, P.; Mazza, S. Omega-3 fatty acids and antioxidants in neurological and psychiatric diseases: An overview. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2006, 31, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Liao, J.X.; Liu, Y.Y.; Luo, H.L.; Zhang, W.J. Potential therapeutic effect of olfactory ensheathing cells in neurological diseases: Neurodegenerative diseases and peripheral nerve injuries. Front. Immunol. 2023, 14, 1280186. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Brown, R.E.; Zhang, P.C.; Zhao, Y.T.; Ju, X.; Song, C. DHA, EPA and their combination at various ratios differently modulated Aβ25-35-induced neurotoxicity in SH-SY5Y cells. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 85–94. [Google Scholar] [CrossRef]
- Park, G.; Nhan, H.S.; Tyan, S.H.; Kawakatsu, Y.; Zhang, C.; Navarro, M.; Koo, E.H. Caspase activation and caspase-mediated cleavage of APP is associated with amyloid beta-protein-induced synapse loss in Alzheimer’s disease. Cell Rep. 2020, 31, 107839. [Google Scholar] [CrossRef]
- Perez, M.J.; Ibarra-García-Padilla, R.; Tang, M.; Porter, J.A., Jr.; Johnson, G.V.W.; Quintanilla, R.A. Caspase-3 cleaved tau impairs mitochondrial function through the opening of the mitochondrial permeability transition pore. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 166898. [Google Scholar] [CrossRef]
- Gendron, T.F.; Petrucelli, L. The role of tau in neurodegeneration. Mol. Neurodegener. 2009, 4, 13. [Google Scholar] [CrossRef]
- Eckert, G.P.; Lipka, U.; Muller, W.E. Omega-3 fatty acids in neurodegenerative diseases: Focus on mitochondria. Prostaglandins Leukot. Essent. Fatty Acids 2013, 88, 105–114. [Google Scholar] [CrossRef]
- Lee, S.Y.; Surbeck, J.W.; Drake, M.; Saunders, A.; Jin, H.D.; Shah, V.A.; Rajala, R.V. Increased glial fibrillary acid protein and vimentin in vitreous fluid as a biomarker for proliferative vitreoretinopathy. Investig. Ophthalmol. Vis. Sci. 2020, 61, 22. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmsson, U.; Pozo-Rodrigalvarez, A.; Kalm, M.; de Pablo, Y.; Widestrand, Å.; Pekna, M.; Pekny, M. The role of GFAP and vimentin in learning and memory. Biol. Chem. 2019, 400, 1147–1156. [Google Scholar] [CrossRef]
- Osborn, L.M.; Kamphuis, W.; Wadman, W.J.; Hol, E.M. Astrogliosis: An integral player in the pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2016, 144, 121–141. [Google Scholar] [CrossRef] [PubMed]
- Tyrtyshnaia, A.; Konovalova, S.; Bondar, A.; Ermolenko, E.; Sultanov, R.; Manzhulo, I. Anti-inflammatory activity of N-docosahexaenoylethanolamine and N-eicosapentaenoylethanolamine in a mouse model of lipopolysaccharide-induced neuroinflammation. Int. J. Mol. Sci. 2021, 22, 10728. [Google Scholar] [CrossRef]
- Joffre, C.; Dinel, A.L.; Chataigner, M.; Pallet, V.; Layé, S. n-3 Polyunsaturated Fatty Acids and Their Derivates Reduce Neuroinflammation during Aging. Nutrients 2020, 12, 647. [Google Scholar] [CrossRef]
- Pellitteri, R.; Catania, M.V.; Bonaccorso, C.M.; Ranno, E.; Dell’Albani, P.; Zaccheo, D. Viability of olfactory ensheathing cells after hypoxia and serum deprivation: Implication for therapeutic transplantation: Role of Growth Factors in Hypoxic OECs. J. Neurosci. Res. 2014, 92, 1757–1766. [Google Scholar] [CrossRef]
- Campisi, A.; Spatuzza, M.; Russo, A.; Raciti, G.; Vanella, A.; Stanzani, S.; Pellitteri, R. Expression of Tissue Transglutaminase on Primary Olfactory Ensheathing Cells Cultures Exposed to Stress Conditions. Neurosci. Res. 2012, 72, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Huynh, T.N.; Duong, M.T.; Gow, J.G.; Chang, C.C.Y.; Chang, T.Y. ACAT1/SOAT1 Blockade Suppresses LPS-Mediated Neuroinflammation by Modulating the Fate of Toll-like Receptor 4 in Microglia. Int. J. Mol. Sci. 2023, 24, 5616. [Google Scholar] [CrossRef]
- La Cognata, V.; Golini, E.; Iemmolo, R.; Balletta, S.; Morello, G.; De Rosa, C.; Villari, A.; Marinelli, S.; Vacca, V.; Bonaventura, G.; et al. CXCR2 increases in ALS cortical neurons and its inhibition prevents motor neuron degeneration in vitro and improves neuromuscular function in SOD1G93A mice. Neurobiol. Dis. 2021, 160, 105538. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pellitteri, R.; La Cognata, V.; Russo, C.; Patti, A.; Sanfilippo, C. Protective Role of Eicosapentaenoic and Docosahexaenoic and Their N-Ethanolamide Derivatives in Olfactory Glial Cells Affected by Lipopolysaccharide-Induced Neuroinflammation. Molecules 2024, 29, 4821. https://doi.org/10.3390/molecules29204821
Pellitteri R, La Cognata V, Russo C, Patti A, Sanfilippo C. Protective Role of Eicosapentaenoic and Docosahexaenoic and Their N-Ethanolamide Derivatives in Olfactory Glial Cells Affected by Lipopolysaccharide-Induced Neuroinflammation. Molecules. 2024; 29(20):4821. https://doi.org/10.3390/molecules29204821
Chicago/Turabian StylePellitteri, Rosalia, Valentina La Cognata, Cristina Russo, Angela Patti, and Claudia Sanfilippo. 2024. "Protective Role of Eicosapentaenoic and Docosahexaenoic and Their N-Ethanolamide Derivatives in Olfactory Glial Cells Affected by Lipopolysaccharide-Induced Neuroinflammation" Molecules 29, no. 20: 4821. https://doi.org/10.3390/molecules29204821
APA StylePellitteri, R., La Cognata, V., Russo, C., Patti, A., & Sanfilippo, C. (2024). Protective Role of Eicosapentaenoic and Docosahexaenoic and Their N-Ethanolamide Derivatives in Olfactory Glial Cells Affected by Lipopolysaccharide-Induced Neuroinflammation. Molecules, 29(20), 4821. https://doi.org/10.3390/molecules29204821







