Olive Oil Industry By-Products as a Novel Source of Biophenols with a Promising Role in Alzheimer Disease Prevention
Abstract
:1. Introduction
2. Olive Tree Bioactive Phenolic Compounds
3. Olive Oil Production and By-Products
3.1. Overview of Olive Oil Production
3.2. Types of By-Products Generated by the Olive Oil Industry
3.2.1. Olive Pomace
3.2.2. Olive Mill Wastewater
3.2.3. Olive Leaves
3.3. Extraction Methods
4. Polyphenols and Alzheimer’s Disease
4.1. Overview of Alzheimer’s Disease
4.1.1. Oxidative Stress
4.1.2. Autophagy
4.1.3. Chronic Neuroinflammation
4.1.4. Gut Microbiota
4.1.5. Synaptic Hypothesis in AD
4.2. Preclinical Models for Assessing Polyphenol Therapeutics in Alzheimer’s Disease
4.2.1. Induced Pluripotent Stem Cells (iPSC)-Derived Neurons
4.2.2. Primary Neuronal Cells
4.2.3. APP/PS1 Mice
4.2.4. APPSwe/PS1dE9 Mouse
4.2.5. Senescence-Accelerated Mouse Prone 8 (SAMP8)
4.2.6. Sprague–Dawley Rats
4.2.7. Caenorhabditis Elegans
4.2.8. Drosophila Melanogaster
| Polyphenol | Chemical Formula | Plant Origin | Experimental Model | Health Effects | Reference |
|---|---|---|---|---|---|
| Flavonoids | |||||
| Apigenin | C15H10O5 | Some of the primary sources of apigenin include: Parsley (Petroselinum crispum) Celery (Apium graveolens) Chamomile (Matricaria chamomilla) Thyme (Thymus vulgaris) Peppermint (Mentha piperita) Oregano (Origanum vulgare) Onions (Allium cepa) Oranges (Citrus sinensis) Artichokes (Cynara scolymus) | Human-induced pluripotent stem cell (iPSC)-derived neurons. For the inflammation-based assays, neurons were treated with 50 μM apigenin or a vehicle control for 24 h. Following this treatment, the media was replaced with conditioned media from activated microglia for 48 h. In addition to the inflammation assays, the neurons were subjected to oxidative stress by treatment with 300 μM H2O2 or 10 μM SNAP for 24 h, after a 24 h pre-treatment with 50 μM apigenin. | Apigenin: Significant anti-inflammatory and neuroprotective effects. Protects neurons from neurite retraction induced by inflammatory stress. Reduces the release of NO and various pro-inflammatory cytokines. Protects neurons from apoptosis by reducing caspase-3/7 activity. Reduces neuronal hyper-excitability and disturbances in calcium signaling. Decreases both oxidative and nitrosative stress levels. | [184] |
| Cyanidin-3-O-glucoside Malvidin-3-O-glucoside Pelargonidin-3-O-glucoside Peonidin | Cyanidin-3-O-glucoside: C21H21O11 Malvidin-3-O-glucoside: C23H25O12 Pelargonidin-3-O-glucoside: C21H21O10 Peonidin chloride (Peonidin): C16H13ClO6 | SK-N-SH cell line (human neuroblastoma cell line). The polyphenol formulation tested was MAF14001, an equimolar mixture of cyanidin-3-O-glucoside chloride, malvidin-3-O-glucoside chloride, pelargonidin-3-O-glucoside chloride, and peonidin chloride. This formulation was tested at concentrations ranging from 5 to 20 µM. The administration mode involved direct addition of MAF14001 to the cell culture medium. The cells were incubated with the formulation for 24 h to assess the protective effects against Aβ peptide-induced toxicity. | MAF14001, a mixture of anthocyanins and anthocyanidins: Protects SK-N-SH cells against Aβ-induced toxicity. Prevents Aβ-induced oxidative stress, mitochondrial dysfunction, and apoptosis Interacts with Aβ to prevent its aggregation, a key process in Aβ-induced oxidative stress. Decreases TAU phosphorylation induced by Aβ. | [185] | |
| Epicatechin (EC) Catechin (CE) | Theobroma cacao | Mouse hippocampal cell lines HT-22. Primary neuronal cultures. The cells were treated with Aβ oligomers (12.5 µM) for 24 h to mimic AD. Differentiated cells were treated with cocoa extract containing 30 µg/mL EC, 10 µg/mL CE, and 170 µg/mL total polyphenols for 4 days. | Cocoa polyphenolic extract: Exerts neuroprotective effects by activating the BDNF survival pathway. This activation counteracted neurite dystrophy induced by Aβ plaques and oligomers, suggesting the potential of cocoa polyphenols as preventive agents against neurodegenerative diseases, such as AD by reducing oxidative stress and promoting neuronal survival. | [186] | |
| Epicatechin (EC) Catechin (CE) Procyanidins | EC: C15H14O6 CE: C15H14O6 Procyanidins: Varies depending on the degree of polymerization (DP) | Theobroma cacao | Mouse brain hippocampal slices. Slices were exposed to 25 µM and 100 µM concentrations of cocoa extracts. The hippocampal slices were initially acclimated in oxygenated artificial cerebrospinal fluid and subsequently incubated with the cocoa extracts at 32 °C for a duration of 1 h. | Cocoa polyphenolic extract: Reduces Aβ oligomer formation. Prevents synaptic deficits induced by Aβ oligomers. Restores long-term potentiation reduced by oligomeric Aβ. | |
| Epigallocatechin-3-gallate (EGCG) | C22H18O11 | Camellia sinensis | Human SH-SY5Y neuroblastoma cells and Chinese hamster ovary. Cells transfected with human APP695 were treated with 1–10 μM EGCG for 2 days. | EGCG: Demonstrates potent iron-chelating activity comparable to that of desferrioxamine. Significantly reduces both APP and toxic Aβ peptide production Promotes non-amyloidogenic APP processing through increased secretion of soluble APP-α and activation of PKC. | [187] |
| Epigallocatechin-3-gallate (EGCG) | C22H18O11 | Camellia sinensis | Cholinergic-like neurons (ChLNs) derived from umbilical cord mesenchymal stem cells with either wild-type or PSEN1 E280A mutation. The cells were exposed to varying concentrations of EGCG, ranging from 5 to 50 µM, with 50 µM selected for further experiments based on its efficacy in previous tests. The EGCG was administered directly into the regular culture medium for a duration of 4 days post-transdifferentiation. | EGCG: Inhibits APP aggregation. Blocks the phosphorylation of TAU (p-TAU), increases mitochondrial membrane potential (∆Ψm), and decreases oxidation of DJ-1 at the residual Cys106-SH. Inhibits the activation of transcription factors c-JUN and P53, as well as PUMA and CASPASE-3, in mutant ChLNs compared to WT. Reverses Ca2+ influx dysregulation in response to ACh stimuli in PSEN1 E280A ChLNs. Inhibits the activation of the transcription factor NF-κB and reduces the secretion of the pro-inflammatory cytokine IL-6 in wild-type astrocyte-like cells when exposed to the culture supernatant from mutant ChLNs. | [188] |
| Luteolin (LUT) | C15H10O6 | Various plants including parsley, thyme, and celery | Human neuroblastoma BE(2)-M17 cells Cells were pre-treated DHA, LUT, and urolithin A (UA), each at a concentration of 5 µM in combination (D5L5U5) and individually at 30 µM, 20 µM, and 30 µM respectively. The pre-treatment lasted for 24 h, after which the cells were exposed to 20 µM of oligomeric Aβ1–42 for various durations ranging from 4 to 72 h | DHA, LUT, and UA in combination (D5L5U5): Have potent inhibitory effects against Aβ1–42-induced toxicity through protecting mitochondria. These effects include minimizing oxidative stress, increasing ATP levels, and inducing mitophagy and mitobiogenesis. | [189] |
| Quercetin Thymol | Quercetin: C15H10O7 Thymol: C10H14O | Quercetin: found in Allium cepa, asparagus, red leaf lettuce, apple, and berries. Thymol: major component of Thymus vulgaris. | PC12 cell model The concentrations of the polyphenols used were 40 µM for quercetin and 35 µg/mL for thymol, while the concentrations of Allium cepa extract were 1000 µg/mL and Thymus vulgaris essential oil was 0.4 ppm. The incubation period for these treatments was 48 h. The cell model was created using formaldehyde at a final concentration of 0.35 mM to induce AD-like conditions in the PC12 cells. | Quercetin: Reduces the rate of apoptosis in AD cells significantly better than other compounds. Increases the expression of genes related to AD, such as PP2A, GSK3, and NMDAR. Thymol: Shows anti-AD and antioxidant effects, increases cell survival rate, and reduces apoptosis rate. Both thymol and Thymus vulgaris essential oil significantly increase the expression of the PP2A gene. | [190] |
| Phlorotannins | |||||
| Eckol, 6,6′-Bieckol, 8,8′-Bieckol Dieckol Phlorofucofuroeckol-A (PFF-A) | Eckol: C18H12O9 6,6′-Bieckol and 8,8′-Bieckol: C36H22O20 Dieckol: C36H22O18 Phlorofucofuroeckol-A: C30H18O13 | Ecklonia cava | SK-N-SH neuroblastoma cells. Cells were exposed to 2 μL of 50 mM of each polyphenol for 24 h. | Ecklonia cava extract: Inhibits AChE activity (IC50 from 16.0 to 96.3 μM). Shows a potent BuChE inhibitory activity, particularly phlorofucofuroeckol-A (PFF-A) with an IC50 of 0.95 μM. Inhibits GSK-3β, which is involved in the formation of hyper p-TAU and generation of Aβ. Bieckol and PFF-A inhibit APP biosynthesis. PFF-A exhibits strong β-BACE-1 inhibitory activity. | [191] |
| Phenylpropanoids, Flavonoids, and Hydroxycinnamic acids | |||||
| Phenylpropanoids Flavonoids Hydroxycinnamic acids | Arabidopsis thaliana mutants, including prn1, cop1, and xpf3 | Primary mixed glial cultures derived from the cerebral cortex of neonatal APOE-targeted-replacement (APOE-TR) and APOE-knock-out (KO) mice. These cultures contained approximately 95% astrocytes and 5% microglia. Cells were treated with the plant extracts at a concentration of 150 µg/mL, either with or without inflammatory stimuli (LPS or Aβ oligomers). Incubation times included pre-treatment periods followed by inflammatory stimulation, with TNFα levels measured after 16 h. | Polyphenol-enriched extracts from Arabidopsis thaliana mutants: Exhibits anti-inflammatory effects, especially the xpf3 mutant. Attenuates TNF-α secretion in mixed glial cultures, with a notable reduction observed in APOE4 cultures compared to APOE3 and APOE2. Could serve as a potential therapeutic agent for AP-OE-modulated neuroinflammation, a characteristic of AD. | [192] | |
| Stilbenes | |||||
| Resveratrol | C14H12O3 | Grapes and red wine | HEK293 cells stably transfected with human APP695. N2a cells stably transfected with wild-type or Swedish mutant human APP695 cDNAs. Cells were treated with 20–40 µM resveratrol for 24 h or 10 or 20 µM resveratrol for 12, 48, and 72 h. | Resveratrol: Decreases levels of secreted and intracellular Aβ peptides in the cell lines. Does not inhibit Aβ production but promotes intracellular degradation of Aβ via a mechanism involving proteasome involvement. Decreases in Aβ could be prevented by selective proteasome inhibitors and by siRNA-directed silencing of the proteasome subunit β5. Demonstrates a proteasome-dependent anti-amyloidogenic activity, suggesting therapeutic potential in AD. | [193] |
| Resveratrol | C14H12O3 | Resveratrol is derived from grapes | Rat glioma cell line C6. These cells are classified as part of the astrocyte lineage. The cells were treated with LPS at a concentration of 1 μg/mL. The treatments included exposure to LPS alone for 6 and 24 h, and combinations of LPS with resveratrol (25 μM) for 24 h. | Resveratrol: Decreases TAU hyperphosphorylation induced by LPS. Increases APP expression. Has a potential therapeutic role in reducing neuroinflammation and modifying the expression of proteins associated with AD. | [194] |
| Trans ε-viniferin Resveratrol. | Trans ε-viniferin—C28H22O6 Resveratrol—C14H12O3 | Vine shoots (Vitis vinifera) | Murine primary co-cultures of neurons and astrocytes were prepared from the hippocampus and cortex of embryonic day 18 (E18) mice. The cultures were first pre-treated with either 1 μM of trans ε-viniferin or resveratrol, or with 0.001% DMSO as a vehicle control, in a medium with serum for 24 h. Following this pre-treatment, the cultures were then treated for 48 h in a serum-free medium with 20 μM Aβ42, which had been previously aggregated by incubation for 72 h at 37 °C, and 200 pg/mL IL-1β to mimic the inflammatory context of AD. The selection of the 1 μM concentration for the polyphenols was based on preliminary cytotoxicity assays that showed higher concentrations of trans ε-viniferin were cytotoxic, whereas 1 μM was not. | Trans ε-viniferin: Induces the disaggregation of Aβ peptide and rescues inflammation. These effects were higher than those of resveratrol, making trans ε-viniferin a promising multi-target therapeutic candidate for AD. | [195] |
| Tannins | |||||
| Tannic acid (TA) Epigallocatechin gallate (EGCG) | TA: C76H52O46 EGCG: C22H18O11 | TA: Found in various plant species, including oak, sumac, and witch hazel. Epigallocatechin gallate (EGCG): Major component of green tea leaves (Camellia sinensis) | SH-SY5Y neuronal cells Cells were treated with 20 μM concentrations of TA and EGCG | TA: Acts as a dual-acting therapeutic agent against AD and ferroptosis. Modulates Aβ42 and TAU aggregation. Chelates metal ions, reducing oxidative stress. Rescues mitochondrial function. Activates and enhances GPX4 levels, inhibiting ferroptosis. Activates Nrf2, regulating ferroptosis in neuronal cells. Inhibits lipid peroxidation and protein oxidation, providing neuroprotective effects. EGCG: Inhibits Aβ42 aggregation. Shows antioxidant properties, reducing oxidative stress. Does not directly activate GPX4 but inhibits RSL3-induced GPX4 inhibition. | [196] |
| Polyphenol | Chemical Formula | Plant Origin | Experimental Model | Health Effects | Reference |
|---|---|---|---|---|---|
| Flavonoids | |||||
| Catechin Epicatechin Proanthocyanidins | Vitis vinifera (Grape seed polyphenol extract—GSPE) | Male Sprague–Dawley rats. Rates aged 12 weeks and weighing between 250–260 g. GSPE was administered at doses of 25 or 250 mg/kg BW. The mode of administration was oral gavage, carried out once daily over a period of 11 days. | GSPE-derived phenolic acids, specifically 3-hydroxybenzoic acid (3-HBA) and 3-(3′-hydroxyphenyl) propionic acid (3-HPP): Interfere with the assembly of Aβ peptides into neurotoxic aggregates. This suggests potential therapeutic effects in modulating AD pathogenesis by preventing the formation of Aβ oligomers and fibrils. | [197] | |
| Baicalin Icariin Dihydromyricetin (DHM) Hesperidin | Baicalin: C21H18O11 Icariin: C33H40O15 Dihydromyricetin (DHM): C15H12O8 Hesperidin: C28H34O15 | Baicalin: Scutellaria baicalensis Icariin: Epimedium species Dihydromyricetin (DHM): Hovenia dulcis Hesperidin: Citrus sinensis and Citrus reticulata | Heterozygous male transgenic APP/PS1-(21) mice, bred with female wild-type C57BL/6J mice. Each polyphenol (baicalin, icariin, DHM, hesperidin) was administered at a dose of 100 mg/kg BW. The polyphenols were administered orally via gavage, dissolved in 1% carboxymethylcellulose (CMC). The treatment period was ten days, with daily administration of the polyphenols. | Hesperidin and icariin: Restore behavioral deficits. Reduce Aβ deposits in both the cortex and hippocampus of the transgenic mice. Baicalin and DHM: No substantial effects on the above parameters. | [198] |
| Gallic acid Catechin Epicatechin Proanthocyanidins | Gallic acid: C7H6O5 Catechin: C15H14O6 Epicatechin: C15H14O6 Proanthocyanidins: C31H28O | Vitis vinifera L. (grape seed extract (GSE) is rich in polyphenols) | APPSwe/PS1dE9 transgenic mice. Mice were administered daily polyphenols with a total polyphenolic content of 592.5 mg/g DW, including gallic acid (49 mg/g DW), catechin (41 mg/g DW), epicatechin (66 mg/g DW), and proanthocyanidins (436.6 mg catechin Eq./g DW). The daily consumption of polyphenols was approximately 1.2–1.7 mg per gram of BW, which is equivalent to about 5.9 g per day for a 60 kg human. | GSE: Reduces the Aβ burden in the brain and blood. Prevents Aβ deposition. Attenuates microgliosis and inflammation. Reduces brain Aβ levels by 33%, serum Aβ levels by 44%, amyloid plaques by 49%, and microgliosis by 70%. | [199] |
| Stilbenes | |||||
| Resveratrol | C14H12O3 | Grapes, red wine, and mulberry (Morus atropurpurea L.) | Senescence-accelerated mouse prone 8 (SAMP8) and senescence-accelerated mouse resistant 1 (SAMR1). The diet was supplemented with trans-resveratrol at a concentration of 1 g/kg and administered ad libitum to mice, starting at 2 months of age and maintained on this regimen until 9 months of age. | Resveratrol: Increases lifespan and reduces neurodegenerative markers in SAMP8 mice. Reduces levels of Aβ peptides. Decreases tau hyperphosphorylation. Enhances cognitive function and memory in animal models. Activates SIRT1 and AMPK pathways, promoting non-amyloidogenic processing of APP. Reduces oxidative stress. | [200] |
| Resveratrol | C14H12O3 | Double-transgenic AβPP/PS1 mice, which express a chimeric mouse/human Aβ protein precursor (Mo/Hu AβPP695swe) and a mutant human presenilin 1 (PS1-dE9). Mice were fed a chow diet containing 1% resveratrol, which translated to a daily resveratrol consumption of approximately 4 mg/kg/day, over a period of 10 months starting from 2 months of age. | Resveratrol: Prevents memory loss as measured by the object recognition test. Reduces the Aβ burden. Increases mitochondrial complex IV protein levels. These effects were mediated by increased activation of SIRT1 and AMPK pathways. Promotes changes in inflammatory processes (increased IL1β and TNF gene expression). | [201] | |
| Resveratrol | C14H12O3 | Male wild-type (WT) and AD transgenic 5xFAD mice. Mice were subjected to a high-fat diet (HFD) and resveratrol supplementation for 16 weeks, starting from 2 months of age to 6 months. The HFD constituted 60% of calories from fat, while the resveratrol-supplemented diet included 1 g/kg of trans-resveratrol (0.1% w/w). This concentration was chosen based on previous studies demonstrating its neuroprotective effects. The mice received approximately 120 mg/kg BW of trans-resveratrol per day. | Resveratrol: Neuroprotective effects against HFD-induced amyloid pathology, reducing amyloid burden and tau pathology in the cerebral cortex. Improves memory deficits and enhanced brain resilience against neurodegeneration through proteostasis enhancement and tau deacetylation mediated by SIRT1. | [202] | |
| Mix of polyphenols | |||||
| Grape seed polyphenol extract (GSPE) | Vitis vinifera L. (Grape seeds) | TMHT mouse model of AD. Mice were treated with 200 mg/kg/day of GSPE, starting at 4 months of age and continued for 2 months. | GSPE: Interferes with the assembly of tau peptides into neurotoxic aggregates Reduces the development of AD-type tau neuropathology. Decreases tau phosphorylation at specific sites, thereby preventing the formation of NFTs and reducing ERK 1/2 activity. Led to a significant reduction in the accumulation of insoluble tau and hyperphosphorylation of tau in the brains of TMHT mice. | [203] | |
| Flavonoids Phenolic acids Anthocyanins | Muscadine wine was generated from Vitis rotundifolia (cv. Noble grapes). Cabernet Sauvignon wine was generated from Vitis vinifera L. grapes. | Tg2576 AD mouse model mice. Mice consumed approximately 4 mL of wine-adulterated water per day over a period of 10 months, with the concentration of polyphenols in the muscadine wine measured as 1.731 mg/L (as gallic acid). | Muscadine wine polyphenols: Interfere with the aggregation of Aβ peptides into high-molecular-weight oligomeric species, which are implicated in cognitive dysfunction in AD. Cabernet Sauvignon wine polyphenols: Promote non-amyloidogenic α-secretase activity, reducing Aβ neuropathology. | [204] | |
| Anthocyanins Flavonoids Organic acids (malic acid, oxalic acid, and tartaric acid) | Vitis vinifera L. (red grape), | Wistar rats (12–15 weeks old, weighing 250–300 g) AD was induced by orally administering AlCl3 at a dose of 17 mg/kg BW daily for four consecutive weeks. After the induction period, the rats were divided into five groups: normal healthy controls, normal rats receiving Vitis vinifera leaf polyphenol (VLP) extract, AD-induced positive controls, AD-induced rats treated with VLP extract, and AD-induced rats treated with rivastigmine (RIVA). The VLP extract was administered orally at a dose of 100 mg/kg BW daily for 21 days, whereas RIVA was administered at a dose of 0.3 mg/kg BW daily for the same duration. | VLP: Neurorestorative activity. Antiapoptotic activity. Anti-inflammatory activity. Anticholinesterase activity. Antioxidative activity. Anti-amnesic activity. Improves behavioral outcomes in T-maze tests. Reduces brain damage as evidenced by histopathological investigations. | [205] | |
| Anthocyanins Hydroxycinnamic acid derivatives (e.g., caffeic acid) Hydrolyzable tannins (e.g., ellagic acid, quercetin-3-O-glucoside, punicalin) Hydroxybenzoic acids (e.g., gallic acid, protocatechuic acid) Hydroxycyclohexane carboxylic acids (e.g., quinic acid) Hydroxyphenyls (e.g., kaempferol, catechin) | Punica granatum | APPsw/Tg2576 transgenic mice were used alongside wild-type control mice. The experimental groups consisted of wild-type controls on a regular diet, transgenic controls on a regular diet, and transgenic mice fed with a 4% pomegranate-enriched diet. The pomegranate supplementation was administered for 15 months | Pomegranate: Attenuates oxidative damage, as evidenced by decreased lipid peroxidation and protein carbonyl levels. Restores the activities of antioxidant enzymes (superoxide dismutase, catalase, glutathione peroxidase, glutathione, and glutathione S transferase) in the brain. This suggests that the therapeutic potential of pomegranate in the treatment of AD might be associated with its ability to counteract oxidative stress. | [206] | |
| Cranberry extract (CBE) | Vaccinium macrocarpon | Caenorhabditis elegans (C. elegans) transgenic strain CL4176. The polyphenol concentration used was 2 mg/mL of CBE. The administration of CBE was performed through supplementation in the nematode growth medium (NGM) plates. For the preventive treatment, CBE supplementation began from egg hatching and continued until the L3 larval stage, before the induction of Aβ expression via heat-shock. For the therapeutic treatment, CBE was administered starting from the L3 stage after Aβ expression induction. The incubation/administration time varied accordingly: in the preventive treatment, CBE exposure was until the L3 stage prior to Aβ induction, whereas in the therapeutic treatment, it was from L3 stage until paralysis occurred. Both protocols aimed to assess the efficacy of CBE in alleviating Aβ toxicity and its potential preventive versus therapeutic effects in the C. elegans AD model. | CBE: Delays the body paralysis triggered by Aβ toxicity. Reduces Aβ expression at both RNA and protein levels. Improves memory health. | [207] | |
| Dark chocolate (DC) containing 4% total polyphenol content | One of the primary polyphenols in dark chocolate is epicatechin: C15H14O6 | Theobroma cacao | Sprague–Dawley rat pups. Rats were divided into four groups: control rats (Ctrl), control rats treated with dark chocolate (Ctrl Choc), non-transgenic AD (NTAD) model rats induced by monosodium glutamate (MSG), and NTAD rats treated with dark chocolate (MSG Choc). The dark chocolate (DC) used contained 70% cocoa solids and 4% total polyphenol content, administered orally at a dosage of 500 mg/kg BW per day. The polyphenol content in the dark chocolate used in the study was determined to be 20 mg of total polyphenol content (TPC) in 500 mg of dark chocolate | Dark chocolate: Reduces hyperglycemia in the treated rats. Inhibits the cholinesterase activity in the hippocampal tissue homogenates. Enhances cognitive performance in the spatial memory-related Barnes maze task. Increases cell volume in the CA3 region of the hippocampus. Improves cognitive function and cholinergic activity in the hippocampus while correcting metabolic disturbances in aged NTAD rats. | [208] |
| Harrisonia abyssinica | Quercetin (C15H10O) Kaempferol (C15H10O6) Apigenin (C15H10O5) | Harrisonia abyssinica | Male Wistar albino rats. The rats were divided into five groups: a control group, an AlCl:treated group, a rivastigmine-treated group, and two groups treated with different doses of Harrisonia abyssinica extract. AlCl3 was administered at a dose of 100 mg/kg BW per day orally. Rivastigmine was administered at a dose of 0.3 mg/kg BW intraperitoneally. Harrisonia abyssinica extract was administered at two doses: 100 mg/kg BW and 200 mg/kg BW per day by oral gavage. The treatments were administered for a period of 3 weeks. | Harrisonia abyssinica: Improves memory and learning performance as assessed by the passive avoidance test. Reduces hippocampal levels of AChE and extracellular regulated kinase, both of which were elevated by AlCl3. Restores normal levels of neurotransmitters (noradrenaline, dopamine, serotonin) in the brain. Decreases oxidative stress markers, pro-inflammatory cytokines, and apoptotic markers. Prevents Aβ plaque deposition and restoration of normal histological appearance of the hippocampus. | [209] |
| Anthocyanin | Ccyanidin-3-glucosylrutinoside (C27H31O15), Cyanidin-3-rutinoside (C27H31O15), and Cyanidin-3-glucoside (C21H21O11) | Montmorency cherries | 5xFAD transgenic mouse. The study administered a proprietary product, Total Body Rhythm (TBR), composed of tart cherry extract rich in anthocyanins (426.7 μg/mg) and omega fatty acids, to both 6-month-old and 12-month-old mice. The TBR treatment, dosed at 60 mg/kg, was delivered via oral gavage every other day for two months, with a 0.5% methyl-cellulose PBS solution as the vehicle | TBR: Reduces memory deficits in the MWM and NOR tests. Decreases anxiety levels in the OF task primarily in 6-month-old male mice. Protects against neuron loss, reduces activation of astrocytes and microglia primarily in 6-month-old mice, and attenuates Aβ deposition. | [210] |
| Cyanidin 3,5-O-diglucoside Cyanidin 3-O-glucoside Cyanidin 3-O-rutinoside (+)-Catechin (−)-Epicatechin Procyanidin dimer B1, B3, B4, and B5 Procyanidin trimer C1, C2, EEC, and T2 Kaempferol 3,7-O-diglucoside Kaempferol 3-O-(6″-acetyl-galactoside) 7-O-rhamnoside Kaempferol 3-O-galactoside Kaempferol 3-O-galactoside 7-O-rhamnoside | Cyanidin 3,5-O-diglucoside: C27H31O16 Cyanidin 3-O-glucoside: C21H21O11 Cyanidin 3-O-rutinoside: C27H31O15 (+)-Catechin: C15H14O6 (−)-Epicatechin: C15H14O6 Procyanidin dimer B1, B3, B4, and B5: C30H26O12 Procyanidin trimer C1, C2, EEC, and T2: C45H38O18 Kaempferol 3,7-O-diglucoside: C27H30O16 Kaempferol 3-O-(6″-acetyl-galactoside) 7-O-rhamnoside: C29H32O16 Kaempferol 3-O-galactoside: C21H20O11 Kaempferol 3-O-galactoside 7-O-rhamnoside: C27H30O15 | Rosa x hybrida | C. elegans that express human Aβ peptide, a key protein involved in AD. Concentration used: 100 μg/mL. Worms were exposed to the extract in their growth medium. | Rosa x hybrida: Reduces paralysis rate significantly. Significant improvements in mobility were observed at 36, 38, 40, and 42 h post-induction. Increases the chemotactic index, which measures the worms’ ability to move towards a chemical stimulus. No toxicity was observed, as indicated by normal metabolism, reproduction, and lifespan parameters even after exposure to the Rosa x hybrida extract at a concentration of 100 μg/mL. | [211] |
| Cocoa polyphenols | Catechin: C15H14O6 Epicatechin: C15H14O6 Procyanidin B2: C30H26O12 Theobromine: C7H8N4O2 | Theobroma cacao | Caenorhabditis elegans, the specific transgenic strain employed expressed Aβ in neurons, demonstrating middle-age onset behavioral dysfunction similar to human AD. For the polyphenol treatment, a concentration of 5 mg/mL cocoa powder, which contains 27.01 mg GAE/g of total phenolics and 10.13 mg CE/g of total flavonoids, was used. The administration method involved suspending the cocoa powder in M9 buffer with concentrated Escherichia coli OP50, which was then added to NGM plates. The worms were exposed to the cocoa treatment starting from the L1 larval stage and continued daily transfers onto fresh plates to avoid progeny production until they stopped laying eggs around day 9. Samples were collected at three different time points: days 4, 8, and 12, to represent young, middle, and old age, respectively. | Cocoa: Reduces Aβ-induced cognitive deficits. Reduces elevated levels of proline and asparagine in young transgenic worms, which are associated with cognitive deficits. Normalizes hypoxanthine levels in middle-aged transgenic worms, which is linked to memory deficits. Alters urea cycle metabolites, reducing ornithine levels in transgenic worms expressing Aβ, indicating potential modulation of AD pathology. | [212] |
| Ellagic acid Pelargonidin-3-glucoside Chrysanthemin Kaempferol-3-O-D-glucoside Pelargonidin-3-rutinoside | Ellagic acid: C14H6O8 Pelargonidin-3-glucoside: C21H21ClO10 Chrysanthemin: C21H21O11 Kaempferol-3-O-D-glucoside:C21H20O11 Pelargonidin-3-rutinoside: C27H31O14 | Strawberry variety Fragaria × ananassa cv. Romina | Caenorhabditis elegans. The specific strains utilized included N2 Bristol (wild-type), CL4176 (expressing human amyloid β1–42 peptide), and others. Concentrations of 100, 500, and 1000 µg/mL of strawberry methanolic extract were used. Worms were exposed to these concentrations for various durations, depending on the specific assay, ranging from 15 min to several days. | Strawberry: Reduces Aβ-protein induced paralysis. Decreases Aβ aggregation. Prevents oxidative stress. The effects were mediated through the DAF-16/FOXO and SKN-1/NRF2 signaling pathways. | [213] |
| Desmodium elegans: Gallic acid Coumaric acid Chlorogenic acid Caffeic acid p-Coumaroylhexose 3-O-Caffeoylquinic acid 4-O-Caffeoylquinic acid (+)-Catechin Quercetin-3-rutinoside Quercetin-3-O-glucuronide Kaempferol-7-O-glucuronide Isorhamnetin-7-O-glucuronide Isorhamnetin-3-O-rutinoside | Gallic acid: C7H6O5 Coumaric acid: C9H8O3 Chlorogenic acid: C16H18O9 Caffeic acid: C9H8O4 p-Coumaroylhexose: C15H18O8 3-O-Caffeoylquinic acid: C16H18O9 4-O-Caffeoylquinic acid: C16H18O9 (+)-Catechin: C15H14O6 Quercetin-3-rutinoside: C27H30O16 Quercetin-3-O-glucuronide: C21H20O13 Kaempferol-7-O-glucuronide: C21H18O13 Isorhamnetin-7-O-glucuronide: C22H20O13 Isorhamnetin-3-O-rutinoside: C28H32O16 | Desmodium elegans, which was collected from Ganajeer, Malamjaba Swat, Khyber Pakhtunkhwa, Pakistan | Mice. The mice were administered the test sample at a dose of 1 mg/kg per day (i.p.). | Desmodium elegans extracts: Improves cognitive performance, showing anxiolytic effects and enhancement in spatial memory. Improves the escape latency in the shallow water maze test, indicating enhanced memory and learning abilities in the treated mice compared to the disease control group. | [214] |
| Gallic acid Ferulic acid p-Coumaric acid | Gallic acid: C7H6O5 Ferulic acid: C10H10O4 p-Coumaric acid: C9H8O3 | Solanum lycopersicum (tomato), Cenostigma pluviosum, and Peltophorum dubium represented 76.66% of the pollen types sampled | Drosophila melanogaster AD model (AD-like) Drosophila were treated with different concentrations of the methanolic pollen extract (0.1 mg/mL, 0.04 mg/mL, 0.02 mg/mL, and 0.004 mg/mL) over a period of up to 21 days. The administration was carried out using an enriched puree medium refreshed every two days. | Pollen: Enhances climbing ability of Drosophila melanogaster AD-like model flies. Reduces neurodegeneration index in histopathological analysis. Improves survival rate. Exhibits significant antioxidant response. Reduces damage in brain tissue. | [215] |
| Phenolic acids | |||||
| Chlorogenic acid (CGA) | C16H18O9 | Tea, coffee, roasted green beans, berries, cocoa, citrus fruits, apples, and many vegetables | Male albino Swiss mice weighing 25–35 g. The mice received ICV-STZ injections at a concentration of 3 mg/kg on days 1 and 3 to induce SAD. Following the second STZ injection, CGA was administered at a concentration of 5 mg/kg orally, starting 2 h after the second STZ administration and continued daily for 26 days. The administration mode for CGA was oral gavage. | CGA: Alleviates memory deficits induced by ICV-STZ. Protects against an increase in nitrite/nitrate and TBARS levels in the brain. Preserves the number of viable cells in the prefrontal cortex and hippocampus. Prevents the depletion of BDNF in the prefrontal cortex and hippocampus. Mitigates astrogliosis and microgliosis in the prefrontal cortex and hippocampus. Exhibits neuroprotective effects, suggesting its potential as a therapeutic agent in the treatment of SAD. | [216] |
| Curcuminoid | |||||
| Curcumin (Cur) and Nanocurcumin (NC) | C21H20O6 | Curcuma longa | B6SJL-Tg (5xFAD) mice. Cur and NC were dissolved in methanol and diluted with PBS to a final concentration of 50 mg/kg BW. These solutions were injected intraperitoneally once daily for either 2 or 5 days to one-year-old B6SJL-Tg (5xFAD) mice. The concentrations tested ranged from 1 mM to 1 nM to determine the minimum effective dose for labeling Aβ plaques. | Curc: Binds to Aβ plaques. Inhibits misfolded Aβ aggregation Reduces oxidative damage. Decreases Aβ plaques in the brain. NC: Shows enhanced permeability into brain tissue and binds to Aβ plaques more effectively than dietary Cu. Both Cu and NC can label Aβ plaques in postmortem and in vivo brain tissue, suggesting potential for monitoring Aβ plaque load after anti-amyloid therapy. | [217] |
| Polyprenol or isoprenoid alcohol | |||||
| Ropren®, | H-(C5H8)n-OH | Picea abies (L.) Karst | Male Wistar rats, aged 3–4 months and weighing between 180–200 g. The polyphenol being focusing on was Ropren®, a substance containing polyprenols extracted from the green verdure of Picea abies. Ropren® was administered orally at a dosage of 8.6 mg/kg BW daily for 28 days. Additionally, testosterone propionate (TP) was administered subcutaneously at a dose of 0.5 mg/kg BW daily for the same period. The experimental procedure began with the induction of an AD model through the intracerebroventricular injection of A peptide (25–35) at a concentration of 3 μg/μL, followed by a 14-day recovery period. Subsequently, the rats underwent gonadectomy and another 14-day recovery period before starting the daily administration of Ropren®, TP, or oil solvent. | Ropren®: Ameliorates cognitive impairment induced by Aβ (25–35) peptide injection. Shows memory-enhancing effects Increases locomotor activity, rearing and grooming events, and completely restores impaired cognitive performance. Restores testosterone levels in the GDX/Aβ rats. | [218] |
| Lignan | |||||
| Magnolol (MN) | C22H18O11 | Magnolia officinalis | TgCRND8 transgenic mice. Mice were fed with MN at concentrations of 20 mg/kg and 40 mg/kg via oral gavage. Donepezil, a commonly used AD medication, was administered at 5 mg/kg as a positive control. The treatments were carried out daily for a period of four consecutive months. The polyphenol and donepezil were dissolved in 0.5% sodium carboxymethyl cellulose (CMC-Na) for MN and in normal saline for donepezil. | MN: Ameliorates cognitive deficits. Suppresses neuroinflammation and synaptic dysfunction. Inhibits Aβ deposition. Modulates PI3K/Akt/GSK-3β and NF-κB pathways. Improves cognitive function through increased expression of synaptic proteins and anti-inflammatory cytokines. | [219] |
| Ellagitannins | |||||
| Ellagic acid (EA) | C14H6O8 | Berries, nuts, and other fruits | Swiss albino male mice aged 8–12 weeks and weighing 26–30 g. Mice were housed under standard conditions and provided with food and water ad libitum. The mice were randomly divided into three groups: Control (vehicle), STZ-sAD (AD model), and STZ-sAD treated with EA. The sporadic AD model was induced through ICV injection of STZ at a dose of 3 mg/kg BW. EA was administered orally at a dose of 75 mg/kg BW for 28 days. | EA: Reverses the upregulation of AD biomarkers caused by STZ. Improves recognition memory as evidenced by the NORT test. Modulates genes involved in synaptic plasticity, such as AMPAR, and its scaffolding proteins. Reduces oxidative stress and neuronal loss. Enhances antioxidant enzyme activity and reduce lipid peroxidation. Downregulates apoptotic markers. | [220] |
| Polyphenol | Chemical Formula | Plant Origin | Experimental Model | Health Effects | Reference |
|---|---|---|---|---|---|
| Flavonoids | |||||
| Epigallocatechin-3-gallate (EGCG) | C22H18O11 | Camellia sinensis | SweAPP N2a cells transfected with the human APP gene. Cells were treated with various nanoparticle formulations and controls (25 µM: 3 µM) for 18 h. Male Sprague Dawley rats, pre-cannulated and weighing between 200–250 g. Rats were administered 100 mg EGCG/kg BW via oral gavage, with blood samples collected over an 8 h period. | EGCG: Promotes non-amyloidogenic processing of APP by upregulating α-secretase, preventing brain Aβ plaque formation. | [221] |
| Epigallocatechin-3-gallate (EGCG) Luteolin Apigenin Naringenin Diosmin Flavone | EGCG: C22H18O11 Luteolin: C15H10O6 Apigenin: C15H10O5 Naringenin: C15H12O5 Diosmin: C28H32O15 Flavone: C15H10O2 | EGCG: Camellia sinensis Luteolin: Many fruits, vegetables, and medicinal herbs Apigenin: Parsley, celery, and chamomile Naringenin: Citrus fruits Diosmin: Citrus fruits Flavone: Various plants including parsley and celery | N2a cells were stably transfected with the human APP-695 gene harboring the “Swedish” mutation (APPsw) Cells were treated with 1 µM EGCG for 48 h. APP/PS-1 double-mutant transgenic mice. EGCG was administered to these mice at a concentration of 10 mg/mL in their drinking water, resulting in an approximate dose of 37.1 ± 1.6 mg/kg/day. The treatment was carried out over a period of 5.5 months | EGCG: Reduces Aβ levels and plaques. Provides cognitive benefits in AD transgenic mice. Restores mitochondrial respiratory rates, membrane potential, ROS production, and ATP levels in brain mitochondria. Luteolin: Decreases ROS production. Restores mitochondrial membrane potential and ATP levels. Apigenin, naringenin, diosmin, and flavone: Reduce oxidative stress. Restore mitochondrial function including respiratory rates, membrane potential, and ATP levels. | [222] |
| Epigallocatechin (EGC) Epicatechin-3-gallate (ECG) | EGC: C15H14O7 ECG: C22H18O11 | Camellia sinensis | Neuro-2a cells. Cells were treated with Aβ40 and Cu2+/Zn2+-Aβ40 aggregates to induce neurotoxicity. EGC and ECG were used at a concentration of 20 μM, maintaining a ratio of [Aβ40]:[Cu2+/Zn2+]:[EGC/ECG] at 1:2:2 with an incubation time of 24 h. APP/PS1 transgenic mice. Micewere administered ECG intravenously at a dose of 100 mg/kg during a study period of 2 months. | EGC and ECG: Reduce the aggregation of Aβ40 induced by Cu2+ and Zn2+. Inhibit the formation of β-sheet-rich Aβ40 aggregates. Alleviate the Cu2+- and Zn2+-induced neurotoxicity on N2a cells by reducing ROS production. ECG cross the BBB and reduce Aβ plaques in the brains of APP/PS1 mice, protecting neurons from damage. | [223] |
| Trilobatin (TLB) | C21H24O10 | Lithocarpus polystachyus | HT22 hippocampal cells. The cells were treated with various concentrations of TLB, specifically 1, 5, and 10 μM. The incubation time for these treatments was 24 h. C57BL/6J wild-type (WT) mice and 3xFAD transgenic AD mice. Animals were treated with TLB dissolved in saline and administered via gavage. The TLB concentrations used were 10 mg/kg and 20 mg/kg, administered once daily for 12 weeks. | TLB: Protects 3xFAD AD model mice against Aβ burden, neuroinflammation, tau hyperphosphorylation, synaptic degeneration, hippocampal neuronal loss, and memory impairment. Suppresses glial activation by inhibiting the TLR4-MYD88-NFκB pathway, leading to a reduction in inflammatory factors TNF-α, IL-1β, and IL-6. Ameliorates cognitive deficits. Reduces tau and Aβ pathology. Modulates spine plasticity. Protects against neuronal loss, and inhibited gliosis. | [224] |
| Trilobatin (TLB) | C21H24O10 | Lithocarpus polystachyus | BV2 microglial cells BV2 cells were treated with Aβ25-35 to induce an AD model. TLB was administered at concentrations of 12.5, 25, and 50 μM. The treatment period for the experiments involving BV2 cells was not explicitly detailed but was sufficient to measure cell viability and cytotoxicity. Two animal models were used: APP/PS1 transgenic mice. Mice were administered TLB at doses of 4 and 8 mg/kg/day via intragastric (i.g.) administration for a period of 3 months. Rats subjected to intracerebroventricular (ICV) injection of Aβ25-35: These rats were subsequently administered TLB at doses of 2.5, 5, and 10 mg/kg/day via i.g. administration for 14 days. | TLB: Improves cognitive deficits in both animal models. Ameliorates neuroinflammation and oxidative stress by inhibiting the HMGB1/TLR4/NF-κB signaling pathway and activating the SIRT3/SOD2 pathway, which helps to restore redox homeostasis and suppress neuroinflammation. | [225] |
| Curcuminoid | |||||
| Curcumin | C21H20O6 | Curcuma longa | Differentiated SK-N-SH human neuroblastoma cells. Cells were treated with varying doses of NanoCurc™ at concentrations of 250 nM, 500 nM, 1 μM, 2.5 μM, and 5 μM. These cells were co-treated with 100 μM H2O2 simultaneously for 24 h. Athymic mice Mice were administered NanoCurc™ intraperitoneally at a dose of 25 mg/kg twice daily for four weeks. | Curcumin: Reduces oxidative damage and amyloid pathology in AD models. Shows anti-inflammatory properties, inhibits pro-inflammatory transcription factors, and may disaggregate Aβ plaques, reducing neuroinflammation and protecting against AD progression. NanoCurc™: Protects neuronally differentiated human SK-N-SH cells from ROS (H2O2) mediates insults and rescues cells previously insulted with H2O2. In vivo, decreases levels of H2O2, caspase 3, and caspase 7 activities in the brain, and increases glutathione concentrations. | [226] |
| Anthocyanins and Related Compounds | |||||
| Delphinidin 3-galactoside (Del) and cyanidin 3-galactoside (Cya) | Del: C21H21O12 Cya: C21H21O11 | Vaccinium myrtillus | Mouse neuroblastoma Neuro2a cells. The cells were exposed to Aβ samples incubated with or without VMA for either 24 or 48 h. Specifically, Aβ1–40 solutions were incubated at 37 °C for either 48 or 96 h, and Aβ1–42 solutions were incubated for either 24 or 48 h before being added to the cell cultures. The VMA concentrations used in the cell culture studies were typically prepared at a 2.5-fold molar ratio relative to the Aβ peptides. The incubation times varied depending on the specific assay but generally included intervals up to 96 h for Aβ1–40 and 48 h for Aβ1–42 Double-transgenic mice expressing human APP with the Swedish mutation (K670N/M671L) and human presenilin-2 proteins containing the N141I mutation. The DT mice were fed a diet supplemented with 1% VMA. The administration started at 60 days after birth and continued throughout the study period. Additionally, a lower concentration of 0.25% VMA was also tested in some groups of DT mice. | VMA Prevents cognitive degeneration in AD mice. However, this cognitive improvement was paradoxically associated with an increase in insoluble Aβ deposits in the brain, suggesting that VMA might promote the formation of non-toxic Aβ aggregates, diverting them from forming toxic species. | [227] |
| Myricetin, Quercetin, and Anthocyanin-rich extracts | Myricetin: C15H10O8 Quercetin: C15H10O7 | The polyphenols were derived from blackcurrants and bilberries | Human SH-SY5Y neuroblastoma cells stably overexpressing the APP751 isoform. Myricetin: 2 and 20 μM Quercetin: 10 μM Anthocyanin-rich extracts: 8 and 31 /mLμg/mL. Incubation time: 24 h for normal growth conditions and assessments of viability and APP processing, 60 min for ROS measurement under menadione-induced stress conditions. Male APdE9 mice and age-matched wild-type littermates. Bilberry extract: 1.53 mg/g. Blackcurrant extract: 1.43 mg/g | Bilberry and blackcurrant anthocyanin-rich extracts: Decrease APP C-terminal fragments levels Alleviate spatial working memory deficits and hyperactivity in the APdE9 mouse model of AD. These findings suggest that dietary polyphenols from bilberries and blackcurrants might have potential benefits in modulating neurodegenerative processes associated with AD. | [228] |
| Mix of polyphenols | |||||
| Grape Seed Polyphenol Extract (GSPE) Resveratrol | Resveratrol: C14H12O3 | GSPE: Derived from grape seeds. Resveratrol: Found in grapes and wine. | Paired helical filaments (PHFs) isolated from AD brains. PHFs were treated with 100 µM GSPE for varying incubation times, ranging from 5 s to 24 h, with a standard incubation time of 1 h generally used to ensure robust results. TMHT mouse model of tauopathy. GSPE at concentrations ranging from 1 µM to 100 µM, and resveratrol at a concentration of 100 µM, was administered to the mice at a daily dose of 200 mg/kg BW for the duration of the animal experiment, which lasted four weeks. | GSPE: Disrupts and disintegrates the ultrastructure of native PHFs in AD brain. Inhibits tau aggregation and promotes dissociation from already assembled filaments. Significantly inhibits tau-mediated neuropathology in the TMHT mouse model. Suggested as a potential therapeutic agent for tau-mediated neurodegenerative conditions, like AD. Resveratrol: Ineffective in altering the ultrastructure of PHFs as compared to GSPE. Not effective in inducing width expansion of filaments. | [229] |
| Punicalagin and ellagic acid, both components of pomegranate extract (POMx) | Punicalagin: C48H28O30 Ellagic Acid: C14H6O8 | Punica granatum | HeLa/NFAT stable cell line. Polyphenol concentration: 0.1 mmol/L to 100 mmol/L for both punicalagin and ellagic acid. Incubation time: 30 min pre-treatment followed by 6 h of stimulation with TPA and ionomycin. Male C57BL/6 APPswe/PS1dE9 transgenic mice. POMx at 6.25 mL/L in drinking water. Oral administration via drinking water for 3 months. | POMx: Improves behavioral performance. Reduces microgliosis. Decreases NFAT activity and lowers TNF-α concentrations in the brains of the APP/PS1 mice. These effects suggest that POMx has anti-inflammatory properties that may attenuate the progression of AD by reducing neuroinflammation and improving cognitive functions. | [230] |
| 3-O-quercetin glucopyranoside Rutin 3-O-Quercetin rhamnopyranoside Chlorogenic acid | 3-O-Quercetin Glucopyranoside (Isoquercetin): C21H20O12 Rutin (Quercetin-3-rutinoside): C27H30O16 Ursolic Acid: C30H48O3 Oleanolic Acid: C30H48O3 3-O-Quercetin Rhamnopyranoside (Quercitrin): C21H20O11 Chlorogenic Acid: C16H18O9 Scopoletin: C10H8O4 | Bouvardia ternifolia (BtHA) | Human neuroblastoma cell line SH-SY5Y. Cells were treated with various concentrations of fibrillar Aβ (Ab1-42) peptide in the presence of BtHA or its specific fractions. The primary bioactive constituents of BtHA included 3-O-quercetin glucopyranoside (415 mg/g), rutin (229.9 mg/g), ursolic acid (54 mg/g), oleanolic acid (20.8 mg/g), 3-O-quercetin rhamnopyranoside (12.8 mg/g), chlorogenic acid (9.5 mg/g), and scopoletin (1.38 mg/g). Male ICR-strain mice were employed to assess the anti-inflammatory activity using the TPA-induced ear edema assay. The BtHA extract and its fractions were administered topically at a dose of 6.5 mg per ear. The extract and fractions were applied 15 min after TPA treatment. | The study found that Bouvardia ternifolia extract exhibited significant neuroprotection against Aβ peptide, with anti-inflammatory, antioxidant, and anti-acetylcholinesterase effects. These effects are attributed to its content of polyphenols, coumarins, and triterpenes, suggesting potential as a therapeutic agent in the treatment of AD | [231] |
| Proanthocyanidins Hecogenin Ferulic acid Catechin Gallic acid Epicatechin Epicatechin gallate | Ferulic acid: C10H10O4 Catechin: C15H14O6 Gallic acid: C7H6O5 Epicatechin: C15H14O6 | Fagopyrum dibotrys (FDE) | SH-SY5Y cells. Cells were treated with FDE at concentrations of 2.5 mg/mL, 5 mg/mL, and 10 mg/mL. The cells were incubated with these concentrations for 24 h to assess the neuroprotective effects against Aβ-induced toxicity. APP/PS1 transgenic mice. Mice were administered a diet containing 0.65% FDE for a period of nine months. The amount of FDE consumed by the mice was approximately 0.103 mg/kg/day, ensuring consistent and long-term exposure to the extract. | FDE: Cleans Aβ deposits in the brain. Decreases Aβ burden in the plasma. Inhibits microhemorrhage. Reduces reactive microglia. Promotes Aβ fibrils disaggregation. Inhibits neurotoxicity induced by Aβ in SH-SY5Y cells. | [232] |
| Caffeic acid Trans-ferulic acid Isorhamnetin Irilin B | Caffeic Acid: C9H8O4 Trans-Ferulic Acid: C10H10O4 Acanthoside B: C34H44O20 Isorhamnetin: C16H12O7 Irilin B: (requires specific details for accurate formula) | Salicornia europaea L. | BV-2 microglial cells. The cells were pre-treated with SE-EE at concentrations of 20, 100, and 200 µg/mL for 1 h, followed by LPS stimulation (200 ng/mL) for either 6 or 24 h. Male C57BL/6N mice, aged 8–9 weeks and weighing 24–27 g, were used. The mice were divided into five groups, receiving either 0.9% saline, scopolamine (2 mg/kg), SE-EE (50 or 100 mg/kg), or tacrine (10 mg/kg). SE-EE and tacrine were administered orally for 7 days prior to scopolamine injection. The total phenolic content in SE-EE was measured as 51.29 mg gallic acid equivalent per gram of sample, and the flavonoid content was 19.87 mg rutin equivalent per gram of sample. | SE-EE: Dose-dependently attenuates LPS-induced inflammation in BV-2 cell Improves cognitive function in scopolamine-induced amnesic mice by suppressing oxidative stress markers, regulating inflammatory cytokines and associated proteins expression, ameliorating p-CREB/BDNF levels, and promoting neurogenesis and neuron proliferation. | [233] |
| Caffeic acid Quercetin derivatives (Quercetin-exoside-rhamnoside, Quercetin-dirhamnoside) Kaempferol derivatives (Kaempferol-3-O-glucoside-7-O-rhamnoside, Kaempferol-3,7-O-dirhamnoside) Isorhamnetin-hexoside-rhamnoside Sinapic acid Luteolin Di-O-sinapoyl-β-glucose isomers | Caffeic acid: C9H8O4 Quercetin-rhamnoside-hexoside: C27H30O16 Kaempferol-3-O-glucoside-7-O-rhamnoside: C27H30O15 Quercetin-dirhamnoside: C27H30O15 Isorhamnetin-hexoside-rhamnoside: C28H32O16 Kaempferol-3,7-di-O-rhamnoside: C27H30O14 Sinapic acid: C11H12O5 Di-O-sinapoyl-β-glucose isomers: C28H32O14 Luteolin: C15H10O6 | Arabidopsis thaliana | BV2 murine microglial cells. Cells were treated with 25 µM of aggregated Aβ25-35 peptide in the presence or absence of a polyphenolic extract from Arabidopsis thaliana. The extract was administered at a concentration of 20 µL/mL, derived from seedlings grown for seven days and cold-pressed to preserve the polyphenolic content. The incubation times for the treatments were set at 2 and 24 h. Drosophila melanogaster flies expressing human Aβ1–42 were employed. The flies were fed a diet supplemented with 40 µL/mL of the Arabidopsis extract throughout their developmental period. | Arabidopsis thaliana: Has significant anti-inflammatory effects both in vitro and in vivo. In BV2 cells, the extract reduces the expression of pro-inflammatory cytokines (IL-6, IL-1β, TNF-α) and increases the expression of anti-inflammatory cytokines (IL-4, IL-10, IL-13). These effects were observed after 24 h of treatment, indicating a robust anti-inflammatory response. The extract also activated the Nrf2-antioxidant response element signaling pathway, leading to upregulation of heme oxygenase-1 (HO-1) mRNA and increased NAD(P)H oxidoreductase 1 (NQO1) activity, which are indicators of enhanced cellular antioxidant defenses. In the Drosophila model, the polyphenolic extract significantly improves the impaired climbing ability of the AD flies expressing human Aβ1–42, confirming its neuroprotective effects in vivo. This suggests that the extract could mitigate the neurotoxic effects of Aβ, potentially offering a protective strategy against neurodegenerative diseases, like AD. These results highlight the potential of Arabidopsis thaliana polyphenolic extract as a therapeutic agent with anti-inflammatory and neuroprotective properties, warranting further investigation in more complex organisms to fully understand its impact on neuroinflammation and neurodegeneration. | [234] |
| Feruloylquinic acid p-Coumaroylquinic acid Rutin Quercetin-3(6″malonyl)-neohesperioside B-type procyanidin dimers Chlorogenic acids (CGAs) Flavan-3-ol glycosides Procyanidins | Feruloylquinic acid: C17H20O9 p-Coumaroylquinic acid: C16H18O8 Rutin: C27H30O16 Quercetin-3(6″malonyl)-neohesperioside: C29H34O17 Procyanidins: (C15H14O6)n Chlorogenic acid: C16H18O9 | Humulus lupulus L. | Human neuroblastoma SH-SY5Y cells. Cells were treated with different concentrations of hop extracts or specific polyphenolic fractions. The polyphenol concentration used was 0.25 mg/mL for the total extract and concentrations varied for different fractions (e.g., 0.03 mg/mL for fraction B). The administration mode was direct incubation with the cell culture medium. For the cytotoxicity assay, SH-SY5Y cells were incubated with Aβ1–42 and hop extracts for 24 h. Caenorhabditis elegans model (CL2006) expressing human Aβ3-42. The nematodes were treated with hop extracts at concentrations ranging from 10 to 250 /mLμg/mL. The administration mode was oral feeding, and the incubation/administration time was 120 h. | Humulus lupulus: Prevents Aβ1–42 aggregation and cytotoxicity. Exhibits antioxidant properties. Enhances autophagy, promoting the clearance of misfolded and aggregated proteins in SH-SY5Y cells. In vivo efficacy in reducing Aβ-induced toxicity in C. elegans models. | [235] |
| Polyphenol-rich fungi | Phellinus linteus, Ganoderma lucidum, and Inonotus obliquus | PC12 cells. These cells were treated with Zn2+ to induce Aβ aggregation. The cells were pretreated with different concentrations of the aqueous extract of mixed medicinal mushroom mycelia (MMMM) at 10 µg/mL (MMMM-L) and 100 µg/mL (MMMM-H) for 16 h before being exposed to 50 µM Zn2+ for 3 h. 5xAD transgenic mice. These mice were orally administered 30 mg/kg/day of MMMM for 8 weeks. Behavioral tests, including the Y-maze test (Y-MT) and the passive avoidance test (PAT), were conducted to assess memory function. | MMMM: In PC12 cells, MMMM treatment reduces Aβ aggregation, oxidative stress, and apoptosis while enhancing cell viability and antioxidant enzyme activity. In 5xFAD mice, MMMM treatment ameliorates memory impairments, reduced Aβ plaque accumulation, and decreased neuroinflammation in the hippocampus. | [236] | |
| Hydroxycinnamic acids | |||||
| Rosmarinic Acid (RA) | C18H16O8 | Lamiaceae family, including rosemary and lemon balm. | BBB model using brain capillary endothelial cell monolayers. The experiments included controls, like caffeine and sodium fluorescein, to establish permeability benchmarks. For the permeability assay, the cells were incubated with RA, and permeability was measured over a period of 20 min at intervals. 5-month-old female Tg2576 mice (AD model) and 7–10-week-old female C57BL/6J mice. The Tg2576 mice were divided into control and RA-fed groups for a period of 10 months. The C57BL/6J mice were divided into a control group and a group fed with a diet containing 0.5% RA for 7 weeks. Tg2576 mice: RA was included in the diet at an unspecified concentration for 10 months. C57BL/6J mice: 0.5% RA was added to the diet and administered for 7 weeks. The administration was oral through dietary inclusion. | RA: Increases the concentration of monoamines (dopamine, norepinephrine, 3,4-dihydroxyphenylacetic acid, and levodopa) in the cerebral cortex of mice. Downregulates the expression of MAO B in the substantia nigra and ventral tegmental area, regions involved in dopamine synthesis. These changes were linked to a suppression of Aβ aggregation in the brains of the AD model mice, suggesting that RA has a potential therapeutic effect against AD by enhancing the dopamine-signaling pathway and inhibiting Aβ aggregation. | [237] |
| p-Coumaric acid | C9H8O3 | Many fruits, vegetables, and cereals | PC12 neuronal cells. The concentration of pCA used was 50 µg/mL, and the cells were incubated for three days with Aβ42 (10 µM) at 37 °C. Drosophila melanogaster (fruit fly). Various concentrations of pCA (50 µM, 100 µM, 500 µM, and 1000 µM) were administered through feeding. The administration was performed using a capillary feeder assay, and the effects were observed over a period corresponding to the lifespan of the flies, with specific attention paid to survival rate and locomotive ability. | p-Coumaric acid: Reduces Aβ42 fibrillation and decreases Aβ42-induced cell mortality in PC12 neuronal cells. In the Drosophila AD model, p-Coumaric acid partially reverses the rough eye phenotype, significantly lengthened lifespan, and enhanced mobility in a sex-dependent manner. | [238] |
| Tannins | |||||
| Tannic Acid (TA) | C76H52O46 | TA is a plant-derived hydrolyzable tannin polyphenol found in numerous herbaceous and woody plants. | SweAPP N2a cells. These cells were treated with varying concentrations of TA (1.563, 3.125, 6.25, 12.5, or 25 μM) for 12 h to evaluate the effects on Aβ production and APP metabolism. Transgenic PSAPP mouse model. These mice were orally administered TA at a concentration of 30 mg/kg/day via gavage for 6 months. The study assessed cognitive function and AD-like pathology in these mice, including behavioral impairments, brain amyloid deposits, and neuroinflammation. | TA: Prevents behavioral impairments, such as hyperactivity, decreased object recognition, and defective spatial reference memory. reduces brain parenchymal and cerebral vascular amyloid deposits, and mitigates neuroinflammation. Decreases the production of various Aβ species, including oligomers. These effects were linked to the inhibition of BACE1 expression and activity, and a shift towards non-amyloidogenic APP processing pathways, suggesting that TA might serve as a potential prophylactic treatment for AD. | [239] |
| Xanthones | |||||
| Alpha-mangostin | C24H26O6 | Garcinia mangostana | BV-2 microglial cells, Chang liver cells, and bEnd.3 mouse brain endothelial cells. Cells were treated with α-mangostin (α-M) or its nanoparticle formulation (NP(α-M)). The concentrations used were 25, 50, and 100 ng/mL for BV-2 cells and 50, 500, and 1000 ng/mL for Chang liver cells. These cells were treated for 24 h, followed by incubation with 2 μg/mL Aβ 1-42 (Aβ1–42) in serum-free DMEM for an additional 24 h. Male SAMP8 mice, female APP/PS1 transgenic mice, and Kun-Ming mice. Animals were administered intravenous injections of either α-M solution or NP(α-M) at a dosage of 1 mg/kg/day over a period of four weeks. | α-mangostin: Upregulates LDLR expression. Increases cellular uptake and degradation of Aβ1–42. Enhances brain clearance of Aβ1–42. Reduces Aβ deposition. Attenuates neuroinflammatory responses. Ameliorates neurologic changes. Reverses behavioral deficits in AD model mice. | [240] |
| Avenanthramides | |||||
| Avenanthramide-C (Avn-C) | C17H15NO4 | Avena sativa | Hippocampal slices prepared from wild-type (C57BL/6J) and AD transgenic mice (Tg2576 and 5xFAD). The slices were treated with different concentrations of Avn-C (specifically 50 µM) and exposed to 0.5 µM oligomeric Aβ42 to examine the effects on long-term potentiation (LTP). The slices were incubated in artificial cerebrospinal fluid (aCSF) perfused with a gas mixture of 95% O2 and 5% CO2 at room temperature. The treatment duration for Avn-C was 2 h, with a 0.5-h pretreatment period before the addition of Aβ42 Wild-type C57BL/6J mice, Tg2576, and 5xFAD transgenic mouse models of AD. Male mice aged 7–8 months for Tg2576 and 5–6 months for 5xFAD Avn-C was administered orally at a concentration of 6 mg/kg per day for a period of 2 weeks. | Avn-C: Improves memory and cognitive functions in the transgenic mouse models of AD. Reverses the impaired LTP in both ex vivo- and in vivo-treated AD mice hippocampus. Improves recognition and spatial memory. Reduces caspase-3 cleavage. Reverses neuroinflammation. Increases levels of phosphorylated glycogen synthase kinase-3β (pS9GSK-3β) and IL-10. These beneficial effects are mediated through the binding of Avn-C to α1A adrenergic receptors, stimulating AMPK. | [241] |
4.3. Preclinical Discoveries: Polyphenol Activity in Alzheimer’s Models
4.4. Preclinical Discoveries: Olive-Derived Polyphenol Activity in Alzheimer’s Models
4.5. Clinical Trials
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gómez-Cruz, I.; Del Mar Contreras, M.; Romero, I.; Castro, E. Towards the Integral Valorization of Olive Pomace-Derived Biomasses through Biorefinery Strategies. ChemBioEng Rev. 2024, 11, 253–277. [Google Scholar] [CrossRef]
- Enaime, G.; Dababat, S.; Wichern, M.; Lübken, M. Olive mill wastes: From wastes to resources. Environ. Sci. Pollut. Res. 2024, 31, 20853–20880. [Google Scholar] [CrossRef] [PubMed]
- International Olive Council. IOC—STATISTICS. 2023. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2022/12/IOC-Olive-Oil-Dashboard-2.html#production-2data (accessed on 1 August 2024).
- Garriga, J.M. Production Costs and Drought Are Affecting Spain’s Agrifood Sector, Agrifood Sector. Available online: https://www.caixabankresearch.com/en/sector-analysis/agrifood/production-costs-and-drought-are-affecting-spains-agrifood-sector (accessed on 1 October 2024).
- International Olive Oil Council. Available online: https://www.internationaloliveoil.org/wp-content/uploads/2023/12/HO-CE901-13-12-2023-P.pdf (accessed on 8 October 2024).
- Khwaldia, K.; Attour, N.; Matthes, J.; Beck, L.; Schmid, M. Olive byproducts and their bioactive compounds as a valuable source for food packaging applications. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1218–1253. [Google Scholar] [CrossRef]
- Sebastian, S.A.; Padda, I.; Johal, G. Long-term impact of mediterranean diet on cardiovascular disease prevention: A systematic review and meta-analysis of randomized controlled trials. Curr. Probl. Cardiol. 2024, 49, 102509. [Google Scholar] [CrossRef] [PubMed]
- Nucci, D.; Sommariva, A.; Degoni, L.M.; Gallo, G.; Mancarella, M.; Natarelli, F.; Savoia, A.; Catalini, A.; Ferranti, R.; Pregliasco, F.E.; et al. Association between Mediterranean diet and dementia and Alzheimer disease: A systematic review with meta-analysis. Aging Clin. Exp. Res. 2024, 36, 77. [Google Scholar] [CrossRef]
- Filardo, S.; Roberto, M.; Di Risola, D.; Mosca, L.; Di Pietro, M.; Sessa, R. Olea europaea L-derived secoiridoids: Beneficial health effects and potential therapeutic approaches. Pharmacol. Ther. 2024, 254, 108595. [Google Scholar] [CrossRef]
- Amiot, M.-J.; Fleuriet, A.; Macheix, J.-J. Accumulation of oleuropein derivatives during olive maturation. Phytochemistry 1989, 28, 67–69. [Google Scholar] [CrossRef]
- Fernandes, S.; Ribeiro, C.; Paiva-Martins, F.; Catarino, C.; Santos-Silva, A. Protective effect of olive oil polyphenol phase II sulfate conjugates on erythrocyte oxidative-induced hemolysis. Food Funct. 2020, 11, 8670–8679. [Google Scholar] [CrossRef]
- Costa, V.; Costa, M.; Videira, R.A.; Andrade, P.B.; Paiva-Martins, F. Anti-Inflammatory Activity of Olive Oil Polyphenols—The Role of Oleacein and Its Metabolites. Biomedicines 2022, 10, 2990. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: Metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Kaddoumi, A. Extra-Virgin Olive Oil in Alzheimer’s Disease: A Comprehensive Review of Cellular, Animal, and Clinical Studies. Int. J. Mol. Sci. 2024, 25, 1914. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S. Olive Oil Phenols as Promising Multi-targeting Agents Against Alzheimer’s Disease. Adv. Exp. Med. Biol. 2015, 863, 1–20. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Association, A. Alzheimer’s disease facts and figures. Alzheimer’s Dement 2019, 15, 321–387. [Google Scholar]
- Segade, M.; Bermejo, R.; Silva, A.; Paiva-Martins, F.; Gil-Longo, J.; Campos-Toimil, M. Involvement of endothelium in the vasorelaxant effects of 3,4-DHPEA-EA and 3,4-DHPEA-EDA, two major functional bioactives in olive oil. J. Funct. Foods 2016, 23, 637–646. [Google Scholar] [CrossRef]
- Romero-Márquez, J.M.; Navarro-Hortal, M.D.; Jiménez-Trigo, V.; Muñoz-Ollero, P.; Forbes-Hernández, T.Y.; Esteban-Muñoz, A.; Giampieri, F.; Delgado Noya, I.; Bullón, P.; Vera-Ramírez, L.; et al. An Olive-Derived Extract 20% Rich in Hydroxytyrosol Prevents β-Amyloid Aggregation and Oxidative Stress, Two Features of Alzheimer Disease, via SKN-1/NRF2 and HSP-16.2 in Caenorhabditis elegans. Antioxidants 2022, 11, 629. [Google Scholar] [CrossRef]
- Costa, M.; Paiva-Martins, F. Olive oil phenolic compounds as antioxidants in functional foods: Description, sources and stability. In Lipid Oxidation in Food and Biological Systems: A Physical Chemistry Perspective; Springer: Berlin/Heidelberg, Germany, 2022; pp. 427–453. [Google Scholar]
- Costa, M.; Costa, V.; Lopes, M.; Paiva-Martins, F. A biochemical perspective on the fate of virgin olive oil phenolic compounds in vivo. Crit. Rev. Food Sci. Nutr. 2024, 64, 1403–1428. [Google Scholar] [CrossRef]
- Jurišić Grubešić, R.; Nazlić, M.; Miletić, T.; Vuko, E.; Vuletić, N.; Ljubenkov, I.; Dunkić, V. Antioxidant Capacity of Free Volatile Compounds from Olea europaea L. cv. Oblica Leaves Depending on the Vegetation Stage. Antioxidants 2021, 10, 1832. [Google Scholar] [CrossRef]
- Salvador, M.D.; Aranda, F.; Gómez-Alonso, S.; Fregapane, G. Influence of extraction system, production year and area on Cornicabra virgin olive oil: A study of five crop seasons. Food Chem. 2003, 80, 359–366. [Google Scholar] [CrossRef]
- Termentzi, A.; Halabalaki, M.; Skaltsounis, A.L. 6—From Drupes to Olive Oil: An Exploration of Olive Key Metabolites. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2015; pp. 147–177. [Google Scholar]
- Khlif, I.; Jellali, K.; Michel, T.; Halabalaki, M.; Skaltsounis, A.L.; Allouche, N. Characteristics, Phytochemical Analysis and Biological Activities of Extracts from Tunisian Chetoui Olea europaea Variety. J. Chem. 2015, 2015, 418731. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De la Rosa, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT—Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Cecchi, L.; Migliorini, M.; Cherubini, C.; Innocenti, M.; Mulinacci, N. Whole Lyophilized Olives as Sources of Unexpectedly High Amounts of Secoiridoids: The Case of Three Tuscan Cultivars. J. Agric. Food Chem. 2015, 63, 1175–1185. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Gomes, L.; Leitão, F.; Bronze, M.; Coelho, A.V.; Boas, L.V. Secoiridoids in olive seed: Characterization of nüzhenide and 11-methyl oleosides by liquid chromatography with diode array and mass spectrometry. Grasas Aceites 2010, 61, 157–164. [Google Scholar] [CrossRef]
- Abu-Izneid, T.; Rauf, A.; Shariati, M.A.; Khalil, A.A.; Imran, M.; Rebezov, M.; Uddin, M.S.; Mahomoodally, M.F.; Rengasamy, K.R.R. Sesquiterpenes and their derivatives-natural anticancer compounds: An update. Pharmacol. Res. 2020, 161, 105165. [Google Scholar] [CrossRef]
- Frangipane, M.T.; Cecchini, M.; Massantini, R.; Monarca, D. Extra Virgin Olive Oil from Destoned Fruits to Improve the Quality of the Oil and Environmental Sustainability. Foods 2022, 11, 1479. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.M.; Maestri, D.M. The effects of genotype and extraction methods on chemical composition of virgin olive oils from Traslasierra Valley (Córdoba, Argentina). Food Chem. 2006, 96, 507–511. [Google Scholar] [CrossRef]
- Soares, T.F.; Alves, R.C.; Oliveira, M.B.P.P. From Olive Oil Production to By-Products: Emergent Technologies to Extract Bioactive Compounds. Food Rev. Int. 2024, 1–28. [Google Scholar] [CrossRef]
- Doula, M.K.; Moreno-Ortego, J.L.; Tinivella, F.; Inglezakis, V.J.; Sarris, A.; Komnitsas, K. Chapter 2—Olive mill waste: Recent advances for the sustainable development of olive oil industry. In Olive Mill Waste; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 29–56. [Google Scholar]
- Abbattista, R.; Ventura, G.; Calvano, C.D.; Cataldi, T.R.I.; Losito, I. Bioactive Compounds in Waste By-Products from Olive Oil Production: Applications and Structural Characterization by Mass Spectrometry Techniques. Foods 2021, 10, 1236. [Google Scholar] [CrossRef]
- Moreno, A.D.; Ballesteros, M.; Negro, M.J. 5—Biorefineries for the valorization of food processing waste. In The Interaction of Food Industry and Environment; Galanakis, C., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 155–190. [Google Scholar]
- Kapellakis, I.E.; Tsagarakis, K.P.; Crowther, J.C. Olive oil history, production and by-product management. Rev. Environ. Sci. Bio/Technol. 2008, 7, 1–26. [Google Scholar] [CrossRef]
- Obied, H.K.; Prenzler, P.D.; Robards, K. Potent antioxidant biophenols from olive mill waste. Food Chem. 2008, 111, 171–178. [Google Scholar] [CrossRef]
- Agabo-García, C.; Repetto, G.; Albqmi, M.; Hodaifa, G. Evaluation of the olive mill wastewater treatment based on advanced oxidation processes (AOPs), flocculation, and filtration. J. Environ. Chem. Eng. 2023, 11, 109789. [Google Scholar] [CrossRef]
- Muscolo, A.; Papalia, T.; Settineri, G.; Romeo, F.; Mallamaci, C. Three different methods for turning olive pomace in resource: Benefits of the end products for agricultural purpose. Sci. Total Environ. 2019, 662, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Expósito-Díaz, A.; Miho, H.; Ledesma-Escobar, C.A.; Moral, J.; Díez, C.M.; Priego-Capote, F. Influence of genetic and interannual factors on bioactive compounds of olive pomace determined through a germplasm survey. Food Chem. 2022, 378, 132107. [Google Scholar] [CrossRef]
- Zhao, H.; Kim, Y.; Avena-Bustillos, R.J.; Nitin, N.; Wang, S.C. Characterization of California olive pomace fractions and their in vitro antioxidant and antimicrobial activities. LWT 2023, 180, 114677. [Google Scholar] [CrossRef]
- Freitas, L.; Simões, R.; Miranda, I.; Peres, F.; Ferreira-Dias, S. Optimization of Autohydrolysis of Olive Pomaces to Obtain Bioactive Oligosaccharides: The Effect of Cultivar and Fruit Ripening. Catalysts 2022, 12, 788. [Google Scholar] [CrossRef]
- Antónia Nunes, M.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive pomace as a valuable source of bioactive compounds: A study regarding its lipid- and water-soluble components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef]
- Messad, S.; Bensmail, S.; Salhi, O.; Djouahra Fahem, D. Effect of Extraction Method on Organoleptic, Physicochemical Properties and Some Biological Activities of Olive Oil from the Algerian Chemlal Variety. Eur. J. Biol. 2022, 81, 58–67. [Google Scholar] [CrossRef]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Pimentel, F.B.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Olive by-products for functional and food applications: Challenging opportunities to face environmental constraints. Innov. Food Sci. Emerg. Technol. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic compounds from olive mill wastes: Health effects, analytical approach and application as food antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Skaltsounis, A.-L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. 11—Recovery of High Added Value Compounds from Olive Tree Products and Olive Processing Byproducts. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Champaign, IL, USA, 2015; pp. 333–356. [Google Scholar]
- Romeu, M.F.C.; Bernardo, J.; Daniel, C.I.; Costa, N.; Crespo, J.G.; Silva Pinto, L.; Nunes Da Ponte, M.; Nunes, A.V.M. Hydroxytyrosol recovery from olive pomace: A simple process using olive mill industrial equipment and membrane technology. J. Food Sci. Technol. 2024, 61, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Cayuela, M.L.; Millner, P.D.; Meyer, S.L.F.; Roig, A. Potential of olive mill waste and compost as biobased pesticides against weeds, fungi, and nematodes. Sci. Total Environ. 2008, 399, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.; Bakhouche, A.; Lozano-Sánchez, J.s.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Literature review on production process to obtain extra virgin olive oil enriched in bioactive compounds. Potential use of byproducts as alternative sources of polyphenols. J. Agric. Food Chem. 2013, 61, 5179–5188. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Kamboj, M.; Ajlouni, S.; Fang, Z. Incorporation of salmon bone gelatine with chitosan, gallic acid and clove oil as edible coating for the cold storage of fresh salmon fillet. Food Control 2021, 125, 107994. [Google Scholar] [CrossRef]
- Cifuentes-Cabezas, M.; Galinha, C.F.; Crespo, J.G.; Cinta Vincent-Vela, M.; Antonio Mendoza-Roca, J.; Álvarez-Blanco, S. Nanofiltration of wastewaters from olive oil production: Study of operating conditions and analysis of fouling by 2D fluorescence and FTIR spectroscopy. Chem. Eng. J. 2023, 454, 140025. [Google Scholar] [CrossRef]
- Huertas-Alonso, A.J.; Gonzalez-Serrano, D.J.; Hadidi, M.; Salgado-Ramos, M.; Orellana-Palacios, J.C.; Sánchez-Verdú, M.P.; Xia, Q.; Simirgiotis, M.J.; Barba, F.J.; Dar, B.N.; et al. Table Olive Wastewater as a Potential Source of Biophenols for Valorization: A Mini Review. Fermentation 2022, 8, 215. [Google Scholar] [CrossRef]
- Shabir, S.; Ilyas, N.; Saeed, M.; Bibi, F.; Sayyed, R.Z.; Almalki, W.H. Treatment technologies for olive mill wastewater with impacts on plants. Environ. Res. 2023, 216, 114399. [Google Scholar] [CrossRef] [PubMed]
- Bombino, G.; Andiloro, S.; Folino, A.; Lucas-Borja, M.E.; Zema, D.A. Short-term effects of olive oil mill wastewater application on soil water repellency. Agric. Water Manag. 2021, 244, 106563. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Saadaoui, N.; Kiai, H.; Raiti, J.; Hafidi, A. Potential applications of olive mill wastewater as biopesticide for crops protection. Sci. Total Environ. 2017, 576, 10–21. [Google Scholar] [CrossRef]
- Daâssi, D.; Lozano-Sánchez, J.; Borrás-Linares, I.; Belbahri, L.; Woodward, S.; Zouari-Mechichi, H.; Mechichi, T.; Nasri, M.; Segura-Carretero, A. Olive oil mill wastewaters: Phenolic content characterization during degradation by Coriolopsis gallica. Chemosphere 2014, 113, 62–70. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Zyad, A.; Amechrouq, A.; Oukerrou, M.A.; Mouse, H.A.; Mbarki, M. Identification and characterisation of phenolic compounds extracted from Moroccan olive mill wastewater. Food Sci. Technol. 2014, 34, 249–257. [Google Scholar] [CrossRef]
- Foti, P.; Romeo, F.V.; Russo, N.; Pino, A.; Vaccalluzzo, A.; Caggia, C.; Randazzo, C.L. Olive Mill Wastewater as Renewable Raw Materials to Generate High Added-Value Ingredients for Agro-Food Industries. Appl. Sci. 2021, 11, 7511. [Google Scholar] [CrossRef]
- Azzam, M.O.J.; Hazaimeh, S.A. Olive mill wastewater treatment and valorization by extraction/concentration of hydroxytyrosol and other natural phenols. Process Saf. Environ. Prot. 2021, 148, 495–523. [Google Scholar] [CrossRef]
- Benincasa, C.; Pellegrino, M.; Romano, E.; Claps, S.; Fallara, C.; Perri, E. Qualitative and Quantitative Analysis of Phenolic Compounds in Spray-Dried Olive Mill Wastewater. Front. Nutr. 2022, 8, 782693. [Google Scholar] [CrossRef]
- Bernini, R.; Campo, M.; Cassiani, C.; Fochetti, A.; Ieri, F.; Lombardi, A.; Urciuoli, S.; Vignolini, P.; Villanova, N.; Vita, C. Polyphenol-Rich Extracts from Agroindustrial Waste and Byproducts: Results and Perspectives According to the Green Chemistry and Circular Economy. J. Agric. Food Chem. 2024, 72, 12871–12895. [Google Scholar] [CrossRef]
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of by-products from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Elhrech, H.; Aguerd, O.; El Kourchi, C.; Gallo, M.; Naviglio, D.; Chamkhi, I.; Bouyahya, A. Comprehensive Review of Olea europaea: A Holistic Exploration into Its Botanical Marvels, Phytochemical Riches, Therapeutic Potentials, and Safety Profile. Biomolecules 2024, 14, 722. [Google Scholar] [CrossRef]
- Razmpoosh, E.; Abdollahi, S.; Mousavirad, M.; Clark, C.C.T.; Soltani, S. The effects of olive leaf extract on cardiovascular risk factors in the general adult population: A systematic review and meta-analysis of randomized controlled trials. Diabetol. Metab. Syndr. 2022, 14, 151. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.A.; Norhayati, M.N.; Mohamad, N. Olive leaf extract effect on cardiometabolic profile among adults with prehypertension and hypertension: A systematic review and meta-analysis. PeerJ 2021, 9, e11173. [Google Scholar] [CrossRef]
- Laveriano-Santos, E.P.; Vallverdú-Queralt, A.; Bhat, R.; Tresserra-Rimbau, A.; Gutiérrez-Alcalde, E.; Campins-Machado, F.M.; Lamuela-Raventós, R.M.; Pérez, M. Unlocking the potential of olive residues for functional purposes: Update on human intervention trials with health and cosmetic products. J. Sci. Food Agric. 2024, 104, 3816–3822. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Gonçalves, L.; Marto, J.; Martins, A.M.; Silva, A.N.; Pinto, P.; Martins, M.; Fraga, C.; Ribeiro, H.M. Investigations of Olive Oil Industry By-Products Extracts with Potential Skin Benefits in Topical Formulations. Pharmaceutics 2021, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- da Silva, A.C.P.; Paiva, J.P.; Diniz, R.R.; dos Anjos, V.M.; Silva, A.B.S.M.; Pinto, A.V.; dos Santos, E.P.; Leitão, A.C.; Cabral, L.M.; Rodrigues, C.R.; et al. Photoprotection assessment of olive (Olea europaea L.) leaves extract standardized to oleuropein: In vitro and in silico approach for improved sunscreens. J. Photochem. Photobiol. B Biol. 2019, 193, 162–171. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Ng, J.N.C.; Junsuwan, N.; Phaitoonwattanakij, S.; Phothong, W.; Eimpunth, S.; Manuskiatti, W. Efficacy of olive leaf extract–containing cream for facial rejuvenation: A pilot study. J. Cosmet. Dermatol. 2020, 19, 1662–1666. [Google Scholar] [CrossRef]
- Martínez-Navarro, M.E.; Cebrián-Tarancón, C.; Alonso, G.L.; Salinas, M.R. Determination of the Variability of Bioactive Compounds and Minerals in Olive Leaf along an Agronomic Cycle. Agronomy 2021, 11, 2447. [Google Scholar] [CrossRef]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; Contreras, M.d.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Content of phenolic compounds and mannitol in olive leaves extracts from six Spanish cultivars: Extraction with the Soxhlet method and pressurized liquids. Food Chem. 2020, 320, 126626. [Google Scholar] [CrossRef]
- Abdel-Razek, A.; Badr, A.; Shehata, M. Characterization of Olive Oil By-products: Antioxidant Activity, Its Ability to Reduce Aflatoxigenic Fungi Hazard and Its Aflatoxins. Annu. Res. Rev. Biol. 2017, 14, 1–14. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.G.A.; Figueiredo, C.; Santos, C.; Silva, A.M.S. Phenolic and lipophilic metabolite adjustments in Olea europaea (olive) trees during drought stress and recovery. Phytochemistry 2021, 185, 112695. [Google Scholar] [CrossRef]
- Taamalli, A.; Arráez-Román, D.; Barrajón-Catalán, E.; Ruiz-Torres, V.; Pérez-Sánchez, A.; Herrero, M.; Ibañez, E.; Micol, V.; Zarrouk, M.; Segura-Carretero, A.; et al. Use of advanced techniques for the extraction of phenolic compounds from Tunisian olive leaves: Phenolic composition and cytotoxicity against human breast cancer cells. Food Chem. Toxicol. 2012, 50, 1817–1825. [Google Scholar] [CrossRef]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the Antimicrobial Activity of Olive Leaf Extract Against Foodborne Bacterial Pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Calvano, C.D.; Tamborrino, A. Valorization of Olive By-Products: Innovative Strategies for Their Production, Treatment and Characterization. Foods 2022, 11, 768. [Google Scholar] [CrossRef] [PubMed]
- Qosa, H.; Batarseh, Y.S.; Mohyeldin, M.M.; El Sayed, K.A.; Keller, J.N.; Kaddoumi, A. Oleocanthal Enhances Amyloid-β Clearance from the Brains of TgSwDI Mice and in Vitro across a Human Blood-Brain Barrier Model. ACS Chem. Neurosci. 2015, 6, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Paiva-Martins, F.; Gordon, M.H. Isolation and characterization of the antioxidant component 3, 4-dihydroxyphenylethyl 4-formyl-3-formylmethyl-4-hexenoate from olive (Olea europaea) leaves. J. Agric. Food Chem. 2001, 49, 4214–4219. [Google Scholar] [CrossRef]
- Paiva-Martins, F.; Pinto, M. Isolation and characterization of a new hydroxytyrosol derivative from olive (Olea europaea) leaves. J. Agric. Food Chem. 2008, 56, 5582–5588. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Alfaleh, A.A.; Sindi, H.A. Systematic study on date palm seeds (Phoenix dactylifera L.) extraction optimisation using natural deep eutectic solvents and ultrasound technique. Sci. Rep. 2024, 14, 16622. [Google Scholar] [CrossRef]
- Grisales-Mejía, J.F.; Cedeño-Fierro, V.; Ortega, J.P.; Torres-Castañeda, H.G.; Andrade-Mahecha, M.M.; Martínez-Correa, H.A.; Álvarez-Rivera, G.; Mendiola, J.A.; Cifuentes, A.; Ibañez, E. Advanced NADES-based extraction processes for the recovery of phenolic compounds from Hass avocado residues: A sustainable valorization strategy. Sep. Purif. Technol. 2024, 351, 128104. [Google Scholar] [CrossRef]
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Olive Pomace-Derived Biomasses Fractionation through a Two-Step Extraction Based on the Use of Ultrasounds: Chemical Characteristics. Foods 2021, 10, 111. [Google Scholar] [CrossRef] [PubMed]
- de Melo, M.M.R.; Silvestre, A.J.D.; Silva, C.M. Supercritical fluid extraction of vegetable matrices: Applications, trends and future perspectives of a convincing green technology. J. Supercrit. Fluids 2014, 92, 115–176. [Google Scholar] [CrossRef]
- da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. TrAC Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef]
- Otero, P.; Quintana, S.E.; Reglero, G.; Fornari, T.; García-Risco, M.R. Pressurized Liquid Extraction (PLE) as an Innovative Green Technology for the Effective Enrichment of Galician Algae Extracts with High Quality Fatty Acids and Antimicrobial and Antioxidant Properties. Mar. Drugs 2018, 16, 156. [Google Scholar] [CrossRef]
- Durante, M.; Ferramosca, A.; Treppiccione, L.; Di Giacomo, M.; Zara, V.; Montefusco, A.; Piro, G.; Mita, G.; Bergamo, P.; Lenucci, M.S. Application of response surface methodology (RSM) for the optimization of supercritical CO2 extraction of oil from patè olive cake: Yield, content of bioactive molecules and biological effects in vivo. Food Chem. 2020, 332, 127405. [Google Scholar] [CrossRef]
- Irakli, M.; Chatzopoulou, P.; Ekateriniadou, L. Optimization of ultrasound-assisted extraction of phenolic compounds: Oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018, 124, 382–388. [Google Scholar] [CrossRef]
- Martínez-Patiño, J.C.; Gullón, B.; Romero, I.; Ruiz, E.; Brnčić, M.; Žlabur, J.Š.; Castro, E. Optimization of ultrasound-assisted extraction of biomass from olive trees using response surface methodology. Ultrason. Sonochemistry 2019, 51, 487–495. [Google Scholar] [CrossRef]
- İlbay, Z.; Şahin, S.; Büyükkabasakal, K. A novel approach for olive leaf extraction through ultrasound technology: Response surface methodology versus artificial neural networks. Korean J. Chem. Eng. 2014, 31, 1661–1667. [Google Scholar] [CrossRef]
- Lama-Muñoz, A.; del Mar Contreras, M.; Espínola, F.; Moya, M.; de Torres, A.; Romero, I.; Castro, E. Extraction of oleuropein and luteolin-7-O-glucoside from olive leaves: Optimization of technique and operating conditions. Food Chem. 2019, 293, 161–168. [Google Scholar] [CrossRef]
- Ghoreishi, S.M.; Shahrestani, R.G. Subcritical water extraction of mannitol from olive leaves. J. Food Eng. 2009, 93, 474–481. [Google Scholar] [CrossRef]
- Schievano, A.; Adani, F.; Buessing, L.; Botto, A.; Casoliba, E.N.; Rossoni, M.; Goldfarb, J.L. An integrated biorefinery concept for olive mill waste management: Supercritical CO2 extraction and energy recovery. Green Chem. 2015, 17, 2874–2887. [Google Scholar] [CrossRef]
- Caballero, A.S.; Romero-García, J.M.; Castro, E.; Cardona, C.A. Supercritical fluid extraction for enhancing polyphenolic compounds production from olive waste extracts. J. Chem. Technol. Biotechnol. 2020, 95, 356–362. [Google Scholar] [CrossRef]
- Martín-García, B.; Pimentel-Moral, S.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Box-Behnken experimental design for a green extraction method of phenolic compounds from olive leaves. Ind. Crops Prod. 2020, 154, 112741. [Google Scholar] [CrossRef]
- Vásquez-Villanueva, R.; Plaza, M.; García, M.C.; Marina, M.L. Recovery and determination of cholesterol-lowering compounds from Olea europaea seeds employing pressurized liquid extraction and gas chromatography-mass spectrometry. Microchem. J. 2020, 156, 104812. [Google Scholar] [CrossRef]
- Fernandez-Pastor, I.; Fernandez-Hernandez, A.; Perez-Criado, S.; Rivas, F.; Martinez, A.; Garcia-Granados, A.; Parra, A. Microwave-assisted extraction versus Soxhlet extraction to determine triterpene acids in olive skins. J. Sep. Sci. 2017, 40, 1209–1217. [Google Scholar] [CrossRef]
- Macedo, G.A.; Santana, Á.L.; Crawford, L.M.; Wang, S.C.; Dias, F.F.G.; de Moura Bell, J.M.L.N. Integrated microwave- and enzyme-assisted extraction of phenolic compounds from olive pomace. LWT 2021, 138, 110621. [Google Scholar] [CrossRef]
- Rosa, G.S.d.; Martiny, T.R.; Dotto, G.L.; Vanga, S.K.; Parrine, D.; Gariepy, Y.; Lefsrud, M.; Raghavan, V. Eco-friendly extraction for the recovery of bioactive compounds from Brazilian olive leaves. Sustain. Mater. Technol. 2021, 28, e00276. [Google Scholar] [CrossRef]
- Nichols, E.; Vos, T. The estimation of the global prevalence of dementia from 1990-2019 and forecasted prevalence through 2050: An analysis for the Global Burden of Disease (GBD) study 2019. Alzheimer’s Dement. 2021, 17, e051496. [Google Scholar] [CrossRef]
- O’Connor, A.; Weston, P.S.J.; Pavisic, I.M.; Ryan, N.S.; Collins, J.D.; Lu, K.; Crutch, S.J.; Alexander, D.C.; Fox, N.C.; Oxtoby, N.P. Quantitative detection and staging of presymptomatic cognitive decline in familial Alzheimer’s disease: A retrospective cohort analysis. Alzheimer’s Res. Ther. 2020, 12, 126. [Google Scholar] [CrossRef]
- Chang, W.P.; Huang, X.; Downs, D.; Cirrito, J.R.; Koelsch, G.; Holtzman, D.M.; Ghosh, A.K.; Tang, J. β-Secretase inhibitor GRL-8234 rescues age-related cognitive decline in APP transgenic mice. FASEB J. 2011, 25, 775–784. [Google Scholar] [CrossRef]
- Patow, G.; Stefanovski, L.; Ritter, P.; Deco, G.; Kobeleva, X.; for the Alzheimer’s Disease Neuroimaging Initiative. Whole-brain modeling of the differential influences of amyloid-beta and tau in Alzheimer’s disease. Alzheimer’s Res. Ther. 2023, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.; Oasa, S.; Muntsant Soria, A.; Tiiman, A.; Söderberg, L.; Amandius, E.; Möller, C.; Lannfelt, L.; Terenius, L.; Giménez-Llort, L.; et al. The interwoven fibril-like structure of amyloid-beta plaques in mouse brain tissue visualized using super-resolution STED microscopy. Cell Biosci. 2023, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Yates, D.; McLoughlin, D.M. The molecular pathology of Alzheimer’s disease. Psychiatry 2008, 7, 1–5. [Google Scholar] [CrossRef]
- Motta, C.; Di Donna, M.G.; Bonomi, C.G.; Assogna, M.; Chiaravalloti, A.; Mercuri, N.B.; Koch, G.; Martorana, A. Different associations between amyloid-βeta 42, amyloid-βeta 40, and amyloid-βeta 42/40 with soluble phosphorylated-tau and disease burden in Alzheimer’s disease: A cerebrospinal fluid and fluorodeoxyglucose-positron emission tomography study. Alzheimer’s Res. Ther. 2023, 15, 144. [Google Scholar] [CrossRef]
- Kumar, A.; Scarpa, M.; Nordberg, A. Tracing synaptic loss in Alzheimer’s brain with SV2A PET-tracer UCB-J. Alzheimer’s Dement. 2024, 20, 2589–2605. [Google Scholar] [CrossRef]
- Tran, K.M.; Kawauchi, S.; Kramár, E.A.; Rezaie, N.; Liang, H.Y.; Sakr, J.S.; Gomez-Arboledas, A.; Arreola, M.A.; Cunha, C.D.; Phan, J.; et al. A Trem2R47H mouse model without cryptic splicing drives age- and disease-dependent tissue damage and synaptic loss in response to plaques. Mol. Neurodegener. 2023, 18, 12. [Google Scholar] [CrossRef]
- Emmerson, J.T.; Malcolm, J.C.; Do Carmo, S.; Nguyen, P.; Breuillaud, L.; Martinez-Trujillo, J.C.; Cuello, A.C. Neuronal loss and inflammation preceding fibrillary tau pathology in a rat model with early human-like tauopathy. Neurobiol. Dis. 2023, 187, 106317. [Google Scholar] [CrossRef]
- Wang, H.; Shang, Y.; Wang, E.; Xu, X.; Zhang, Q.; Qian, C.; Yang, Z.; Wu, S.; Zhang, T. MST1 mediates neuronal loss and cognitive deficits: A novel therapeutic target for Alzheimer’s disease. Prog. Neurobiol. 2022, 214, 102280. [Google Scholar] [CrossRef]
- Guerrero-Carrasco, M.; Targett, I.; Olmos-Alonso, A.; Vargas-Caballero, M.; Gomez-Nicola, D. Low-grade systemic inflammation stimulates microglial turnover and accelerates the onset of Alzheimer’s-like pathology. Glia 2024, 72, 1340–1355. [Google Scholar] [CrossRef]
- Ennerfelt, H.; Frost, E.L.; Shapiro, D.A.; Holliday, C.; Zengeler, K.E.; Voithofer, G.; Bolte, A.C.; Lammert, C.R.; Kulas, J.A.; Ulland, T.K.; et al. SYK coordinates neuroprotective microglial responses in neurodegenerative disease. Cell 2022, 185, 4135–4152.e22. [Google Scholar] [CrossRef]
- Fontana, I.C.; Kumar, A.; Okamura, N.; Nordberg, A. Multitracer Approach to Understanding the Complexity of Reactive Astrogliosis in Alzheimer’s Brains. ACS Chem. Neurosci. 2024, 15, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Ballweg, A.; Klaus, C.; Vogler, L.; Katzdobler, S.; Wind, K.; Zatcepin, A.; Ziegler, S.I.; Secgin, B.; Eckenweber, F.; Bohr, B.; et al. [18F]F-DED PET imaging of reactive astrogliosis in neurodegenerative diseases: Preclinical proof of concept and first-in-human data. J. Neuroinflammation 2023, 20, 68. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Lei, H.; Xu, Z.; Li, S.; Yu, H.; Xie, J.; Zhang, Z.; Liu, G.; Zhang, Y.; et al. A novel transgenic mouse line with hippocampus-dominant and inducible expression of truncated human tau. Transl. Neurodegener. 2023, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.S.; Marschallinger, J.; Kaindl, J.; Höfling, C.; Rossner, S.; Heneka, M.T.; Van Der Linden, A.; Aigner, L. Early Changes in Hippocampal Neurogenesis in Transgenic Mouse Models for Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 5796–5806. [Google Scholar] [CrossRef]
- Vanderlinden, G.; Ceccarini, J.; Vande Casteele, T.; Michiels, L.; Lemmens, R.; Triau, E.; Serdons, K.; Tournoy, J.; Koole, M.; Vandenbulcke, M.; et al. Spatial decrease of synaptic density in amnestic mild cognitive impairment follows the tau build-up pattern. Mol. Psychiatry 2022, 27, 4244–4251. [Google Scholar] [CrossRef]
- Soni, N.; Hohsfield, L.A.; Tran, K.M.; Kawauchi, S.; Walker, A.; Javonillo, D.; Phan, J.; Matheos, D.; Da Cunha, C.; Uyar, A.; et al. Genetic diversity promotes resilience in a mouse model of Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, 2794–2816. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, L.; Chen, C.; Wei, X.; Ma, Y.; Ma, Y. Investigating the causal relationship between immune cell and Alzheimer’s disease: A mendelian randomization analysis. BMC Neurol. 2024, 24, 98. [Google Scholar] [CrossRef]
- Nava Catorce, M.; Acero, G.; Gevorkian, G. Age- and sex-dependent alterations in the peripheral immune system in the 3xTg-AD mouse model of Alzheimer’s disease: Increased proportion of CD3+CD4-CD8- double-negative T cells in the blood. J. Neuroimmunol. 2021, 360, 577720. [Google Scholar] [CrossRef]
- Doorduijn, A.S.; Van De Rest, O.; Van Der Flier, W.M.; Visser, M.; De Van Der Schueren, M.A.E. Energy and Protein Intake of Alzheimer’s Disease Patients Compared to Cognitively Normal Controls: Systematic Review. J. Am. Med. Dir. Assoc. 2019, 20, 14–21. [Google Scholar] [CrossRef]
- Lokesh, M.; Bandaru, L.J.M.; Rajanna, A.; Rao, J.S.; Challa, S. Unveiling Potential Neurotoxic Mechansisms: Pb-Induced Activation of CDK5-p25 Signaling Axis in Alzheimer’s Disease Development, Emphasizing CDK5 Inhibition and Formation of Toxic p25 Species. Mol. Neurobiol. 2024, 61, 3090–3103. [Google Scholar] [CrossRef]
- Lyons, C.E.; Graves, S.I.; Razzoli, M.; Jeganathan, K.; Mansk, R.P.; McGonigle, S.; Sabarinathan, N.; van Deursen, J.M.; Baker, D.J.; Bartolomucci, A. Chronic Social and Psychological Stress Impact Select Neuropathologies in the PS19 Mouse Model of Tauopathy. Psychosom. Med. 2024, 86, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.H.; Zhang, S.; Simon, J.M.; McLarnan, S.M.; Chung, W.K.; Pearson, B.L. Environmental carcinogens disproportionally mutate genes implicated in neurodevelopmental disorders. Front. Neurosci. 2023, 17, 1106573. [Google Scholar] [CrossRef] [PubMed]
- Kulminski, A.M.; Jain-Washburn, E.; Loiko, E.; Loika, Y.; Feng, F.; Culminskaya, I. Associations of the APOE ε2 and ε4 alleles and polygenic profiles comprising APOE-TOMM40-APOC1 variants with Alzheimer’s disease biomarkers. Aging 2022, 14, 9782–9804. [Google Scholar] [CrossRef] [PubMed]
- Pettigrew, C.; Nazarovs, J.; Soldan, A.; Singh, V.; Wang, J.; Hohman, T.; Dumitrescu, L.; Libby, J.; Kunkle, B.; Gross, A.L.; et al. Alzheimer’s disease genetic risk and cognitive reserve in relationship to long-term cognitive trajectories among cognitively normal individuals. Alzheimer’s Res. Ther. 2023, 15, 66. [Google Scholar] [CrossRef]
- Lanoiselée, H.-M.; Nicolas, G.; Wallon, D.; Rovelet-Lecrux, A.; Lacour, M.; Rousseau, S.; Richard, A.-C.; Pasquier, F.; Rollin-Sillaire, A.; Martinaud, O.; et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017, 14, e1002270. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Pradhan, A.; Heesch, A.; Helbig, A.; Blennow, K.; Koch, C.; Bertgen, L.; Koo, E.H.; Brinkmalm, G.; Zetterberg, H.; et al. Differential effects of familial Alzheimer’s disease-causing mutations on amyloid precursor protein (APP) trafficking, proteolytic conversion, and synaptogenic activity. Acta Neuropathol. Commun. 2023, 11, 87. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, H.; Liu, X.; Zhang, W.; Zhang, S.; Jiao, B. APP, PSEN1, and PSEN2 Variants in Alzheimer’s Disease: Systematic Re-evaluation According to ACMG Guidelines. Front. Aging Neurosci. 2021, 13, 695808. [Google Scholar] [CrossRef]
- Lewkowicz, E.; Nakamura, M.N.; Rynkiewicz, M.J.; Gursky, O. Molecular modeling of apoE in complexes with Alzheimer’s amyloid-β fibrils from human brain suggests a structural basis for apolipoprotein co-deposition with amyloids. Cell. Mol. Life Sci. 2023, 80, 376. [Google Scholar] [CrossRef]
- Zhou, M.; Jiao, Q.; Wu, Z.; Li, W.; Liu, G.; Wang, R.; Tang, Y. Uncovering the Oxidative Stress Mechanisms and Targets in Alzheimer’s Disease by Integrating Phenotypic Screening Data and Polypharmacology Networks. J. Alzheimers Dis. 2024, 99, S139–S156. [Google Scholar] [CrossRef]
- Tang, Z.; Chen, Z.; Guo, M.; Peng, Y.; Xiao, Y.; Guan, Z.; Ni, R.; Qi, X. NRF2 Deficiency Promotes Ferroptosis of Astrocytes Mediated by Oxidative Stress in Alzheimer’s Disease. Mol. Neurobiol. 2024, 61, 7517–7533. [Google Scholar] [CrossRef]
- Ashleigh, T.; Swerdlow, R.H.; Beal, M.F. The role of mitochondrial dysfunction in Alzheimer’s disease pathogenesis. Alzheimer’s Dement. 2023, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Walter, E.; Wohleb, E.; Fan, Y.; Wang, C. ATG5 (autophagy related 5) in microglia controls hippocampal neurogenesis in Alzheimer disease. Autophagy 2024, 20, 847–862. [Google Scholar] [CrossRef] [PubMed]
- Yun, Q.; Ma, S.-F.; Zhang, W.-N.; Gu, M.; Wang, J. FoxG1 as a Potential Therapeutic Target for Alzheimer’s Disease: Modulating NLRP3 Inflammasome via AMPK/mTOR Autophagy Pathway. Cell. Mol. Neurobiol. 2024, 44, 35. [Google Scholar] [CrossRef] [PubMed]
- Ou, G.Y.; Lin, W.W.; Zhao, W.J. Construction of Long Noncoding RNA-Associated ceRNA Networks Reveals Potential Biomarkers in Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 82, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.W.; Blumenschine, R.J.; Adams, D.B. Ratio of central nervous system to body metabolism in vertebrates: Its constancy and functional basis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1981, 241, R203–R212. [Google Scholar] [CrossRef]
- Hamilton, J.A.; Hillard, C.J.; Spector, A.A.; Watkins, P.A. Brain Uptake and Utilization of Fatty Acids, Lipids and Lipoproteins: Application to Neurological Disorders. J. Mol. Neurosci. 2007, 33, 2–11. [Google Scholar] [CrossRef]
- Karelson, E.; Bogdanovic, N.; Garlind, A.; Winblad, B.; Zilmer, K.; Kullisaar, T.; Vihalemm, T.; Kairane, C.; Zilmer, M. The Cerebrocortical Areas in Normal Brain Aging and in Alzheimer’s Disease: Noticeable Differences in the Lipid Peroxidation Level and in Antioxidant Defense. Neurochem. Res. 2001, 26, 353–361. [Google Scholar] [CrossRef]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Lorente, L.; Martín, M.M.; González-Rivero, A.F.; Pérez-Cejas, A.; Abreu-González, P.; Ramos, L.; Argueso, M.; Cáceres, J.J.; Solé-Violán, J.; Alvarez-Castillo, A.; et al. Association Between DNA and RNA Oxidative Damage and Mortality of Patients with Traumatic Brain Injury. Neurocritical Care 2020, 32, 790–795. [Google Scholar] [CrossRef]
- Rao, Y.L.; Ganaraja, B.; Marathe, A.; Manjrekar, P.A.; Joy, T.; Ullal, S.; Pai, M.M.; Murlimanju, B.V. Comparison of malondialdehyde levels and superoxide dismutase activity in resveratrol and resveratrol/donepezil combination treatment groups in Alzheimer’s disease induced rat model. 3 Biotech 2021, 11, 329. [Google Scholar] [CrossRef]
- Pamphlett, R.; Bishop, D.P. The toxic metal hypothesis for neurological disorders. Front. Neurol. 2023, 14, 1173779. [Google Scholar] [CrossRef] [PubMed]
- Stefanatos, R.; Sanz, A. The role of mitochondrial ROS in the aging brain. FEBS Lett. 2018, 592, 743–758. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Gu, G.J.; Shen, X.; Zhang, Q.; Wang, G.M.; Wang, P.J. Neural stem cell transplantation enhances mitochondrial biogenesis in a transgenic mouse model of Alzheimer’s disease-like pathology. Neurobiol. Aging 2015, 36, 1282–1292. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, W.; Lin, Z.; Wu, Y.; Li, Y.; Xia, X. Panax notoginseng saponins prevent dementia and oxidative stress in brains of SAMP8 mice by enhancing mitophagy. BMC Complement. Med. Ther. 2024, 24, 144. [Google Scholar] [CrossRef]
- Xie, Y.; Ke, X.; Ye, Z.; Li, X.; Chen, Z.; Liu, J.; Wu, Z.; Liu, Q.; Du, X. Se-methylselenocysteine ameliorates mitochondrial function by targeting both mitophagy and autophagy in the mouse model of Alzheimer’s disease. Food Funct. 2024, 15, 4310–4322. [Google Scholar] [CrossRef] [PubMed]
- Lecca, D.; Jung, Y.J.; Scerba, M.T.; Hwang, I.; Kim, Y.K.; Kim, S.; Modrow, S.; Tweedie, D.; Hsueh, S.C.; Liu, D.; et al. Role of chronic neuroinflammation in neuroplasticity and cognitive function: A hypothesis. Alzheimer’s Dement. 2022, 18, 2327–2340. [Google Scholar] [CrossRef]
- Arranz, A.M.; De Strooper, B. The role of astroglia in Alzheimer’s disease: Pathophysiology and clinical implications. Lancet Neurol. 2019, 18, 406–414. [Google Scholar] [CrossRef]
- Lana, D.; Branca, J.J.V.; Delfino, G.; Giovannini, M.G.; Casamenti, F.; Nardiello, P.; Bucciantini, M.; Stefani, M.; Zach, P.; Zecchi-Orlandini, S.; et al. Morphofunctional Investigation in a Transgenic Mouse Model of Alzheimer’s Disease: Non-Reactive Astrocytes Are Involved in Aβ Load and Reactive Astrocytes in Plaque Build-Up. Cells 2023, 12, 2258. [Google Scholar] [CrossRef]
- Prasad, H.; Rao, R. Amyloid clearance defect in ApoE4 astrocytes is reversed by epigenetic correction of endosomal pH. Proc. Natl. Acad. Sci. USA 2018, 115, E6640–E6649. [Google Scholar] [CrossRef]
- Ioannou, M.S.; Jackson, J.; Sheu, S.-H.; Chang, C.-L.; Weigel, A.V.; Liu, H.; Pasolli, H.A.; Xu, C.S.; Pang, S.; Matthies, D.; et al. Neuron-Astrocyte Metabolic Coupling Protects against Activity-Induced Fatty Acid Toxicity. Cell 2019, 177, 1522–1535.e14. [Google Scholar] [CrossRef]
- Santiago-Balmaseda, A.; Aguirre-Orozco, A.; Valenzuela-Arzeta, I.E.; Villegas-Rojas, M.M.; Pérez-Segura, I.; Jiménez-Barrios, N.; Hurtado-Robles, E.; Rodríguez-Hernández, L.D.; Rivera-German, E.R.; Guerra-Crespo, M.; et al. Neurodegenerative Diseases: Unraveling the Heterogeneity of Astrocytes. Cells 2024, 13, 921. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xu, Z.; Zhang, L.; Zhang, C.; Zhao, X.; Mao, Y.; Zhang, H.; Liang, X.; Wu, J.; Yang, Y.; et al. Gut-derived β-amyloid: Likely a centerpiece of the gut–brain axis contributing to Alzheimer’s pathogenesis. Gut Microbes 2023, 15, 2167172. [Google Scholar] [CrossRef]
- Cochran, J.N.; Hall, A.M.; Roberson, E.D. The dendritic hypothesis for Alzheimer’s disease pathophysiology. Brain Res. Bull. 2014, 103, 18–28. [Google Scholar] [CrossRef]
- Paterno, G.; Bell, B.M.; Gorion, K.-M.M.; Prokop, S.; Giasson, B.I. Reassessment of Neuronal Tau Distribution in Adult Human Brain and Implications for Tau Pathobiology. Acta Neuropathol. Commun. 2022, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Weston, P.S.J.; Coath, W.; Harris, M.J.; Malone, I.B.; Dickson, J.; Aigbirhio, F.I.; Cash, D.M.; Zhang, H.; Schott, J.M. Cortical tau is associated with microstructural imaging biomarkers of neurite density and dendritic complexity in Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 2750–2754. [Google Scholar] [CrossRef]
- Guptarak, J.; Scaduto, P.; Tumurbaatar, B.; Zhang, W.R.; Jupiter, D.; Taglialatela, G.; Fracassi, A. Cognitive integrity in Non-Demented Individuals with Alzheimer’s Neuropathology is associated with preservation and remodeling of dendritic spines. Alzheimer’s Dement. 2024, 20, 4677–4691. [Google Scholar] [CrossRef]
- Penney, J.; Ralvenius, W.T.; Tsai, L.-H. Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 2020, 25, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Frankowski, H.; Yeboah, F.; Berry, B.J.; Kinoshita, C.; Lee, M.; Evitts, K.; Davis, J.; Kinoshita, Y.; Morrison, R.S.; Young, J.E. Knock-Down of HDAC2 in Human Induced Pluripotent Stem Cell Derived Neurons Improves Neuronal Mitochondrial Dynamics, Neuronal Maturation and Reduces Amyloid Beta Peptides. Int. J. Mol. Sci. 2021, 22, 2526. [Google Scholar] [CrossRef]
- Sharlow, E.R.; Llaneza, D.C.; Mendelson, A.J.; Mingledorff, G.A.; Porterfield, V.M.; Salvati, K.; Beenhakker, M.; Bloom, G.S.; Lazo, J.S. Extracellular cues accelerate neurogenesis of induced pluripotent stem cell derived neurons. bioRxiv 2021. [Google Scholar] [CrossRef]
- Faria-Pereira, A.; Temido-Ferreira, M.; Morais, V.A. BrainPhys Neuronal Media Support Physiological Function of Mitochondria in Mouse Primary Neuronal Cultures. Front. Mol. Neurosci. 2022, 15, 837448. [Google Scholar] [CrossRef] [PubMed]
- Pollio, G.; Roncarati, R.; Seredenina, T.; Terstappen, G.C.; Caricasole, A. A reporter assay for target validation in primary neuronal cultures. J. Neurosci. Methods 2008, 172, 34–37. [Google Scholar] [CrossRef] [PubMed]
- Struzyna, L.A.; Watt, M.L. The Emerging Role of Neuronal Organoid Models in Drug Discovery: Potential Applications and Hurdles to Implementation. Mol. Pharmacol. 2021, 99, 256–265. [Google Scholar] [CrossRef]
- Okouchi, M.; Ekshyyan, O.; Maracine, M.; Aw, T.Y. Neuronal Apoptosis in Neurodegeneration. Antioxid. Redox Signal. 2007, 9, 1059–1096. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Yan, J.; Zhou, Q.; Wang, X. Recent Progress in Research on Mechanisms of Action of Natural Products against Alzheimer’s Disease: Dietary Plant Polyphenols. Int. J. Mol. Sci. 2022, 23, 13886. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Nabavi, S.M.; Orhan, I.E.; Skalicka-Woźniak, K.; D’Onofrio, G.; Aiello, F. Chapter 3—Natural Compounds and Their Derivatives as Multifunctional Agents for the Treatment of Alzheimer Disease. In Discovery and Development of Neuroprotective Agents from Natural Products; Brahmachari, G., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 63–102. [Google Scholar]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef]
- Balducci, C.; Forloni, G. APP Transgenic Mice: Their Use and Limitations. NeuroMol. Med. 2011, 13, 117–137. [Google Scholar] [CrossRef]
- Garcia-Alloza, M.; Robbins, E.M.; Zhang-Nunes, S.X.; Purcell, S.M.; Betensky, R.A.; Raju, S.; Prada, C.; Greenberg, S.M.; Bacskai, B.J.; Frosch, M.P. Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiol. Dis. 2006, 24, 516–524. [Google Scholar] [CrossRef]
- Ruan, L.; Kang, Z.; Pei, G.; Le, Y. Amyloid deposition and inflammation in APPswe/PS1dE9 mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 531–540. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Poon, H.F. The senescence-accelerated prone mouse (SAMP8): A model of age-related cognitive decline with relevance to alterations of the gene expression and protein abnormalities in Alzheimer’s disease. Exp. Gerontol. 2005, 40, 774–783. [Google Scholar] [CrossRef]
- Flood, J.F.; Morley, J.E. Learning and memory in the SAMP8 mouse. Neurosci. Biobehav. Rev. 1997, 22, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.F.; Goldman, H.; Altman, H.J. Age-related changes in regional cerebral blood flow and behavior in Sprague-Dawley rats. Neurobiol. Aging 1988, 9, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Prüßing, K.; Voigt, A.; Schulz, J.B. Drosophila melanogaster as a model organism for Alzheimer’s disease. Mol. Neurodegener. 2013, 8, 35. [Google Scholar] [CrossRef]
- Balez, R.; Steiner, N.; Engel, M.; Muñoz, S.S.; Lum, J.S.; Wu, Y.; Wang, D.; Vallotton, P.; Sachdev, P.; O’Connor, M.; et al. Neuroprotective effects of apigenin against inflammation, neuronal excitability and apoptosis in an induced pluripotent stem cell model of Alzheimer’s disease. Sci. Rep. 2016, 6, 31450. [Google Scholar] [CrossRef]
- Belkacemi, A.; Ramassamy, C. Innovative anthocyanin/anthocyanidin formulation protects SK-N-SH cells against the amyloid-β peptide-induced toxicity: Relevance to alzheimer’s disease. Cent. Nerv. Syst. Agents Med. Chem. 2016, 16, 37–49. [Google Scholar] [CrossRef]
- Cimini, A.; Gentile, R.; D’Angelo, B.; Benedetti, E.; Cristiano, L.; Avantaggiati, M.L.; Giordano, A.; Ferri, C.; Desideri, G. Cocoa powder triggers neuroprotective and preventive effects in a human Alzheimer’s disease model by modulating BDNF signaling pathway. J. Cell. Biochem. 2013, 114, 2209–2220. [Google Scholar] [CrossRef]
- Reznichenko, L.; Amit, T.; Zheng, H.; Avramovich-Tirosh, Y.; Youdim, M.B.H.; Weinreb, O.; Mandel, S. Reduction of iron-regulated amyloid precursor protein and β-amyloid peptide by (–)-epigallocatechin-3-gallate in cell cultures: Implications for iron chelation in Alzheimer’s disease. J. Neurochem. 2006, 97, 527–536. [Google Scholar] [CrossRef]
- Soto-Mercado, V.; Mendivil-Perez, M.; Velez-Pardo, C.; Jimenez-Del-Rio, M. (−)-Epigallocatechin-3-Gallate Diminishes Intra-and Extracellular Amyloid-Induced Cytotoxic Effects on Cholinergic-like Neurons from Familial Alzheimer’s Disease PSEN1 E280A. Biomolecules 2021, 11, 1845. [Google Scholar] [CrossRef]
- Jayatunga, D.P.W.; Hone, E.; Fernando, W.M.A.D.B.; Garg, M.L.; Verdile, G.; Martins, R.N. Mitoprotective Effects of a Synergistic Nutraceutical Combination: Basis for a Prevention Strategy Against Alzheimer’s Disease. Front. Aging Neurosci. 2022, 13, 781468. [Google Scholar] [CrossRef]
- Foroughi, S.; Shahanipour, K.; Monajemi, R.; Ahadi, A.M. Investigating the effects of Thymus vulgaris essential oil, Allium cepa extract, and their active compounds (thymol and quercetin) on expression profile of genes related to Alzheimer’s disease in PC12 model cell. Brain Res. 2024, 1838, 148966. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.W.; Lee, H.S.; Shin, H.C.; Lee, B.H. Multifunctional activity of polyphenolic compounds associated with a potential for alzheimer’s disease therapy from ecklonia cava. Phytother. Res. 2015, 29, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Ghura, S.; Tai, L.; Zhao, M.; Collins, N.; Che, C.-T.; Warpeha, K.M.; LaDu, M.J. Arabidopsis thaliana extracts optimized for polyphenols production as potential therapeutics for the APOE-modulated neuroinflammation characteristic of Alzheimer’s disease in vitro. Sci. Rep. 2016, 6, 29364. [Google Scholar] [CrossRef] [PubMed]
- Marambaud, P.; Zhao, H.; Davies, P. Resveratrol Promotes Clearance of Alzheimer’s Disease Amyloid-β Peptides. J. Biol. Chem. 2005, 280, 37377–37382. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, J.A.; Hernández-Aguilar, M.E.; Rojas-Durán, F.; Herrera-Covarrubias, D.; García-Hernández, L.I.; Mestizo-Gutiérrez, S.L.; Aranda-Abreu, G.E. Expression of proteins linked to Alzheimer’s disease in C6 rat glioma cells under the action of lipopolysaccharide (LPS), nimesulide, resveratrol and citalopram. Turk. J. Biochem. 2020, 45, 793–801. [Google Scholar] [CrossRef]
- Vion, E.; Page, G.; Bourdeaud, E.; Paccalin, M.; Guillard, J.; Rioux Bilan, A. Trans ε-viniferin is an amyloid-β disaggregating and anti-inflammatory drug in a mouse primary cellular model of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 88, 1–6. [Google Scholar] [CrossRef]
- Baruah, P.; Moorthy, H.; Ramesh, M.; Padhi, D.; Govindaraju, T. A natural polyphenol activates and enhances GPX4 to mitigate amyloid-β induced ferroptosis in Alzheimer’s disease. Chem. Sci. 2023, 14, 9427–9438. [Google Scholar] [CrossRef]
- Wang, D.; Ho, L.; Faith, J.; Ono, K.; Janle, E.M.; Lachcik, P.J.; Cooper, B.R.; Jannasch, A.H.; D’Arcy, B.R.; Williams, B.A.; et al. Role of intestinal microbiota in the generation of polyphenol-derived phenolic acid mediated attenuation of Alzheimer’s disease β-amyloid oligomerization. Mol. Nutr. Food Res. 2015, 59, 1025–1040. [Google Scholar] [CrossRef]
- Li, C.; Meng, P.; Zhang, B.; Kang, H.; Wen, H.; Schluesener, H.; Cao, Z.; Zhang, Z. Computer-aided identification of protein targets of four polyphenols in Alzheimer’s disease (AD) and validation in a mouse AD model. J. Biomed. Res. 2019, 33, 101. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Thomas, P.; Zhong, J.-H.; Bi, F.-F.; Kosaraju, S.; Pollard, A.; Fenech, M.; Zhou, X.-F. Consumption of Grape Seed Extract Prevents Amyloid-β Deposition and Attenuates Inflammation in Brain of an Alzheimer’s Disease Mouse. Neurotox. Res. 2009, 15, 3–14. [Google Scholar] [CrossRef]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. AGE 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Griñán-Ferré, C.; Ferrer, I.; Camins, A.; Sanfeliu, C.; Del Valle, J.; Pallàs, M. Neuroprotective Role of Trans-Resveratrol in a Murine Model of Familial Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Sarroca, S.; Gatius, A.; Rodríguez-Farré, E.; Vilchez, D.; Pallàs, M.; Griñán-Ferré, C.; Sanfeliu, C.; Corpas, R. Resveratrol confers neuroprotection against high-fat diet in a mouse model of Alzheimer’s disease via modulation of proteolytic mechanisms. J. Nutr. Biochem. 2021, 89, 108569. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Santa-Maria, I.; Ho, L.; Ksiezak-Reding, H.; Ono, K.; Teplow, D.B.; Pasinetti, G.M. Grape derived polyphenols attenuate Tau neuropathology in a mouse model of alzheimer’s disease. J. Alzheimer’s Dis. 2010, 22, 653–661. [Google Scholar] [CrossRef]
- Ho, L.; Chen, L.H.; Wang, J.; Zhao, W.; Talcott, S.T.; Ono, K.; Teplow, D.; Humala, N.; Cheng, A.; Percival, S.S.; et al. Heterogeneity in red wine polyphenolic contents differentially influences Alzheimer’s disease-type neuropathology and cognitive deterioration. J. Alzheimer’s Dis. 2009, 16, 59–72. [Google Scholar] [CrossRef]
- Borai, I.H.; Ezz, M.K.; Rizk, M.Z.; Aly, H.F.; El-Sherbiny, M.; Matloub, A.A.; Fouad, G.I. Therapeutic impact of grape leaves polyphenols on certain biochemical and neurological markers in AlCl3-induced Alzheimer’s disease. Biomed. Pharmacother. 2017, 93, 837–851. [Google Scholar] [CrossRef]
- Subash, S.; Essa, M.; Al-Asmi, A.; Al-Adawi, S.; Vaishnav, R.; Braidy, N.; Manivasagam, T.; Guillemin, G. Pomegranate from oman alleviates the brain oxidative damage in transgenic mouse model of alzheimer′s disease. J. Tradit. Complement. Med. 2014, 4, 232–238. [Google Scholar] [CrossRef]
- Guo, H.; Dong, Y.Q.; Ye, B.P. Cranberry extract supplementation exerts preventive effects through alleviating Aβ toxicity in Caenorhabditis elegans model of Alzheimer’s disease. Chin. J. Nat. Med. 2016, 14, 427–433. [Google Scholar] [CrossRef]
- Madhavadas, S.; Kapgal, V.K.; Kutty, B.M.; Subramanian, S. The Neuroprotective Effect of Dark Chocolate in Monosodium Glutamate-Induced Nontransgenic Alzheimer Disease Model Rats: Biochemical, Behavioral, and Histological Studies. J. Diet. Suppl. 2016, 13, 449–460. [Google Scholar] [CrossRef]
- Anwar, H.M.; Georgy, G.S.; Hamad, S.R.; Badr, W.K.; El Raey, M.A.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. A Leaf Extract of Harrisonia abyssinica Ameliorates Neurobehavioral, Histological and Biochemical Changes in the Hippocampus of Rats with Aluminum Chloride-Induced Alzheimer’s Disease. Antioxidants 2021, 10, 947. [Google Scholar] [CrossRef]
- Bowers, Z.; Maiti, P.; Bourcier, A.; Morse, J.; Jenrow, K.; Rossignol, J.; Dunbar, G. Tart Cherry Extract and Omega Fatty Acids Reduce Behavioral Deficits and Gliosis in the 5xFAD Mouse Model of Alzheimer’s Disease. Brain Sci. 2021, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Quiles, J.L.; Roma-Rodrigues, C.; Raposo, L.R.; Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Esteban-Muñoz, A.; Varela-López, A.; García, L.C.; Cianciosi, D.; et al. Rosa x hybrida extracts with dual actions: Antiproliferative effects against tumour cells and inhibitor of Alzheimer disease. Food Chem. Toxicol. 2021, 149, 112018. [Google Scholar] [CrossRef] [PubMed]
- Munasinghe, M.; Afshari, R.; Heydarian, D.; Almotayri, A.; Dias, D.A.; Thomas, J.; Jois, M. Effects of cocoa on altered metabolite levels in purine metabolism pathways and urea cycle in Alzheimer’s disease in C. elegans. Transl. Med. Aging 2022, 6, 14–24. [Google Scholar] [CrossRef]
- Navarro-Hortal, M.D.; Romero-Márquez, J.M.; Esteban-Muñoz, A.; Sánchez-González, C.; Rivas-García, L.; Llopis, J.; Cianciosi, D.; Giampieri, F.; Sumalla-Cano, S.; Battino, M.; et al. Strawberry (Fragaria × ananassa cv. Romina) methanolic extract attenuates Alzheimer’s beta amyloid production and oxidative stress by SKN-1/NRF and DAF-16/FOXO mediated mechanisms in C. elegans. Food Chem. 2022, 372, 131272. [Google Scholar] [CrossRef] [PubMed]
- Mahnashi, M.H.; Ashraf, M.; Alhasaniah, A.H.; Ullah, H.; Zeb, A.; Ghufran, M.; Fahad, S.; Ayaz, M.; Daglia, M. Polyphenol-enriched Desmodium elegans DC. ameliorate scopolamine-induced amnesia in animal model of Alzheimer’s disease: In Vitro, In Vivo and In Silico approaches. Biomed. Pharmacother. 2023, 165, 115144. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.C.L.; Malta, S.M.; Franco, R.R.; Silva, H.C.G.; Silva, M.H.; Rodrigues, T.S.; De Oliveira, R.M.; Araújo, T.N.; Augusto, S.C.; Espindola, F.S.; et al. Antioxidant and anti-Alzheimer’s potential of Tetragonisca angustula (Jataí) stingless bee pollen. Sci. Rep. 2024, 14, 308. [Google Scholar] [CrossRef]
- Bezerra, J.R.; de Souza Nascimento, T.; Tavares, J.; de Aguiar, M.S.S.; Maia, M.V.V.; de Andrade, G.M. Neuroprotective Effect of Chlorogenic Acid in an Animal Model of Sporadic Alzheimer’s Disease Induced by Streptozotocin. Mol. Neurobiol. 2024. [Google Scholar] [CrossRef]
- Maiti, P.; Hall, T.C.; Paladugu, L.; Kolli, N.; Learman, C.; Rossignol, J.; Dunbar, G.L. A comparative study of dietary curcumin, nanocurcumin, and other classical amyloid-binding dyes for labeling and imaging of amyloid plaques in brain tissue of 5×-familial Alzheimer’s disease mice. Histochem. Cell Biol. 2016, 146, 609–625. [Google Scholar] [CrossRef]
- Fedotova, J.; Soultanov, V.; Nikitina, T.; Roschin, V.; Ordyan, N.; Hritcu, L. Cognitive-enhancing activities of the polyprenol preparation Ropren® in gonadectomized β-amyloid (25–35) rat model of Alzheimer’s disease. Physiol. Behav. 2016, 157, 55–62. [Google Scholar] [CrossRef]
- Xian, Y.F.; Qu, C.; Liu, Y.; Ip, S.P.; Yuan, Q.J.; Yang, W.; Lin, Z.X. Magnolol Ameliorates Behavioral Impairments and Neuropathology in a Transgenic Mouse Model of Alzheimer’s Disease. Oxidative Med. Cell. Longev. 2020, 2020, 5920476. [Google Scholar] [CrossRef]
- Singh, N.A.K.; Prasad, S. Ellagic Acid Reverses Alterations in the Expression of AMPA Receptor and Its Scaffolding Proteins in the Cerebral Cortex and Memory Decline in STZ-sporadic Alzheimer’ s Disease Mouse Model. Psychopharmacology 2024, 241, 2117–2131. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Giunta, B.; Bickford, P.C.; Fountain, M.; Tan, J.; Shytle, R.D. Nanolipidic particles improve the bioavailability and α-secretase inducing ability of epigallocatechin-3-gallate (EGCG) for the treatment of Alzheimer’s disease. Int. J. Pharm. 2010, 389, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Dragicevic, N.; Smith, A.; Lin, X.; Yuan, F.; Copes, N.; Delic, V.; Tan, J.; Cao, C.; Shytle, R.D.; Bradshaw, P.C. Green tea epigallocatechin-3-gallate (EGCG) and other flavonoids reduce Alzheimer’s amyloid-induced mitochondrial dysfunction. J. Alzheimer’s Dis. 2011, 26, 507–521. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Y.; Zhu, S.; Lu, Y.; Zhu, L.; Wang, Y.; Wang, X. Inhibition of Aβ aggregates in Alzheimer’s disease by epigallocatechin and epicatechin-3-gallate from green tea. Bioorganic Chem. 2020, 105, 104382. [Google Scholar] [CrossRef]
- Ding, J.; Huang, J.; Yin, D.; Liu, T.; Ren, Z.; Hu, S.; Ye, Y.; Le, C.; Zhao, N.; Zhou, H.; et al. Trilobatin Alleviates Cognitive Deficits and Pathologies in an Alzheimer’s Disease Mouse Model. Oxidative Med. Cell. Longev. 2021, 2021, 3298400. [Google Scholar] [CrossRef]
- Gao, J.-M.; Zhang, X.; Shu, G.-T.; Chen, N.-N.; Zhang, J.-Y.; Xu, F.; Li, F.; Liu, Y.-G.; Wei, Y.; He, Y.-Q.; et al. Trilobatin rescues cognitive impairment of Alzheimer’s disease by targeting HMGB1 through mediating SIRT3/SOD2 signaling pathway. Acta Pharmacol. Sin. 2022, 43, 2482–2494. [Google Scholar] [CrossRef]
- Ray, B.; Bisht, S.; Maitra, A.; Maitra, A.; Lahiri, D.K. Neuroprotective and neurorescue effects of a novel polymeric nanoparticle formulation of curcumin (NanoCurc™) in the neuronal cell culture and animal model: Implications for Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 23, 61–77. [Google Scholar] [CrossRef]
- Yamakawa, M.Y.; Uchino, K.; Watanabe, Y.; Adachi, T.; Nakanishi, M.; Ichino, H.; Hongo, K.; Mizobata, T.; Kobayashi, S.; Nakashima, K.; et al. Anthocyanin suppresses the toxicity of Aβ deposits through diversion of molecular forms in in vitro and in vivo models of Alzheimer’s disease. Nutr. Neurosci. 2016, 19, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Vepsäläinen, S.; Koivisto, H.; Pekkarinen, E.; Mäkinen, P.; Dobson, G.; McDougall, G.J.; Stewart, D.; Haapasalo, A.; Karjalainen, R.O.; Tanila, H.; et al. Anthocyanin-enriched bilberry and blackcurrant extracts modulate amyloid precursor protein processing and alleviate behavioral abnormalities in the APP/PS1 mouse model of Alzheimer’s disease. J. Nutr. Biochem. 2013, 24, 360–370. [Google Scholar] [CrossRef]
- Ksiezak-Reding, H.; Ho, L.; Santa-Maria, I.; Diaz-Ruiz, C.; Wang, J.; Pasinetti, G.M. Ultrastructural alterations of Alzheimer’s disease paired helical filaments by grape seed-derived polyphenols. Neurobiol. Aging 2012, 33, 1427–1439. [Google Scholar] [CrossRef]
- Rojanathammanee, L.; Puig, K.L.; Combs, C.K. Pomegranate Polyphenols and Extract Inhibit Nuclear Factor of Activated T-Cell Activity and Microglial Activation In Vitro and in a Transgenic Mouse Model of Alzheimer Disease. J. Nutr. 2013, 143, 597–605. [Google Scholar] [CrossRef] [PubMed]
- García-Morales, G.; Huerta-Reyes, M.; González-Cortazar, M.; Zamilpa, A.; Jiménez-Ferrer, E.; Silva-García, R.; Román-Ramos, R.; Aguilar-Rojas, A. Anti-inflammatory, antioxidant and anti-acetylcholinesterase activities of Bouvardia ternifolia: Potential implications in Alzheimer’s disease. Arch. Pharmacal Res. 2015, 38, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Yuan, J.P.; Ding, T.; Yan, L.; Ling, L.; Zhou, X.F.; Zeng, Y.Q. Neuroprotective Effect of Fagopyrum dibotrys Extract against Alzheimer’s Disease. Evid.-Based Complement. Altern. Med. 2017, 2017, 3294586. [Google Scholar] [CrossRef]
- Karthivashan, G.; Park, S.-Y.; Kweon, M.-H.; Kim, J.; Haque, M.E.; Cho, D.-Y.; Kim, I.-S.; Cho, E.-A.; Ganesan, P.; Choi, D.-K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; D’Erme, M.; Trovato, M.; Mancini, P.; Piacentini, L.; Casale, A.M.; Wessjohann, L.; Gazzino, R.; Costantino, P.; et al. Anti-Inflammatory Activity of A Polyphenolic Extract from Arabidopsis thaliana in In Vitro and In Vivo Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 708. [Google Scholar] [CrossRef]
- Palmioli, A.; Mazzoni, V.; De Luigi, A.; Bruzzone, C.; Sala, G.; Colombo, L.; Bazzini, C.; Zoia, C.P.; Inserra, M.; Salmona, M.; et al. Alzheimer’s Disease Prevention through Natural Compounds: Cell-Free, In Vitro, and In Vivo Dissection of Hop (Humulus lupulus L.) Multitarget Activity. ACS Chem. Neurosci. 2022, 13, 3152–3167. [Google Scholar] [CrossRef]
- Jeong, J.H.; Hong, G.-L.; Jeong, Y.G.; Lee, N.S.; Kim, D.K.; Park, J.Y.; Park, M.; Kim, H.M.; Kim, Y.E.; Yoo, Y.C.; et al. Mixed Medicinal Mushroom Mycelia Attenuates Alzheimer’s Disease Pathologies In Vitro and In Vivo. Curr. Issues Mol. Biol. 2023, 45, 6775–6789. [Google Scholar] [CrossRef] [PubMed]
- Hase, T.; Shishido, S.; Yamamoto, S.; Yamashita, R.; Nukima, H.; Taira, S.; Toyoda, T.; Abe, K.; Hamaguchi, T.; Ono, K.; et al. Rosmarinic acid suppresses Alzheimer’s disease development by reducing amyloid β aggregation by increasing monoamine secretion. Sci. Rep. 2019, 9, 8711. [Google Scholar] [CrossRef]
- Tan, F.H.P.; Najimudin, N.; Watanabe, N.; Shamsuddin, S.; Azzam, G. p-Coumaric acid attenuates the effects of Aβ42 in vitro and in a Drosophila Alzheimer’s disease model. Behav. Brain Res. 2023, 452, 114568. [Google Scholar] [CrossRef]
- Mori, T.; Rezai-Zadeh, K.; Koyama, N.; Arendash, G.W.; Yamaguchi, H.; Kakuda, N.; Horikoshi-Sakuraba, Y.; Tan, J.; Town, T. Tannic Acid Is a Natural β-Secretase Inhibitor That Prevents Cognitive Impairment and Mitigates Alzheimer-like Pathology in Transgenic Mice. J. Biol. Chem. 2012, 287, 6912–6927. [Google Scholar] [CrossRef]
- Yao, L.; Gu, X.; Song, Q.; Wang, X.; Huang, M.; Hu, M.; Hou, L.; Kang, T.; Chen, J.; Chen, H.; et al. Nanoformulated alpha-mangostin ameliorates Alzheimer’s disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J. Control. Release 2016, 226, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, V.S.; Samidurai, M.; Park, H.J.; Wang, M.; Park, R.Y.; Yu, S.Y.; Kang, H.K.; Hong, S.; Choi, W.-S.; Lee, Y.Y.; et al. Avenanthramide-C Restores Impaired Plasticity and Cognition in Alzheimer’s Disease Model Mice. Mol. Neurobiol. 2020, 57, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Naomi, R.; Yazid, M.D.; Teoh, S.H.; Balan, S.S.; Shariff, H.; Kumar, J.; Bahari, H.; Embong, H. Dietary Polyphenols as a Protection against Cognitive Decline: Evidence from Animal Experiments; Mechanisms and Limitations. Antioxidants 2023, 12, 1054. [Google Scholar] [CrossRef] [PubMed]
- Nájera-Maldonado, J.M.; Salazar, R.; Alvarez-Fitz, P.; Acevedo-Quiroz, M.; Flores-Alfaro, E.; Hernández-Sotelo, D.; Espinoza-Rojo, M.; Ramírez, M. Phenolic Compounds of Therapeutic Interest in Neuroprotection. J. Xenobiotics 2024, 14, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Nishal, S.; Phaugat, P.; Bazaad, J.; Dhaka, R.; Khatkar, S.; Khatkar, A.; Khayatkashani, M.; Alizadeh, P.; Haghighi, S.M.; Mehri, M. A Concise Review of Common Plant-derived Compounds as a Potential Therapy for Alzheimer’s Disease and Parkinson’s Disease: Insight into Structure-Activity-Relationship. CNS Neurol. Disord.-Drug Targets (Former. Curr. Drug Targets-CNS Neurol. Disord.) 2023, 22, 1057–1069. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Wang, J. Cocoa extracts reduce oligomerization of amyloid-β: Implications for cognitive improvement in Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2014, 41, 643–650. [Google Scholar] [CrossRef]
- Mandel, S.; Weinreb, O.; Amit, T.; Youdim, M.B.H. Cell signaling pathways in the neuroprotective actions of the green tea polyphenol (-)-epigallocatechin-3-gallate: Implications for neurodegenerative diseases. J. Neurochem. 2004, 88, 1555–1569. [Google Scholar] [CrossRef]
- Weinreb, O.; Amit, T.; Mandel, S.; Youdim, M.B.H. Neuroprotective molecular mechanisms of (−)-epigallocatechin-3-gallate: A reflective outcome of its antioxidant, iron chelating and neuritogenic properties. Genes Nutr. 2009, 4, 283–296. [Google Scholar] [CrossRef]
- Kelsey, N.A.; Wilkins, H.M.; Linseman, D.A. Nutraceutical Antioxidants as Novel Neuroprotective Agents. Molecules 2010, 15, 7792–7814. [Google Scholar] [CrossRef]
- Singh, N.A.; Mandal, A.K.A.; Khan, Z.A. Potential neuroprotective properties of epigallocatechin-3-gallate (EGCG). Nutr. J. 2015, 15, 60. [Google Scholar] [CrossRef] [PubMed]
- Hugel, H.M.; Jackson, N. Redox chemistry of green tea polyphenols: Therapeutic benefits in neurodegenerative diseases. Mini Rev. Med. Chem. 2012, 12, 380. [Google Scholar] [CrossRef] [PubMed]
- Leri, M.; Bertolini, A.; Stefani, M.; Bucciantini, M. EVOO Polyphenols Relieve Synergistically Autophagy Dysregulation in a Cellular Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 7225. [Google Scholar] [CrossRef]
- Maiuolo, J.; Costanzo, P.; Masullo, M.; D’Errico, A.; Nasso, R.; Bonacci, S.; Mollace, V.; Oliverio, M.; Arcone, R. Hydroxytyrosol–Donepezil Hybrids Play a Protective Role in an In Vitro Induced Alzheimer’s Disease Model and in Neuronal Differentiated Human SH-SY5Y Neuroblastoma Cells. Int. J. Mol. Sci. 2023, 24, 13461. [Google Scholar] [CrossRef]
- Alaziqi, B.; Beckitt, L.; Townsend, D.J.; Morgan, J.; Price, R.; Maerivoet, A.; Madine, J.; Rochester, D.; Akien, G.; Middleton, D.A. Characterization of Olive Oil Phenolic Extracts and Their Effects on the Aggregation of the Alzheimer’s Amyloid-β Peptide and Tau. ACS Omega 2024, 9, 32557–32578. [Google Scholar] [CrossRef]
- Abuznait, A.H.; Qosa, H.; Busnena, B.A.; El Sayed, K.A.; Kaddoumi, A. Olive-Oil-Derived Oleocanthal Enhances β-Amyloid Clearance as a Potential Neuroprotective Mechanism against Alzheimer’s Disease: In Vitro and in Vivo Studies. ACS Chem. Neurosci. 2013, 4, 973–982. [Google Scholar] [CrossRef] [PubMed]
- Tajmim, A.; Cuevas-Ocampo, A.K.; Siddique, A.B.; Qusa, M.H.; King, J.A.; Abdelwahed, K.S.; Sonju, J.J.; El Sayed, K.A. (-)-Oleocanthal Nutraceuticals for Alzheimer’s Disease Amyloid Pathology: Novel Oral Formulations, Therapeutic, and Molecular Insights in 5xFAD Transgenic Mice Model. Nutrients 2021, 13, 1702. [Google Scholar] [CrossRef]
- Lauretti, E.; Nenov, M.; Dincer, O.; Iuliano, L.; Praticò, D. Extra virgin olive oil improves synaptic activity, short-term plasticity, memory, and neuropathology in a tauopathy model. Aging Cell 2020, 19, e13076. [Google Scholar] [CrossRef]
- Dinda, B.; Dinda, M.; Kulsi, G.; Chakraborty, A.; Dinda, S. Therapeutic potentials of plant iridoids in Alzheimer’s and Parkinson’s diseases: A review. Eur. J. Med. Chem. 2019, 169, 185–199. [Google Scholar] [CrossRef]
- Grossi, C.; Ed Dami, T.; Rigacci, S.; Stefani, M.; Luccarini, I.; Casamenti, F. Employing Alzheimer Disease Animal Models for Translational Research: Focus on Dietary Components. Neurodegener. Dis. 2014, 13, 131–134. [Google Scholar] [CrossRef]
- Grossi, C.; Rigacci, S.; Ambrosini, S.; Ed Dami, T.; Luccarini, I.; Traini, C.; Failli, P.; Berti, A.; Casamenti, F.; Stefani, M. The Polyphenol Oleuropein Aglycone Protects TgCRND8 Mice against Aß Plaque Pathology. PLoS ONE 2013, 8, e71702. [Google Scholar] [CrossRef] [PubMed]
- Diomede, L.; Rigacci, S.; Romeo, M.; Stefani, M.; Salmona, M. Oleuropein aglycone protects transgenic C. elegans strains expressing Aβ42 by reducing plaque load and motor deficit. PLoS ONE 2013, 8, e58893. [Google Scholar] [CrossRef]
- Luccarini, I.; Pantano, D.; Nardiello, P.; Cavone, L.; Lapucci, A.; Miceli, C.; Nediani, C.; Berti, A.; Stefani, M.; Casamenti, F. The polyphenol oleuropein aglycone modulates the PARP1-SIRT1 interplay: An in vitro and in vivo study. J. Alzheimer’s Dis. 2016, 54, 737–750. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, P.; Bian, H.; Jin, S.; Liu, J.; Yu, N.; Cui, H.; Sun, F.; Qian, X.; Qiu, W.; et al. Reduced neurogenesis in human hippocampus with Alzheimer’s disease. Brain Pathol. 2024, 34, e13225. [Google Scholar] [CrossRef] [PubMed]
- Colizzi, C. The protective effects of polyphenols on Alzheimer’s disease: A systematic review. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2019, 5, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Kaddoumi, A.; Denney, T.S.; Deshpande, G.; Robinson, J.L.; Beyers, R.J.; Redden, D.T.; Praticò, D.; Kyriakides, T.C.; Lu, B.; Kirby, A.N.; et al. Extra-Virgin Olive Oil Enhances the Blood–Brain Barrier Function in Mild Cognitive Impairment: A Randomized Controlled Trial. Nutrients 2022, 14, 5102. [Google Scholar] [CrossRef]
- Arab, H.; Mahjoub, S.; Hajian-Tilaki, K.; Moghadasi, M. The effect of green tea consumption on oxidative stress markers and cognitive function in patients with Alzheimer’s disease: A prospective intervention study. Casp. J. Intern. Med. 2016, 7, 188. [Google Scholar]
- Ide, K.; Yamada, H.; Takuma, N.; Park, M.; Wakamiya, N.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Green Tea Consumption Affects Cognitive Dysfunction in the Elderly: A Pilot Study. Nutrients 2014, 6, 4032–4042. [Google Scholar] [CrossRef]
- Ide, K.; Yamada, H.; Takuma, N.; Kawasaki, Y.; Harada, S.; Nakase, J.; Ukawa, Y.; Sagesaka, Y.M. Effects of green tea consumption on cognitive dysfunction in an elderly population: A randomized placebo-controlled study. Nutr. J. 2015, 15, 49. [Google Scholar] [CrossRef]
- Baum, L.; Lam, C.W.K.; Cheung, S.K.-K.; Kwok, T.; Lui, V.; Tsoh, J.; Lam, L.; Leung, V.; Hui, E.; Ng, C.; et al. Six-Month Randomized, Placebo-Controlled, Double-Blind, Pilot Clinical Trial of Curcumin in Patients With Alzheimer Disease. J. Clin. Psychopharmacol. 2008, 28, 110–113. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Desideri, G.; Kwik-Uribe, C.; Grassi, D.; Necozione, S.; Ghiadoni, L.; Mastroiacovo, D.; Raffaele, A.; Ferri, L.; Bocale, R.; Lechiara, M.C.; et al. Benefits in Cognitive Function, Blood Pressure, and Insulin Resistance Through Cocoa Flavanol Consumption in Elderly Subjects With Mild Cognitive Impairment. Hypertension 2012, 60, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Escudero, J.; Baquero, M.; Cebrián, M.; Carbonell-Asíns, J.A.; Muñoz, J.E.; Satorres, E.; Meléndez, J.C.; Ferrer-Rebolleda, J.; Cózar-Santiago, M.d.P.; et al. Genistein effect on cognition in prodromal Alzheimer’s disease patients. The GENIAL clinical trial. Alzheimer’s Res. Ther. 2022, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Nash, T.A.; Shidler, M.D.; Shukitt-Hale, B.; Joseph, J.A. Concord grape juice supplementation improves memory function in older adults with mild cognitive impairment. Br. J. Nutr. 2010, 103, 730–734. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Pouchieu, C.; Pourtau, L.; Gaudout, D.; Pallet, V.; Drummond, P.D. Effects of a polyphenol-rich grape and blueberry extract (Memophenol™) on cognitive function in older adults with mild cognitive impairment: A randomized, double-blind, placebo-controlled study. Front. Psychol. 2023, 14, 1144231. [Google Scholar] [CrossRef]
- Morillas-Ruiz, J.M.; Rubio-Perez, J.M.; Albaladejo, M.D.; Zafrilla, P.; Parra, S.; Vidal-Guevara, M.L. Effect of an antioxidant drink on homocysteine levels in Alzheimer’s patients. J. Neurol. Sci. 2010, 299, 175–178. [Google Scholar] [CrossRef]
- Gleason, C.E.; Fischer, B.L.; Dowling, N.M.; Setchell, K.D.R.; Atwood, C.S.; Carlsson, C.M.; Asthana, S. Cognitive Effects of Soy Isoflavones in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 47, 1009–1019. [Google Scholar] [CrossRef]
- Tsolaki, M.; Lazarou, E.; Kozori, M.; Petridou, N.; Tabakis, I.; Lazarou, I.; Karakota, M.; Saoulidis, I.; Melliou, E.; Magiatis, P. A randomized clinical trial of greek high phenolic early harvest extra virgin olive oil in mild cognitive impairment: The MICOIL pilot study. J. Alzheimer’s Dis. 2020, 78, 801–817. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, J.M.; Castellano, J.M.; Garcia-Rodriguez, S.; Quintero-Flórez, A.; Carrasquilla, N.; Perona, J.S. Lipophilic Bioactive Compounds Transported in Triglyceride-Rich Lipoproteins Modulate Microglial Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 7706. [Google Scholar] [CrossRef]
- Borén, J.; Taskinen, M.-R.; Björnson, E.; Packard, C.J. Metabolism of triglyceride-rich lipoproteins in health and dyslipidaemia. Nat. Rev. Cardiol. 2022, 19, 577–592. [Google Scholar] [CrossRef]
- Peng, J.; Luo, F.; Ruan, G.; Peng, R.; Li, X. Hypertriglyceridemia and atherosclerosis. Lipids Health Dis. 2017, 16, 233. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, Z.J.; Lam, V.; Takechi, R.; Nesbit, M.; Vaccarezza, M.; Mamo, J.C.L. Peripheral metabolism of lipoprotein-amyloid beta as a risk factor for Alzheimer’s disease: Potential interactive effects of APOE genotype with dietary fats. Genes Nutr. 2023, 18, 2. [Google Scholar] [CrossRef] [PubMed]
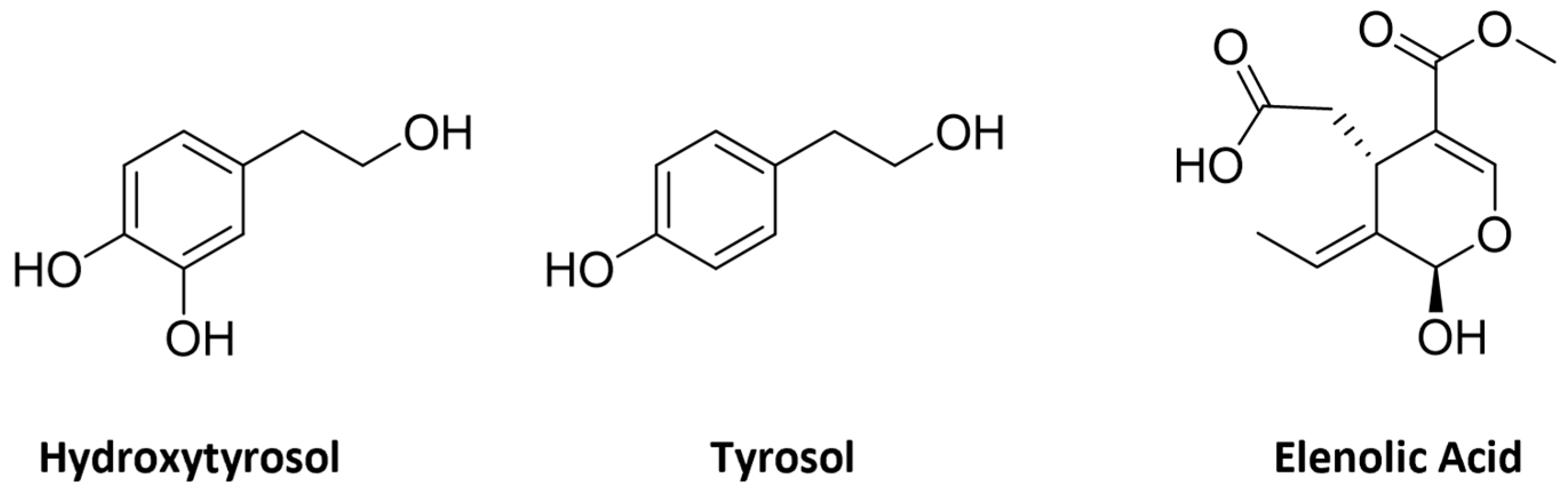

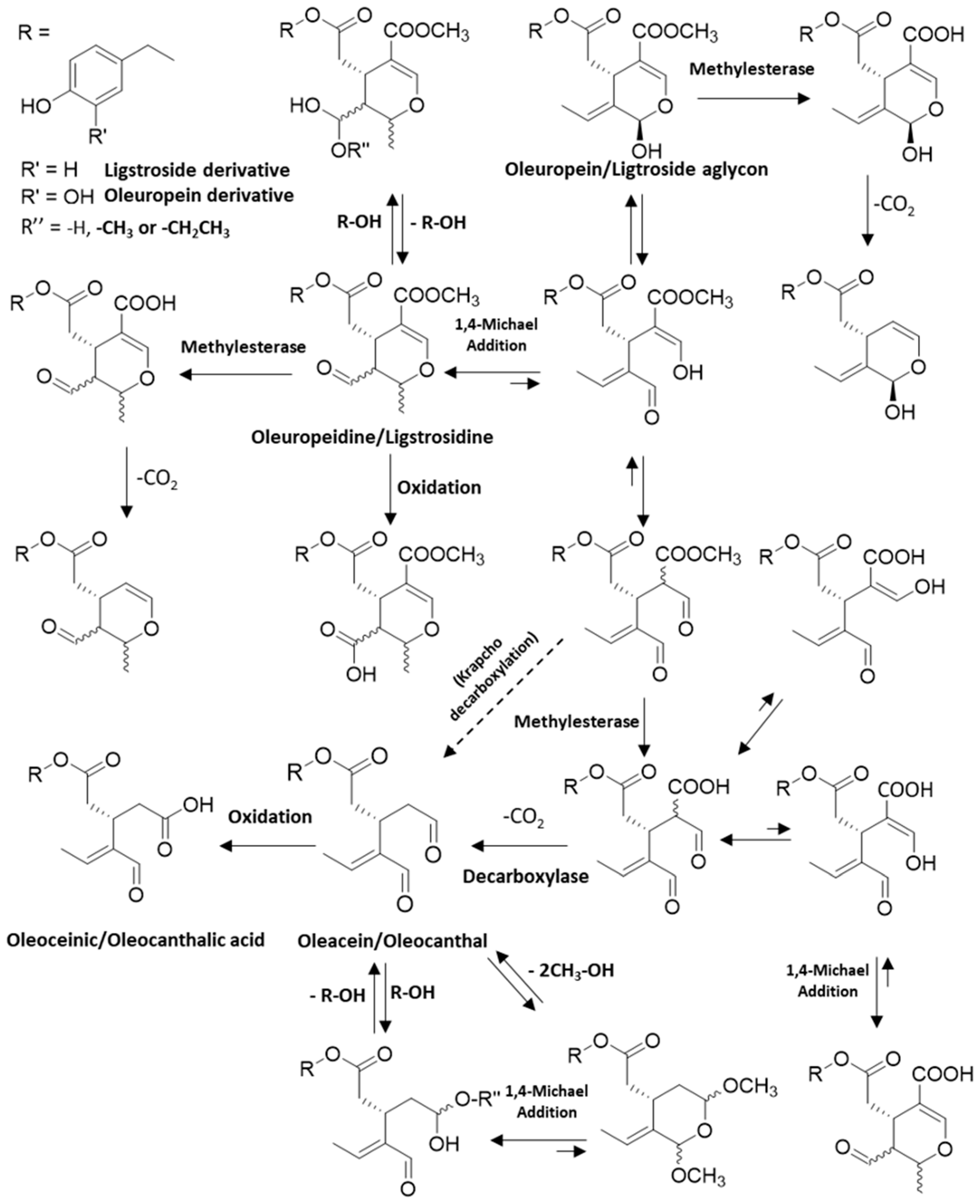
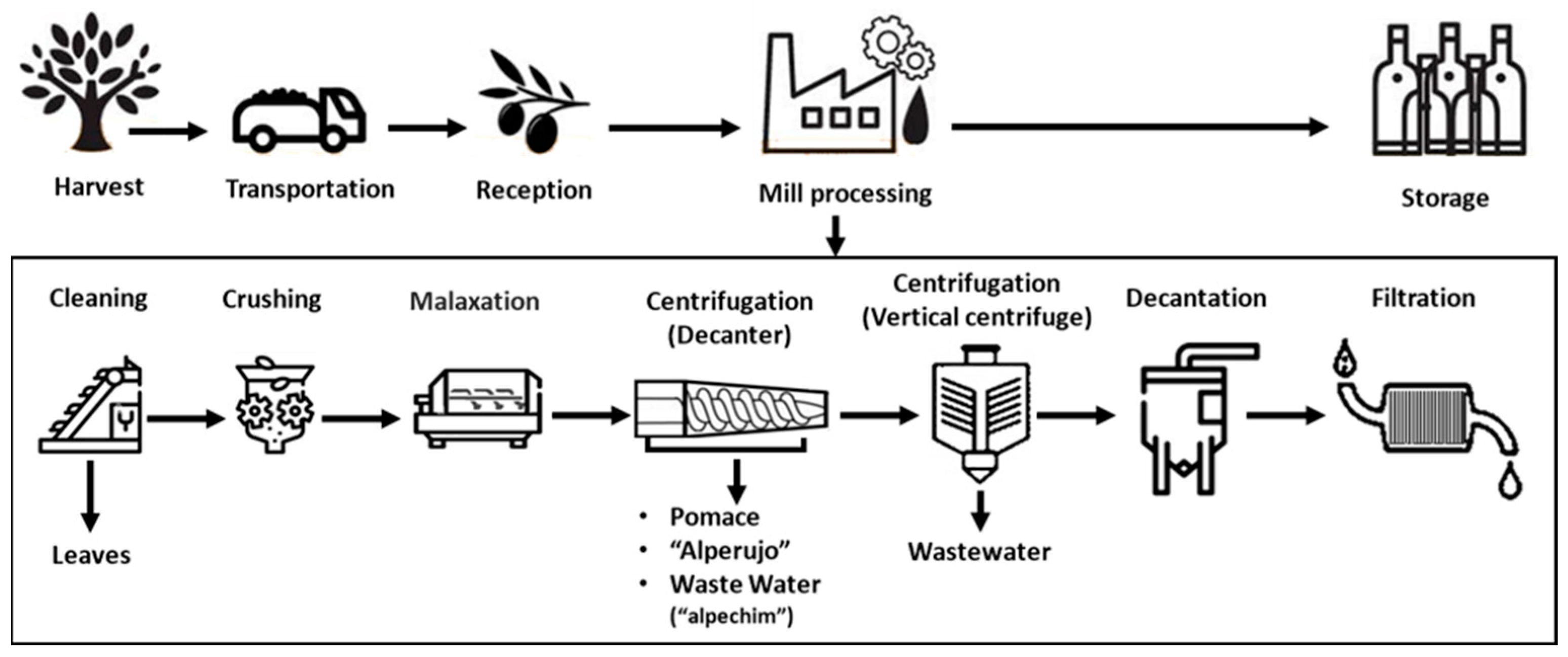

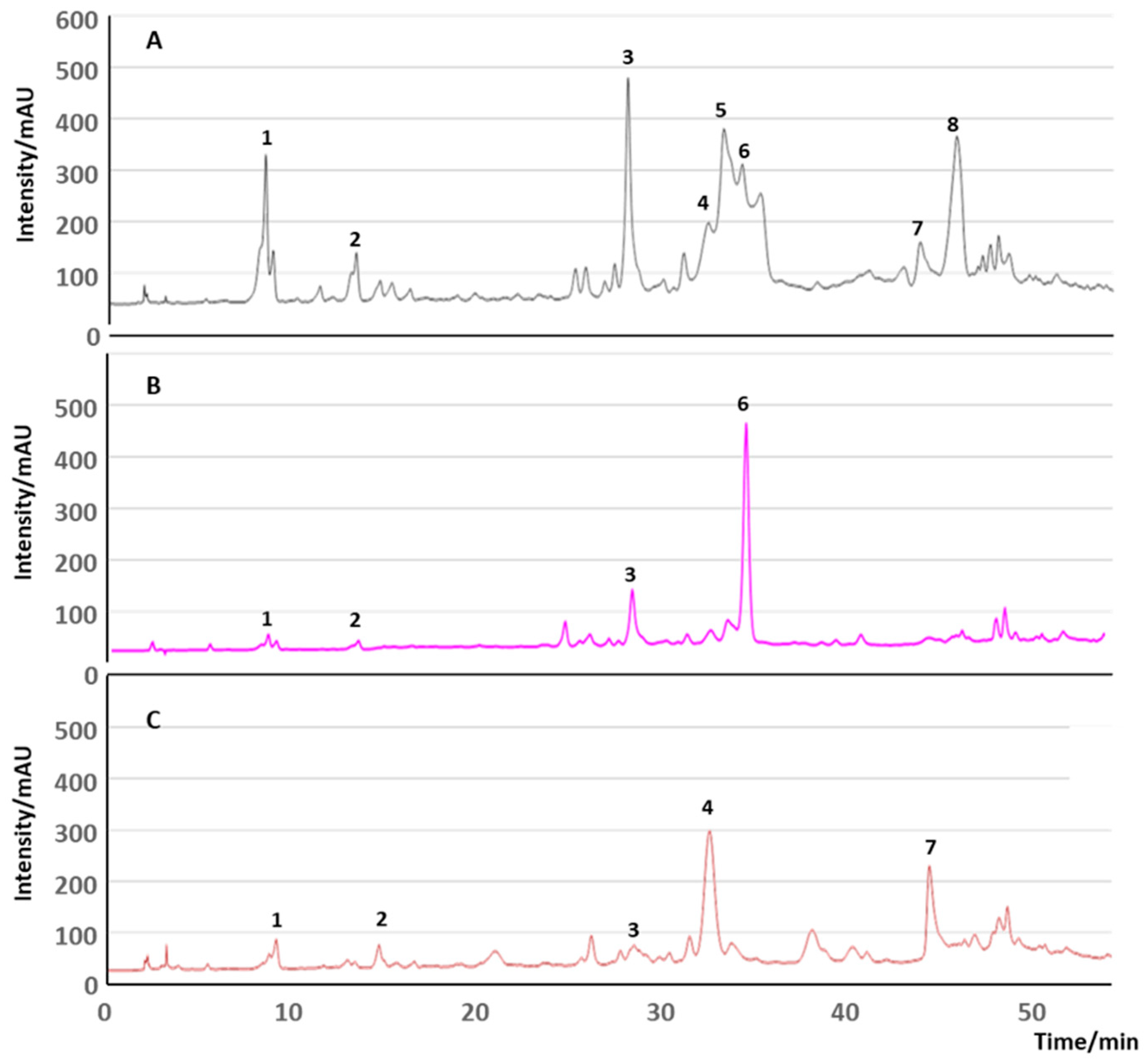
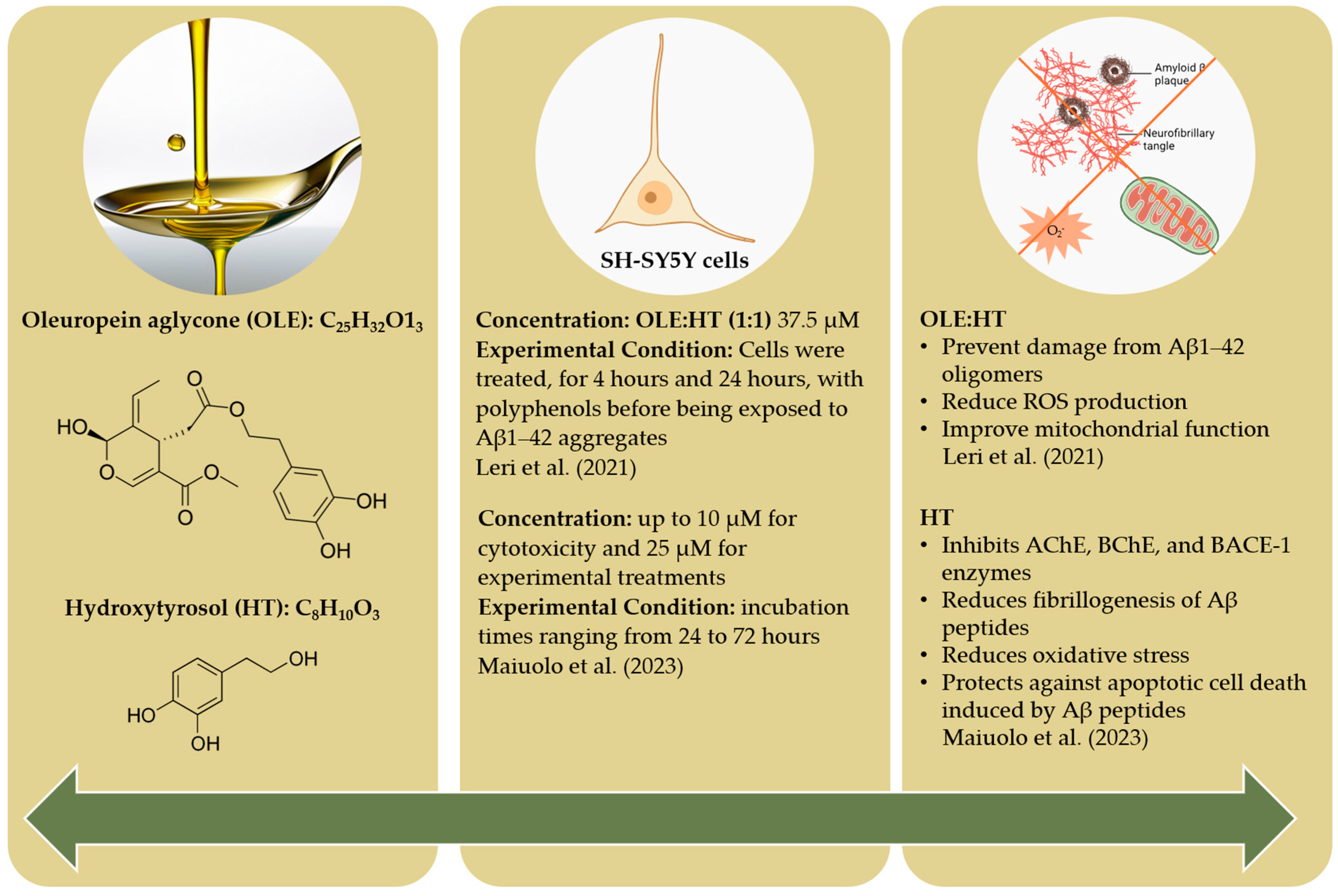
| Study Title | Participants | Intervention | Objective | Clinical Trial ID |
|---|---|---|---|---|
| Does extra virgin olive oil induce gene and metabolic changes in healthy subjects | 40 (healthy with family history of Alzheimer’s disease | 30 mL extra virgin olive oil daily for 6 months | Assess the impact of extra virgin olive oil on the molecular and biological mechanisms associated with AD in healthy individuals with a family history of the disease. | NCT05929924 |
| Pilot Study About Extra Virgin Olive Oil “Coratina” in Mild Cognitive Impairment and Alzheimer’s Disease Patients (EVOCAD) | 24 mild cognitive impairment and Alzheimer’s disease patients | Extra virgin olive oil ‘Coratina’ vs. biophenol low-dose olive oil for 12 months | Assess and compare the effects of extra virgin olive oil and biophenol low-dose olive oil on brain and cardiovascular functions. | NCT04229186 |
| Auburn University research on olive oil for Alzheimer’s disease (AU-ROOAD) | 24 Mild cognitive impairment patients | Extra-virgin olive oil that is rich with oleocanthal versus phenols and olive oil low in oleocanthal and other phenols, daily for 6 months | Evaluate the effect of extra-virgin olive oil in MCI participants and compare its effect with refined olive oil (ROO; null in phenolic fraction) using a variety of metrics: (i) the blood brain barrier integrity obtained from contrast-enhanced MRI imaging, and brain function and network connectivity obtained by functional MRI (fMRI), (ii) cognitive function assessed using neuropsychological evaluation, and (iii) blood Aβ, tau, phospho-tau 181, and neurofilament light levels. | NCT03824197 |
| Greek high-phenolic early harvest extra virgin olive oil in mild cognitive impairment: the MICOIL Pilot Study | 50 mild cognitive impairment patients | High phenolic early-harvest extra virgin olive oil (HP-EH-EVOO) versus moderate-phenolic (MP-EVOO), 50 mL/day, for 12 months | Evaluate the beneficial effect of extra virgin olive oil in comparison to freshly pressed extra virgin olive oil on patients diagnosed with mild cognitive impairment | NCT03362996 |
| Attenuation of Postprandial Inflammatory Processes in Alzheimer’s Disease Patients by Consumption of Pomace Oil (CORDIAL) | 80 (40 early-stage Alzheimer’s disease patients, 40 healthy individuals | Olive pomace oil versus high-oleic sunflower oil in the other | Evaluate the effect on triglyceride-rich lipoproteins and neuroinflammation via microglia inactivation | NCT06245616 |
| Management of dementia with olive oil leaves (GOLDEN) | 100 mild cognitive impairment patients | Beverage of olive oil from leaves versus dietary supplement: Mediterranean diet, for 24 months | Evaluate and compare the effects of the olive-leaf beverage and Mediterranean diet on memory and cognitive function in individuals with mild dementia. | NCT04440020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, M.; Costa, M.; Paiva-Martins, F.; Silva, P. Olive Oil Industry By-Products as a Novel Source of Biophenols with a Promising Role in Alzheimer Disease Prevention. Molecules 2024, 29, 4841. https://doi.org/10.3390/molecules29204841
Gonçalves M, Costa M, Paiva-Martins F, Silva P. Olive Oil Industry By-Products as a Novel Source of Biophenols with a Promising Role in Alzheimer Disease Prevention. Molecules. 2024; 29(20):4841. https://doi.org/10.3390/molecules29204841
Chicago/Turabian StyleGonçalves, Marta, Marlene Costa, Fátima Paiva-Martins, and Paula Silva. 2024. "Olive Oil Industry By-Products as a Novel Source of Biophenols with a Promising Role in Alzheimer Disease Prevention" Molecules 29, no. 20: 4841. https://doi.org/10.3390/molecules29204841
APA StyleGonçalves, M., Costa, M., Paiva-Martins, F., & Silva, P. (2024). Olive Oil Industry By-Products as a Novel Source of Biophenols with a Promising Role in Alzheimer Disease Prevention. Molecules, 29(20), 4841. https://doi.org/10.3390/molecules29204841









