Novel Thiourea Ligands—Synthesis, Characterization and Preliminary Study on Their Coordination Abilities
Abstract
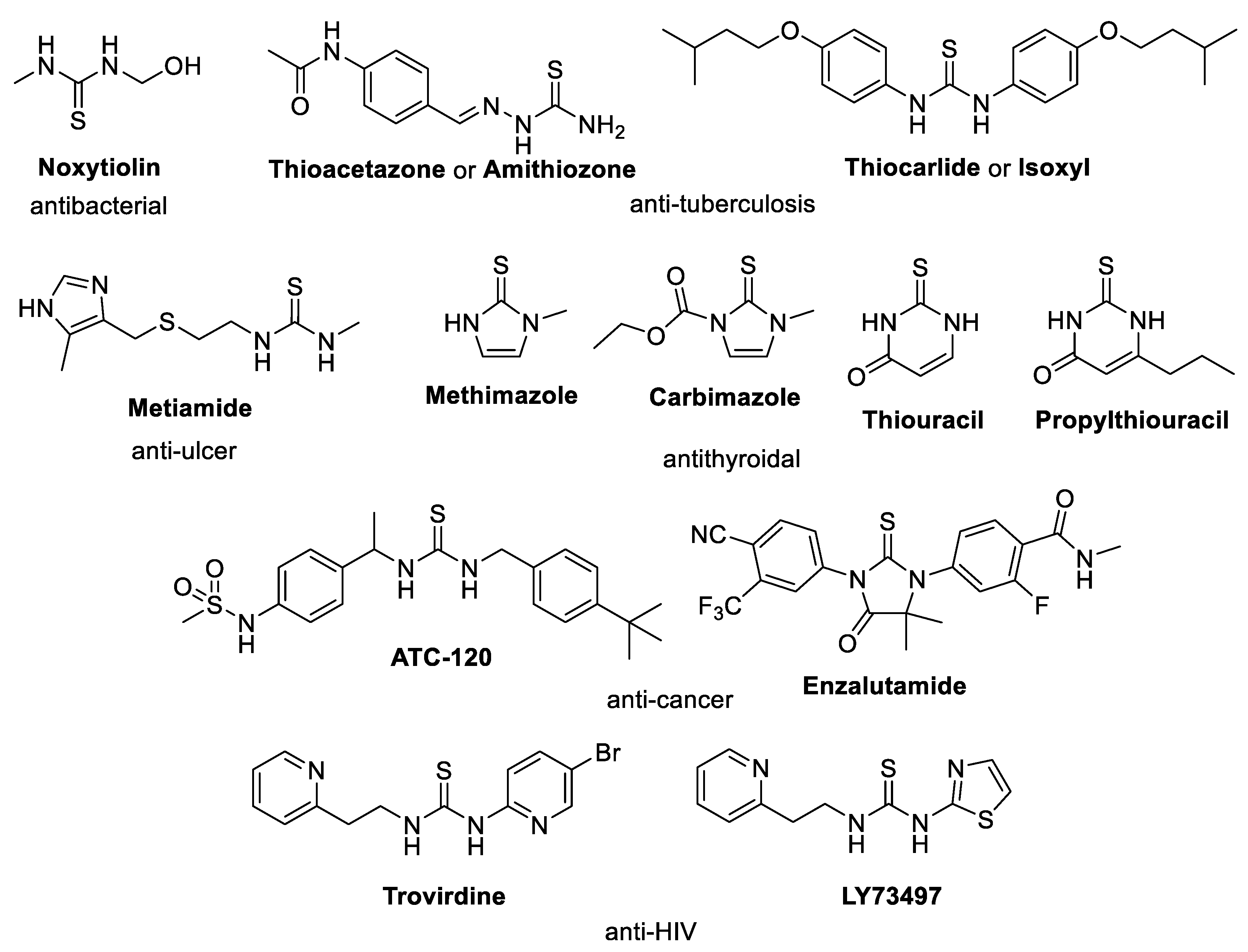
:1. Introduction
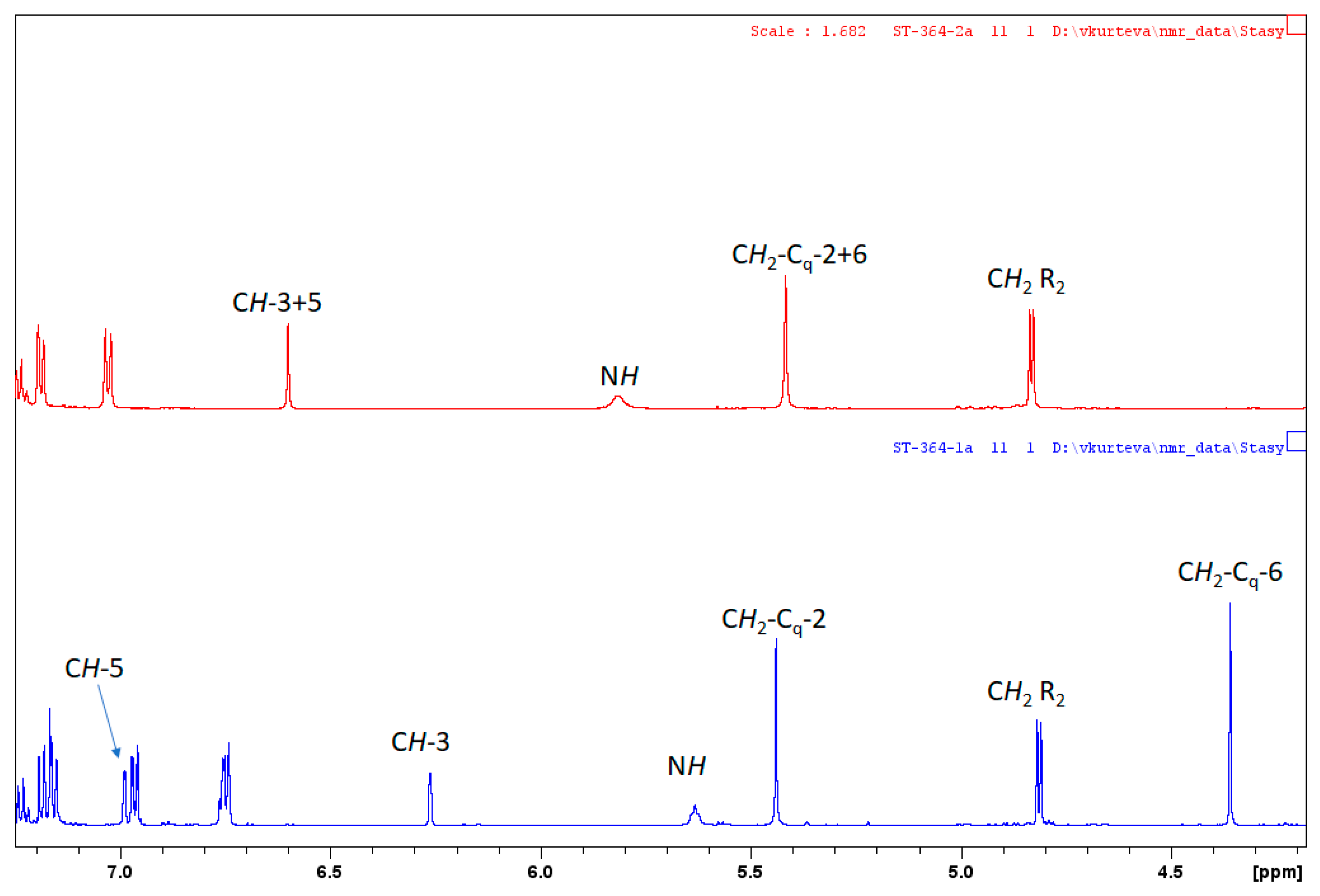
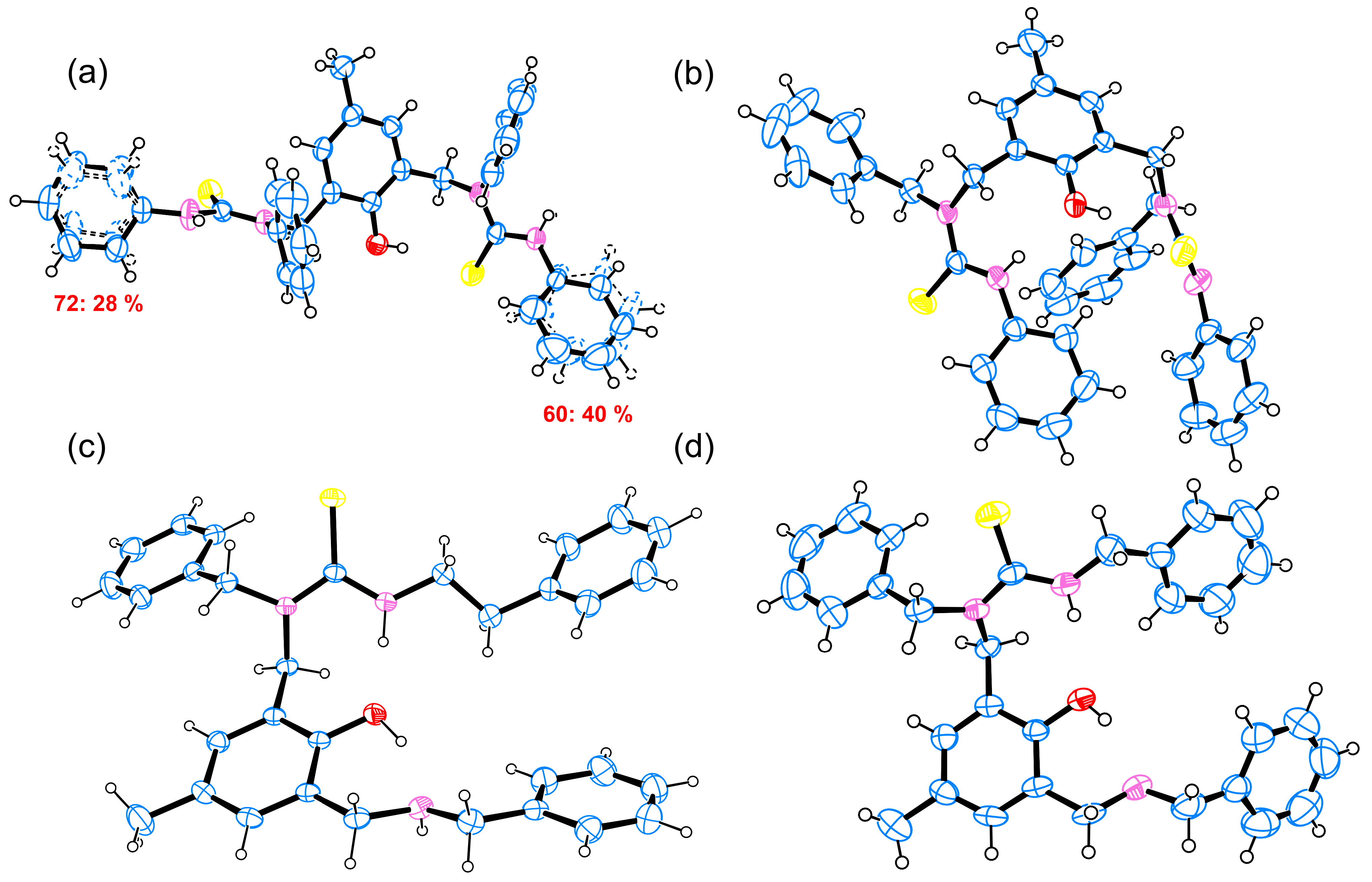
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. General Procedure for the Preparation of Ligands 3 and 4
3.3. Crystallography
3.4. Isothermal Titration Calorimetry (ITC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schroeder, D.C. Thioureas. Chem. Rev. 1955, 55, 181–228. [Google Scholar] [CrossRef]
- Aly, A.A.; Ahmed, E.K.; El-Mokadem, K.M.; Hegazy, M.E.F. Update survey on aroyl substituted thioureas and their applications. J. Sulfur Chem. 2007, 28, 73–93. [Google Scholar] [CrossRef]
- Saeed, A.; Flörke, U.; Erben, M.F. A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J. Sulfur Chem. 2014, 35, 318–355. [Google Scholar] [CrossRef]
- Zahra, U.; Saeed, A.; Abdul Fattah, T.; Flörke, U.; Erben, M.F. Recent trends in chemistry, structure, and various applications of 1-acyl-3-substituted thioureas: A detailed review. RSC Adv. 2022, 12, 12710–12745. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V. Urea/Thiourea based optical sensors for toxic analytes: A convenient path for detection of first nerve agent (Tabun). Bull. Chem. Soc. Jpn. 2021, 94, 309–326. [Google Scholar] [CrossRef]
- Muhammad, M.; Khan, S.; Shehzadi, S.A.; Gul, Z.; Al-Saidi, H.M.; Kamran, A.W.; Alhumaydhi, F.A. Recent advances in colorimetric and fluorescent chemosensors based on thiourea derivatives for metallic cations: A review. Dyes Pigm. 2022, 205, 110477. [Google Scholar] [CrossRef]
- Al-Saidi, H.M.; Khan, S. Recent Advances in Thiourea based colorimetric and fluorescent chemosensors for detection of anions and neutral analytes: A review. Crit. Rev. Anal. Chem. 2024, 54, 93–109. [Google Scholar] [CrossRef]
- George, M.; Tan, G.; John, V.T.; Weiss, R.G. Urea and thiourea derivatives as low molecular-mass organogelators. Chem. Eur. J. 2005, 11, 3243–3254. [Google Scholar] [CrossRef]
- Fatima, S.; Sharma, R.; Asghar, F.; Kamal, A.; Badshah, A.; Kraatz, H.-B. Study of new amphiphiles based on ferrocene containing thioureas as efficient corrosion inhibitors: Gravimetric, electrochemical, SEM and DFT studies. J. Ind. Eng. Chem. 2019, 76, 374–387. [Google Scholar] [CrossRef]
- Bennett, E.; Greenberg, M.W.; Jordan, A.J.; Hamachi, L.S.; Banerjee, S.; Billinge, S.J.L.; Owen, J.S. Size dependent optical properties and structure of ZnS nanocrystals prepared from a library of thioureas. Chem. Mater. 2022, 34, 706–717. [Google Scholar] [CrossRef]
- Lambert, W.T.; Goldsmith, M.E.; Sparks, T.C. Insecticidal activity of novel thioureas and isothioureas. Pest Manag. Sci. 2016, 73, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Bosch, A.; Bettiol, M.; González, D.L.N.; Erben, M.F.; Lamberti, Y. Novel guanidine compound against multidrug-resistant cystic fibrosis-associated bacterial species. Molecules 2018, 23, 1158. [Google Scholar] [CrossRef]
- Ronchetti, R.; Moroni, G.; Carotti, A.; Gioiello, A.; Camaioni, E. Recent advances in urea- and thiourea-containing compounds: Focus on innovative approaches in medicinal chemistry and organic synthesis. RSC Med. Chem. 2021, 12, 1046–1064. [Google Scholar] [CrossRef] [PubMed]
- Roman, R.; Pintilie, L.; Căproiu, M.T.; Dumitrașcu, F.; Nuță, D.C.; Zarafu, I.; Ioniță, P.; Chifiriuc, M.C.; Chiriță, C.; Moroșan, A.; et al. New N-acyl thiourea derivatives: Synthesis, standardized quantification method and in vitro evaluation of potential biological activities. Antibiotics 2023, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, H.; Takemoto, Y. Discovery and application of asymmetric reaction by multi-functional thioureas. Bull. Chem. Soc. Jpn. 2008, 81, 785–795. [Google Scholar] [CrossRef]
- Dawood, K.M. Bis-thiourea derivatives and their utility in synthesis of mono-heterocyclic, bis-heterocyclic, and fused heterocyclic systems. J. Heterocycl. Chem. 2019, 56, 1701–1721. [Google Scholar] [CrossRef]
- Steppeler, F.; Iwan, D.; Wojaczyńska, E.; Wojaczyński, J. Chiral thioureas—Preparation and significance in asymmetric synthesis and medicinal chemistry. Molecules 2020, 25, 401. [Google Scholar] [CrossRef]
- Gandhi, S.; Sivadas, V.; Baire, B. Thiourea–tertiary amine promoted cascade catalysis: A tool for complexity generation. Eur. J. Org. Chem. 2021, 2021, 220–234. [Google Scholar] [CrossRef]
- Takemoto, Y. Development of chiral thiourea catalysts and its application to asymmetric catalytic reactions. Chem. Pharm. Bull. 2010, 58, 593–601. [Google Scholar] [CrossRef]
- Li, J.; Shi, L.-L.; Chen, J.; Gong, J.; Yang, Z. Thioureas as ligands in organometallic reactions. Synthesis 2014, 46, 2007–2023. [Google Scholar]
- Limnios, D.; Kokotos, C.G. Ureas and Thioureas as Asymmetric Organocatalysts. In Sustainable Catalysis: Without Metals or Other Endangered Elements, Part 2, ed.; North, M., North, M., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2015; Chapter 19; pp. 196–255. [Google Scholar]
- Park, Y.; Harper, K.C.; Kuhl, N.; Kwan, E.E.; Liu, R.Y.; Jacobsen, E.N. Macrocyclic bis-thioureas catalyse stereospecific glycosylation reactions. Science 2017, 355, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Parvin, T.; Yadav, R.; Choudhury, L.H. Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs). Org. Biomol. Chem. 2020, 18, 5513–5532. [Google Scholar] [CrossRef] [PubMed]
- Jain, I.; Malik, P. Advances in urea and thiourea catalyzed ring opening polymerization: A brief overview. Eur. Polym. J. 2020, 133, 109791. [Google Scholar] [CrossRef]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badshah, A. Thiourea derivatives in drug design and medicinal chemistry: A short review. J. Drug Des. Med. Chem. 2016, 2, 10–20. [Google Scholar] [CrossRef]
- Wahid, A.; Basra, S.M.A.; Farooq, M. Thiourea: A molecule with immense biological significance for plants. Int. J. Agric. Biol. 2017, 19, 911–920. [Google Scholar] [CrossRef]
- Goncalves, I.L.; de Azambuja, G.O.; Kawano, D.F.; Eifler-Lima, V.L. Thioureas as building blocks for the generation of heterocycles and compounds with pharmacological activity: An overview. Mini-Rev. Org. Chem. 2018, 15, 28–35. [Google Scholar] [CrossRef]
- Mishra, A.; Batra, S. Thiourea and guanidine derivatives as antimalarial and antimicrobial agents. Curr. Top. Med. Chem. 2013, 13, 2011–2025. [Google Scholar] [CrossRef]
- Ravichandran, V.; Shalini, S.; Kumar, K.S.; Rajak, H.; Agrawal, R.K. Design, synthesis and evaluation of thiourea derivatives as antimicrobial and antiviral agents. Lett. Drug Des. Discov. 2019, 16, 618–624. [Google Scholar] [CrossRef]
- Özgeriş, B. Synthesis of substituted phenethylamine-based thioureas and their antimicrobial and antioxidant properties. Russ. J. Org. Chem. 2021, 57, 422–429. [Google Scholar] [CrossRef]
- Brown, J.R.; North, E.J.; Hurdle, J.G.; Morisseau, C.; Scarborough, J.S.; Sun, D.; Korduláková, J.; Scherman, M.S.; Jones, V.; Grzegorzewicz, A.; et al. The structure-activity relationship of urea derivatives as anti-tuberculosis agents. Bioorg. Med. Chem. 2011, 19, 5585–5595. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Qazi, S.; Yiv, S.; Uckun, F.M. A novel vaginal microbicide containing the rationally designed anti-HIV compound HI-443 (N′-[2-(2-thiophene)ethyl]-N′-[2-(5-bromopyridyl)] thiourea]). Expert Opin. Investig. Drugs 2012, 21, 265–279. [Google Scholar] [CrossRef] [PubMed]
- Tong, K.K.H.; Hanif, M.; Lovett, J.H.; Hummitzsch, K.; Harris, H.H.; Söhnel, T.; Jamieson, S.M.F.; Hartinger, C.G. Thiourea-derived chelating ligands and their organometallic compounds: Investigations into their anticancer activity. Molecules 2020, 25, 3661. [Google Scholar] [CrossRef]
- Canudo-Barreras, G.; Ortego, L.; Izaga, A.; Marzo, I.; Herrera, R.P.; Gimeno, M.C. Synthesis of new thiourea-metal complexes with promising anticancer properties. Molecules 2021, 26, 6891. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, W. Advancement and recent trends in seeking less toxic and more active anti-cancer drugs: Insights into thiourea based molecules. Main Group Chem. 2022, 21, 885–901. [Google Scholar] [CrossRef]
- Gilmore, O.J.; Reid, C. Noxytiolin and peritoneal adhesion formation. Br. J. Surg. 1976, 63, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Zmrhal, J.; Nezádalová, E. Intraperitoneal administration of noxythiolin VUFB in the prevention and therapy of inflammatory postoperative complications in gynecology. Cesk Gynekol. 1992, 57, 71–73. [Google Scholar]
- Phetsuksiri, B.; Jackson, M.; Scherman, H.; McNeil, M.; Besra, G.S.; Baulard, A.R.; Slayden, R.A.; DeBarber, A.E.; Barry III, C.E.; Baird, M.S.; et al. Unique mechanism of action of the thiourea drug isoxyl on Mycobacterium tuberculosis. J. Biol. Chem. 2003, 278, 53123–53130. [Google Scholar] [CrossRef]
- Falzon, D.; Hill, G.; Pal, S.N.; Suwankesawong, W.; Jaramillo, E. Pharmacovigilance and tuberculosis: Applying the lessons of Thioacetazone. Bull. World Health Organ. 2014, 92, 918–919. [Google Scholar] [CrossRef]
- de Freitas Paulo, T.; Duhayon, C.; de França Lopes, L.G.; Sousa, E.H.S.; Chauvin, R.; Bernardes-Génisson, V. Further Insights into the oxidative pathway of thiocarbonyl-type antitubercular prodrugs: Ethionamide, Thioacetazone, and Isoxyl. Chem. Res. Toxicol. 2021, 34, 1879–1889. [Google Scholar] [CrossRef]
- Black, J.W.; Duncan, W.A.; Emmett, J.C.; Ganellin, C.R.; Hesselbo, T.; Parsons, M.E.; Wyllie, J.H. Metiamide—An orally active histamine H2-receptor antagonist. Agents Actions 1973, 3, 133–137. [Google Scholar] [CrossRef]
- Diav-Citrin, O.; Ornoy, A. Teratogen update: Antithyroid drugs—Methimazole, Carbimazole, and Propylthiouracil. Teratology 2002, 65, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.D.A.; Kung, J. Propylthiouracil, and methimazole, and carbimazole-related hepatotoxicity. Expert Opin. Drug Saf. 2014, 13, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- Durant, G.J.; Emmett, J.C.; Ganellin, C.R.; Miles, P.D.; Parsons, M.E.; Prain, H.D.; White, G.R. Cyanoguanidine-thiourea equivalence in the development of the histamine H2-receptor antagonist, cimetidine. J. Med. Chem. 1977, 20, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jee, J.G. Repositioning of Thiourea-Containing Drugs as Tyrosinase Inhibitors. Int. J. Mol. Sci. 2015, 16, 28534–28548. [Google Scholar] [CrossRef]
- Schalken, J.; Fitzpatrick, J.M. Enzalutamide: Targeting the androgen signalling pathway in metastatic castration-resistant prostate cancer. BJU Int. 2016, 117, 215–225. [Google Scholar] [CrossRef]
- Ahgren, C.; Backro, K.; Bell, F.W.; Cantrell, A.S.; Clemens, M.; Colacino, J.M.; Deeter, J.B.; Engelhardt, J.A.; Hogberg, M.; Jaskunas, S.R.; et al. The PETT series, a new class of potent nonnucleoside inhibitors ofhuman immunodeficiency virus type 1 reverse transcriptase. Antimicrob. Agents Chemother. 1995, 39, 1329–1335. [Google Scholar] [CrossRef]
- De Clercq, E. In search of a selective antiviral chemotherapy. Clin. Microbiol. Rev. 1997, 10, 674–693. [Google Scholar] [CrossRef]
- De Clercq, E. Hamao Umezawa memorial award lecture: An Odyssey in the viral chemotherapy field. Int. J. Antimicrob. Agents 2001, 18, 309–328. [Google Scholar] [CrossRef]
- Petz, W. 40 Years of transition-metal thiocarbonyl chemistry and the related CSe and CTe compounds. Coord. Chem. Rev. 2008, 252, 1689–1733. [Google Scholar] [CrossRef]
- Schenk, W.A. The coordination chemistry of small sulfur-containing molecules: A personal perspective. Dalton Trans. 2011, 40, 1209–1219. [Google Scholar] [CrossRef]
- Maiti, B.K.; Almeida, R.M.; Moura, I.; Moura, J.J.G. Rubredoxins derivatives: Simple sulphur-rich coordination metal sites and its relevance for biology and chemistry. Coord. Chem. Rev. 2017, 352, 379–397. [Google Scholar] [CrossRef]
- Haiduc, I. Inverse coordination metal complexes with oxalate and sulfur, selenium and nitrogen analogues as coordination centers. Topology and systematization. J. Coord. Chem. 2020, 73, 1619–1700. [Google Scholar] [CrossRef]
- Gilbert-Bass, K.; Stennett, C.R.; Grotjahn, R.; Ziller, J.W.; Furche, F.; Evans, W.J. Exploring sulfur donor atom coordination chemistry with La(II), Nd(II), and Tm(II) using a terphenylthiolate ligand. Chem. Commun. 2024, 60, 4601–4604. [Google Scholar] [CrossRef]
- Rangel-Garcia, J.; Rivas, C.E.; Serrano, O.; Cristobal, C. AcSac, SacSac, and SacNac, the forgotten sulfur-based ligands and their reactivity in the formation of main and transition metal complexes. Eur. J. Inorg. Chem. 2024, 27, e202400118. [Google Scholar] [CrossRef]
- Hollmann, K.; Oppermann, A.; Witte, M.; Li, S.; Amen, M.; Flörke, U.; Egold, H.; Henkel, G.; Herres-Pawlis, S. Copper(I) complexes with thiourea derivatives as ligands: Revealing secrets of their bonding scheme. Eur. J. Inorg. Chem. 2017, 2017, 1266–1279. [Google Scholar] [CrossRef]
- Mohapatra, R.K.; Das, P.K.; Pradhan, M.K.; El-Ajaily, M.M.; Das, D.; Salem, H.F.; Mahanta, U.; Badhei, G.; Parhi, P.K.; Maihub, A.A.; et al. Recent advances in urea- and thiourea-based metal complexes: Biological, sensor, optical, and corroson inhibition studies. Comments Inorganic Chem. 2019, 39, 27–187. [Google Scholar] [CrossRef]
- Nkabyo, H.A.; Barnard, I.; Koch, K.R.; Luckay, R.C. Recent advances in the coordination and supramolecular chemistry of monopodal and bipodal acylthiourea-based ligands. Coord. Chem. Rev. 2021, 427, 213588. [Google Scholar] [CrossRef]
- Ullah, S.A.; Saeed, A.; Azeem, M.; Haider, M.B.; Erben, M.F. Exploring the latest trends in chemistry, structure, coordination, and diverse applications of 1-acyl-3-substituted thioureas: A comprehensive review. RSC Adv. 2024, 14, 18011–18063. [Google Scholar] [CrossRef]
- Karmakar, A.; Hazra, S.; Pombeiro, A.J.L. Urea and thiourea based coordination polymers and metal-organic frameworks: Synthesis, structure and applications. Coord. Chem. Rev. 2022, 453, 214314. [Google Scholar] [CrossRef]
- Ray, D.A.; Baniasadi, M.; Graves, J.E.; Greenwood, A.; Farnaud, S. Thiourea leaching: An update on a sustainable approach for gold recovery from E-waste. J. Sust. Metall. 2022, 8, 597–612. [Google Scholar] [CrossRef]
- Chen, T.-C.; Chung, A.; Hsu, C.-J.; His, H.-C. Selective adsorption/reduction of Au in electronic waste recycling wastewater by self-assembled thiourea-crosslinked reduced graphene oxide framework ball. Sep. Pur. Technol. 2025, 353, 128374. [Google Scholar] [CrossRef]
- Borda, J.; Torres, R. Prospects for thiourea as a leaching agent in colombian gold small-scale mining: A comprehensive review. J. Sustain. Min. 2022, 21, 298–308. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Chu, X.; Li, W.-H.; Zhao, S.-S.; Zhang, J.-X.; Qin, Z.-Q.; Li, H.-Y.; Xue, W. Four cyclometalated iridium(III) complex-based chemosensors for turn-on response to thiourea in fruit juice and their application in test strips. Dyes Pigm. 2023, 217, 111427. [Google Scholar] [CrossRef]
- Wei, S.; Ma, C.; Liu, X.; Liu, N.; Yuan, M.; Xiao, K.; Yan, W.; Xin, H. A facile synthesis of a copper(I) thiourea sulphate complex and its application for highly efficient chalcopyrite solar cells. Chem. Commun. 2023, 59, 9848–9851. [Google Scholar] [CrossRef]
- Moradi, P.; Kikhavani, T.; Abbasi Tyula, Y. A new samarium complex of 1,3-bis(pyridin-3-ylmethyl)thiourea on boehmite nanoparticles as a practical and recyclable nanocatalyst for the selective synthesis of tetrazoles. Sci. Rep. 2023, 13, 5902. [Google Scholar] [CrossRef] [PubMed]
- Tyula, Y.A.; Moradi, P.; Nikoorazm, M. A New neodymium complex on boehmite nanoparticles with 1,3-bis(pyridine-3-ylmethyl)thiourea as a practical and reusable nanocatalyst for the chemoselective synthesis of tetrazoles. ChemistrySelect 2023, 8, e202301674. [Google Scholar] [CrossRef]
- Banerjee, I.; Bhattacharjee, J.; Kumar, R.; Pal, K.; Panda, T.K. Synthesis, characterization and catalytic activities of Zn(II) and Cd(II) complexes supported by unsymmetrical aryl thiourea ligands. Z. Anorg. Allg. Chem. 2023, 649, e202200340. [Google Scholar] [CrossRef]
- Uysal, M.E.; Solmaz, U.; Arslan, H. Ruthenium(III) acyl thiourea complex: A catalyst for transfer hydrogenation of nitroarenes. Polyhedron 2024, 247, 116707. [Google Scholar] [CrossRef]
- Rakhshani, S.; Rezvani, A.R.; Dušek, M.; Eigner, V. Design and fabrication of novel thiourea coordination compounds as potent inhibitors of bacterial growth. J. Antibiot. 2019, 72, 260–270. [Google Scholar] [CrossRef]
- Nongpiur, C.G.L.; Diengdoh, D.F.; Banothu, V.; Gannon, P.M.; Kaminsky, W.; Kollipara, M.R. Variable coordination behavior of rhodium metal complexes towards thiourea derivative ligands in comparison to its ruthenium and iridium analogs: Synthesis and biological studies. J. Organomet. Chem. 2023, 999, 122823. [Google Scholar] [CrossRef]
- Al-Halbosy, A.T.F.; Hamada, A.A.; Faihan, A.S.; Saleh, A.M.; Yousef, T.A.; Abou-Krisha, M.M.; Alhalafi, M.H.; Al-Janabi, A.S.M. Thiourea derivative metal complexes: Spectroscopic, anti-microbial evaluation, ADMET, toxicity, and molecular docking studies. Inorganics 2023, 11, 390. [Google Scholar] [CrossRef]
- Al-Salim, Y.M.; Al-Asadi, R.H. Tropical, Synthesis, anti-breast cancer activity, and molecular docking studies of thiourea benzamide derivatives and their complexes with copper ion. J. Nat. Prod. Res. 2023, 7, 3158–3167. [Google Scholar]
- Noor, A.; Qayyum, S.; Ali, Z.; Muhammad, N. Syntheses and structural characterization of divalent metal complexes (Co, Ni, Pd and Zn) of sterically hindered thiourea ligand and a theoretical insight of their interaction with SARS-CoV-2 enzyme. J. Mol. Struct. 2023, 1274, 134442. [Google Scholar] [CrossRef] [PubMed]
- Javadzade, T.; Rzayeva, I.; Demukhamedova, S.; Akverdieva, G.; Farzaliyev, V.; Sujayev, A.; Chiragov, F. Synthesis, structural analysis, DFT study, antioxidant activity of metal complexes of N-substituted thiourea. Polyhedron 2023, 231, 116274. [Google Scholar] [CrossRef]
- Thangavel, S.K.; Mohamed Kasim, M.S.; Rengan, R. Promoting the anticancer activity with multidentate furan-2-carboxamide functionalized aroyl thiourea chelation in binuclear half-sandwich ruthenium(II) complexes. Inorg. Chem. 2024, 63, 7520–7539. [Google Scholar] [CrossRef]
- Borges, A.P.; Obata, M.M.S.; Libardi, S.H.; Trevisan, R.O.; Deflon, V.M.; Abram, U.; Ferreira, F.B.; Costa, L.A.S.; Patrocínio, A.O.T.; da Silva, M.V.; et al. Gold(I) and SILver(I) complexes containing hybrid sulfonamide/thiourea ligands as potential leishmanicidal agents. Pharmaceutics 2024, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Todorova, S.E.; Rusew, R.I.; Shivachev, B.L.; Kurteva, V.B. Polydentate N,O-ligands possessing unsymmetrical urea fragments attached to a p-cresol scaffold. Molecules 2023, 28, 6540. [Google Scholar] [CrossRef]
- Rigaku, O.D. Crysalis Pro; Rigaku Oxford Diffraction Ltd.: Oxfordshire, UK, 2015. [Google Scholar]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]

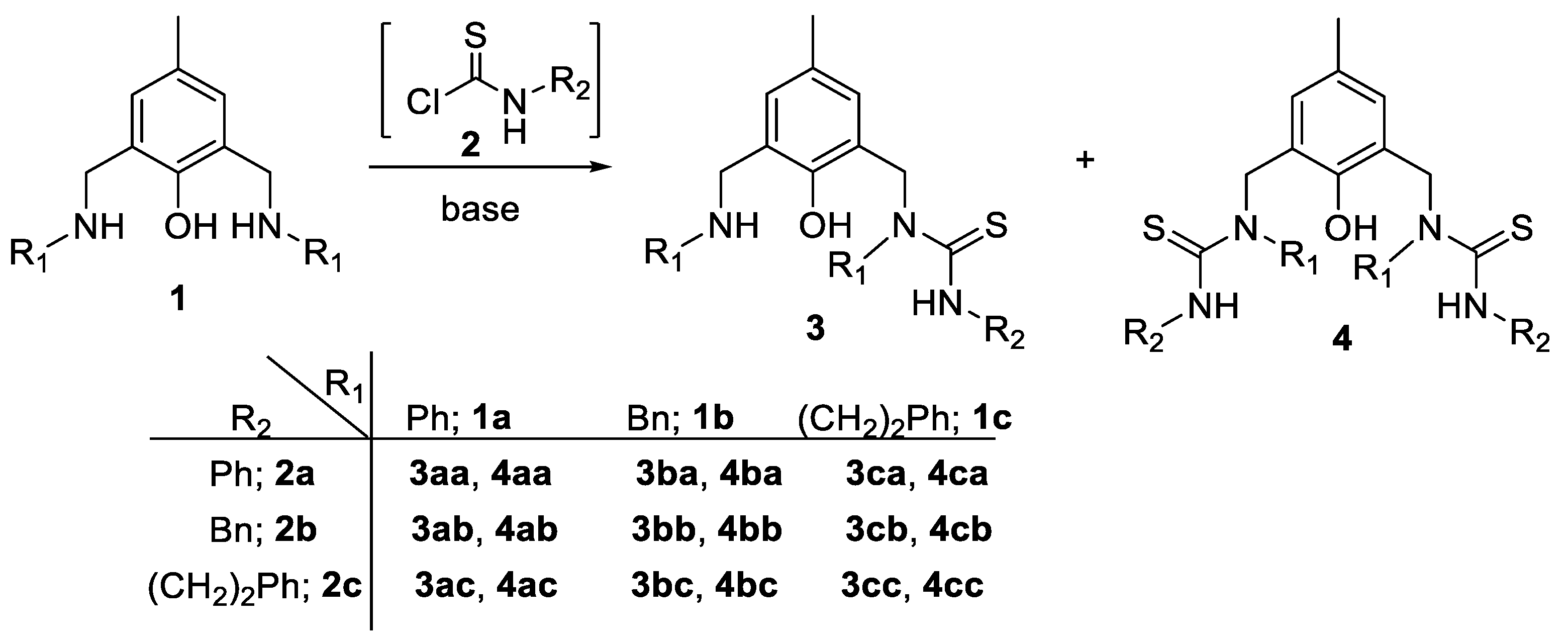
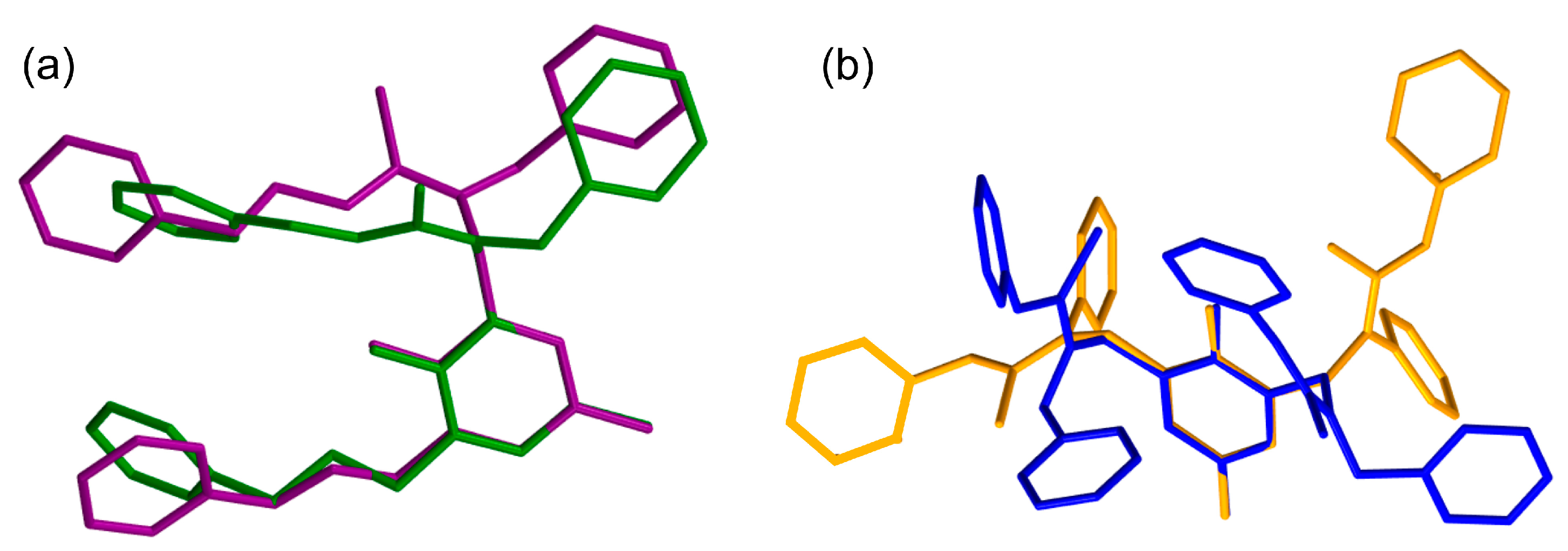
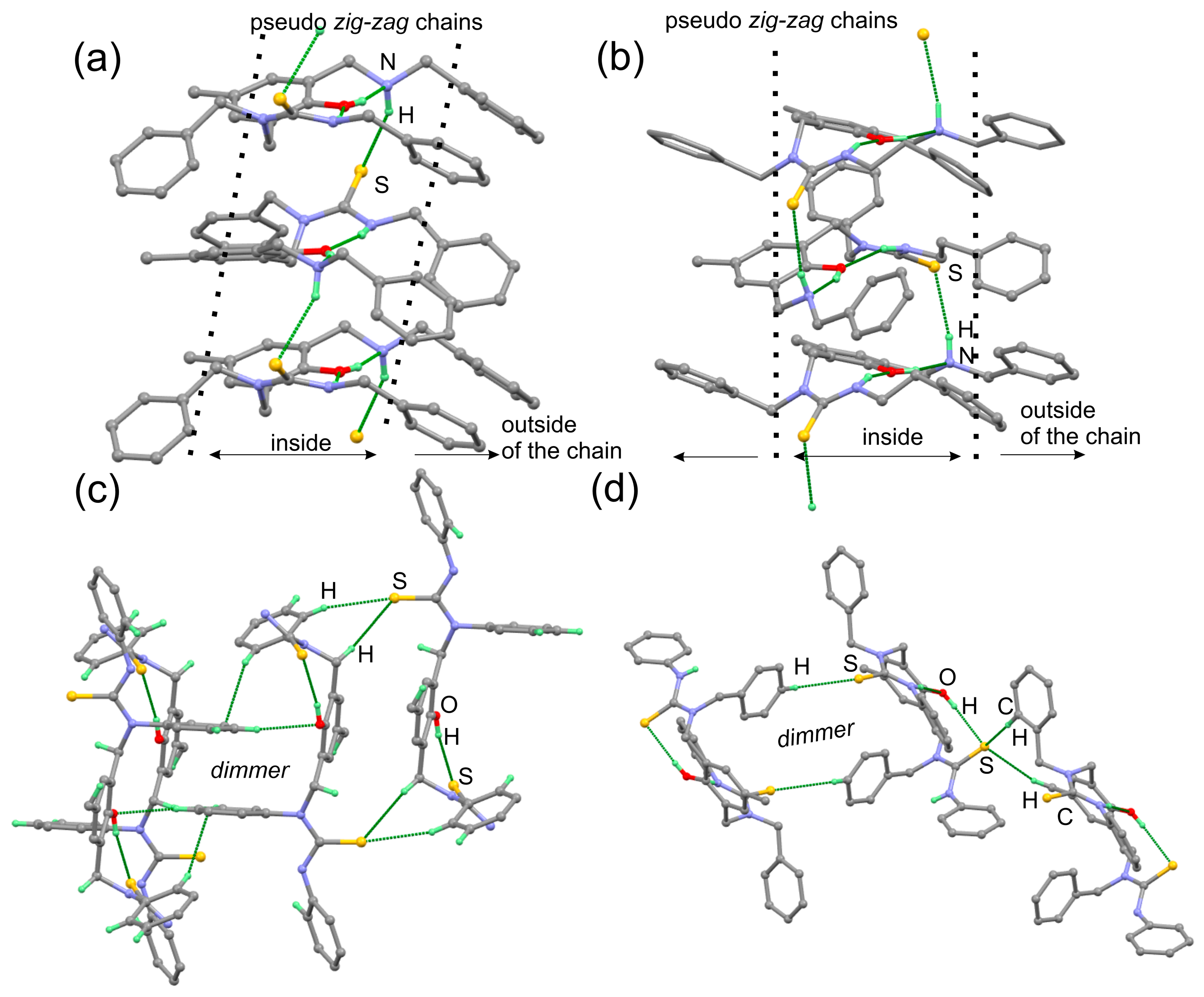
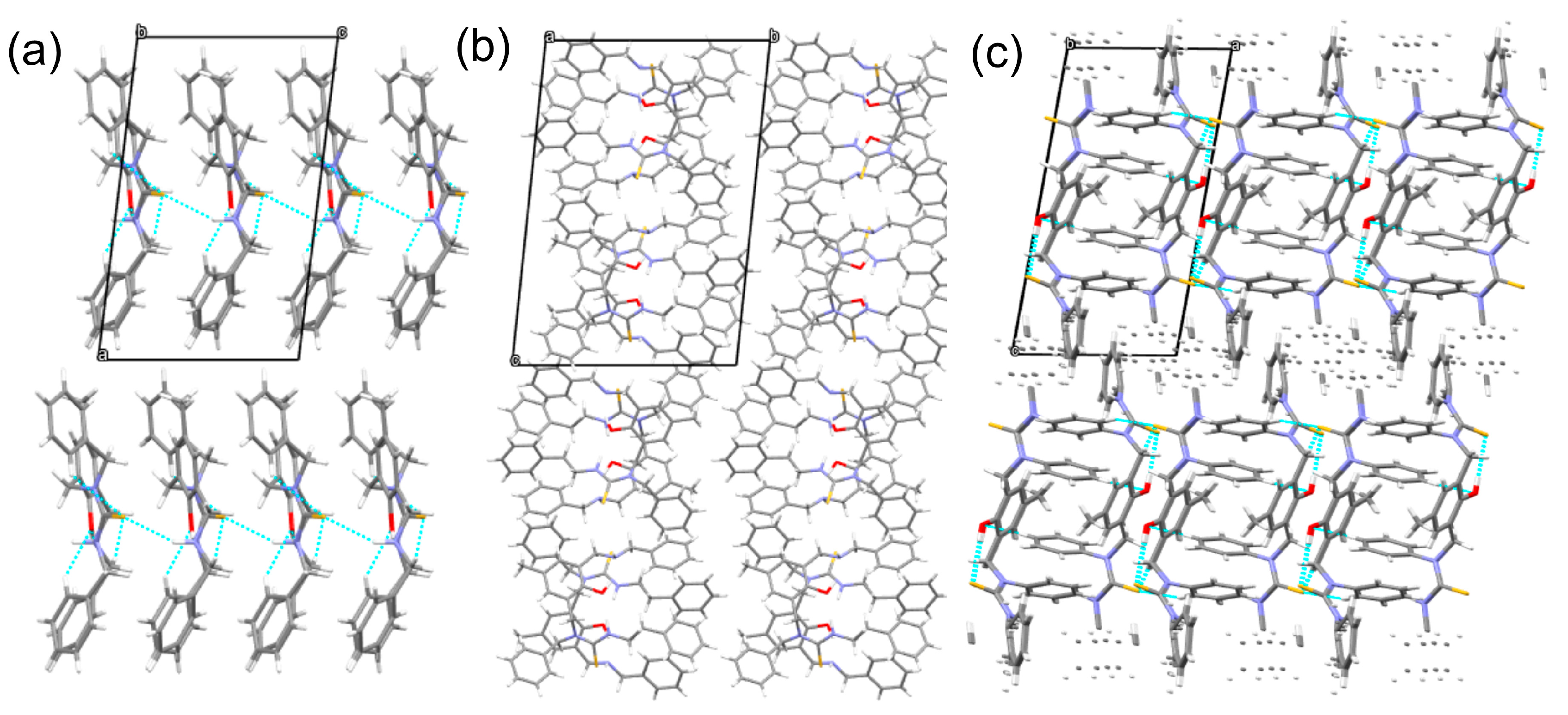


| Entry | Conditions a | Products | ||||
|---|---|---|---|---|---|---|
| 3 | Yield, % | 4 | Yield, % | Total, % | ||
| 1 | 1a:2a 1:1 | 3aa | - | 4aa | 14 | 14 |
| 2 | 1a:2a 1:2 | - | 58 | 58 | ||
| 3 | 1a:2a 1:3 | - | 66 | 66 | ||
| 4 | 1a:2b 1:1 | 3ab | 36 | 4ab | - | 36 |
| 5 | 1a:2b 1:2 | 24 | 11 | 35 | ||
| 6 | 1a:2b 1:3 | - | 38 | 38 | ||
| 7 | 1a:2c 1:1 | 3ac | 24 | 4ac | 17 | 41 |
| 8 | 1a:2c 1:2 | 20 | 31 | 51 | ||
| 9 | 1a:2c 1:3 | 16 | 23 | 39 | ||
| 10 | 1b:2a 1:1 | 3ba | 32 | 4ba | - | 32 |
| 11 | 1b:2a 1:2 | 23 | 32 | 55 | ||
| 12 | 1b:2a 1:3 | - | 54 | 54 | ||
| 13 | 1b:2b 1:1 | 3bb | 59 | 4bb | 3 | 62 |
| 14 | 1b:2b 1:2 | 2 | 34 | 36 | ||
| 15 | 1b:2b 1:3 | 2 | 39 | 41 | ||
| 16 | 1b:2c 1:1 | 3bc | 33 | 4bc | 11 | 44 |
| 17 | 1b:2c 1:2 | - | 62 | 62 | ||
| 18 | 1b:2c 1:3 | - | 60 | 60 | ||
| 19 | 1c:2a 1:1 | 3ca | - | 4ca | 27 | 27 |
| 20 | 1c:2a 1:2 | - | 47 | 47 | ||
| 21 | 1c:2a 1:3 | - | 48 | 48 | ||
| 22 | 1c:2b 1:1 | 3cb | 25 | 4cb | - | 25 |
| 23 | 1c:2b 1:2 | - | 16 | 16 | ||
| 24 | 1c:2b 1:3 | - | 19 | 19 | ||
| 25 | 1c:2c 1:1 | 3cc | 42 | 4cc | 5 | 47 |
| 26 | 1c:2c 1:2 | 21 | 24 | 45 | ||
| 27 | 1c:2c 1:3 | - | 24 | 24 | ||
| Ligand | CH-3/CH-3 | CH-5/CH-5 | CH2-Cq-2/CH2-Cq-2 | CH2-Cq-6/CH2-Cq-6 |
|---|---|---|---|---|
| 4aa | 6.622/131.69 | 5.472/54.36 | ||
| 3ab | 6.263/131.38 | 6.991/130.09 | 5.441/55.67 | 4.360/45.29 |
| 4ab | 6.601/131.16 | 5.418/54.45 | ||
| 3ac | 6.978/130.01 | 6.210/131.37 | 5.365/55.31 | 4.344/45.06 |
| 4ac | 6.559/130.95 | 5.349/54.05 | ||
| 3ba | 6.850/131.15 | 6.830/129.68 | 4.645 br * | 4.005/51.35 |
| 4ba | 6.941/133.00 | 5.017 br * | ||
| 3bb | 6.770/129.88 | 6.713/129.26 | 4.518 br * | 3.785/51.24 |
| 4bb | 6.824/131.77 | 4.893 br * | ||
| 3bc | 6.763 (common)/129.82 (common) | 4.497 br * | 5.213 br * | |
| 4bc | 6.776/131.75 | 4.836 br * | ||
| 4ca | 7.027/132.78 | 4.956 br * | ||
| 3cb | 6.725/129.32 | 6.895/129.60 | 3.805/51.72 | 4.187/55.46 |
| 4cb | 6.878/131.39 | 4.779/50.44 | ||
| 3cc | 6.736/128.93 | 6.823/128.81 | 3.898/51.99 | 4.444/48.30 |
| 4cc | 6.823/131.26 | 4.725/50.86 | ||
| π…π Type | Ring A | Ring B | Cg…Cg | Shift | |
|---|---|---|---|---|---|
| Å | Å | ||||
| 3bb | T-shape | C20-C21-C23-C22-C18-C19 | C28-C27-C29-C31-C30-C26 1 | 4.084 | – |
| T-shape | C20-C21-C23-C22-C18-C19 | C20-C21-C23-C22-C18-C19 1 | 3.909 | – | |
| 3bc | T-shape | C151-C101-C121-C141-C131-C111 | C52-C62-C42-C72-C32-C22 2 | 3.726 | – |
| T-shape | C191-C201-C241-C211-C221-C231 | C302-C292-C272-C322-C312-C282 2 | 3.791 | – | |
| 4aa | Parallel displaced | C2-C3-C4-C5-C6-C7 | C2-C3-C4-C5-C6-C7 3 | 3.807 | 1.357 |
| 4ba | T-shape | C32-C36-C35-C37-C34-C33 | C30-C28-C29-C27-C26-C25 4 | 3.919 | – |
| Cell Contents (µM) | Syringe Contents (µM) | Ka. 106 (M−1) | N * |
|---|---|---|---|
| 3ab 0.25 | KCl 1.25 | 1.89 ± 0.52 | 2.114 |
| 3ac 0.25 | KCl 1.25 | 1.02 ± 0.41 | 1.996 |
| 3ba 0.25 | KCl 1.25 | 1.57 ± 0.57 | 2.070 |
| 3bb 0.25 | KCl 1.25 | 2.31 ± 0.84 | 2.193 |
| 3bc 0.25 | KCl 1.25 | 0.87 ± 0.61 | 1.919 |
| 4aa 0.25 | KCl 1.25 | 1.22 ± 0.31 | 2.165 |
| 4ac 0.25 | KCl 1.25 | 2.29 ± 0.58 | 1.582 |
| 4ba 0.25 | KCl 1.25 | 1.92 ± 0.65 | 2.364 |
| 4bc 0.25 | KCl 1.25 | 0.98 ± 0.64 | 2.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Todorova, S.E.; Rusew, R.I.; Petkova, Z.S.; Shivachev, B.L.; Kurteva, V.B. Novel Thiourea Ligands—Synthesis, Characterization and Preliminary Study on Their Coordination Abilities. Molecules 2024, 29, 4906. https://doi.org/10.3390/molecules29204906
Todorova SE, Rusew RI, Petkova ZS, Shivachev BL, Kurteva VB. Novel Thiourea Ligands—Synthesis, Characterization and Preliminary Study on Their Coordination Abilities. Molecules. 2024; 29(20):4906. https://doi.org/10.3390/molecules29204906
Chicago/Turabian StyleTodorova, Stanislava E., Rusi I. Rusew, Zhanina S. Petkova, Boris L. Shivachev, and Vanya B. Kurteva. 2024. "Novel Thiourea Ligands—Synthesis, Characterization and Preliminary Study on Their Coordination Abilities" Molecules 29, no. 20: 4906. https://doi.org/10.3390/molecules29204906
APA StyleTodorova, S. E., Rusew, R. I., Petkova, Z. S., Shivachev, B. L., & Kurteva, V. B. (2024). Novel Thiourea Ligands—Synthesis, Characterization and Preliminary Study on Their Coordination Abilities. Molecules, 29(20), 4906. https://doi.org/10.3390/molecules29204906







