Recovery of Low-Concentration Tungsten from Acidic Solution Using D318 Macroporous Resin
Abstract
:1. Introduction
2. Results and Discussion
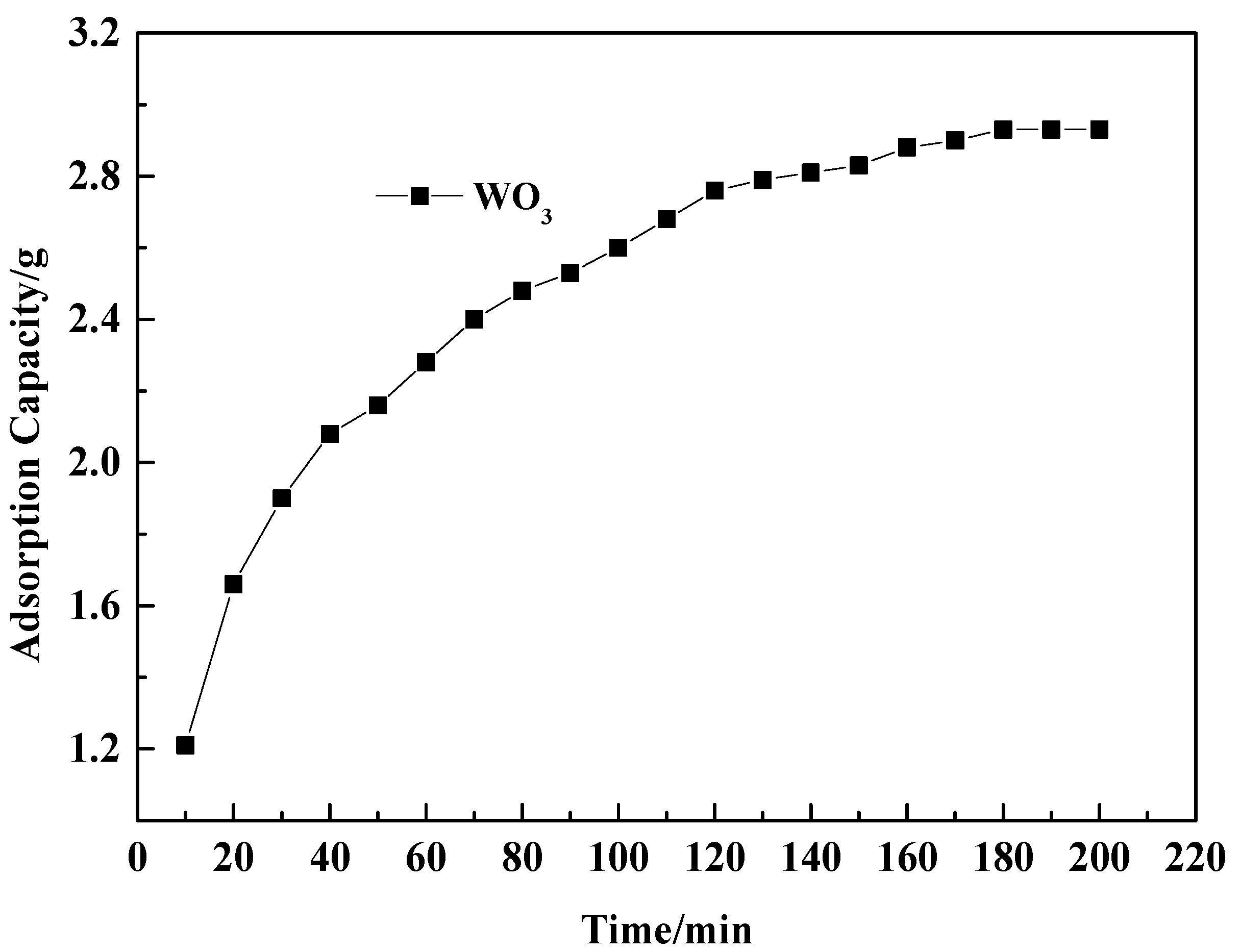
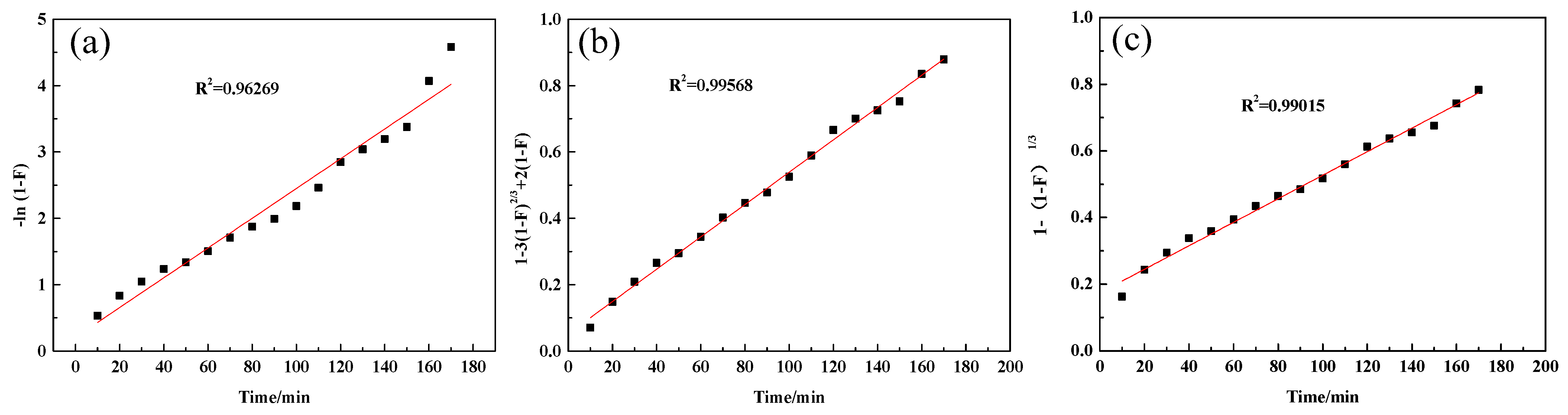
2.1. Static Adsorption Kinetics
2.2. Static Adsorption Thermodynamics
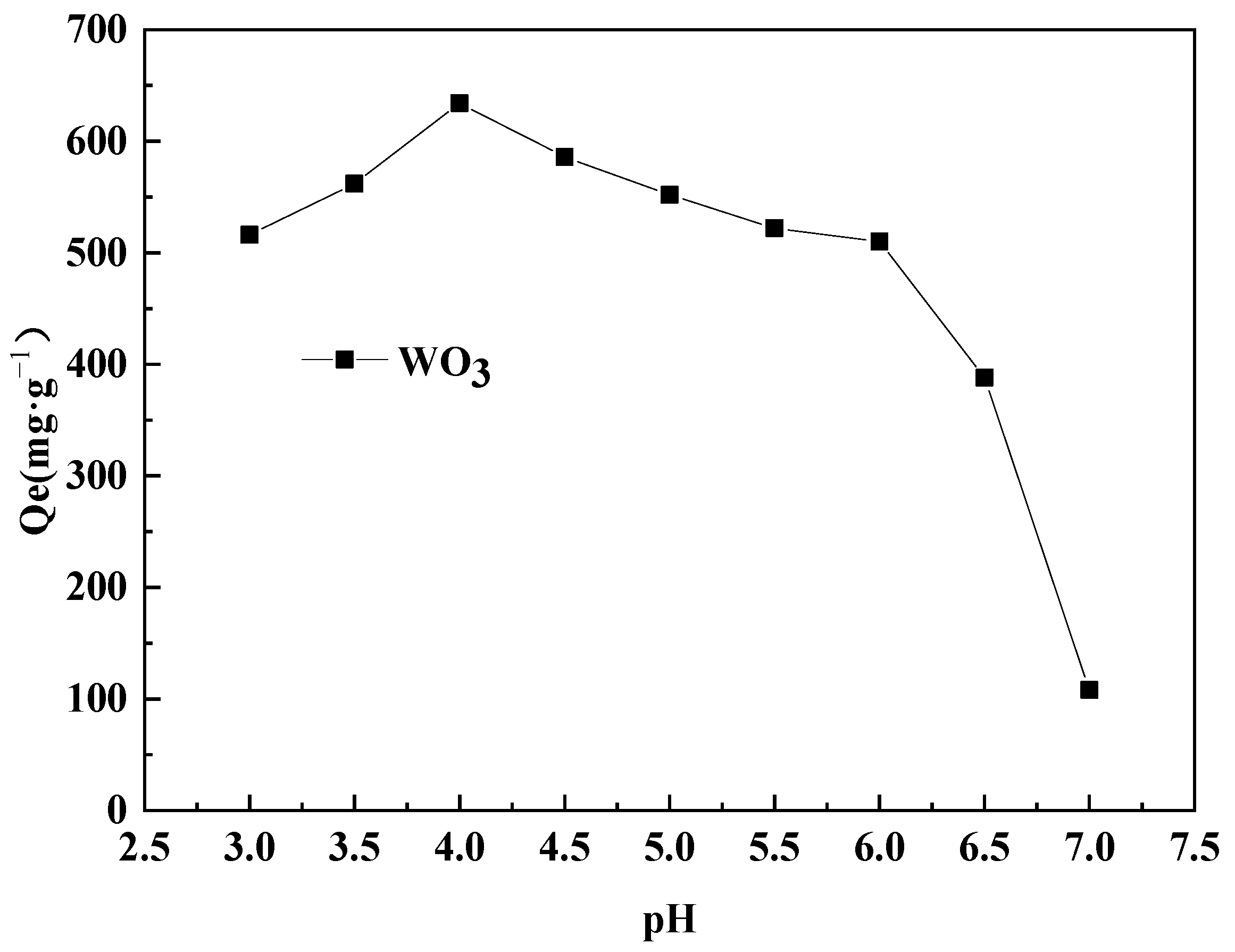
2.2.1. Effect of pH on Static Adsorption Capacity of WO3
2.2.2. Static Adsorption Isotherm
2.2.3. Adsorption Thermodynamics
2.3. Dynamic Adsorption Experiment
2.3.1. Effect of WO3 Concentration in Acidic Solution on Dynamic Adsorption Capacity
2.3.2. Effect of Adsorption Flow Rate on Dynamic Adsorption Capacity
2.4. Dynamic Desorption Experiment
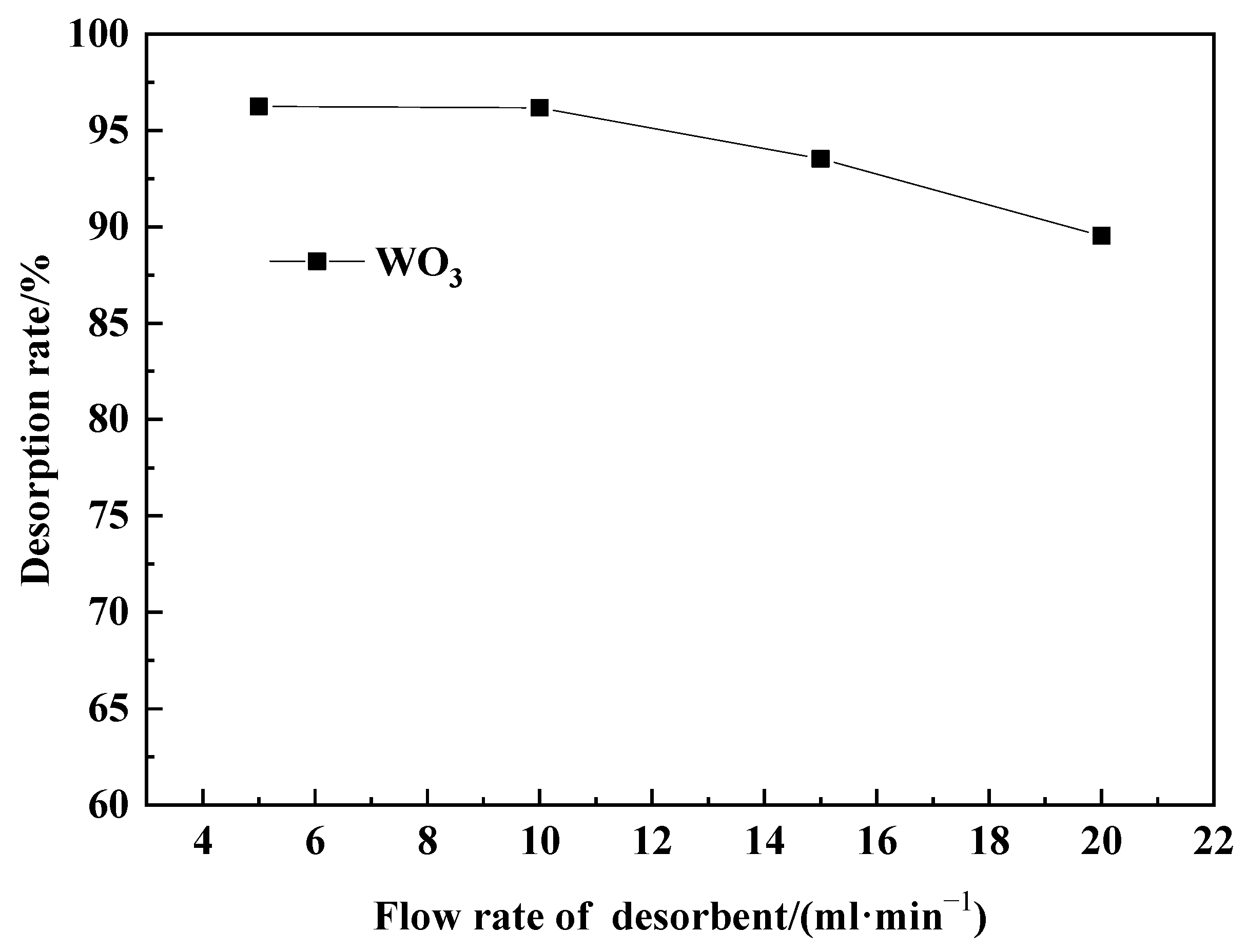
2.4.1. Effect of Desorption Agent Flow Rate on WO3 Desorption Efficiency
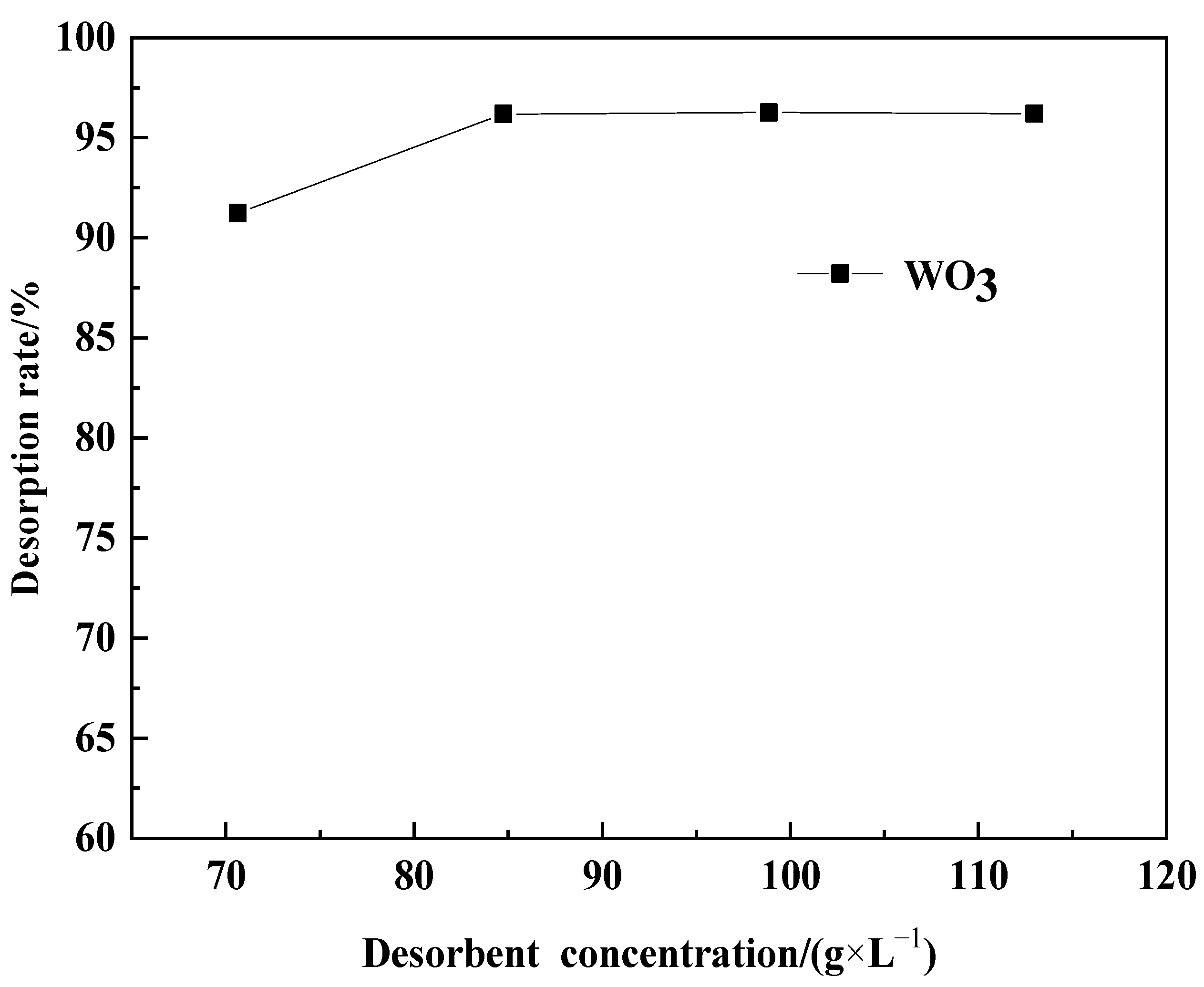
2.4.2. Effect of Desorption Agent Concentration on WO3 Desorption Efficiency
2.4.3. Effect of Desorption Method on WO3 Desorption Efficiency
2.4.4. Effect of Desorption Agent Type on WO3 Desorption Efficiency
2.5. Analysis of Adsorption Mechanism
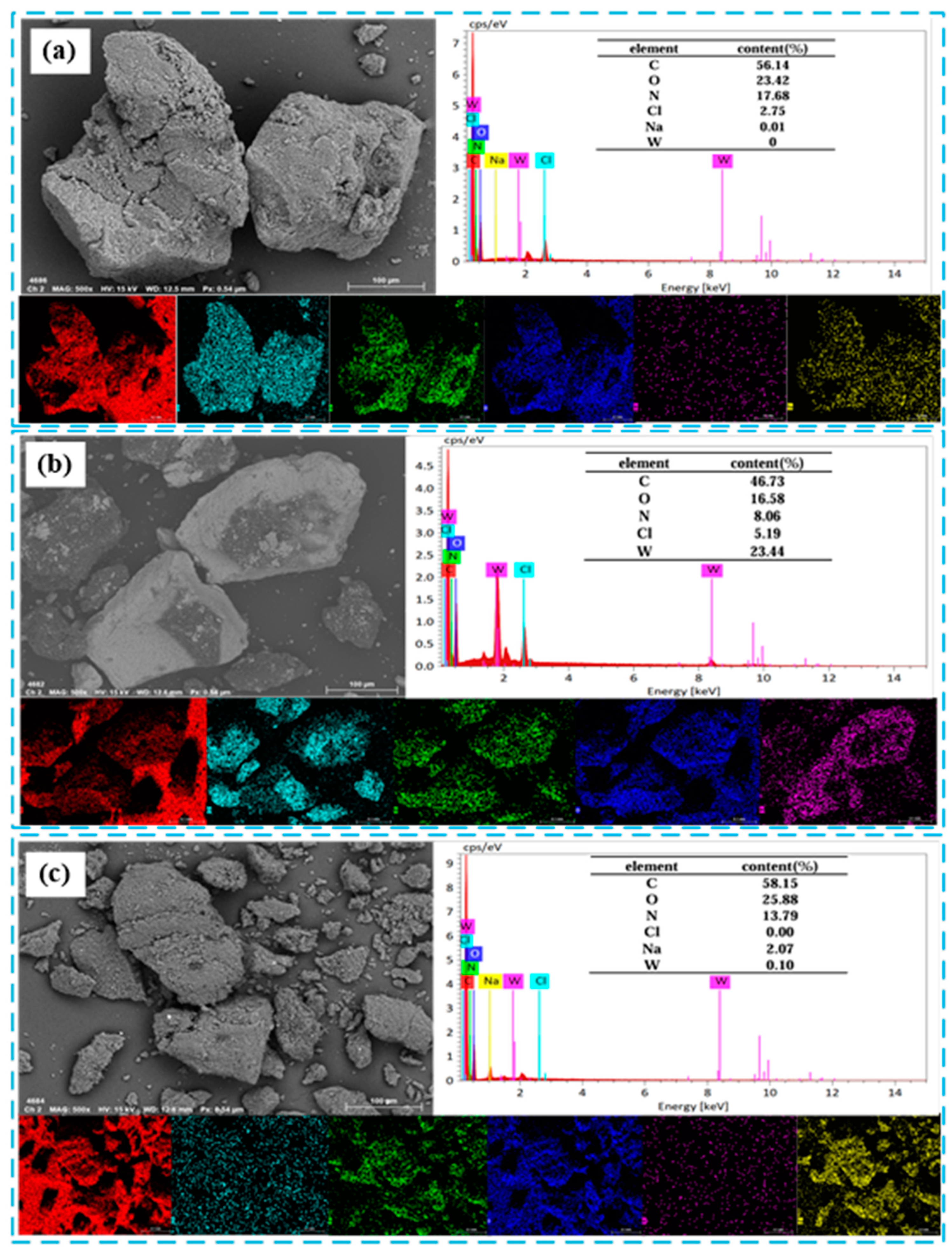
2.5.1. SEM-EDS Test Analysis
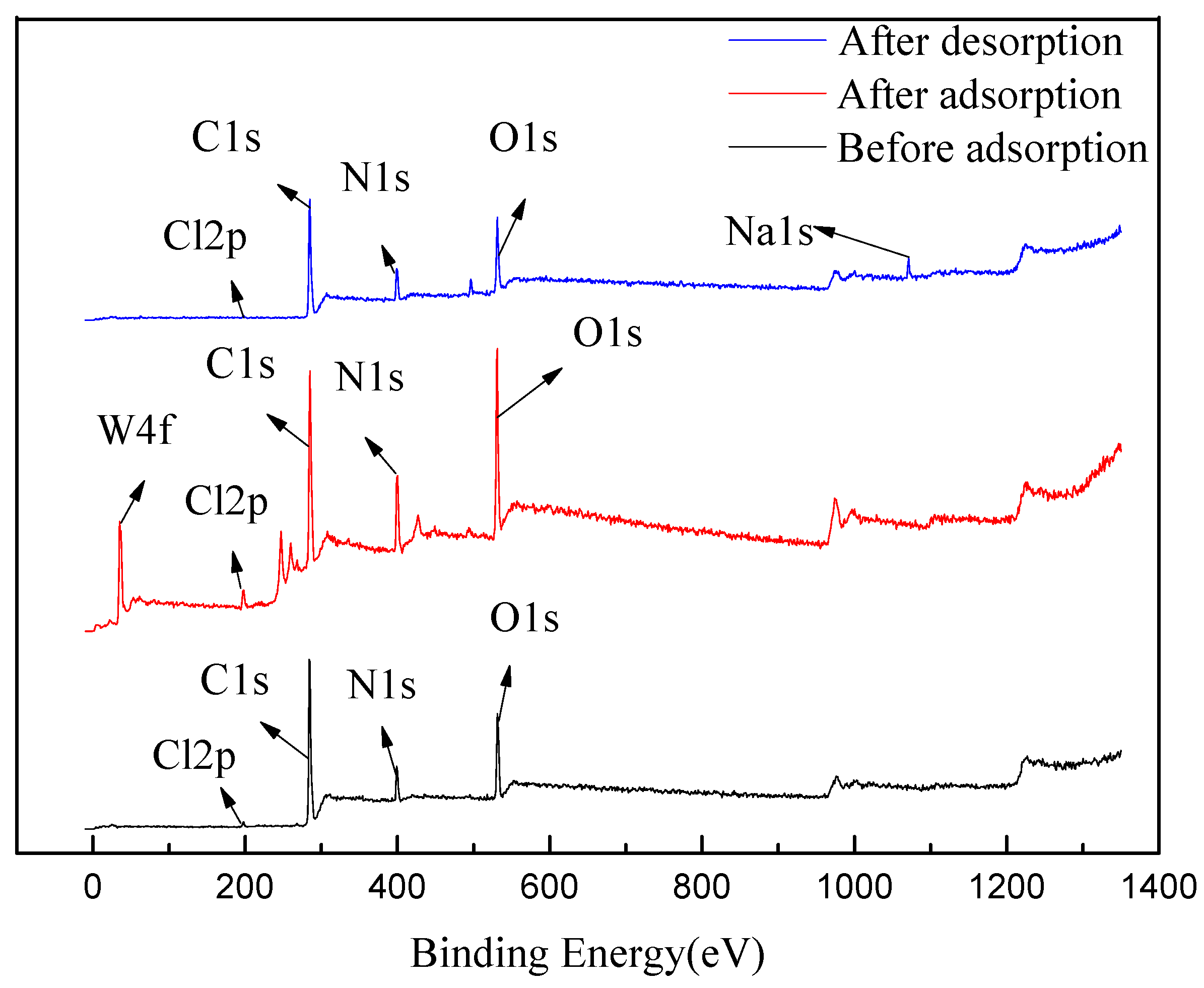
2.5.2. XPS Test Analysis
3. Experimental
3.1. Materials
3.2. Resin Pretreatment
3.3. Static Adsorption Experiment
3.4. Method for Dynamic Adsorption Experiments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Li, J.; Zhao, Z.; Huang, S.; Chen, X.; Hu, K. Recovery and separation of W and Mo from high-molybdenum synthetic scheelite in HCl solutions containing H2O2. Hydrometallurgy 2015, 155, 1–5. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, Y.; Che, J.; Wang, C.; Ma, B. Green leaching of tungsten from synthetic scheelite with sulfuric acid-hydrogen peroxide solution to prepare tungstic acid. Sep. Purif. Technol. 2020, 241, 116752. [Google Scholar] [CrossRef]
- Gong, D.; Zhou, K.; Peng, C.; Li, J.; Chen, W. Sequential extraction of tungsten from scheelite through roasting and alkaline leaching. Miner. Eng. 2019, 132, 238–244. [Google Scholar] [CrossRef]
- Chen, X.; Guo, F.; Chen, Q.; Liu, X.; Zhao, Z. Leaching tungsten and rare earth elements from scheelite through H2SO4–H3PO4 mixed acid decomposition. Miner. Eng. 2020, 156, 106526. [Google Scholar] [CrossRef]
- Xiao, L.; Ji, L.; Yin, C.; Chen, A.; Chen, X.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Tungsten extraction from scheelite hydrochloric acid decomposition residue by hydrogen peroxide. Miner. Eng. 2022, 179, 107461. [Google Scholar] [CrossRef]
- Meng, X.; Zeng, P.; Lin, S.; Bao, H.; Wu, M.; Yang, L.; Jing, G.; Han, H.; Zhang, C.; Jiang, X.; et al. Removal of chemical oxygen demand and ammonia nitrogen from high salinity tungsten smelting wastewater by one-step electrochemical oxidation: From bench-scale test, pilot-scale test, to industrial test. J. Environ. Manag. 2023, 340, 117983. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, C.; Dai, Y.; Wang, X.; Wang, M. Separation of vanadium and chromium in solution after vanadium precipitation by co-extraction with N263 and selective stripping with NaOH solution. Hydrometallurgy 2022, 208, 105798. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, X.; Zhao, Z.; Liu, X.; Li, J.; He, L.; Sun, F. Pressure leaching of wolframite using a sulfuric-phosphoric acid mixture. Miner. Eng. 2021, 169, 106941. [Google Scholar] [CrossRef]
- Li, J.-T.; Gao, L.-L.; Zhao, Z.-W.; Liu, X.-H.; Chen, X.-Y. Efficient recovery of tungsten from scheelite concentrates using a sulfur-phosphorus mixed acid leaching system. Tungsten 2024, 6, 821–832. [Google Scholar] [CrossRef]
- Martins, J.I.; Lima, J.L.F.C.; Moreira, A.; Costa, S.C. Tungsten recovery from alkaline leach solutions as synthetic scheelite. Hydrometallurgy 2007, 85, 110–115. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Y.; Li, H. Kinetics of sodium hydroxide leaching of scheelite. Int. J. Refract. Met. Hard Mater. 2011, 29, 289–292. [Google Scholar] [CrossRef]
- Chen, Y.; Huo, G.; Guo, X.; Wang, Q. Sustainable extraction of tungsten from the acid digestion product of tungsten concentrate by leaching-solvent extraction together with raffinate recycling. J. Clean. Prod. 2022, 375, 133924. [Google Scholar] [CrossRef]
- Plattes, M.; Bertrand, A.; Schmitt, B.; Sinner, J.; Verstraeten, F.; Welfring, J. Removal of tungsten oxyanions from industrial wastewater by precipitation, coagulation and flocculation processes. J. Hazard. Mater. 2007, 148, 613–615. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.-J.; Yoo, H.; Jeon, J.H.; Kim, H.S.; Lee, J.-Y. Differential adsorption of vanadium(V) and tungsten(W) on ion exchange resins: A novel approach for separation and recovery of spent catalyst leachate. J. Clean. Prod. 2023, 426, 139157. [Google Scholar] [CrossRef]
- Shi, T.; Ma, L.; Xi, X.; Nie, Z. Preparation of functional polytertiary amine macroporous resin and its adsorption and separation properties for tungsten and molybdenum. Sep. Purif. Technol. 2024, 332, 125759. [Google Scholar] [CrossRef]
- Yi, X.; Huo, G.; Pu, H.; Zhang, Z.; Chen, P.; Liu, J. Improving the washing and desorption of tungsten-adsorbed weak base resin. Hydrometallurgy 2020, 192, 105258. [Google Scholar] [CrossRef]
- Jeon, J.H.; Sola, A.B.C.; Lee, J.-Y.; Koduru, J.R.; Jyothi, R.K. Separation of vanadium and tungsten from synthetic and spent catalyst leach solutions using an ion-exchange resin. RSC Adv. 2022, 12, 3635–3645. [Google Scholar] [CrossRef]
- Zhao, Z.; Hu, F.; Hu, Y.; Wang, S.; Sun, P.; Huo, G.; Li, H. Adsorption behaviour of WO42− onto 201×7 resin in highly concentrated tungstate solutions. Int. J. Refract. Met. Hard Mater. 2010, 28, 633–637. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, J.; Chen, X.; Liu, X.; Li, J.; Zhang, W. Separation of tungsten and molybdenum using macroporous resin: Equilibrium adsorption for single and binary systems. Hydrometallurgy 2013, 140, 120–127. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Chen, X.; Li, J.; Zhao, Z. Separation of tungsten and molybdenum using macroporous resin: Competitive adsorption kinetics in binary system. Hydrometallurgy 2014, 144–145, 77–85. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Xiao, Q.-G.; Gao, Y.-Y.; Ning, P.-G.; Xu, H.-B.; Zhang, Y. Direct extraction of Mo(VI) from acidic leach solution of molybdenite ore by ion exchange resin: Batch and column adsorption studies. Trans. Nonferrous Met. Soc. China 2018, 28, 1660–1669. [Google Scholar] [CrossRef]
- Zhu, X.-Z.; Huo, G.-S.; Ni, J.; Song, Q. Tungsten removal from molybdate solutions using chelating ion-exchange resin: Equilibrium adsorption isotherm and kinetics. J. Cent. South Univ. 2016, 23, 1052–1057. [Google Scholar] [CrossRef]
- Zhao, Z.-W.; Xu, X.-Y.; Chen, X.-Y.; Huo, G.-S.; Chen, A.-L.; Liu, X.-H.; Xu, H. Thermodynamics and kinetics of adsorption of molybdenum blue with D301 ion exchange resin. Trans. Nonferrous Met. Soc. China 2012, 22, 686–693. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, C.; Wang, X. Adsorption behavior of molybdenum onto D314 ion exchange resin(Article). J. Cent. South Univ. 2014, 21, 4445–4449. [Google Scholar] [CrossRef]
- Luo, B.; Liu, X.; Li, J.; Chen, X.; He, L.; Sun, F. Kinetics of Tungstate and Phytate Adsorption by D201 Resin. Jom 2021, 73, 1337–1343. [Google Scholar] [CrossRef]
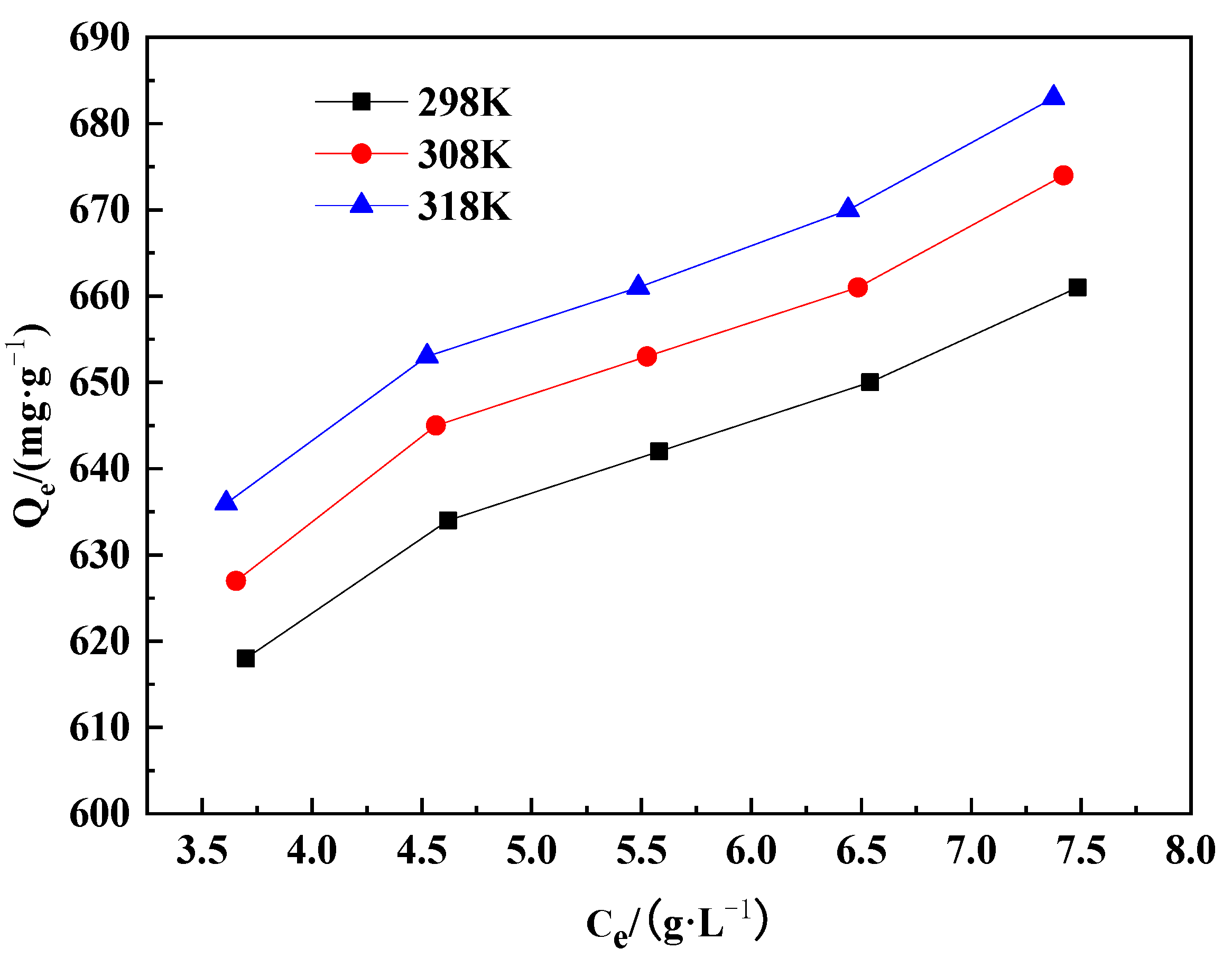



| T/K | Fitted Equation | Qm | KL | R2 |
|---|---|---|---|---|
| 298 | Ce/Qe = 1.41 Ce + 0.75 | 0.7206 | 1.41 | 0.9996 |
| 308 | Ce/Qe = 1.38 Ce + 0.77 | 0.7424 | 1.38 | 0.9995 |
| 318 | Ce/Qe = 1.36 Ce + 0.75 | 0.7543 | 1.36 | 0.9995 |
| T/K | Fitted Equation | n | Kf | R2 |
|---|---|---|---|---|
| 298 | ln(Qe) = 0.090ln(Ce) + 6.309 | 0.1904 | 0.090 | 0.9857 |
| 308 | ln(Qe) = 0.095ln(Ce) + 6.319 | 0.1912 | 0.095 | 0.9750 |
| 318 | ln(Qe) = 0.094ln(Ce) + 6.335 | 0.1886 | 0.094 | 0.9806 |
| T/K | ΔG/(KJ·mol−1) | ΔH/(KJ·mol−1) | ΔS/(J·mol−1·K−1) |
|---|---|---|---|
| 298 | −12.686 | 2.095 | 49.601 |
| 308 | −13.182 | ||
| 318 | −13.678 |
| Desorption Methods | Desorption Solution Volume/L | WO3 Concentration of Desorption Solution/(g·L−1) | Washing Solution Volume/L | WO3 Concentration of Washing Solution/(g·L−1) | Desorption Rate/% |
|---|---|---|---|---|---|
| Conventional desorption | 0.645 | 184.29 | 0.615 | 14.89 | 96.18 |
| Dry desorption | 0.639 | 188.76 | 0.625 | 13.33 | 96.87 |
| Cyclic desorption | 0.642 | 191.31 | 0.615 | 12.87 | 98.21 |
| Desorbent | Desorption Solution Volume/L | WO3 Concentration of Desorption Solution/(g·L−1) | Washing Solution Volume/L | WO3 Concentration of Washing Solution/(g·L−1) | Desorption Rate/% |
|---|---|---|---|---|---|
| Sodium hydroxide solution | 0.642 | 191.31 | 0.615 | 12.87 | 98.21 |
| Ammonia solution | 0.635 | 187.86 | 0.618 | 13.35 | 95.80 |
| C1s | O1s | N1s | Cl2p | W4f | Na1s | |
|---|---|---|---|---|---|---|
| Before adsorption | 73.36 | 16.7 | 9.06 | 0.87 | 0 | 0 |
| After adsorption | 58.28 | 21.24 | 15.42 | 2.45 | 2.61 | 0 |
| After desorption | 69.16 | 16.89 | 10.79 | 0.71 | 0 | 2.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, X.; Zeng, B.; Liang, B.; Zhang, K.; Huang, L.; Liu, X.; Huang, W. Recovery of Low-Concentration Tungsten from Acidic Solution Using D318 Macroporous Resin. Molecules 2024, 29, 4946. https://doi.org/10.3390/molecules29204946
Zeng X, Zeng B, Liang B, Zhang K, Huang L, Liu X, Huang W. Recovery of Low-Concentration Tungsten from Acidic Solution Using D318 Macroporous Resin. Molecules. 2024; 29(20):4946. https://doi.org/10.3390/molecules29204946
Chicago/Turabian StyleZeng, Xiangrong, Bin Zeng, Binjun Liang, Kuifang Zhang, Lijinhong Huang, Xinzhe Liu, and Wanfu Huang. 2024. "Recovery of Low-Concentration Tungsten from Acidic Solution Using D318 Macroporous Resin" Molecules 29, no. 20: 4946. https://doi.org/10.3390/molecules29204946
APA StyleZeng, X., Zeng, B., Liang, B., Zhang, K., Huang, L., Liu, X., & Huang, W. (2024). Recovery of Low-Concentration Tungsten from Acidic Solution Using D318 Macroporous Resin. Molecules, 29(20), 4946. https://doi.org/10.3390/molecules29204946






