New Materials for Thin-Film Solid-Phase Microextraction (TF-SPME) and Their Use for Isolation and Preconcentration of Selected Compounds from Aqueous, Biological and Food Matrices
Abstract
:1. Introduction
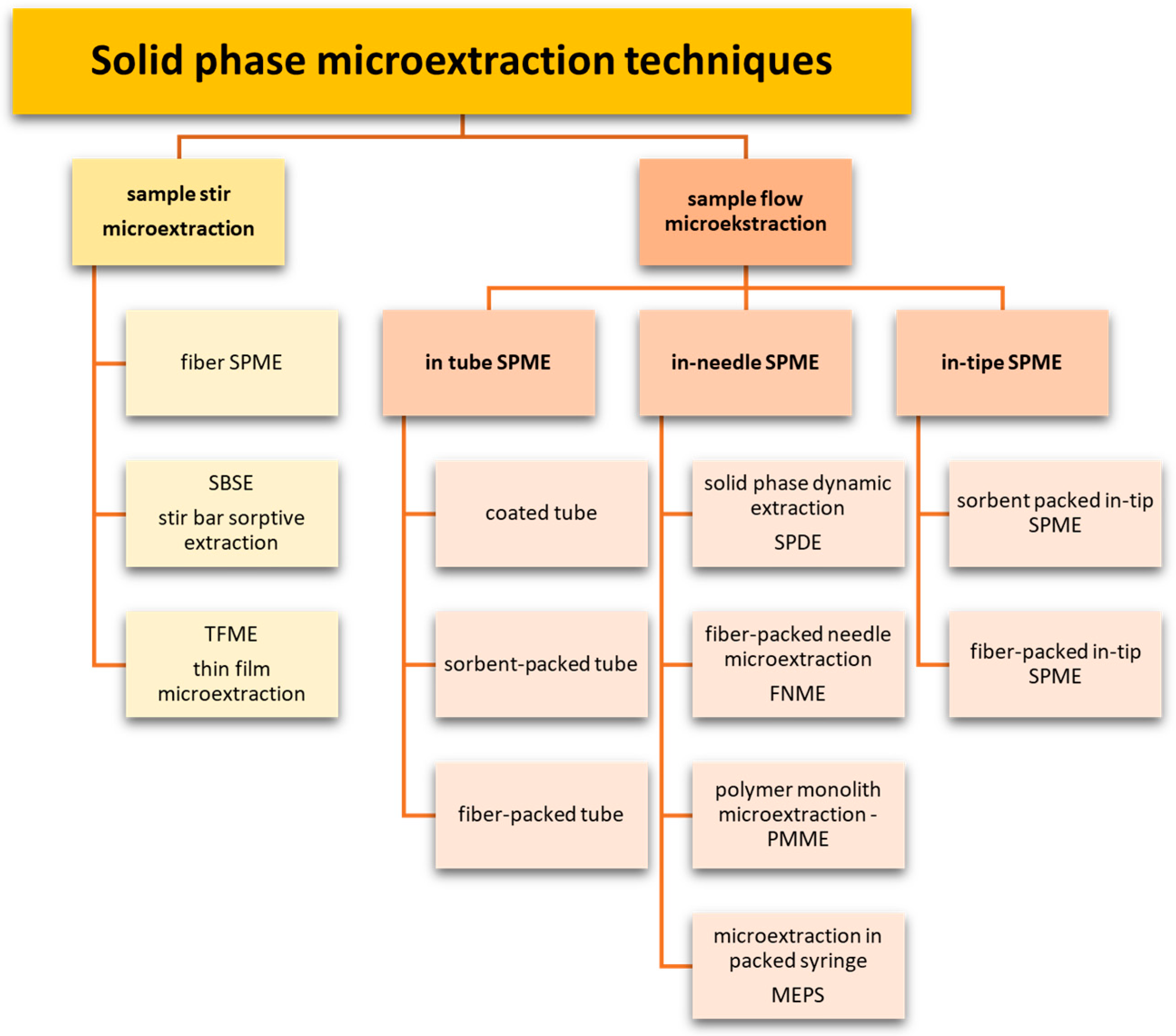
2. Microextraction Techniques
- Thin glass fiber or metal rod (fiber SPME);
- Rotating magnetic dipole (SBSE);
- Solid substrate in the form of a thin film (TF-SPME).
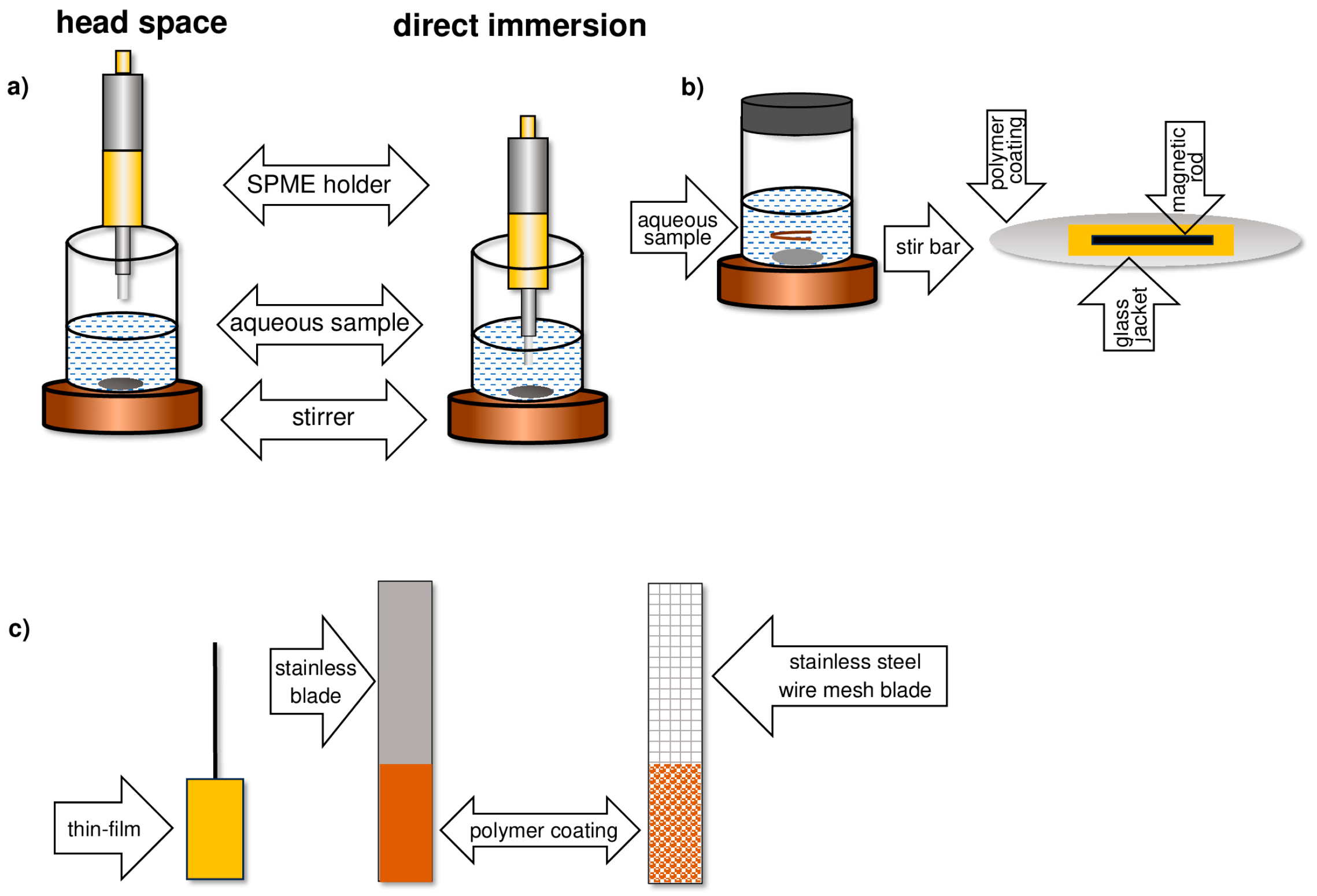
3. Solid-Phase Microextraction SPME
- Complete elimination of organic solvents from the analytical workflow;
- Short time of the analyte extraction step;
- Simplicity and speed of performing the analysis;
- The possibility of sampling in situ and in vivo systems [13];
- High sensitivity of substance determination at the ppt level;
- An ability to automate the analytical procedure [14];
- The possibility of desorption of analytes directly in the dispenser of the measuring device.
- In a direct immersion (DI) manner;
- As adsorption from a headspace (HS) phase;
4. Thin-Film Microextraction—TFME
- The rate at which the system reaches equilibrium;
- The sorption capacity of the solid phase.
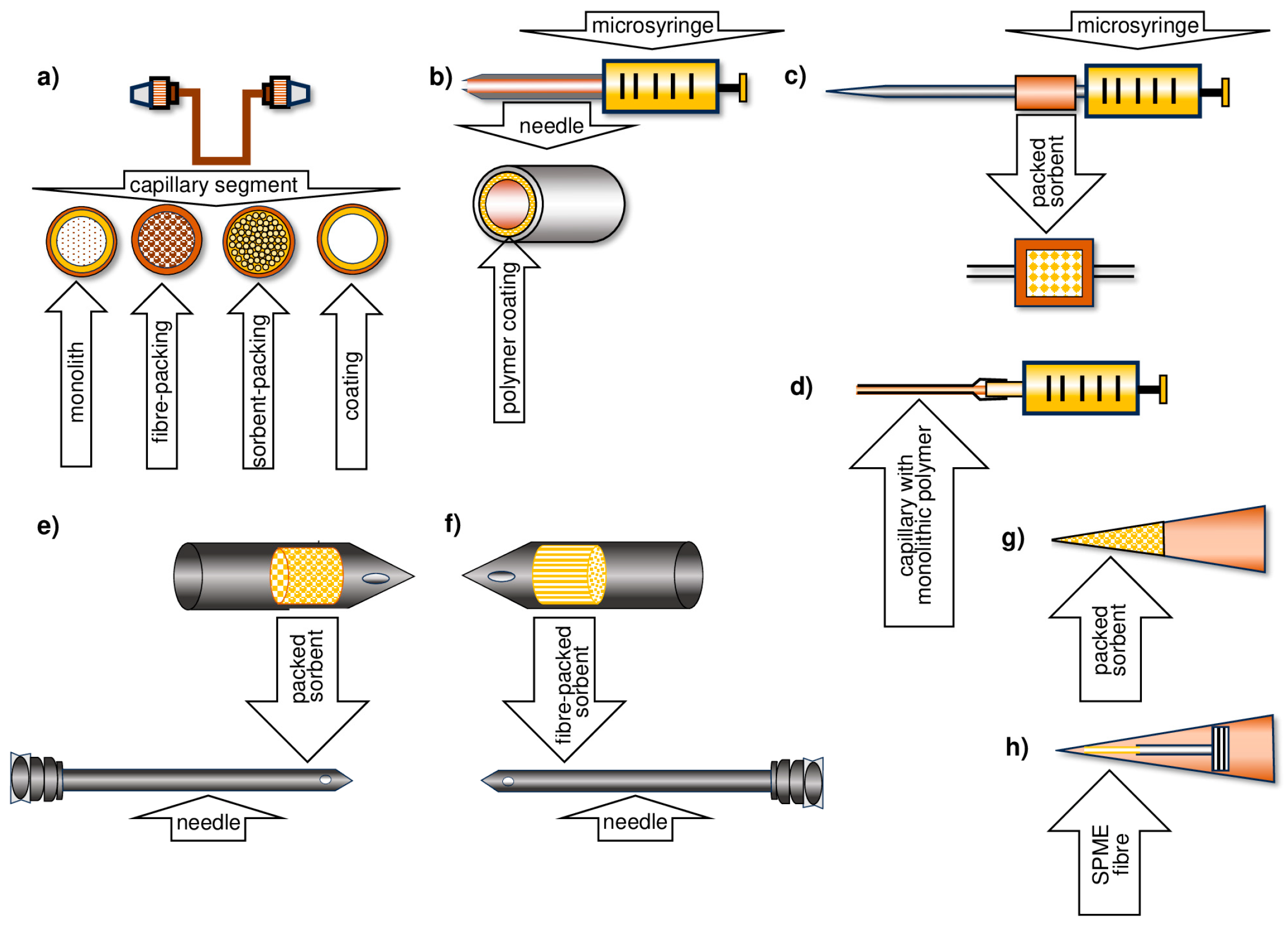
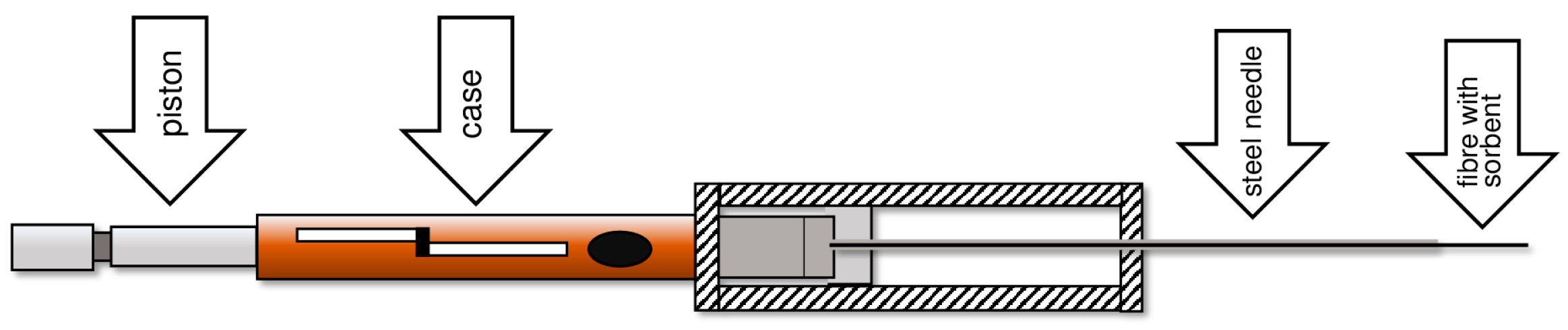
5. Development of the TF-SPME Technique
- More than 1000 times larger surface-area-to-volume ratio of the sorption phase;
- A smaller volume of the extraction phase.
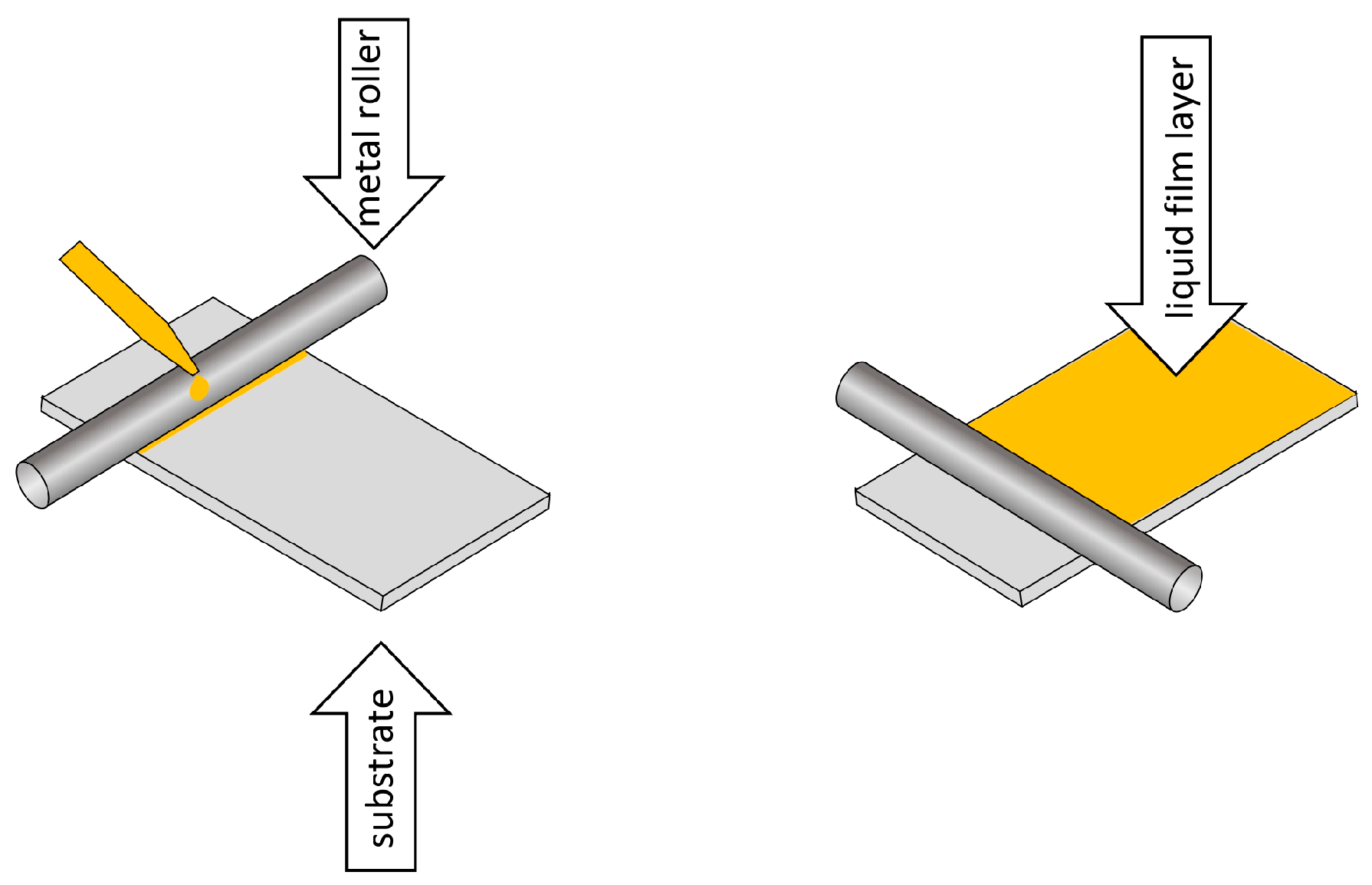
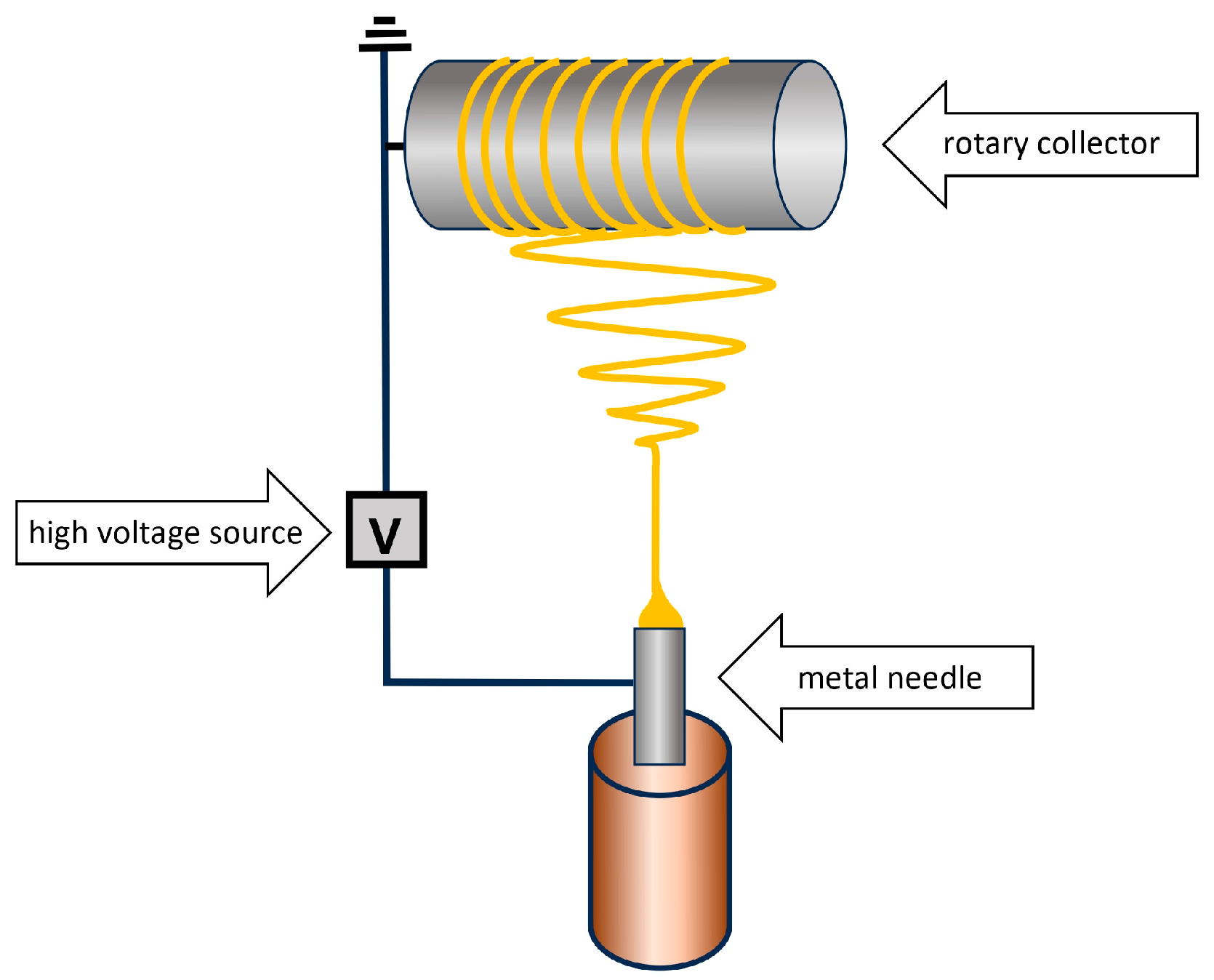
6. Methods for Obtaining Active Coatings in TFME
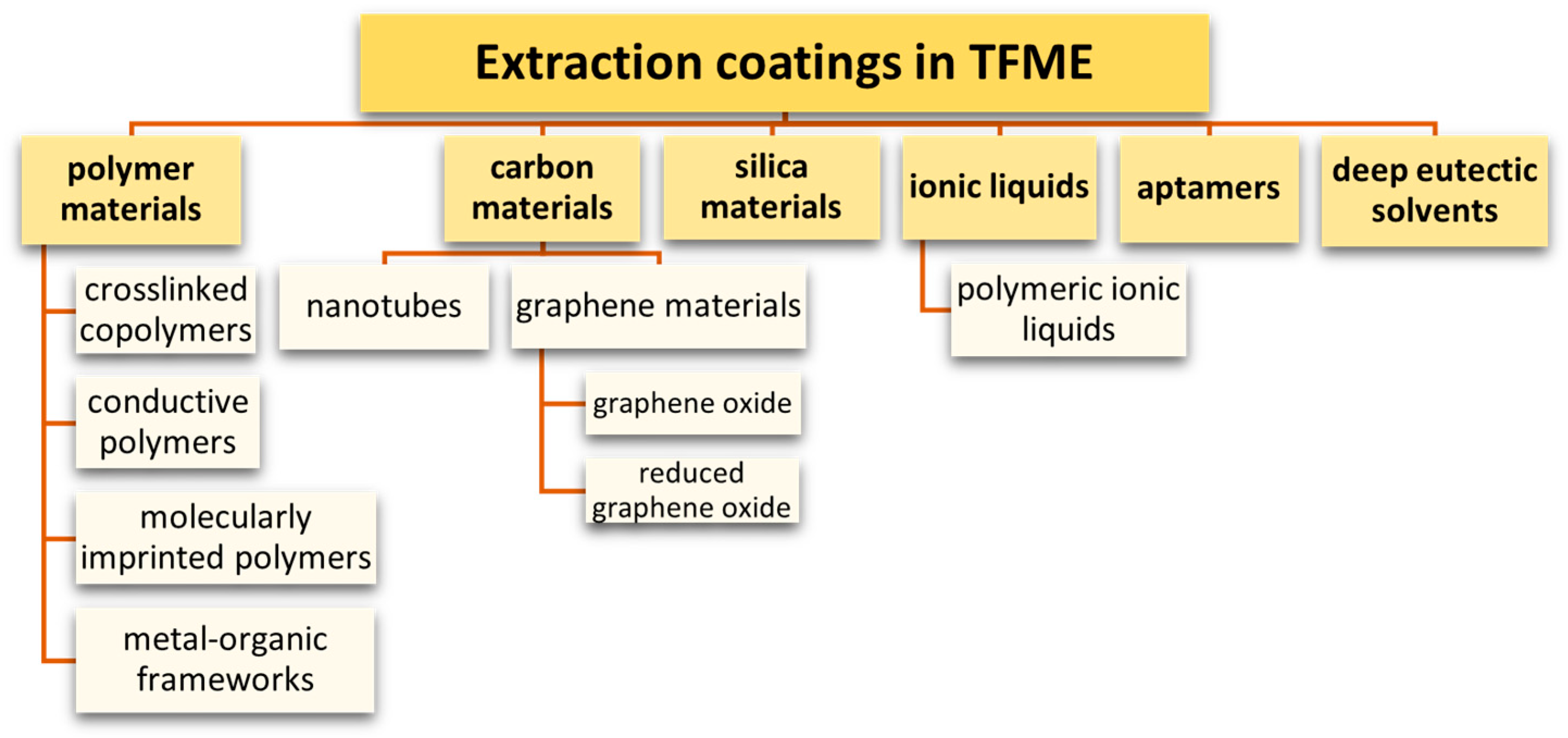
7. Sorbents Used in the TFME Technique
7.1. Polymeric Adsorbents
- High thermal resistance;
- The ease with which it undergoes desorption;
- Chemical and physiological inertness;
- Flexibility and mechanical strength;
- Low chemical reactivity.
7.2. Conductive Polymers
- Polyacetylene (PA) and polyphenylacetylene (PPA), containing double bonds;
- Polyfluorene (PF) and polyparaphenylene (PPP), polyaniline (PANI), and polyphenylenevinylene (PPV), containing aromatic rings in the polymer chain;
- Heterocyclic polymers with a nitrogen atom: polypyrrole (PPy) and polypyridine (PPY);
- Heterocyclic polymers with a sulfur atom: polythiophene (PTh) and polyethylene dioxythiophene (PEDOT), polyfuran (PFu), as well as polycyanamide (PCN) and polyvinylferrocene (pVFc).
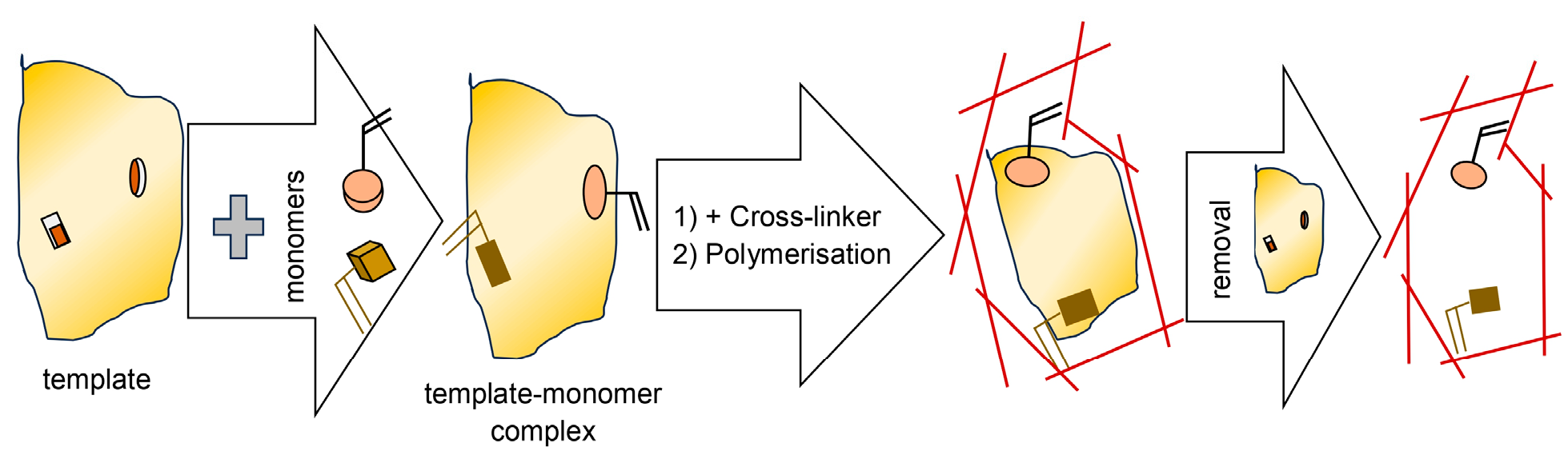
7.3. Molecularly Imprinted Polymers
7.4. Metal-Organic Frameworks
7.5. Carbon Nanomaterials
7.5.1. Graphene Materials
7.5.2. Carbon Nanotubes
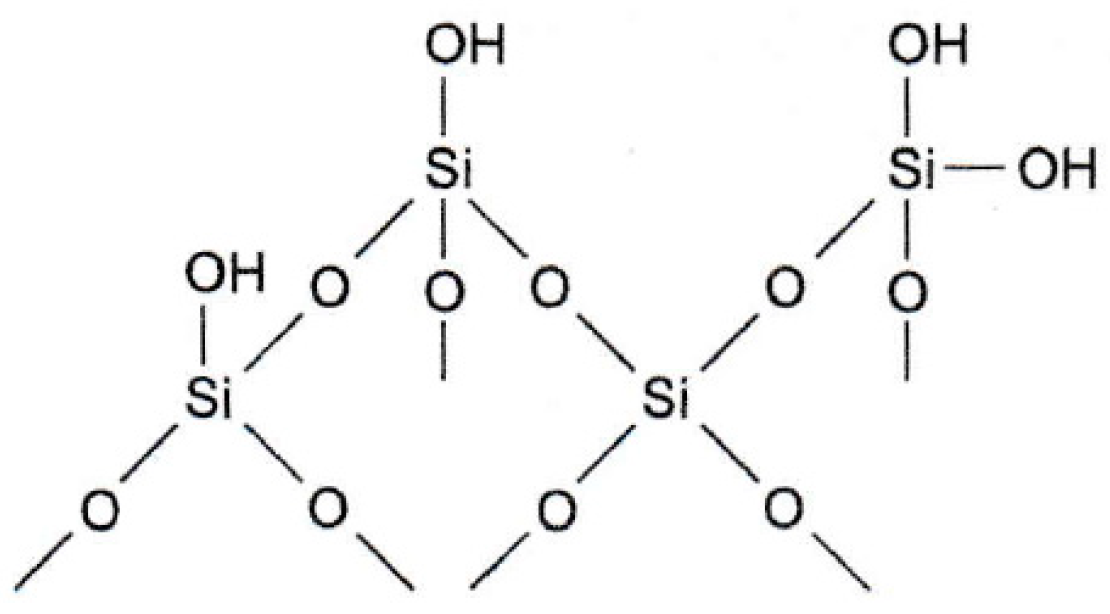
7.6. Silica Materials
- Ester, formed in the reaction of a silanol group with an alcohol:≡Si-OH + R-OH→≡Si-O-R + H2O
- Carbon, formed in the reaction of a silanol group with thionyl chloride and then with an organometallic compound:≡Si-OH + SOCl2→≡Si-Cl+ SO2+HCl≡Si-Cl + R-Li→≡Si-R+ SO2+LiCl
- Siloxane, formed by the reaction of the silanol group with organ chlorosilanes:≡Si-OH + Cl-SiR3→≡Si-O-SiR3 + HCl.
7.7. Aptamers
7.8. Ionic Liquids and Poly(Ionic Liquids)
7.9. Deep Eutectic Solvents
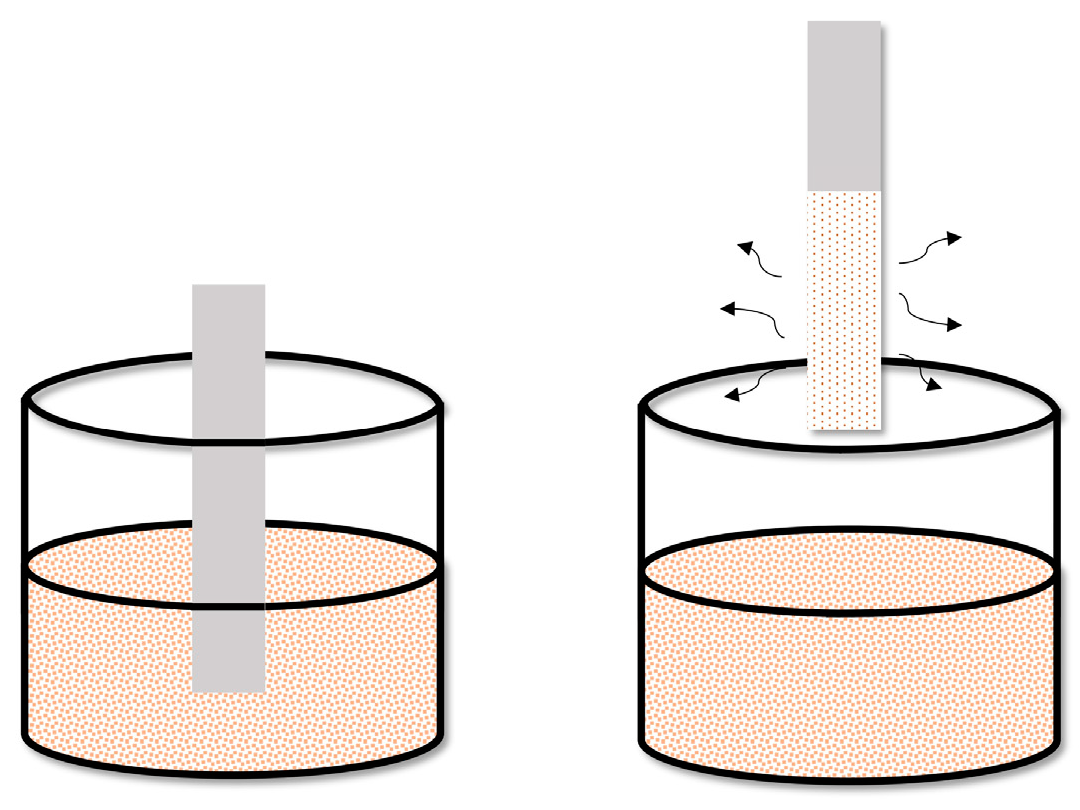
8. TF-SPME with Thermal and Solvent Desorption
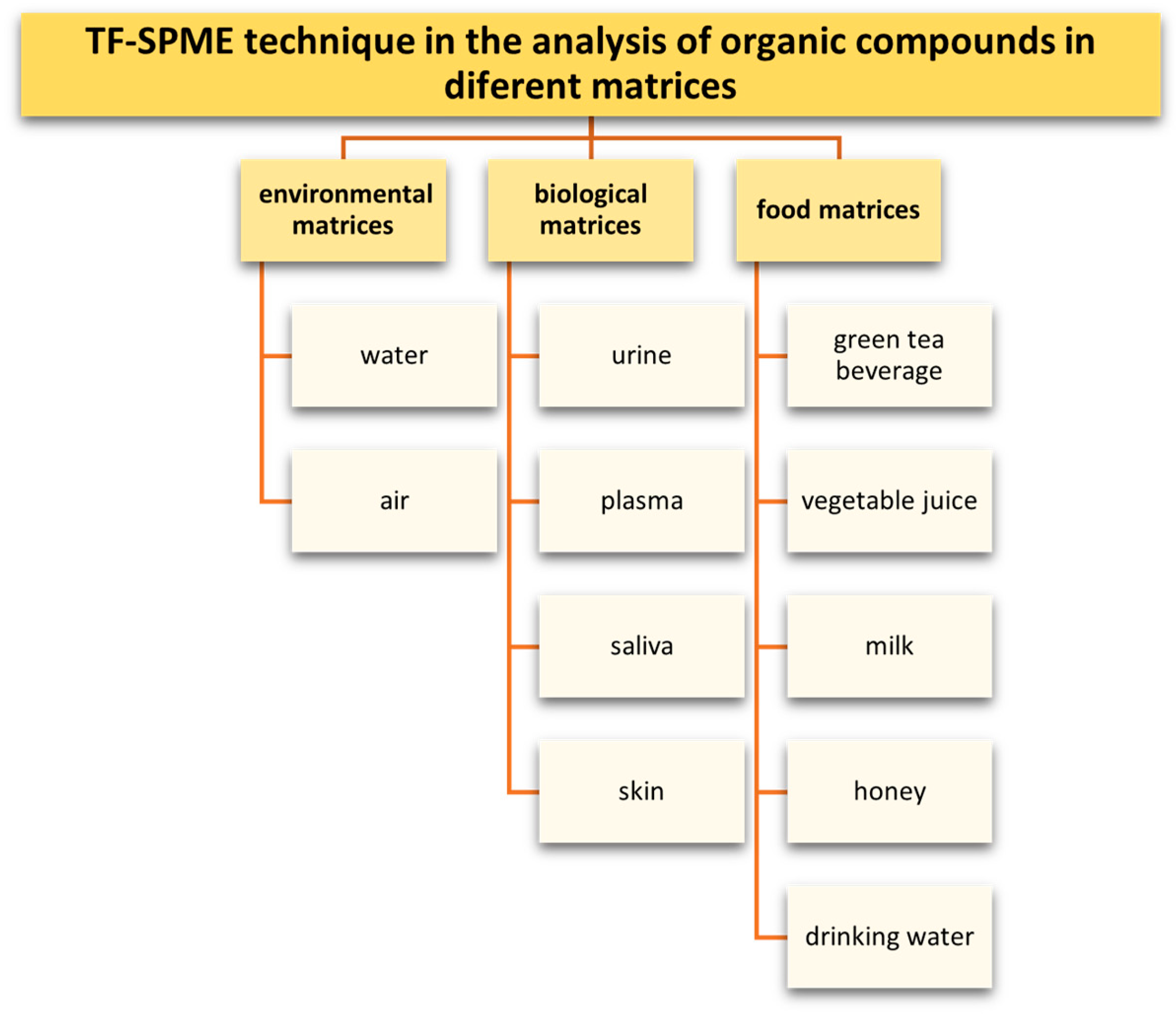
9. Application of the TFME Technique in the Analysis of Organic Compounds
- Compounds that are environmental contaminants in aqueous and food matrices (polycyclic aromatic hydrocarbons, polycyclic aromatic sulfur heterocycles (PASHs), plant protection products, phenols, chlorophenols, alkylphenols, bisphenol A);
- Preservatives determined in aqueous matrices (parabens and personal care products PCPs);
- Biologically active compounds determined in aqueous and biologic matrices (drugs from various therapeutic groups including nonsteroidal anti-inflammatory drugs, antibiotics, antidepressants, tranquilizers, opioids, anti-cancer drugs, sex hormones, doping agents, narcotics).
| Analyte | Sorption Phase | Detection | LOD | Recovery [%] | RSD [%] | Ref. |
|---|---|---|---|---|---|---|
| PAHs | PDMS | GC-MS | - | - | 6–11 | [34] |
| MWCNTs/CTA | HPLC-UV | 0.02–0.09 ng/mL | 99–101 | 1–8 | [100] | |
| Polycyclic aromatic sulfur heterocycles | MIP | GC-MS | 0.029–0.166 µg L−1 | - | ≤6.0 | [59] |
| Pesticides/fungicides | DVB/PDMS | GC-MS | 0.01–0.25 μg L−1 | 90–130 | 2–20 | [42] |
| GC-MS | 1.0–4.0 ng L−1 | 71–124 | 3–20 | [43] | ||
| Pesticides/fungicides | PPy/PA | GC-MS | 50 ng L−1 | 96–98 | 4 | [52] |
| amino-Zr-MOF/PAN | CD-IMS | 0.6 μg L−1 | - | 11 | [74] | |
| MWCNTs/CTA | GC-MS | 1 μg L−1 | - | ≤20 | [96] | |
| MIP | LC-MS/MS | 0.002–0.02 μg L−1 | 90–110 | <15 | [60] | |
| PAMAM@GO-PVDF | HPLC-UV | 0.12–0.20 μg L−1 | 98–99 | - | [89] | |
| MOF/PU | GC-MS | 0.005–0.1 μg L−1 | 72–110 | 4–5 | [128] | |
| DES/CTA | GC-MS | 0.4–1.3 μg L−1 | 69–103 | 3–14 | [127] | |
| PVA/CA/C)/AV | HPLC-UV | - | 86–97 | 6–7 | [159] | |
| DES | HPLC-UV | - | 72–94 | 3–11 | [160] | |
| DVB/PDMS | GC-TMS | - | - | - | [40] | |
| VOCs/SVOCs | GC-MS | - | - | - | [24] | |
| N-nitrosamines | DVB/PDMS | GC-MS | 3 ng L−1 | - | 8 | [22] |
| Chlorobenzenes | PANI-N6 | GC-MS | 19–33 ng L−1 | 93–103 | 5–14 | [54] |
| Phenols | MIP | UHPLC-PDA | 0.1–2 μg L−1 | 85–100 | 1–14 | [61] |
| LC-MS | - | - | - | [63] | ||
| MWCNTs-COOH/PDMS | HPLC-UV | 1–2 μg L−1 | 64–90 | - | [97] | |
| Polychlorinated biphenyls | MWCNTs-COOH-Ch/PP | GC-MS | <0.60 ng L−1 | 86–104 | 0.17–5.01 | [99] |
| Bisphenol A | PVA/PVP/PES | FS | 0.3 ng mL−1 | 84–96 | 5–10 | [136] |
| Phthalates, alkylphenols, bisphenols | Polyamide-coated paper | HPLC-DAD | 1.5–7.6 μg L−1 | - | ≤24% | [137] |
| Sulfonamides | p-Poly-(MMA-IL) | HPLC-DAD | 0.14–0.52 µg L−1 | 90–110 | 10 | [116] |
| Aniline | poly-(MMA-BVImBr) | LC-MS/MS | 0.5 μg L−1 | 91–96 | 8.3 | [117] |
| Parabens | DES | HPLC-UV | 0.018–0.055 ng L−1 | 68–94 | 4–7 | [119] |
| Parabens | PDMS/DES | HPLC-UV | 0.023–0.062 ng mL−1 | 79–88 | 3–6 | [121] |
| CA-MIL-101(Cr)@CNFs | HPLC-DAD | 11 ng L−1 | 92–100 | <5 | [141] | |
| Formaldehyde | DES | HPLC-UV-Vis | 0.15 ng mL−1 | 78–99 | 3–5 | [122] |
| Personal care products | MIL-100(Fe)/PS/cellulose | HPLC-PDA | 7.5 µg·L−1 | 78–128 | 11 | [75] |
| Flame retardants: | ZIF-8@N-rGO | HPLC | 0.03–0.14 ng L−1 | 89–106 | - | [161] |
| Nonsteroidal anti-inflammatory drugs | MWCNT/Agr-Ch | HPLC-UV | 0.89–8.05 ng mL−1 | - | <5 | [94] |
| Estrogens | C18/PAN | LC-UV | 1.2–1.6 ng mL−1 | 87–109 | 3–6 | [47] |
| Glucocorticoids | SDS-MWCNTs/PP | UHPLC-MS | 0.019–0.098 ng mL−1 | - | 2–4 | [93] |
| Carbamazepine | C18/SCX | DESI-MS | <ng L−1 | - | - | [104] |
| Triclosan | C18/SCX | DESI-MS | <ng L−1 | - | - | [104] |
| Antibiotics: sulfonamides, tetracyclines, fluoroquinolones, penicillin, macrolides | p-Poly-(MMA-IL)FP | LC-MS/MS | 0.05–4.52 μgL−1 | 79–127 | 1–12 | [147] |
| Antibiotics: Amoxicillin, enrofloxacin, tetracycline, doxycycline | PVA-SA-βCD | HPLC-UV | 0.02–0.03 μgL−1 | 70–100 | 1–2 | [148] |
| Illicit drugs: methamphetamine ketamine methaqualone | DVB/PDMS | GC-MS | For methamphetamine: 5.5 ng L−1 For ketamine: 2.0 ng L−1 For methaqualone: 1.1 ng L−1 | 95–111 | <6 | [162] |
| Chlorpyrifos, triclosan, tonalide | CTA with plasticizers | GC-MS | 0.05–0.42 μgL−1 | >80 | <10 | [163] |
| Haloacetic acids | PDMS HLB/PDMS Carboxen/PDMS | GC-ECD | - | 51–92 | 7–19 | [164] |
| Sexual hormones: 17β-estradiol, 17α-ethinylestradiol, estrone, progesterone, medroxyprogesterone acetate, hydroxyprogesterone | CTA/NPOE CTA/DBS | HPLC–MS/MS | 0.1–5.7 ng L−1 | >60 | <12 | [165] |
| Organic pollutants: benzene, 2-hexanone, hexanal, α-pinene, limonene, eucalyptol, 2-nona- none, 2-nonanol, 2-undecanone, ethyl nonanoate, 1-undecanol, ethyl undecanoate | GO/PS-DVB s | GC-MS | 0.4–8.1 ng L−1 | 78–111 | 2–6 | [166] |
| Matrix | Analyte | Sorption Phase | Detection | LOD | Recovery [%] | RSD [%] | Ref. |
|---|---|---|---|---|---|---|---|
| Green tea beverage | PAHs | MWCNTs/Agr | HPLC-UV | 0.1–50 ng L−1 | 91–107 | - | [95] |
| Vegetable juice | Codeine, acetamiprid | Aptamer/cellulose | ESI-IMS | 1.8–3.7 ng mL−1 | - | 2–6 | [106] |
| Honey, pork, chicken, milk | Sulfonamides: sulfathiazole, sulfamerazine, sulfadimidine, sulfamethoxazole, sulfisoxazole | MIL-101(Cr)/CC | HPLC-PDA | 2.5–4.5 μg mL−1 | 82–114 | <9 | [167] |
| Milk | Sulfathiazole | HVImBr/MMA | SF | 0.07–0.23 | 84–107 | [114] | |
| Phthalates | C18-FMSNs/PAN | HPLC | 0.096–0.26 ng mL−1 | 86–110 | <7 | [168] | |
| Honey | Macrolides, lincosamides | ZIF-8@GO | UPLC-MS/MS | 0.1–04 μg kg−1 | 68–107 | [88] | |
| Honey, tea | Chlorophenols | ACGO | HPLC-UV | 0.03–0.13 μg mL−1 | - | 3–6 | [138] |
| Drinking water | Anti-inflammatory antibacterial drugs | DVB/PAN | LC-ESI-MS/MS | ng L−1 | - | [41] | |
| Cod liver oil | Polychlorinated n-alkanes | HLB/PDMS | GC-MS | 0.07–0.22 μg/g | - | 2–12 | [169] |
| Apple, tomato | Benzoylurea insecticides | PAN/ZIF8@E coli | HPLC-UV | 0.12–0.15 μgL−1 | 93–110 | ≤8 | [170] |
| Fruit juice, black tea | Flavonoids: morin, quercetin | Co3O4@GO-Nylon-6 | HPLC-UV | For morin: 1.3 μgL−1 For quercetin: 1.6 μgL−1 | For morin: 73 For quercetin: 64 | <5 | [171] |
| Fruit and tea beverages | Pesticides | polyurethane | GC-ECD | 0.001–0.015 μgL−1 | 77–106 | - | [172] |
| Apple juice | HLB/PTFE AF | GC-MS | 1.0–5.0 ng mL−1 | - | ≤20 | [129] | |
| Cereal | PVA/MCS/HC-POF | HPLC-UV | ≤4.0 ng mL−1 | 63–79 | ≤7 | [130] | |
| Vegetables and fruits | POM@UIO-66-NH2/GO | HPLC-UV | 0.31–0.34 μgL−1 | 89–102 | 2–4 | [131] | |
| Vegetables and fruits | UiO-66/PS | GC | 1.5–3 μg kg−1 | 88–96 | 5–7 | [132] | |
| Carrot juice, apple juice, strawberry juice | phosphotungstic acid/polyvinylidene fluoride membrane. | HPLC-UV-Vis | 0.29–0.31 μgL−1 | 96–105 | 4–6 | [173] | |
| Fruit juice, tea | Neonicotinoid insecticides | PU/PMMA | UPLC-MS/MS | 0.001–0.1 μgL−1 | 81–108 | - | [133] |
| Fruit juice | Thiram fungicide | Silver nano network/silicon wafer | SERS | 0.01 μgL−1 | - | 7 | [174] |
| Milk, honey, fruits, vegetables | Conazole fungicides | MIL-88A@CNTs | CD-IMS | For penconazole: 0.30 ng mL−1 For propiconazole: 0.50 ng mL−1 | 86–97 | 5–7 | [175] |
| Dry chili, chili powder, dry Sichuan pepper, Sichuan pepper powder | Rhodamine B | COF-117-PTFE | HPLC-FLD | 0.007 μgL−1 | 68–71 | 7 | [176] |
| Matrix | Analyte | Sorption Phase | Detection | LOD | Recovery [%] | RSD [%] | Ref. |
|---|---|---|---|---|---|---|---|
| Urine | Doping agents | C18/PAN | LC-MS | 0.25–10 ng mL−1 LOQ | 85–130 | <20 | [45,46] |
| Hormones | PANI | HPLC-FLD | 0.30–3.03 μg L−1 | 71–115 | ≤12 | [53] | |
| Steroidal hormones | C18/PAN PS/DVB | UHPLC-ESI-QTOF/MS | - | 74–99 | - | [149] | |
| Estrogens | MIL-53(Al)/PVDF MIL-53(Fe) /PVDF MIL-100(Fe) /PVDF MIL-101(Cr) /PVDF UiO-66(Zr) /PVDF | HPLC-FLD | 0.005–1 ng mL−1 | 80–103 | ≤11.4 | [72] | |
| Urinary androgens | - | HPLC-QqQ/MS | 0.04–0.09 ng mL−1 | - | - | [177] | |
| Caffeine | ZIF-8/LDH/GO/PVDF | HPLC-UV | - | - | [73] | ||
| Aldehydes | MOF-199/PS | HPLC- VWD | 4.2–17.3 nmol L−1 | 82–112 | 2–13 | [76] | |
| Non-steroidal anti-inflammatory drugs | CY-GO-LDH | HPLC-UV | 0.25 μg L−1 | - | 6 | [82] | |
| Urine | Diclofenac | LDH/GO/PVDF | HPLC-UV | 0.14 μg L−1 in water 0.23 μg L−1 in urine 0.57 μg L−1 plasma samples | 89–93 | 7 7 7 | [84] |
| Benzodiazepines | C18/glue | HPLC-MS | 0.05−0.15 ng mL−1 | - | 5−7 | [25] | |
| Codeine acetamiprid | Aptamer/cellulose | ESI-IMS | 3.7 ng mL−1 | 87–91 | 2–6 | [106] | |
| Methamphetamine | Aptamer/CDs/ cellulose | ESI-IMS | 0.45 ng·mL−1 | 87–108 | <8 | [107] | |
| Codeine | Aptamer/cellulose | ESI-IMS | 3.4 ng·mL−1 | 90 | 7 | [108] | |
| Nonsteroidal anti-inflammatory drugs | p-PIL-AcO | LC-MS/MS. | 3.8 μg L−1 for indomethacin 7.2 μg L−1 for diclofenac 6.8 μg L−1 for tolmetin 9.4 μg L−1 for ketoprofen 15.7 μg L−1 for naproxen 5.1 μg L−1 for ibuprofen | 72–95 | 1–13 | [115] | |
| Nonsteroidal anti-inflammatory drugs: naproxen, aspirin, tolmetin, celecoxib | MOF-5 | HPLC-UV | 0.57–0.77 μg L−1 | 94–108 | 4–6 | [143] | |
| Endocrine-disrupting compounds | DES | LC-MS/MS | 0.01–1.15 ng mL−1 | - | 3–10 | [178] | |
| Tramadol | Ni(DMG)2-NiO-Cell | HPLC-UV | 0.1–1.0 ng mL−1 | 86 | 6–8 | [156] | |
| Carvedilol blocker | g-C3N4/N6 NC | FS | 1.0 ng mL−1 | 83 | 4 | [158] | |
| Fentanyl, methadone, zolpidem | Octyl-cyanopropyl/PAN | HPLC-MS/MS | 4.0–17.4 ng mL−1 | 43–76 | <15 | [157] | |
| Urine | Fluoxetine | GO/CS | HPLC-UV-Vis | 1.0 ng mL−1 | 82 | ≤9 | [152] |
| Tricyclic antidepressants | Ni-Co MOFs-PAN | HPLC-UV | 0.06–0.3 µg L−1 | 91–100 | <5 | [153] | |
| Biogenic monoamines | HLB/PAN | UPLC-MS/MS | 36–75 | <9 | [179] | ||
| Plasma | Tricyclic antidepressants | MIP | LC-MS/MS | 1.0–5.0 ng mL−1 | 90–110 | 15 | [62] |
| Antidepressant drugs: Clomipramine, Clozapine, Trimipramine | PVA/CA/β-cyclodextrin/Bi2S3@g-C3N4 | GC-FID | 0.03–0.15 ng mL−1 | 78–95 | 5–7 | [154] | |
| Mycophenolic acid | MIP | UPLC | 0.3 ng mL−1 | - | 4 | [64] | |
| Benzodiazepines | C18-TEOS | LC-MS/MS | 0.4–0.7 ng mL−1 | 11–83 | 4–8 | [101] | |
| C18/PAN | LC-MS/MS | 0.08–0.2 ng mL−1 | 83–98 | <9 | [44] | ||
| Anti-cancer drugs | Co-MOF-74/polyfam | HPLC-UV | 0.03–0.20 µg L−1 | - | 3–9 | [71] | |
| polylactic acid PLA | HPLC | 0.03 µg L−1 | - | 8 | [180] | ||
| Tramadol | Ni(DMG)2-NiO-Cell | HPLC-UV | 0.1–1.0 ng mL−1 | 92 | 6–8 | [156] | |
| Plasma | Carvedilol | g-C3N4/N6 NC | FS | 1.0 ng mL−1 | 87 | 3.6 | [158] |
| Fentanyl, methadone, zolpidem | Octyl-cyanopropyl/PAN | HPLC-MS/MS | 4.3–8.3 ng mL−1 | 34–62 | <15 | [157] | |
| Fluoxetine | GO/CS | HPLC-UV-Vis | 1.6 ng mL−1 | 87 | ≤9 | [152] | |
| Oral fluid | Fentanyl, methadone, zolpidem | Octyl-cyanopropyl/PAN | HPLC-MS/MS | 4.8–9.6 ng mL−1 | 27–38 | <15 | [157] |
| Saliva | β-blockers | GO/PEG | LC-MS/MS | 1.25–8.00 nmol L−1 | 80–109 | 4–13 | [87] |
| Methamphetamine | aptamer/CDs/cellulose | ESI-IMS | 0.6 ng·mL−1 | 87–108 | 6 | [107] | |
| Exhaled air condensate | Aldehydes | PS/G | HPLC | 3.8 nmol L−1 | 80–106 | 16 | [86] |
| Skin | Volatile organic compounds | PDMS | GC-MS | - | - | <9 | [37] |
| Fish tissue | Polychlorinated biphenyls | PDMS | LC-MS | - | - | - | [35] |
| Pharmaceuticals | C18/PAN | LC/MS-MS | 0.08–0.21 ng g−1 | - | 9–18 | [102] | |
| Fish plasma | Steroid hormones | LC-MS/MS | 0.006–0.150 ng mL−1 | - | ≤6 ≤15 | [103] |
10. Summary
Funding
Conflicts of Interest
Abbreviations
| AAS | Atomic absorption spectroscopy |
| ACGO | Graphene oxide-coated agarose/chitosan |
| AES | Atomic emission spectroscopy |
| Agr | Agarose |
| AV | Aloe vera gel |
| BVImBr | 1-butyl-3-vinylimidazolium bromide |
| CA | Citric acid |
| CA | Citric acid |
| CC | Carbon cloth |
| CD-IMS | Corona discharge ion mobility spectrometry |
| CDs | Carbon dots |
| Ch | Chitosan |
| CNMs | Carbon nanomaterials |
| CNT | Carbon nanotube |
| CPs | Conductive polymers |
| CS | Chitosan |
| CTA | Cellulose triacetate |
| CVD | Chemical vapor deposition |
| CY | Cotton yarn |
| CY-GO-LDH | Cotton yarn–graphene oxide-layered double hydroxide composite |
| DAD | Diode array detector |
| DBS | Dibutyl sebacate |
| DCBI-MS | Desorption corona beam ionization mass spectrometry |
| DES | Deep eutectic solvents |
| DESI-MS | Desorption electrospray ionization mass spectrometry |
| DI-SDME | Direct immersion single-drop microextraction |
| DLLME | Dispersive liquid–liquid microextraction |
| DNA | Deoxyribonucleic acid |
| ESD | Electrospray deposition technique |
| ESI-IMS | Electrospray ionization ion mobility spectrometer |
| FAAS | Flame atomic absorption spectroscopy |
| FID | Flame ionization detector |
| FMSNs | Fibrous mesoporous silica nanospheres |
| FS | Fluorescence spectrometry |
| G | Graphene |
| GBMs | Graphene-based materials |
| GC | Gas chromatography |
| GC-MS | Gas chromatography–mass spectrometry |
| GC-TMS | Gas chromatography–toroidal ion trap mass spectrometry |
| GO | Graphene oxide |
| HBD | Hydrogen bond donor |
| HF-LPME | Hollow-fiber liquid phase microextraction |
| HLB | Hydrophilic–lipophilic balance |
| HPLC | High-performance liquid chromatography |
| HPLC-VWD | High-performance liquid chromatography system with a variable wavelength ultraviolet detector |
| HPLC-DAD | High-performance liquid chromatography system with a diode array detector |
| HPLC-FLD | High-performance liquid chromatography with fluorescence detection |
| HPLC-MS | High-performance liquid chromatography with mass spectrometry |
| HPLC-PDA | High-performance liquid chromatography with photodiode array detector; |
| HPLC-UV | High-performance liquid chromatography with ultraviolet spectrophotometry |
| HPLC-UV-Vis | High-performance liquid chromatography with ultraviolet–visible spectrophotometry |
| HS-GC-SCD | Headspace gas chromatography sulfur chemiluminescence detection |
| HS-SDME | Headspace single-drop microextraction |
| HVImBr | 1-hexyl-3-vinylimidazolium bromide |
| ILs | Ionic liquids |
| IMS | Ion mobility spectrometry |
| LC/MS–MS | Liquid chromatography–tandem mass spectrometry |
| LC-ESI-MS/MS | Liquid chromatography/electrospray ionization–tandem mass spectrometry |
| LC-MS | Liquid chromatography and mass spectrometry |
| LC-UV | Liquid chromatography with ultraviolet spectrophotometry |
| LIBS | Laser-induced breakdown spectroscopy |
| LLE | Liquid–liquid extraction |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| LPME | Liquid-phase microextraction |
| MALDI-TOF MS | Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry |
| MEPS | Microextraction in packed syringe |
| METs | Microextraction techniques |
| MIPs | Molecularly imprinted polymers |
| MMA | Methylmethacrylate |
| MOFs | Metal–organic frameworks |
| MPA | Mycophenolic acid |
| MWCNT | Multi-wall carbon nanotubes |
| NPOE | Nitrophenyl octyl ether |
| OCTNs | Oxidized carbon nanotubes |
| OPPs | Organophosphorus pesticides |
| PA | Polyacetylene |
| PAMAM | Poly(amidoamine) |
| PAN | Polyacrylonitrile |
| PANI | Polyaniline |
| PASHs | Polycyclic aromatic sulfur heterocycles |
| PCBs | Polychlorinated biphenyls |
| PCN | Polycyanamide |
| PCPs | Personal care products |
| PDMS | Polydimethylsiloxane |
| PEDOT | Polyethylenedioxytiofene |
| PEG | Polyethylene glycol |
| PES | Polyether sulfone |
| PF | Polyfluorene |
| PFu | Polyfuran |
| PILs | Poly(ionic liquids) |
| PLOT | Porous layer open tubular |
| PMMA | Polymethyl methacrylate |
| PMME | Polymer monolith microextraction |
| PP | Polypropylene |
| PPA | Polyphenylacetylene |
| p-PIL-AcO | Pil-coated paper with acetate |
| p-Poly-(MMA-IL)FP | Paper-based polymeric ionic liquid |
| PPP | Polyparaphenylene |
| PPV | Polyphenylenevinylene |
| PPy | Polypyrrole |
| PPY | Polypyridine |
| PS | Polystyrene |
| PTFE AF | Polytetrafluoroethylene amorphous fluoropolymer |
| PTh | Polythiophene |
| PU | Polyurethane |
| PVA | Polyvinyl alcohol |
| PVA/MCS/HC-POF | Polyvinyl alcohol/modified chitosan/hydroxy-containing porous organic framework |
| PVA-SA-βCD | Polyvinyl alcohol doped with beta cyclodextrin and alginate |
| PVDF | Poly(vinylidene fluoride) |
| pVFc | Polyvinylferrocene |
| PVP | Polyvinyl pyrrolidone |
| rGO | Reduced graphene oxide |
| RNA | Ribonucleic acid |
| SBSE | Stir bar sorptive extraction |
| SBU | Secondary building units |
| SCOT | Support-coated open tubular |
| SDME | Single-drop microextraction |
| SDS | Sodium dodecyl sulfate |
| SELEX | Systematic evolution of ligands by exponential enrichment |
| SEM | Scanning electron microscope |
| SERS | Surface-enhanced Raman scattering |
| SF | Spectrofluorometry |
| SPDE | Solid-phase dynamic extraction |
| SPE | Solid-phase extraction |
| SPME | Solid-phase microextraction |
| SVOCs | Semi-volatile organic compounds |
| SWCNT | Single-wall carbon nanotubes |
| TCAs | Tricyclic antidepressant drugs |
| TEM | Transmission electron microscope |
| TEOS | Tetraethoxysilane |
| TFME | Thin-film microextraction |
| TXRF | Total reflection X-ray fluorescence spectrometry |
| UHPLC-MS | Ultra-high-performance liquid chromatography with mass spectrometry |
| UHPLC-PDA | Ultra-high-performance liquid chromatography with photodiode array detector |
| UHPLC-UV/VIS | Ultra-high-performance liquid chromatography with ultraviolet–visible spectrophotometry |
| UPLC | Ultra-performance liquid chromatography |
| UPLC–MS/MS | Ultra-performance liquid chromatography with tandem mass spectrometry |
| VOCs | Volatile organic compounds |
| WA | Aliphatic hydrocarbons |
| WCOT | Wall-coated open tubular |
| WWA | Polycyclic aromatic hydrocarbons |
| ZIFs | Zeolitic imidazolate frameworks |
References
- Piri-Moghadam, H.; Ahmadi, F.; Pawliszyn, J. A Critical Review of Solid Phase Microextraction for Analysis of Water Samples. TrAC—Trends Anal. Chem. 2016, 85, 133–143. [Google Scholar] [CrossRef]
- Marín-San Román, S.; Rubio-Bretón, P.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Advancement in Analytical Techniques for the Extraction of Grape and Wine Volatile Compounds. Food Res. Int. 2020, 137, 109712. [Google Scholar] [CrossRef] [PubMed]
- Drabińska, N.; Marcinkowska, M.A.; Wieczorek, M.N.; Jeleń, H.H. Application of Sorbent-Based Extraction Techniques in Food Analysis. Molecules 2023, 28, 7985. [Google Scholar] [CrossRef]
- Leszczyńska, D.; Hallmann, A.; Treder, N.; Bączek, T.; Roszkowska, A. Recent Advances in the Use of SPME for Drug Analysis in Clinical, Toxicological, and Forensic Medicine Studies. Talanta 2024, 270, 125613. [Google Scholar] [CrossRef]
- Hou, X.; Wang, L.; Guo, Y. Recent Developments in Solid-Phase Microextraction Coatings for Environmental and Biological Analysis. Chem. Lett. 2017, 46, 1444–1455. [Google Scholar] [CrossRef]
- Llompart, M.; Celeiro, M.; García-Jares, C.; Dagnac, T. Environmental Applications of Solid-Phase Microextraction. TrAC—Trends Anal. Chem. 2019, 112, 1–12. [Google Scholar] [CrossRef]
- Mirnaghi, F.S.; Hein, D.; Pawliszyn, J. Thin-Film Microextraction Coupled with Mass Spectrometry and Liquid Chromatography-Mass Spectrometry. Chromatographia 2013, 76, 1215–1223. [Google Scholar] [CrossRef]
- Olcer, Y.A.; Tascon, M.; Eroglu, A.E.; Boyacı, E. Thin Film Microextraction: Towards Faster and More Sensitive Microextraction. TrAC—Trends Anal. Chem. 2019, 113, 93–101. [Google Scholar] [CrossRef]
- Emmons, R.V.; Tajali, R.; Gionfriddo, E. Development, Optimization and Applications of Thin Film Solid Phase Microextraction (TF-SPME) Devices for Thermal Desorption: A Comprehensive Review. Separations 2019, 6, 39. [Google Scholar] [CrossRef]
- Kataoka, H.; Ishizaki, A.; Saito, K. Recent Progress in Solid-Phase Microextraction and Its Pharmaceutical and Biomedical Applications. Anal. Methods 2016, 8, 5773–5788. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid Phase Microextraction with Thermal Desorption Using Fused Silica Optical Fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Yu, X.; Yuan, H.; Gorecki, T.; Pawliszyn, J. Determination of Lead in Blood and Urine by SPME/GC. Anal. Chem. 1999, 71, 2998–3002. [Google Scholar] [CrossRef]
- Zhang, X.; Oakes, K.D.; Wang, S.; Servos, M.R.; Cui, S.; Pawliszyn, J.; Metcalfe, C.D. In Vivo Sampling of Environmental Organic Contaminants in Fish by Solid-Phase Microextraction. TrAC—Trends Anal. Chem. 2012, 32, 31–39. [Google Scholar] [CrossRef]
- Eisert, R.; Pawliszyn, J. Design of Automated Solid-Phase Microextraction for Trace Analysis of Organic Compounds in Aqueous Samples. J. Chromatogr. A 1997, 776, 293–303. [Google Scholar] [CrossRef]
- Ouyang, G.; Jiang, R. Solid Phase Microextraction: Recent Developments and Applications; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier: London, UK, 2012; ISBN 9780124160170. [Google Scholar]
- Fontanals, N.; Marcé, R.M.; Borrull, F. New Materials in Sorptive Extraction Techniques for Polar Compounds. J. Chromatogr. A 2007, 1152, 14–31. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-Film Microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef]
- Wilcockson, J.B.; Gobas, F.A.P.C. Thin-Film Solid-Phase Extraction to Measure Fugacities of Organic Chemicals with Low Volatility in Biological Samples. Environ. Sci. Technol. 2001, 35, 1425–1431. [Google Scholar] [CrossRef]
- Bragg, L.; Qin, Z.; Alaee, M.; Pawliszyn, J. Field Sampling with a Polydimethylsiloxane Thin-Film. J. Chromatogr. Sci. 2006, 44, 317–323. [Google Scholar] [CrossRef]
- Rodil, R.; von Sonntag, J.; Montero, L.; Popp, P.; Buchmeiser, M.R. Glass-Fiber Reinforced Poly(Acrylate)-Based Sorptive Materials for the Enrichment of Organic Micropollutants from Aqueous Samples. J. Chromatogr. A 2007, 1138, 1–9. [Google Scholar] [CrossRef]
- Riazi Kermani, F.; Pawliszyn, J. Sorbent Coated Glass Wool Fabric as a Thin Film Microextraction Device. Anal. Chem. 2012, 84, 8990–8995. [Google Scholar] [CrossRef]
- Jiang, R.; Pawliszyn, J. Preparation of a Particle-Loaded Membrane for Trace Gas Sampling. Anal. Chem. 2014, 86, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Grandy, J.J.; Singh, V.; Lashgari, M.; Gauthier, M.; Pawliszyn, J. Development of a Hydrophilic Lipophilic Balanced Thin Film Solid Phase Microextraction Device for Balanced Determination of Volatile Organic Compounds. Anal. Chem. 2018, 90, 14072–14080. [Google Scholar] [CrossRef] [PubMed]
- Cudjoe, E.; Vuckovic, D.; Hein, D.; Pawliszyn, J. Investigation of the Effect of the Extraction Phase Geometry on the Performance of Automated Solid-Phase Microextraction. Anal. Chem. 2009, 81, 4226–4232. [Google Scholar] [CrossRef]
- Murata, M.; Oizumi, T.; Gi, M.; Tsuji, R.; Arita, M.; Fujii, A.; Ozaki, M. Highly (100)-Oriented CH3NH3PbI3 Thin Film Fabricated by Bar-Coating Method and Its Additive Effect of Ammonium Chloride. Sol. Energy Mater. Sol. Cells 2020, 208, 110409. [Google Scholar] [CrossRef]
- Electrospinning Services | NEI Corporation. Available online: https://www.neicorporation.com/services/electrospinning/ (accessed on 23 November 2023).
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of Nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Frederichi, D.; Scaliante, M.H.N.O.; Bergamasco, R. Structured Photocatalytic Systems: Photocatalytic Coatings on Low-Cost Structures for Treatment of Water Contaminated with Micropollutants—A Short Review. Environ. Sci. Pollut. Res. 2021, 28, 23610–23633. [Google Scholar] [CrossRef]
- Lashgari, M.; Yamini, Y. An Overview of the Most Common Lab-Made Coating Materials in Solid Phase Microextraction. Talanta 2019, 191, 283–306. [Google Scholar] [CrossRef]
- Reyes-Garcés, N.; Gionfriddo, E.; Gómez-Ríos, G.A.; Alam, M.N.; Boyacl, E.; Bojko, B.; Singh, V.; Grandy, J.; Pawliszyn, J. Advances in Solid Phase Microextraction and Perspective on Future Directions. Anal. Chem. 2018, 90, 302–360. [Google Scholar] [CrossRef]
- Suhas; Gupta, V.K.; Carrott, P.J.M.; Singh, R.; Chaudhary, M.; Kushwaha, S. Cellulose: A Review as Natural, Modified and Activated Carbon Adsorbent. Bioresour. Technol. 2016, 216, 1066–1076. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A Review on Modification Methods to Cellulose-Based Adsorbents to Improve Adsorption Capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef]
- Qin, Z.; Bragg, L.; Ouyang, G.; Niri, V.H.; Pawliszyn, J. Solid-Phase Microextraction under Controlled Agitation Conditions for Rapid on-Site Sampling of Organic Pollutants in Water. J. Chromatogr. A 2009, 1216, 6979–6985. [Google Scholar] [CrossRef] [PubMed]
- Jahnke, A.; Mayer, P.; Broman, D.; McLachlan, M.S. Possibilities and Limitations of Equilibrium Sampling Using Polydimethylsiloxane in Fish Tissue. Chemosphere 2009, 77, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Rönkkö, T. Recent Developments in Solid Phase Microextraction Techniques. Master’s Thesis, University of Helsinki, Helsinki, Finland, 2015. [Google Scholar]
- Jiang, R.; Cudjoe, E.; Bojko, B.; Abaffy, T.; Pawliszyn, J. A Non-Invasive Method for in Vivo Skin Volatile Compounds Sampling. Anal. Chim. Acta 2013, 804, 111–119. [Google Scholar] [CrossRef]
- Eom, I.Y.; Risticevic, S.; Pawliszyn, J. Simultaneous Sampling and Analysis of Indoor Air Infested with Cimex lectularius L. (Hemiptera: Cimicidae) by Solid Phase Microextraction, Thin Film Microextraction and Needle Trap Device. Anal. Chim. Acta 2012, 716, 2–10. [Google Scholar] [CrossRef]
- Guerra-Diaz, P.; Gura, S.; Almirall, J.R. Dynamic Planar Solid Phase Microextraction-Ion Mobility Spectrometry for Rapid Field Air Sampling and Analysis of Illicit Drugs and Explosives. Anal. Chem. 2010, 82, 2826–2835. [Google Scholar] [CrossRef]
- Grandy, J.J.; Boyaci, E.; Pawliszyn, J. Development of a Carbon Mesh Supported Thin Film Microextraction Membrane As a Means to Lower the Detection Limits of Benchtop and Portable GC/MS Instrumentation. Anal. Chem. 2016, 88, 1760–1767. [Google Scholar] [CrossRef]
- Di Carro, M.; Lluveras-Tenorio, A.; Benedetti, B.; Magi, E. An Innovative Sampling Approach Combined with Liquid Chromatography–Tandem Mass Spectrometry for the Analysis of Emerging Pollutants in Drinking Water. J. Mass Spectrom. 2020, 55, e4608. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Gionfriddo, E.; Rodriguez-Lafuente, A.; Grandy, J.J.; Lord, H.L.; Obal, T.; Pawliszyn, J. Inter-Laboratory Validation of a Thin Film Microextraction Technique for Determination of Pesticides in Surface Water Samples. Anal. Chim. Acta 2017, 964, 74–84. [Google Scholar] [CrossRef]
- Piri-Moghadam, H.; Gionfriddo, E.; Grandy, J.J.; Alam, M.N.; Pawliszyn, J. Development and Validation of Eco-Friendly Strategies Based on Thin Film Microextraction for Water Analysis. J. Chromatogr. A 2018, 1579, 20–30. [Google Scholar] [CrossRef]
- Moradi, E.; Ebrahimzadeh, H.; Mehrani, Z. Electrospun Acrylonitrile Butadiene Styrene Nanofiber Film as an Efficient Nanosorbent for Head Space Thin Film Microextraction of Polycyclic Aromatic Hydrocarbons from Water and Urine Samples. Talanta 2019, 205, 120080. [Google Scholar] [CrossRef]
- Boyaci, E.; Gorynski, K.; Rodriguez-Lafuente, A.; Bojko, B.; Pawliszyn, J. Introduction of Solid-Phase Microextraction as a High-Throughput Sample Preparation Tool in Laboratory Analysis of Prohibited Substances. Anal. Chim. Acta 2014, 809, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Garcés, N.; Bojko, B.; Pawliszyn, J. High Throughput Quantification of Prohibited Substances in Plasma Using Thin Film Solid Phase Microextraction. J. Chromatogr. A 2014, 1374, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Cai, P.S.; Li, D.; Chen, J.; Xiong, C.M.; Ruan, J.L. Comparison of Two Thin-Film Microextractions for the Analysis of Estrogens in Aqueous Tea Extract and Environmental Water Samples by High Performance Liquid Chromatography-Ultraviolet Detection. Food Chem. 2015, 173, 1158–1166. [Google Scholar] [CrossRef]
- Bahrani, S.; Hashemi, S.A.; Mousavi, S.M.; Arjmand, M.; Ghalamfarsa, F.; Ghaedi, M. Conductive Polymers in Green Analytical Chemistry. ACS Symp. Ser. 2022, 1405, 1–37. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yang, X.; Chen, J.; Fu, H.; Cheng, T.; Wang, Y. Conducting Polymers in Environmental Analysis. TrAC—Trends Anal. Chem. 2012, 39, 163–179. [Google Scholar] [CrossRef]
- Bagheri, H.; Ayazi, Z.; Naderi, M. Conductive Polymer-Based Microextraction Methods: A Review. Anal. Chim. Acta 2013, 767, 1–13. [Google Scholar] [CrossRef]
- Mehrani, Z.; Ebrahimzadeh, H.; Asgharinezhad, A.A.; Moradi, E. Determination of Copper in Food and Water Sources Using Poly M-Phenylenediamine/CNT Electrospun Nanofiber. Microchem. J. 2019, 149, 103975. [Google Scholar] [CrossRef]
- Bagheri, H.; Aghakhani, A.; Akbari, M.; Ayazi, Z. Electrospun Composite of Polypyrrole-Polyamide as a Micro-Solid Phase Extraction Sorbent. Anal. Bioanal. Chem. 2011, 400, 3607–3613. [Google Scholar] [CrossRef]
- Turazzi, F.C.; Morés, L.; Carasek, E.; Barra, G.M. de O. Polyaniline-Silica Doped with Oxalic Acid as a Novel Extractor Phase in Thin Film Solid-Phase Microextraction for Determination of Hormones in Urine. J. Sep. Sci. 2023, 46, 2300280. [Google Scholar] [CrossRef]
- Bagheri, H.; Aghakhani, A. Polyaniline-Nylon-6 Electrospun Nanofibers for Headspace Adsorptive Microextraction. Anal. Chim. Acta 2012, 713, 63–69. [Google Scholar] [CrossRef]
- Turiel, E.; Martín-Esteban, A. Molecularly Imprinted Polymers-Based Microextraction Techniques. TrAC—Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Yan, H.; Kyung, H.R. Characteristic and Synthetic Approach of Molecularly Imprinted Polymer. Int. J. Mol. Sci. 2006, 7, 155–178. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular Imprinting: Perspectives and Applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef]
- Díaz-Álvarez, M.; Turiel, E.; Martín-Esteban, A. Recent Advances and Future Trends in Molecularly Imprinted Polymers-Based Sample Preparation. J. Sep. Sci. 2023, 46, 2300157. [Google Scholar] [CrossRef]
- Hijazi, H.Y.; Bottaro, C.S. Molecularly Imprinted Polymer Thin-Film as a Micro-Extraction Adsorbent for Selective Determination of Trace Concentrations of Polycyclic Aromatic Sulfur Heterocycles in Seawater. J. Chromatogr. A 2020, 1617, 460824. [Google Scholar] [CrossRef]
- Azizi, A.; Shahhoseini, F.; Langille, E.A.; Akhoondi, R.; Bottaro, C.S. Micro-Gel Thin Film Molecularly Imprinted Polymer Coating for Extraction of Organophosphorus Pesticides from Water and Beverage Samples. Anal. Chim. Acta 2021, 1187, 339135. [Google Scholar] [CrossRef]
- Abu-Alsoud, G.F.; Bottaro, C.S. Porous Thin-Film Molecularly Imprinted Polymer Device for Simultaneous Determination of Phenol, Alkylphenol and Chlorophenol Compounds in Water. Talanta 2021, 223, 121727. [Google Scholar] [CrossRef]
- Shahhoseini, F.; Langille, E.A.; Azizi, A.; Bottaro, C.S. Thin Film Molecularly Imprinted Polymer (TF-MIP), a Selective and Single-Use Extraction Device for High-Throughput Analysis of Biological Samples. Analyst 2021, 146, 3157–3168. [Google Scholar] [CrossRef]
- Gryshchenko, A.O.; Bottaro, C.S. Development of Molecularly Imprinted Polymer in Porous Film Format for Binding of Phenol and Alkylphenols from Water. Int. J. Mol. Sci. 2014, 15, 1338–1357. [Google Scholar] [CrossRef]
- Langille, E.; Bottaro, C.S. Development and Application of a Thin-Film Molecularly Imprinted Polymer for the Measurement of Mycophenolic Acid in Human Plasma. J. Clin. Lab. Anal. 2023, 37, e24864. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Moler, D.B.; Li, H.; Chen, B.; Reineke, T.M.; Michael, O.; Omar, M.Y. Modular Chemistry: Secondary Building Units as a Basis ForBuilding Units as a Basis for Robust Metal-Organic Carboxylate Frameworks. Acc. Chem. Res 2001, 34, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Cheetham, A.K.; Rao, C.N.R.; Feller, R.K. Structural Diversity and Chemical Trends in Hybrid Inorganic-Organic Framework Materials. Chem. Commun. 2006, 46, 4780–4795. [Google Scholar] [CrossRef]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and Synthesis of an Exceptionally Stable and Highly. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Pacheco-Fernández, I.; Pasán, J.; Pino, V. Are Metal-Organic Frameworks Able to Provide a New Generation of Solid-Phase Microextraction Coatings?—A Review. Anal. Chim. Acta 2016, 939, 26–41. [Google Scholar] [CrossRef]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to Metal−Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef]
- Gutiérrez-Serpa, A.; Pasán, J.; Jiménez-Abizanda, A.I.; Kaskel, S.; Senkovska, I.; Pino, V. Thin-Film Microextraction Using the Metal-Organic Framework DUT-52 for Determining Endocrine Disrupting Chemicals in Cosmetics. Microchem. J. 2022, 181, 107685. [Google Scholar] [CrossRef]
- Khodayari, P.; Jalilian, N.; Ebrahimzadeh, H.; Amini, S. Trace-Level Monitoring of Anti-Cancer Drug Residues in Wastewater and Biological Samples by Thin-Film Solid-Phase Micro-Extraction Using Electrospun Polyfam/Co-MOF-74 Composite Nanofibers Prior to Liquid Chromatography Analysis. J. Chromatogr. A 2021, 1655, 462484. [Google Scholar] [CrossRef]
- Gao, G.; Li, S.; Li, S.; Zhao, L.; Wang, T.; Hou, X. Development and Application of Vortex-Assisted Membrane Extraction Based on Metal–Organic Framework Mixed-Matrix Membrane for the Analysis of Estrogens in Human Urine. Anal. Chim. Acta 2018, 1023, 35–43. [Google Scholar] [CrossRef]
- Jafari, Z.; Hadjmohammadi, M.R. In Situ Growth of Zeolitic Imidazolate Framework-8 on a GO-PVDF Membrane as a Sorbent for Thin-Film Microextraction of Caffeine Followed by Quantitation through High-Performance Liquid Chromatography. Anal. Methods 2020, 12, 1736–1743. [Google Scholar] [CrossRef]
- Bahrami, H.; Rezaei, B.; Jafari, M.T. Coupling of a Novel Electrospun Polyacrylonitrile/Amino-Zr-MOF Nanofiber as a Thin Film for Microextraction-Corona Discharge-Ion Mobility Spectrometry for the Analysis of Chlorpyrifos in Water Samples. Anal. Methods 2019, 11, 1073–1079. [Google Scholar] [CrossRef]
- Guerra-Martín, I.; Gutiérrez-Serpa, A.; Jiménez-Abizanda, A.I.; Pasán, J.; Pino, V. Thin Films Using the Greenly Prepared Metal-Organic Framework MIL-100(Fe) and Recycled Polystyrene Supported onto Cellulose for the Microextraction of Personal Care Products from Waters and Cosmetics. Microchem. J. 2023, 195, 109502. [Google Scholar] [CrossRef]
- Liu, F.; Xu, H. Development of a Novel Polystyrene/Metal-Organic Framework-199 Electrospun Nanofiber Adsorbent for Thin Film Microextraction of Aldehydes in Human Urine. Talanta 2017, 162, 261–267. [Google Scholar] [CrossRef]
- Valenzuela, E.F.; de Paula, F.G.F.; Teixeira, A.P.C.; Menezes, H.C.; Cardeal, Z.L. A New Carbon Nanomaterial Solid-Phase Microextraction to Pre-Concentrate and Extract Pesticides in Environmental Water. Talanta 2020, 217, 121011. [Google Scholar] [CrossRef] [PubMed]
- Maciel, E.V.S.; Mejía-Carmona, K.; Jordan-Sinisterra, M.; da Silva, L.F.; Vargas Medina, D.A.; Lanças, F.M. The Current Role of Graphene-Based Nanomaterials in the Sample Preparation Arena. Front. Chem. 2020, 8, 664. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Jiang, G. Application of Graphene in Analytical Sample Preparation. TrAC—Trends Anal. Chem. 2012, 37, 1–11. [Google Scholar] [CrossRef]
- Valcárcel, M.; Cárdenas, S.; Simonet, B.M.; Moliner-Martínez, Y.; Lucena, R. Carbon Nanostructures as Sorbent Materials in Analytical Processes. TrAC—Trends Anal. Chem. 2008, 27, 34–43. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Lu, Q.; Qu, Q. Graphene-Based Materials: Fabrication and Application for Adsorption in Analytical Chemistry. J. Chromatogr. A 2014, 1362, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.; Haghdoostnejad, K. Woven Cotton Yarn-Graphene Oxide-Layered Double Hydroxide Composite as a Sorbent for Thin Film Microextraction of Nonsteroidal Anti-Inflammatory Drugs Followed by Quantitation through High Performance Liquid Chromatography. Anal. Chim. Acta 2020, 1097, 94–102. [Google Scholar] [CrossRef]
- de la Calle, I.; Ruibal, T.; Lavilla, I.; Bendicho, C. Direct Immersion Thin-Film Microextraction Method Based on the Sorption of Pyrrolidine Dithiocarbamate Metal Chelates onto Graphene Membranes Followed by Total Reflection X-Ray Fluorescence Analysis. Spectrochim. Acta-Part B At. Spectrosc. 2019, 152, 14–24. [Google Scholar] [CrossRef]
- Ghani, M.; Ghoreishi, S.M.; Azamati, M. Magnesium-Aluminum-Layered Double Hydroxide-Graphene Oxide Composite Mixed-Matrix Membrane for the Thin-Film Microextraction of Diclofenac in Biological Fluids. J. Chromatogr. A 2018, 1575, 11–17. [Google Scholar] [CrossRef]
- Ripoll, L.; Legnaioli, S.; Palleschi, V.; Hidalgo, M. Evaluation of Electrosprayed Graphene Oxide Coatings for Elemental Analysis by Thin Film Microextraction Followed by LIBS Detection (TFME-LIBS). Spectrochim. Acta-Part B At. Spectrosc. 2021, 183, 106267. [Google Scholar] [CrossRef]
- Huang, J.; Deng, H.; Song, D.; Xu, H. Electrospun Polystyrene/Graphene Nanofiber Film as a Novel Adsorbent of Thin Film Microextraction for Extraction of Aldehydes in Human Exhaled Breath Condensates. Anal. Chim. Acta 2015, 878, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Karimiyan, H.; Hadjmohammadi, M.R.; Kunjali, K.L.; Moein, M.M.; Dutta, J.; Abdel-Rehim, M. Graphene Oxide/Polyethylene Glycol-Stick for Thin Film Microextraction of β-Blockers from Human Oral Fluid by Liquid Chromatography-Tandem Mass Spectrometry. Molecules 2019, 24, 3664. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Wu, H.; Wang, J.; Zhao, H.; Ji, X.; Xu, Y.; Li, R.; Zhang, H.; Yang, H.; et al. High-Throughput Method Based on a Novel Thin-Film Microextraction Coating for Determining Macrolides and Lincosamides in Honey. Food Chem. 2021, 346, 128920. [Google Scholar] [CrossRef]
- Jafari, Z.; Hadjmohammadi, M.R. Polyvinylidene Difluoride Film with Embedded Poly(Amidoamine) Modified Graphene Oxide for Extraction of Chlorpyrifos and Diazinon. Microchim. Acta 2021, 188, 37. [Google Scholar] [CrossRef]
- Song, X.Y.; Chen, J.; Shi, Y.P. Different Configurations of Carbon Nanotubes Reinforced Solid-Phase Microextraction Techniques and Their Applications in the Environmental Analysis. TrAC—Trends Anal. Chem. 2017, 86, 263–275. [Google Scholar] [CrossRef]
- Wepasnick, K.A.; Smith, B.A.; Bitter, J.L.; Fairbrother, D.H. Chemical and Structural Characterization of Carbon Nanotube Surfaces. Anal. Bioanal. Chem. 2011, 396, 1003–1014. [Google Scholar] [CrossRef]
- Hussain, C.M.; Mitra, S. Micropreconcentration Units Based on Carbon Nanotubes (CNT). Anal. Bioanal. Chem. 2011, 399, 75–89. [Google Scholar] [CrossRef]
- Goh, S.X.L.; Goh, E.X.Y.; Lee, H.K. Sodium Dodecyl Sulfate-Multi-Walled Carbon Nanotubes-Coated-Membrane Solid Phase Extraction of Glucocorticoids in Aqueous Matrices. Talanta 2021, 221, 121624. [Google Scholar] [CrossRef]
- Wan Ibrahim, W.N.; Sanagi, M.M.; Mohamad Hanapi, N.S.; Kamaruzaman, S.; Yahaya, N.; Wan Ibrahim, W.A. Solid-Phase Microextraction Based on an Agarose-Chitosan-Multiwalled Carbon Nanotube Composite Film Combined with HPLC–UV for the Determination of Nonsteroidal Anti-Inflammatory Drugs in Aqueous Samples. J. Sep. Sci. 2018, 41, 2942–2951. [Google Scholar] [CrossRef]
- Loh, S.H.; Sanagi, M.M.; Wan Ibrahim, W.A.; Hasan, M.N. Multi-Walled Carbon Nanotube-Impregnated Agarose Film Microextraction of Polycyclic Aromatic Hydrocarbons in Green Tea Beverage. Talanta 2013, 106, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla, I.; Perelló, C.; Merlo, F.; Profumo, A.; Fontàs, C.; Anticó, E. Multiwalled Carbon Nanotubes Embedded in a Polymeric Matrix as a New Material for Thin Film Microextraction (TFME) in Organic Pollutant Monitoring. Polymers 2023, 15, 314. [Google Scholar] [CrossRef] [PubMed]
- Kueseng, P.; Pawliszyn, J. Carboxylated Multiwalled Carbon Nanotubes/Polydimethylsiloxane, a New Coating for 96-Blade Solid-Phase Microextraction for Determination of Phenolic Compounds in Water. J. Chromatogr. A 2013, 1317, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, W.N.W.; Sanagi, M.M.; Hanapi, N.S.M.; Hadzir, N.M.; Yahaya, N.; Kamaruzaman, S. Agarose-Chitosan-Intergrated Multiwalled Carbon Nanotubes Film Solid Phase Microextraction Combined with High Performance Liquid Chromatography for the Determination of Tricyclic Antidepressant Drugs in Aqueous Samples. Malaysian J. Anal. Sci. 2020, 24, 33–41. [Google Scholar]
- Ge, D.; Lee, H.K. Polypropylene Membrane Coated with Carbon Nanotubesfunctionalized with Chitosan: Application in the Microextraction Ofpolychlorinated Biphenyls and Polybrominated Diphenyl Ethers from Environmental Water Samples. J. Chromatogr. A 2015, 1408, 56–62. [Google Scholar] [CrossRef]
- Mukhtar, N.H.; See, H.H. Carbonaceous Nanomaterials Immobilised Mixed Matrix Membrane Microextraction for the Determination of Polycyclic Aromatic Hydrocarbons in Sewage Pond Water Samples. Anal. Chim. Acta 2016, 931, 57–63. [Google Scholar] [CrossRef]
- Mirnaghi, F.S.; Monton, M.R.N.; Pawliszyn, J. Thin-Film Octadecyl-Silica Glass Coating for Automated 96-Blade Solid-Phase Microextraction Coupled with Liquid Chromatography-Tandem Mass Spectrometry for Analysis of Benzodiazepines. J. Chromatogr. A 2012, 1246, 2–8. [Google Scholar] [CrossRef]
- Togunde, O.P.; Oakes, K.D.; Servos, M.R.; Pawliszyn, J. Optimization of Solid Phase Microextraction for Non-Lethal in Vivo Determination of Selected Pharmaceuticals in Fish Muscle Using Liquid Chromatography-Mass Spectrometry. J. Chromatogr. A 2012, 1261, 99–106. [Google Scholar] [CrossRef]
- Maciążek-Jurczyk, M.; Bessonneau, V.; Ings, J.; Bragg, L.; McMaster, M.; Servos, M.R.; Bojko, B.; Pawliszyn, J. Development of a Thin-Film Solid-Phase Microextraction (TF-SPME) Method Coupled to Liquid Chromatography and Tandem Mass Spectrometry for High-Throughput Determination of Steroid Hormones in White Sucker Fish Plasma. Anal. Bioanal. Chem. 2020, 412, 4183–4194. [Google Scholar] [CrossRef]
- Strittmatter, N.; Düring, R.A.; Takáts, Z. Analysis of Wastewater Samples by Direct Combination of Thin-Film Microextraction and Desorption Electrospray Ionization Mass Spectrometry. Analyst 2012, 137, 4037–4044. [Google Scholar] [CrossRef]
- Pichon, V. Aptamer-Based and Immunosorbents; Elsevier Inc.: Amsterdam, The Netherlands, 2019; ISBN 9780128169063. [Google Scholar]
- Zargar, T.; Khayamian, T.; Jafari, M.T. Immobilized Aptamer Paper Spray Ionization Source for Ion Mobility Spectrometry. J. Pharm. Biomed. Anal. 2017, 132, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Zargar, T.; Khayamian, T.; Jafari, M.T. Aptamer-Modified Carbon Nanomaterial Based Sorption Coupled to Paper Spray Ion Mobility Spectrometry for Highly Sensitive and Selective Determination of Methamphetamine. Microchim. Acta 2018, 185, 103. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, Z.; Khayamian, T.; Saraji, M. Anticodeine Aptamer Immobilized on a Whatman Cellulose Paper for Thin-Film Microextraction of Codeine from Urine Followed by Electrospray Ionization Ion Mobility Spectrometry. Anal. Bioanal. Chem. 2014, 407, 1615–1623. [Google Scholar] [CrossRef]
- Patinha, D.J.S.; Silvestre, A.J.D.; Marrucho, I.M. Poly(Ionic Liquids) in Solid Phase Microextraction: Recent Advances and Perspectives. Prog. Polym. Sci. 2019, 98, 101148. [Google Scholar] [CrossRef]
- Acree, W.E.; Grubbs, L.M. Analytical Applications of Ionic Liquids. In Encyclopedia of Analytical Chemistry: Applications, Theory and Instrumentation; Wiley: Hoboken, NJ, USA, 2012; ISBN 9780470027318. [Google Scholar]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep Eutectic Solvents: Syntheses, Properties and Applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Aguilera-Herrador, E.; Lucena, R.; Cárdenas, S.; Valcárcel, M. The Roles of Ionic Liquids in Sorptive Microextraction Techniques. TrAC—Trends Anal. Chem. 2010, 29, 602–616. [Google Scholar] [CrossRef]
- Mei, M.; Huang, X.; Chen, L. Recent Development and Applications of Poly (Ionic Liquid)s in Microextraction Techniques. TrAC—Trends Anal. Chem. 2019, 112, 123–134. [Google Scholar] [CrossRef]
- Sadeghi, S.; Oliaei, S. Microextraction of Sulfathiazole from Milk and Honey Samples Using a Polymeric Ionic Liquid Membrane Followed by Fluorometric Determination. J. Food Compos. Anal. 2021, 97, 103774. [Google Scholar] [CrossRef]
- Ríos-Gómez, J.; García-Valverde, M.T.; López-Lorente, Á.I.; Toledo-Neira, C.; Lucena, R.; Cárdenas, S. Polymeric Ionic Liquid Immobilized onto Paper as Sorptive Phase in Microextraction. Anal. Chim. Acta 2020, 1094, 47–56. [Google Scholar] [CrossRef]
- Shahriman, M.S.; Mohamad, S.; Mohamad Zain, N.N.; Alias, Y.; Chandrasekaram, K.; Raoov, M. Paper-Based Polymeric Ionic Liquid for Thin Film Micro Extraction of Sulfonamides in Environmental Water Samples Prior to HPLC-DAD Analysis. Microchem. J. 2021, 171, 106798. [Google Scholar] [CrossRef]
- Cai, M.Q.; Wei, X.Q.; Du, C.H.; Ma, X.M.; Jin, M.C. Novel Amphiphilic Polymeric Ionic Liquid-Solid Phase Micro-Extraction Membrane for the Preconcentration of Aniline as Degradation Product of Azo Dye Orange G under Sonication by Liquid Chromatography-Tandem Mass Spectrometry. J. Chromatogr. A 2014, 1349, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Werner, J.; Zgoła-Grześkowiak, A.; Grześkowiak, T. Development of Novel Thin-Film Solid-Phase Microextraction Materials Based on Deep Eutectic Solvents for Preconcentration of Trace Amounts of Parabens in Surface Waters. J. Sep. Sci. 2022, 45, 1374–1384. [Google Scholar] [CrossRef]
- Nakhle, L.; Kfoury, M.; Mallard, I.; Landy, D.; Greige-Gerges, H. Microextraction of Bioactive Compounds Using Deep Eutectic Solvents: A Review. Environ. Chem. Lett. 2021, 19, 3747–3759. [Google Scholar] [CrossRef]
- Werner, J.; Grześkowiak, T.; Zgoła-Grześkowiak, A. A Polydimethylsiloxane/Deep Eutectic Solvent Sol-Gel Thin Film Sorbent and Its Application to Solid-Phase Microextraction of Parabens. Anal. Chim. Acta 2022, 1202, 339666. [Google Scholar] [CrossRef]
- Werner, J.; Zgoła-Grześkowiak, A.; Grześkowiak, T. Deep Eutectic Solvent-Based Coating Sorbent for Preconcentration of Formaldehyde by Thin-Film Solid-Phase Microextraction Technique. Processes 2022, 10, 828. [Google Scholar] [CrossRef]
- Riazanskaia, S.; Blackburn, G.; Harker, M.; Taylor, D.; Thomas, C.L.P. The Analytical Utility of Thermally Desorbed Polydimethylsilicone Membranes for In-Vivo Sampling of Volatile Organic Compounds in and on Human Skin. Analyst 2008, 133, 1020–1027. [Google Scholar] [CrossRef]
- Yu, M.; Roszkowska, A.; Pawliszyn, J. In Vivo Solid-Phase Microextraction and Applications in Environmental Sciences. ACS Environ. Au 2022, 2, 30–41. [Google Scholar] [CrossRef]
- Bessonneau, V.; Boyaci, E.; Maciazek-Jurczyk, M.; Pawliszyn, J. In Vivo Solid Phase Microextraction Sampling of Human Saliva for Non-Invasive and on-Site Monitoring. Anal. Chim. Acta 2015, 856, 35–45. [Google Scholar] [CrossRef]
- Choi, H.; Jedrychowski, W.; Spengler, J.; Camann, D.E.; Whyatt, R.M.; Rauh, V.; Tsai, W.Y.; Perera, F.P. International Studies of Prenatal Exposure to Polycyclic Aromatic Hydrocarbons and Fetal Growth. Environ. Health Perspect. 2006, 114, 1744–1750. [Google Scholar] [CrossRef]
- Quintanilla, I.; Fontàs, C.; Anticó, E. Deep Eutectic Solvents Incorporated in a Polymeric Film for Organophosphorus Pesticide Microextraction from Water Samples. Anal. Chim. Acta 2024, 1318, 342940. [Google Scholar] [CrossRef] [PubMed]
- Afsordeh, A.; Arbabsadeghi, A.; Javanmardi, H.; Bagheri, H. Incorporation of Cu-TATAB Metal-Organic Framework within Polyurethane Nanocomposite for Enhanced Thin Film Microextraction of Some Chlorinated Pesticides. J. Chromatogr. A 2024, 1730, 465061. [Google Scholar] [CrossRef] [PubMed]
- Kahremanoğlu, K.; Akpınar, Y.; Boyaci, E. Development of Thin Film Microextraction Method for the Multi-Residue Analysis of Selected Pesticides. Adv. Sample Prep. 2023, 6, 100061. [Google Scholar] [CrossRef]
- Majd, M.; Gholami, M.; Fathi, A.; Sedghi, R.; Nojavan, S. Thin-Film Solid-Phase Microextraction of Pesticides from Cereal Samples Using Electrospun Polyvinyl Alcohol/Modified Chitosan/Porous Organic Framework Nanofibers. Food Chem. 2024, 444, 138647. [Google Scholar] [CrossRef] [PubMed]
- Hosseinian, S.B.; Ghani, M.; Raoof, J.B. UIO-66-NH2 Encapsulated Polyoxometalate Loaded on Graphene Oxide Accommodated on Mixed Cellulose Esters (MCE) Paper for Thin Film Microextraction of Pesticides besides Their Quantification via High-Performance Liquid Chromatography-Ultraviolet Detection. Microchem. J. 2024, 199, 110031. [Google Scholar] [CrossRef]
- Ayazi, Z.; Ekhteraei, M.; Pashayi, S.; Seyed Ahmadian, S.M. Zr-Based Metal–Organic Framework Incorporated Polystyrene Nanocomposite as a Novel Sorbent for Ultrasound Assisted-Thin Film Microextraction of Organophosphorus Pesticides from Complex Samples. Food Chem. 2022, 393, 133343. [Google Scholar] [CrossRef]
- Liu, Z.; Li, W.; Zhu, X.; Hua, R.; Wu, X.; Xue, J. Combination of Polyurethane and Polymethyl Methacrylate Thin Films as a Microextraction Sorbent for Rapid Adsorption and Sensitive Determination of Neonicotinoid Insecticides in Fruit Juice and Tea by Ultra High Performance Liquid Chromatography with Tand. J. Chromatogr. A 2021, 1659, 462646. [Google Scholar] [CrossRef]
- Michałowicz, J.; Duda, W. Phenols-Sources and Toxicity. Polish J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Thomas, K.V.; Balaam, J.; Hurst, M.R.; Thain, J.E. Identification of in Vitro Estrogen and Androgen Receptor Agonists in North Sea Offshore Produced Water Discharges. Environ. Toxicol. Chem. 2004, 23, 1156–1163. [Google Scholar] [CrossRef]
- Ayazi, Z.; Safarpour, M.; Ahmadi, F. Monolithic Polyethersulfone Membrane Modified with PVA and PVP as a Novel Extracting Media for Thin Film Microextraction of Bisphenol A from Aquatic Samples. Microchem. J. 2022, 175, 107143. [Google Scholar] [CrossRef]
- Scur, R.; Dagnoni Huelsmann, R.; Carasek, E. Polyamide-Coated Paper-Based Sorptive Phase Applied in High-Throughput Thin Film Microextraction Designed by 3D Printing. Microchem. J. 2023, 189, 108515. [Google Scholar] [CrossRef]
- Ghani, M.; Jafari, Z.; Raoof, J.B. Porous Agarose/Chitosan/Graphene Oxide Composite Coupled with Deep Eutectic Solvent for Thin Film Microextraction of Chlorophenols. J. Chromatogr. A 2023, 1694, 463899. [Google Scholar] [CrossRef] [PubMed]
- Ocaña-González, J.A.; Villar-Navarro, M.; Ramos-Payán, M.; Fernández-Torres, R.; Bello-López, M.A. New Developments in the Extraction and Determination of Parabens in Cosmetics and Environmental Samples. A Review. Anal. Chim. Acta 2015, 858, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Błedzka, D.; Gromadzińska, J.; Wasowicz, W. Parabens. From Environmental Studies to Human Health. Environ. Int. 2014, 67, 27–42. [Google Scholar] [CrossRef]
- Jakavula, S.; Nqombolo, A.; Mpupa, A.; Ren, J.; Nomngongo, P.N. Hybrid Porous Material Supported in a Cellulose Acetate Polymeric Membrane for the Direct Immersion Thin-Film Microextraction of Parabens in Water. J. Chromatogr. A 2023, 1705, 464187. [Google Scholar] [CrossRef]
- Lin, A.Y.C.; Yu, T.H.; Lateef, S.K. Removal of Pharmaceuticals in Secondary Wastewater Treatment Processes in Taiwan. J. Hazard. Mater. 2009, 167, 1163–1169. [Google Scholar] [CrossRef]
- Nahandast, M.; Darvishnejad, F.; Raoof, J.B.; Ghani, M. Modification of Cellulose Substrate by in Situ Synthesis of Metal-Organic Framework-5 for Thin Film Microextraction of Some Non-Steroidal Anti-Inflammatory Drugs and Their Measurement by High-Performance Liquid Chromatography-Ultraviolet Detector. J. Chromatogr. A 2024, 1724, 464924. [Google Scholar] [CrossRef]
- Isobe, T.; Sato, K.; Joon-Woo, K.; Tanabe, S.; Suzuki, G.; Nakayama, K. Determination of Natural and Synthetic Glucocorticoids in Effluent of Sewage Treatment Plants Using Ultrahigh Performance Liquid Chromatography-Tandem Mass Spectrometry. Environ. Sci. Pollut. Res. 2015, 22, 14127–14135. [Google Scholar] [CrossRef]
- Chen, Q.; Jia, A.; Snyder, S.A.; Gong, Z.; Lam, S.H. Glucocorticoid Activity Detected by in Vivo Zebrafish Assay and in Vitro Glucocorticoid Receptor Bioassay at Environmental Relevant Concentrations. Chemosphere 2016, 144, 1162–1169. [Google Scholar] [CrossRef]
- Lalone, C.A.; Villeneuve, D.L.; Olmstead, A.W.; Medlock, E.K.; Kahl, M.D.; Jensen, K.M.; Durhan, E.J.; Makynen, E.A.; Blanksma, C.A.; Cavallin, J.E.; et al. Effects of a Glucocorticoid Receptor Agonist, Dexamethasone, on Fathead Minnow Reproduction, Growth, and Development. Environ. Toxicol. Chem. 2012, 31, 611–622. [Google Scholar] [CrossRef]
- Shahriman, M.S.; Mohamad, S.; Mohamad Zain, N.N.; Raoov, M. Poly-(MMA-IL) Filter Paper: A New Class of Paper-Based Analytical Device for Thin-Film Microextraction of Multi-Class Antibiotics in Environmental Water Samples Using LC-MS/MS Analysis. Talanta 2023, 254, 124188. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Mousavi Rad, N. Bacterial Cellulose-Supported Dual-Layered Nanofibrous Adsorbent for Thin-Film Micro-Solid-Phase Extraction of Antibiotics in Municipal Wastewaters. Talanta 2024, 276, 126198. [Google Scholar] [CrossRef] [PubMed]
- Struck-Lewicka, W.; Karpińska, B.; Rodzaj, W.; Nasal, A.; Wielgomas, B.; Markuszewski, M.J.; Siluk, D. Development of the Thin Film Solid Phase Microextraction (TF-SPME) Method for Metabolomics Profiling of Steroidal Hormones from Urine Samples Using LC-QTOF/MS. Front. Mol. Biosci. 2023, 10, 1074263. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.Y.; McCalley, D. Quantitative Analysis of Estrogens in Human Urine Using Gas Chromatography/Nagative Chemical Ionisation Mass Spectrometry. Rapid Commun. Mass Spectrom. 2000, 14, 1991–2001. [Google Scholar] [CrossRef]
- Morés, L.; Dias, A.N.; Carasek, E. Development of a High-Throughput Method Based on Thin-Film Microextraction using a 96-Well Plate System with a Cork Coating for the Extraction of Emerging Contaminants in River Water Samples. J. Sep. Sci. 2018, 41, 697–703. [Google Scholar] [CrossRef]
- Ayazi, Z.; Farshineh Saei, S.; Pashayi Sarnaghi, S. A Novel Self-Supportive Thin Film Based on Graphene Oxide Reinforced Chitosan Nano-Biocomposite for Thin Film Microextraction of Fluoxetine in Biological and Environmental Samples. J. Pharm. Biomed. Anal. 2023, 236, 115678. [Google Scholar] [CrossRef]
- Moosavi, N.S.; Yamini, Y. Growth of Bimetallic Ni-Co MOFs on a Skeleton of Electrospun PAN Nanofibers and Coating on a Thin Film for SPME of Amitriptyline and Nortriptyline in Urine and Plasma Samples. J. Pharm. Biomed. Anal. 2023, 236, 115755. [Google Scholar] [CrossRef]
- Hosseini, F.S.; Kharazmi, F.; Davarani, S.S.H.; Ebrahimzadeh, H. Development of Electrospun Nanofibers Based on Poly (Vinyl Alcohol) for Thin Film Solid-Phase Microextraction of Antidepressant Drugs in Biological Samples. J. Chromatogr. A 2023, 1697, 463984. [Google Scholar] [CrossRef]
- Silberstein, S.D.; Mccrory, D.C. Health Policy; USA Gov.: New York, NY, USA, 2000; pp. 854–864.
- Ayazi, Z.; Hobbivand, S.; Sarnaghi, S.P. Nickel Oxide Nanoparticles Modified with Dimethylglyoxime Grafted on Cellulose Surface as an Efficient Adsorbent for Thin Film Microextraction of Tramadol in Biological Fluids Followed by Its Determination Using HPLC. Anal. Methods 2024, 16, 5710–5722. [Google Scholar] [CrossRef]
- Goryński, K.; Sobczak, Ł.; Kołodziej, D. Developing and Evaluating the Greenness of a Reliable, All-in-One Thin-Film Microextraction Protocol for Determining Fentanyl, Methadone, and Zolpidem in Plasma, Urine, and Oral Fluid. Molecules 2024, 29, 335. [Google Scholar] [CrossRef]
- Ayazi, Z.; Pourtaghi, E.; Pashayi Sarnaghi, S. Graphitic Carbon Nitride Reinforced Nylon 6 Nanocomposite-Based Monolithic Thin Film for Microextraction of Carvedilol from Biological Samples. Chem. Pap. 2024, 78, 3747–3760. [Google Scholar] [CrossRef]
- Hejabri Kandeh, S.; Amini, S.; Ebrahimzadeh, H. Development of Poly(Vinyl Alcohol)/Chitosan/Aloe Vera Gel Electrospun Composite Nanofibers as a Novel Sorbent for Thin-Film Micro-Extraction of Pesticides in Water and Food Samples Followed by HPLC-UV Analysis. New J. Chem. 2022, 46, 2431–2440. [Google Scholar] [CrossRef]
- Werner, J.; Świtek, J.; Frankowski, R.; Zgoła-Grześkowiak, A. Development of a Green Deep Eutectic Solvent-Based Thin Film Solid Phase Microextraction Technique for the Preconcentration of Chlorophenoxy Acid Herbicides in Drainage Ditches and River Waters Using a Central Composite Design. Microchem. J. 2022, 183, 108101. [Google Scholar] [CrossRef]
- Zeng, L.; Cheng, D.; Mao, Z.; Zhou, Y.; Jing, T. ZIF-8/Nitrogen-Doped Reduced Graphene Oxide as Thin Film Microextraction Adsorbents for Simultaneous Determination of Novel Halogenated Flame Retardants in Crayfish-Aquaculture Water Systems. Chemosphere 2022, 287, 132408. [Google Scholar] [CrossRef]
- Chen, X.; Liu, S.; Jiang, R.; Luan, T.; Ouyang, G. Rapid Detection and Speciation of Illicit Drugs via a Thin-Film Microextraction Approach for Wastewater-Based Epidemiology Study. Sci. Total Environ. 2022, 842, 156888. [Google Scholar] [CrossRef]
- Merlo, F.; Profumo, A.; Fontàs, C.; Anticó, E. Preparation of New Polymeric Phases for Thin-Film Liquid Phase Microextraction (TF-LPME) of Selected Organic Pollutants. Microchem. J. 2022, 175, 107120. [Google Scholar] [CrossRef]
- van der Merwe, P.; Forbes, P. Comparison of Three Sorbents for Thin Film Solid Phase Microextraction of Haloacetic Acids from Water. Anal. Methods 2024, 16, 5154–5165. [Google Scholar] [CrossRef]
- Merlo, F.; Quarta, V.; Speltini, A.; Profumo, A.; Fontàs, C.; Anticó, E. Sexual Hormones Monitoring in Surface Waters and Wastewaters from Northern Italy by Thin Film Microextraction Coupled with HPLC–MS/MS. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef]
- Murtada, K.; Pawliszyn, J. Evaluation of Thin Film Microextraction Based on Graphene Oxide/ Polymer Composite: Experimental and Theoretical Insights. Talanta 2024, 274, 126032. [Google Scholar] [CrossRef]
- Sun, J.-C.; Pang, Y.-H.; Yang, C.; Shen, X.-F. Metal-Organic Framework Modified Carbon Cloth for Electric Field Enhanced Thin Film Microextraction of Sulfonamides in Animal-Derived Food. J. Chromatogr. A 2022, 1674, 463120. [Google Scholar] [CrossRef]
- Yan, K.; Liu, X.; Liu, J.; He, C.; Li, J.; Bai, Q. Octadecyl-Fibrous Mesoporous Silica Nanospheres Coated 96-Blade Thin-Film Microextraction for High-Throughput Analysis of Phthalic Acid Esters in Food and Migration from Food Packages. J. Chromatogr. A 2024, 1716, 464636. [Google Scholar] [CrossRef] [PubMed]
- Gruszecka, D.; Grandy, J.; Gionfriddo, E.; Singh, V.; Pawliszyn, J. Direct Immersion Thin Film Solid Phase Microextraction of Polychlorinated N-Alkanes in Cod Liver Oil. Food Chem. 2021, 353, 129244. [Google Scholar] [CrossRef]
- Ghasemi, S.; Raoof, J.B.; Ghani, M.; Ojani, R. Bacteria-Templated ZIF-8 Embedded in Polyacrylonitrile Nanofibers as a Novel Sorbent for Thin Film Microextraction of Benzoylurea Insecticides. Talanta 2024, 269, 125403. [Google Scholar] [CrossRef]
- Darvishnejad, F.; Bakhsh Raoof, J.; Ghani, M. Thin Film Microextraction Based on Co3O4@GO-Nylon-6 Polymeric Membrane to Extract Morin and Quercetin and Determining Them through High Performance Liquid Chromatography-Ultraviolet Detection. Microchem. J. 2021, 170, 106684. [Google Scholar] [CrossRef]
- Li, W.; Gu, Y.; Liu, Z.; Hua, R.; Wu, X.; Xue, J. Development of a Polyurethane-Coated Thin Film Solid Phase Microextraction Device for Multi-Residue Monitoring of Pesticides in Fruit and Tea Beverages. J. Sep. Sci. 2023, 46, 2200661. [Google Scholar] [CrossRef]
- Darvishnejad, F.; Raoof, J.B.; Ghani, M.; Ojani, R. Keggin-Type Polyoxometalate Embedded Polyvinylidene Fluoride for Thin Film Microextraction of Organophosphorus Pesticides. Food Chem. X 2023, 19, 100857. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, P.; Guan, Q.; Yan, X.; Yu, L.; Wu, G.; Hong, Y.; Wang, C. Combining Thin-Film Microextraction and Surface Enhanced Raman Spectroscopy to Sensitively Detect Thiram Based on 3D Silver Nanonetworks. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 287, 122073. [Google Scholar] [CrossRef]
- Giyahban, F.; Amini, S.; Kandeh, S.H.; Ebrahimzadeh, H. Development of Poly(Vinyl Alcohol)/Citric Acid/MIL-88A@CNTs Electrospun Nanofibers for Thin-Film Micro-Extraction of Conazole Fungicides Followed by CD-IMS Analysis. Chem. Pap. 2022, 76, 4705–4718. [Google Scholar] [CrossRef]
- Cao, H.-L.; Yang, C.; Qian, H.-L.; Yan, X.-P. Urea-Linked Covalent Organic Framework Functionalized Polytetrafluoroethylene Film for Selective and Rapid Thin Film Microextraction of Rhodamine B. J. Chromatogr. A 2022, 1673, 463133. [Google Scholar] [CrossRef]
- Rajska, A.; Raczak-Gutknecht, J.; Struck-Lewicka, W.; Buszewska-Forajta, M.; Wityk, P.; Verding, P.; Kowalewska, A.; Siluk, D.; Rachoń, D.; Jan Markuszewski, M. Determination of Urinary Androgens in Women with Polycystic Ovary Syndrome Using LC-QqQ/MS and the Application of Thin Film Solid-Phase Microextraction (TF-SPME). J. Chromatogr. A 2024, 1718, 464735. [Google Scholar] [CrossRef]
- Chabowska, A.; Werner, J.; Zgoła-Grześkowiak, A.; Płatkiewicz, J.; Frankowski, R.; Płotka-Wasylka, J. Development of Thin Film SPME Sorbents Based on Deep Eutectic Solvents and Their Application for Isolation and Preconcentration of Endocrine-Disrupting Compounds Leaching from Diapers to Urine. Microchem. J. 2024, 199, 110023. [Google Scholar] [CrossRef]
- Chen, L.; Singh, V.; Rickert, D.; Khaled, A.; Pawliszyn, J. High Throughput Determination of Free Biogenic Monoamines and Their Metabolites in Urine Using Thin-Film Solid Phase Microextraction. Talanta 2021, 232, 122438. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, H.; Matin, A.A.; Mohammadnejad, M. Cold Atmospheric Plasma Treated 3D Printed Polylactic Acid Film; Application in Thin Film Solid Phase Microextraction of Anticancer Drugs. Talanta 2024, 266, 125064. [Google Scholar] [CrossRef]





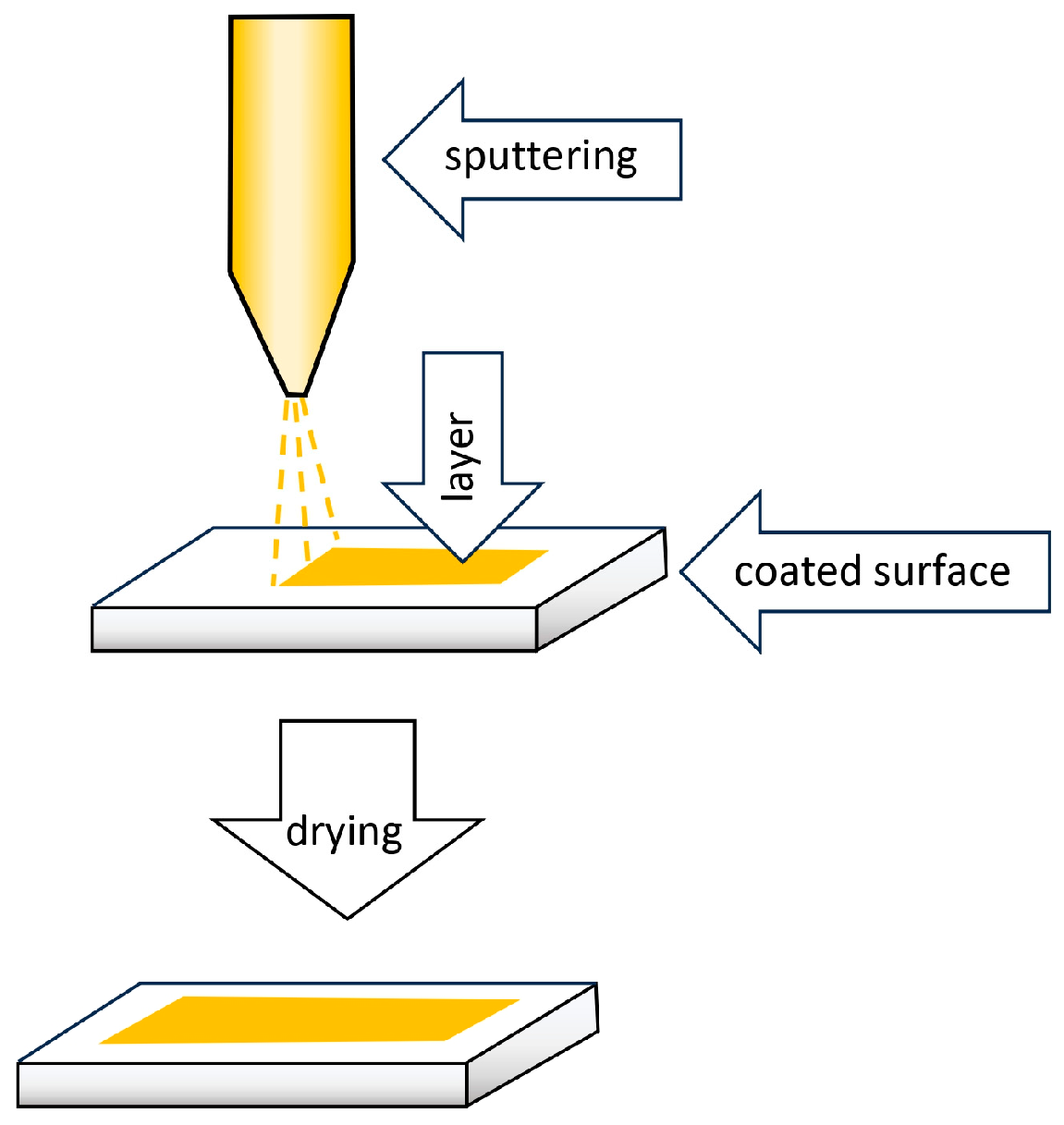



| Technique | Film Thickness Achieved | Advantages | Disadvantages |
|---|---|---|---|
| Dip coating | Low μm range |
|
|
| Spin coating | Hundreds of μm |
|
|
| Electrospinning coating | nm–μm range |
|
|
| Bar coating | Hundreds of μm |
|
|
| Spray coating | μm–mm range |
|
|
| Sorption Phase | Analyte | Matrix | Detection | Reference |
|---|---|---|---|---|
| PDMS | PAHs | water | GC-MS | [34] |
| polychlorinated biphenyls | fish tissue | LC-MS | [35] | |
| pesticides | water | DCBI-MS | [36] | |
| volatile organic compounds | skin | GC-MS | [37] | |
| insect pheromones | air | GC-MS | [38] | |
| drugs and explosive substances | standards in different solvents | IMS | [39] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Reference |
|---|---|---|---|---|---|
| DVB/PDMS | bar coating | VOCs/SVOCs | water | GC-TMS | [40] |
| DVB/PDMS | spin coating | N-nitrosamines | water | GC-MS | [22] |
| DVB/PDMS | bar coating | VOCs | water | GC-MS | [24] |
| DVB/PAN | ready for use | anti-inflammatory antibacterial drugs | drinking water | LC-ESI-MS/MS | [41] |
| DVB/PDMS | bar coating | pesticides | water | GC-MS | [42] |
| DVB/PDMS | bar coating | pesticides | water | GC-MS | [43] |
| C18/PAN | dip coating spray coating | benzodiazepines | blood plasma | LC-MS/MS | [44] |
| C18/PAN | spray coating | doping agents | urine, blood plasma | LC-MS | [45,46] |
| C18/PAN | spray coating | estrogens | water | LC-UV | [47] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Reference |
|---|---|---|---|---|---|
| PmPDA/CNT | electrospinning coating | copper | water, food | FAAS | [51] |
| PPy/PA | electrospinning coating | pesticides | water | GC-MS | [52] |
| PANI | gluing | hormones | urine | HPLC-FLD | [53] |
| PANI-N6 | electrospinning coating | chlorobenzenes | water | GC-MS | [54] |
| Template | Functional Monomer | Crosslinking Reagent | Analyte | Matrix | Detection | Ref. |
|---|---|---|---|---|---|---|
| 2-thiophenocarboxyaldehyde | 1-vinylimidazole | bisphenol dimethacrylate | PASHs | seawater | GC–MS | [59] |
| 2-{[diethoxy(sulfanylidene)-λ-phosphanyl]amino}acetic acid | methacrylic acid | ethylene glycol dimethacrylate | OPPs | water | LC-MS/MS | [60] |
| catechol | 4-vinyl benzoic acid | ethylene glycol dimethacrylate | phenols | water | UHPLC-PDA | [61] |
| (3-(10,11-dihydro-5H-dibenzo[b,f]azepin-5-yl)propyl)(methyl) carbamate | methacrylic acid | ethylene glycol dimethacrylate | TCAs | plasma | LC-MS/MS | [62] |
| phenol | itaconic acid, 4-vinylpyridine, styrene | ethylene glycol, dimethacrylate, triethylene glycol dimethacrylate, pentaerythritol triacrylate | phenols | water | LC-MS | [63] |
| mycophenolate mofetil | 4-vinylpyridine | ethylene glycol dimethacrylate | MPA | plasma | UPLC | [64] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Reference |
|---|---|---|---|---|---|
| DUT-52/PVDF | bar coating | PCPs | cosmetics | UHPLC-UV/VIS | [70] |
| Co-MOF-74/polyfam | electrospinning coating | anti-cancer drugs | water sewage, plasma | HPLC-UV | [71] |
| MIL-53(Al)/PVDF MIL-53(Fe)/PVDF MIL-100(Fe)/PVDF MIL-101(Cr)/PVDF UiO-66(Zr)/PVDF | bar coating | estrogens | urine | HPLC-FLD | [72] |
| ZIF-8/LDH/GO/PVDF | bar coating | caffeine | urine | HPLC-UV | [73] |
| amino-Zr-MOF/PAN | electrospinning coating | pesticides | water | CD-IMS | [74] |
| MIL-100(Fe)/PS/cellulose | dip coating | PCPs | pool water, cosmetics | HPLC-PDA | [75] |
| MOF-199/PS | electrospinning coating | aldehydes | urine | HPLC-VWD | [76] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Ref. |
|---|---|---|---|---|---|
| CY-GO-LDH | dip coating | non-steroidal anti-inflammatory drugs | plasma, urine | HPLC-UV | [82] |
| graphene membrane | drop casting | metal ions | water | TXRF | [83] |
| LDH/GO/PVDF | application to a Petri dish | diclofenac | urine | HPLC-UV | [84] |
| GO | ESD | metal ions | water | LIBS | [85] |
| PS/G | electrospinning coating | aldehydes | exhaled air condensate | HPLC | [86] |
| GO/PEG | dip coating | β-blockers | saliva | LC-MS/MS | [87] |
| ZIF-8@GO | dip coating | macrolides, lincosamides | honey | UPLC-MS/MS | [88] |
| PAMAM@GO-PVDF | application to a Petri dish | OPPs | water | HPLC-UV | [89] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Ref. |
|---|---|---|---|---|---|
| SDS-MWCNTs/PP | application on PP substrate | glucocorticoids | water | UHPLC-MS | [93] |
| MWCNT/Agr-Ch | application to a Petri dish | non-steroidal anti-inflammatory drugs | water | HPLC-UV | [94] |
| MWCNTs/Agr | application to a Petri dish | PAHs | green tea drink | HPLC-UV | [95] |
| MWCNTs/CTA | application to a Petri dish | fungicides personal care products PCPs | water | GC-MS | [96] |
| MWCNTs- COOH/PDMS | dip coating | phenolic compounds | water | HPLC-UV | [97] |
| Agr-Ch-MWCNTs | application to a Petri dish | TCAs | water | HPLC-UV-Vis | [98] |
| MWCNTs-COOH-Ch/PP | dip coating | PCBs | water | GC-MS | [99] |
| MWCNTs/CTA | application to a Petri dish | PAHs | water | HPLC-UV | [100] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Reference |
|---|---|---|---|---|---|
| C18-TEOS | dip coating | benzodiazepines | plasma | LC–MS/MS | [101] |
| C18/PAN | spray coating | pharmaceuticals | fish tissue | LC/MS–MS | [102] |
| C18/PAN | spray coating | steroid hormones | fish plasma | LC–MS/MS | [103] |
| C18/SCX | commercial strips | carbamazepine triclosan | water | DESI-MS | [104] |
| C18/glue | dip coating | benzodiazepines | urine | HPLC-MS | [25] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Reference |
|---|---|---|---|---|---|
| aptamer/cellulose | dip coating | codeine acetamiprid | urine, vegetable juice water | ESI-IMS | [106] |
| aptamer/CDs/cellulose | dip coating | methamphetamine | urine, plasma saliva | ESI-IMS | [107] |
| aptamer/cellulose | dip coating | codeine | urine | ESI-IMS | [108] |
| Sorption Phase | Preparation | Analyte | Matrix | Detection | Reference |
|---|---|---|---|---|---|
| HVImBr/MMA | application to the Petri dish | sulfathiazole | milk, honey | SF | [114] |
| p-PIL-AcO | dip coating | nonsteroidal anti-inflammatory drugs | urine | LC-MS/MS. | [115] |
| p-Poly-(MMA-IL) | dip coating | sulfonamides | water | HPLC-DAD | [116] |
| poly-(MMA-BVImBr) | application to the Petri dish | aniline | water | LC-MS/MS | [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krumplewski, W.; Rykowska, I. New Materials for Thin-Film Solid-Phase Microextraction (TF-SPME) and Their Use for Isolation and Preconcentration of Selected Compounds from Aqueous, Biological and Food Matrices. Molecules 2024, 29, 5025. https://doi.org/10.3390/molecules29215025
Krumplewski W, Rykowska I. New Materials for Thin-Film Solid-Phase Microextraction (TF-SPME) and Their Use for Isolation and Preconcentration of Selected Compounds from Aqueous, Biological and Food Matrices. Molecules. 2024; 29(21):5025. https://doi.org/10.3390/molecules29215025
Chicago/Turabian StyleKrumplewski, Witold, and Iwona Rykowska. 2024. "New Materials for Thin-Film Solid-Phase Microextraction (TF-SPME) and Their Use for Isolation and Preconcentration of Selected Compounds from Aqueous, Biological and Food Matrices" Molecules 29, no. 21: 5025. https://doi.org/10.3390/molecules29215025
APA StyleKrumplewski, W., & Rykowska, I. (2024). New Materials for Thin-Film Solid-Phase Microextraction (TF-SPME) and Their Use for Isolation and Preconcentration of Selected Compounds from Aqueous, Biological and Food Matrices. Molecules, 29(21), 5025. https://doi.org/10.3390/molecules29215025







