Encapsulation of ɣ-Aminobutyric Acid Compounds Extracted from Germinated Brown Rice by Freeze-Drying Technique
Abstract
:1. Introduction
2. Results and Discussion
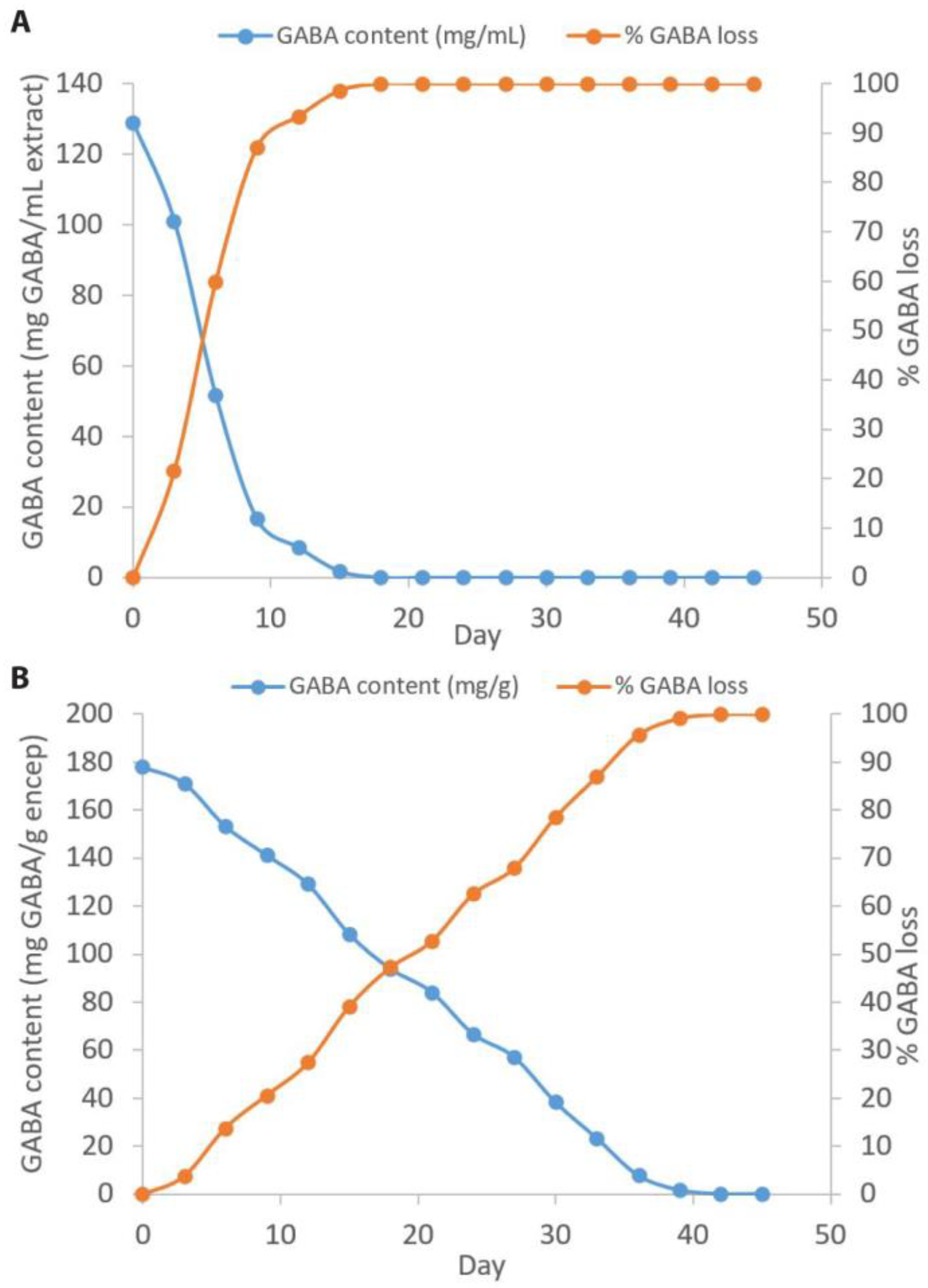
2.1. GABA Content
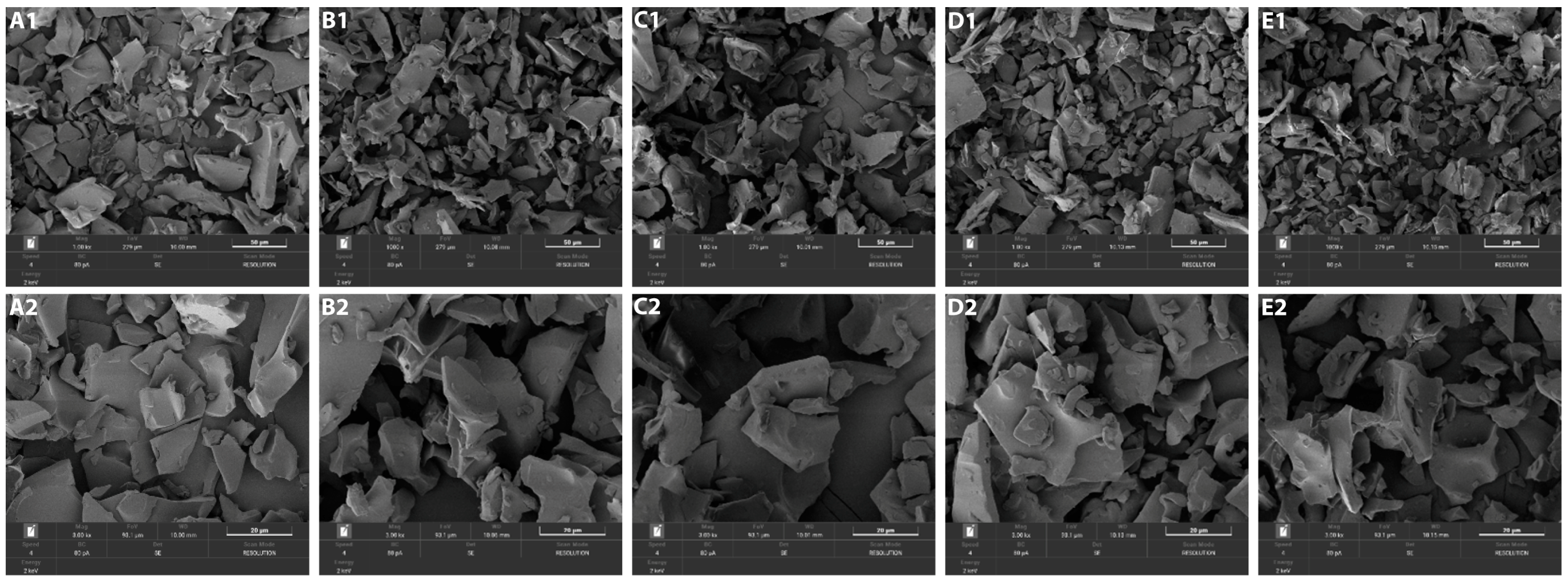
2.2. Morphology of Encapsulated GABA
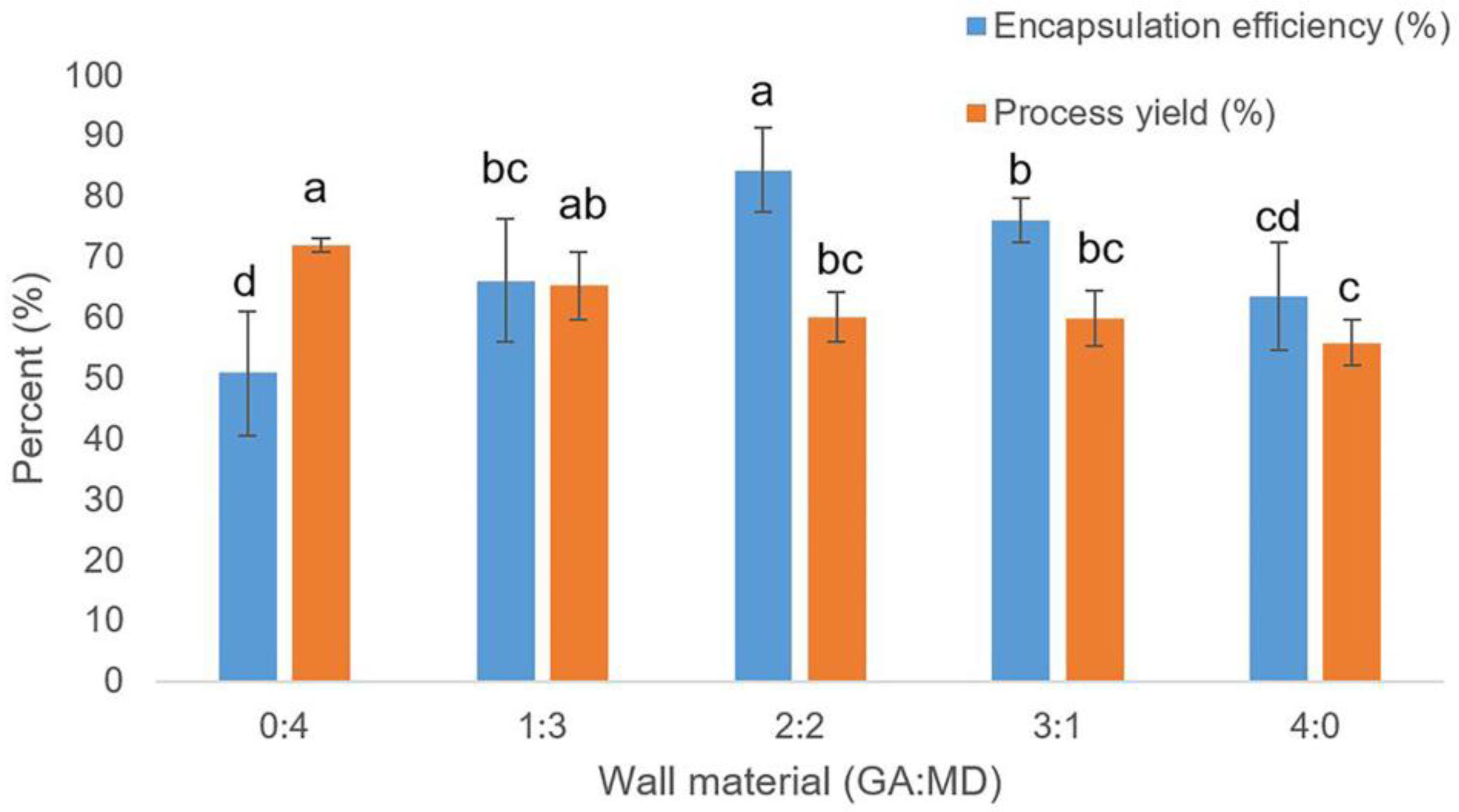
2.3. Encapsulation Efficiency and Process Yield
2.4. Physical Properties of Encapsulated GABA Powder
2.5. Stability of Encapsulated GABA Powder
3. Materials and Methods
3.1. Raw Material Preparation and Chemicals
3.2. GABA Extraction
3.3. Preparation of Encapsulated Powders
3.4. Determination of GABA Content
3.5. Determination of Antioxidant Capacity
3.6. Determination of Encapsulation Efficiency
3.7. Process Yield Determination
3.8. Moisture Content and Water Activity Measurements
3.9. Solubility Measurement
3.10. Bulk Density and Flowability Measurements
3.11. Morphology and Stability of the Encapsulated GABA Powder Analyses
3.12. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tanamool, V.; Hongsachart, P.; Soemphol, W. Screening and characterisation of gamma-aminobutyric acid (GABA) producing lactic acid bacteria isolated from Thai fermented fish (Plaa-som) in Nong Khai and its application in Thai fermented vegetables (Som-pak). Food Sci. Technol. 2019, 40, 483–490. [Google Scholar] [CrossRef]
- Li, L.; Dou, N.; Zhang, H.; Wu, C. The versatile GABA in plants. Plant Signal. Behav. 2021, 16, 1862565. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Mishra, H.N. Co-microencapsulation of γ-aminobutyric acid (GABA) and probiotic bacteria in thermostable and biocompatible exopolysaccharides matrix. LWT 2021, 136, 110293. [Google Scholar] [CrossRef]
- Khan, W.; Bhatt, P.C.; Panda, B.P. Degradation Kinetics of Gamma Amino Butyric Acid in M onascus-Fermented rice. J. Food Qual. 2015, 38, 123–129. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Haque, M.A.; Adhikari, B. Encapsulation in the food industry: A brief historical overview to recent developments. Food Nutr. Sci. 2020, 11, 481–508. [Google Scholar] [CrossRef]
- Boostani, S.; Jafari, S.M. A comprehensive review on the controlled release of encapsulated food ingredients; fundamental concepts to design and applications. Trends Food Sci. Technol. 2021, 109, 303–321. [Google Scholar] [CrossRef]
- Huang, Y.; Stonehouse, A.; Abeykoon, C. Encapsulation methods for phase change materials—A critical review. Int. J. Heat Mass Transf. 2023, 200, 123458. [Google Scholar] [CrossRef]
- Wilkowska, A.; Ambroziak, W.; Czyżowska, A.; Adamiec, J. Effect of microencapsulation by spray drying and freeze drying technique on the antioxidant properties of blueberry (Vaccinium myrtillus) juice polyphenolic compounds. Pol. J. Food Nutr. Sci. 2016, 66, 11–16. [Google Scholar] [CrossRef]
- Li, X.-Y.; Wu, M.-B.; Xiao, M.; Lu, S.-H.; Wang, Z.-M.; Yao, J.-M.; Yang, L.-R. Microencapsulated β-carotene preparation using different drying treatments. J. Zhejiang Univ. Sci. B 2019, 20, 901. [Google Scholar] [CrossRef]
- Pan-Utai, W.; Iamtham, S. Enhanced microencapsulation of C-phycocyanin from Arthrospira by freeze-drying with different wall materials. Food Technol. Biotechnol. 2020, 58, 423–432. [Google Scholar] [CrossRef]
- Xiao, Z.; Xia, J.; Zhao, Q.; Niu, Y.; Zhao, D. Maltodextrin as wall material for microcapsules: A review. Carbohydr. Polym. 2022, 298, 120113. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamayda, A.; Abu-Jdayil, B.; Ayyash, M.; Tannous, J. Advances in microencapsulation techniques using Arabic gum: A comprehensive review. Ind. Crops Prod. 2023, 205, 117556. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tamaddon, A.M.; Fazaeli, M. Double encapsulation of fucoxanthin using porous starch through sequential coating modification with maltodextrin and gum Arabic. Food Sci. Nutr. 2020, 8, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Lv, Y. Degradation kinetic of anthocyanins from rose (Rosa rugosa) as prepared by microencapsulation in freeze-drying and spray-drying. Int. J. Food Prop. 2019, 22, 2009–2021. [Google Scholar] [CrossRef]
- Vanavichit, A.; Kamolsukyeunyong, W.; Siangliw, M.; Siangliw, J.L.; Traprab, S.; Ruengphayak, S.; Chaichoompu, E.; Saensuk, C.; Phuvanartnarubal, E.; Toojinda, T. Thai Hom Mali Rice: Origin and breeding for subsistence rainfed lowland rice system. Rice 2018, 11, 20. [Google Scholar] [CrossRef]
- Hazra, A.; Das, S. The molecular and metabolic events behind different germination stages of rice seeds: A metabolomics perspective. JSFA Rep. 2024, 4, 118–134. [Google Scholar] [CrossRef]
- Khwanchai, P.; Chinprahast, N.; Pichyangkura, R.; Chaiwanichsiri, S. Gamma-aminobutyric acid and glutamic acid contents, and the GAD activity in germinated brown rice (Oryza sativa L.): Effect of rice cultivars. Food Sci. Biotechnol. 2014, 23, 373–379. [Google Scholar] [CrossRef]
- Rezende, Y.R.R.S.; Nogueira, J.P.; Narain, N. Microencapsulation of extracts of bioactive compounds obtained from acerola (Malpighia emarginata DC) pulp and residue by spray and freeze drying: Chemical, morphological and chemometric characterization. Food Chem. 2018, 254, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Marksa, M.; Sosnowska, K.; Winnicka, K.; Morkuniene, R.; Bernatoniene, J. Freeze-drying technique for microencapsulation of Elsholtzia ciliata ethanolic extract using different coating materials. Molecules 2020, 25, 2237. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of antioxidant phenolic compounds extracted from spent coffee grounds by freeze-drying and spray-drying using different coating materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Hussain, S.A.; Hameed, A.; Nazir, Y.; Naz, T.; Wu, Y.; Suleria, H.A.R.; Song, Y. Microencapsulation and the characterization of polyherbal formulation (PHF) rich in natural polyphenolic compounds. Nutrients 2018, 10, 843. [Google Scholar] [CrossRef] [PubMed]
- Stabrauskiene, J.; Pudziuvelyte, L.; Bernatoniene, J. Optimizing Encapsulation: Comparative Analysis of Spray-Drying and Freeze-Drying for Sustainable Recovery of Bioactive Compounds from Citrus x paradisi L. Peels. Pharmaceuticals 2024, 17, 596. [Google Scholar] [CrossRef]
- Lee, D.S.; Robertson, G.L. Interactive influence of decision criteria, packaging film, storage temperature and humidity on shelf life of packaged dried vegetables. Food Packag. Shelf Life 2021, 28, 100674. [Google Scholar] [CrossRef]
- Sidlagatta, V.; Chilukuri, S.V.V.; Devana, B.R.; Dasi, S.D.; Rangaswamy, L. Effect of maltodextrin concentration and inlet air temperature on properties of spray dried powder from reverse osmosis concentrated sweet orange juice. Braz. Arch. Biol. Technol. 2020, 63, e20190538. [Google Scholar] [CrossRef]
- Suhag, R.; Kellil, A.; Razem, M. Factors Influencing Food Powder Flowability. Powders 2024, 3, 65–76. [Google Scholar] [CrossRef]
- Zareifard, M.R.; Niakousari, M.; Shokrollahi, Z.; Javadian, S. A feasibility study on the drying of lime juice: The relationship between the key operating parameters of a small laboratory spray dryer and product quality. Food Bioprocess Technol. 2012, 5, 1896–1906. [Google Scholar] [CrossRef]
- Goula, A.M.; Adamopoulos, K.G. A new technique for spray drying orange juice concentrate. Innov. Food Sci. Emerg. Technol. 2010, 11, 342–351. [Google Scholar] [CrossRef]
- Selim, K.A.; Alharthi, S.S.; Abu El-Hassan, A.M.; Elneairy, N.A.; Rabee, L.A.; Abdel-Razek, A.G. The Effect of Wall Material Type on the Encapsulation Efficiency and Oxidative Stability of Fish Oils. Molecules 2021, 26, 6109. [Google Scholar] [CrossRef]
- Sripakdee, T.; Sriwicha, A.; Jansam, N.; Mahachai, R.; Chanthai, S. Determination of total phenolics and ascorbic acid related to an antioxidant activity and thermal stability of the Mao fruit juice. Int. Food Res. J. 2015, 22, 618. [Google Scholar]
- Borompichaichartkul, C.; Hamad, A.; Suriyarak, S. Encapsulation of curcumin by spray drying using the combination of tween 80 and chitosan. J. Food Technol. Siam Univ. 2020, 15, 96–109. [Google Scholar]
- AgbangbaI, C.; Aide, E.; Honfo, H.; Kakai, R. On the use of post-hoc tests in environmental and biological sciences: A critical review. Heliyon 2024, 10, e25131. [Google Scholar] [CrossRef]
| Sample | GABA Content |
|---|---|
| KDML 105 brown rice (mg/g DW) | 13.08 ± 0.05 a |
| KDML 105 germinated brown rice (mg/g DW) | 30.24 ± 0.10 b |
| Germinated brown rice extract (mg/mL) | 129.12 ± 1.83 c |
| Wall Material (GA:MD, w/w) | GABA Content of Encapsulated GABA (mg/g DW) | DPPH (IC50) |
|---|---|---|
| 0:4 | 54.75 ± 1.38 e | 1.09 ± 0.49 a |
| 1:3 | 71.15 ± 1.16 c | 1.41 ± 0.51 a |
| 2:2 | 90.77 ± 1.90 a | 1.80 ± 0.29 a |
| 3:1 | 81.82 ± 1.34 b | 1.63 ± 0.53 a |
| 4:0 | 68.34 ± 1.58 d | 1.36 ± 0.73 a |
| Wall Material (GA:MD, w/w) | Moisture Content (%) | Water Activity (aw) | Solubility (%) | Bulk Density (g/mL) |
|---|---|---|---|---|
| 0:4 | 6.40 ± 0.46 a | 0.58 ± 0.05 a | 84.11 ± 4.83 c | 0.48 ± 0.04 a |
| 1:3 | 5.48 ± 0.82 b | 0.44 ± 0.11 a | 84.56 ± 1.64 b | 0.49 ± 0.05 a |
| 2:2 | 5.06 ± 0.74 bc | 0.42 ± 0.13 b | 90.62 ± 6.84 a | 0.47 ± 0.05 a |
| 3:1 | 4.54 ± 0.85 c | 0.38 ± 0.04 a | 80.36 ± 4.28 c | 0.49 ± 0.03 a |
| 4:0 | 3.79 ± 0.59 d | 0.36 ± 0.11 a | 81.24 ± 4.22 b | 0.50 ± 0.03 a |
| Wall Material (GA:MD, w/w) | Carr’s Index (CI) | Hausner Ratio (HR) | Flowability |
|---|---|---|---|
| 0:4 | 44.56 ± 4.97 c | 1.82 ± 0.16 d | Very very poor |
| 1:3 | 41.02 ± 4.21 c | 1.70 ± 0.13 cd | Very very poor |
| 2:2 | 36.72 ± 4.85 b | 1.59 ± 0.12 bc | Very poor |
| 3:1 | 35.14 ± 3.96 b | 1.55 ± 0.96 b | Very poor |
| 4:0 | 29.72 ± 4.40 a | 1.42 ± 0.09 a | poor |
| Flowability | Carr’s Index | Hausner Ratio |
|---|---|---|
| Excellent | 0–10 | 1.00–1.11 |
| Good | 11–15 | 1.12–1.18 |
| Fair | 16–20 | 1.19–1.25 |
| Possible | 21–25 | 1.26–1.34 |
| Poor | 26–30 | 1.35–1.45 |
| Very poor | 32–37 | 1.46–1.59 |
| Very, very poor | >38 | >1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nilkamheang, T.; Thanaseelangkoon, C.; Sangsue, R.; Parisaka, S.; Nghiep, L.K.; Wanyo, P.; Toontom, N.; Tudpor, K. Encapsulation of ɣ-Aminobutyric Acid Compounds Extracted from Germinated Brown Rice by Freeze-Drying Technique. Molecules 2024, 29, 5119. https://doi.org/10.3390/molecules29215119
Nilkamheang T, Thanaseelangkoon C, Sangsue R, Parisaka S, Nghiep LK, Wanyo P, Toontom N, Tudpor K. Encapsulation of ɣ-Aminobutyric Acid Compounds Extracted from Germinated Brown Rice by Freeze-Drying Technique. Molecules. 2024; 29(21):5119. https://doi.org/10.3390/molecules29215119
Chicago/Turabian StyleNilkamheang, Tarinee, Chanikarn Thanaseelangkoon, Rawinan Sangsue, Sarunya Parisaka, Le Ke Nghiep, Pitchaporn Wanyo, Nitchara Toontom, and Kukiat Tudpor. 2024. "Encapsulation of ɣ-Aminobutyric Acid Compounds Extracted from Germinated Brown Rice by Freeze-Drying Technique" Molecules 29, no. 21: 5119. https://doi.org/10.3390/molecules29215119
APA StyleNilkamheang, T., Thanaseelangkoon, C., Sangsue, R., Parisaka, S., Nghiep, L. K., Wanyo, P., Toontom, N., & Tudpor, K. (2024). Encapsulation of ɣ-Aminobutyric Acid Compounds Extracted from Germinated Brown Rice by Freeze-Drying Technique. Molecules, 29(21), 5119. https://doi.org/10.3390/molecules29215119








