Application of Fenton’s Reaction for Removal of Organic Matter from Groundwater
Abstract
1. Introduction
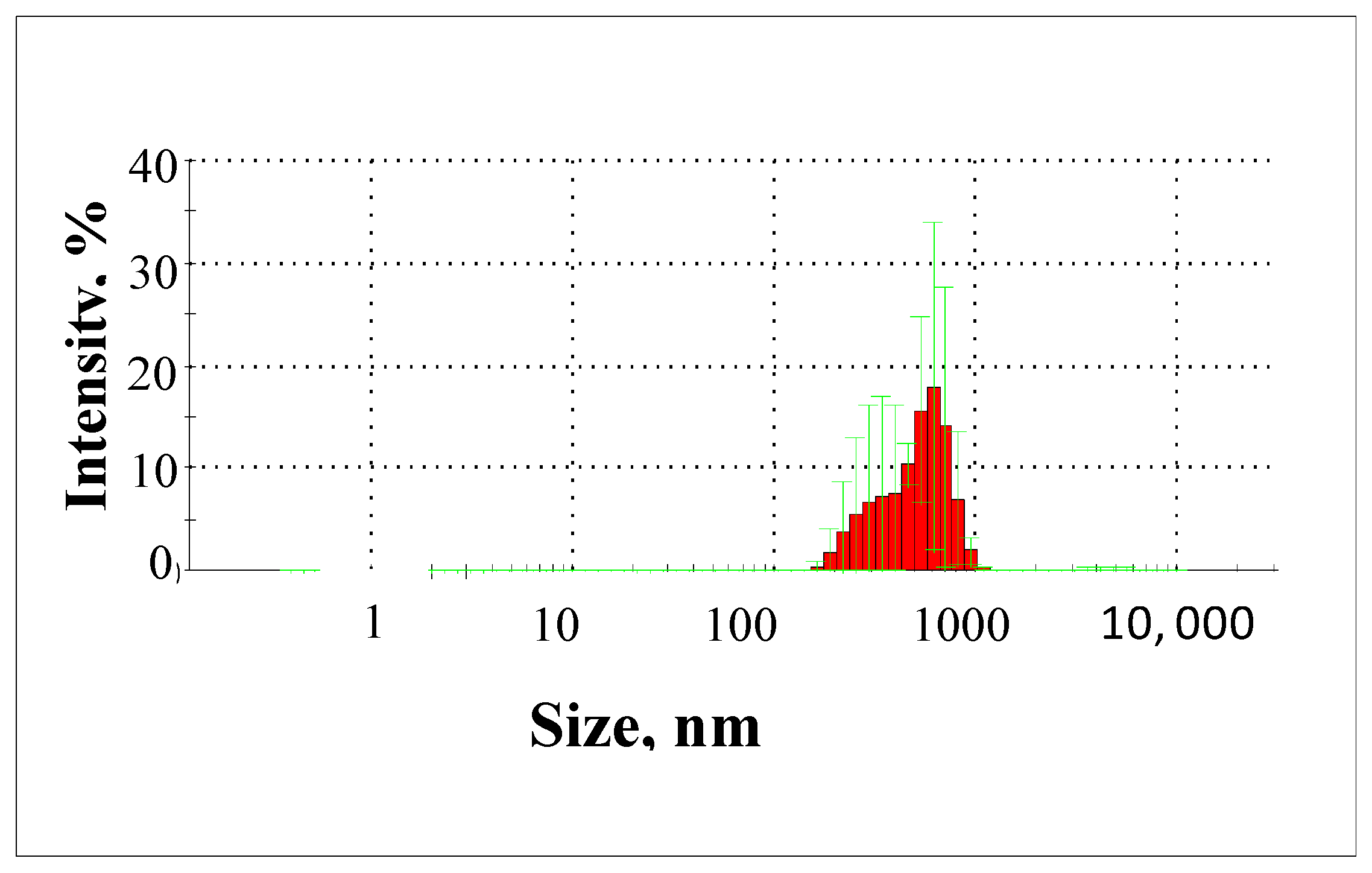
2. Results and Discussion
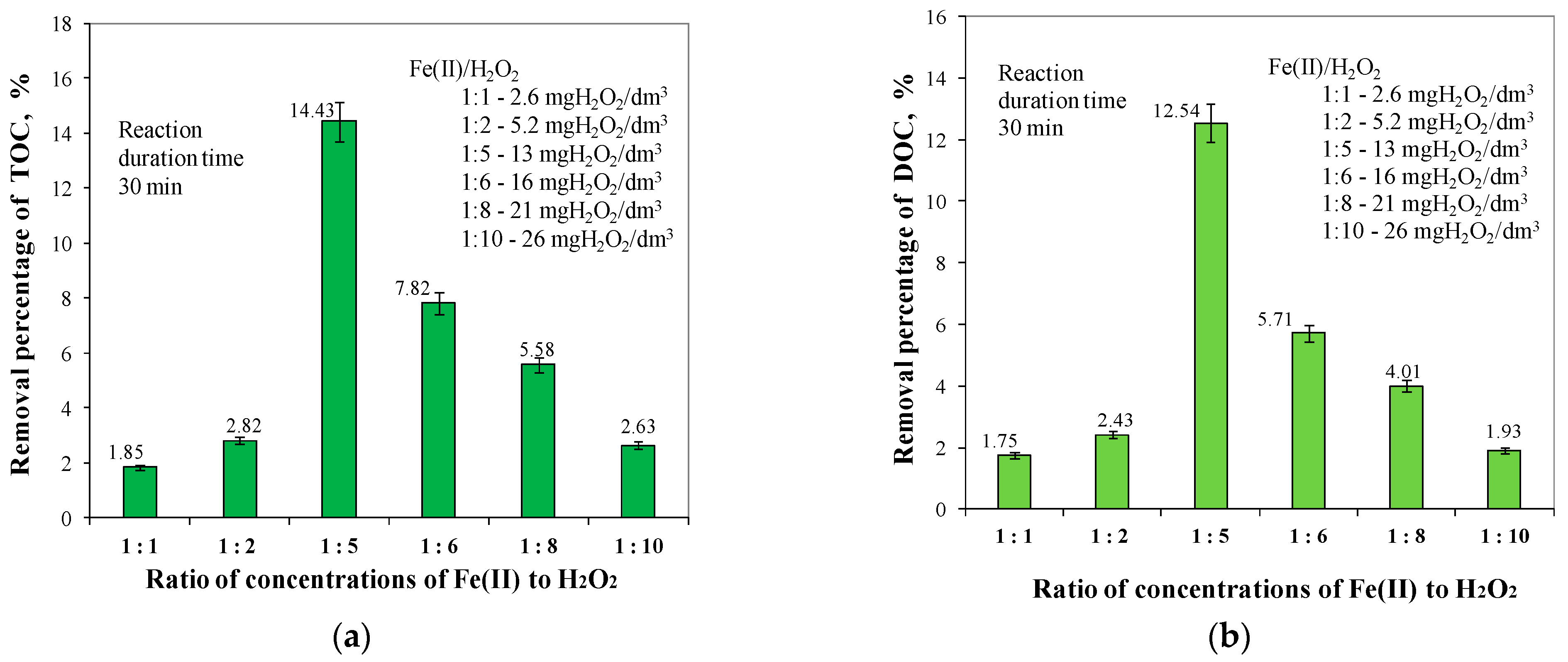
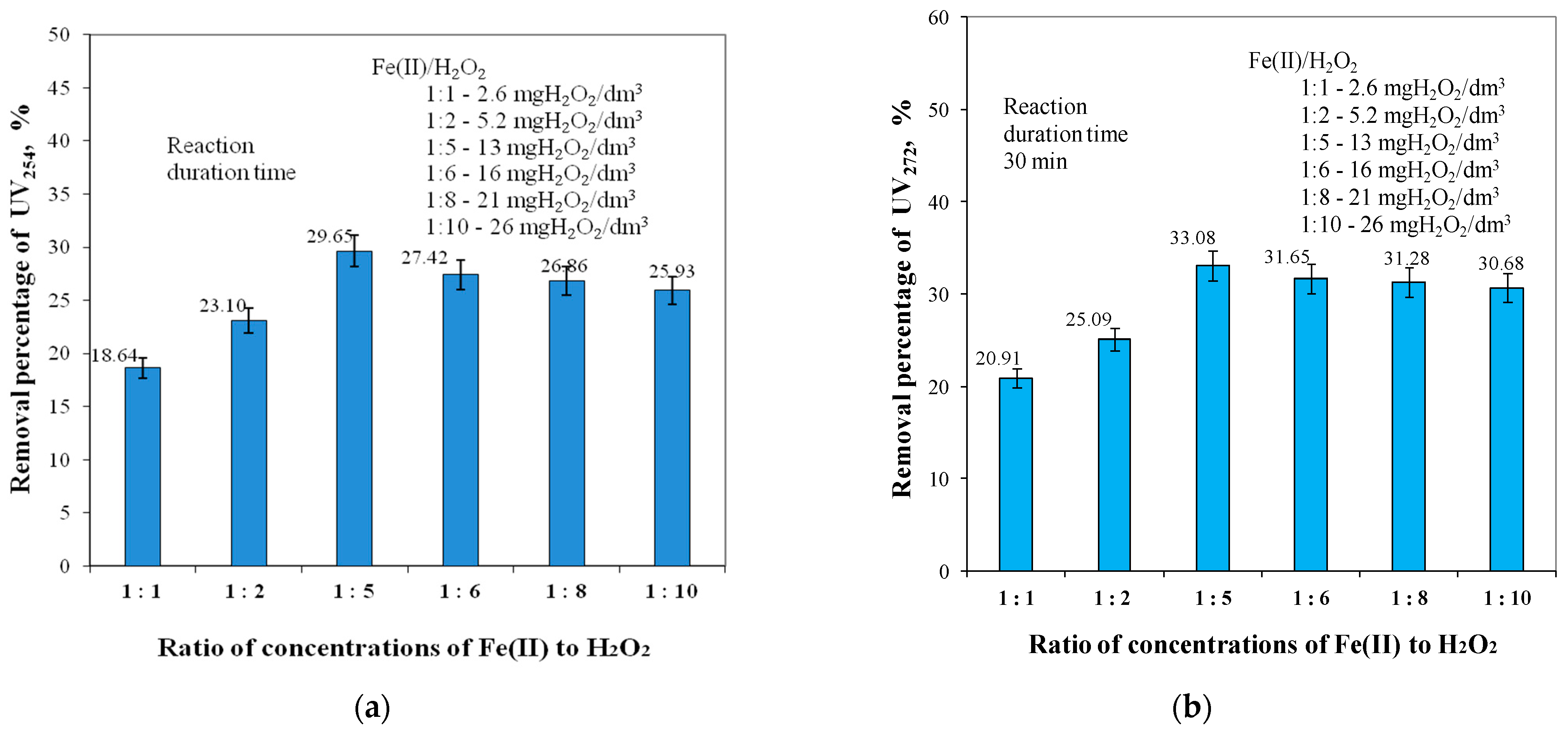
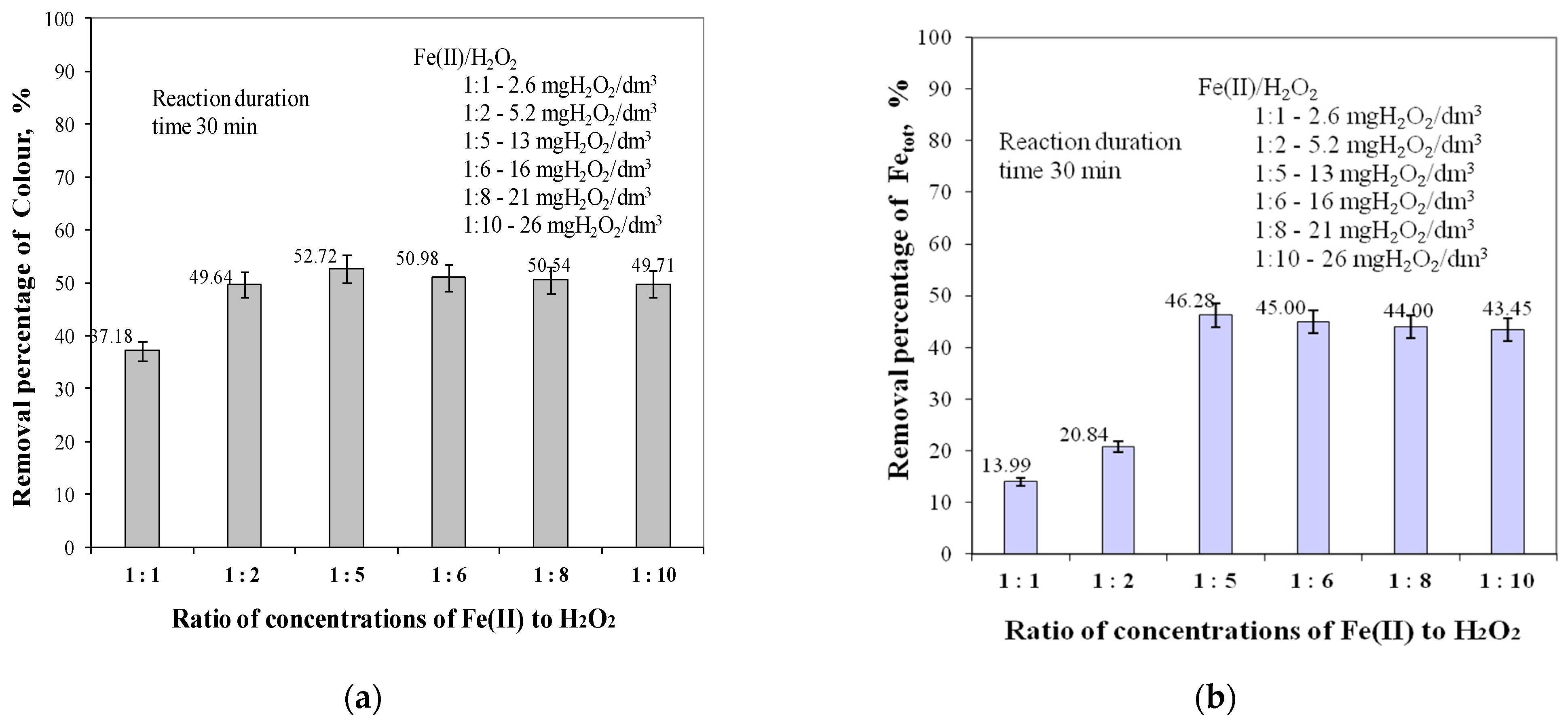
2.1. Ratios of Fe(II) to H2O2 Concentrations
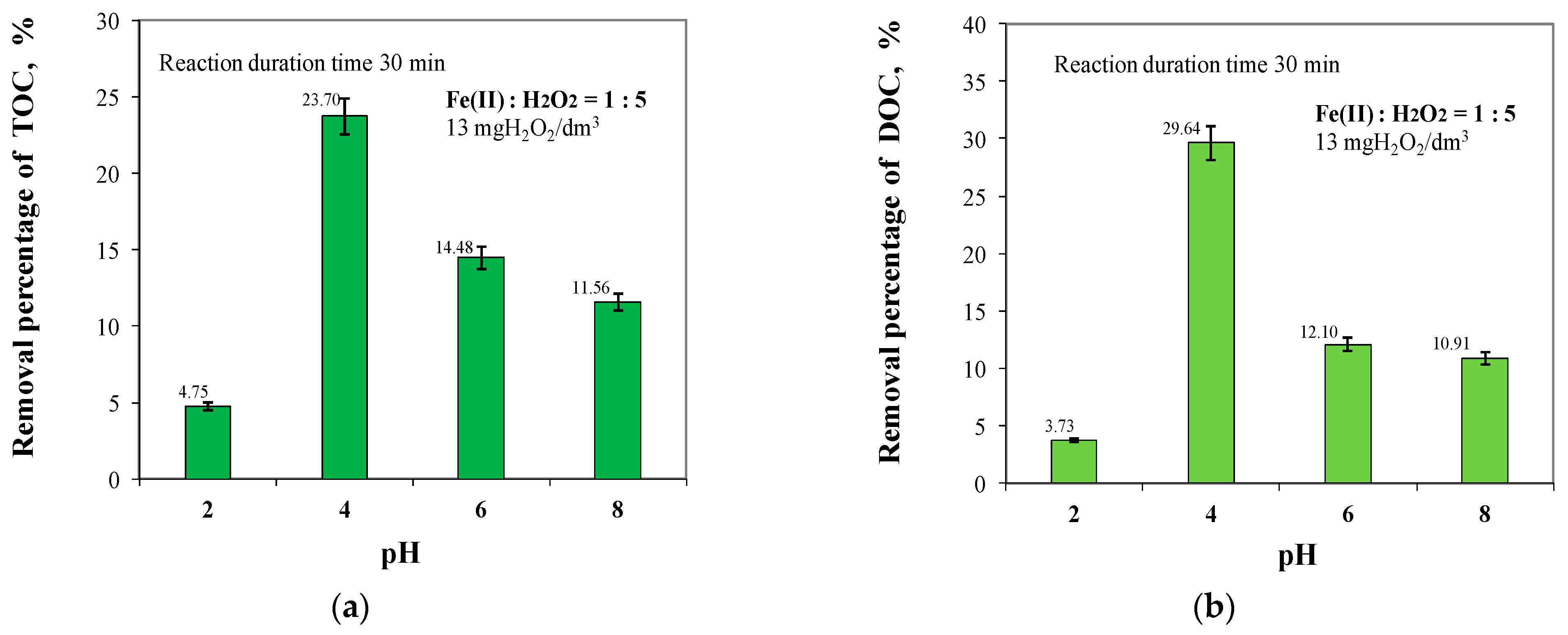
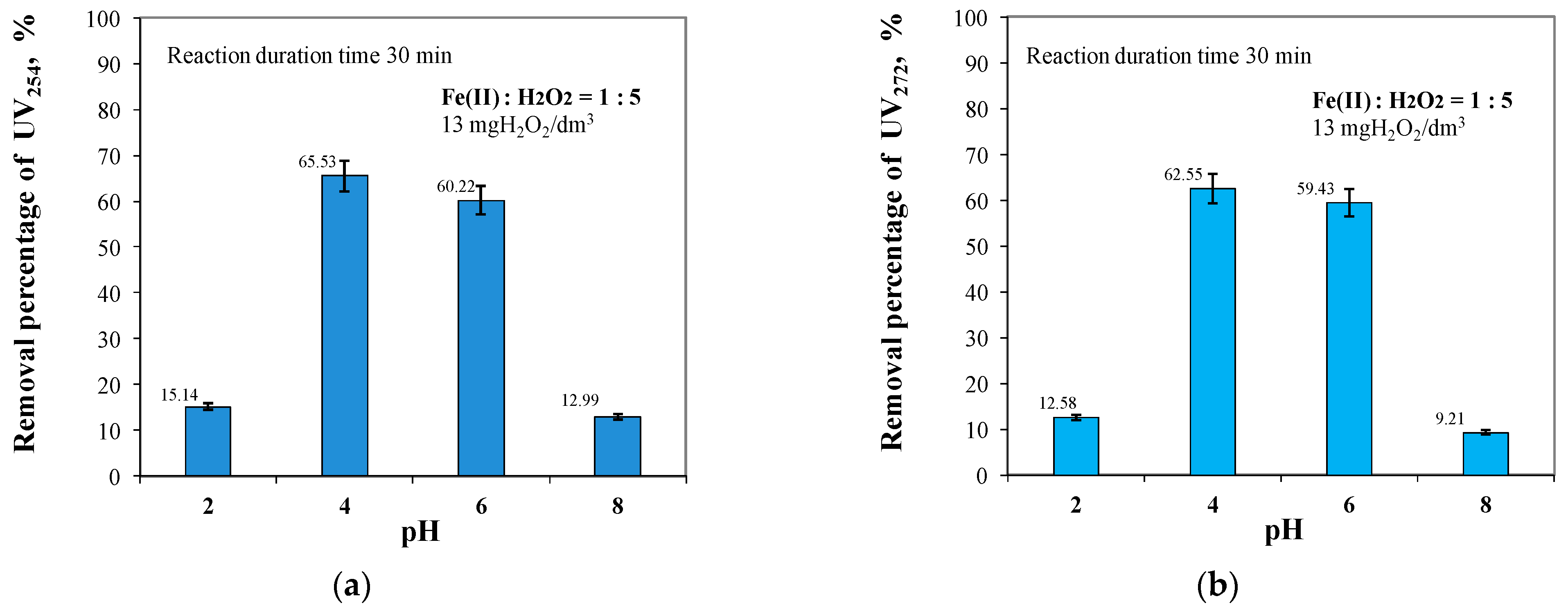
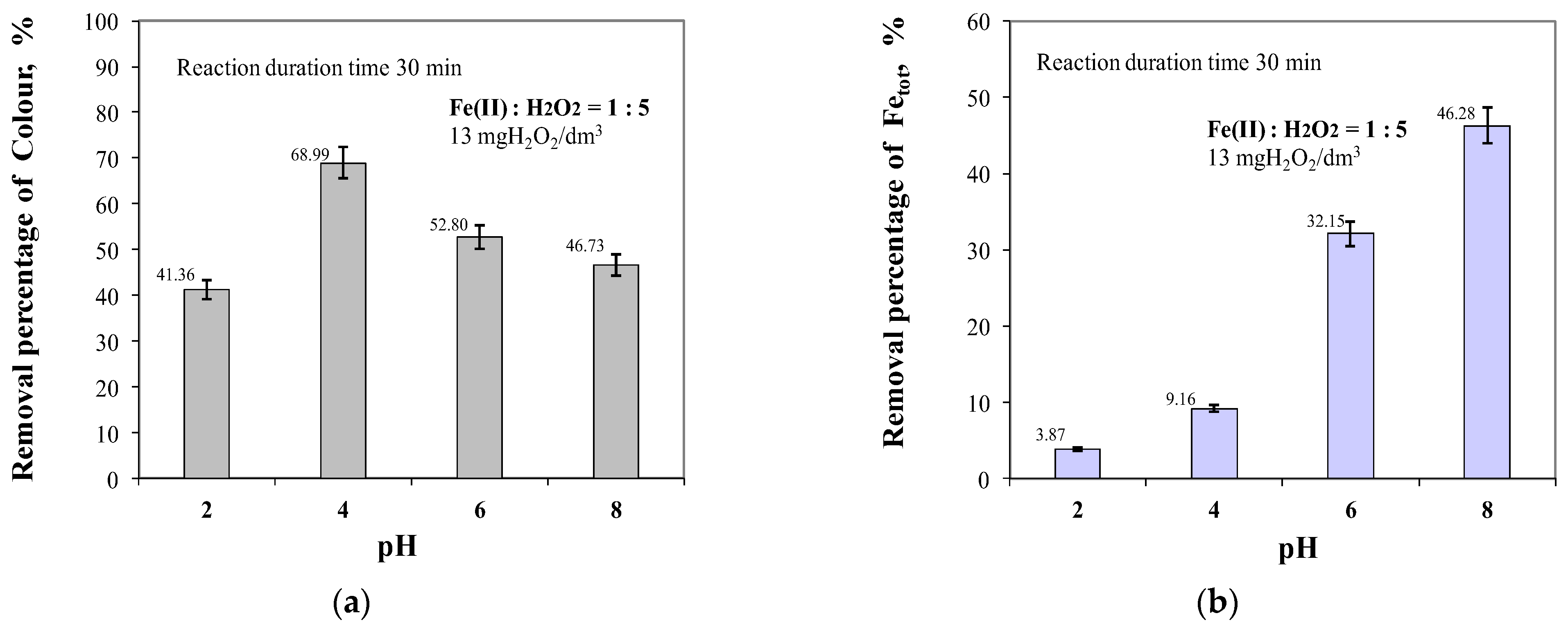
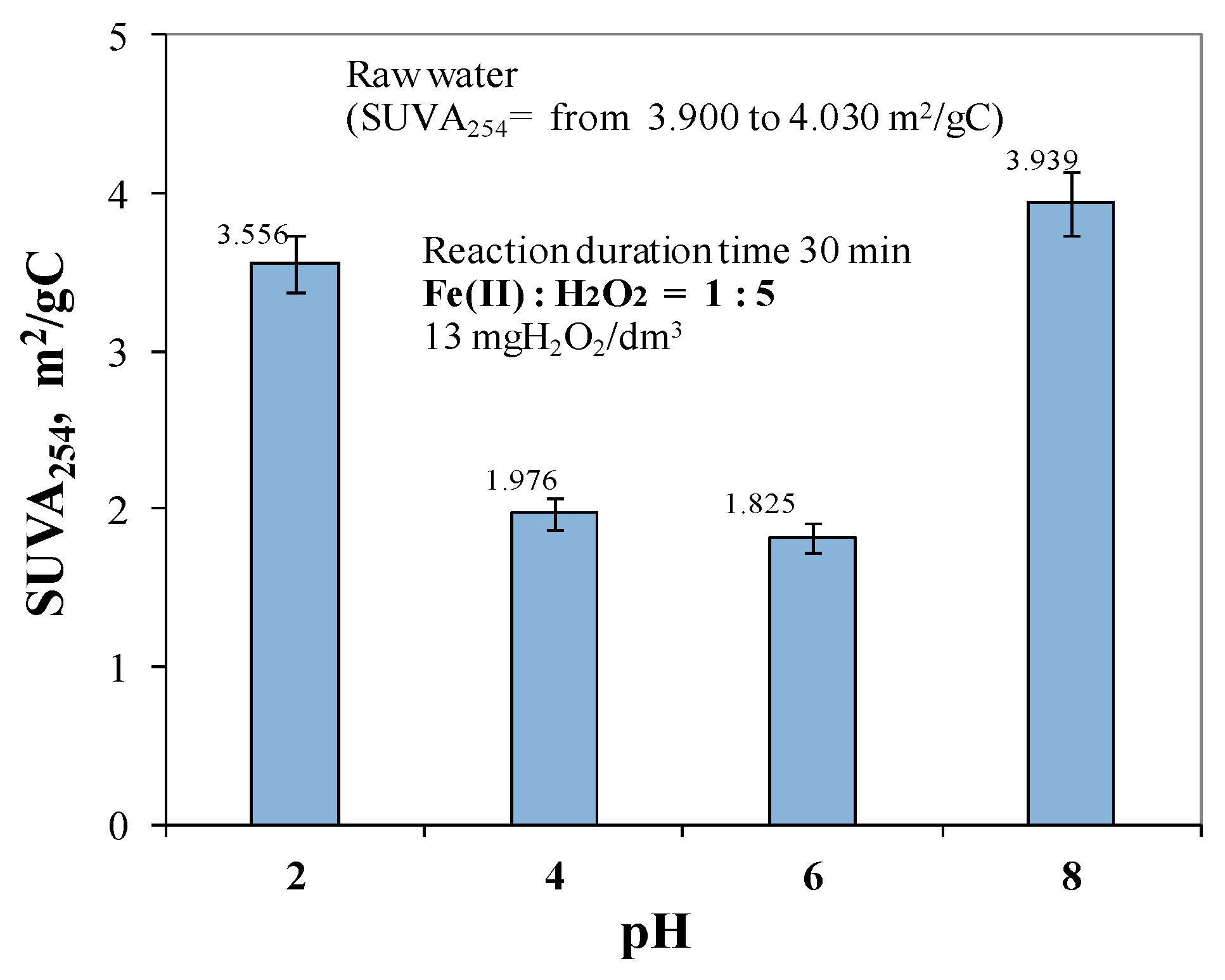
2.2. Effect of pH in the Range of 2 to 8
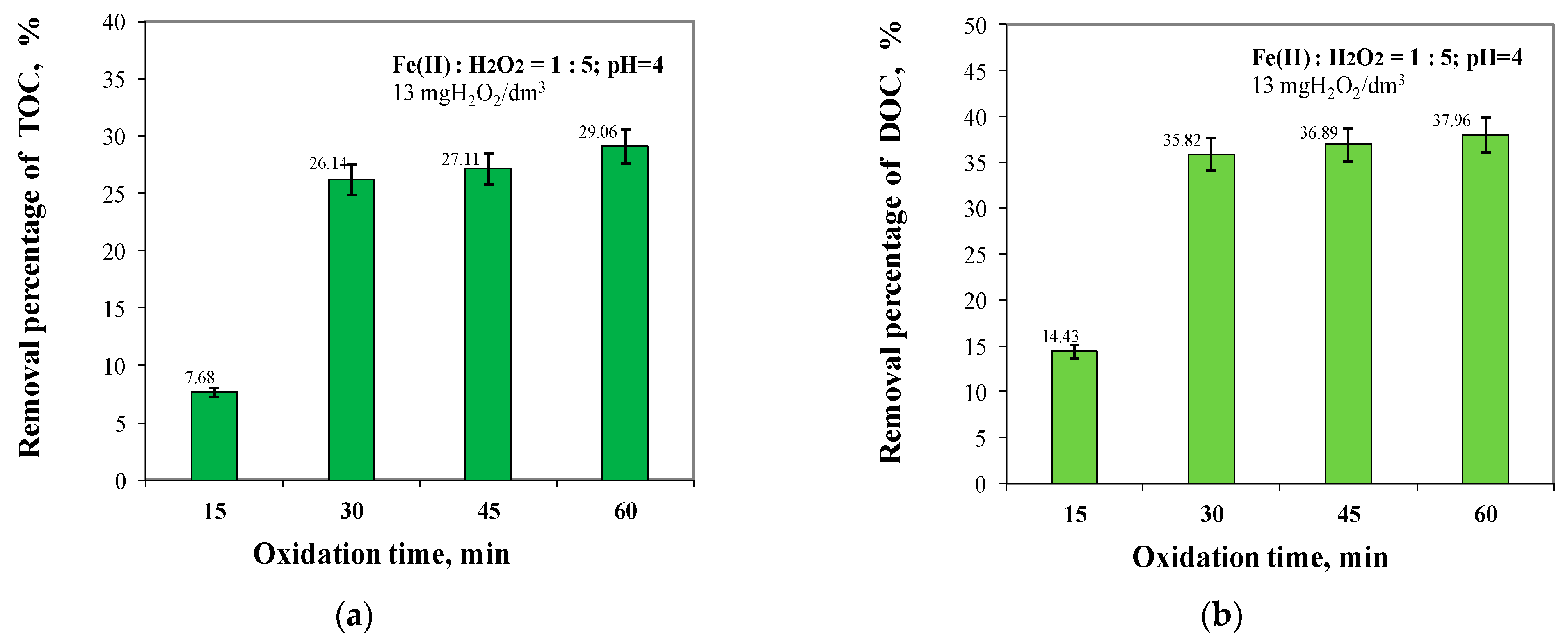
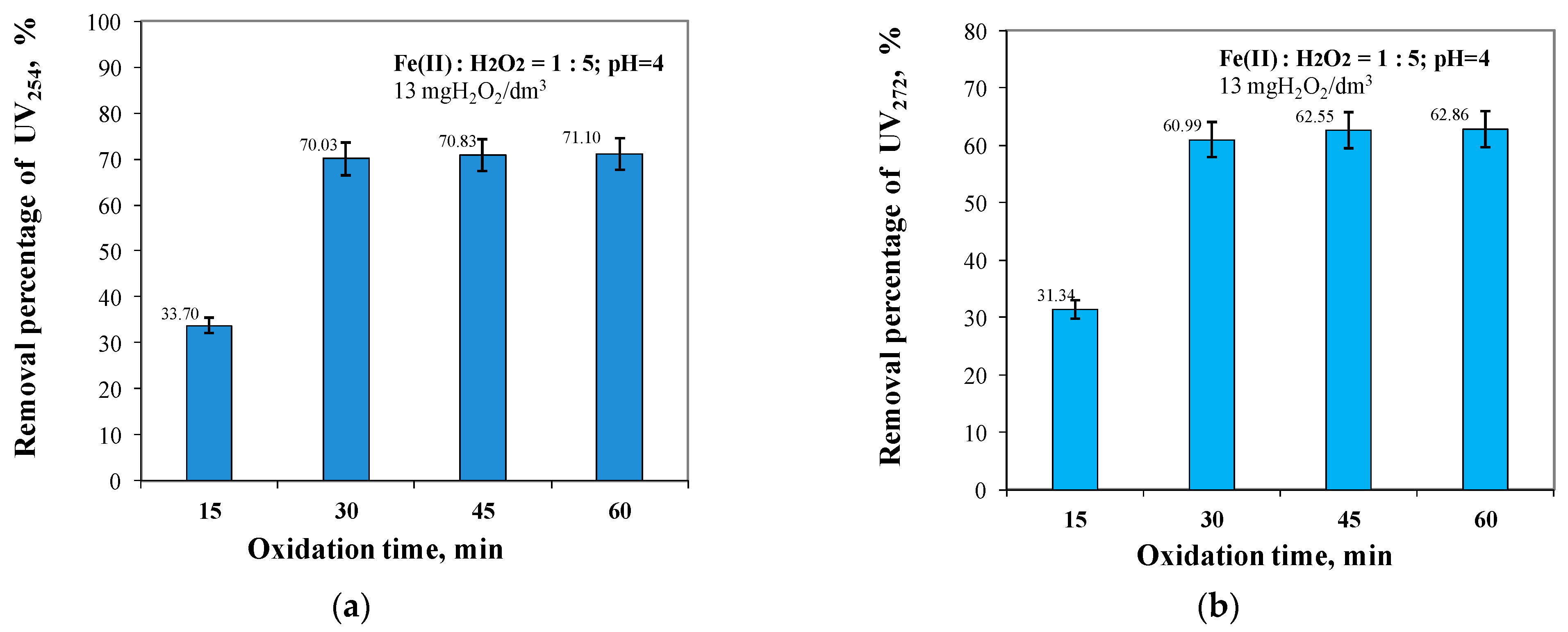
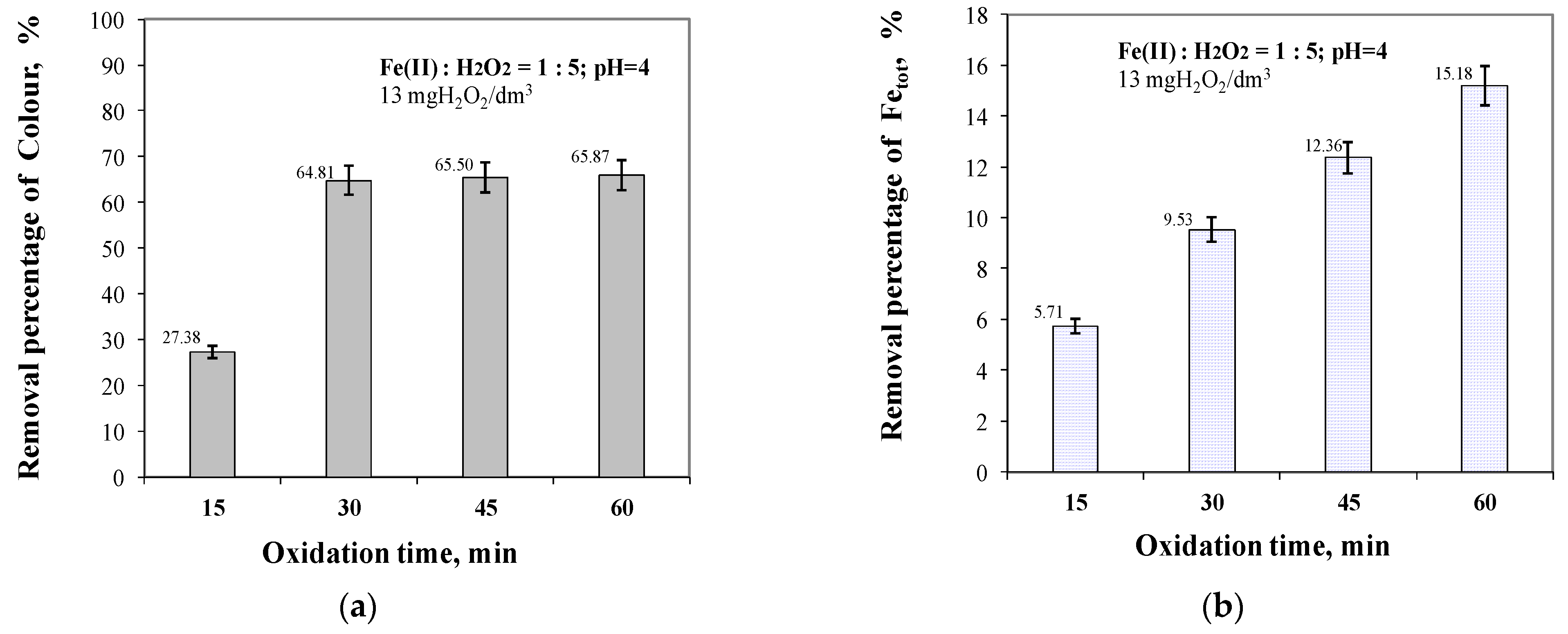
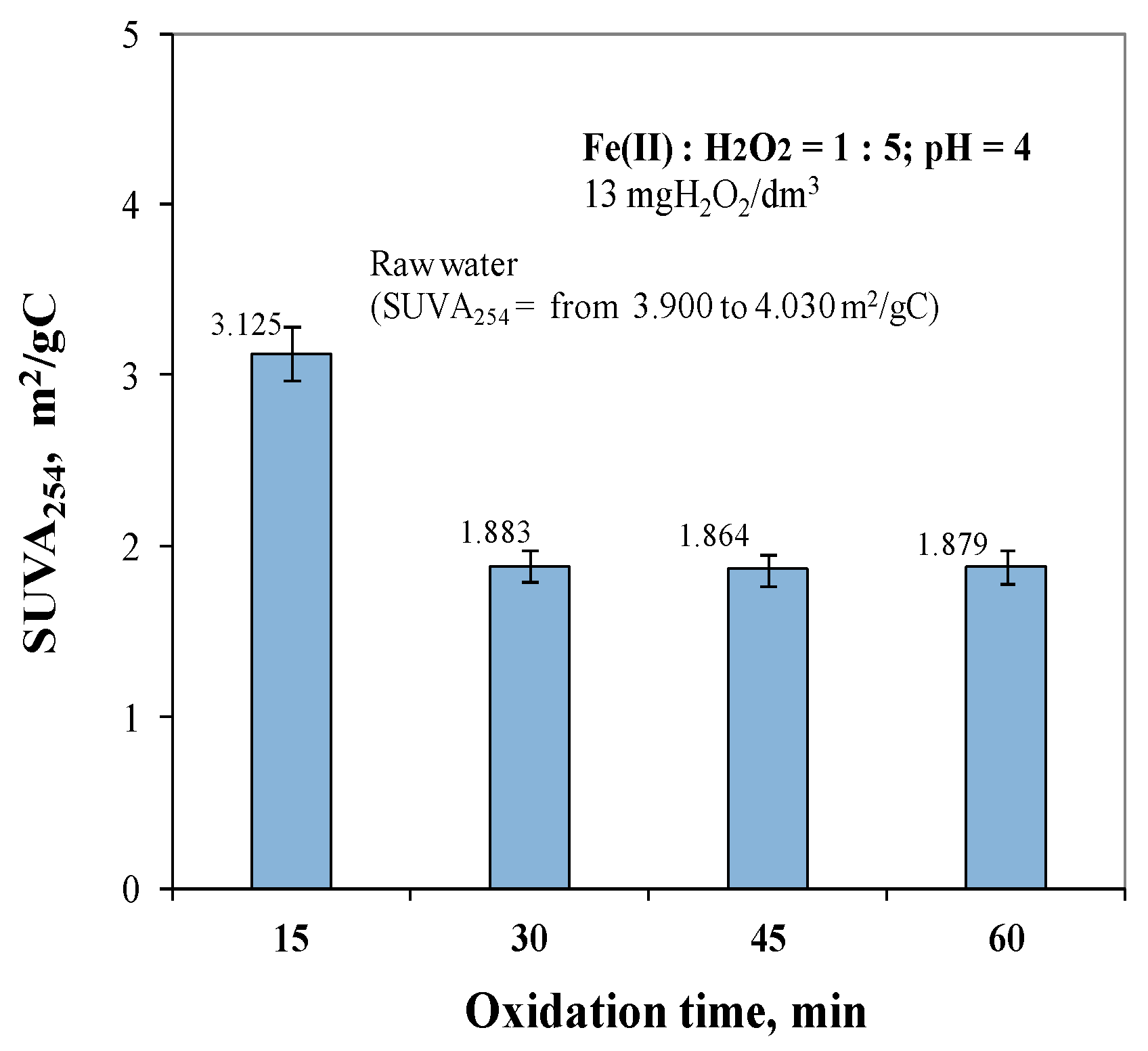
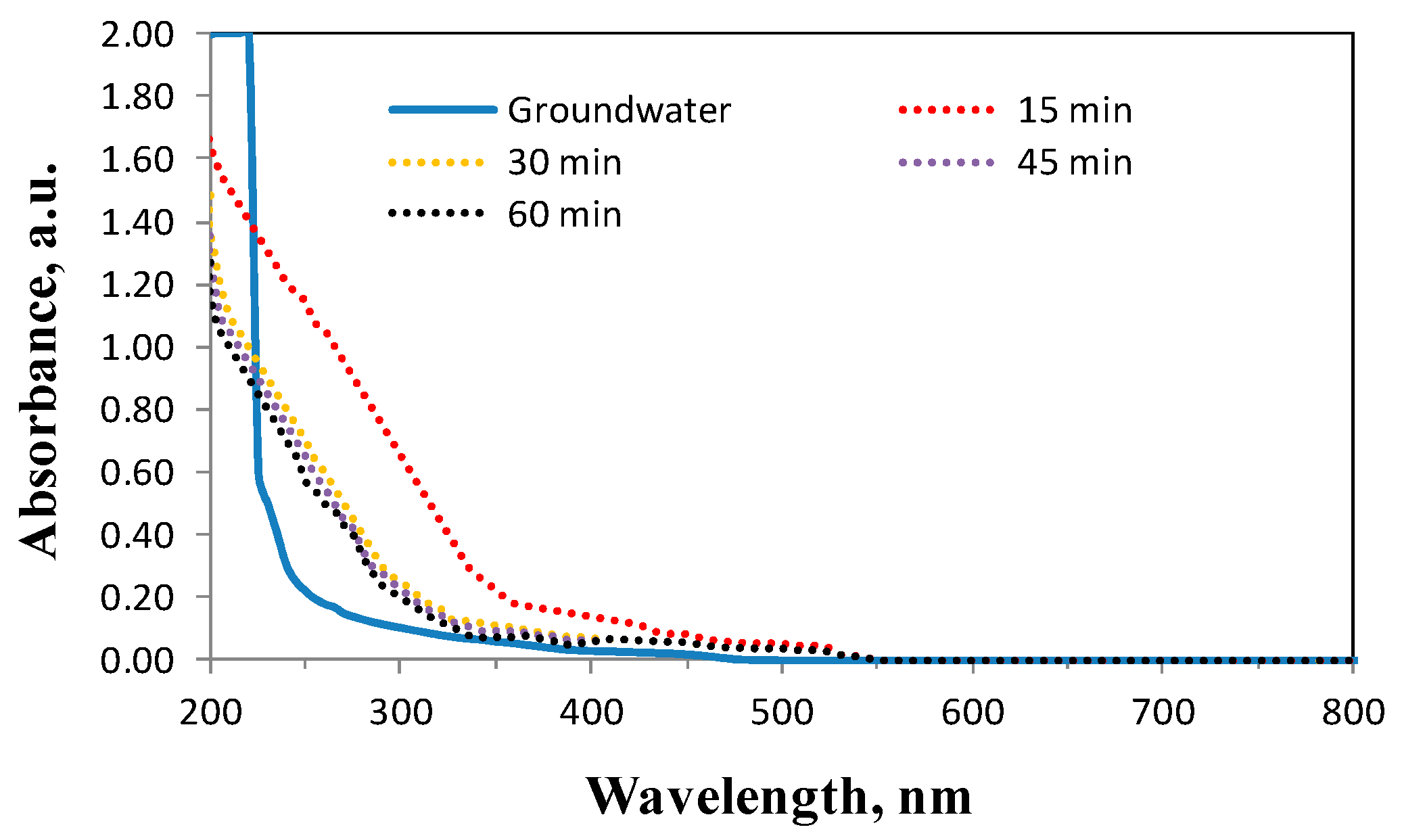
2.3. Effect of Oxidation Time from 15 to 60 min
3. Materials and Methods
3.1. Water Samples
3.2. Technological Research
Reagents and Materials
3.3. Analytical Methods
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krupińska, I. The impact of the oxidising agent type and coagulant type on the effectiveness of coagulation in the removal of pollutants from underground water with an increased content of organic substances. J. Environ. Eng. Landsc. 2016, 24, 70–78. [Google Scholar] [CrossRef]
- Płuciennik-Koropczuk, E.; Kumanowska, P. Chemical stability of water in the water supply network—Preliminary research. Civ. Environ. Eng. Rep. 2018, 28, 79–89. [Google Scholar] [CrossRef]
- Krupinska, I. Removing iron and organic substances from water over the course of its treatment with the application of average and highly alkaline polyaluminium chlorides. Molecules 2021, 26, 1367. [Google Scholar] [CrossRef] [PubMed]
- Krupińska, I. Removal of natural organic matter from groundwater by coagulation using prehydrolysed and non-prehydrolysed coagulants. Desalin. Water Treat. 2018, 132, 244–252. [Google Scholar] [CrossRef]
- Thurman, E.M. Organic Geochemistry of Natural Waters; Springer International Publishing: Dordrecht, The Netherlands, 1985. [Google Scholar]
- World Health Organization. Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Ijpelaar, G.F.; Groenendijk, M.; Hopman, R.; Kruithof, J.C. Advanced oxidation technologies for the degradation of pesticides in ground water and surface water. Water Sci. Technol. Water Supply 2002, 2, 129–138. [Google Scholar] [CrossRef]
- Murray, C.A.; Parsons, S.A. Advanced oxidation processes: Flow sheet options for bulk natural organic matter removal. Water Sci. Technol. Water Supply 2004, 4, 113–119. [Google Scholar] [CrossRef]
- Tubić, A.; Agbaba, J.; Dalmacija, B.; Molnar, J.; Maletić, S.; Watson, M.; Perović, S. Insight into changes during coagulation in NOM reactivity for trihalomethanes and haloacetic acid formation. J. Environ. Manag. 2013, 118, 153–160. [Google Scholar] [CrossRef]
- Chiang, P.C.; Chang, E.E.; Chang, P.C.; Huang, C.P. Effects of pre-ozonation on the removal of THM precursors by coagulation. Sci. Total Environ. 2009, 407, 5735–5742. [Google Scholar] [CrossRef]
- Kitis, M.; Karanfil, T.; Wigton, A.; Kilduff, J.E. Isolation of dissolved organic matter (DOM) fromsurface waters using reverse osmosis and its impact on the reactivity of DOM to formation and speciation of disinfection by-products. Water Res. 2002, 35, 2225–2234. [Google Scholar] [CrossRef]
- Liang, L.; Singer, P.C. Factors influencing the formation and relative distribution of haloacetic acids and trihalomethanes in drinking water. Environ. Sci. Technol. 2003, 37, 2920–2928. [Google Scholar] [CrossRef]
- Wolska, M. Removal of precursors of chlorinated organic compounds in selected water treatment processes. Desalin. Water Treat. 2018, 52, 3938–3946. [Google Scholar] [CrossRef]
- Płuciennik-Koropczuk, E.; Myszograj, S. Changes in quantity of non-biodegradable organic micropollutants in municipal wastewater based on COD fractionation. Desalin. Water Treat. 2020, 186, 334–340. [Google Scholar] [CrossRef]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Uyguner, C.; Suphandag, S.; Kerc, A.; Bekbolet, M. Evaluation of adsorption and coagulation characteristics of humic acids preceded by alternative advanced oxidation techniques. Desalination 2007, 210, 183–193. [Google Scholar] [CrossRef]
- Goslan, E.; Gurses, F.; Banks, J.; Parsons, S. An investigation into reservoir NOM reduction by UV photolysis and advanced oxidation processes. Chemosphere 2006, 65, 1113–1119. [Google Scholar] [CrossRef]
- Sanly, L.M.; Chiang, K.; Amal, R.; Fabris, R.; Chow, C.; Drikas, M. A study on the removal of humic acid using advanced oxidation process. Sep. Sci. Technol. 2007, 42, 1391–1404. [Google Scholar] [CrossRef]
- Machi, J.; Molczan, M. Methods for natural organic matter characterization in water taken and treated for human consumption. Ochr. Sr. 2016, 38, 25–32. [Google Scholar]
- Yamazaki, I.; Piette, L.H. ESR spin-trapping studies on the reaction of Fe2+ ions with H2O2 -reactive species on oxygen toxicity on biology. J. Biol. Chem. 1990, 265, 13589–13594. [Google Scholar] [CrossRef]
- Murray, C.A.; Parsons, S.A. Removal of NOM from drinking water: Fenton’s and photo-Fenton’s processes. Chemosphere 2004, 54, 1017–1023. [Google Scholar] [CrossRef]
- Watts, R.J.; Teel, A.M. Chemistry of Modified Fenton’s Reagent (Catalyzed H2O2 Propagations-CHP) for In Situ Soil and Groundwater Remediation. J. Environ. Eng. 2005, 131, 612–622. [Google Scholar] [CrossRef]
- Ciottia, C.; Baciocchi, R.; Tuhkanen, T. Influence of the operating conditions on highly oxidative radicals generation in Fenton’s systems. J. Hazard. Mater. 2009, 161, 402–408. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Abdelshafy, A.M.; Hu, Q.; Luo, Z.; Ban, Z.; Li, L. Hydrogen Peroxide from Traditional Sanitizer to Promising Disinfection Agent in Food Industry. Food Rev. Int. 2023, 40, 658–690. [Google Scholar] [CrossRef]
- Jing, G.; Wang, Y.; Wu, M.; Liu, W.; Xiong, S.; Yu, J.; Jiang, Y. Photocatalytic Degradation and Pathway from Mycotoxins in Food: A Review. Food Rev. Int. 2023, 40, 276–292. [Google Scholar] [CrossRef]
- Galapate, R.P.; Baes, A.U.; Okada, M. Transformation of dissolved organic matter during ozonation: Effects on trihalomethane formation potential. Water Res. 2001, 9, 2201. [Google Scholar] [CrossRef]
- Wolska, M.; Machi, J.; Szerzyna, S.; Mołczan, M.; Adamski, M.; Wisniewski, J. Effect of ozonation on organic substance removal efficiency during adsorption. Desalin. Water Treat. 2018, 117, 101–107. [Google Scholar] [CrossRef]
- Ijpelaar, G.F.; Groenendijk, M.; Kruithof, J.C.; Schippers, J.C. Fenton process for the combined removal of iron and organic micropollutants in groundwater treatment. Water Sci. Technol. Water Supply 2002, 2, 229–236. [Google Scholar] [CrossRef]
- Stasinakis, A.S. Use of selected advanced oxidation processes (AOPs) for wastewater treatment—A mini rewiev. Glob. NEST J. 2008, 10, 376–385. [Google Scholar]
- Kastanek, F.; Spacilova, M.; Krystynik, P.; Dlaskova, M.; Solcova, O. Fenton Reaction-Unique but Still Mysterious. Processes 2023, 11, 432. [Google Scholar] [CrossRef]
- Torres, G.F.; Ortega Méndez, J.A.; Tinoco, D.L.; Marin, E.D.; Araña, J.; Herrera-Melián, J.A.; Doña Rodrígez, J.M.; Pérez Peña, J. Detoxification of synthetic and real groundwater contaminated with gasoline and diesel using Fenton, photo-Fenton, and biofilters. Desalin. Water Treat. 2016, 57, 23760–23769. [Google Scholar] [CrossRef]
- Lipczynska-Kochany, E.; Kochany, J. Effect of humic substances on the Fenton treatment of wastewater at acidic and neutral pH. Chemosphere 2008, 73, 745–750. [Google Scholar] [CrossRef]
- Kochany, J.; Lipczynska-Kochany, E. Fenton reaction in the presence of humates. Treatment of highly contaminated wastewater at neutral pH. Environ. Technol. 2007, 28, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Molnar, J.; Agbaba, J.; Watson, M.; Tubić, A.; Kragulj, M.; Maletić, S.; Dalmacija, B. Groundwater treatment using the Fenton process: Changes in natural organic matter characteristics and arsenic removal. Int. J. Environ. Res. 2015, 9, 467–474. [Google Scholar]
- Moncayo-Lasso, A.; Pulgarin, C.; Benítez, N. Degradation of DBPs’ precursors in river water before and after slow sand filtration by photo-Fenton process at pH 5 in solar CPC reactor. Water Res. 2008, 42, 4125–4132. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Leal, M.; Li, Q. Degradation of natural organic matter by TiO2 photocatalytic oxidation and its effect on fouling of low-pressure membranes. Water Res. 2008, 42, 1142–1150. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.; Chiang, K.; Drikas, M.; Amal, R. Removal of humic acid using TiO2 photocatalytic process—Fractionation and molecular weight characterization studies. Chemosphere 2008, 72, 263–271. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.; Drikas, M.; Amal, R. Comparison of photocatalytic degradation of natural organic matter in two Australian surface waters using multiple analytical techniques. Org. Geochem. 2010, 41, 124–129. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Sanabria, J.; Pulgarin, C.; Benítez, N. Simultaneous E. coli inactiva- tion and NOM degradation in river water via photo-Fenton process at naturalpH in solar CPC reactor: A new way for enhancingsolar disinfection of natural water. Chemosphere 2009, 77, 296–300. [Google Scholar] [CrossRef]
- Moncayo-Lasso, A.; Rincon, A.-G.; Pulgarin, C.; Benitez, N. Significant decrease of THMs generated during chlorination of river water by previous photo-Fenton treatment at near neutral pH. J. Photochem. Photobiol. A Chem. 2012, 229, 46–52. [Google Scholar] [CrossRef]
- Nie, Y.; Hu, C.; Zhou, L.; Qu, J.; Wei, Q.; Wang, D. Degradation characteristics of humic acid over iron oxides/Fe0 core-shell nanoparticles with UVA/H2O2. J. Hazard. Mater. 2010, 173, 474–479. [Google Scholar] [CrossRef]
- Buchanan, W.; Roddick, F.; Porter, N. Formation of hazardous by-products resulting from irradiation of natural organic matter: Comparison between UV and VUV irradiation. Chemosphere 2006, 63, 1130–1141. [Google Scholar] [CrossRef]
- Xiao, J.Y.; Guo, S.F.; Wang, D.; An, Q. Fenton-Like Reaction: Recent Advances and New Trends. Chemistry 2024, 30, 25. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk-Makuła, M.; Myszograj, S.; Włodarczyk, M. Removal of Organic Micro-Pollutants from Wastewater in Electrochemical Processes—Review. Energies 2023, 16, 5591. [Google Scholar] [CrossRef]
- Che, H.C.; Tian, X.K.; Xu, X.D.; Nie, Y.L.; Yang, C.; Li, Y.; Lu, L.Q. A promising evaluation method for the detoxification effect of Fenton oxidation technology via tracking by-products of pollutants and corresponding acute toxicity. Sep. Purif. Technol. 2024, 353, 128438. [Google Scholar] [CrossRef]
- Sillanpaa, M.; Matilainen, A. Chapter 8—NOM removal by advanced oxidation processes. In Natural Organic Matter in Water, 2nd ed.; Mika, S., Yuri, P., Eds.; Butterworth-Heinemann: Oxford, UK, 2023; pp. 225–265. [Google Scholar]
- Włodarczyk-Makuła, M.; Wiśniowska, E. Halogenated organic compounds in water and in wastewater. Civ. Environ. Eng. Rep. 2019, 29, 236–247. [Google Scholar] [CrossRef]
- Teh Fu, Y. Chemical Processes for Environmental Engineering; Imperial College Press: London, UK, 2007. [Google Scholar]
- Anielak, A. Highly Efficient Water Treatment Methods; PWN: Warszawa, Poland, 2015. [Google Scholar]
- Kowal, A.L.; Świderska-Bróż, M.; Wolska, M. Water Treatment; PWN: Warszawa, Poland, 2022. [Google Scholar]
- Moel, P.J.; Verberk, J.Q.J.C.; Van Dijk, J.C. Drinking Water. In Principles and Practices; World Scientific Publishing Co. Pte. Ltd.: London, UK, 2007. [Google Scholar]
- Cao, W.; Yan, G.; Hofmann, H.; Scheuermann, A. State of the Art on Fe Precipitation in Porous Media: Hydrogeochemical Processes and Evolving Parameters. J. Mar. Sci. Eng. 2024, 12, 690. [Google Scholar] [CrossRef]
- Krupińska, I. Impact of the Oxidant Type on the Efficienty of the Oxidation and Removal of Iron Compounds from Groundwater Containing Humic Substances. Molecules 2020, 25, 3380. [Google Scholar] [CrossRef]
- Krupińska, I. Importance of Humic Substances for Methods of Groundwater Treatment. Pol. J. Soil Sci. 2016, 48, 161. [Google Scholar] [CrossRef]
- Chowdhury, S.; Champagne, P.; McLellan, P.J. Models for Predicting Disinfection Byproduct (DBP) Formation in Drinking Waters: A Chronological Review. Sci. Total Environ. 2009, 407, 4189–4206. [Google Scholar] [CrossRef]
- Ding, S.; Deng, Y.; Bond, T.; Fang, C.; Cao, Z.; Chu, W. Disinfection Byproduct Formation during Drinking Water Treatment and Distribution: A Review of Unintended Effects of Engineering Agents and Materials. Water Res. 2019, 160, 313–329. [Google Scholar] [CrossRef]
- Hua, G.; Reckhow, D.A. Characterization of Disinfection Byproduct Precursors Based on Hydrophobicity and Molecular Size. Environ. Sci. Technol. 2007, 41, 3309–3315. [Google Scholar] [CrossRef]
- Regulation of the Minister of Health Dated 7 December 2017 Amending the Regulation on the Quality of Drinking Water Mean for Human Consumption. Available online: http://isap.sejm.gov.pl/isap.nsf/DocDetails.xsp?id=WDU20170002294 (accessed on 30 July 2024).
- Zhang, Y.M.; Zhou, M. A critical review of the application of chelating agents to enable Fenton and Fenton-like reactionsat high pH values. J. Hazard. Mater. 2019, 362, 436–450. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhao, G.; Yang, Z.; Li, Q. Molecular comparison of organic matter removal from shale gas flowback wastewater: Ozonation versus Fenton process. Sci. Total Environ. 2023, 905, 167147. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Shi, J.; Xie, W.; Wang, G.; Wang, Y.; Tang, J.; Lu, H. Enhancing the Fenton-like degradation of organic contaminants with silicate-iron in aquifer media by ascorbic acid. Colloids Surf. A Physicochem. Eng. Asp. 2024, 685, 133307. [Google Scholar] [CrossRef]
- Ungwanen, J.; Raymond, A.; Wuana, A.U.; Itodo, R.; Sha’Ato, R.; Dantas, F. A review on the use of chelating agents as an alternative to promote photo-Fenton at neutral pH: Current trends, knowledge gap and future studies. Sci. Total Environ. 2020, 710, 134872. [Google Scholar]
- Simić, M.D.; Brdarić, T.; Branislava, G.; Rosić, B.; Švorc, L.; Relić, D.; Aćimović, D. Degradation of bisphenol A via the electro–Fenton process using nanostructured carbon-metal oxide anodes: Intermediates and reaction mechanisms study. J. Environ. Chem. Eng. 2024, 12, 113369. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Lee, J.; Xing, L. A review of application mechanism and research progress of Fe/montmorillonite-based catalysts in heterogeneous Fenton reactions. J. Environ. Chem. 2024, 12, 112152. [Google Scholar] [CrossRef]
- Wang, Q.; Qin, H.; Fan, J.; Xie, H. New insight into the mechanism of ferric hydroxide-based heterogeneous Fenton-like reaction. J. Hazard. Mater. 2023, 443, 130278. [Google Scholar] [CrossRef]
- Janda, A.; Marcinkowski, T. Fenton Process Modifi cation Possibilities for Oxidation Effectiveness of Hard Degradable Organic Pollutants. Ochr. Sr. 2019, 41, 47–53. [Google Scholar]
- Mołczan, M.; Szlachta, M.; Karpińska, A.; Biłyk, A. Application of specific ultraviolet absorbance (SUVA) in water quality assessment. Ochr. Sr. 2006, 28, 11–16. [Google Scholar]
- Chen, L.; Zhou, M.H.; Lei, L.C. Evidence against hydroxyl radical mechanism in photo-Fenton degradation of p-chlorophenol. J. Environ. Sci. 2006, 18, 388–391. [Google Scholar]
- Wang, C.; Zhang, S.; Sakai, Y.; Zhang, Z. Analysis of a complex produced in the Fenton oxidation process. Water Sci. Technol. 2014, 69, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Khorassani, H.E.; Trebuchon, P.; Bitar, H.; Tomas, O. A simple UV spectrophotometric procedure for the survey of industrial sewage system. Water Sci. Technol. 1999, 39, 77–82. [Google Scholar] [CrossRef]
- Lei, C.; Sun, Y.; Tsang, D.C.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2018, 232, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.D.; Kanmani, S. Textile dye degradation using nano zero valent iron: A review. J. Environ. Manag. 2016, 177, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Colombo, A.; Dragonetti, C.; Magni, M.; Roberto, D. Degradation of toxic halogenated organic compounds by iron-containing mono-, bi- and tri-metallic particles in water. Inorg. Chim. Acta 2015, 431, 48–60. [Google Scholar] [CrossRef]
- PN-ISO 6332; Water Quality—Determination of Iron. Spectrophotometric Method with 1,10 Phenanthroline. International Organization for Standardization: Geneva, Switzerland, 2001.
- Poter, B.B.; Wimsatt, J.C. Determination of Total Organic Carbon and Specific UV Absorbance at 254 nm in Source Water and Drinking Water; EPA/600/R-09/112. Method 415.3; United States Environmental Protection Agency: Washington, DC, USA, 2009. [Google Scholar]
- Karanfil, T.; Schlautman, M.A.; Erdogan, I. Survey of DOC and UV measurement practices with implications for SUVA determination. Am. Water Work. Assoc. 2002, 94, 68–80. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.-Q. Advances in the Characterization and Monitoring of Natural Organic Matter Using Spectroscopic Approaches. Water Res. 2021, 190, 116759. [Google Scholar] [CrossRef]
- APHA. Standard Method for the Examination of Water and Wastewater, 23rd ed.; American Public Health Association/Water Works Association: Washington, DC, USA, 2017. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krupińska, I. Application of Fenton’s Reaction for Removal of Organic Matter from Groundwater. Molecules 2024, 29, 5150. https://doi.org/10.3390/molecules29215150
Krupińska I. Application of Fenton’s Reaction for Removal of Organic Matter from Groundwater. Molecules. 2024; 29(21):5150. https://doi.org/10.3390/molecules29215150
Chicago/Turabian StyleKrupińska, Izabela. 2024. "Application of Fenton’s Reaction for Removal of Organic Matter from Groundwater" Molecules 29, no. 21: 5150. https://doi.org/10.3390/molecules29215150
APA StyleKrupińska, I. (2024). Application of Fenton’s Reaction for Removal of Organic Matter from Groundwater. Molecules, 29(21), 5150. https://doi.org/10.3390/molecules29215150





