Determination of Photosensitizing Potential of Lapachol for Photodynamic Inactivation of Bacteria
Abstract
:1. Introduction
2. Results
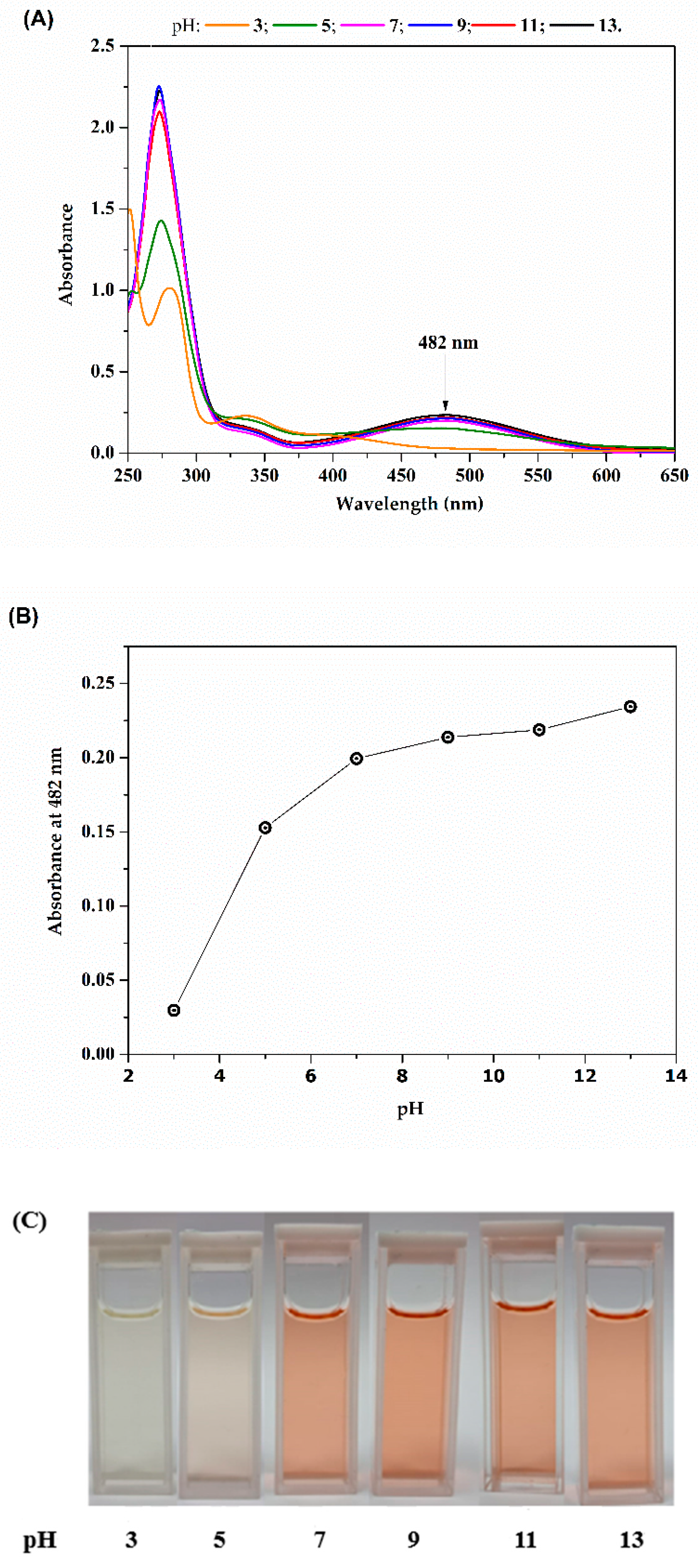
2.1. Optical Characterization of Lapachol
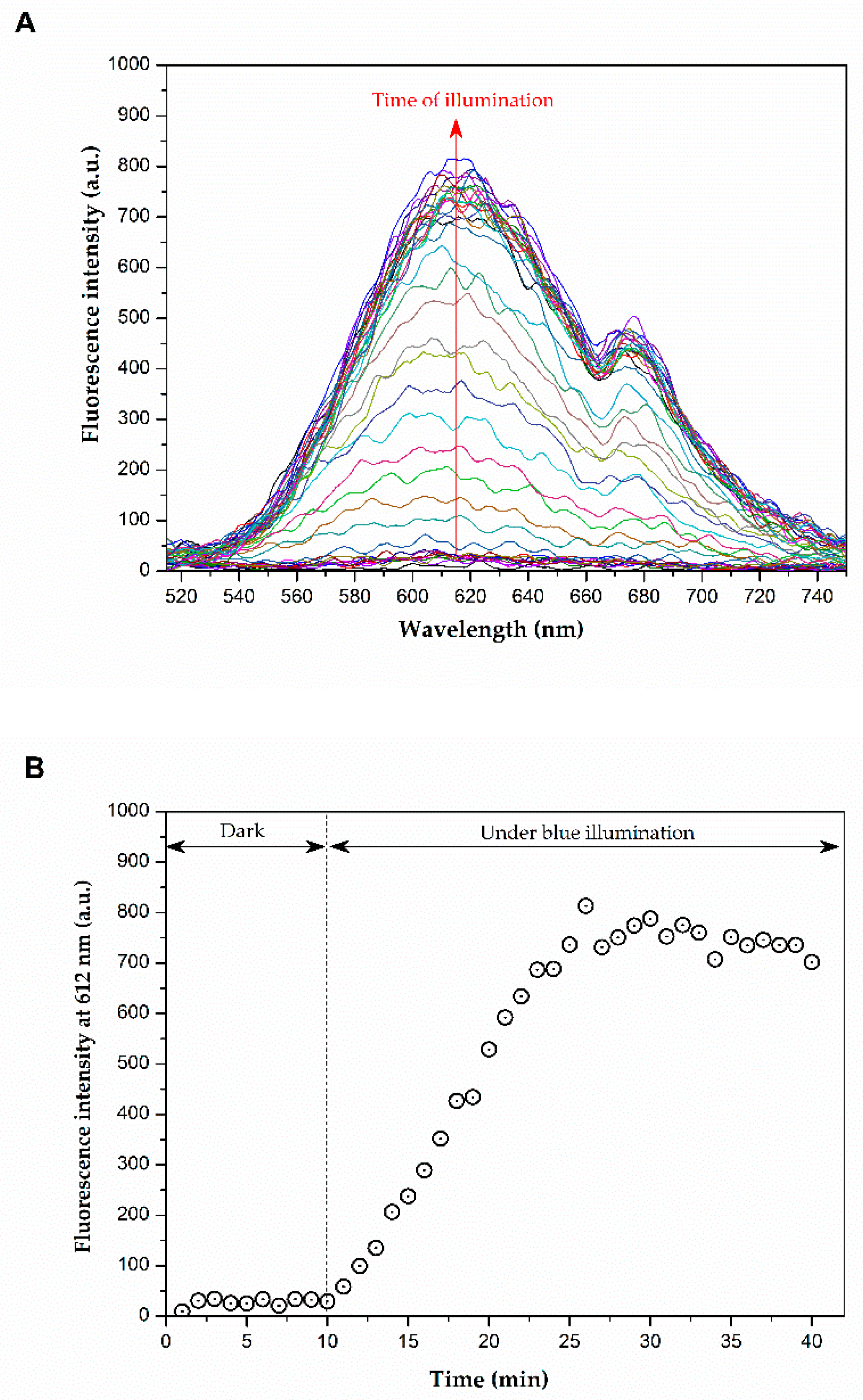
2.2. Production of Reactive Oxygen Species (ROS)
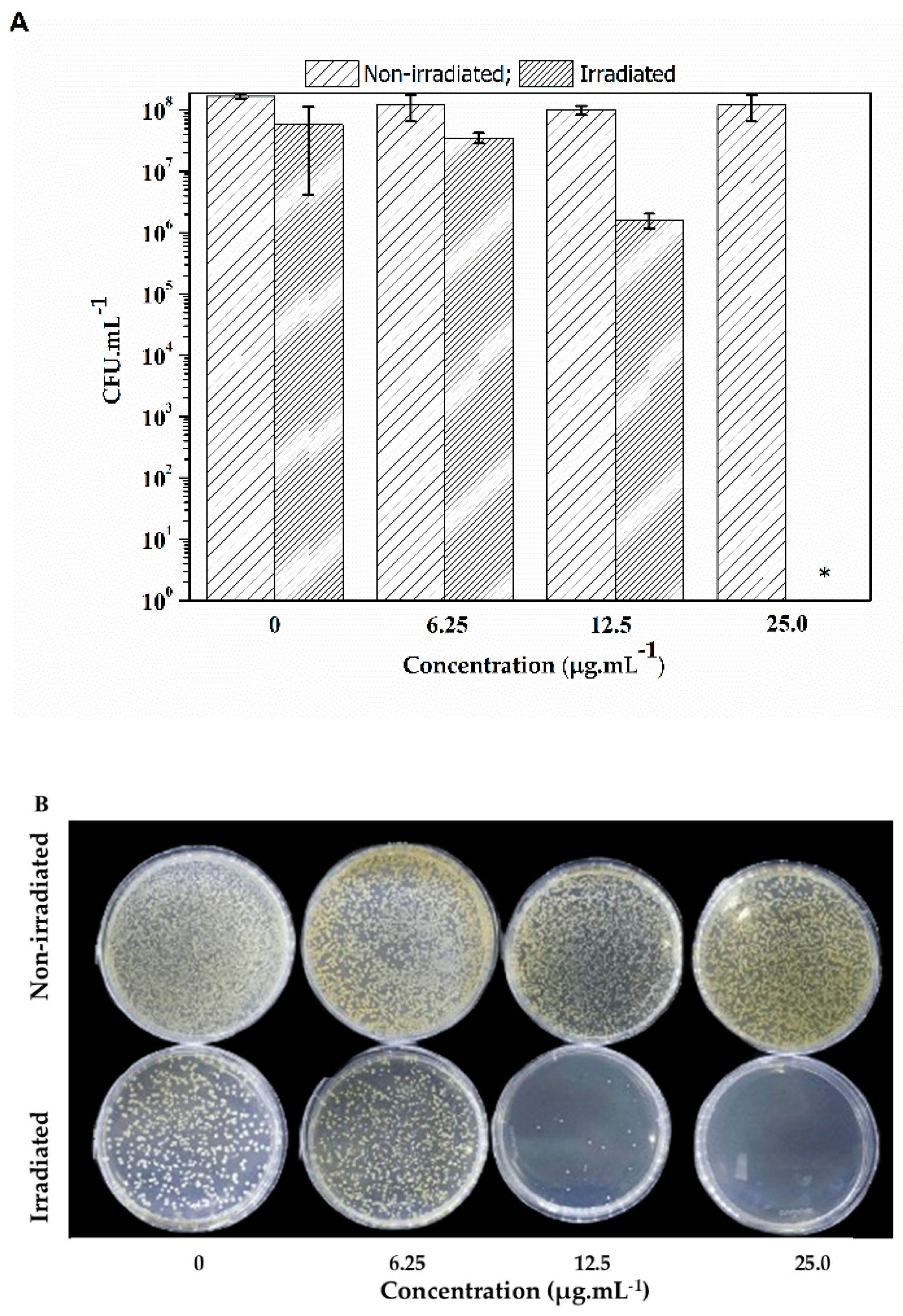
2.3. Photoinactivation Assay
3. Discussion
4. Materials and Methods
4.1. Photosensitizer
4.2. UV-Vis Absorption
4.3. Determination of Reactive Oxygen Species Production
4.4. Photoinactivation Assay
4.5. Scanning Electron Microscopy (SEM)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Runcie, H. Infection in a Pre-Antibiotic Era. J. Anc. Dis. Prev. Remedies 2015, 3, 125. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. Antibiotic-Resistant Bugs in the 21st Century—A Clinical Super-Challenge. New Engl. J. Med. 2009, 360, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Review on Antimicrobial Resistance: Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; Wellcome Trust: London. UK, 2016. [Google Scholar]
- Baron, S. Medical Microbiology, 4th ed.; University of Texas Medical Branch at Galveston: Galveston, TX, USA, 1996; ISBN 10:0-9631172-1-1. [Google Scholar]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic Resistance—The Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.C.D. Bacteria: Staphylococcus Aureus. In Encyclopedia of Food Safety; Elsevier: Amsterdam, The Netherlands, 2014; pp. 530–534. [Google Scholar]
- Sperandio, F.; Huang, Y.-Y.; Hamblin, M. Antimicrobial Photodynamic Therapy to Kill Gram-Negative Bacteria. Recent. Pat. Antiinfect. Drug Discov. 2013, 8, 108–120. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Hasan, T. Photodynamic Therapy: A New Antimicrobial Approach to Infectious Disease? Photochem. Photobiol. Sci. 2004, 3, 436–450. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, R.; Zaat, S.A.; Breukink, E.; Heger, M. Antibacterial Photodynamic Therapy: Overview of a Promising Approach to Fight Antibiotic-Resistant Bacterial Infections. J. Clin. Transl. Res. 2015, 1, 140. [Google Scholar] [CrossRef]
- Vilaça, R.M.A.; Sodré, G.P.; Ferreira, I.S.; Da Costa, G.D.; Dias, L.D. Fotossensibilizadores de Origem Natural: Extração, Caracterização e Recentes Avanços Na Fotoinativação Bacteriana. Braz. J. Health Rev. 2023, 6, 23436–23457. [Google Scholar] [CrossRef]
- Ludačka, P.; Kubát, P.; Bosáková, Z.; Mosinger, J. Antibacterial Nanoparticles with Natural Photosensitizers Extracted from Spinach Leaves. ACS Omega 2022, 7, 1505–1513. [Google Scholar] [CrossRef]
- Dias, L.D.; Blanco, K.C.; Mfouo-Tynga, I.S.; Inada, N.M.; Bagnato, V.S. Curcumin as a Photosensitizer: From Molecular Structure to Recent Advances in Antimicrobial Photodynamic Therapy. J. Photochem. Photobiol. C Photochem. Rev. 2020, 45, 100384. [Google Scholar] [CrossRef]
- Rajendran, M. Quinones as Photosensitizer for Photodynamic Therapy: ROS Generation, Mechanism and Detection Methods. Photodiagnosis Photodyn. Ther. 2016, 13, 175–187. [Google Scholar] [CrossRef]
- Hussain, H.; Krohn, K.; Ahmad, V.U.; Miana, G.A.; Green, I.R. Lapachol: An Overview. Arkivoc 2007, 2007, 145–171. [Google Scholar] [CrossRef]
- Silva, C.C.; Oliveira Paiva, R.; Costa, G.L.; Echevarria, A. Anti-Bacterial Activity of New 2-Amino-1, 4-Naphthoquinones. Braz. J. Dev. 2021, 7, 54215–54229. [Google Scholar] [CrossRef]
- Januário, S.R.; Silvério-lopes, S. O Poder Terapêutico Do Ipê Roxo e Seu Uso Na Terapia Complementar Ao Tratamento de Neoplasias. Rev. Bras. Saúde 2014, 5, 9–14. [Google Scholar] [CrossRef]
- Araújo, E.L.; Alencar, J.R.B.; Neto, P.J.R. Lapachol: Segurança e Eficácia Na Terapêutica. Rev. Bras. Farmacogn. 2002, 12, 57–59. [Google Scholar] [CrossRef]
- Lira, A.A.M.; Sester, E.A.; Carvalho, A.L.M.; Strattmann, R.R.; Albuquerque, M.M.; Wanderley, A.G.; Santana, D.P. Development of Lapachol Topical Formulation: Anti-Inflammatory Study of a Selected Formulation. Am. Assoc. Pharm. Sci. 2008, 9, 163–168. [Google Scholar] [CrossRef]
- Chen, Q.; Bai, L.; Zhou, X.; Xu, P.; Li, X.; Xu, H.; Zheng, Y.; Zhao, Y.; Lu, S.; Xue, M. Development of Long-Circulating Lapachol Nanoparticles: Formation, Characterization, Pharmacokinetics, Distribution and Cytotoxicity. RSC Adv. 2020, 10, 30025–30034. [Google Scholar] [CrossRef]
- Linzner, N.; Fritsch, V.N.; Busche, T.; Tung, Q.N.; Loi, V.V.; Bernhardt, J.; Kalinowski, J.; Antelmann, H. The Plant-Derived Naphthoquinone Lapachol Causes an Oxidative Stress Response in Staphylococcus Aureus. Free Radic. Biol. Med. 2020, 158, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Nepomuceno, J.C. Lapachol and Its Derivatives as Potential Drugs for Cancer Treatment. In Plants and Crop the Biology and Biotechnology Research; 2014; pp. 1–20. [Google Scholar]
- Salustiano, E.J.S.; Netto, C.D.; Fernandes, R.F.; Silva, A.J.M.d.; Bacelar, T.S.; Castro, C.P.; Buarque, C.D.; Maia, R.C.; Rumjanek, V.M.; Costa, P.R.R. Comparison of the Cytotoxic Effect of Lapachol, α-Lapachone and Pentacyclic 1, 4-Naphthoquinones. Investig. New Drugs 2010, 28, 139–144. [Google Scholar] [CrossRef]
- Souza, M.A.; Johann, S.; Alves, L.; Lima, S.; Campos, F.F.; Mendes, I.C.; Beraldo, H.; Souza-fagundes, E.M.D.; Cisalpino, P.S.; Rosa, C.A.; et al. The Antimicrobial Activity of Lapachol and Its Thiosemicarbazone and Semicarbazone Derivatives. Mem. Inst. Oswaldo Cruz 2013, 108, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.G.T.; Miranda, F.F.; Ferreira, V.F.; Freitas, C.C.; Rabello, R.F.; Carballido, J.M.; Corrêa, L.C.D. Synthesis and Antimicrobial Evaluation of 3-Hydrazino-Naphthoquinones as Analogs of Lapachol. J. Braz. Chem. Soc. 2001, 12, 339–345. [Google Scholar] [CrossRef]
- Eyong, K.O.; Kumar, S.P.; Kuete, V.; Folefoc, G.N.; Langmi, H.; Meyer, M.J.J.; Lall, N.; Baskaran, S. Cobalt Mediated Ring Contraction Reaction of Lapachol and Initial Antibacterial Evaluation of Naphthoquinones Derived from Lapachol. Med. Chem. Res. 2012, 21, 2117–2122. [Google Scholar] [CrossRef]
- Guo, J.; Dai, J.; Peng, X.; Wang, Q.; Wang, S.; Lou, X.; Xia, F.; Zhao, Z.; Tang, B.Z. 9,10-Phenanthrenequinone: A Promising Kernel to Develop Multifunctional Antitumor Systems for Efficient Type I Photodynamic and Photothermal Synergistic Therapy. ACS Nano 2021, 15, 20042–20055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Kuang, H.; Xu, Y.; Shi, L.; Cao, W.; Zhu, K.; Xu, L.; Ma, J. Rational Design of a High-Performance Quinoxalinone-Based AIE Photosensitizer for Image-Guided Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 42551–42557. [Google Scholar] [CrossRef]
- Jadhao, M.; Ahirkar, P.; Joshi, R.; Kumar, H.; Ghosh, S.K. Interaction of Aluminum Phthalocyanine with Aziridinyl Quinone in Biomimicking Micellar Microenvironment for the Application in Photodynamic Therapy: Effect of Micellar Hydration. J. Photochem. Photobiol. A Chem. 2016, 316, 62–68. [Google Scholar] [CrossRef]
- Rodrigues, B.M.; Diniz, C.C.; da Rocha, V.N.; Köhler, M.H.; Brandão, G.P.; Machado, L.A.; da Silva Júnior, E.N.; Iglesias, B.A. First Report of Trans.-A2B-Corrole Derived from a Lapachone Derivative: Photophysical, TD-DFT and Photobiological Assays. RSC Adv. 2023, 13, 11121–11129. [Google Scholar] [CrossRef]
- da SS Campanholi, K.; Gerola, A.P.; Vilsinski, B.H.; de Oliveira, É.L.; de Morais, F.A.P.; Rabello, B.R.; Braga, G.; Calori, I.R.; Silva, E.L.; Hioka, N.; et al. Development of Pluronic® Nanocarriers Comprising Pheophorbide, Zn-Pheophorbide, Lapachol and β-Lapachone Combined Drugs: Photophysical and Spectroscopic Studies. Dye. Pigment. 2018, 157, 238–250. [Google Scholar] [CrossRef]
- Singh, I.; Ogata, R.T.; Moore, R.E.; Chang, C.W.J.; Scheuer, P.J. Electronic Spectra of Substituted Naphthoquinones. Tetrahedron 1968, 24, 6053–6073. [Google Scholar] [CrossRef]
- Farfán, R.A.; Espíndola, J.A.; Gomez, M.I.; de Jiménez, M.C.L.; Martínez, M.A.; Piro, O.E.; Castellano, E.E. Structural and Spectroscopic Properties of Two New Isostructural Complexes of Lapacholate with Cobalt and Copper. Int. J. Inorg. Chem. 2012, 2012, 973238. [Google Scholar] [CrossRef]
- Segoloni, E.; Di Maria, F. UV–VIS Spectral and GC–MS Characterization of Handroanthus Serratifolius (Vahl.) Grose (a.k.a. Tabebuia Serratifolia (Vahl.) Nichols/Lapacho) Heartwood Main Extractives: A Comparison of Protocols Aimed at a Practical Evaluation of Lapachol and Dehydro-α-Lapachone Content. Eur. J. Wood Wood Prod. 2018, 76, 1547–1561. [Google Scholar] [CrossRef]
- Caires, C.S.A.; Silva, C.M.; Lima, A.R.; Alves, L.M.; Lima, T.H.N.; Rodrigues, A.C.S.; Chang, M.R.; Oliveira, S.L.; Whitby, C.; Nascimento, V.A.; et al. Photodynamic Inactivation of Methicillin-Resistant Staphylococcus Aureus by a Natural Food Colorant (e-141II). Molecules 2020, 25, 4464. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, Y.; Gutterman, M.; Malik, Z.; Ehrenberg, B. Inactivation of Gram-Negative Bacteria by Photosensitized Porphyrins. Photochem. Photobiol. 1992, 55, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Durantini, E. Photodynamic Inactivation of Bacteria. Curr. Bioact. Compd. 2006, 2, 127–142. [Google Scholar] [CrossRef]
- Caires, C.S.A.; Lima, A.R.; Lima, T.H.N.; Silva, C.M.; Araujo, L.O.; Aguilera, L.F.; Nascimento, V.A.; Caires, A.R.L.; Oliveira, S.L. Photodynamic Inactivation of Methicillin-Resistant Staphylococcus Aureus by Using Giemsa Dye as a Photosensitizer. Photodiagnosis Photodyn. Ther. 2024, 45, 103952. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, R.G.; Flores, R.S.; Miessi, G.; Pulcherio, J.H.V.; Aguilera, L.F.; Araujo, L.O.; Oliveira, S.L.; Caires, A.R.L. Determination of Photosensitizing Potential of Lapachol for Photodynamic Inactivation of Bacteria. Molecules 2024, 29, 5184. https://doi.org/10.3390/molecules29215184
Lima RG, Flores RS, Miessi G, Pulcherio JHV, Aguilera LF, Araujo LO, Oliveira SL, Caires ARL. Determination of Photosensitizing Potential of Lapachol for Photodynamic Inactivation of Bacteria. Molecules. 2024; 29(21):5184. https://doi.org/10.3390/molecules29215184
Chicago/Turabian StyleLima, Regiane G., Raphael S. Flores, Gabriella Miessi, Jhoenne H. V. Pulcherio, Laís F. Aguilera, Leandro O. Araujo, Samuel L. Oliveira, and Anderson R. L. Caires. 2024. "Determination of Photosensitizing Potential of Lapachol for Photodynamic Inactivation of Bacteria" Molecules 29, no. 21: 5184. https://doi.org/10.3390/molecules29215184
APA StyleLima, R. G., Flores, R. S., Miessi, G., Pulcherio, J. H. V., Aguilera, L. F., Araujo, L. O., Oliveira, S. L., & Caires, A. R. L. (2024). Determination of Photosensitizing Potential of Lapachol for Photodynamic Inactivation of Bacteria. Molecules, 29(21), 5184. https://doi.org/10.3390/molecules29215184








