Detection of Silver and Mercury Ions Using Naphthalimide-Based Fluorescent Probe
Abstract
1. Introduction
2. Results and Discussion
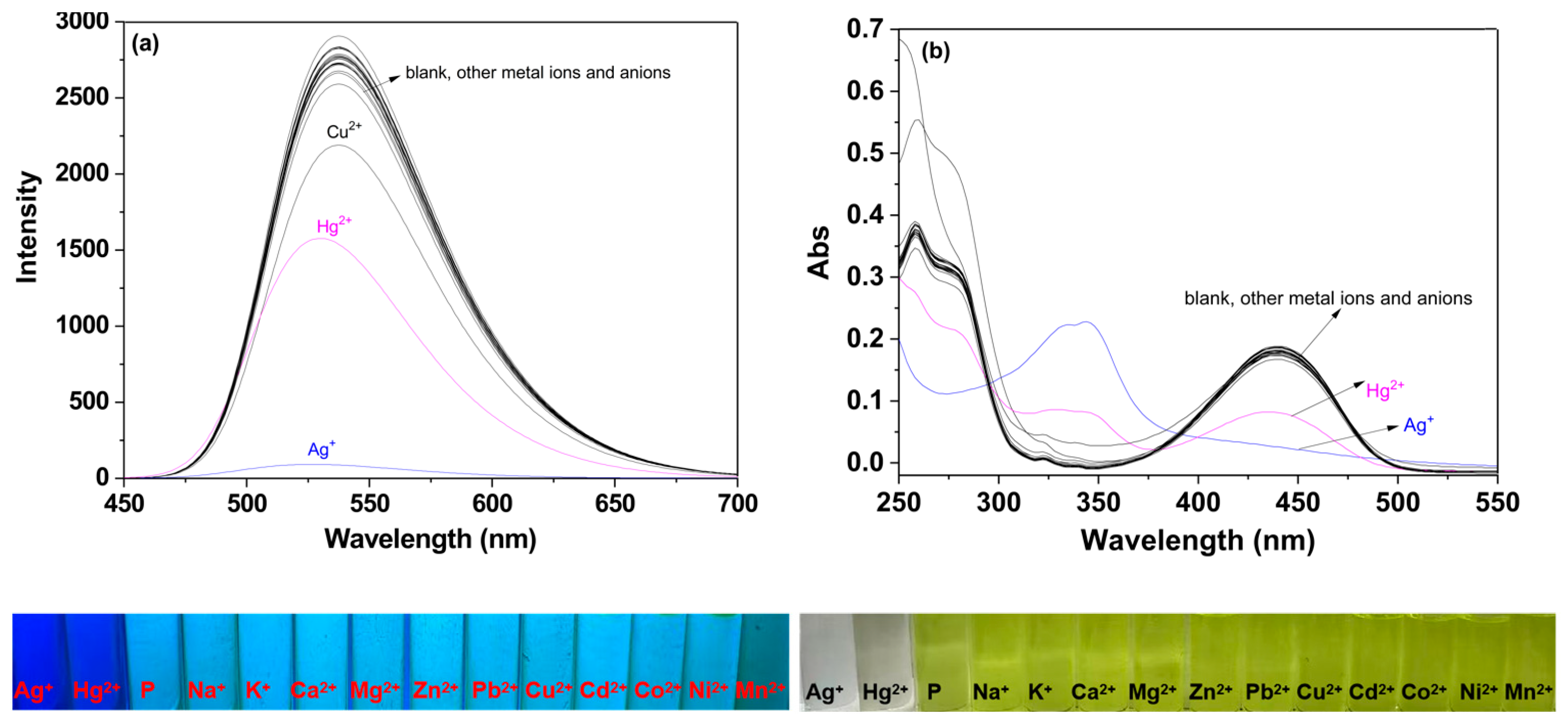
2.1. Selective Analysis of P to Metal Ions and Anions
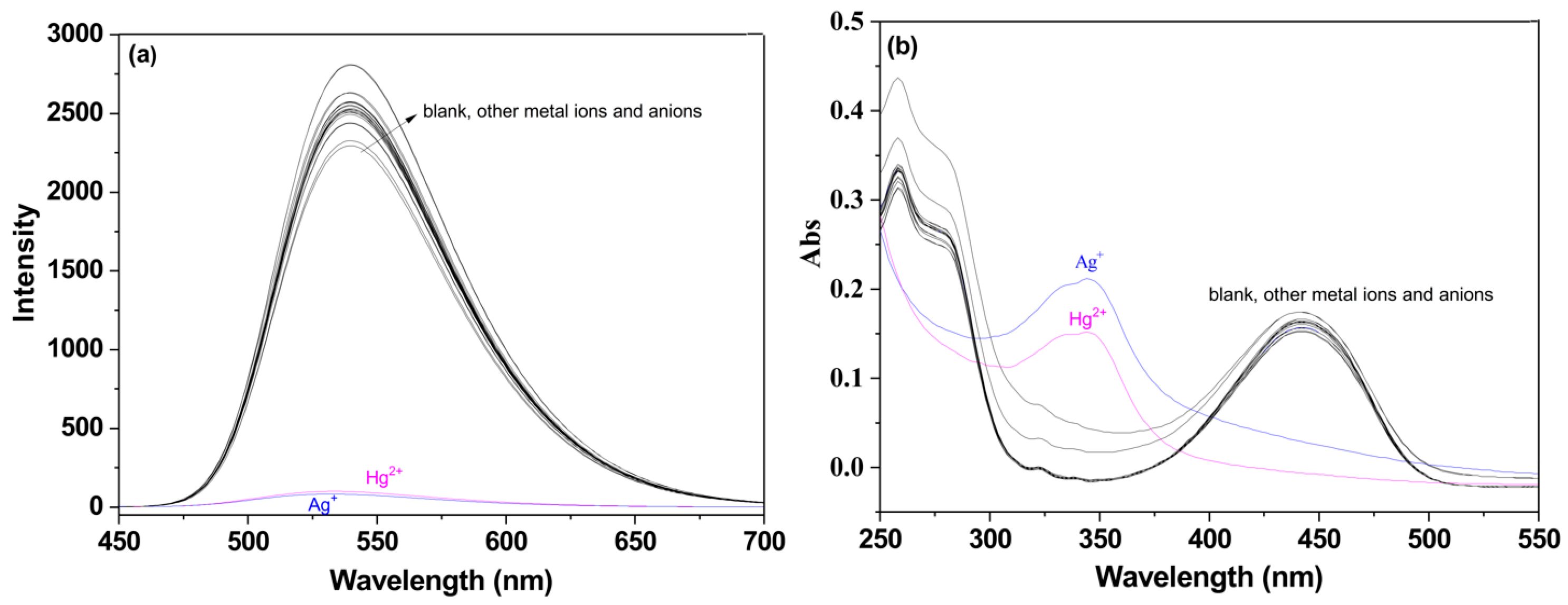
2.2. Competition Analysis of P for Detecting Ag+/Hg2+
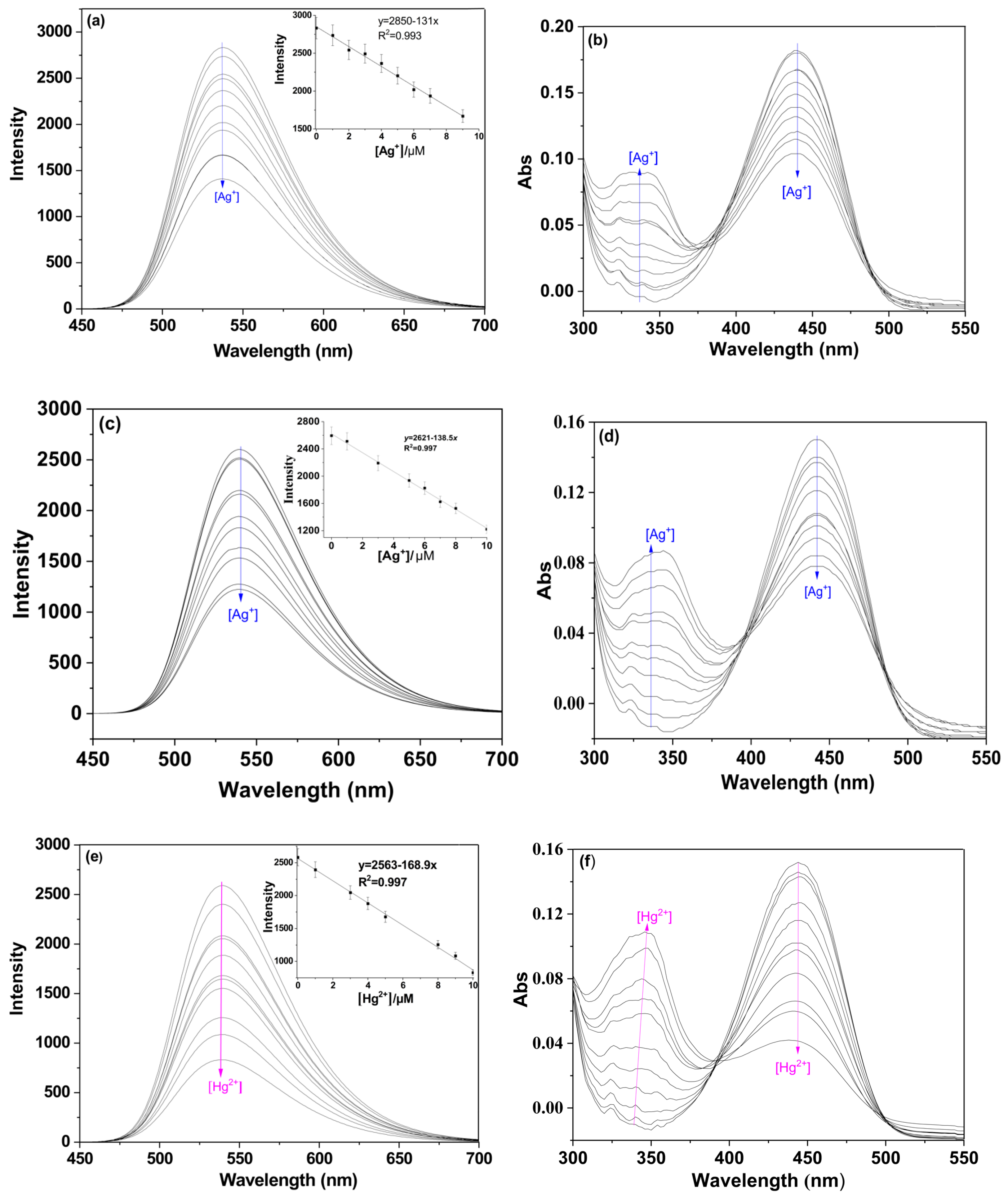
2.3. Sensitivity Analysis of P for Detecting Ag+/Hg2+
2.4. Effect of Solvents, pH, and Time on P to Ag+/Hg2+
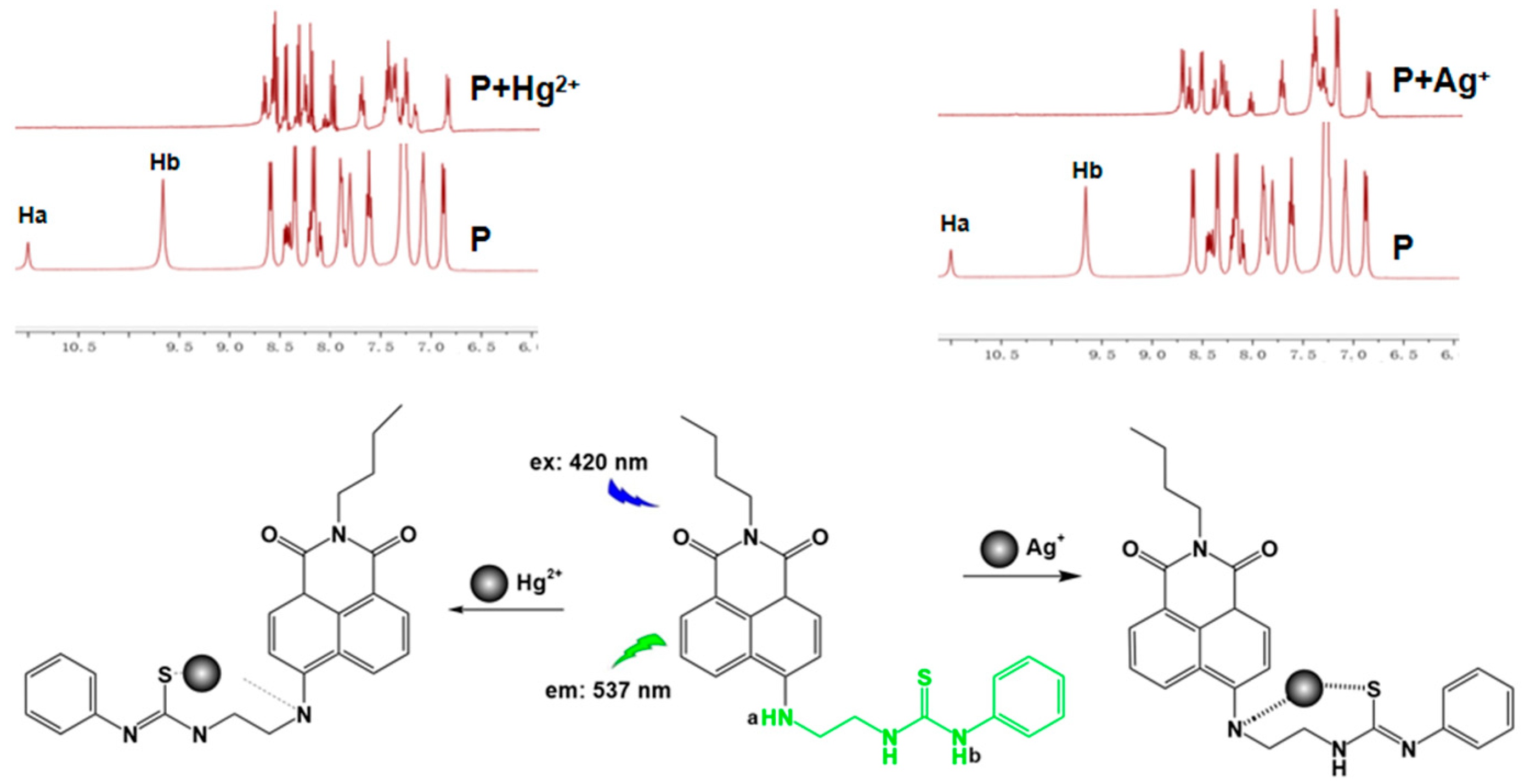
2.5. Mechanism Analysis of P for the Recognition of Ag+/Hg2+
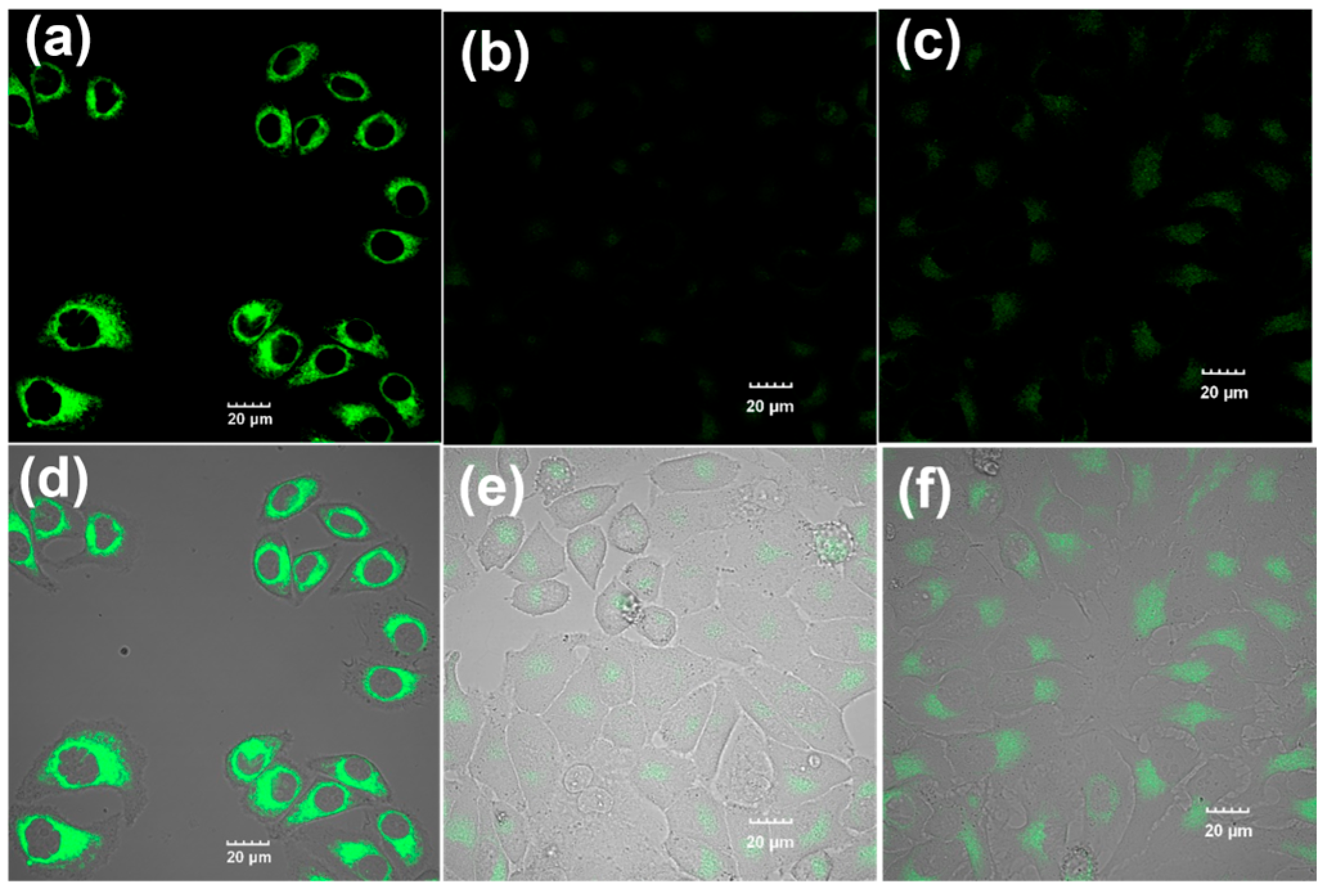
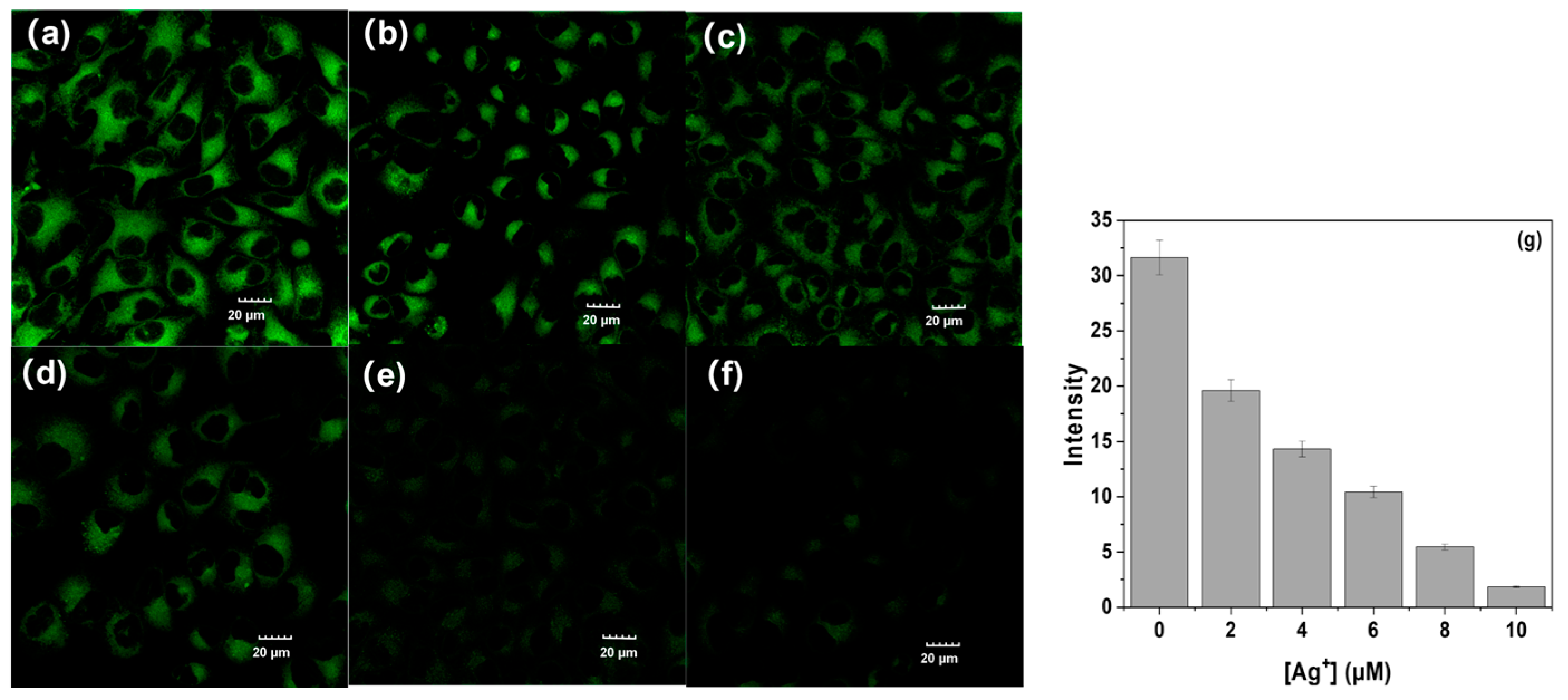
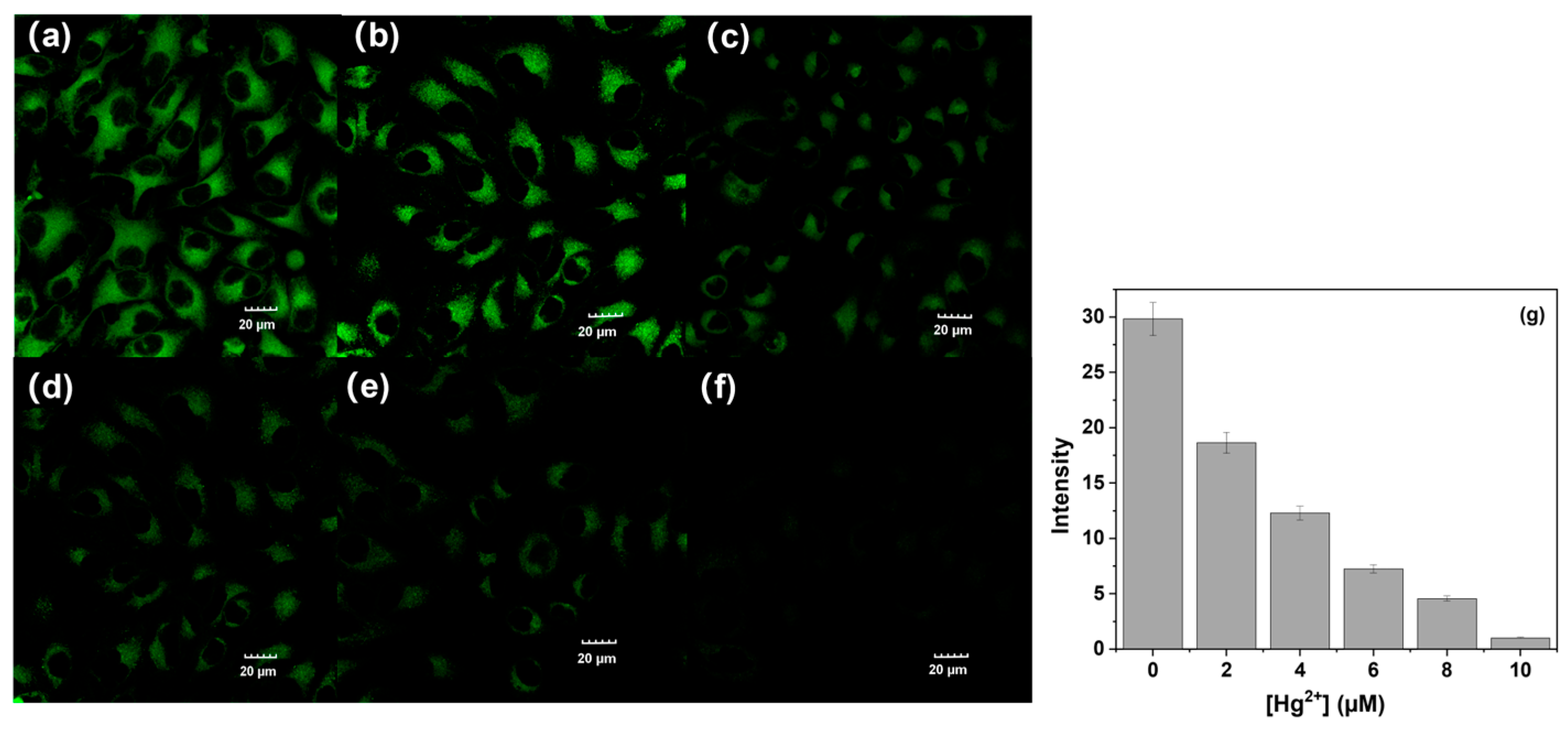
2.6. Cell Imaging
3. Experimental Section
3.1. Reagents and Instruments
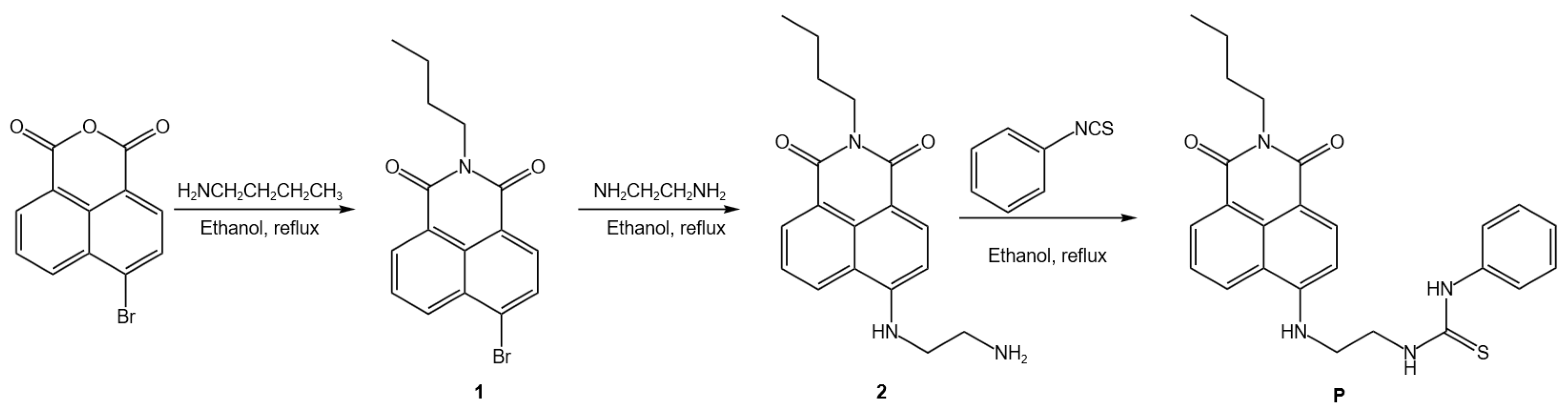
3.2. Synthesis
3.3. General Spectroscopic Methods
3.4. Reagent Addition
3.5. Cell Incubation and Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.J.; Zuo, G.C.; Pan, X.H.; Wei, W.; Qi, X.L.; Su, T.; Dong, W. Nitrogen-doped carbon dots as a fluorescent probe for the highly sensitive detection of Ag+ and cell imaging. Luminescence 2018, 33, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Chew, E.H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system: A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [PubMed]
- Coelho, G.D.; Fontana, K.B.; Maranhao, T.A.; Borges, D.L.G. Dielectric barrier discharge-assisted determination of methylmercury in particulate matter by atomic absorption spectrometry. Anal. Methods 2022, 14, 1371–1377. [Google Scholar] [CrossRef] [PubMed]
- Amiri, N.; Rofouei, M.K.; Ghasemi, J.B. Multivariate optimization, preconcentration and determination of mercury ions with (1-(p-acetyl phenyl)-3-(o-methyl benzoate)) triazene in aqueous samples using ICP-AES. Anal. Methods 2016, 8, 1111–1119. [Google Scholar] [CrossRef]
- Guo, W.; Hu, S.; Zhang, J.; Zhang, H. Elimination of oxide interferences and determination of ultra-trace silver in soils by ICP-MS with ion–molecule reactions. Sci. Total Environ. 2011, 409, 2981–2986. [Google Scholar] [CrossRef]
- Xie, H.F.; Yu, C.J.; Huang, Y.L.; Xu, H.; Zhang, Q.L.; Sun, X.H.; Feng, X.; Redshaw, C. A turn-off fluorescent probe for the detection of Cu2+ based on a tetraphenylethylene-functionalized salicylaldehyde Schiff-base. Mater. Chem. Front. 2020, 4, 1500–1506. [Google Scholar] [CrossRef]
- Pournaki, M.; Fallah, A.; Gü, H.O.; Gazi, M.A. A novel chitosan based fluorescence chemosensor for selective detection of Fe (III) ion in acetic aqueous medium. Mater. Technol. 2021, 36, 91–96. [Google Scholar] [CrossRef]
- Kashyap, K.S.; Kumar, A.; Hira, S.K.; Dey, S. Recognition of Al3+ through the off-on mechanism as a proficient driving force for the hydrolysis of BODIPY conjugated Schiff base and its application in bio-imaging. Inorg. Chim. Acta 2019, 498, 119157. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Han, X.Y.; Zhang, L.W.; Wang, X.Y.; Chen, L.X. Fluorescent probe for mercury ion imaging analysis: Strategies and applications. Chem. Eng. J. 2021, 406, 127166. [Google Scholar] [CrossRef]
- Bahta, M.; Ahmed, N. An AIEE active 1,8-naphthalimide-sulfamethizole probe for ratiometric fluorescent detection of Hg2+ ions in aqueous media. J. Photochem. Photobiol. A 2020, 391, 112354. [Google Scholar]
- Samanta, T.; Shunmugam, R. Colorimetric and fluorometric probes for the optical detection of environmental Hg(II) and As(III) ions. Mater. Adv. 2021, 2, 64–95. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, S.; Yoon, J.; Spring, D.R. Discovery of a highly selective turn-on fluorescent probe for Ag+. Analyst 2010, 135, 2554–2559. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Liu, J.G.; Tan, H.Y.; Yan, J.W.; Zhang, L. A colorimetric and far-red fluorescent probe for the highly sensitive detection of silver (I). RSC Adv. 2017, 7, 55567–55570. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, C.W.; Lu, G.; Fu, Q.Y.; Li, N.; Ji, Y.X. Ag+-selective “off-on” probe based on napthalimide derivative. New J. Chem. 2012, 36, 819–822. [Google Scholar] [CrossRef]
- Huang, Y.; Li, C.F.; Shi, W.J.; Tan, H.Y.; He, Z.Z.; Zheng, L.Y.; Liu, F.G.; Yan, J.W. A near-infrared BODIPY-based fluorescent probe for ratiometric and discriminative detection of Hg2+ and Cu2+ ions in living cells. Talanta 2019, 198, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Yang, Y.; Wang, Y.Z.; Qiu, F.Z.; Song, X.R.; Tang, X.L.; Zhang, G.L.; Liu, W.S. Dual-functional colorimetric fluorescent probe for sequential Cu2+ and S2- detection in bio-imaging. Sens. Actuators B Chem. 2019, 288, 27–37. [Google Scholar] [CrossRef]
- Liao, Z.; Liu, Y.; Han, S.F.; Wang, D.; Zheng, J.Q.; Zheng, X.J.; Jin, L.P. A novel acylhydrazone-based derivative as dual-mode chemosensor for Al3+, Zn2+ and Fe3+ and its applications in cell imaging. Sens. Actuators B Chem. 2017, 244, 914–921. [Google Scholar] [CrossRef]
- Sharma, S.; Chayawan; Debnath, J.; Ghosh, K.S. Method for highly selective, ultrasensitive fluorimetric detection of Cu2+ and Al3+ by Schiff bases containing o-phenylenediamine and o-aminophenol. Methods 2023, 217, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, Z.L.; Li, M.X.; Song, J.; Yang, Y.Q.; Xu, X.; Xu, H.J.; Wang, S.F. A nopinone based multi-functional probe for colorimetric detection of Cu2+ and ratiometric detection of Ag+. Photochem. Photobiol. Sci. 2020, 19, 49–55. [Google Scholar] [CrossRef]
- Rajamohan, R.; Raj, M.R.; Selvamani, T.; Krishnan, M.M.; Govindasamy, C.; Murugan, M.; Lee, Y.R. Chemosensor material as a metal–organic framework with potassium-based perylene tetracarboxylic acid for copper and lead detection. J. Mol. Liq. 2024, 408, 125376. [Google Scholar] [CrossRef]
- Zhang, X.C.; Shi, W.; Chen, X.; Xie, Z.F. Isocyano-functionalized, 1, 8-naphthalimide-based chromophore as efficient ratiometric fluorescence probe for Hg2+ in aqueous medium. Sens. Actuators B Chem. 2018, 255, 3074–3084. [Google Scholar] [CrossRef]
- Kumari, R.; Sunil, D.; Ningthoujam, R.S. Naphthalimides in fluorescent imaging of tumor hypoxia-An up-to-date review. Bioorg. Chem. 2019, 88, 102979. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Zhang, J.; Ding, M.Y.; Chen, L.X. Silver(I) ion detection in aqueous media based on “off-on” fluorescent probe. Anal. Methods 2012, 4, 342–344. [Google Scholar] [CrossRef]
- Song, K.C.; Kim, J.S.; Park, S.M.; Chung, K.C.; Ahn, S.; Chang, S.K. Fluorogenic Hg2+-selective chemodosimeter derived from 8-hydroxyquinoline. Org. Lett. 2006, 8, 3413–3416. [Google Scholar] [CrossRef] [PubMed]
- Haldar, U.; Lee, H.I. BODIPY-derived polymeric chemosensor appended with thiosemicarbazone units for the simultaneous detection and separation of Hg (II) ions in pure aqueous media. ACS Appl. Mater. Interfaces 2019, 11, 13685–13693. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Ji, Y.X.; Wen, S.B.; Zhang, J. Synthesis and characterization of a Mg2+-selective probe based on benzoyl hydrazine derivative and its application in cell imaging. Molecules 2021, 26, 2457. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zheng, T.; Xu, Z.; Li, J.; Li, H.; Tang, J.; Liu, S.; Wang, Y. New NIR spectroscopic probe with a large Stokes shift for Hg2+ and Ag+ detection and living cells imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 271, 120916. [Google Scholar] [CrossRef]
- Li, X.; Liu, K.Q.; Duan, L.; Cheng, X.J. Reaction-based fluorescent silk probes with high sensitivity and selectivity to Hg2+ and Ag+ ions. J. Mater. Chem. C 2021, 9, 4877. [Google Scholar]
- Ye, F.; Liang, X.M.; Xu, K.X.; Pang, X.X.; Chai, Q.; Fu, Y. A novel dithiourea-appended naphthalimide “on-off” fluorescent probe for detecting Hg2+ and Ag+ and its application in cell imaging. Talanta 2019, 200, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Han, X.; Qi, Y.; Jiang, L.; Song, Y.; Ma, Y.; Zhang, J.; Li, H. A novel near-infrared fluorescent and colorimetric probe for selective detection of Ag+ and Hg2+. Color. Technol. 2024, 140, 30–41. [Google Scholar] [CrossRef]
- Singh, R.; Das, G. Fluorogenic detection of Hg2+ and Ag+ ions via two mechanistically discrete signal genres: A paradigm of differentially responsive metal ion sensing. Sens. Actuators B Chem. 2018, 258, 478–483. [Google Scholar] [CrossRef]
- Nandre, J.P.; Patil, S.R.; Sahoo, S.K.; Pradeep, C.P.; Churakov, A.; Yu, F.B.; Chen, L.X.; Redshaw, C.; Patil, A.A.; Patil, U.D. A chemosensor for micro- to nano-molar detection of Ag+ and Hg2+ ions in pure aqueous media and its applications in cell imaging. Dalton Trans. 2017, 46, 14201–14209. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, W.J.; Yan, M.M.; Tu, Q.; Chen, S.W.; Li, T.B.; Yuan, M.S.; Wang, J.Y. 2-Hydroxy benzothiazole modified rhodol: Aggregation-induced emission and dual-channel fluorescence sensing of Hg2+ and Ag+ ions. Sens. Actuators B Chem. 2018, 255, 2086–2094. [Google Scholar] [CrossRef]
- Chen, Z.E.; Zhang, H.; Iqbal, Z. A new thiosemicarbazone fluorescent probe based on 9,9′-anthracece for Hg2+ and Ag+. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 215, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Cáceres, M.I.; Agbaria, R.A.; Warner, I.M. Fluorescence of metal-ligand complexes of mono-and di-substituted naphthalene derivatives. J. Fluoresc. 2005, 15, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.W.; Wen, Y.Y.; Qin, X.; Zhang, J. A fluorescent ratiometric Cu2+ probe based on FRET by naphthalimide-appended rhodamine derivatives. Anal. Methods 2014, 6, 9825–9830. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, C.; Li, X.; Yang, M.; Xie, Y.; Zhang, J. Detection of Silver and Mercury Ions Using Naphthalimide-Based Fluorescent Probe. Molecules 2024, 29, 5196. https://doi.org/10.3390/molecules29215196
Yu C, Li X, Yang M, Xie Y, Zhang J. Detection of Silver and Mercury Ions Using Naphthalimide-Based Fluorescent Probe. Molecules. 2024; 29(21):5196. https://doi.org/10.3390/molecules29215196
Chicago/Turabian StyleYu, Chunwei, Xiangxiang Li, Mei Yang, Yinghao Xie, and Jun Zhang. 2024. "Detection of Silver and Mercury Ions Using Naphthalimide-Based Fluorescent Probe" Molecules 29, no. 21: 5196. https://doi.org/10.3390/molecules29215196
APA StyleYu, C., Li, X., Yang, M., Xie, Y., & Zhang, J. (2024). Detection of Silver and Mercury Ions Using Naphthalimide-Based Fluorescent Probe. Molecules, 29(21), 5196. https://doi.org/10.3390/molecules29215196




