A Novel Delivery System for the Combined Use of Natural Ingredients: The Preparation of Berberine Hydrochloride–Matrine Liposomes and Preliminary Exploration of Their Anti-Tumor Activity
Abstract
1. Introduction
2. Results
2.1. The Results of Preparation of Liposomes
2.1.1. Screening Results of Preparation Methods of BH-MT Liposomes
2.1.2. Single-Factor Screening Results of BH-MT-LP
The Effect of MT Content on EE and DL
The Effect of BH Content on EE and DL
The Effect of Lipid Ratio on EE and DL
The Effect of Oil–Water Volume Ratio on EE and DL
The Effect of Hydration Volume on EE and DL
2.1.3. Box–Behnken Experimental Design (BBD) Method Design and Results Analysis
2.2. Characterization of BH-MT-LP
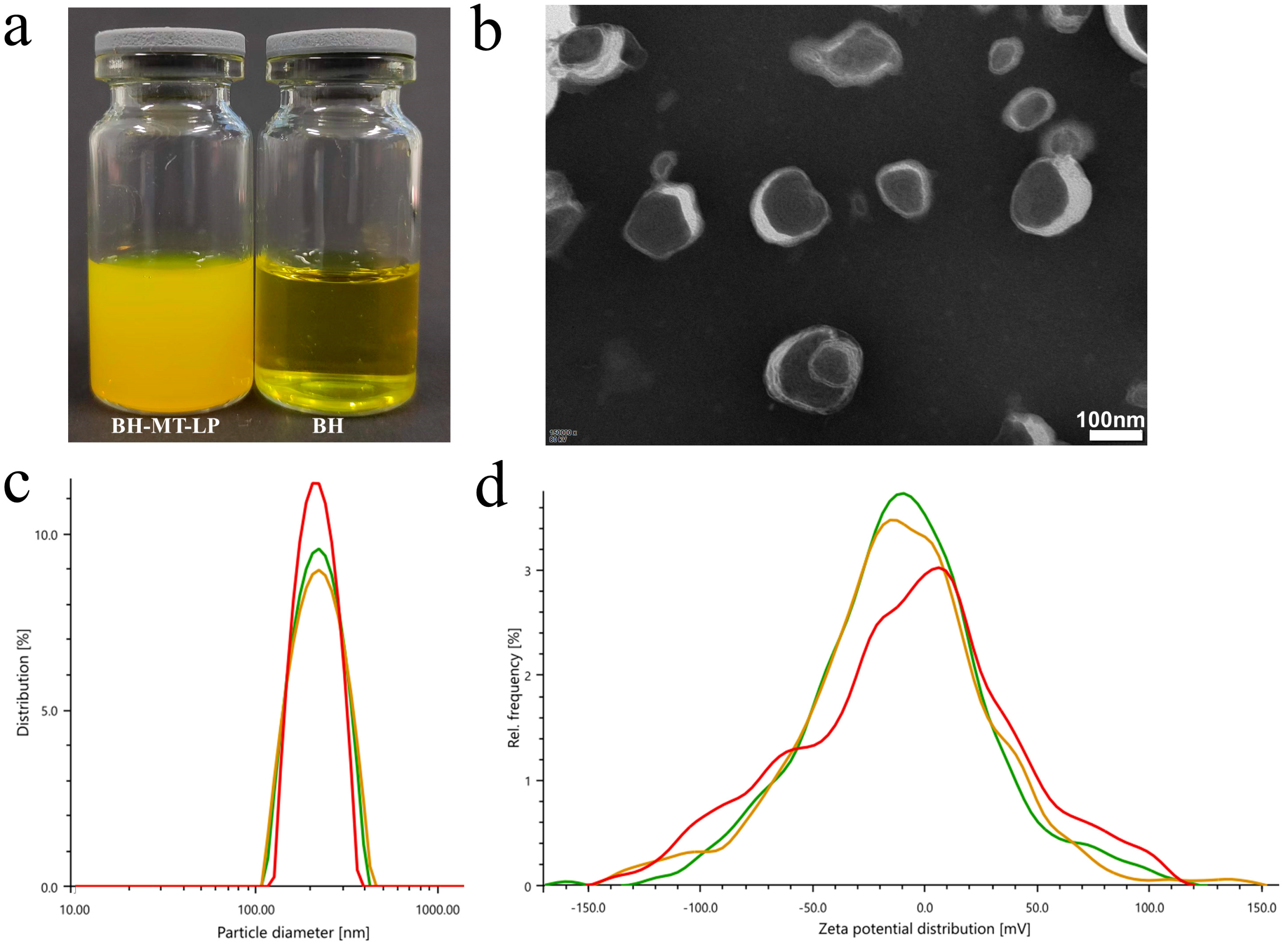
2.2.1. Appearance Form
2.2.2. Morphology Analysis Using Transmission Electron Microscope
2.2.3. Particle Size and Potential
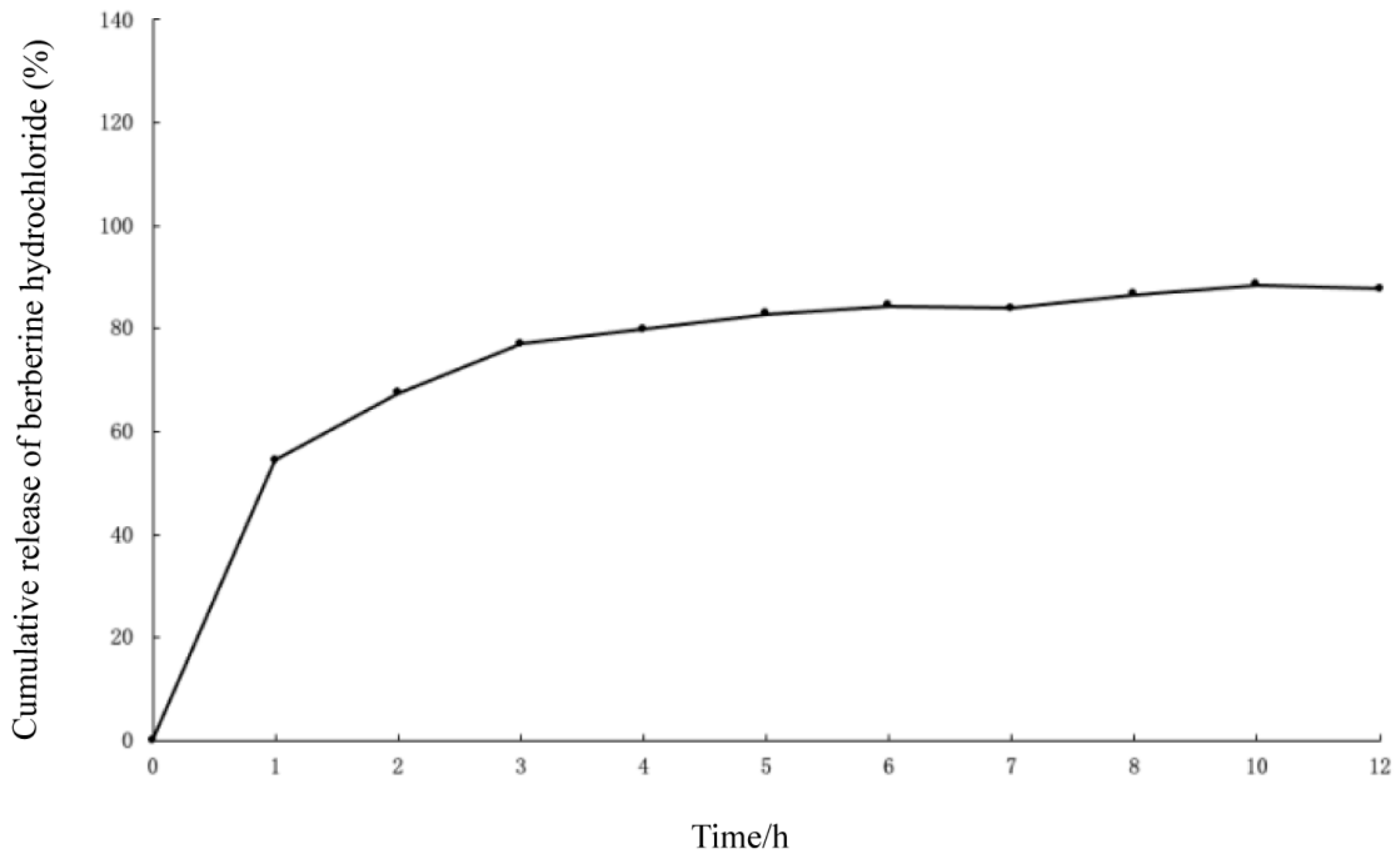
2.3. In Vitro Release of BH-MT-LP
2.4. Anti-Tumor Results
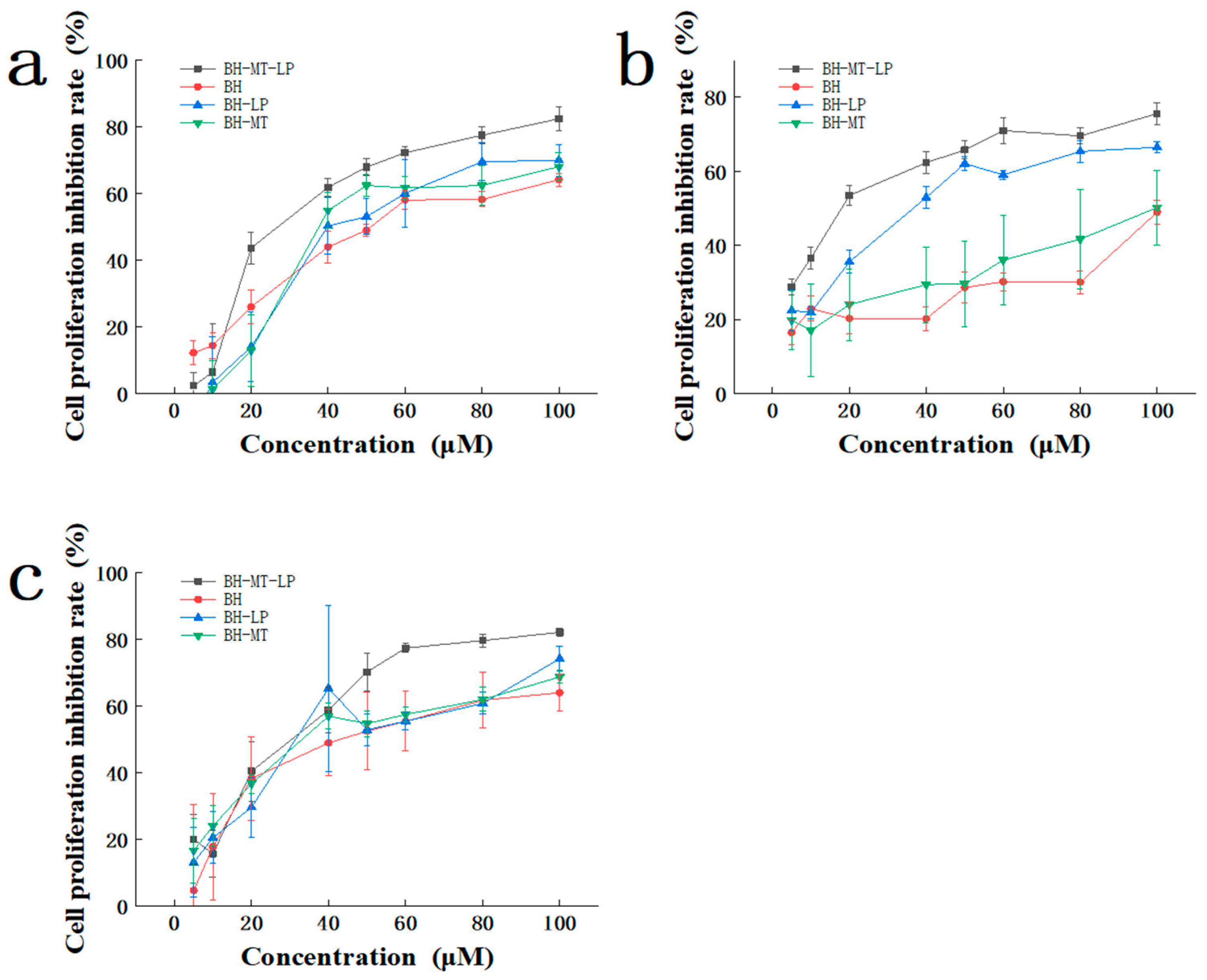
2.4.1. Cytotoxicity of BH-MT-LP in Different Cell Lines
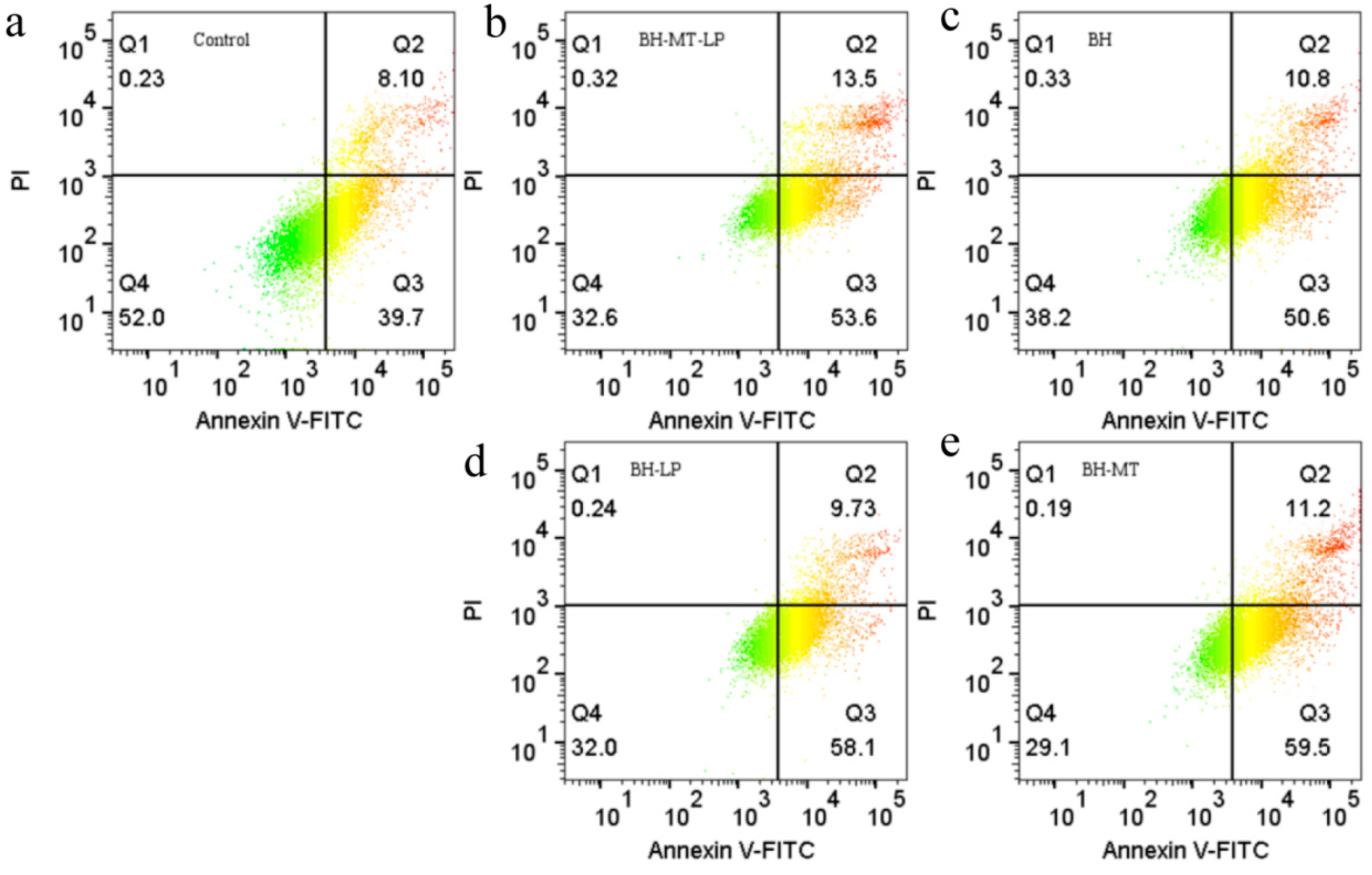
2.4.2. The Effect of BH-MT-LP on Cell Apoptosis
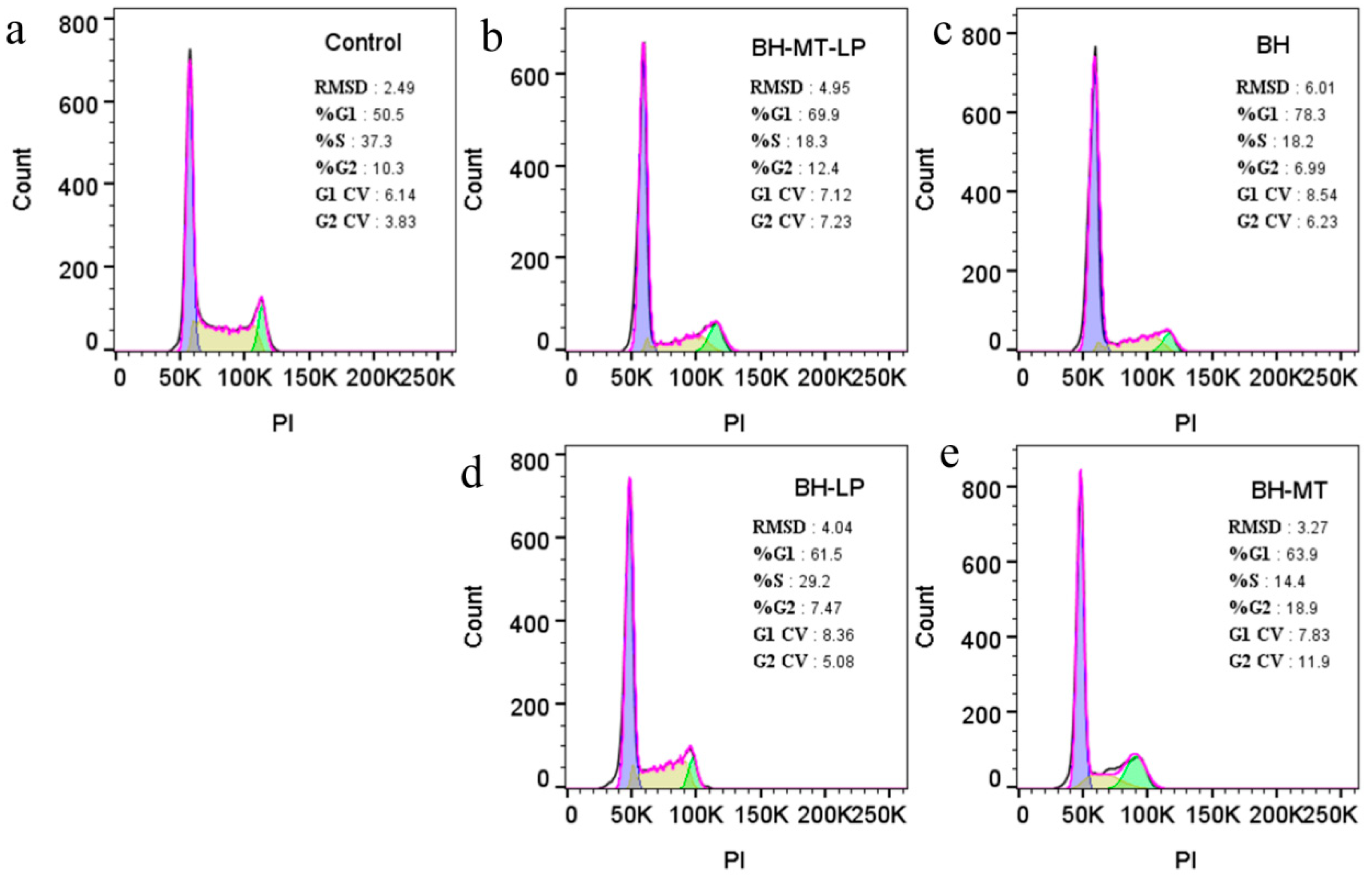
2.4.3. The Effect of BH-MT-LP on Cell Cycle
3. Discussion
3.1. BH-MT-LP Process Method Screening and Optimization
3.2. Results of Anti-Tumor Experiments
3.2.1. Cytotoxicity Experiments
3.2.2. Cell Apoptosis Experiments
3.2.3. Cell Cycle Experiments
4. Materials and Methods
4.1. Materials and Cell Lines
4.2. Preparation of Liposomes
4.2.1. Determination Method for EE and DL
Determination of BH Content by HPLC Method
Determination of Free Drug Content
Total Drug Content Determination
4.2.2. Screening Results of Preparation Methods of BH-MT-LP
Thin-Film Dispersion Method
Reverse Evaporation Method
Ether Injection Method
Ammonium Sulfate Gradient Method
4.2.3. Single-Factor Screening Experiment of BH-MT-LP
The Effect of MT Content on EE and DL
The Effect of BH Content on EE and DL
The Effect of Lipid Ratio on EE and DL
The Effect of Oil–Water Volume Ratio on EE and DL
The Effect of Hydration Volume on EE and DL
4.2.4. BBD Study
4.2.5. Characterization of BH-MT-LP
Appearance Form
Morphology of Transmission Electron Microscope
Particle Size and Potential
In Vitro Release of BH-MT-LP
4.3. Anti-Tumor Experiments
4.3.1. Cytotoxicity Test
4.3.2. Determination of Cell Apoptosis
4.3.3. Cell Cycle Detection
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Metwaly, A.M.; Ghoneim, M.M.; Eissa, I.H.; Elsehemy, I.A.; Dou, D. Traditional ancient Egyptian medicine: A review. Saudi J. Biol. Sci. 2021, 28, 5823–5832. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Li, Y.B.; Wang, Y.L.; Wang, Y.M.; Xu, Y.Y.; Liu, C.X. Research progress and supervision considerations of rationamedication of traditional Chinese medicine based on compatibility and interactions. Chin. Tradit. Herb. Drugs 2023, 54, 375–385. [Google Scholar]
- Wu, P. Shennong’s Herbal Classic; Shanxi Science and Technology Publishing House: Shanxi, China, 2010. [Google Scholar]
- Zhang, S.L. Materia Medica; Shanxi Science and Technology Publishing House: Shanxi, China, 1920. [Google Scholar]
- Commission, C.P. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2020. [Google Scholar]
- Ling, Y.K. Chinese Materia Medica; Shanghai Scientific & Technical Publishers: Shanghai, China, 1984. [Google Scholar]
- Liu, C.; Teng, S. Pu Ji Fang; People’ Medicinal Publishing House: Beijing, China, 1984. [Google Scholar]
- Shen, Z.W. Jiewei Yuansou; Shanghai Scientific & Technical Publishers: Shanghai, China, 1959. [Google Scholar]
- Zhao, M.; Chi, H.J.; Mou, W.L.; Cui, B. Study on antifungal activities of hydrochloric berberine against oralCandida and the inhibitory effects on growth of human tongecarcinoma cells Tca8113 in vitro. J. Pract. Stomatol. 2009, 25, 4. [Google Scholar]
- Tabeshpour, J.; Imenshahidi, M.; Hosseinzadeh, H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran. J. Basic Med. Sci. 2017, 20, 557–568. [Google Scholar] [PubMed]
- Tian, H.; Fu, X.; Wang, H. A study on the effects of berberine hydrochloride on proliferation, apoptosis, autophagy, and MAPK pathway in gastric cancer SGC-7901 cells. Mod. J. Integr. Tradit. Chin. West. Med. 2021, 30, 6. [Google Scholar]
- Tan, H.L.; Chan, K.G.; Pusparajah, P.; Duangjai, A.; Saokaew, S.; Mehmood Khan, T.; Lee, L.H.; Goh, B.H. Rhizoma Coptidis: A Potential Cardiovascular Protective Agent. Front. Pharmacol. 2016, 7, 362. [Google Scholar] [CrossRef]
- Wang, M.C.; Yi, H.X.; Zhan, Z.X.; Feng, Z.T.; Yang, G.G.; Zhang, Y.; Zhang, D.Y. A self-assembled nanomedicine for glucose supply interruption-amplified low-temperature photothermal therapy and anti-prometastatic inflammatory processes of triple-negative breast cancer. Aggregate 2024, e622. [Google Scholar] [CrossRef]
- Zhi, X.; Chen, X.; Su, J.C. Research progress on the pharmacological effects of matrine. J. Chengdu Univ. Tradit. Chin. Med. 2017, 40, 5. [Google Scholar]
- Zhu, J.L. The Effect of Matrine on The Formation of Biofilm of Staphylococcus Epidermidis; Ningxia University: Ningxia, China, 2014. [Google Scholar]
- Zhang, X.; Hou, G.Q.; Liu, A.D.; Xu, H.; Cao, X. Matrine inhibits the development and progression of ovarian cancer by repressing cancer associated phosphorylation signaling pathways. Cell Death Dis. 2019, 10, 770. [Google Scholar] [CrossRef]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef]
- He, W.; Guo, X.X. Comparative Study on Transdermal Absorption of Nimesulide by Liposome and Liposome gel. China Pharm. 2022, 25, 5. [Google Scholar]
- Zhang, D.Y.; Liang, Y.; Wang, M.; Younis, M.R.; Yi, H.; Zhao, X.; Chang, J.; Zheng, Y.; Guo, W.; Yu, X. Self-Assembled Carrier-Free Nanodrugs for Starvation Therapy-Amplified Photodynamic Therapy of Cancer. Adv. Healthc. Mater. 2023, 12, e22031772023. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, P.L.; Guo, W.B.; Huang, X.M.; Lei, H.M. Natural Berberine-Based Chinese Herb Medicine Assembled Nanostructures with Modified Antibacterial Application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.X.; Liu, C.; Hou, Y.; Liu, Y.; Zheao, Z.; Zhao, H.; Xin, X.; Liu, W.; Zhang, X.T.; Chen, L.Q.; et al. Sequential Delivery of Quercetin and Paclitaxel for the Fibrotic Tumor Microenvironment Remodeling and Chemotherapy Potentiation via a Dual-Targeting Hybrid Micelle-in-Liposome System. ACS Appl. Mater. Interfaces 2022, 14, 10102–10116. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.Y.; Lai, Y.F.; Hsieh, Y.L.; Wu, C.H.; Chiu, C.C.; Yang, Y.M. Divergent effects of cholesterol on the structure and fluidity of liposome and catanionic vesicle membranes. FEBS Lett. 2022, 596, 1827–1838. [Google Scholar] [CrossRef]
- Potter, T.D.; Haywood, N.; Teixeira, A.; Hodges, G.; Barrett, E.L.; Miller, M.A. Partitioning into phosphatidylcholine-cholesterol membranes: Liposome measurements, coarse-grained simulations, and implications for bioaccumulation. Environ. Sci. Process. Impacts 2023, 25, 1082–1093. [Google Scholar] [CrossRef]
- Elkady, E.F.; Fouad, M.A.; Mozayad, A.N. Application of Box-Behnken experimental design and response surface methodology for selecting the optimum RP-HPLC conditions for the simultaneous determination of methocarbamol, indomethacin and betamethasone in their pharmaceutical dosage form. BMC Chem. 2022, 16, 114. [Google Scholar] [CrossRef]
- Soliman, N.M.; Shakeel, F.; Haq, N.; Alanazi, F.K.; Alshehri, S.; Bayomi, M.; Alenazi, A.S.M.; Alsarra, I.A. Development and Optimization of Ciprofloxacin HCl-Loaded Chitosan Nanoparticles Using Box-Behnken Experimental Design. Molecules 2022, 27, 4468. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef]
- Wang, H.; Ding, Y.; Zhang, W.; Wei, K.; Pei, Y.; Zou, C.; Zhang, C.; Ding, J.; Fang, H.; Tan, S. Oxymatrine Liposomes for Intervertebral Disc Treatment: Formulation, in vitro and vivo Assessments. Drug Des. Dev. Ther. 2020, 14, 921–931. [Google Scholar] [CrossRef]
- Pandey, R.; Vidi, J.; Riya, M.; Chang, C.M. Experimental Methods for the Biological Evaluation of Nanoparticle-Based Drug Delivery Risks. Pharmaceutics 2023, 15, 612. [Google Scholar] [CrossRef]
- Jamasbi, E.; Hamelian, M.; Hossain, M.A.; Varmira, K. The cell cycle, cancer development and therapy. Mol. Biol. Rep. 2022, 49, 10875–10883. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z. Cell Cycle Progression and Synchronization: An Overview. In Methods in Molecular Biology; Clifton, N.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 2579, pp. 3–23. [Google Scholar] [CrossRef]
- Khan, M.J.; Hafeez, A.; Siddiqui, M.A. Nanocarrier Based Delivery of Berberine: A Critical Review on Pharmaceutical and Preclinical Characteristics of the Bioactive. Curr. Pharm. Biotechnol. 2023, 24, 1449–1464. [Google Scholar] [CrossRef] [PubMed]
- Dymek, M.; Sikora, E. Liposomes as biocompatible and smart delivery systems—The current state. Adv. Colloid Interface Sci. 2022, 309, 102757. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine hydrochloride: Anticancer activity and nanoparticulate delivery system. Int. J. Nanomed. 2011, 6, 1773–1777. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Z.; Dai, Y.H.; Wang, D.K. Research progress in preparation methods and applications of liposomes. Chin. J. Pharm. 2024, 22, 14–24. [Google Scholar]
- Wen, C.; Wu, L.; Fu, L.; Zhang, X.; Zhou, H. Berberine enhances the anti-tumor activity of tamoxifen in drug-sensitive MCF-7 and drug-resistant MCF-7/TAM cells. Mol. Med. Rep. 2016, 14, 2250–2256. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, X.; Cao, S.; Sun, Y.; He, X.; Jiang, B.; Yu, Y.; Duan, J.; Qiu, F.; Kang, N. Berberine represses human gastric cancer cell growth in vitro and in vivo by inducing cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt signaling pathways. Biomed. Pharmacother. 2020, 128, 110245. [Google Scholar] [CrossRef]
- Wang, N.; Feng, Y.; Zhu, M.; Tsang, C.M.; Man, K.; Tong, Y.; Tsao, S.W. Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: The cellular mechanism. J. Cell. Biochem. 2010, 111, 1426–1436. [Google Scholar] [CrossRef]
- Cao, X.; He, Q. Anti-Tumor Activities of Bioactive Phytochemicals in Sophora flavescens for Breast Cancer. Cancer Manag. Res. 2020, 12, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Yu, Y.; Tang, W.; Zhou, S.; Lv, X. Matrine exerts an anti-tumor effect via regulating HN1 in triple breast cancer both in vitro and in vivo. Chem. Biol. Drug Des. 2023, 102, 1469–1477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Liu, J.; Fang, B.; Yao, J.; Cheng, B. Matrine inhibits the invasive and migratory properties of human hepatocellular carcinoma by regulating epithelial-mesenchymal transition. Mol. Med. Rep. 2018, 18, 911–919. [Google Scholar] [CrossRef]
- Luo, C.; Zhong, H.J.; Zhu, L.M.; Wu, X.G.; Ying, J.E.; Wang, X.H.; Lü, W.X.; Xu, Q.; Zhu, Y.L.; Huang, J. Inhibition of matrine against gastric cancer cell line MNK45 growth and its anti-tumor mechanism. Mol. Biol. Rep. 2012, 39, 5459–5464. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yao, L.L.; Wei, K.; Zhou, B.; Bian, M.Q.; Li, H.J.; Zhan, C. Determination of Matrine and Berberine Hydrochloride in KushenGonglao Granules by UPLC. Chin. J. Vet. Drug 2023, 57, 54–59. [Google Scholar]
- Ai, Z.L. The Antioxidative Effects of Baicalin, Matrine or Berberine on L-FABP In Vivo and Vitro; Tsinghua University School of Medicine, Peking Union Medical College, Chinese Academy of Medical Sciences: Beijing, China, 2012. [Google Scholar]
- Xie, G.Y.; Wang, C.Z.; Zhu, L.L.; Sun, Z.X.; Zhang, X.F.; Zhang, H.X.; Cheng, H.J. Effects of Berberine and Matrine against Formation of Mixed Biofilmsof Vulvovaginal Candidiasis. J. Anhui Univ. Chin. Med. 2012, 31, 52–55. [Google Scholar]
- Zhu, Y.; Xie, N.; Chai, Y.; Nie, Y.; Liu, K.; Liu, Y.; Yang, Y.; Su, J.; Zhang, C. Apoptosis Induction, a Sharp Edge of Berberine to Exert Anti-Cancer Effects, Focus on Breast, Lung, and Liver Cancer. Front. Pharmacol. 2022, 13, 803717. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.D.; Hong, J.; Liu, C.; Zhang, X.L.; Zheng, J.P.; Xu, Y.J.; Ou, Z.Y.; Zheng, J.L.; Yu, D.J. Inhibitory Effect of Two Traditional Chinese Medicine Monomers, Berberine and Matrine, on the Quorum Sensing System of Antimicrobial-Resistant Escherichia coli. Front. Microbiol. 2019, 10, 2584. [Google Scholar] [CrossRef]
- Meng, J.; Wang, W.; Ding, J.; Gu, B.; Zhou, F.; Wu, D.; Fu, X.; Qiao, M.; Liu, J. The synergy effect of matrine and berberine hydrochloride on treating colibacillosis caused by an avian highly pathogenic multidrug-resistant Escherichia coli. Poult. Sci. 2024, 103, 104151. [Google Scholar] [CrossRef]
- Duong, T.T.; Isomäki, A.; Paaver, U.; Laidmäe, I.; Tõnisoo, A.; Yen, T.T.H.; Kogermann, K.; Raal, A.; Heinämäki, J.; Pham, T.M. Nanoformulation and Evaluation of Oral Berberine-Loaded Liposomes. Molecules 2021, 26, 2591. [Google Scholar] [CrossRef]
- Tan, X.; Hao, Y.; Ma, N.; Yang, Y.; Jin, W.; Meng, Y.; Zhou, C.; Zheng, W.; Zhang, Y. M6P-modified solid lipid nanoparticles loaded with matrine for the treatment of fibrotic liver. Drug Deliv. 2023, 30, 2219432. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.G.; Huang, S.Y.; Wu, S.X.; Zhou, D.D.; Yang, Z.J.; Saimaiti, A.; Zhao, C.N.; Shang, A.; Zhang, Y.J.; Gan, R.Y.; et al. Anticancer Effects and Mechanisms of Berberine from Medicinal Herbs: An Update Review. Molecules 2022, 27, 4523. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, H.; Chen, W.; Huang, P. Berberine induces apoptosis and arrests the cell cycle in multiple cancer cell lines. Arch. Med. Sci. AMS 2023, 19, 1530–1537. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Chen, J.Z. Chinese Medicinal Chemistry; The Medicine Science and Technology Press of China: Beijing, China, 2024. [Google Scholar]
- Zhou, T.; Liu, Y.; Lei, K.; Liu, J.; Hu, M.; Guo, L.; Guo, Y.; Ye, Q. A “Trojan Horse” Strategy: The Preparation of Bile Acid-Modifying Irinotecan Hydrochloride Nanoliposomes for Liver-Targeted Anticancer Drug Delivery System Study. Molecules 2023, 28, 1577. [Google Scholar] [CrossRef]
- Joshi, S.; Cooke, J.R.; Chan, D.K.; Ellis, J.A.; Hossain, S.S.; Singh-Moon, R.P.; Wang, M.; Bigio, I.J.; Bruce, J.N.; Straubinger, R.M. Liposome size and charge optimization for intraarterial delivery to gliomas. Drug Deliv. Transl. Res. 2016, 6, 225–233. [Google Scholar] [CrossRef]
- Tan, Y.J. Study on Preparation of Cholic Acid-Baicalin Nasal Liposomeand Its Protective Effect on Cerebral Ischemia Reperfusioninjury in Rats; Chengdu University of TCM: Sichuan, China, 2019. [Google Scholar]
- Cazzolla, A.; Mondala, J.R.M.; Wanigasekara, J.; Carroll, J.; Daly, N.; Tiwari, B.; Casey, A.; Curtin, J.F. Synthesis of cationic liposome nanoparticles using a thin film dispersed hydration and extrusion method. PLoS ONE 2024, 19, e03004672024. [Google Scholar] [CrossRef]
- Wang, J.Z.; Li, Y.N.; Xiong, Y.R.; Shen, Y.; Tu, J.S.; Sun, C.M. Preparation of oxaliplatin liposome and in vitro drug-release. J. Pharm. Res. 2016, 35, 6. [Google Scholar]
- Liu, W.Y.; Liu, H.Q. Preparation of Matrine Liposomes by Active Loading Method. Lishizhen Med. Mater. Medica Res. 2007, 18, 2. [Google Scholar]
- Liu, L.C.; Chen, G. Preparation of Berberine Hydrochloride Liposomes and Its Release In Vitro. Pharm. Clin. Res. 2013, 21, 6. [Google Scholar]
- Smith, L.J.; Kukanich, B.K.; Krugner-Higby, L.A.; Schmidt, B.H.; Heath, T.D. Pharmacokinetics of ammonium sulfate gradient loaded liposome-encapsulated oxymorphone and hydromorphone in healthy dogs. Vet. Anaesth. Analg. 2013, 40, 537–545. [Google Scholar] [CrossRef]
- Hamzah, M.; Omranian, S.; Golchin, B.; Hainin, M. Evaluation of effects of extended short-term aging on the rheological properties of asphalt binders at intermediate temperatures using respond surface method. J. Teknol. 2015, 73, 133–139. [Google Scholar] [CrossRef][Green Version]
- Zheng, Y.Z.; Manh, T.; Nam, N.; Gerdroodbary, M.B.; Tlili, I. Optimization of micro Knudsen gas sensor for high precision detection of SO2 in natural gas. Results Phys. 2020, 16, 102933. [Google Scholar] [CrossRef]
- Chua, S.C.; Chong, F.K.; Yen, C.H.; Ho, Y.C. Evaluation and optimization of the coagulation-flocculation process using conventional rice starch in potable water treatment. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 072009. [Google Scholar] [CrossRef]
- Chan, Y.T.; Chin, M.C.; Ling, N. Application of Box-Behnken design in optimization of ultrasound effect on apple pectin as sugar replacer. LWT-Food Sci. Technol. 2019, 115, 108449. [Google Scholar] [CrossRef]
- Ma, X.N.; Yang, S.Q.; Zhang, J.C.; Liu, C. Research Progress on Pharmacological Action of Kushen(Sophorae Flavescentis Radix). J. Liaoning Univ. Tradit. Chin. Med. 2023, 25, 152–156. [Google Scholar] [CrossRef]
- Tchin, D.; Mazamaesso, T.; Toukilnan, D.; Tcharié, E.L.; Gado, N.K. Breast Cancer: Knowledge, Attitudes on Risk Factors and Means of Screening by Medical Students from Lomé, Togo. Adv. Breast Cancer Res. 2020, 9, 127–137. [Google Scholar]
- Zhou, Z.W.; Wu, H.; Yang, R.X.; Xu, A.; Sun, M.J. GSH depletion liposome adjuvant for augmenting the photothermal immunotherapy of breast cancer. Sci. Adv. 2020, 6, eabc4373. [Google Scholar] [CrossRef]
- Wang, X.W.; Wang, X.Y.; Yue, Q.F.; Xu, H.Z.; Cheng, L. Liquid exfoliation of TiN nanodots as novel sonosensitizers for photothermal-enhanced sonodynamic therapy against cancer. Nano Today 2021, 39, 101170. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Wang, X.; Li, J.X.; Hu, J.; Cheng, L.; Yang, X. ROS-based dynamic therapy synergy with modulating tumor cell-microenvironment mediated by inorganic nanomedicine. Coord. Chem. Rev. 2021, 437, 213828. [Google Scholar] [CrossRef]
- Wang, R. Construction and Quality Evaluation of PEG-PCL Nanocarrier System Loaded with Baicalin; Zhengzhou University: Zhengzhou, China.
- Wang, L.; Li, H.T.; Wang, S.P.; Liu, R.; Wu, Z.S.; Wang, C.M.; Wang, Y.T.; Chen, M.W. Enhancing the Antitumor Activity of Berberine Hydrochloride by Solid Lipid Nanoparticle Encapsulation. AAPS PharmSciTech 2014, 15, 834–844. [Google Scholar] [CrossRef]
- Xia, S.; Ma, L.; Wang, G.; Yang, J.; Zhang, M.; Wang, X.; Su, J.; Xie, M. In vitro Antimicrobial Activity and the Mechanism of Berberine Against Methicillin-Resistant Staphylococcus aureus Isolated from Bloodstream Infection Patients. Infect. Drug Resist. 2022, 15, 1933–1944. [Google Scholar] [CrossRef] [PubMed]



| Preparation Method | EE (%) | DL (%) |
|---|---|---|
| Thin-film dispersion method | 86.91 | 6.29 |
| Reverse evaporation method | 94.17 | 6.99 |
| Ether injection method | 91.74 | 6.44 |
| Ammonium sulfate gradient method | 79.33 | 5.94 |
| Group | A | B | C | EE (%) | DL (%) |
|---|---|---|---|---|---|
| 1 | 10 | 1:1 | 3 | 90.12 | 6.42 |
| 2 | 10 | 1:1 | 3 | 89.97 | 6.43 |
| 3 | 5 | 1:2 | 3 | 81.49 | 3.41 |
| 4 | 10 | 3:2 | 5 | 78.58 | 6.05 |
| 5 | 10 | 1:1 | 3 | 92.47 | 6.77 |
| 6 | 10 | 1:2 | 1 | 84.66 | 6.09 |
| 7 | 5 | 3:2 | 3 | 91.9 | 3.61 |
| 8 | 15 | 1:1 | 5 | 76.05 | 7.93 |
| 9 | 10 | 3:2 | 1 | 91.35 | 6.51 |
| 10 | 5 | 1:1 | 1 | 92.93 | 3.95 |
| 11 | 5 | 1:1 | 5 | 90.66 | 3.83 |
| 12 | 15 | 3:2 | 3 | 74.65 | 7.86 |
| 13 | 15 | 1:1 | 1 | 80.11 | 8.31 |
| 14 | 10 | 1:1 | 3 | 87.4 | 6.57 |
| 15 | 10 | 1:1 | 3 | 87.95 | 6.57 |
| 16 | 15 | 1:2 | 3 | 83.61 | 8.98 |
| 17 | 10 | 1:2 | 5 | 88.05 | 6.63 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 524.64 | 9 | 58.29 | 10.59 | 0.0026 |
| A-A | 226.42 | 1 | 226.42 | 41.15 | 0.0004 |
| B-B | 0.2211 | 1 | 0.2211 | 0.0402 | 0.8468 |
| C-C | 30.85 | 1 | 30.85 | 5.61 | 0.0498 |
| AB | 93.8 | 1 | 93.8 | 17.05 | 0.0044 |
| AC | 0.801 | 1 | 0.801 | 0.1456 | 0.7141 |
| BC | 65.29 | 1 | 65.29 | 11.87 | 0.0108 |
| A2 | 57.52 | 1 | 57.52 | 10.45 | 0.0144 |
| B2 | 37.23 | 1 | 37.23 | 6.77 | 0.0354 |
| C2 | 3.79 | 1 | 3.79 | 0.6884 | 0.4341 |
| Residual | 38.52 | 7 | 5.5 | ||
| Lack of Fit | 22.31 | 3 | 7.44 | 1.84 | 0.2809 |
| Pure Error | 16.21 | 4 | 4.05 | ||
| Cor Total | 563.16 | 16 |
| Soruce | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 43.67 | 9 | 4.85 | 79.75 | <0.0001 |
| A-A | 41.77 | 1 | 41.77 | 686.47 | <0.0001 |
| B-B | 0.1458 | 1 | 0.1458 | 2.4 | 0.1656 |
| C-C | 0.0221 | 1 | 0.0221 | 0.3624 | 0.5662 |
| AB | 0.4356 | 1 | 0.4356 | 7.16 | 0.0317 |
| AC | 0.0169 | 1 | 0.0169 | 0.2777 | 0.6145 |
| BC | 0.25 | 1 | 0.25 | 4.11 | 0.0823 |
| A2 | 0.8564 | 1 | 0.8564 | 14.08 | 0.0072 |
| B2 | 0.0779 | 1 | 0.0779 | 1.28 | 0.2952 |
| C2 | 0.0388 | 1 | 0.0388 | 0.6377 | 0.4508 |
| Residual | 0.4259 | 7 | 0.0608 | ||
| Lack of Fit | 0.3455 | 3 | 0.1152 | 5.72 | 0.0626 |
| Pure Error | 0.0805 | 4 | 0.0201 | ||
| Cor Total | 44.1 | 16 |
| Samples | EE (%) | DL (%) |
|---|---|---|
| Verification 1 | 88.87 | 6.95 |
| Verification 2 | 87.95 | 7.22 |
| Verification 3 | 90.84 | 6.84 |
| Average value | 89.22 | 7.03 |
| Predicted value | 89.97 | 7.01 |
| Deviation value | −0.0084 | 0.0028 |
| Time | A | B |
|---|---|---|
| 0–6 min | 90% | 10% |
| 6–20 min | 90–35% | 10–65% |
| 20–25 min | 35–20% | 65–80% |
| 25–35 min | 20% | 80% |
| 35–36 min | 20–90% | 80–10% |
| 36–42 min | 90% | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Ye, Z.; Liu, J.; Zhu, S.; Chen, Y.; Cai, J.; Chen, Y.; Wang, L.; Zhang, L.; Ye, Q. A Novel Delivery System for the Combined Use of Natural Ingredients: The Preparation of Berberine Hydrochloride–Matrine Liposomes and Preliminary Exploration of Their Anti-Tumor Activity. Molecules 2024, 29, 5210. https://doi.org/10.3390/molecules29215210
Xu M, Ye Z, Liu J, Zhu S, Chen Y, Cai J, Chen Y, Wang L, Zhang L, Ye Q. A Novel Delivery System for the Combined Use of Natural Ingredients: The Preparation of Berberine Hydrochloride–Matrine Liposomes and Preliminary Exploration of Their Anti-Tumor Activity. Molecules. 2024; 29(21):5210. https://doi.org/10.3390/molecules29215210
Chicago/Turabian StyleXu, Min, Zhangkai Ye, JunJing Liu, Shunpeng Zhu, Yuchen Chen, Jia Cai, Yangxi Chen, Long Wang, Liang Zhang, and Qiang Ye. 2024. "A Novel Delivery System for the Combined Use of Natural Ingredients: The Preparation of Berberine Hydrochloride–Matrine Liposomes and Preliminary Exploration of Their Anti-Tumor Activity" Molecules 29, no. 21: 5210. https://doi.org/10.3390/molecules29215210
APA StyleXu, M., Ye, Z., Liu, J., Zhu, S., Chen, Y., Cai, J., Chen, Y., Wang, L., Zhang, L., & Ye, Q. (2024). A Novel Delivery System for the Combined Use of Natural Ingredients: The Preparation of Berberine Hydrochloride–Matrine Liposomes and Preliminary Exploration of Their Anti-Tumor Activity. Molecules, 29(21), 5210. https://doi.org/10.3390/molecules29215210






