Photo-Induced Aerobic Oxidation of C–H Bonds
Abstract
1. Introduction
2. Visible-Light Photocatalytic Oxidation Reactions
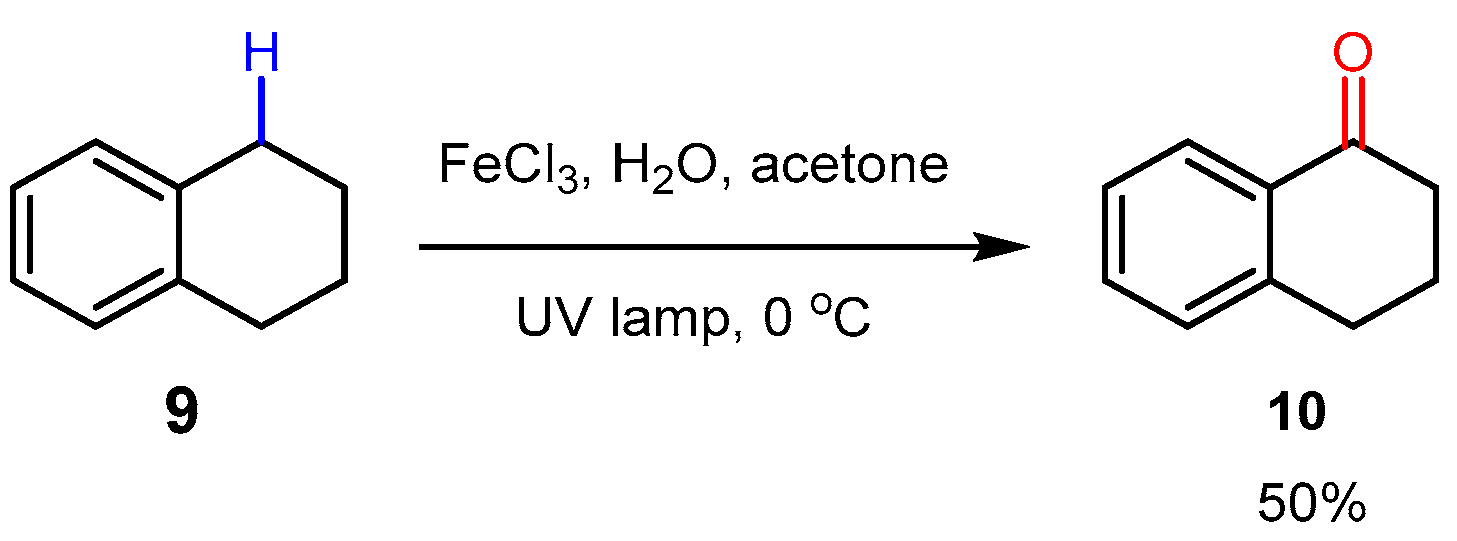
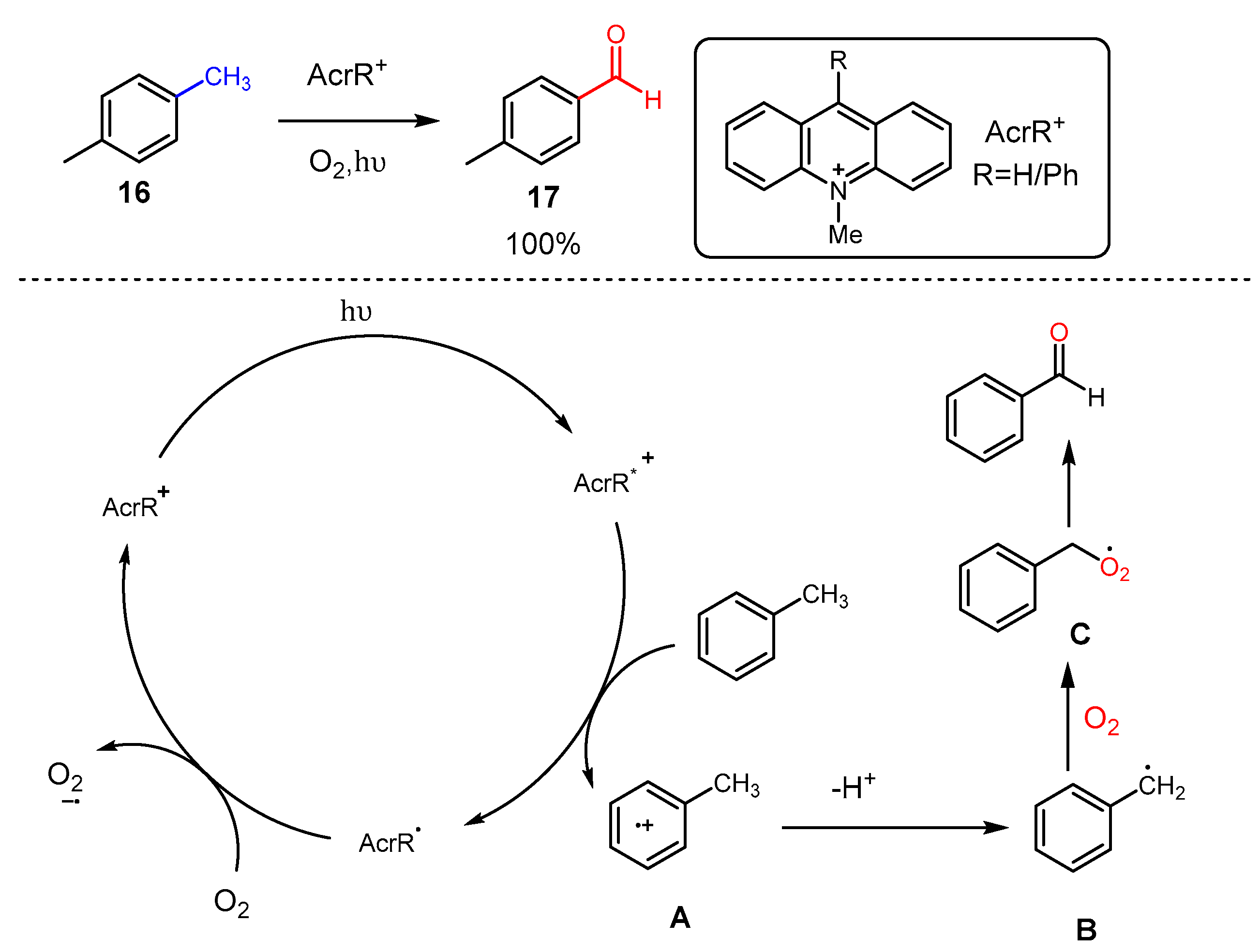
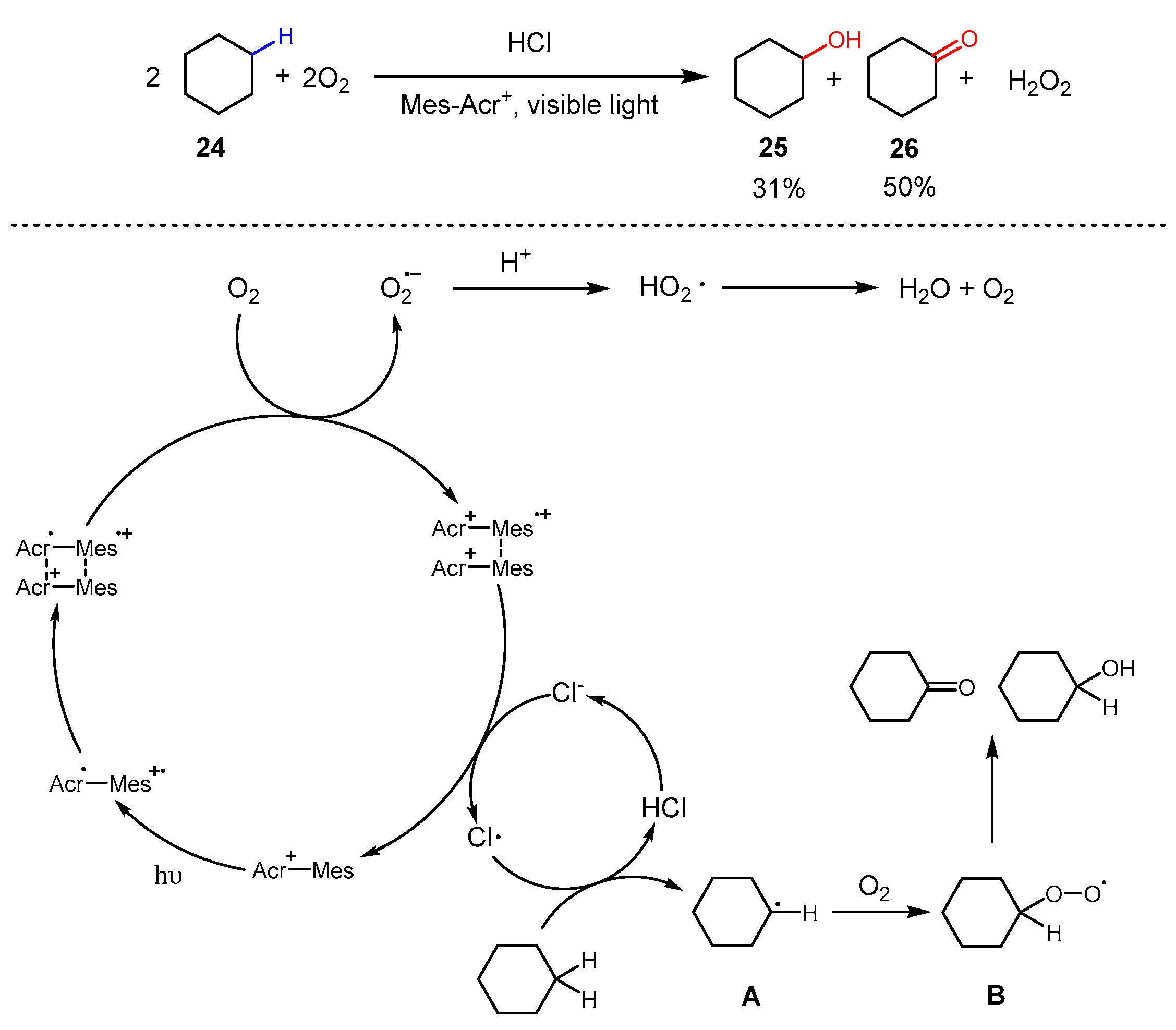
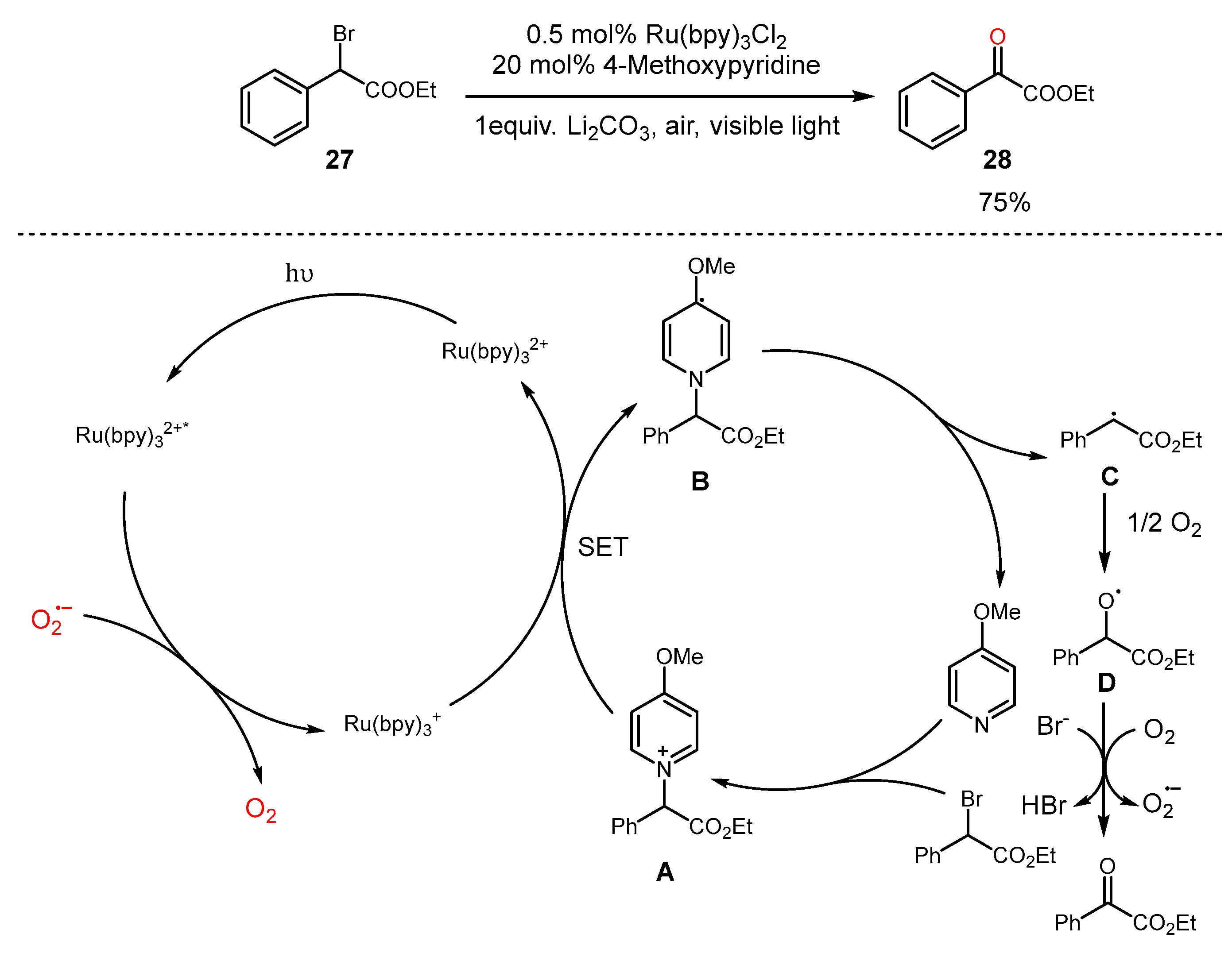
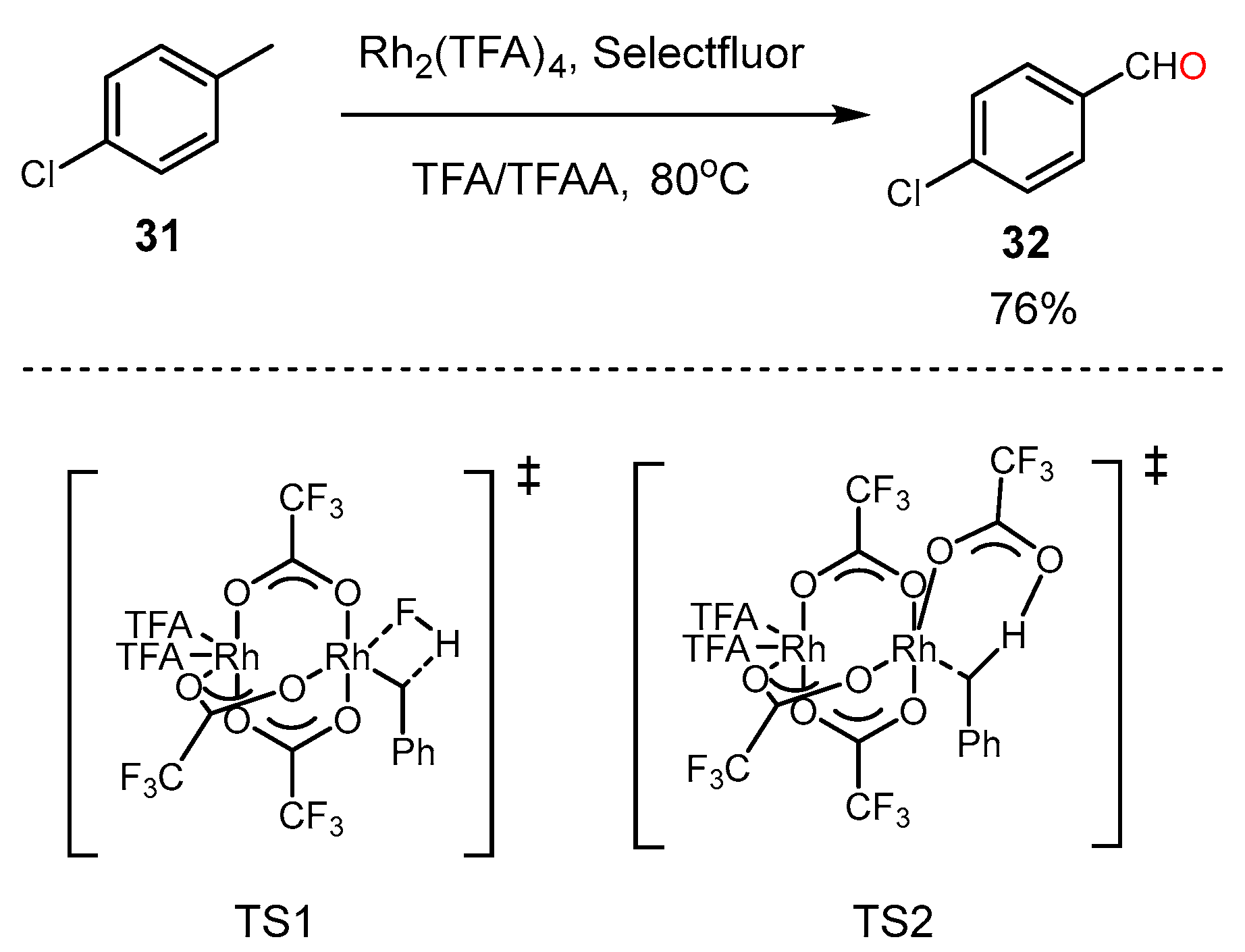
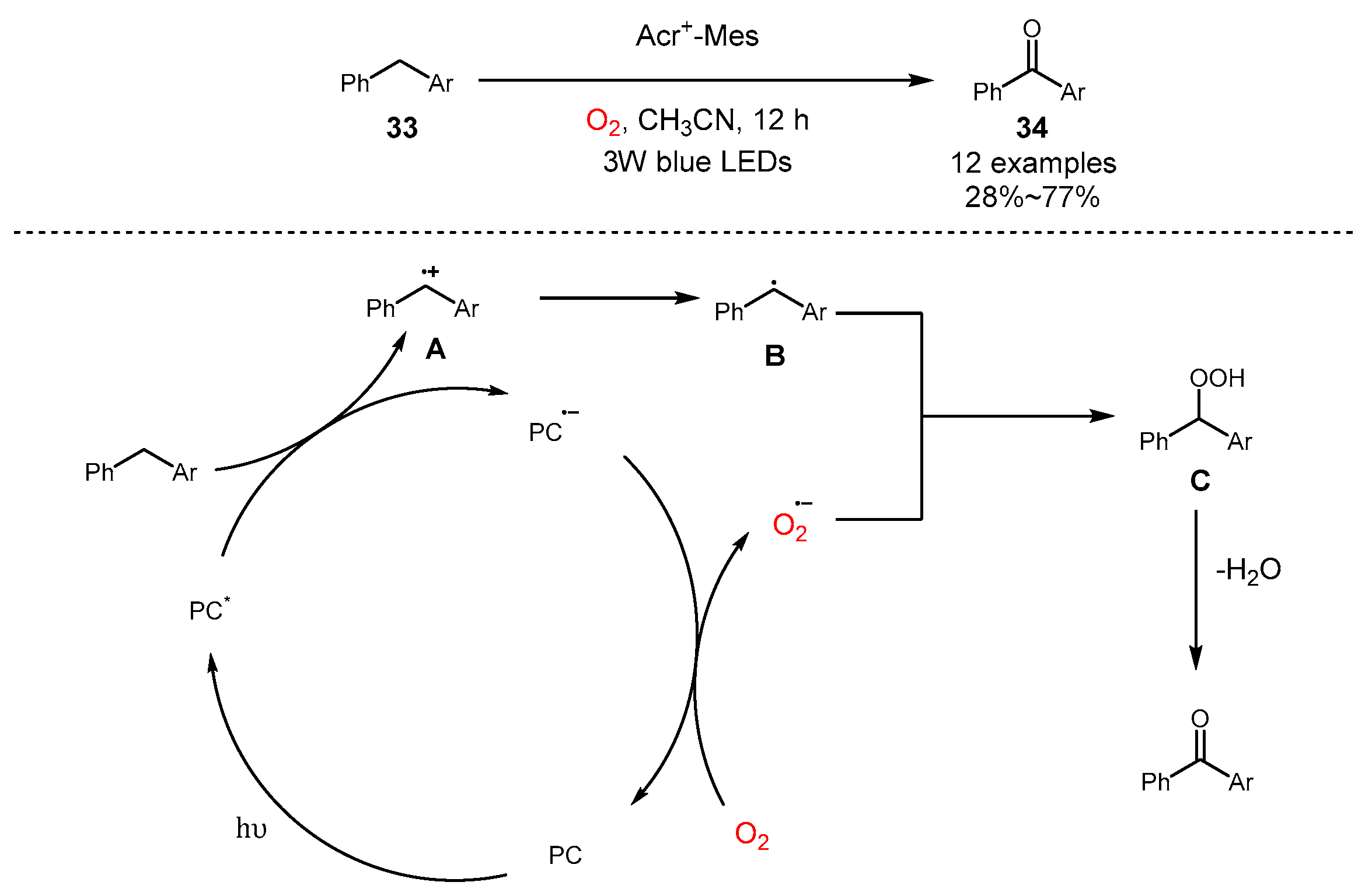
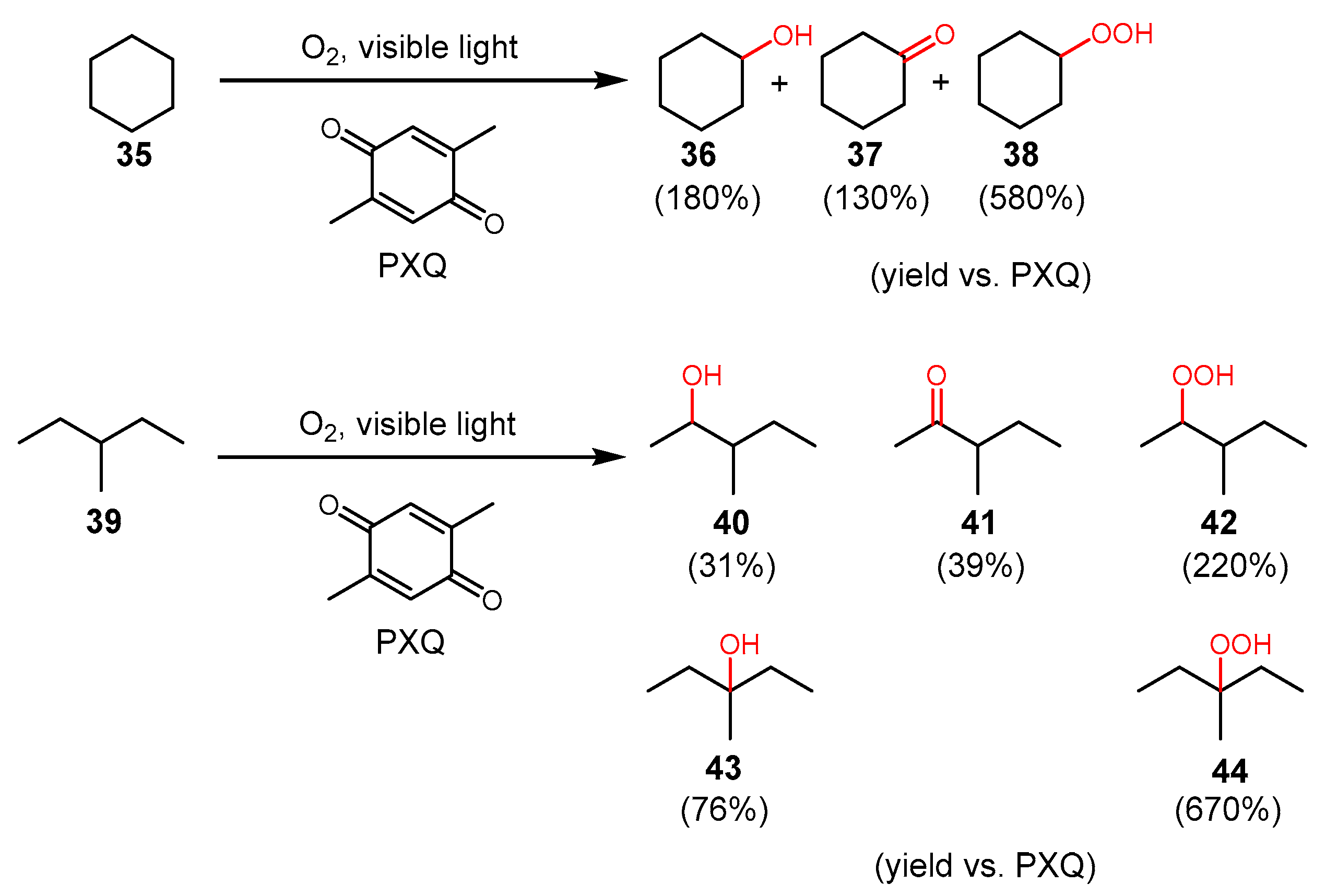
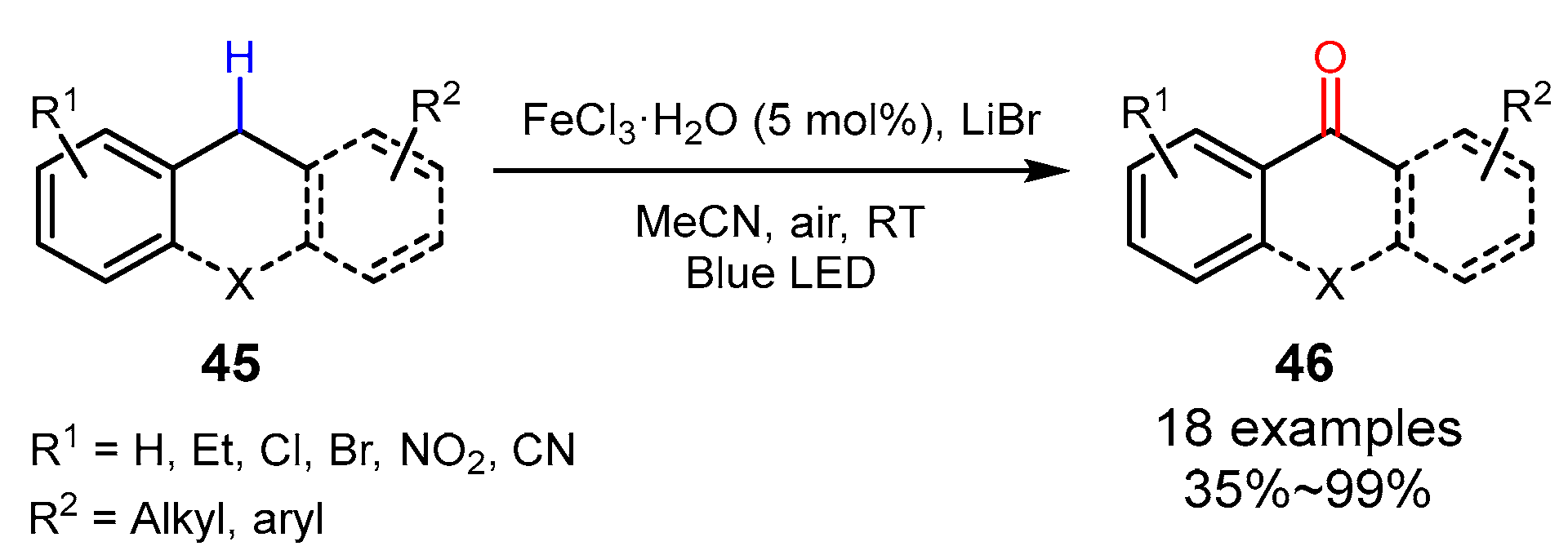
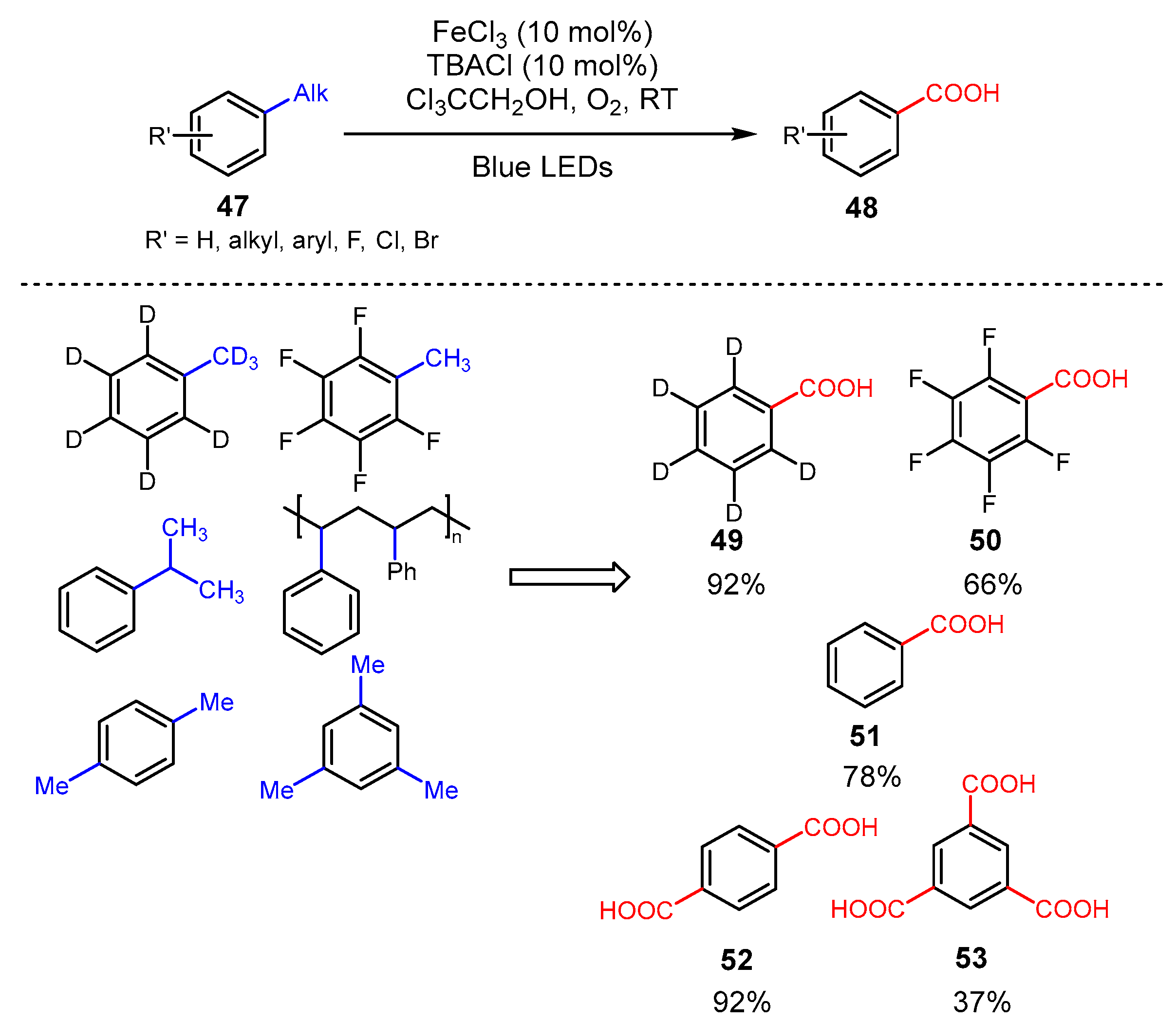
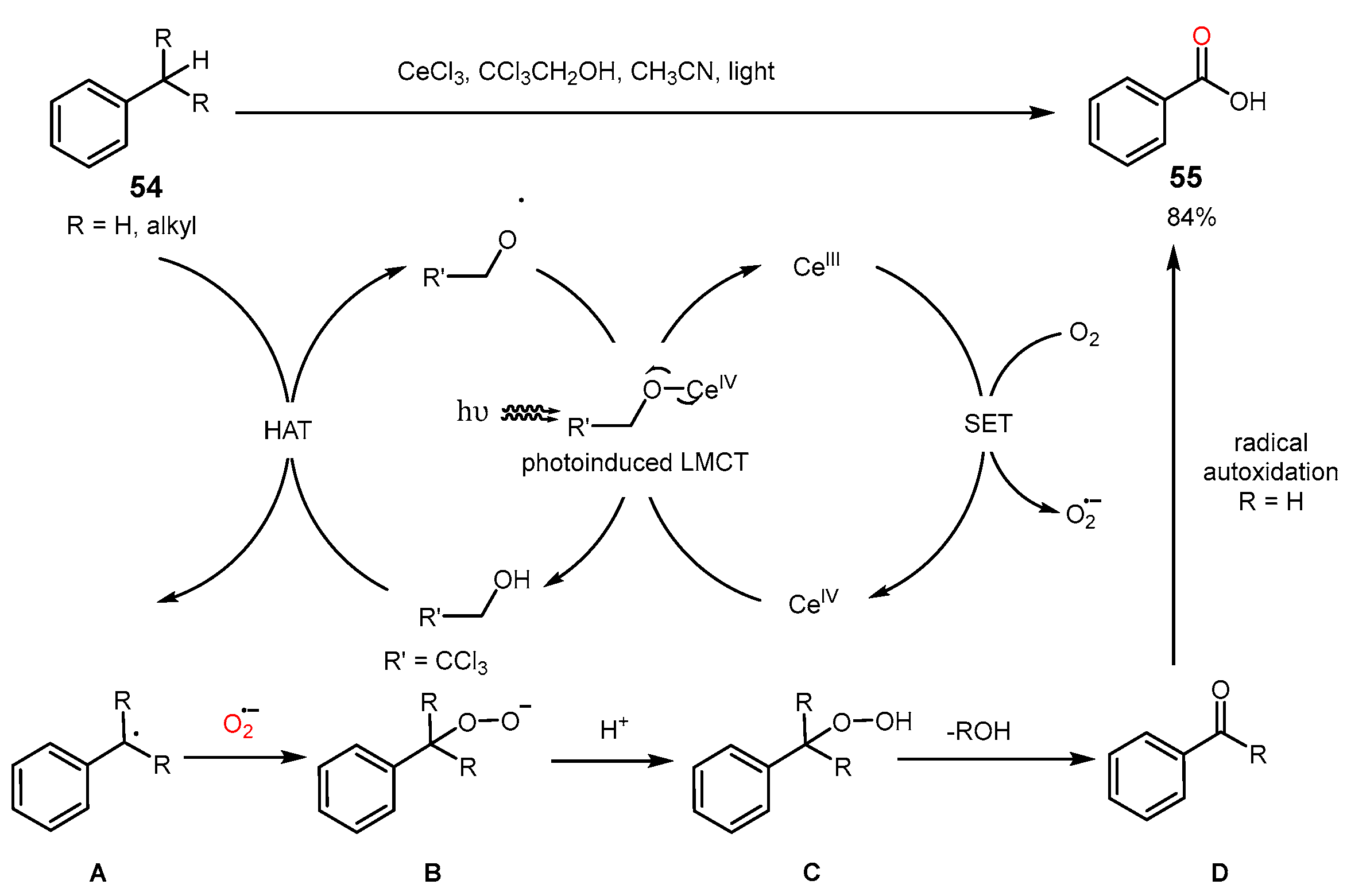
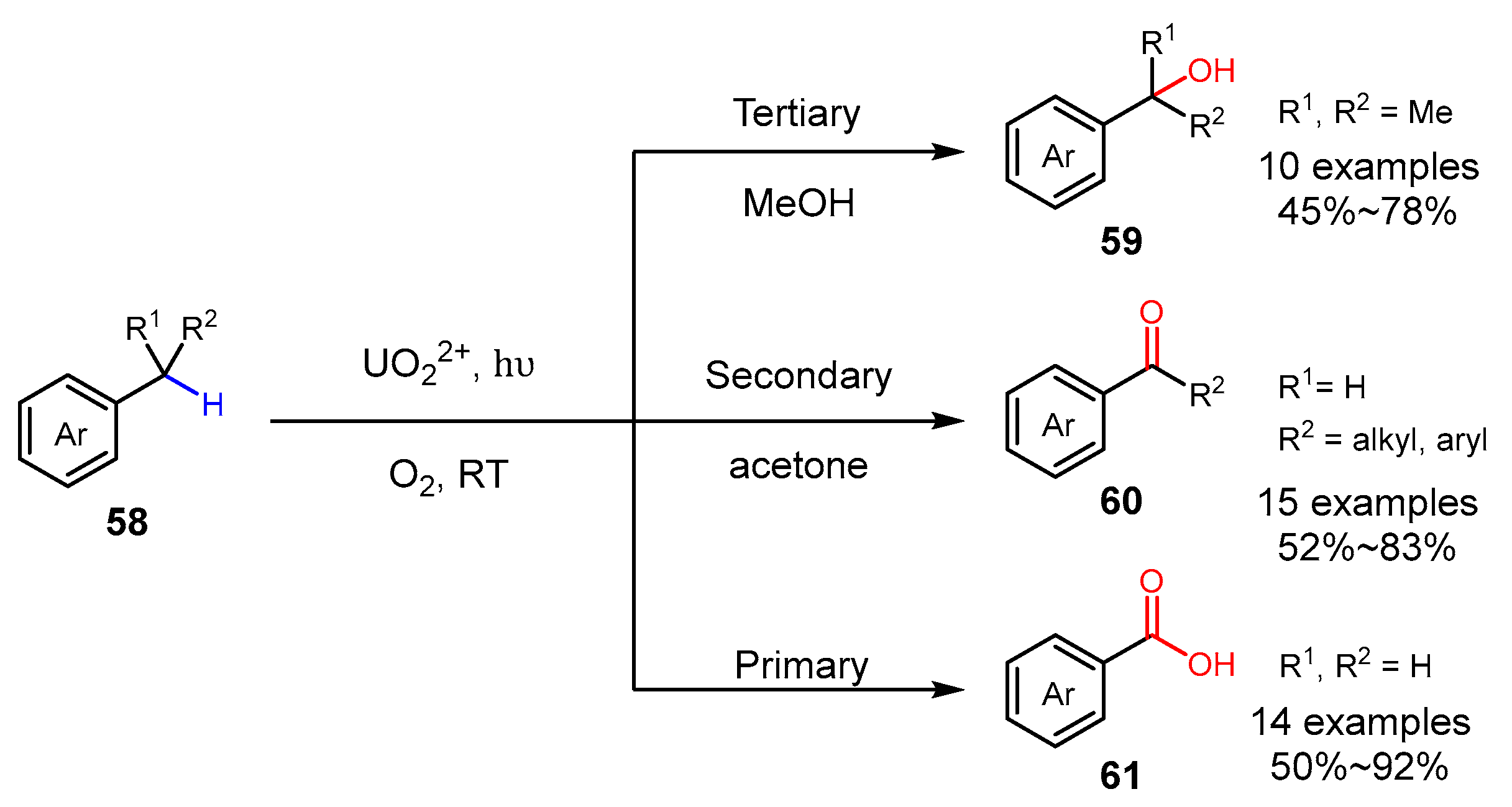
3. Alkane Oxidation
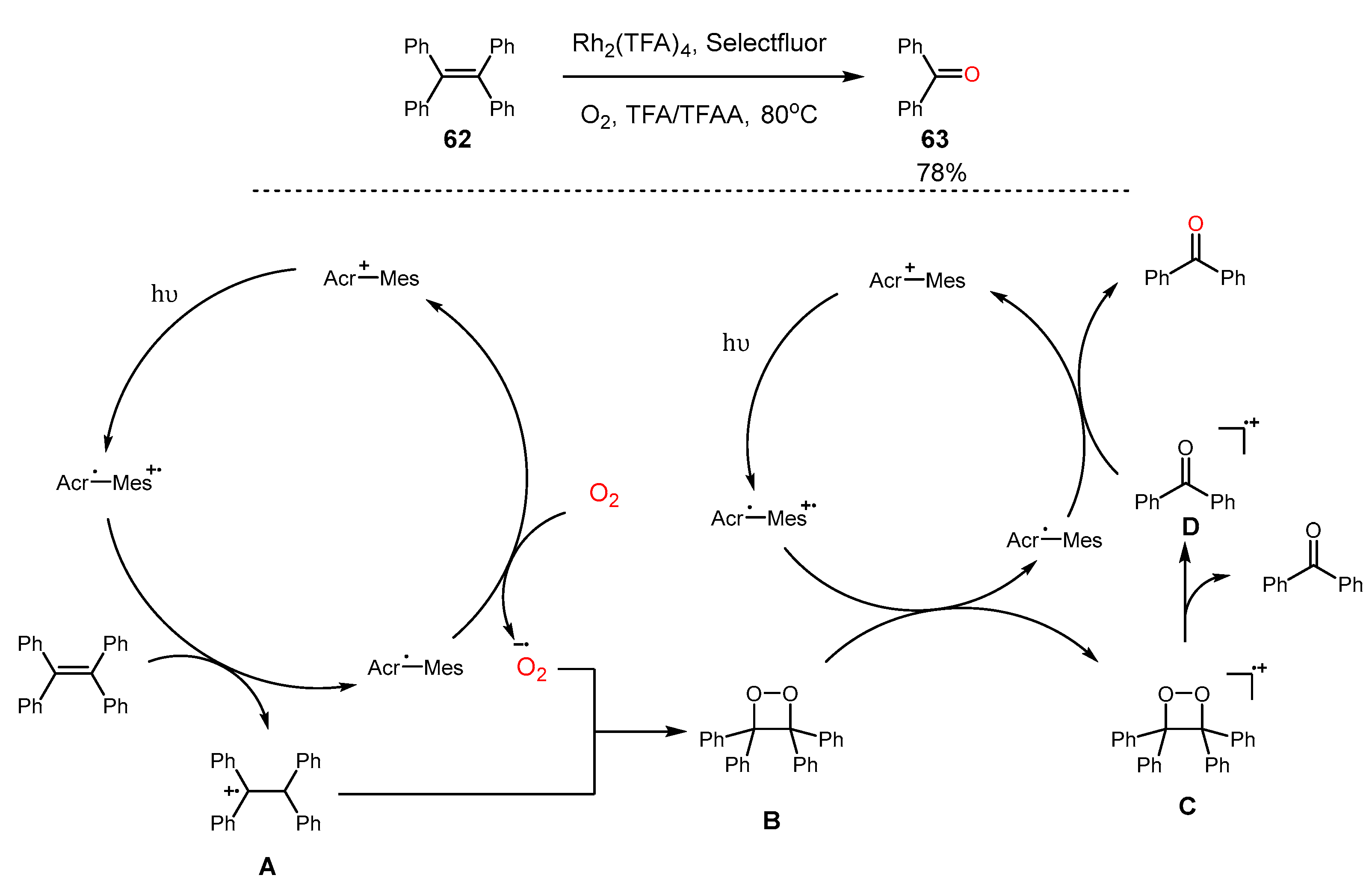
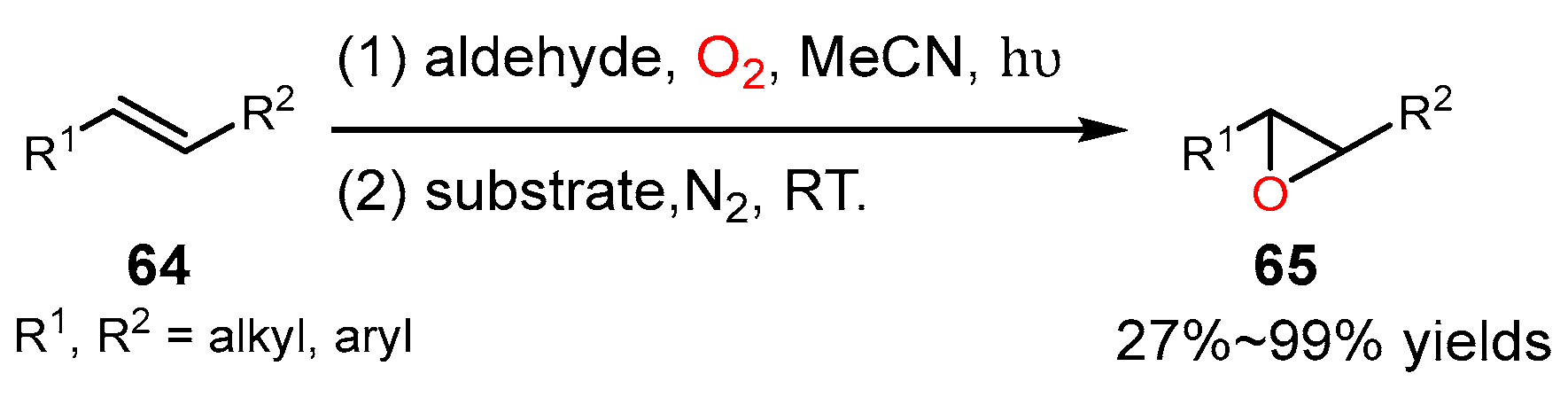
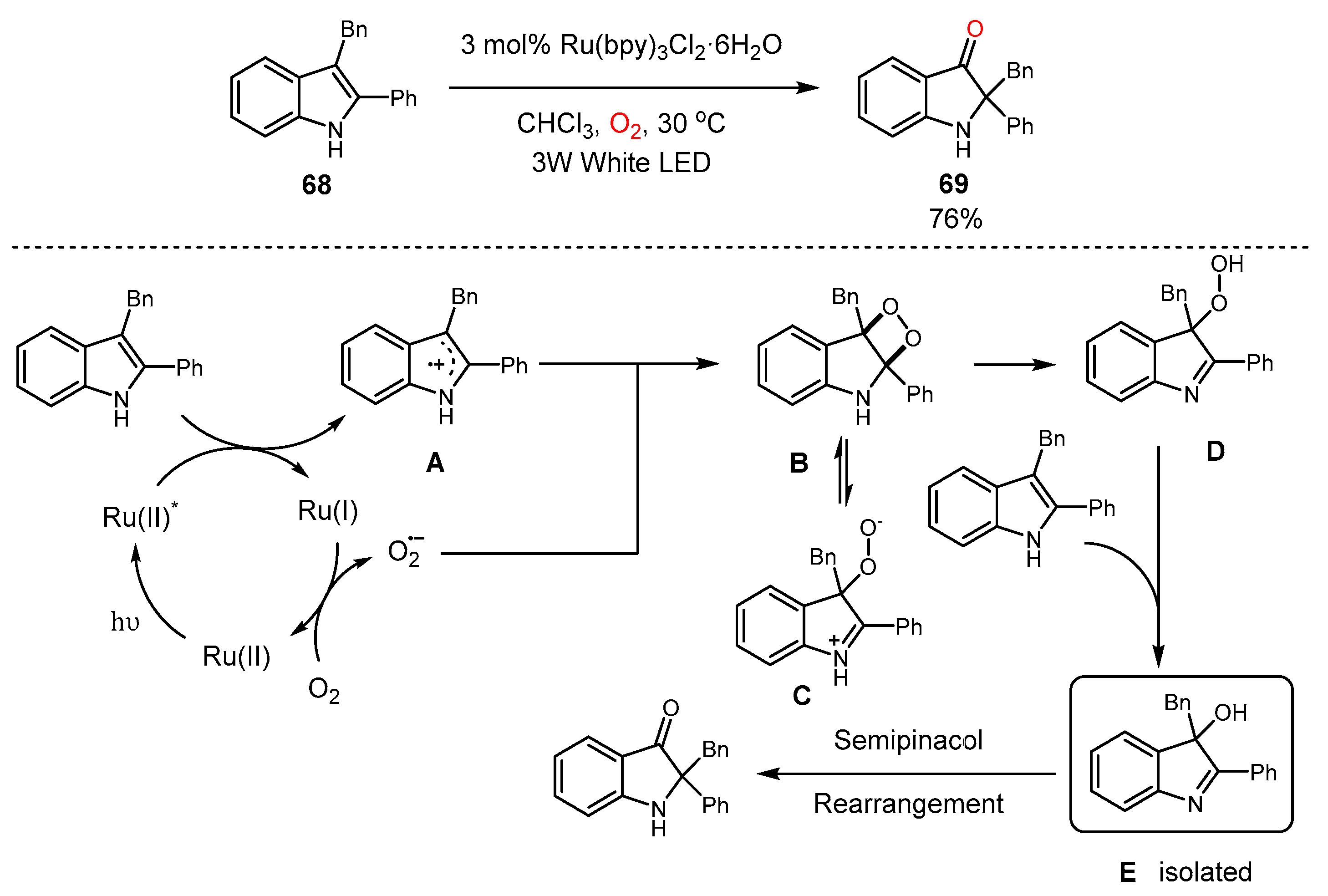
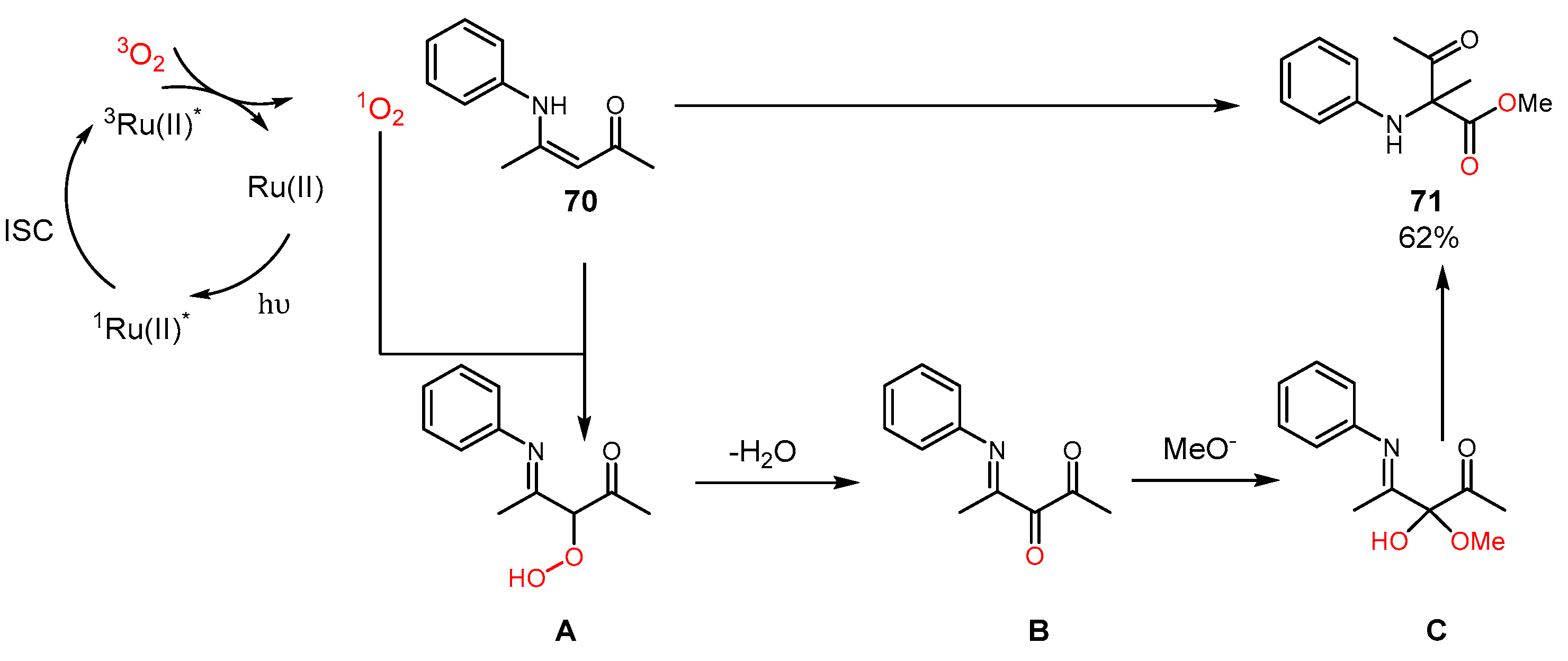
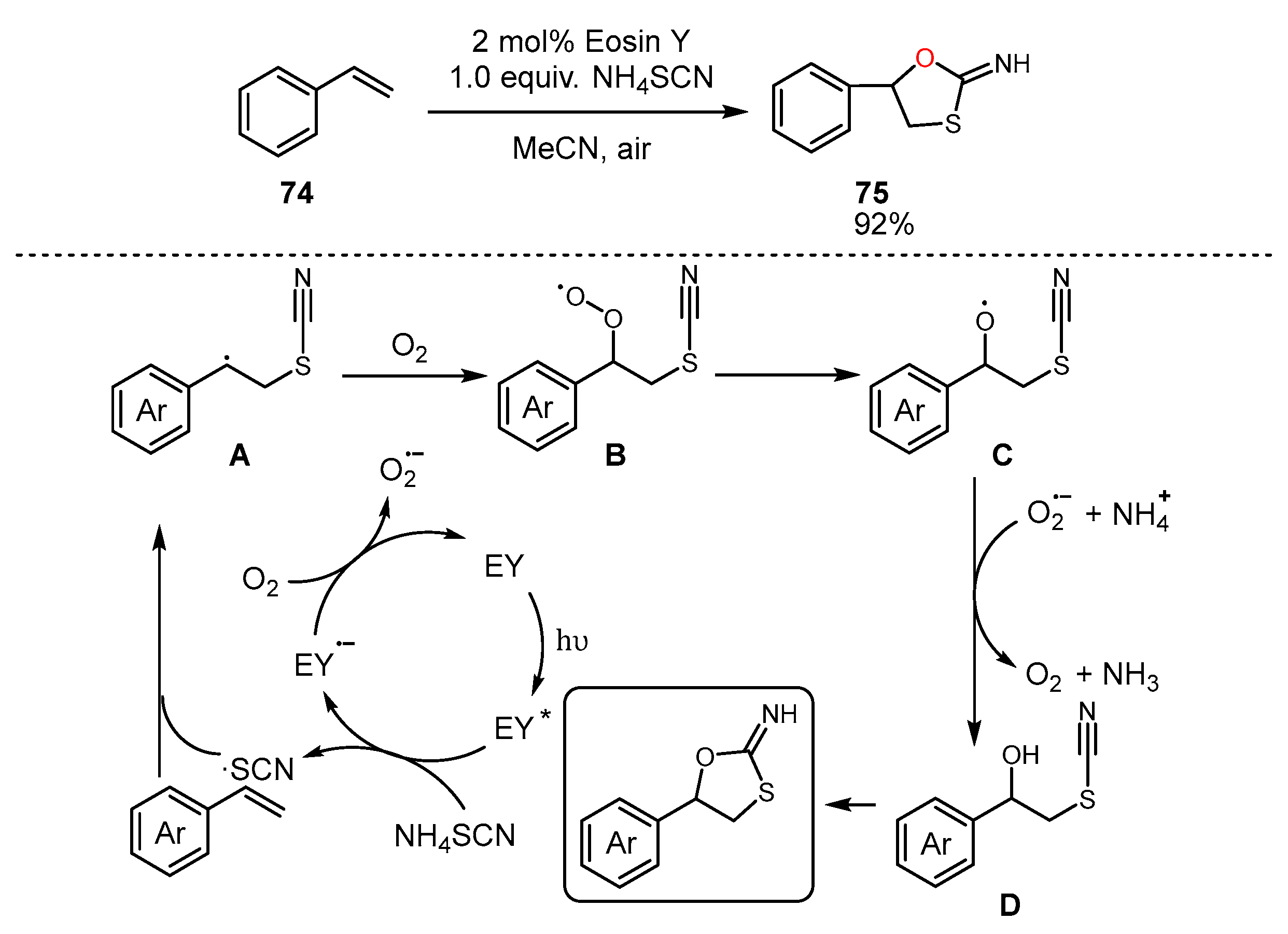
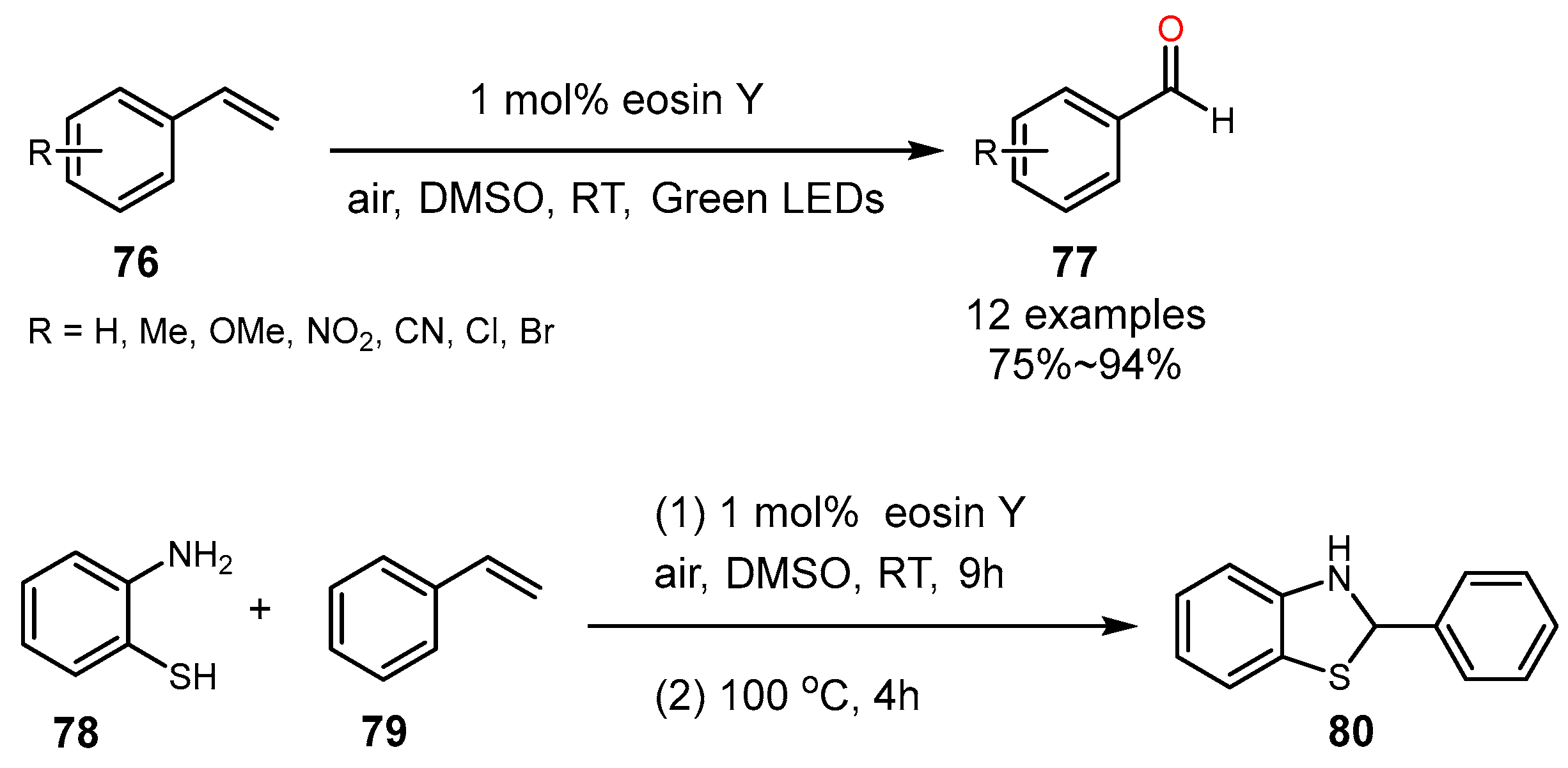
4. Alkene Oxidation
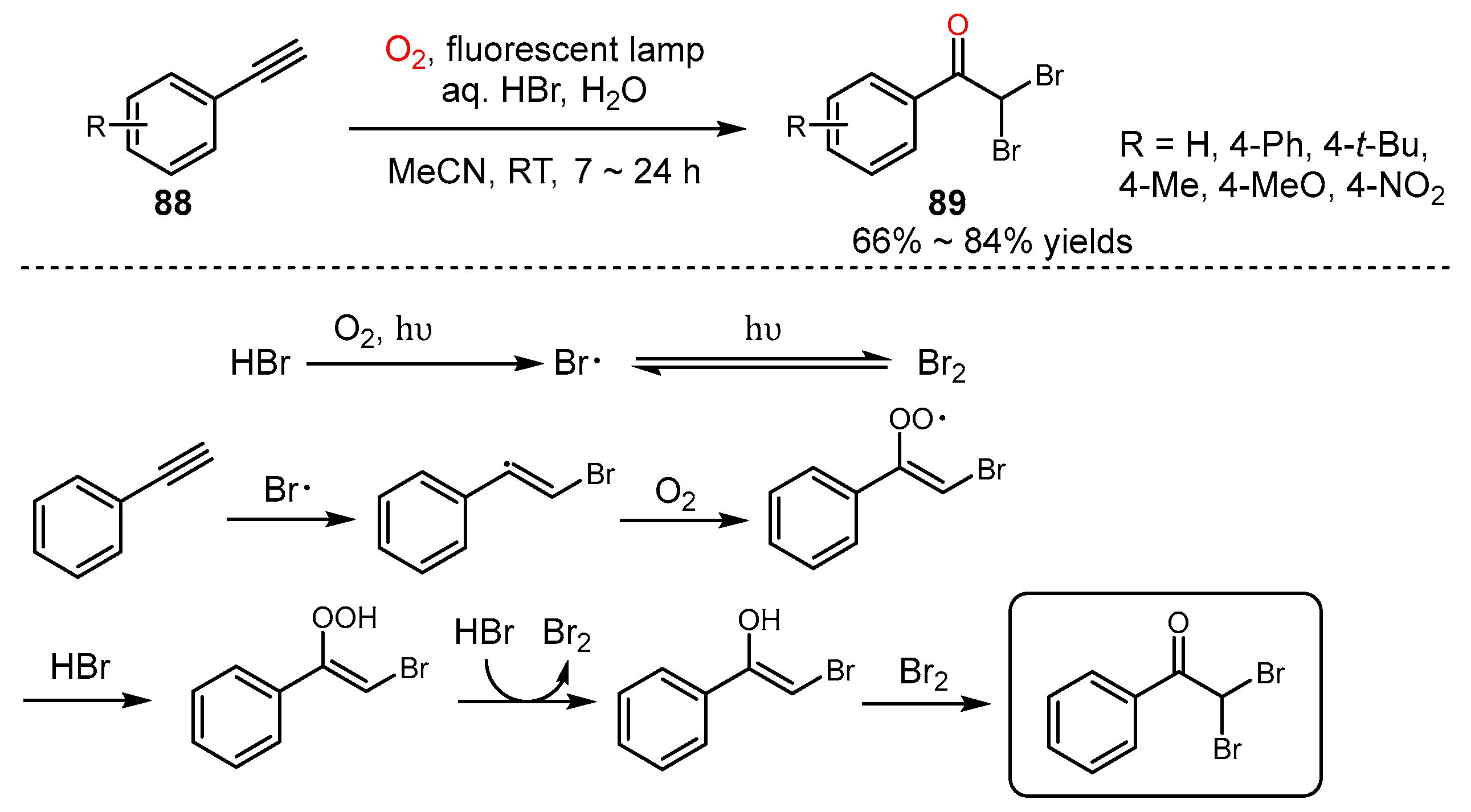
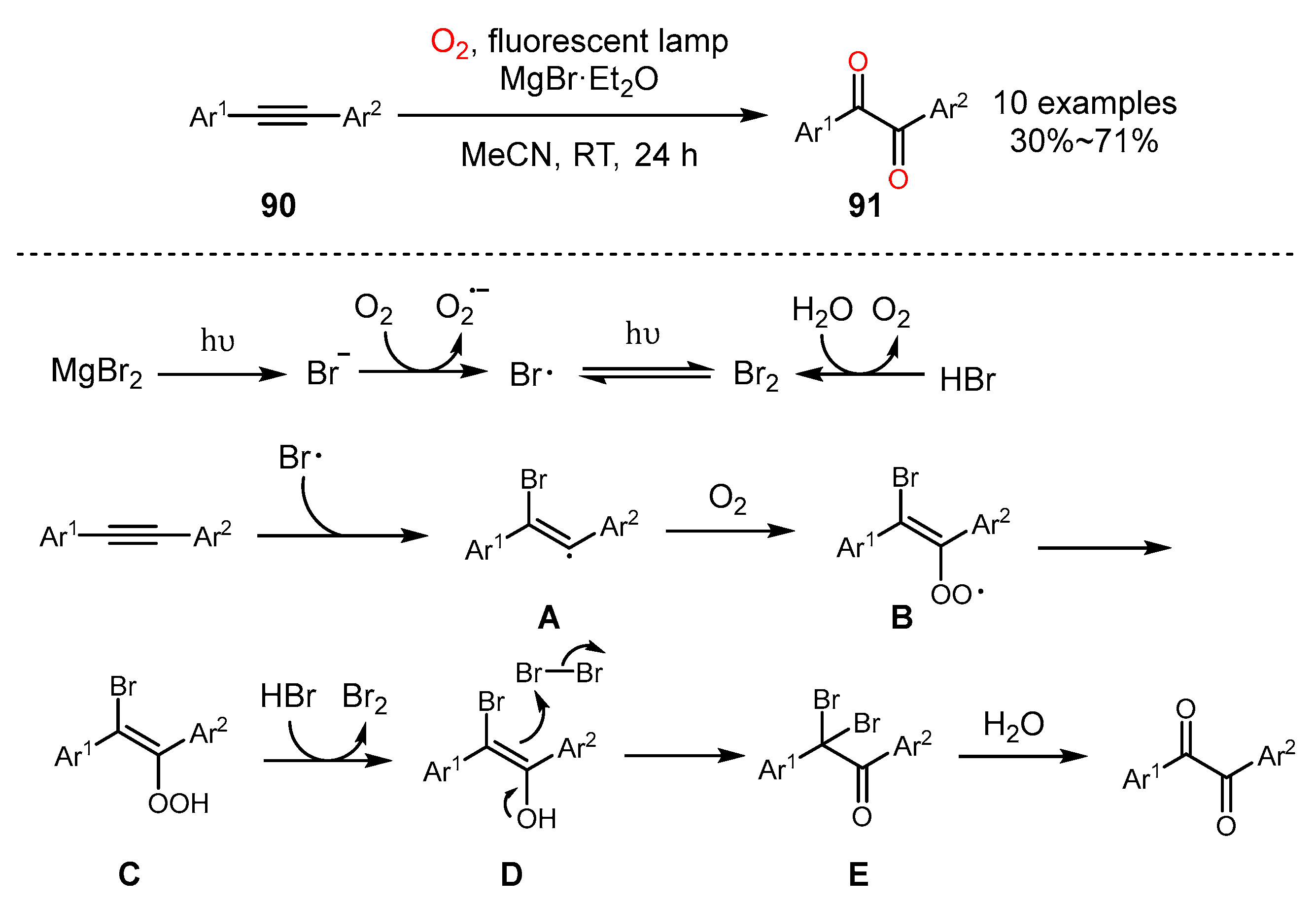
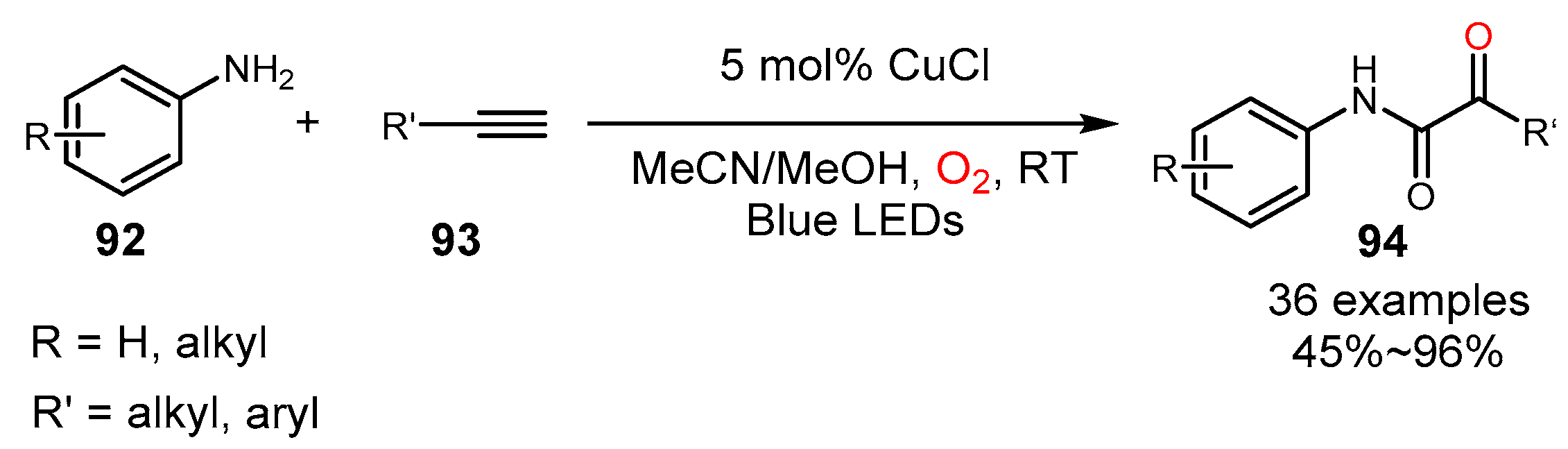
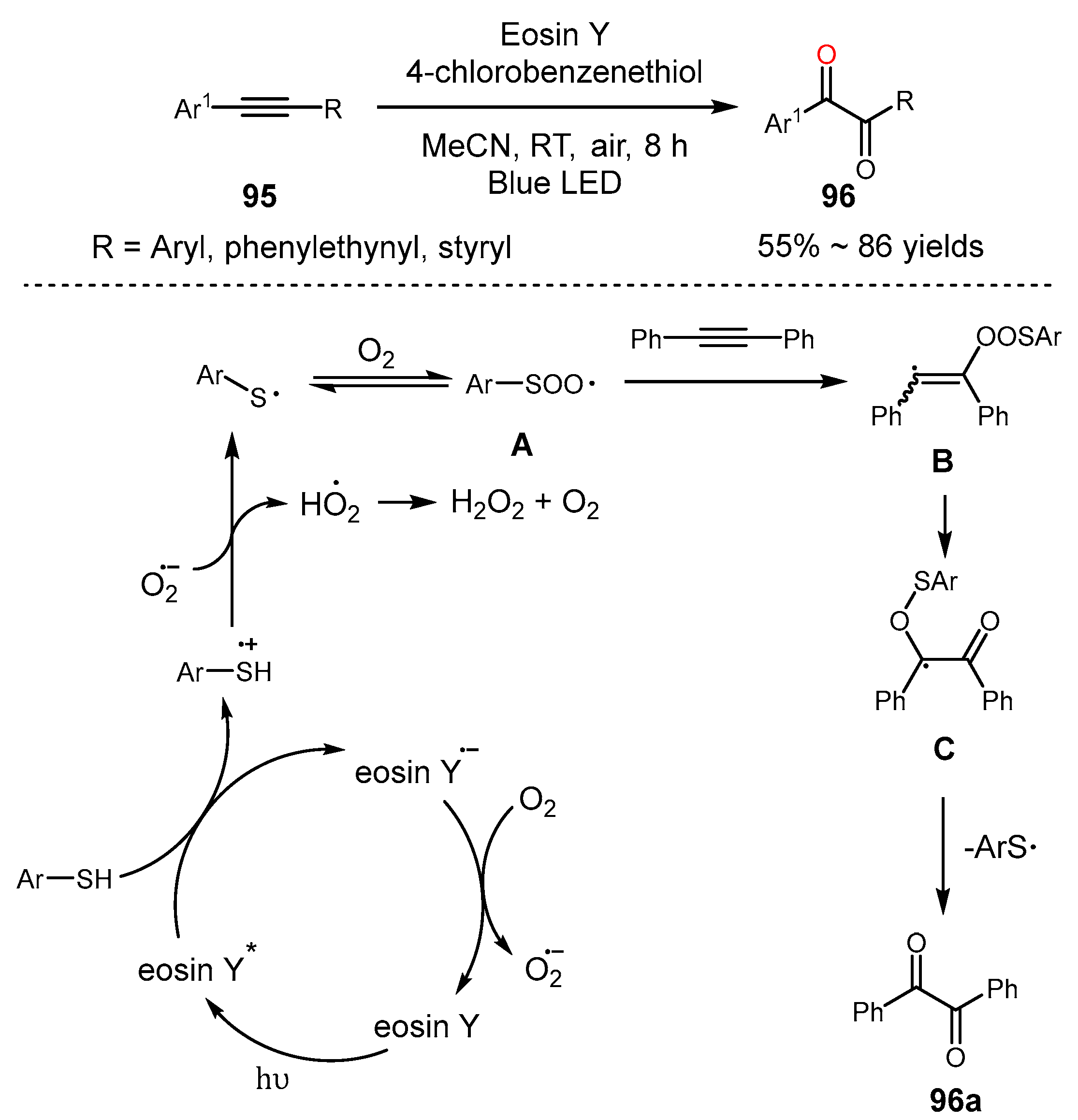
5. Alkyne Oxidation
6. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Jacobsen, E.N. Oxidation Catalysis: Bringing Together the Interests of Academic and Industrial Researchers. Adv. Synth. Catal. 2004, 346, 109. [Google Scholar] [CrossRef]
- Hermans, I.; Spier, E.S.; Neunschwanedr, U.; Turra, N.; Baiker, A. Selective oxidation catalysis: Opportunities and challenges. Top. Catal. 2009, 52, 1162–1174. [Google Scholar] [CrossRef]
- Janey, J.M. Recent Advances in Catalytic, Enantioselectivea-Aminations and a-Oxygenations of Carbonyl Compounds. Angew. Chem. Int. Ed. 2005, 44, 4292–4300. [Google Scholar] [CrossRef]
- Limberg, C. The Role of Radicals in Metal-Assisted Oxygenation Reactions. Angew. Chem. Int. Ed. 2003, 42, 5932–5954. [Google Scholar] [CrossRef]
- Bordeaux, M.; Galareau, A.; Drone, J. Catalytic, Mild, and Selective Oxyfunctionalization of Linear Alkanes: Current Challenges. Angew. Chem. Int. Ed. 2012, 51, 10712–10723. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Hu, X.; Lu, Z. Visible-Light-Promoted Aerobic Homogenous Oxygenation Reactions. Chin. J. Org. Chem. 2017, 31, 251–266. [Google Scholar] [CrossRef][Green Version]
- Stahl, S.S. Palladium Oxidase Catalysis: Selective Oxidation of Organic Chemicals by DirectDioxygen-Coupled Turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef] [PubMed]
- Mallat, T.; Baiker, A. Oxidation of Alcohols with Molecular Oxygen on Solid Catalysts. Chem. Rev. 2004, 104, 3037–3058. [Google Scholar] [CrossRef]
- Zhan, B.-Z.; Thompson, A. Recent Developments in the Aerobic Oxidation of Alcohols. Terahedron 2004, 60, 2917–2935. [Google Scholar] [CrossRef]
- Punniyamurthy, T.; Velusamy, S.; Igbal, J. Recent Advances in Transition Metal Catalyzed Oxidation of Organic Substrates with Molecular Oxygen. Chem. Rev. 2005, 105, 2329–2363. [Google Scholar] [CrossRef]
- Schultz, M.J.; Sigman, M.S. Recent Advances in Homogeneous Transition Metal-Catalyzed Aerobic Alcohol Oxidations. Tetrahedron 2006, 62, 8227–8241. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, C.; Tang, C.; Jiao, N. Recent Advances in Transition-Metal Catalyzed Reactions Using Molecular Oxygen as the Oxidant. Chem. Soc. Rev. 2012, 41, 3381–3430. [Google Scholar] [CrossRef] [PubMed]
- Narayanam, J.M.; Stephenson, C.R. Visible Light Photoredox Catalysis: Applications in Organic Synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef]
- Shi, L.; Xia, W. PhotoredoxFumctionalization of C–H Bonds Adjacent to a Nitrogen Atom. Chem. Soc. Rev. 2012, 41, 7687–7697. [Google Scholar] [CrossRef] [PubMed]
- Ciamician, G. The Photochemistry of the Futre. Science 1912, 36, 385–394. [Google Scholar] [CrossRef]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W. Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef]
- Xuan, J.; Xiao, W.J. Visible-Light Photoredox Catalysis. Angew. Chem. Int. Ed. 2012, 51, 6828–6838. [Google Scholar] [CrossRef]
- Barbier, M. Selective Photoinduced Oxidation of Benzylic Methylen Groups through Uv Irradiation in Presence of Ferric-Chloride. Helv. Chim. Acta 1984, 67, 866–869. [Google Scholar] [CrossRef]
- Mao, Y.; Bakac, A. Photocatalytic oxidation of aromatic hydrocarbons. Inorg. Chem. 1996, 35, 3925–3930. [Google Scholar] [CrossRef]
- Arnold, P.L.; Purkis, J.M.; Rutkauskaite, R.; Kovacs, D.; Love, J.B.; Austin, J. Controlled Photocatalytic Hydrocarbon Oxidation by Uranyl Complexes. ChemCatChem. 2019, 11, 3786–3790. [Google Scholar] [CrossRef]
- Ohkubo, K.; Suga, K.; Morikawa, K.; Fukuzumi, S. Selective Oxygenation of Ring-Substituted Toluenes with Electron-Donating and -Withdrawing Substituents by Molecular Oxygen via Photoinduced Electron Transfer. J. Am. Chem. Soc. 2003, 125, 12850–12859. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Itoh, A. Aerobic photooxidation of benzylamide in the presence of catalytic iodine. Synlett 2008, 2008, 675–678. [Google Scholar]
- Tada, N.; Ban, K.; Yoshida, M.; Hirashima, S.; Miura, T.; Itoh, A. Aerobic photooxidation of benzylamide under visible light irradiation with a combination of 48% aq HBr and Ca(OH)2. Tetrahedron Lett. 2010, 51, 6098–6100. [Google Scholar] [CrossRef]
- Itoh, I.; Matsusaki, Y.; Fujiya, A.; Tada, N.; Miura, T.; Itoh, A. 2-Chloroanthraquinone-catalyzed aerobic photo-oxidative synthesis of diacylamines from benzylamides. Tetrahedron Lett. 2014, 55, 3160–3162. [Google Scholar] [CrossRef]
- Ohkubo, K.; Fujimoto, A.; Fukuzumi, S. Metal-free oxygenation of cyclohexane with oxygen catalyzed by 9-mesityl-10-methylacridinium and hydrogen chloride under visible light irradiation. Chem. Commun. 2011, 47, 8515. [Google Scholar] [CrossRef]
- Su, Y.J.; Zhang, L.R.; Jiao, N. Utilization of Natural Sunlight and Air in the Aerobic Oxidation of Benzyl Halides. Org. Lett. 2011, 13, 2168–2171. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Oisaki, K.; Kanai, M. Chemoselective aerobic photo-oxidation of 9-fluorenes for the synthesis of 9-fluorenones. Tetrahedron Lett. 2014, 55, 4736–4738. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, L.; Lan, Y.; Rao, Y. Development of a Rhodium(II)-Catalyzed Chemoselective C(sp3)-H Oxygenation. Chem-Eur. J. 2015, 21, 14937–14942. [Google Scholar] [CrossRef]
- Yi, H.; Bian, C.; Hu, X.; Niu, L.; Lei, A. Visible light mediated efficient oxidative benzylic sp3 C–H to ketone derivatives obtained under mild conditions using O2. Chem. Commun. 2015, 51, 14046–14049. [Google Scholar] [CrossRef]
- Ohkubo, K.; Hirose, K.; Fukuzumi, S. Photooxygenation of alkanes by dioxygen with p-benzoquinone derivatives with high quantum yields. Photoch. Photobio. Sci. 2016, 15, 731–734. [Google Scholar] [CrossRef]
- Li, S.L.; Zhu, B.; Lee, R.; Qiao, B.K.; Jiang, Z.Y. Visible light-induced selective aerobic oxidative transposition of vinyl halides using a tetrahalogenoferrate(III) complex catalyst. Org. Chem. Front. 2018, 5, 380–385. [Google Scholar] [CrossRef]
- Zhang, G.X.; Zhang, Z.N.; Zeng, R. Photoinduced FeCl3-Catalyzed Alkyl Aromatics Oxidation toward Degradation of Polystyrene at Room Temperature. Chin. J. Chem. 2021, 39, 3225–3230. [Google Scholar] [CrossRef]
- Wang, C.C.; Zhang, G.X.; Zuo, Z.W.; Zeng, R.; Zhai, D.D.; Liu, F.; Shi, Z.J. Photo-induced deep aerobic oxidation of alkyl aromatics. Sci. China Chem. 2021, 64, 1487–1492. [Google Scholar] [CrossRef]
- Niu, K.K.; Shi, X.D.; Ding, L.; Liu, Y.X.; Song, H.J.; Wang, Q.M. HCl-Catalyzed Aerobic Oxidation of Alkylarenes to Carbonyls. ChemSusChem 2022, 15, e202102326. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.Q.; Jiang, X.F. Stepwise benzylic oxygenation uranyl-photocatalysis. Green Chem. 2022, 24, 124–129. [Google Scholar] [CrossRef]
- Ohkubo, K.; Nanjo, T.; Fukuzumi, S. Efficient photocatalytic oxygenation of aromatic alkene to 1,2-dioxetane with oxygen via electron transfer. Org. Lett. 2005, 7, 4265–4268. [Google Scholar] [CrossRef]
- Ohkubo, K.; Nanjo, T.; Fukuzumi, S. Photocatalytic oxygenation of olefins with oxygen isolation of 1,2-dioxetane and the photocatalytic O-O bond cleavage. Catal. Today 2006, 117, 356–361. [Google Scholar] [CrossRef]
- Tada, N.; Okubo, H.; Miura, T.; Itoh, A. Metal-Free Epoxidation of Alkenes with Molecular Oxygen and Benzaldehyde under Visible Light Irradiation. Synlett 2009, 2009, 3024–3026. [Google Scholar] [CrossRef]
- Nobuta, T.; Hirashima, S.; Tada, N.; Miura, T.; Itoh, A. Facile Aerobic Photo-Oxidative Synthesis of Phenacyl Iodides and Bromides from Styrenes Using I2 or Aqueous HBr. Synlett 2010, 2010, 2335–2339. [Google Scholar]
- Ding, W.; Zhou, Q.Q.; Xuan, J.; Li, T.R.; Lu, L.Q.; Xiao, W.J. Photocatalytic aerobic oxidation/semipinacol rearrangement sequence: A concise route to the core of pseudoindoxyl alkaloids. Tetrahedron Lett. 2014, 55, 4648–4652. [Google Scholar] [CrossRef]
- Fan, W.; Li, P. Visible-Light-Mediated 1,2-Acyl Migration: The Reaction of Secondary Enamino Ketones with Singlet Oxygen. Angew. Chem. Int. Ed. 2014, 53, 12201–12204. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.Y.; Lei, T.; Zhao, L.M.; Wu, C.J.; Zhong, J.J.; Gao, X.W.; Tung, C.H.; Wu, L.Z. A Unique 1,2-Acyl Migration for the Construction of Quaternary Carbon by Visible Light Irradiation of Platinum(II) Polypyridyl Complex and Molecular Oxygen. Org. Lett. 2014, 16, 5968–5971. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.K.; Yadav, L.D.S. Visible-light-mediated difunctionalization of styrenes: An unprecedented approach to 5-aryl-2-imino-1,3-oxathiolanes. Green Chem. 2015, 17, 3515–3520. [Google Scholar] [CrossRef]
- Singh, A.K.; Chawla, R.; Yadav, L.D.S. Eosin Y catalyzed visible light mediated aerobic photo-oxidative cleavage of the C-C double bond of styrenes. Tetrahedron Lett. 2015, 56, 653–656. [Google Scholar] [CrossRef]
- Taguchi, M.; Nagasawa, Y.; Yamaguchi, E.; Tada, N.; Miura, T.; Itoh, A. One-pot epoxidation of alkenes using aerobic photoperoxidation of toluenes. Tetrahedron Lett. 2016, 57, 230–232. [Google Scholar] [CrossRef]
- Deng, Y.; Wei, X.J.; Wang, H.; Sun, Y.; Noël, T.; Wang, X. Disulfide-Catalyzed Visible-Light-Mediated Oxidative Cleavage of C=C Bonds and Evidence of an Olefin–Disulfide Charge-Transfer Complex. Angew. Chem. Int. Ed. 2017, 56, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.F.; Zhou, G.L.; Meng, Q.W.; Tang, X.F.; Liu, G.Z.; Yin, H.; Zhao, J.N.; Yang, F.; Yu, Z.Y.; Luo, Y. Visible Light-Induced Aerobic Epoxidation of α,β-Unsaturated Ketones Mediated by Amidines. J. Org. Chem. 2018, 83, 13051–13062. [Google Scholar] [CrossRef]
- Nobuta, T.; Hirashima, S.; Tada, N.; Miura, T.; Itoh, A. Facile aerobic photo-oxidative syntheses of α,α-dibromoacetophenones from aromatic alkynes with 48% aq HBr. Tetrahedron Lett. 2010, 51, 4576–4578. [Google Scholar] [CrossRef]
- Nobuta, T.; Tada, N.; Hattori, K.; Hirashima, S.; Miura, T.; Itoh, A. Facile aerobic photo-oxidative synthesis of α-diketones from alkynes. Tetrahedron Lett. 2011, 52, 875–877. [Google Scholar] [CrossRef]
- Sagadevan, A.; Ragupathi, A.; Lin, C.C.; Hwu, J.R.; Hwang, K.C. Visible-light initiated copper(I)-catalysed oxidative C-N coupling of anilines with terminal alkynes: One-step synthesis of α-ketoamides. Green Chem. 2015, 17, 1113–1119. [Google Scholar] [CrossRef]
- Liu, X.; Cong, T.; Liu, P.; Sun, P.P. Synthesis of 1, 2-diketones via a metal-free, visible-light-induced aerobic photooxidation of alkynes. J. Org. Chem. 2016, 81, 7256–7261. [Google Scholar] [CrossRef] [PubMed]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Liu, F. Photo-Induced Aerobic Oxidation of C–H Bonds. Molecules 2024, 29, 5277. https://doi.org/10.3390/molecules29225277
Chen H, Liu F. Photo-Induced Aerobic Oxidation of C–H Bonds. Molecules. 2024; 29(22):5277. https://doi.org/10.3390/molecules29225277
Chicago/Turabian StyleChen, Haolin, and Feng Liu. 2024. "Photo-Induced Aerobic Oxidation of C–H Bonds" Molecules 29, no. 22: 5277. https://doi.org/10.3390/molecules29225277
APA StyleChen, H., & Liu, F. (2024). Photo-Induced Aerobic Oxidation of C–H Bonds. Molecules, 29(22), 5277. https://doi.org/10.3390/molecules29225277





