Mycophenolate Mofetil, an Inhibitor of Inosine Monophosphate Dehydrogenase, and Tofacitinib, a Janus Kinase Inhibitor, Attenuate Airway Inflammation and Hyperresponsiveness in a Mouse Model of Allergic Asthma
Abstract
1. Introduction
2. Results
2.1. Interpretation of the Results
2.1.1. Ovalbumin(OVA)-Induced Effect
2.1.2. Prevention/Abolishment of OVA-Induced Effect
2.1.3. Reduction/Attenuation of OVA-Induced Effect
2.1.4. Decrease in the Value of Parameter
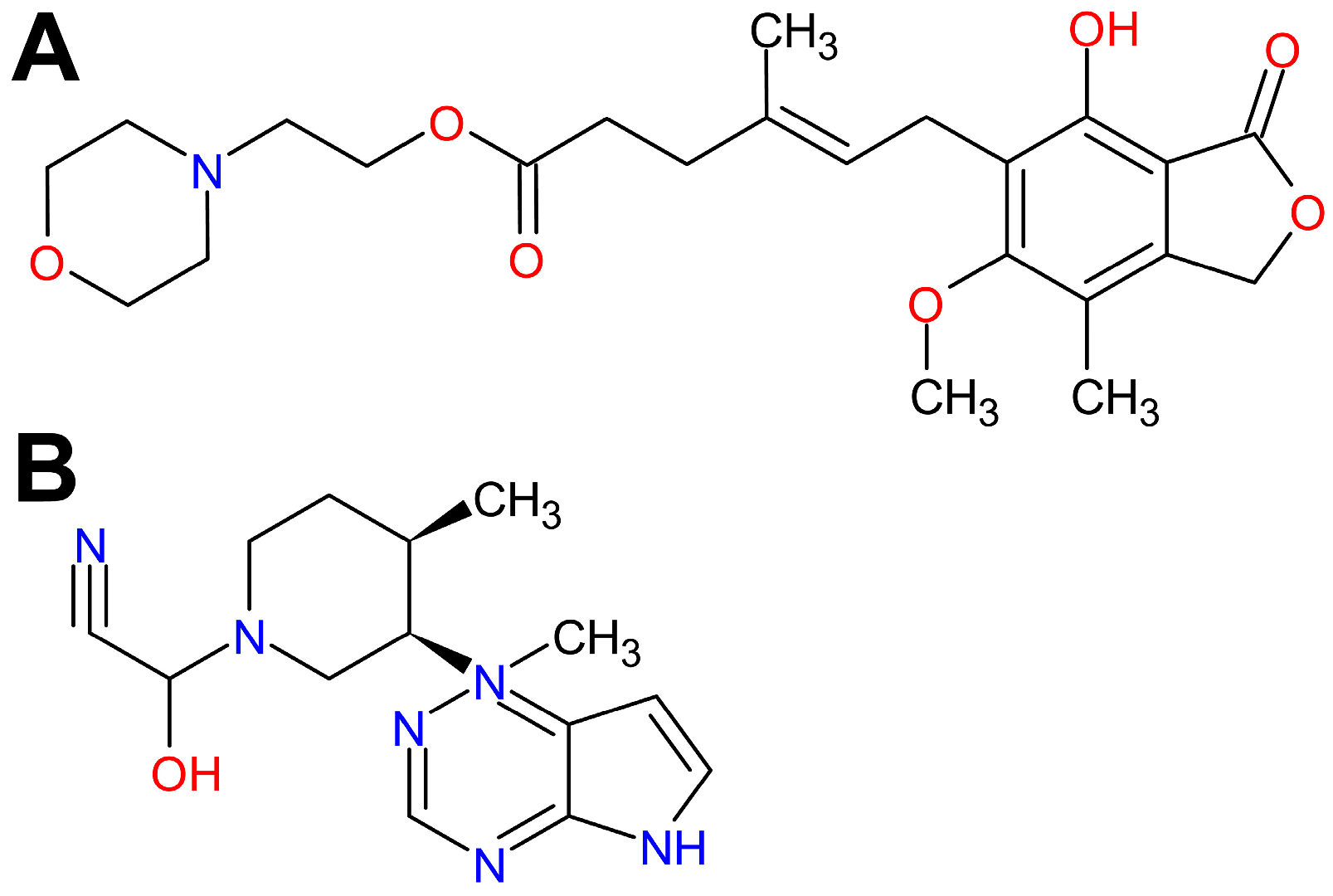
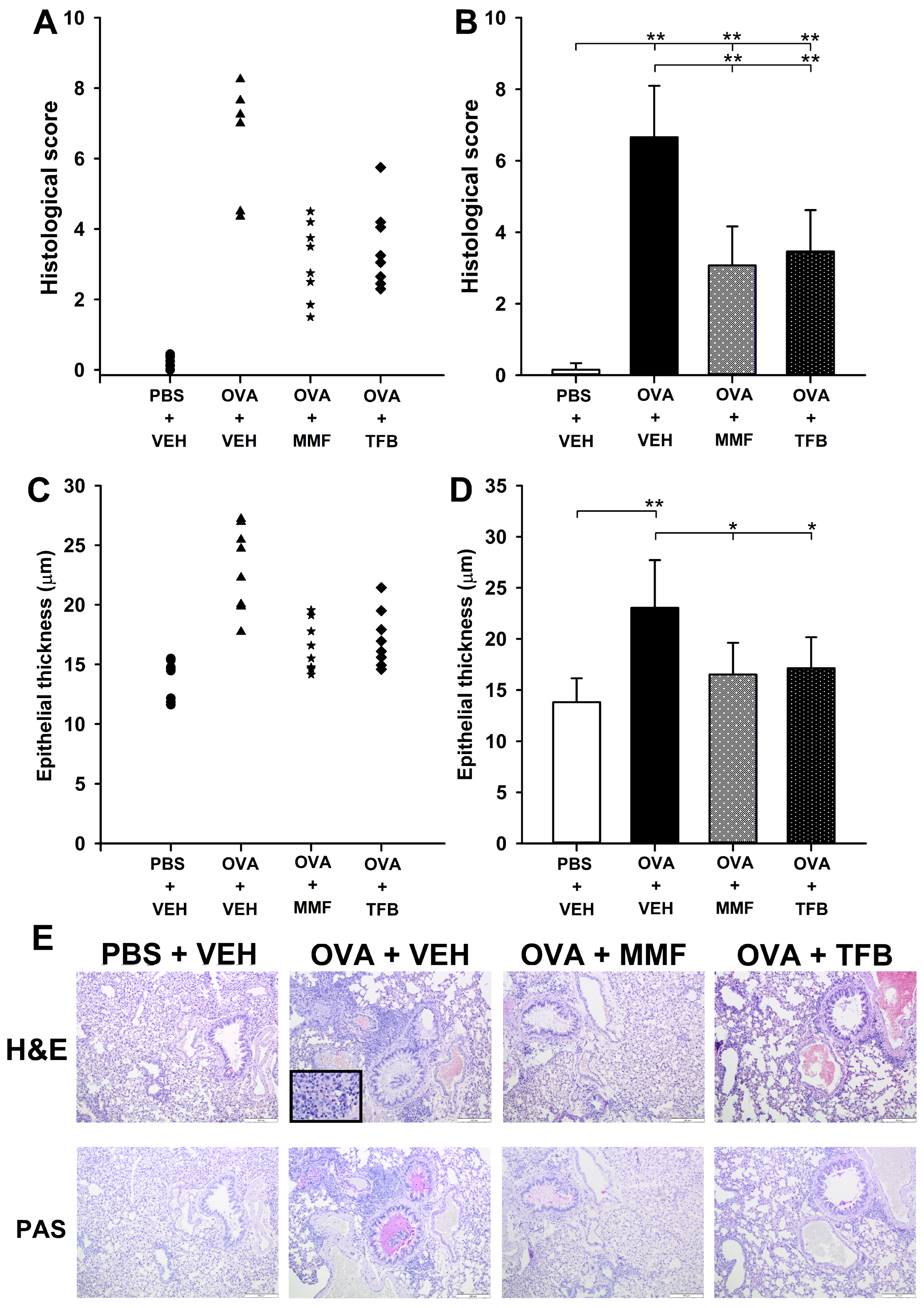
2.2. MMF and TFB Do Not Prevent, but Attenuate OVA-Induced AAI
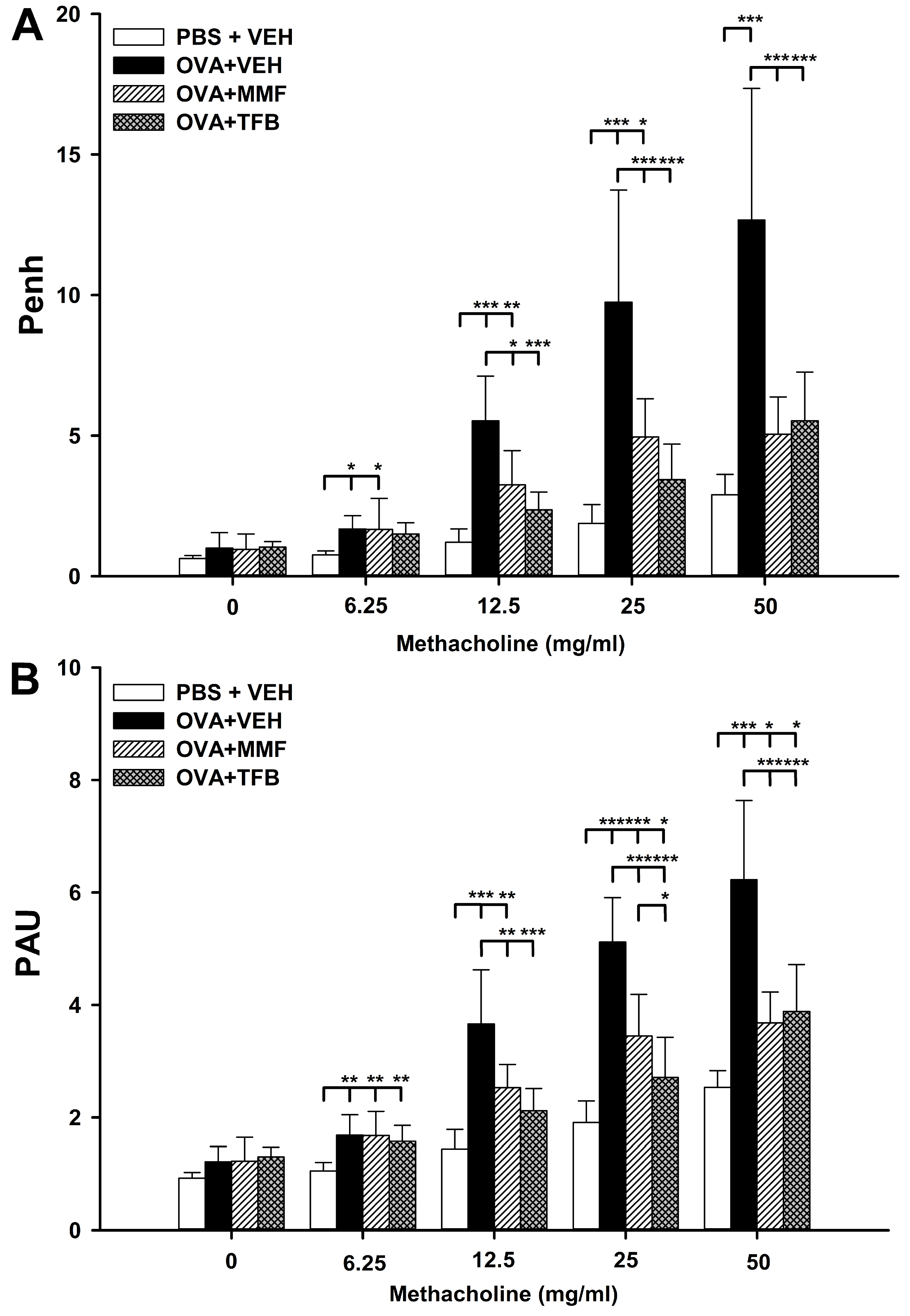
2.3. MMF Reduces OVA-Induced Increases in the Values of Enhanced Pause (Penh) and Pause (PAU), While TFB Prevents and Reduces, Respectively, These Increases
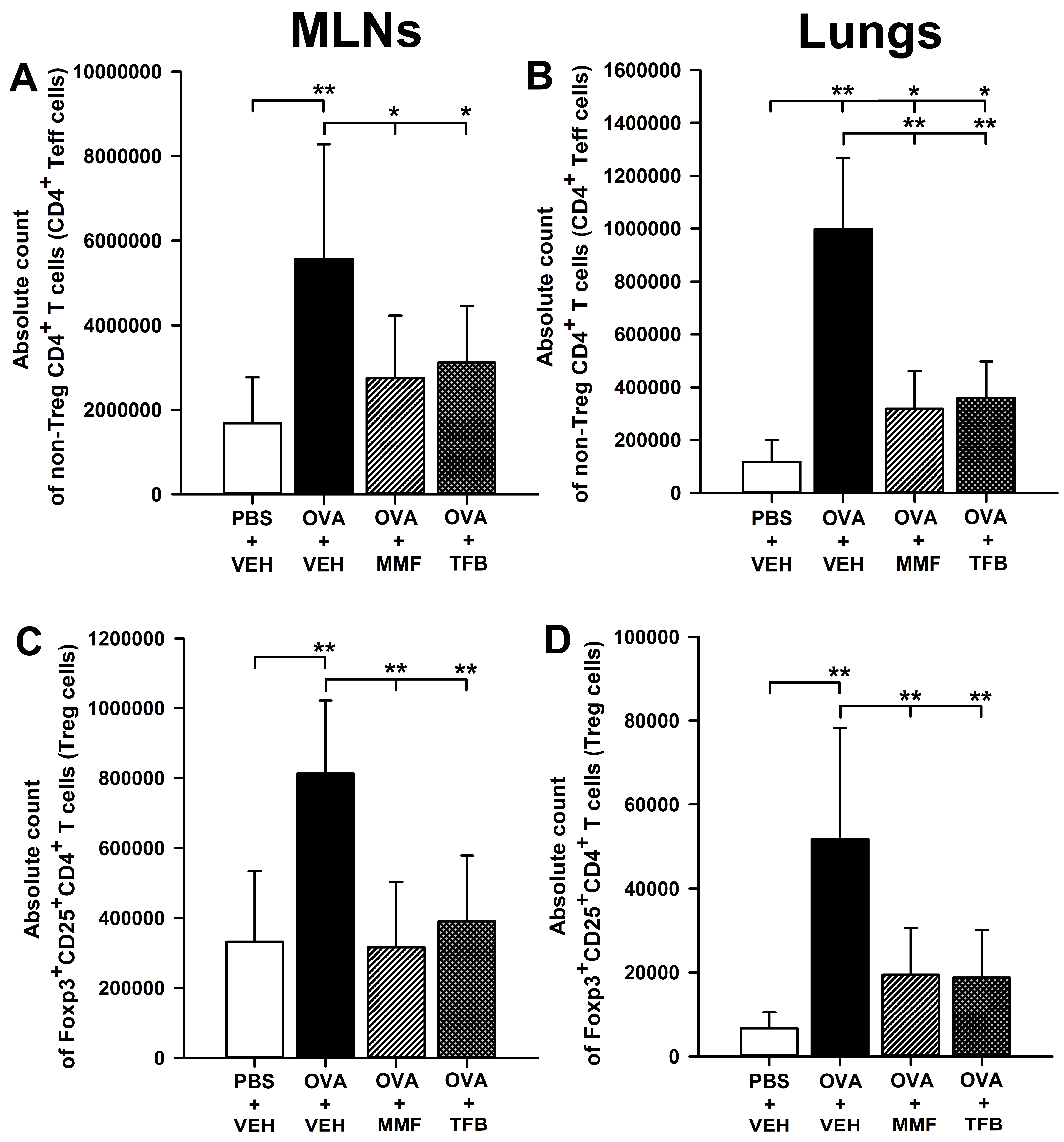
2.4. MMF and TFB Prevents OVA-Induced Increase in the Count of Non-Treg CD4+ T Cells in the Lungs
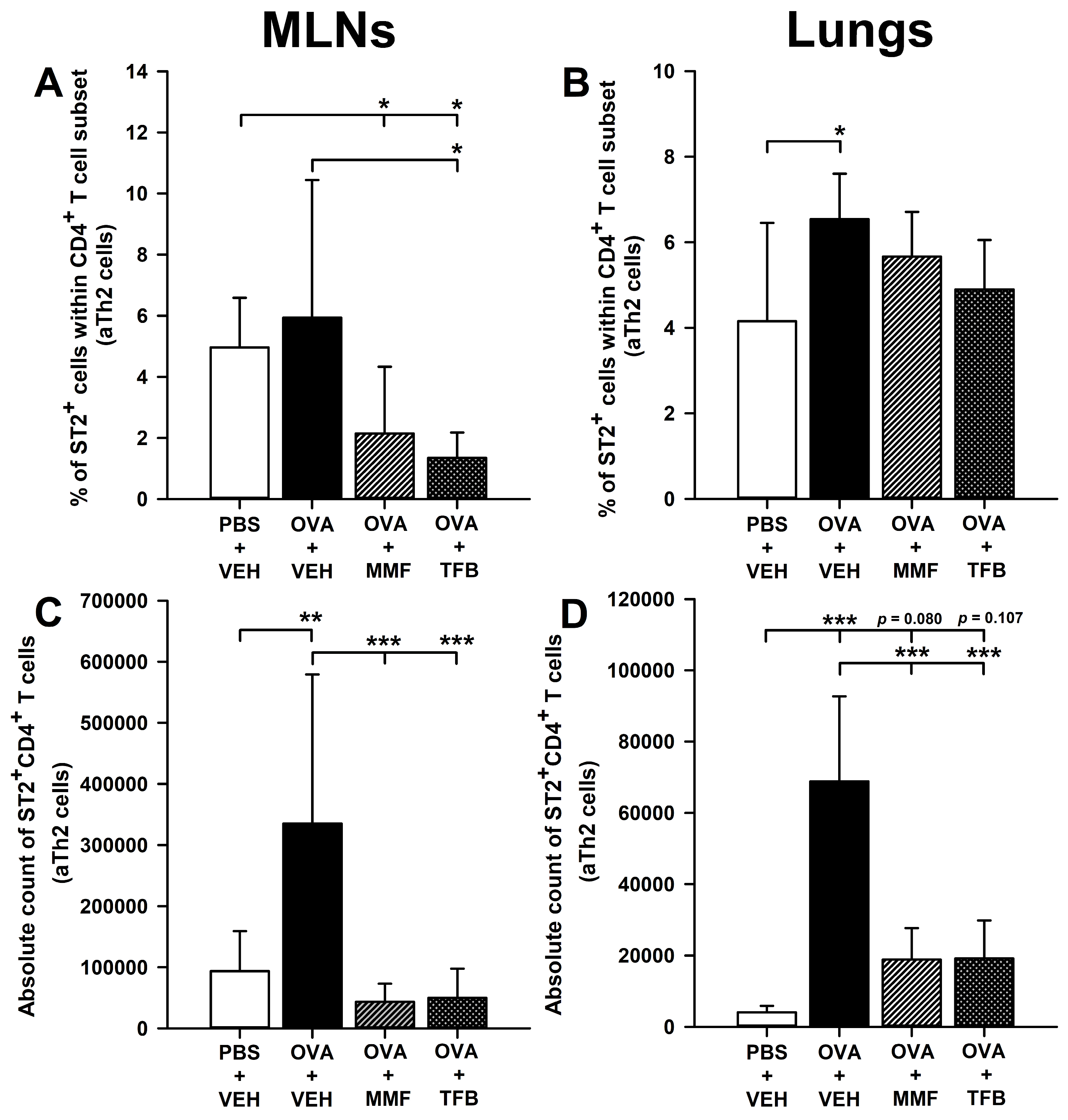
2.5. MMF and TFB Decrease the Percentage of Suppression of Tumorigenicity-2(ST2)+ Cells Within the CD4+ T Cell Subset in the MLNs and Prevent OVA-Induced Increase in the Absolute Number of ST2+CD4+ T Cells in the MLNs and Lungs
3. Discussion
4. Materials and Methods
4.1. Animals
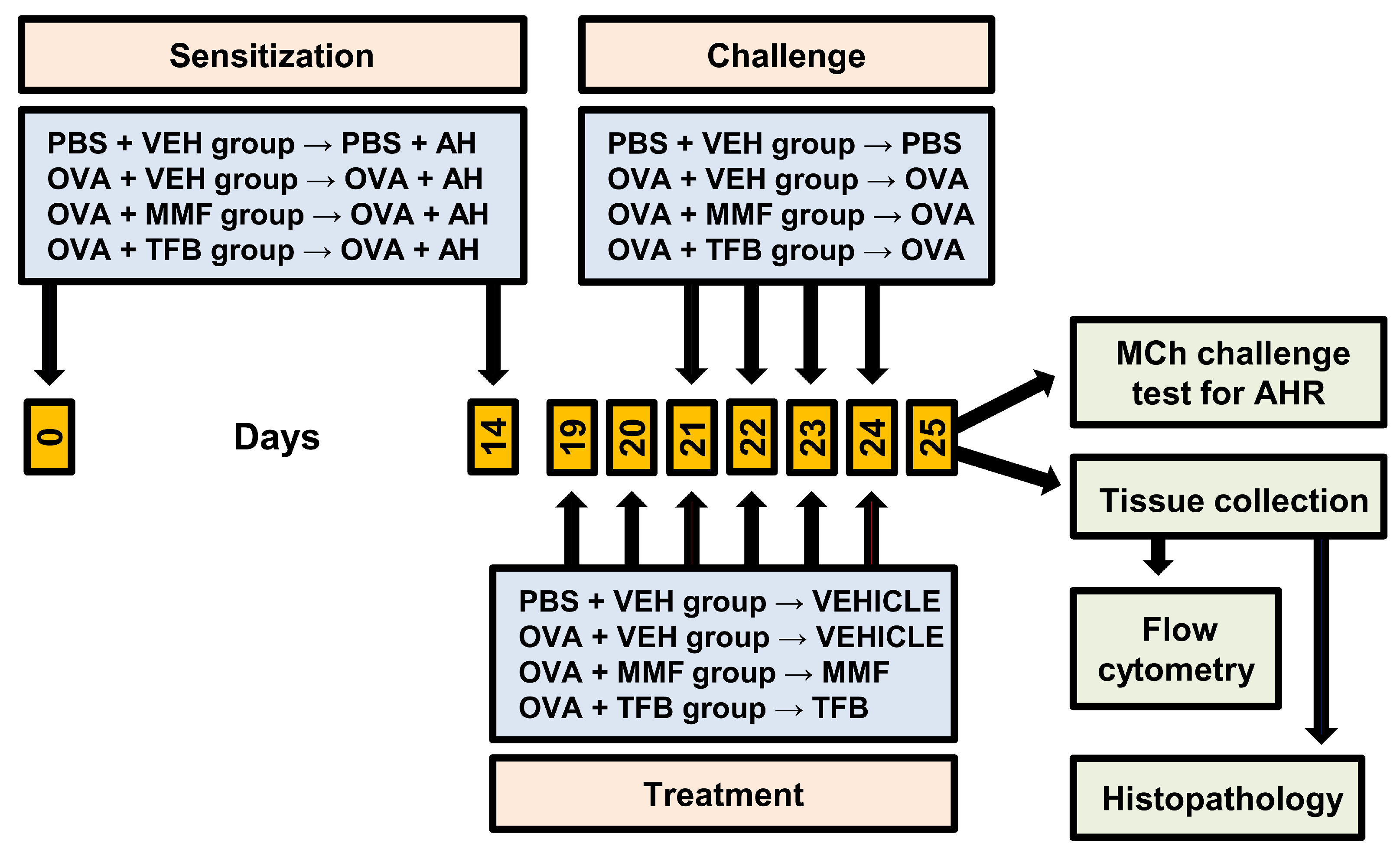
4.2. Antigen Immunization, Airway Challenge and Treatment Protocol
4.3. Measurement of Airway Hyperresponsiveness (AHR)
4.4. Lung Histology
4.5. Isolation of Inductive and Effector Site Lymphocytes
4.5.1. MLNs
4.5.2. Lungs
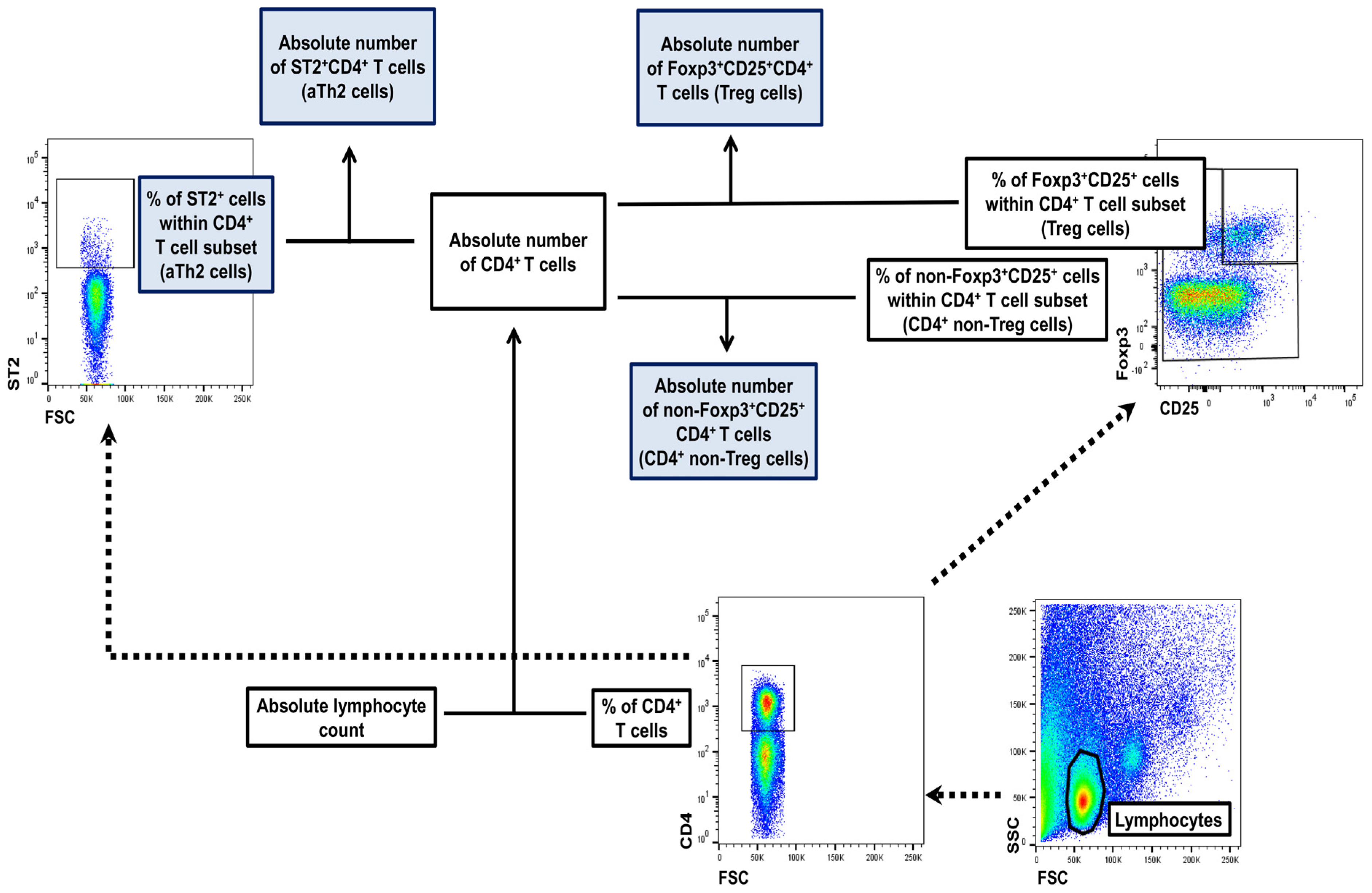
4.6. Flow Cytometry
4.6.1. Extracellular Staining
4.6.2. Intracellular Staining for Foxp3
4.7. FACS Acquisition and Analysis
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir. Med. 2017, 5, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Castillo, E.F.; Zheng, H.; Yang, X.O. Orchestration of epithelial-derived cytokines and innate immune cells in allergic airway inflammation. Cytokine Growth Factor Rev. 2018, 39, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Bryan, S.A.; Leckie, M.J.; Hansel, T.T.; Barnes, P.J. Novel therapy for asthma. Expert Opin. Investig. Drugs 2000, 9, 25–42. [Google Scholar] [PubMed]
- Barnes, P.J.; Adcock, I.M. How do corticosteroids work in asthma? Ann. Intern. Med. 2003, 139, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Hu, C.; Di Nardo, M.; Srinivasan, V.; Adamko, D.J.; Sun, J.; Du, Y.; Zeng, X. Effectiveness of polyvalent bacterial lysate for pediatric asthma control: A retrospective propensity score-matched cohort study. Transl. Pediatr. 2022, 11, 1697–1703. [Google Scholar] [CrossRef]
- Gidaris, D.K.; Stabouli, S.; Bush, A. Beware the inhaled steroids or corticophobia? Swiss. Med. Wkly. 2021, 151, w20450. [Google Scholar] [CrossRef]
- Barnes, P.J. Corticosteroid resistance in patients with asthma and chronic obstructive pulmonary disease. J. Allergy Clin. Immunol. 2013, 131, 636–645. [Google Scholar] [CrossRef]
- Ramamoorthy, S.; Cidlowski, J.A. Exploring the molecular mechanisms of glucocorticoid receptor action from sensitivity to resistance. Endocr. Dev. 2013, 24, 41–56. [Google Scholar]
- Allison, A.C.; Eugui, E.M. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation 2005, 80, 181–190. [Google Scholar] [CrossRef]
- Bremm, M.; Huenecke, S.; Zimmermann, O.; Pfirrmann, V.; Quaiser, A.; Bonig, H.; Soerensen, J.; Klingebiel, T.; Rettinger, E.; Bader, P.; et al. In-vitro influence of mycophenolate mofetil (MMF) and Ciclosporin A (CsA) on cytokine induced killer (CIK) cell immunotherapy. J. Transl. Med. 2016, 14, 264. [Google Scholar] [CrossRef]
- Bhat, R.; Tonutti, A.; Timilsina, S.; Selmi, C.; Gershwin, M.E. Perspectives on Mycophenolate Mofetil in the Management of Autoimmunity. Clin. Rev. Allergy Immunol. 2023, 65, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C. Mechanisms of action of mycophenolate mofetil. Lupus 2005, 14 (Suppl. 1), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Villarino, A.V.; Kanno, Y.; O’Shea, J.J. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat. Immunol. 2017, 18, 374–384. [Google Scholar] [CrossRef]
- Gandolfo, S.; Ciccia, F. JAK/STAT Pathway Targeting in Primary Sjögren Syndrome. Rheumatol. Immunol. Res. 2022, 3, 95–102. [Google Scholar] [CrossRef]
- Georas, S.N.; Donohue, P.; Connolly, M.; Wechsler, M.E. JAK inhibitors for asthma. J. Allergy Clin. Immunol. 2021, 148, 953–963. [Google Scholar] [CrossRef]
- Ashino, S.; Takeda, K.; Li, H.; Taylor, V.; Joetham, A.; Pine, P.R.; Gelfand, E.W. Janus kinase 1/3 signaling pathways are key initiators of TH2 differentiation and lung allergic responses. J. Allergy Clin. Immunol. 2014, 133, 1162–1174. [Google Scholar] [CrossRef] [PubMed]
- Dengler, H.S.; Wu, X.; Peng, I.; Rinderknecht, C.H.; Kwon, Y.; Suto, E.; Kohli, P.B.; Liimatta, M.; Barrett, K.; Lloyd, J.; et al. Lung-restricted inhibition of Janus kinase 1 is effective in rodent models of asthma. Sci. Transl. Med. 2018, 10, eaao2151. [Google Scholar] [CrossRef]
- Li, R.F.; Wang, G.F. JAK/STAT5 signaling pathway inhibitor ruxolitinib reduces airway inflammation of neutrophilic asthma in mice model. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 835–843. [Google Scholar]
- Calbet, M.; Ramis, I.; Calama, E.; Carreño, C.; Paris, S.; Maldonado, M.; Orellana, A.; Calaf, E.; Pauta, M.; De Alba, J.; et al. Novel Inhaled Pan-JAK Inhibitor, LAS194046, Reduces Allergen-Induced Airway Inflammation, Late Asthmatic Response, and pSTAT Activation in Brown Norway Rats. J. Pharmacol. Exp. Ther. 2019, 370, 137–147. [Google Scholar] [CrossRef]
- Kim, J.; Ham, J.; Kang, H.R.; Bae, Y.S.; Kim, T.; Kim, H.Y. JAK3 inhibitor suppresses multipotent ILC2s and attenuates steroid-resistant asthma. Sci. Adv. 2023, 9, eadi3770. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/search?intr=JAK%20Inhibitor (accessed on 24 September 2024).
- Lv, Y.; Qi, J.; Babon, J.J.; Cao, L.; Fan, G.; Lang, J.; Zhang, J.; Mi, P.; Kobe, B.; Wang, F. The JAK-STAT pathway: From structural biology to cytokine engineering. Signal Transduct. Target. Ther. 2024, 9, 221. [Google Scholar] [CrossRef] [PubMed]
- Younis, U.S.; Vallorz, E.; Addison, K.J.; Ledford, J.G.; Myrdal, P.B. Preformulation and Evaluation of Tofacitinib as a Therapeutic Treatment for Asthma. AAPS PharmSciTech 2019, 20, 167. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. Taking our breath away: Dendritic cells in the pathogenesis of asthma. Nat. Rev. Immunol. 2003, 3, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Murdoch, J.R.; Lloyd, C.M. Chronic inflammation and asthma. Mutat. Res. 2010, 690, 24–39. [Google Scholar] [CrossRef]
- Maeshima, K.; Yamaoka, K.; Kubo, S.; Nakano, K.; Iwata, S.; Saito, K.; Ohishi, M.; Miyahara, H.; Tanaka, S.; Ishii, K.; et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-γ and interleukin-17 production by human CD4+ T cells. Arthritis Rheum. 2012, 64, 1790–1798. [Google Scholar] [CrossRef]
- Texler, B.; Zollner, A.; Reinstadler, V.; Reider, S.J.; Macheiner, S.; Jelusic, B.; Pfister, A.; Watschinger, C.; Przysiecki, N.; Tilg, H.; et al. Tofacitinib-Induced Modulation of Intestinal Adaptive and Innate Immunity and Factors Driving Cellular and Systemic Pharmacokinetics. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 383–404. [Google Scholar] [CrossRef]
- Townsend, M.J.; Fallon, P.G.; Matthews, D.J.; Jolin, H.E.; McKenzie, A.N. T1/ST2-deficient mice demonstrate the importance of T1/ST2 in developing primary T helper cell type 2 responses. J. Exp. Med. 2000, 191, 1069–1076. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.; Liu, Z. Role of IL-33-ST2 pathway in regulating inflammation: Current evidence and future perspectives. J. Transl. Med. 2023, 21, 902. [Google Scholar] [CrossRef]
- Tao, B.; Ruan, G.; Wang, D.; Li, Y.; Wang, Z.; Yin, G. Imbalance of peripheral Th17 and regulatory T cells in children with allergic rhinitis and bronchial asthma. Iran. J. Allergy Asthma Immunol. 2015, 14, 273–279. [Google Scholar]
- Tian, M.; Wang, Y.; Lu, Y.; Jiang, Y.H.; Zhao, D.Y. Effects of sublingual immunotherapy for Dermatophagoides farinae on Th17 cells and CD4+CD25+ regulatory T cells in peripheral blood of children with allergic asthma. Int. Forum Allergy Rhinol. 2014, 4, 371–375. [Google Scholar] [CrossRef]
- Demirkiran, A.; Sewgobind, V.D.; van der Weijde, J.; Kok, A.; Baan, C.C.; Kwekkeboom, J.; Tilanus, H.W.; Metselaar, H.J.; van der Laan, L.J. Conversion from calcineurin inhibitor to mycophenolate mofetil-based immunosuppression changes the frequency and phenotype of CD4+FOXP3+ regulatory T cells. Transplantation 2009, 87, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Abadja, F.; Atemkeng, S.; Alamartine, E.; Berthoux, F.; Mariat, C. Impact of mycophenolic acid and tacrolimus on Th17-related immune response. Transplantation 2011, 92, 396–403. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Smeets, R.L.; Koenen, H.J.; Vink, P.M.; Wagenaars, J.; Boots, A.M.; Joosten, I. Mycophenolic acid-mediated suppression of human CD4+ T cells: More than mere guanine nucleotide deprivation. Am. J. Transplant. 2011, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Guzera, M.; Szulc-Dąbrowska, L.; Cywińska, A.; Archer, J.; Winnicka, A. In Vitro Influence of Mycophenolic Acid on Selected Parameters of Stimulated Peripheral Canine Lymphocytes. PLoS ONE 2016, 11, e0154429. [Google Scholar] [CrossRef]
- Slight-Webb, S.; Guthridge, J.M.; Chakravarty, E.F.; Chen, H.; Lu, R.; Macwana, S.; Bean, K.; Maecker, H.T.; Utz, P.J.; James, J.A. Mycophenolate mofetil reduces STAT3 phosphorylation in systemic lupus erythematosus patients. JCI Insight 2019, 4, e124575. [Google Scholar] [CrossRef]
- Zuśka-Prot, M.; Maślanka, T. Effect of inhaled and systemic glucocorticoid treatment on CD4+ regulatory and effector T cells in a mouse model of allergic asthma. Int. Immunopharmacol. 2017, 45, 98–109. [Google Scholar] [CrossRef]
- Maślanka, T.; Otrocka-Domagała, I.; Zuśka-Prot, M.; Gesek, M. Beneficial effects of rosiglitazone, a peroxisome proliferator-activated receptor-γ agonist, in a mouse allergic asthma model is not associated with the recruitment or generation of Foxp3-expressing CD4+ regulatory T cells. Eur. J. Pharmacol. 2019, 848, 30–38. [Google Scholar] [CrossRef]
- Gregorczyk, I.; Maślanka, T. Blockade of RANKL/RANK and NF-κB signalling pathways as novel therapeutic strategies for allergic asthma: A comparative study in a mouse model of allergic airway inflammation. Eur. J. Pharmacol. 2020, 879, 173129. [Google Scholar] [CrossRef]
- Grainge, C.; Jayasekera, N.; Dennison, P.; Rupani, H.; Kurukulaaratchy, R.; Howarth, P. Case series reporting the effectiveness of mycophenolate mofetil in treatment-resistant asthma. Eur. Respir. J. 2013, 42, 1134–1137. [Google Scholar] [CrossRef]
- Mao, H.; Chen, X.; Yi, Q.; Li, S.; Wang, Z.L.; Li, F. Mycophenolate mofetil and triptolide alleviating airway inflammation in asthmatic model mice partly by inhibiting bone marrow eosinophilopoiesis. Int. Immunopharmacol. 2008, 8, 1039–1048. [Google Scholar] [CrossRef]
- Kudlacz, E.; Conklyn, M.; Andresen, C.; Whitney-Pickett, C.; Changelian, P. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur. J. Pharmacol. 2008, 582, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Maślanka, T.; Otrocka-Domagała, I.; Zuśka-Prot, M.; Mikiewicz, M.; Przybysz, J.; Jasiecka, A.; Jaroszewski, J.J. IκB kinase β inhibitor, IMD-0354, prevents allergic asthma in a mouse model through inhibition of CD4+ effector T cell responses in the lung-draining mediastinal lymph nodes. Eur. J. Pharmacol. 2016, 775, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.A.; Piñera, C.; Setién, M.A.; Buelta, L.; de Cos, M.A.; de Francisco, A.L.; Merino, R.; Arias, M. Modulation of autoantibody production by mycophenolate mofetil: Effects on the development of SLE in (NZB x NZW)F1 mice. Nephrol. Dial. Transplant. 2003, 18, 878–883. [Google Scholar] [CrossRef]
- Dalmarco, E.M.; Astolfi, G.; de Liz, R.; de Córdova, C.M.; Fröde, T.S. Modulatory effect of mycophenolate mofetil on carrageenan-induced inflammation in the mouse air pouch model. Int. Immunopharmacol. 2012, 13, 476–482. [Google Scholar] [CrossRef]
- Zhang, L.L.; Pan, H.X.; Wang, Y.X.; Guo, T.; Liu, L. Genome profiling revealed the activation of IL2RG/JAK3/STAT5 in peripheral T-cell lymphoma expressing the ITK-SYK fusion gene. Int. J. Oncol. 2019, 55, 1077–1089. [Google Scholar] [CrossRef]
- Hablot, J.; Ferhat, M.; Lavelle, A.; Salem, F.; Taieb, M.; Medvedovic, J.; Kindt, N.; Reboul, P.; Cailotto, F.; Jouzeau, J.Y.; et al. Tofacitinib treatment alters mucosal immunity and gut microbiota during experimental arthritis. Clin. Transl. Med. 2020, 10, e163. [Google Scholar] [CrossRef]
- Aung, W.W.; Wang, C.; Xibei, J.; Horii, M.; Mizumaki, K.; Kano, M.; Okamura, A.; Kobayashi, T.; Matsushita, T. Immunomodulating role of the JAKs inhibitor tofacitinib in a mouse model of bleomycin-induced scleroderma. J. Dermatol. Sci. 2021, 101, 174–184. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, S.; Holmes, A.P.; Dallas, M.L.; Mahmoud, A.D.; Shipston, M.J.; Peers, C.; Hardie, D.G.; Kumar, P.; Evans, A.M. Publisher Correction: LKB1 is the gatekeeper of carotid body chemosensing and the hypoxic ventilatory response. Commun. Biol. 2022, 5, 765. [Google Scholar] [CrossRef]
- Data Sciences International. DSI FinePointe™ Rodent WBP Application Manual. User Manual. 2024; p. 2003. Available online: https://support.datasci.com/hc/en-us/article_attachments/28816741637011 (accessed on 24 September 2024).
- Wittke, A.; Weaver, V.; Mahon, B.D.; August, A.; Cantorna, M.T. Vitamin D receptor-deficient mice fail to develop experimental allergic asthma. J. Immunol. 2004, 173, 3432–3436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kravčenia, B.; Maślanka, T. Mycophenolate Mofetil, an Inhibitor of Inosine Monophosphate Dehydrogenase, and Tofacitinib, a Janus Kinase Inhibitor, Attenuate Airway Inflammation and Hyperresponsiveness in a Mouse Model of Allergic Asthma. Molecules 2024, 29, 5293. https://doi.org/10.3390/molecules29225293
Kravčenia B, Maślanka T. Mycophenolate Mofetil, an Inhibitor of Inosine Monophosphate Dehydrogenase, and Tofacitinib, a Janus Kinase Inhibitor, Attenuate Airway Inflammation and Hyperresponsiveness in a Mouse Model of Allergic Asthma. Molecules. 2024; 29(22):5293. https://doi.org/10.3390/molecules29225293
Chicago/Turabian StyleKravčenia, Bernard, and Tomasz Maślanka. 2024. "Mycophenolate Mofetil, an Inhibitor of Inosine Monophosphate Dehydrogenase, and Tofacitinib, a Janus Kinase Inhibitor, Attenuate Airway Inflammation and Hyperresponsiveness in a Mouse Model of Allergic Asthma" Molecules 29, no. 22: 5293. https://doi.org/10.3390/molecules29225293
APA StyleKravčenia, B., & Maślanka, T. (2024). Mycophenolate Mofetil, an Inhibitor of Inosine Monophosphate Dehydrogenase, and Tofacitinib, a Janus Kinase Inhibitor, Attenuate Airway Inflammation and Hyperresponsiveness in a Mouse Model of Allergic Asthma. Molecules, 29(22), 5293. https://doi.org/10.3390/molecules29225293




