Mechanistic Study of Atomic Oxygen Erosion on Polyimide Under Electric Fields: A Molecular Dynamics and Density Functional Theory Approach
Abstract
:1. Introduction
2. Results and Discussion
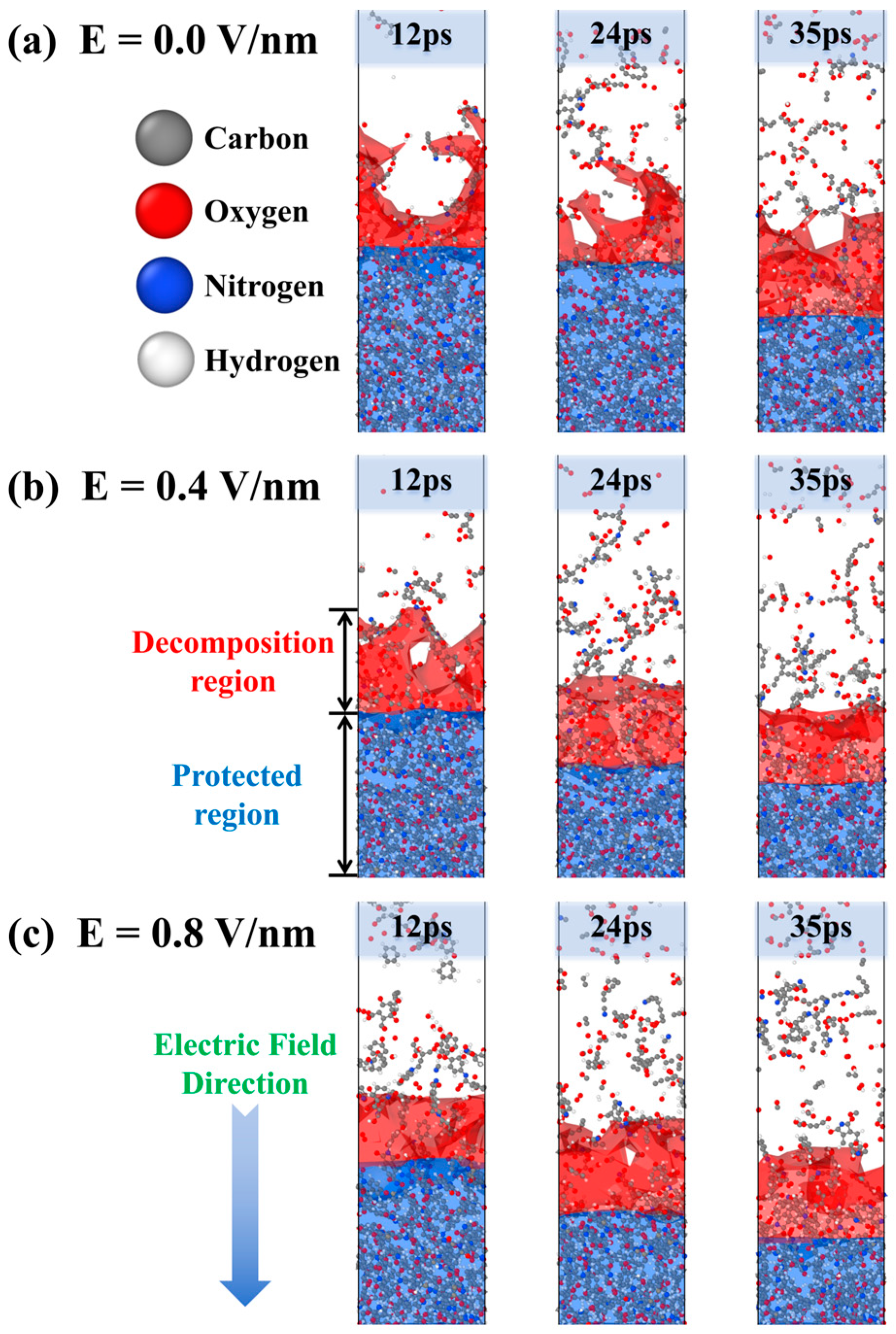
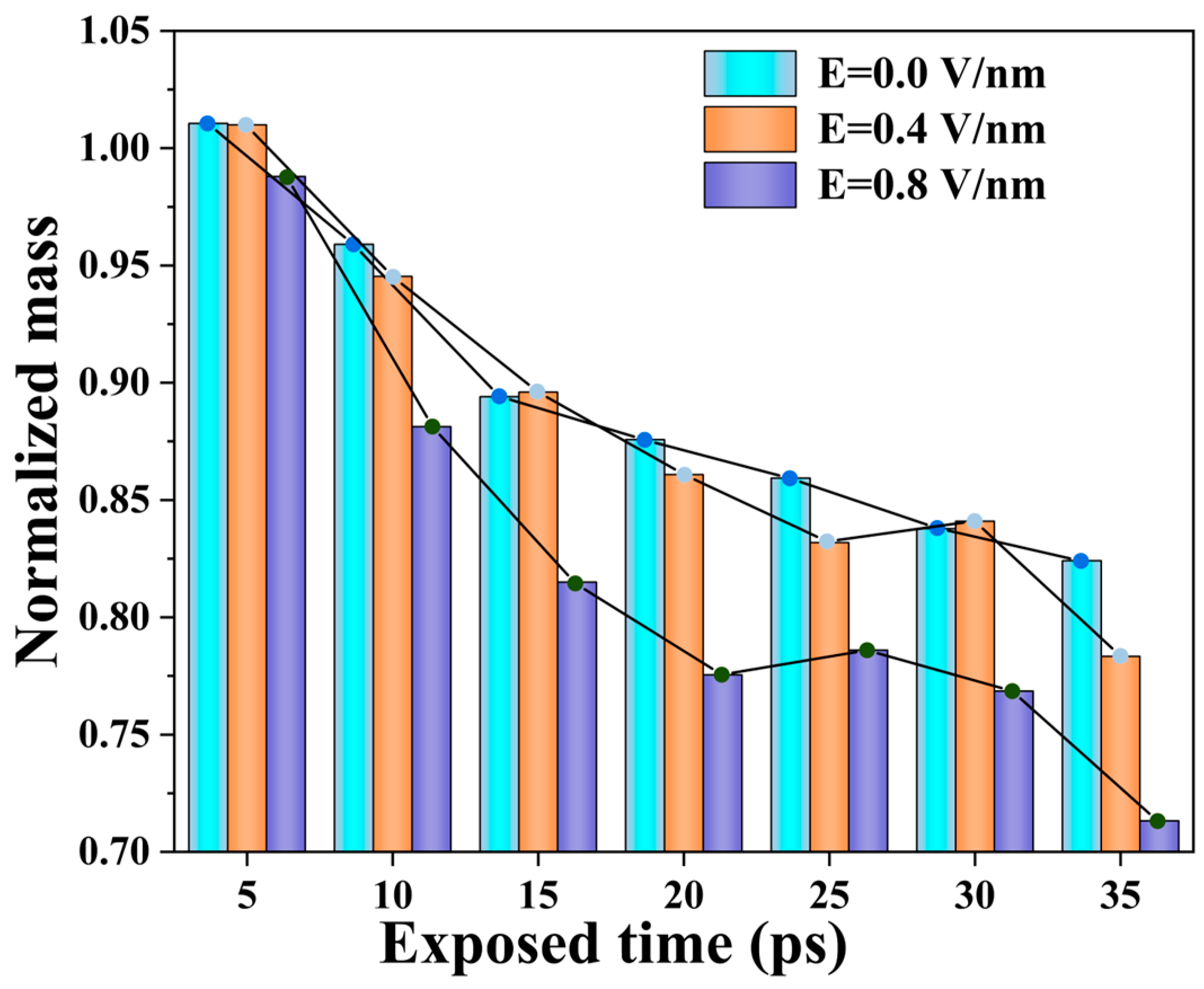
2.1. Polyimide Erosion Kinetics Under Electric Fields
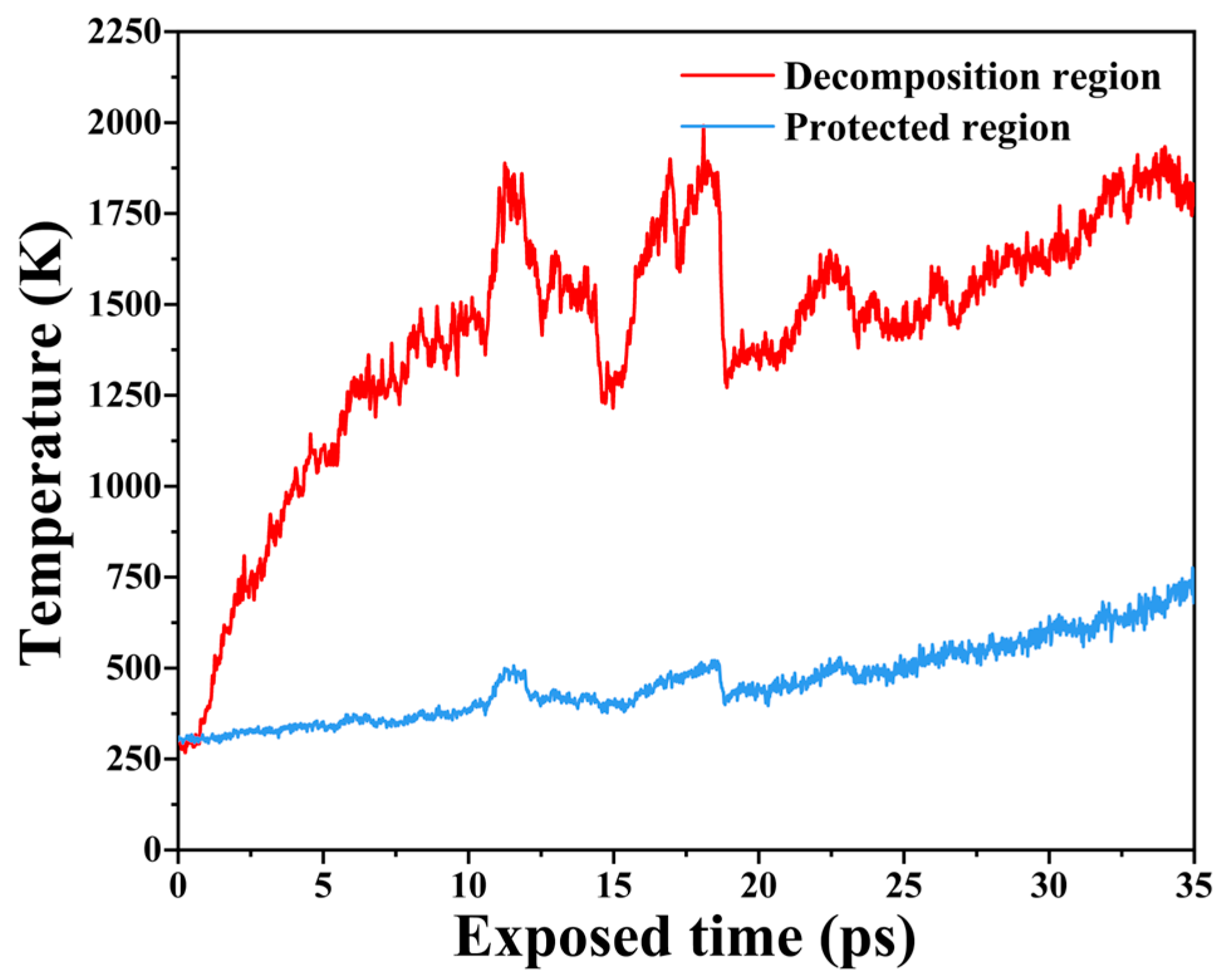
2.2. Free Volume and Temperature Evolution During AO Erosion
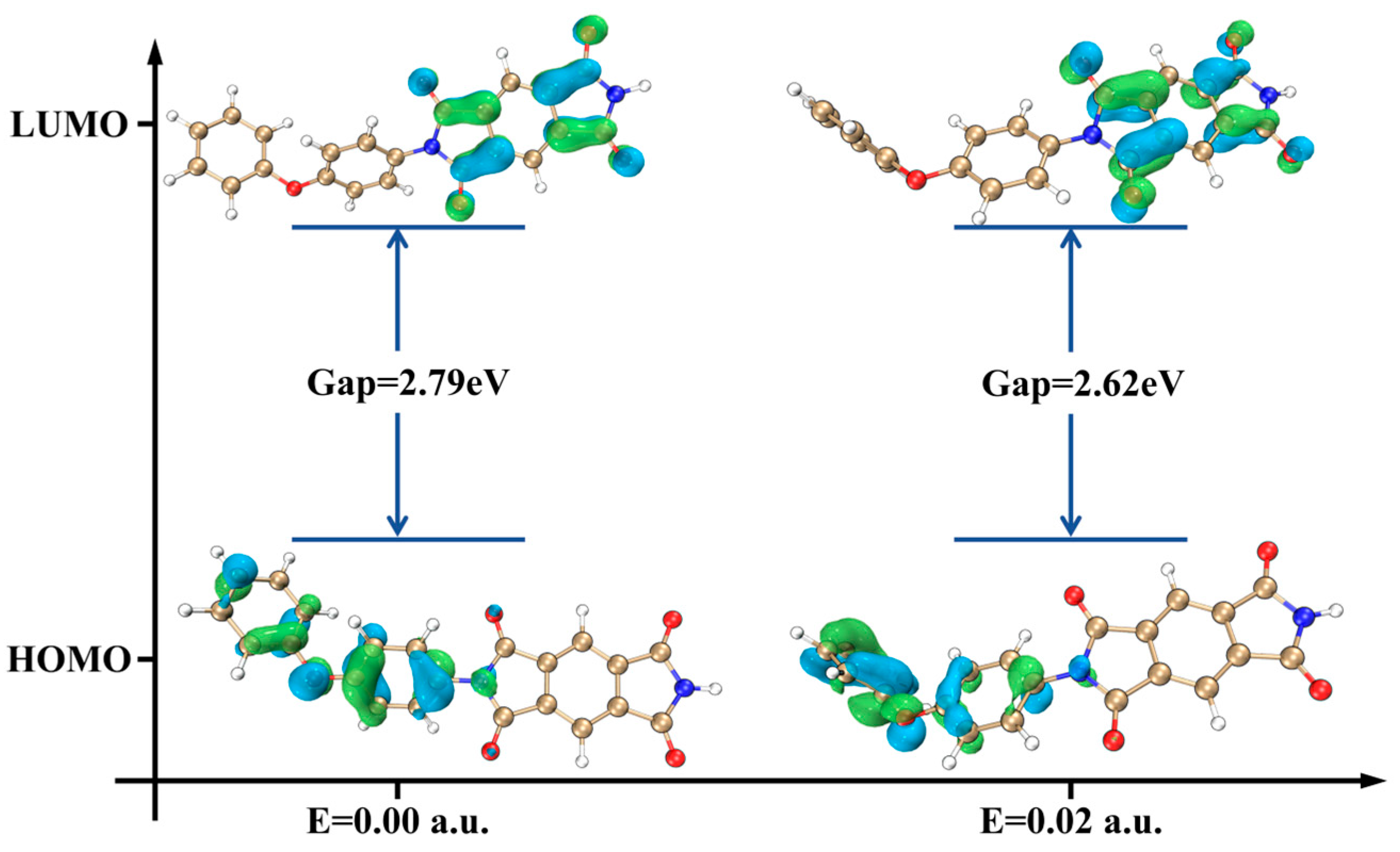
2.3. AO Erosion Product Analysis
3. Methods and Models
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, M.; Niu, H.; Wu, D. Polyimide Fibers with High Strength and High Modulus: Preparation, Structures, Properties, and Applications. Macromol. Rapid Commun. 2018, 20, e1800141. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; He, J.; Yang, H.; Yang, S. Progress in Aromatic Polyimide Films for Electronic Applications: Preparation, Structure and Properties. Polymers 2022, 14, 1269. [Google Scholar] [CrossRef] [PubMed]
- Shao, D.; Xu, C.; Wang, H.; Du, J. Enhancing the Dyeability of Polyimide Fibers with the Assistance of Swelling Agents. Materials 2019, 12, 347. [Google Scholar] [CrossRef] [PubMed]
- Ogbonna, V.E.; Popoola, A.P.I.; Popoola, O.M.; Adeosun, S.O. A Review on Polyimide Reinforced Nanocomposites for Mechanical, Thermal, and Electrical Insulation Applications: Challenges and Recommendations for Future Improvement. Polym. Bull. 2020, 1, 663–695. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional Polyimide/Polyhedral Oligomeric Silsesquioxane Nanocomposites. Polymers 2018, 11, 26. [Google Scholar] [CrossRef]
- Cordill, M.J.; Kreiml, P.; Lassnig, A.; Gebhart, D.D.; Zak, S.; Mitterer, C.; Griesser, T.; Milassin, G. Annealing Effects on Al/Polyimide Adhesion in Flexible Optical Solar Reflectors. IOP Conf. Ser. Mater. Sci. Eng. 2023, 1287, 012006. [Google Scholar] [CrossRef]
- He, J.-J.; Yang, H.-X.; Zheng, F.; Yang, S.-Y. Dielectric Properties of Fluorinated Aromatic Polyimide Films with Rigid Polymer Backbones. Polymers 2022, 14, 649. [Google Scholar] [CrossRef]
- Lan, Z.; Li, C.; Yu, Y.; Wei, J. Colorless Semi-Alicyclic Copolyimides with High Thermal Stability and Solubility. Polymers 2019, 11, 1319. [Google Scholar] [CrossRef]
- Goverapet, S.S.; van Duin, A.C.T. Molecular-Dynamics-Based Study of the Collisions of Hyperthermal Atomic Oxygen with Graphene Using the ReaxFF Reactive Force Field. J. Phys. Chem. A 2011, 46, 13269–13280. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, T.; Zhou, L.; Xu, B.; Ju, Y.; Zhang, Q.; Wu, Z. Radiation Effect of Vacuum Ultraviolet on the Performance of Polyimide Films. J. Macromol. Sci. Part B 2024, 6, 497–511. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, L.; Zou, L.; Ayubi, B.I.; Wang, Y. Mechanism Analysis and Potential Applications of Atomic Oxygen Erosion Protection for Kapton-Type Polyimide Based on Molecular Dynamics Simulations. Polymers 2024, 16, 1687. [Google Scholar] [CrossRef] [PubMed]
- Suliga, A.; Jakubczyk, E.M.; Hamerton, I.; Viquerat, A. Analysis of Atomic Oxygen and Ultraviolet Exposure Effects on Cycloaliphatic Epoxy Resins Reinforced with Octa-Functional POSS. Acta Astronaut. 2018, 142, 103–111. [Google Scholar] [CrossRef]
- Clausi, M.; Santonicola, M.G.; Schirone, L.; Laurenzi, S. Analysis of Ultraviolet Exposure Effects on the Surface Properties of Epoxy/Graphene Nanocomposite Films on Mylar Substrate. Acta Astronaut. 2017, 134, 307–313. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Xu, C.; Luo, Y.; Zhou, Y. A Polysilazane Coating Protecting Polyimide from Atomic Oxygen and Vacuum Ultraviolet Radiation Erosion. Surf. Coat. Technol. 2009, 22, 3338–3343. [Google Scholar] [CrossRef]
- Qi, H.; Shi, Q.; Qian, Y.; Li, Y.; Xu, J.; Xu, C.; Xie, X. The Atomic Oxygen Erosion Resistance Effect and Mechanism of the Perhydropolysilazane-Derived SiOx Coating Used on Polymeric Materials in Space Environment. Polymers 2022, 14, 322. [Google Scholar] [CrossRef]
- Hu, L.; Li, M.; Xu, C.; Luo, Y. Perhydropolysilazane Derived Silica Coating Protecting Kapton from Atomic Oxygen Attack. Thin Solid Films 2011, 3, 1063–1068. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Chen, S.N.; Yan, W.Q.; Li, Q.; Chen, L.; Ou, Y.X.; Liao, B. Protection of Kapton from Atomic Oxygen Attack by SiOx/NiCr Coating. Surf. Coat. Technol. 2021, 423, 127582. [Google Scholar] [CrossRef]
- Jeon, I.; Lee, S.; Yang, S. Hyperthermal Erosion of Thermal Protection Nanocomposites Under Atomic Oxygen and N2 Bombardment. Int. J. Mech. Sci. 2023, 240, 107910. [Google Scholar] [CrossRef]
- Matéo-Vélez, J.-C.; Theillaumas, B.; Sévoz, M.; Andersson, B.; Nilsson, T.; Sarrailh, P.; Payan, D. Simulation and Analysis of Spacecraft Charging Using SPIS and NASCAP/GEO. IEEE Trans. Plasma Sci. 2015, 9, 2808–2816. [Google Scholar] [CrossRef]
- Gupta, S.B.; Kalaria, K.R.; Vaghela, N.P.; Mukherjee, S.; Joshi, R.S.; Puthanveettil, S.E.; Ekkundi, R.S. An Overview of Spacecraft Charging Research in India: Spacecraft Plasma Interaction Experiments—SPIX-II. IEEE Trans. Plasma Sci. 2014, 4, 1072–1077. [Google Scholar] [CrossRef]
- Shang, P.; Zhang, B.; Wu, J.; Wang, P.; Zheng, X.; He, B. Grounding Method and Working Voltage Influence on Deep Dielectric Charging of Polyimide in GEO Environment. IEEE Trans. Plasma Sci. 2021, 6, 1975–1982. [Google Scholar] [CrossRef]
- Diaw, E.H.N.; Le Roy, S.; Teyssèdre, G.; Aubert, E. Current Measurements in High Performance Polymers Used in Aeronautic Cables. IEEE Trans. Dielectr. Electr. Insul. 2020, 27, 2195–2202. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, T.; Wang, Y.; Zhang, L.; Zou, L. Microscopic Pyrolytic and Electric Decomposition Mechanism of Insulating Polyimide/Boron Nitride Nanosheet Composites Based on ReaxFF. Polymers 2022, 14, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, L.; Zou, L.; Wang, G.; Wang, Y. Analysis of Atomic Oxygen Erosion Resistance Mechanism of Polyimide Composite Materials Enhanced by Polyhydroxysilylazane. Appl. Surf. Sci. 2024, 657, 159814. [Google Scholar] [CrossRef]
- Wei, Z.; Li, Y.; Zhang, C.; Yang, L.; Chu, L. Revealing the Mechanism on Steam Co-Gasification of Cellulose and Polyethylene: A Combined ReaxFF and DFT Study. Fuel 2023, 334, 126784. [Google Scholar] [CrossRef]
- Li, G.; Zheng, F.; Huang, Q.; Wang, J.; Niu, B.; Zhang, Y.; Long, D. Molecular Insight into Pyrolysis Processes via Reactive Force Field Molecular Dynamics: A State-of-the-Art Review. J. Anal. Appl. Pyrolysis 2022, 166, 105620. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, L.; Zou, L.; Ayubi, B.I.; Wang, Y. Mechanisms of Atomic Oxygen Erosion in Fluorinated Polyimides Investigated by Molecular Dynamics Simulations. Molecules 2024, 29, 4485. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Zeng, F.; Cui, J. Damage and Degradation of Mechanical Properties of Polyimide-Based Materials Under Atomic Oxygen Attack: A Molecular Dynamics Simulation Study. Comput. Mater. Sci. 2024, 243, 113110. [Google Scholar] [CrossRef]
- Qiao, S.; Jiang, L.; Jiang, H.; Liu, Y.; Xu, Y.; Jiao, Z.; Wang, L. Reactive Molecular Dynamics Simulation on the Disintegration of Kapton and Upilex-S During Atomic Oxygen Impact. Front. Mater. 2023, 10, 1234455. [Google Scholar] [CrossRef]
- Lin, D.; Li, R.; Liu, Y.; Qi, S.; Wu, D. Clarifying the Effect of Moisture Absorption and High-Temperature Thermal Aging on Structure and Properties of Polyimide Film at Molecular Dynamic Level. Polymer 2020, 214, 123251. [Google Scholar] [CrossRef]
- Huang, Y.; Rong, C.; Zhang, R.; Liu, S. Evaluating Frontier Orbital Energy and HOMO/LUMO Gap with Descriptors from Density Functional Reactivity Theory. J. Mol. Model. 2017, 1, 3. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Wei, C.; Wang, S.; Qi, F.; He, Z.; Majeed, U.; Huang, J. Crystal Structure Analysis of Three (E)-N-(3-Methyl, 5-Fluorophenyl)-2-(4-Substituted Benzylidene)Thiosemicarbazone Derivatives: Experimental and Theoretical Studies. J. Mol. Struct. 2022, 1247, 131383. [Google Scholar] [CrossRef]
- Alrub, S.A.; Ali, A.I.; Hussein, R.K.; Alghamdi, S.K.; Eladly, S.A. DFT and TD-DFT Investigations for the Limitations of Lengthening the Polyene Bridge Between N,N-Dimethylanilino Donor and Dicyanovinyl Acceptor Molecules as a D-π-A Dye-Sensitized Solar Cell. Int. J. Mol. Sci. 2024, 25, 5586. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, G.; Pan, Y.; Tian, H.; Niu, B.; Xing, Y.; Long, D. Atomic Oxygen Erosion Mechanism of Polyimide via Reactive Molecular Dynamics Simulation and Density Functional Theory Calculation. Polym. Degrad. Stab. 2024, 228, 110928. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Yang, X.; Meng, Y.; Hu, P.; Huang, C.; Liu, F. From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films. Polymers 2024, 16, 2315. [Google Scholar] [CrossRef]
- Li, X.; Zheng, R.; Wang, C.; Chang, H.; Chen, S.; Wang, L.; Shi, J. Preparation and Properties of Low-Dielectric Polyimide Films Containing Tert-Butyl. Polymers 2024, 16, 984. [Google Scholar] [CrossRef]
- Yang, C.; Xing, X.; Li, Z.; Zhang, S. A Comprehensive Review on Water Diffusion in Polymers Focusing on the Polymer–Metal Interface Combination. Polymers 2020, 12, 138. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, Y.; Zhang, Y.; Cao, B.; Wu, K.; Wang, L. Understanding Water Diffusion Behaviors in Epoxy Resin Through Molecular Simulations and Experiments. Langmuir 2024, 40, 4871–4880. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, D.; Zhang, Q.; Song, M.; Jiao, M.; Zhang, Z. Molecular Dynamics Simulation and Structure Changes of Polyester in Water and Non-Aqueous Solvents. Materials 2022, 15, 2148. [Google Scholar] [CrossRef]
- Han, Z.; Jiao, G.; Ma, C.; Zuo, T.; Han, C.C.; Cheng, H. The Relationship Between Free Volume and Cooperative Rearrangement: From the Temperature-Dependent Neutron Total Scattering Experiment of Polystyrene. Polymers 2021, 13, 3042. [Google Scholar] [CrossRef]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef]
- Akkermans, R.L.C.; Spenley, N.A.; Robertson, S.H. COMPASS III: Automated Fitting Workflows and Extension to Ionic Liquids. Mol. Simul. 2021, 7, 540–551. [Google Scholar] [CrossRef]
- Rahmani, F.; Nouranian, S.; Li, X.; Al-Ostaz, A. Reactive Molecular Simulation of the Damage Mitigation Efficacy of POSS-, Graphene-, and Carbon Nanotube-Loaded Polyimide Coatings Exposed to Atomic Oxygen Bombardment. ACS Appl. Mater. Interfaces 2017, 14, 12802–12811. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Gouzman, I.; Grossman, E.; Verker, R.; Atar, N.; Bolker, A.; Eliaz, N. Advances in Polyimide-Based Materials for Space Applications. Adv. Mater. 2019, 31, e1807738. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G. Numerical Simulation on Atomic Oxygen Undercutting of Kapton Film in Low Earth Orbit. Acta Astronaut. 2010, 67, 388–395. [Google Scholar] [CrossRef]
- Banks, B.A.; de Groh, K.K.; Miller, S.K. Low Earth Orbital Atomic Oxygen Interactions with Spacecraft Materials. MRS Proc. 2004, 851, NN8.1. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Zhang, L.; Zou, L.; Ayubi, B.I.; Wang, Y. Mechanistic Study of Atomic Oxygen Erosion on Polyimide Under Electric Fields: A Molecular Dynamics and Density Functional Theory Approach. Molecules 2024, 29, 5353. https://doi.org/10.3390/molecules29225353
Zhou S, Zhang L, Zou L, Ayubi BI, Wang Y. Mechanistic Study of Atomic Oxygen Erosion on Polyimide Under Electric Fields: A Molecular Dynamics and Density Functional Theory Approach. Molecules. 2024; 29(22):5353. https://doi.org/10.3390/molecules29225353
Chicago/Turabian StyleZhou, Shengrui, Li Zhang, Liang Zou, Bilal Iqbal Ayubi, and Yiwei Wang. 2024. "Mechanistic Study of Atomic Oxygen Erosion on Polyimide Under Electric Fields: A Molecular Dynamics and Density Functional Theory Approach" Molecules 29, no. 22: 5353. https://doi.org/10.3390/molecules29225353
APA StyleZhou, S., Zhang, L., Zou, L., Ayubi, B. I., & Wang, Y. (2024). Mechanistic Study of Atomic Oxygen Erosion on Polyimide Under Electric Fields: A Molecular Dynamics and Density Functional Theory Approach. Molecules, 29(22), 5353. https://doi.org/10.3390/molecules29225353








