First Principle Study on the Z-Type Characteristic Modulation of GaN/g-C3N4 Heterojunction
Abstract
:1. Introduction
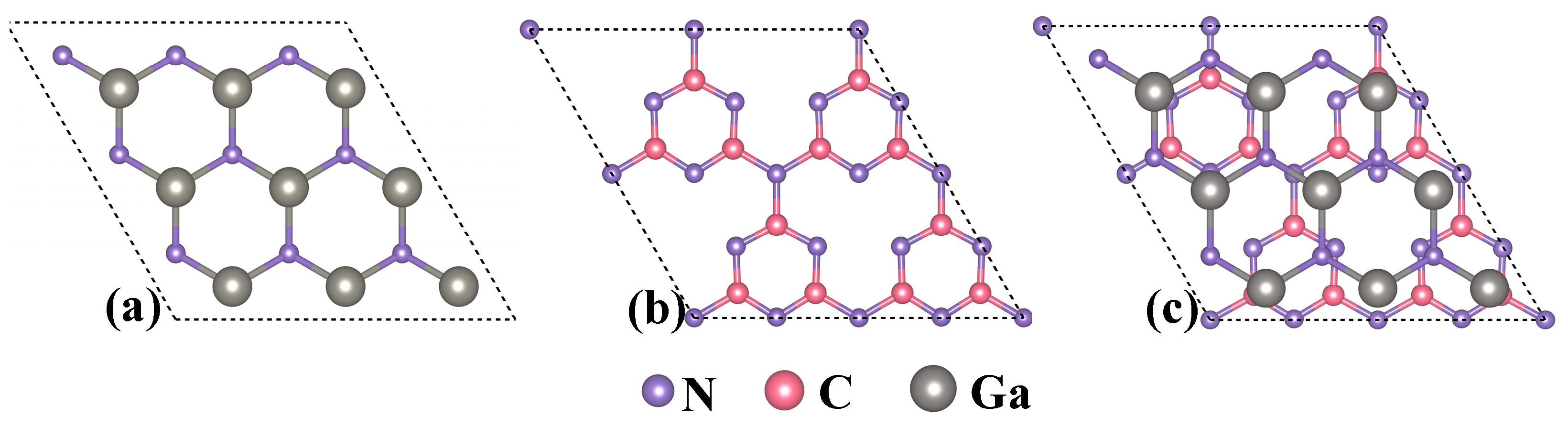
2. Model Structures and Stability
3. Analysis and Discussion
3.1. Electronic Structure and Optical Properties
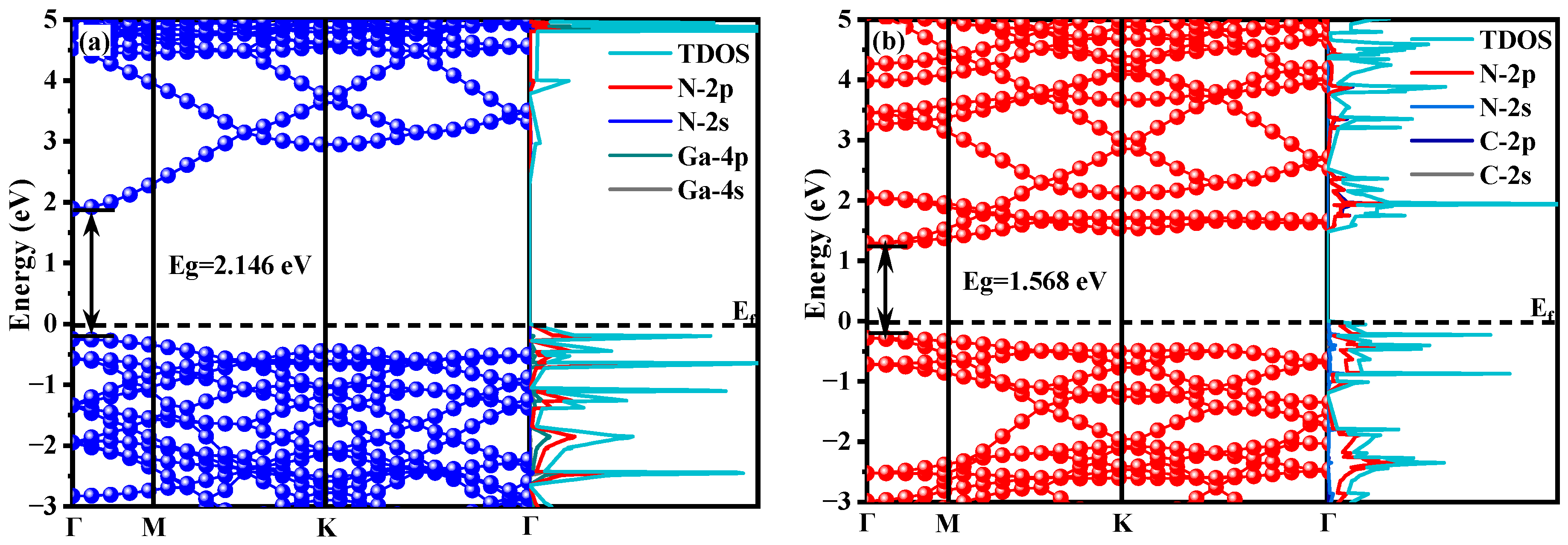
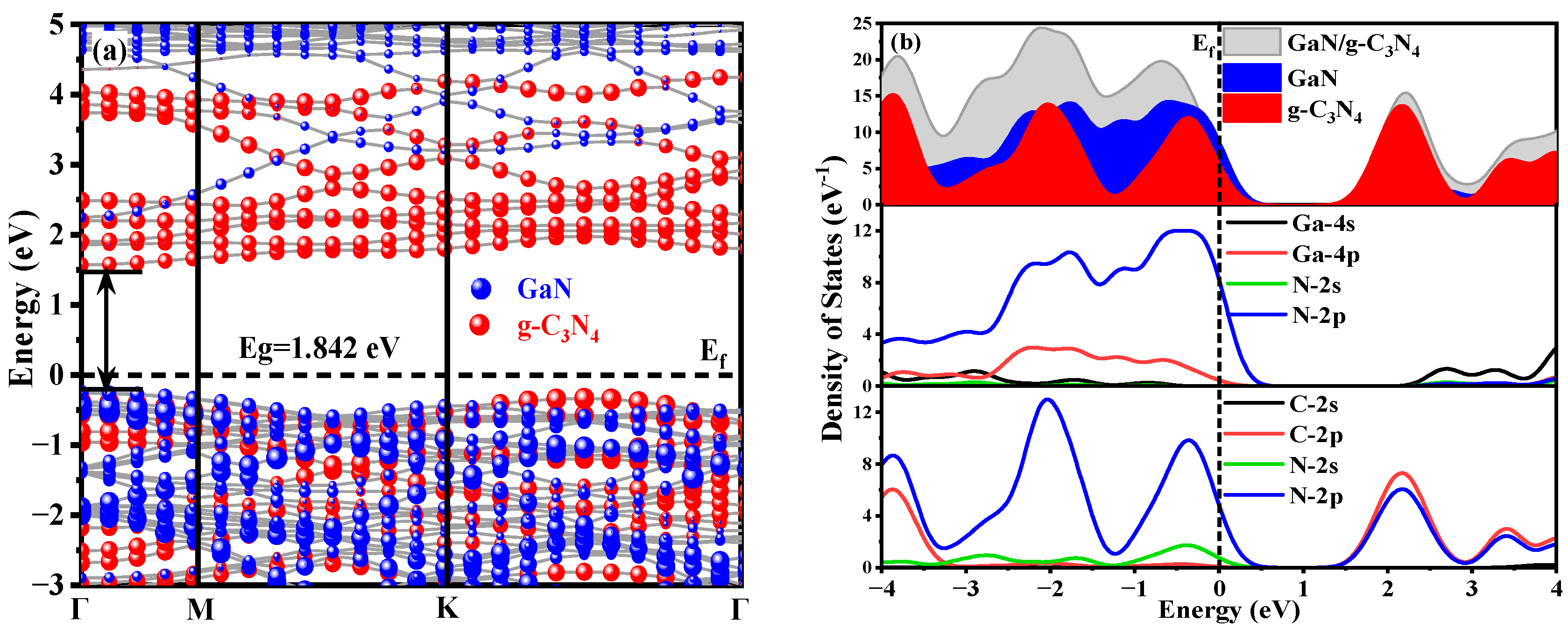
3.1.1. Energy Band Structure and Electronic Density of States
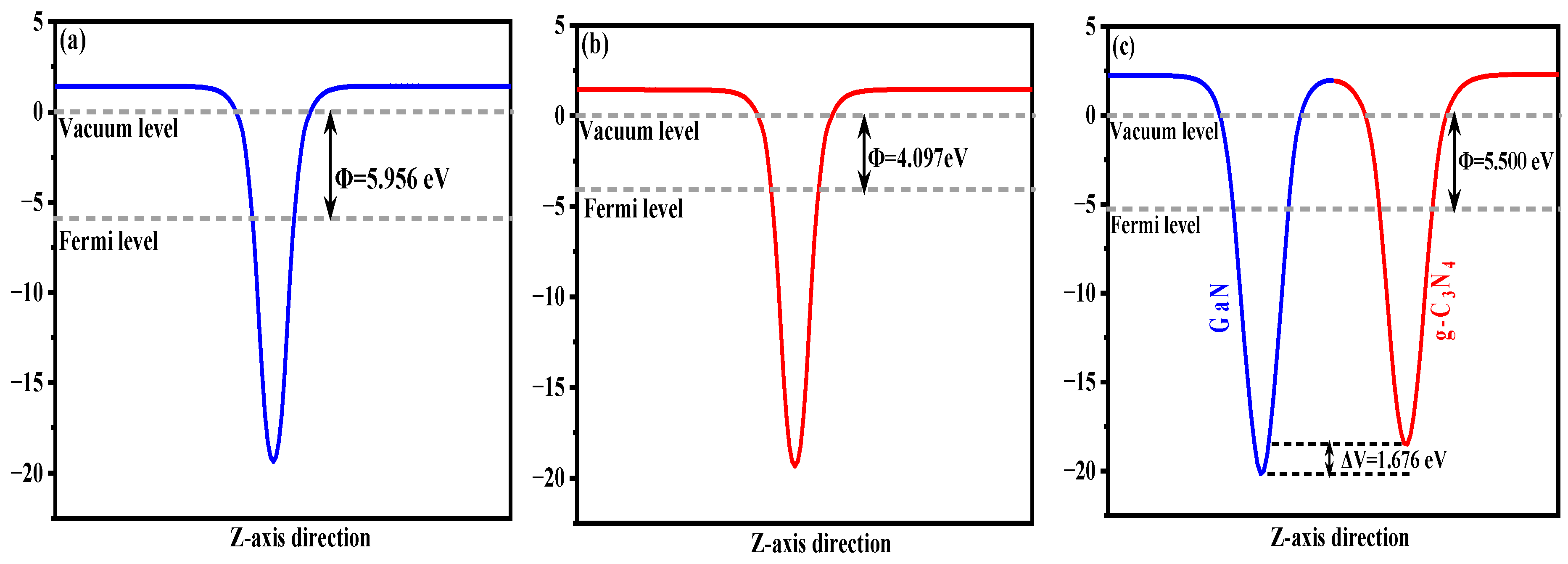
3.1.2. Work Function and Effective Mass
3.1.3. Difference in Charge Density
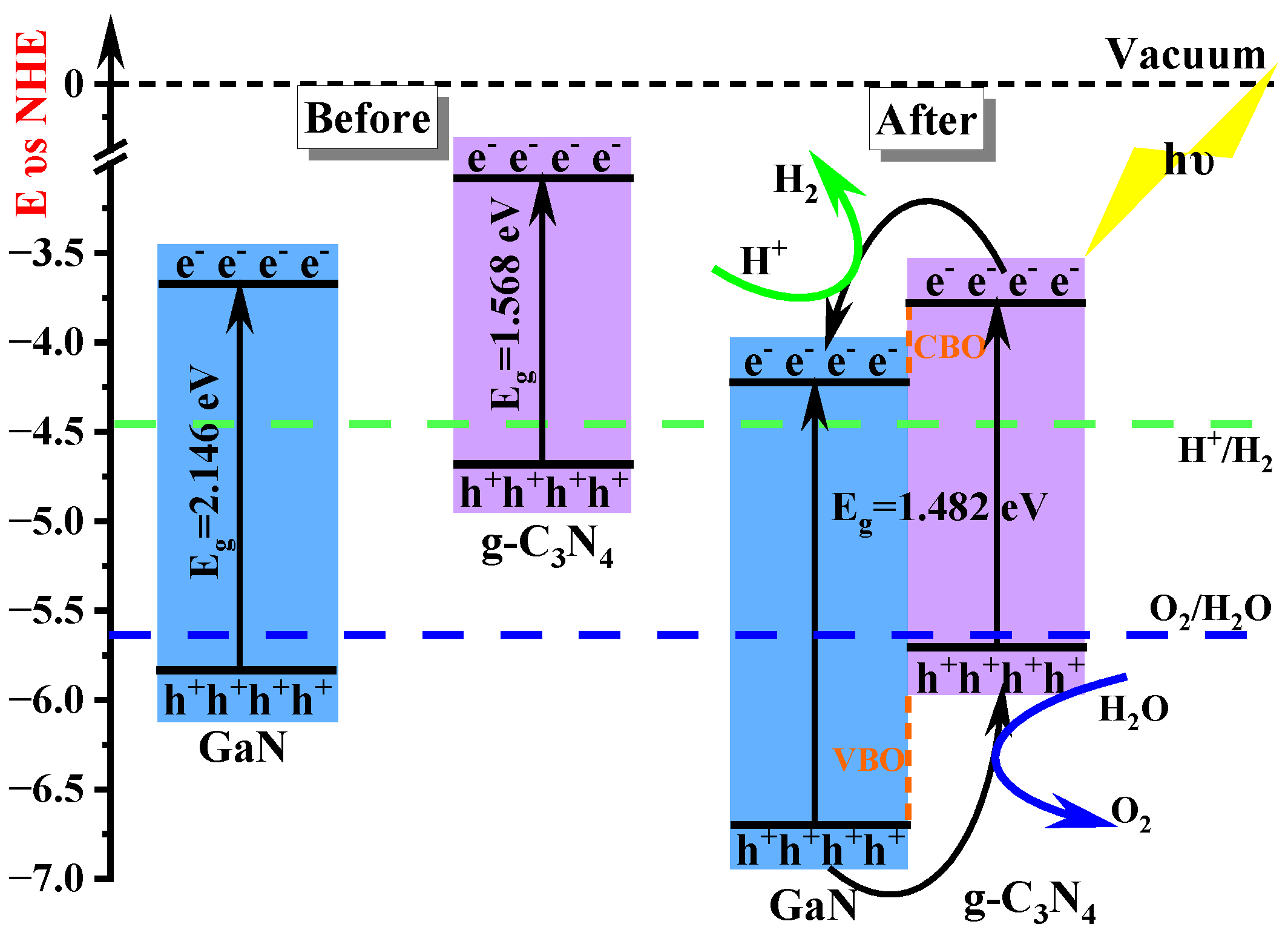
3.2. Photocatalytic Performance
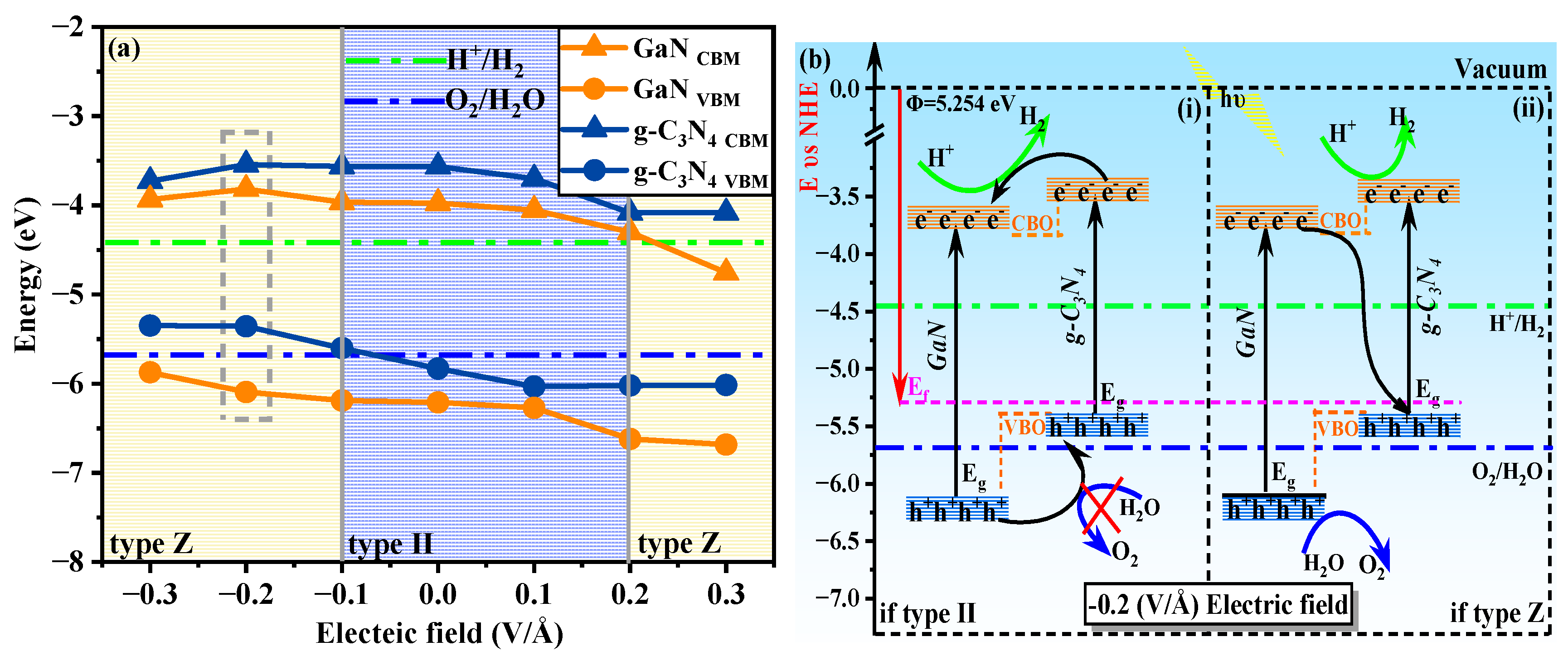
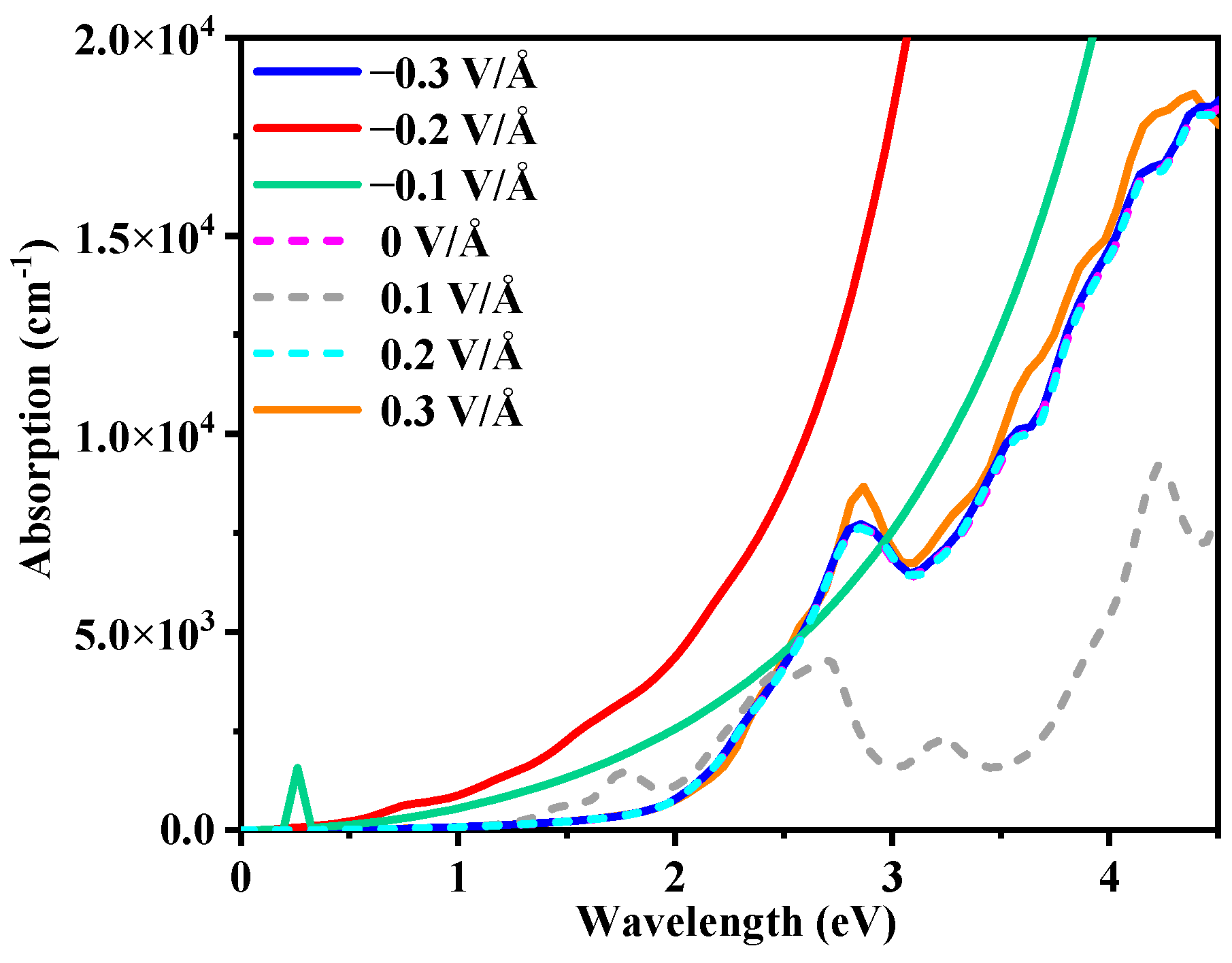
3.3. External Electric Field
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dong, Y.; Zhang, Y.; Liu, S. The Impacts and Instruments of Energy Transition Regulations on Environmental Pollution. Environ. Impact Assess. Rev. 2024, 105, 107448. [Google Scholar] [CrossRef]
- Zheng, D.; Xue, Y.; Wang, J.; Varbanov, P.S.; Klemeš, J.J.; Yin, C. Nanocatalysts in Photocatalytic Water Splitting for Green Hydrogen Generation: Challenges and Opportunities. J. Clean. Prod. 2023, 414, 137700. [Google Scholar] [CrossRef]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-Hydrogen Efficiency of More than 9% in Photocatalytic Water Splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Peiris, S.; De Silva, H.B.; Ranasinghe, K.N.; Bandara, S.V.; Perera, I.R. Recent Development and Future Prospects of TiO2 Photocatalysis. J. Chin. Chem. Soc. 2021, 68, 738–769. [Google Scholar] [CrossRef]
- Hou, Q.; Li, W.; Xu, Z.; Liu, Y.; Sha, S. Study of the Electronic Structure and Absorption Spectrum of Co and H Doped ZnO by First-Principles. Chem. Phys. 2020, 528, 110460. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Song, X.; Huang, B.; Ma, X.; Xing, R. CdS/WO3 S-Scheme Heterojunction with Improved Photocatalytic CO2 Reduction Activity. J. Photochem. Photobiol. B 2022, 233, 112480. [Google Scholar] [CrossRef]
- Yuan, J.; Wang, F.; Zhang, Z.; Song, B.; Yan, S.; Shang, M.-H.; Tong, C.; Zhou, J. Effects of Electric Field and Interlayer Coupling on Schottky Barrier of Germanene/MoSSe Vertical Heterojunction. Phys. Rev. B 2023, 108, 125404. [Google Scholar] [CrossRef]
- Sun, X.; Huang, H.; Zhao, Q.; Ma, T.; Wang, L. Thin-Layered Photocatalysts. Adv. Funct. Mater. 2020, 30, 1910005. [Google Scholar] [CrossRef]
- Dao, Q.D.; Nguyen, T.K.; Dang, T.T.; Kang, S.G.; Nguyen-Phu, H.; Do, L.T.; Van, V.K.; Chung, K.H.; Chung, J.S.; Shin, E.W. Anchoring Highly Distributed Pt Species over Oxidized Graphitic Carbon Nitride for Photocatalytic Hydrogen Evolution: The Effect of Reducing Agents. Appl. Surf. Sci. 2023, 609, 155305. [Google Scholar] [CrossRef]
- Dao, D.Q.; Nguyen, T.K.A.; Kang, S.G.; Shin, E.W. Engineering Oxidation States of a Platinum Cocatalyst over Chemically Oxidized Graphitic Carbon Nitride Photocatalysts for Photocatalytic Hydrogen Evolution. ACS Sustain. Chem. Eng. 2021, 43, 14537–14549. [Google Scholar] [CrossRef]
- Singh, J.; Sharma, P.; Tripathi, N.; Shishkina, D.; Rymzhina, A.; Boltov, E.A.; Platonov, V.; Pavelyev, V.; Volkov, V.S.; Arsenin, A.V.; et al. Synthesis of Highly Sensitive Nanomaterial for Ultra-Fast Photocatalytic Activity: A Detailed Study on Photocatalytic Capabilities of Rod-Shaped TiS3 Nanostructures. Catal. Commun. 2022, 162, 106381. [Google Scholar] [CrossRef]
- Telkhozhayeva, M.; Hirsch, B.; Konar, R.; Teblum, E.; Lavi, R.; Weitman, M.; Malik, B.; Moretti, E.; Nessim, G.D. 2D TiS2 Flakes for Tetracycline Hydrochloride Photodegradation under Solar Light. Appl. Catal. B Environ. 2022, 318, 121872. [Google Scholar] [CrossRef]
- Dai, H.; Cai, X.; Li, X.; Wang, C.; Hou, Y.; Wei, R. First-Principles Calculations on Performance of the g-C3N4/LNS-TiO2(Cr+C) Heterojunction Photocatalyst in Water Splitting Process. Int. J. Hydrogen Energy 2023, 48, 38742–38748. [Google Scholar] [CrossRef]
- Jiang, J.; Xiong, Z.; Wang, H.; Liao, G.; Bai, S.; Zou, J.; Wu, P.; Zhang, P.; Li, X. Sulfur-Doped g-C3N4/g-C3N4 Isotype Step-Scheme Heterojunction for Photocatalytic H2 Evolution. J. Mater. Sci. Technol. 2022, 118, 15–24. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, M.; Cheng, B.; Shao, Y. Recent Advances in g-C3N4-Based Heterojunction Photocatalysts. J. Mater. Sci. Technol. 2020, 56, 1–17. [Google Scholar] [CrossRef]
- Tao, J.; Huang, L.; Xiong, S.; Li, L.-X.; Wang, L.-L.; Xu, L. Two-Dimensional TMDs/MN (M = Al, Ga) van Der Waals Heterojunction Photocatalyst: A First-Principles Study. J. Mater. Sci. 2023, 58, 14080–14095. [Google Scholar] [CrossRef]
- Ren, W.; Yang, J.; Zhang, J.; Li, W.; Sun, C.; Zhao, H.; Wen, Y.; Sha, O.; Liang, B. Recent Progress in SnO2/g-C3N4 Heterojunction Photocatalysts: Synthesis, Modification, and Application. J. Alloys Compd. 2022, 906, 164372. [Google Scholar] [CrossRef]
- Lin, M.; Chen, H.; Zhang, Z.; Wang, X. Engineering Interface Structures for Heterojunction Photocatalysts. Phys. Chem. Chem. Phys. 2023, 25, 4388–4407. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Zhong, J.; Li, J. Facile Construction of CuO/g-C3N4 Heterojunctions with Promoted Photocatalytic Hydrogen Generation Behaviors. Fuel 2023, 353, 129224. [Google Scholar] [CrossRef]
- Pan, J.; Shen, W.; Zhang, Y.; Tang, H.; Sun, H.; Zhong, W.; Yan, X. Metal-Free SiOC/g-C3N4 Heterojunction Composites with Efficient Visible-Light Photocatalytic H2 Production. Appl. Surf. Sci. 2020, 520, 146335. [Google Scholar] [CrossRef]
- Lin, D.; Zhou, Y.; Ye, X.; Zhu, M. Construction of Sandwich Structured Photocatalyst Using Monolayer WS2 Embedded g-C3N4 for Highly Efficient H2 Production. Ceram. Int. 2020, 46, 12933–12941. [Google Scholar] [CrossRef]
- Zhang, X.; Liang, H.; Li, C.; Bai, J. 1 D CeO2/g-C3N4 Type II Heterojunction for Visible-Light-Driven Photocatalytic Hydrogen Evolution. Inorg. Chem. Commun. 2022, 144, 109838. [Google Scholar] [CrossRef]
- Ge, W.; Liu, K.; Deng, S.; Yang, P.; Shen, L. Z-Scheme g-C3N4/ZnO Heterojunction Decorated by Au Nanoparticles for Enhanced Photocatalytic Hydrogen Production. Appl. Surf. Sci. 2023, 607, 155036. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, L.; Shou, Q.; Gao, M.; Wang, H.; Nazir, A.; Huo, P. Fabrication of g-C3N4/SnS2 Type-II Heterojunction for Efficient Photocatalytic Conversion of CO2. J. Mater. Sci. Mater. Electron. 2023, 34, 350. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, X.; Zhang, H.; He, S.; Liu, Y.; Zhao, W.; Liu, H.; Qu, X. Janus MoSH/WSi2N4 van Der Waals Heterostructure: Two-Dimensional Metal/Semiconductor Contact. Molecules 2024, 29, 3554. [Google Scholar] [CrossRef]
- Sarkar, K.; Kumar, P. Activated Hybrid g-C3N4/Porous GaN Heterojunction for Tunable Self-Powered and Broadband Photodetection. Appl. Surf. Sci. 2021, 566, 150695. [Google Scholar] [CrossRef]
- Yi, Y.; Zhou, R.; Zhuang, F.; Ye, X.; Li, H.; Hao, G.; Zhang, R. First-Principles Study on the Electronic Properties and Feasibility of Photocatalytic Water Splitting on Z-Scheme GaN/MoS2 Heterostructure. J. Phys. Chem. Solids 2024, 190, 112006. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, P.; Liu, Q.; Sun, P.; Yi, Y.; Lei, J.; Song, T. The Electronic and Optical Properties of Type-II g-CN/GaGePS van Der Waals Heterostructure Modulated via Biaxial Strain and External Electric Field. Mater. Sci. Semicond. Process. 2024, 171, 107989. [Google Scholar] [CrossRef]
- Xu, L.; Tao, J.; Xiao, B.; Xiong, F.; Ma, Z.; Zeng, J.; Huang, X.; Tang, S.; Wang, L.-L. Two-Dimensional AlN/g-CNs van Der Waals Type-II Heterojunction for Water Splitting. Phys. Chem. Chem. Phys. 2023, 25, 3969–3978. [Google Scholar] [CrossRef]
- Han, S.; Wei, X.; Huang, Y.; Zhang, J.; Zhu, G.; Yang, J. Influence of Strain and External Electric Field on the Performance of PC6/MoSe2 Heterostructure. J. Mater. Sci. 2022, 57, 477–488. [Google Scholar] [CrossRef]
- Guo, R.; Luan, L.; Cao, M.; Zhang, Y.; Wei, X.; Fan, J.; Ni, L.; Liu, C.; Yang, Y.; Liu, J.; et al. Tunable Electronic Properties of GeC/BAs van Der Waals Heterostructure under External Electric Field and Strain. Phys. E Low-Dimens. Syst. Nanostr. 2023, 149, 115628. [Google Scholar] [CrossRef]
- Yang, G.-H.; Zhang, J.-M.; Huang, Y.-H.; Wei, X.-M. Effects of External Electric Field on the Structural and Electronic Properties of SeZrS/SeHfS van Der Waals Heterostructure: A First-Principles Study. Thin Solid Films 2023, 767, 139675. [Google Scholar] [CrossRef]
- Zhao, X.; Niu, W.; Zhang, H.; Dai, X.; Wei, S.; Yang, L. Effects of Interlayer Coupling and Electric Field on the Electronic Properties of PtSe2/ZrSe2 van Der Waals Heterojunctions. Appl. Surf. Sci. 2020, 510, 145316. [Google Scholar] [CrossRef]
- Gao, L.; Zhou, B.; Zhang, J.; Jiang, K.; Shang, L.; Hu, Z.; Chu, J. Strain and Electric Field Tunable Electronic and Optical Properties in Antimonene/C3N van Der Waals Heterostructure. Solid State Sci. 2021, 122, 106771. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, N.; Wang, Y.; Zhao, H.; Chen, H.; Zeng, H.; Zhao, L.; Yang, Q.; Feng, B. Direct Z-Scheme Construction of C2N/Mg(OH)2 Heterojunction and First-Principles Investigation of Photocatalytic Water Splitting. Int. J. Hydrogen Energy 2024, 53, 247–255. [Google Scholar] [CrossRef]
- Sádecký, E.; Brezina, R.; Kazár, J.; Urvölgyi, J. Immunization against Q-Fever of Naturally Infected Dairy Cows. Acta Virol. 1975, 19, 486–488. [Google Scholar]
- Guo, X.; Gu, J.; Lin, S.; Zhang, S.; Chen, Z.; Huang, S. Tackling the Activity and Selectivity Challenges of Electrocatalysts toward the Nitrogen Reduction Reaction via Atomically Dispersed Biatom Catalysts. J. Am. Chem. Soc. 2020, 142, 5709–5721. [Google Scholar] [CrossRef]
- Lv, L.; Shen, Y.; Liu, J.; Meng, X.; Gao, X.; Zhou, M.; Zhang, Y.; Gong, D.; Zheng, Y.; Zhou, Z. Computational Screening of High Activity and Selectivity TM/g-C3N4 Single-Atom Catalysts for Electrocatalytic Reduction of Nitrates to Ammonia. J. Phys. Chem. Lett. 2021, 12, 11143–11150. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Z.; Jia, J.; Robertson, J.; Guo, Y. 2D WSe2/MoSi2N4 Type-II Heterojunction with Improved Carrier Separation and Recombination for Photocatalytic Water Splitting. Appl. Surf. Sci. 2023, 611, 155674. [Google Scholar] [CrossRef]
- Yan, S.; Chen, W.; Xiong, W.; Yang, L.; Luo, R.; Wang, F. Dicarbon Nitride and Janus Transition Metal Chalcogenides van Der Waals Heterojunctions for Photocatalytic Water Splitting. J. Phys. Condens. Matter 2023, 35, 014003. [Google Scholar] [CrossRef]
- Oreshonkov, A.S.; Sukhanova, E.V.; Pankin, D.V.; Popov, Z.I. Electronic Transitions and Vibrational Properties of Bulk and Monolayer g-C3N4, and a g-C3N4/MoS2 Heterostructure from a DFT Study. Phys. Chem. Chem. Phys. 2024, 26, 23023–23031. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-X.; Pang, G.-W.; Pan, D.-Q.; Shi, L.-Q.; Zhang, L.-L.; Lei, B.-C.; Zhao, X.-C.; Huang, Y.-N. First-principles study of influence of electric field on electronic structure and optical properties of GaN/g-C3N4 heterojunction. Acta Phys. 2022, 71, 288–296. [Google Scholar] [CrossRef]
- Zhou, T.; Qian, G.; Huang, S.; Liang, Q.; Luo, X.; Xie, Q. The Effect of Biaxial Strain on the Electronic Structures and Optical Properties of GaS/SSnSe Heterojunction: A First-Principles Calculations. Phys. Lett. A 2023, 480, 128956. [Google Scholar] [CrossRef]
- Lu, P.; Zhao, H.; Li, Z.; Chu, M.; Xie, G.; Xie, T.; Jiang, L. High Photocatalytic Activity of g-C3N4/CdZnS/MoS2 Heterojunction for Hydrogen Production. Int. J. Hydrogen Energy 2024, 82, 776–785. [Google Scholar] [CrossRef]
- Zhu, X.T.; Xu, Y.; Cao, Y.; Zhao, Y.Q.; Sheng, W.; Nie, G.-Z.; Ao, Z. Investigation of the Electronic Structure of Two-Dimensional GaN/Zr2CO2 Hetero-Junction: Type-II Band Alignment with Tunable Bandgap. Appl. Surf. Sci. 2021, 542, 148505. [Google Scholar] [CrossRef]
- Hassan, A.; Nazir, M.A.; Shen, Y.; Guo, Y.; Kang, W.; Wang, Q. First-Principles Study of the Structural, Electronic, and Enhanced Optical Properties of SnS/TaS2 Heterojunction. ACS Appl. Mater. Interfaces 2022, 14, 2177–2184. [Google Scholar] [CrossRef]
- Qiao, F.; Liu, W.; Yang, J.; Liu, Y.; Yuan, J. Fabrication of ZnO/CuInS2 Heterojunction for Boosting Photocatalytic Hydrogen Production. Int. J. Hydrogen Energy 2024, 53, 840–847. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, Y.; Liang, Z.; Gong, Z.; Li, J.; Tang, Z.; Wei, X. Two-Dimensional AlN/PtSSe Heterojunction as A Direct Z-Scheme Photocatalyst for Overall Water Splitting: A DFT Study. J. Phys. Chem. C 2024, 128, 9894–9903. [Google Scholar] [CrossRef]
- Luan, L.; Han, L.; Zhang, D.; Bai, K.; Sun, K.; Xu, C.; Li, L.; Duan, L. AlSb/ZrS2 Heterojunction: A Direct Z-Scheme Photocatalyst with High Solar to Hydrogen Conversion Efficiency and Catalytic Activity across Entire PH Range. Int. J. Hydrogen Energy 2024, 51, 1242–1255. [Google Scholar] [CrossRef]
- Yayak, Y.O.; Topkiran, U.C.; Yagmurcukardes, M.; Sahin, H. Van Der Waals Heterostructures of AlAs and InSe: Stacking-Dependent Raman Spectra and Electric Field Dependence of Electronic Properties. Appl. Surf. Sci. 2024, 654, 159360. [Google Scholar] [CrossRef]
- Wang, G.; Tang, W.; Xu, C.; He, J.; Zeng, Q.; Xie, W.; Gao, P.; Chang, J. Two-Dimensional CdO/PtSSe Heterojunctions Used for Z-Scheme Photocatalytic Water-Splitting. Appl. Surf. Sci. 2022, 599, 153960. [Google Scholar] [CrossRef]
- Dai, Z.-N.; Cao, Y.; Yin, W.J.; Sheng, W.; Xu, Y. Z-Scheme SnC/HfS2 van Der Waals Heterojunction Increases Photocatalytic Overall Water Splitting. J. Phys. Appl. Phys. 2022, 55, 315503. [Google Scholar] [CrossRef]
- Xie, K.-X.; Zhang, Y.; Qiang, Z.-B.; Ding, J.-X.; Nouguiza, H.; Chen, H.-X.; Duan, L.; Fan, J.-B.; Ni, L. A Direct Z-Scheme GeS/GeSe van Der Waals Heterojunction as a Promising Photocatalyst with High Optical Absorption, Solar-to-Hydrogen Efficiency and Catalytic Activity for Overall Water Splitting: First-Principles Prediction. Int. J. Hydrogen Energy 2024, 51, 1381–1391. [Google Scholar] [CrossRef]
- Zhuo, Q.; Zhang, Y.; Fu, Z.; Han, T.; Liu, X.; Ou, J.; Xu, X. First-Principles Study of Photocatalytic Mechanism and Charge Transfer of PtS2/MoSe2 S-Scheme Heterojunction. Appl. Surf. Sci. 2022, 600, 154038. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Tian, W.; Zhang, Y.; Shan, P.; Chen, Y.; Wei, W.; Yuan, H.; Cui, H. Mechanism for Hydrogen Evolution from Water Splitting Based on a MoS2/WSe2 Heterojunction Photocatalyst: A First-Principle Study. RSC Adv. 2020, 10, 41127–41136. [Google Scholar] [CrossRef]
- Holmes, S.T.; Vojvodin, C.S.; Schurko, R.W. Dispersion-Corrected DFT Methods for Applications in Nuclear Magnetic Resonance Crystallography. J. Phys. Chem. A 2020, 124, 10312–10323. [Google Scholar] [CrossRef]
- Fu, S.; Wang, D.; Ma, Z.; Liu, G.; Zhu, X.; Yan, M.; Fu, Y. The First-Principles Study on the Halogen-Doped graphene/MoS2 Heterojunction. Solid State Commun. 2021, 334–335, 114366. [Google Scholar] [CrossRef]
- Sun, Y.; Luan, L.; Zhao, J.; Zhang, Y.; Wei, X.; Fan, J.; Ni, L.; Liu, C.; Yang, Y.; Liu, J.; et al. Tunable Properties of WTe2/GaS Heterojunction and Se-Doped WTe2/GaS Heterojunction. Mater. Sci. Semicond. Process. 2023, 166, 107695. [Google Scholar] [CrossRef]





| Photocatalysts | H (μmol g−1 h−1) | Eg (eV) | Band Structure Type |
|---|---|---|---|
| Layer g-C3N4 [19] | 2.82 | 2.70 | / |
| g-C3N4/SiOC [20] | 1020 | 2.64 | Type II |
| g-C3N4/WS2 [21] | 599.7 | 2.30 | Type II |
| g-C3N4/CeO2 [22] | 229.75 | 2.06 | Type II |
| Methods | Model I (eV) | Model II (eV) | Model III (eV) |
|---|---|---|---|
| TS | −28,134.0639 | −28,134.0486 | −28,134.0806 |
| Grimine | −28,134.0480 | −28,134.0237 | −28,134.0534 |
| System | me* | mh* | D (me*/mh*) |
|---|---|---|---|
| GaN | 0.56 | 0.55 | 0.98 |
| g-C3N4 | 0.58 | 0.62 | 1.07 |
| GaN/g-C3N4 | 0.67 | 1.29 | 1.93 |
| Electric Field (V/Å) | −0.3 | −0.2 | 0.3 | −0.1 | 0 | 0.1 | 0.2 |
| Type | Z | Z | Z | Z | II | II | II |
| ΔP (eV) | 2.544 | 2.550 | 2.620 | 2.525 | 2.245 | 2.369 | 2.335 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, M.-Y.; Zhao, X.-C.; Lei, B.-C.; Huang, Y.-N.; Zhang, L.-L.; Guo, H.; Wang, H.-G. First Principle Study on the Z-Type Characteristic Modulation of GaN/g-C3N4 Heterojunction. Molecules 2024, 29, 5355. https://doi.org/10.3390/molecules29225355
Dai M-Y, Zhao X-C, Lei B-C, Huang Y-N, Zhang L-L, Guo H, Wang H-G. First Principle Study on the Z-Type Characteristic Modulation of GaN/g-C3N4 Heterojunction. Molecules. 2024; 29(22):5355. https://doi.org/10.3390/molecules29225355
Chicago/Turabian StyleDai, Meng-Yao, Xu-Cai Zhao, Bo-Cheng Lei, Yi-Neng Huang, Li-Li Zhang, Hai Guo, and Hua-Gui Wang. 2024. "First Principle Study on the Z-Type Characteristic Modulation of GaN/g-C3N4 Heterojunction" Molecules 29, no. 22: 5355. https://doi.org/10.3390/molecules29225355
APA StyleDai, M.-Y., Zhao, X.-C., Lei, B.-C., Huang, Y.-N., Zhang, L.-L., Guo, H., & Wang, H.-G. (2024). First Principle Study on the Z-Type Characteristic Modulation of GaN/g-C3N4 Heterojunction. Molecules, 29(22), 5355. https://doi.org/10.3390/molecules29225355





