N-Aryl Benzimidazole and Benzotriazole Derivatives and Their Hybrids as Cytotoxic Agents: Design, Synthesis and Structure–Activity Relationship Studies
Abstract
1. Introduction
2. Results
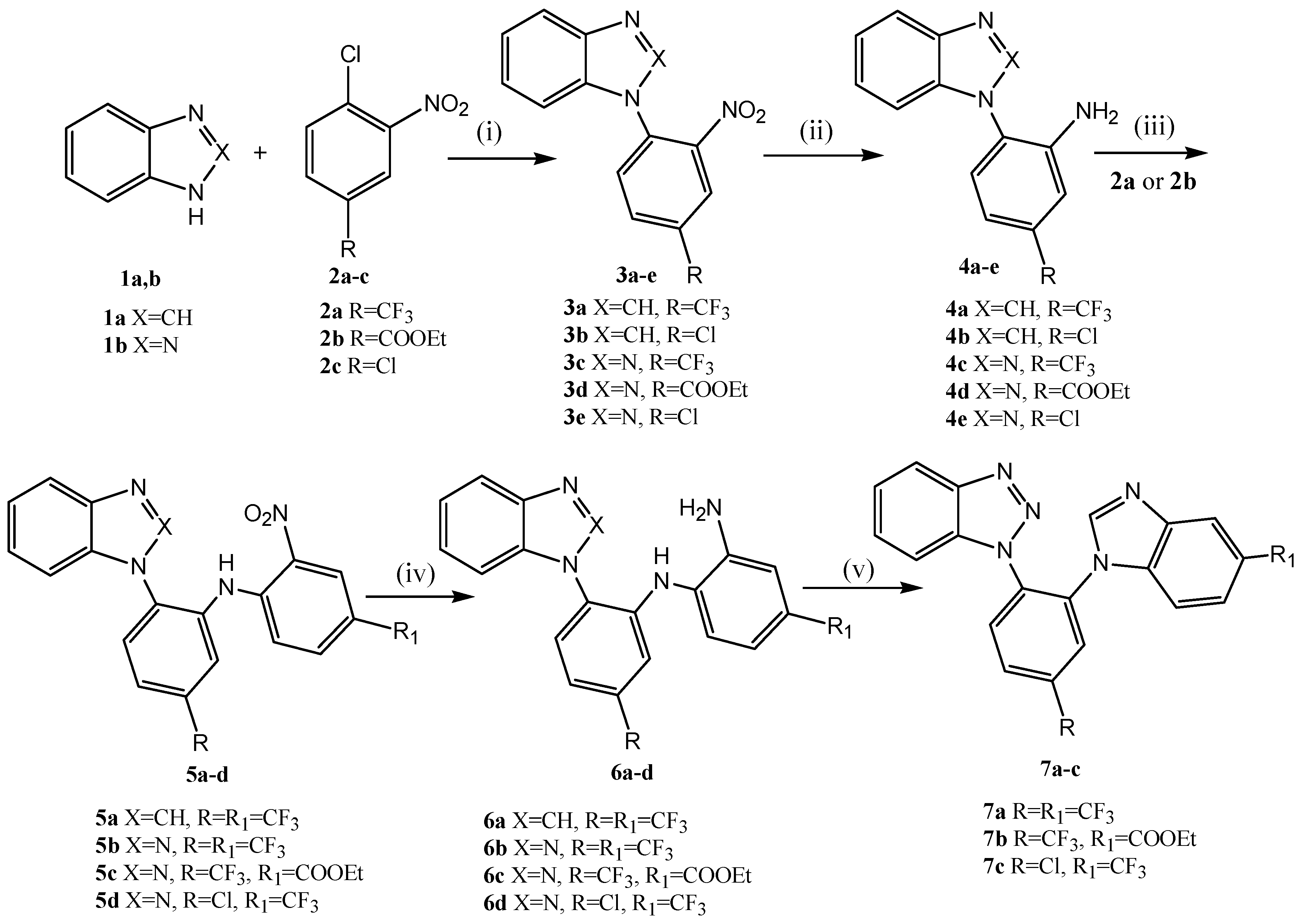
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. Cytotoxic Effect
2.2.2. Antioxidant Activity
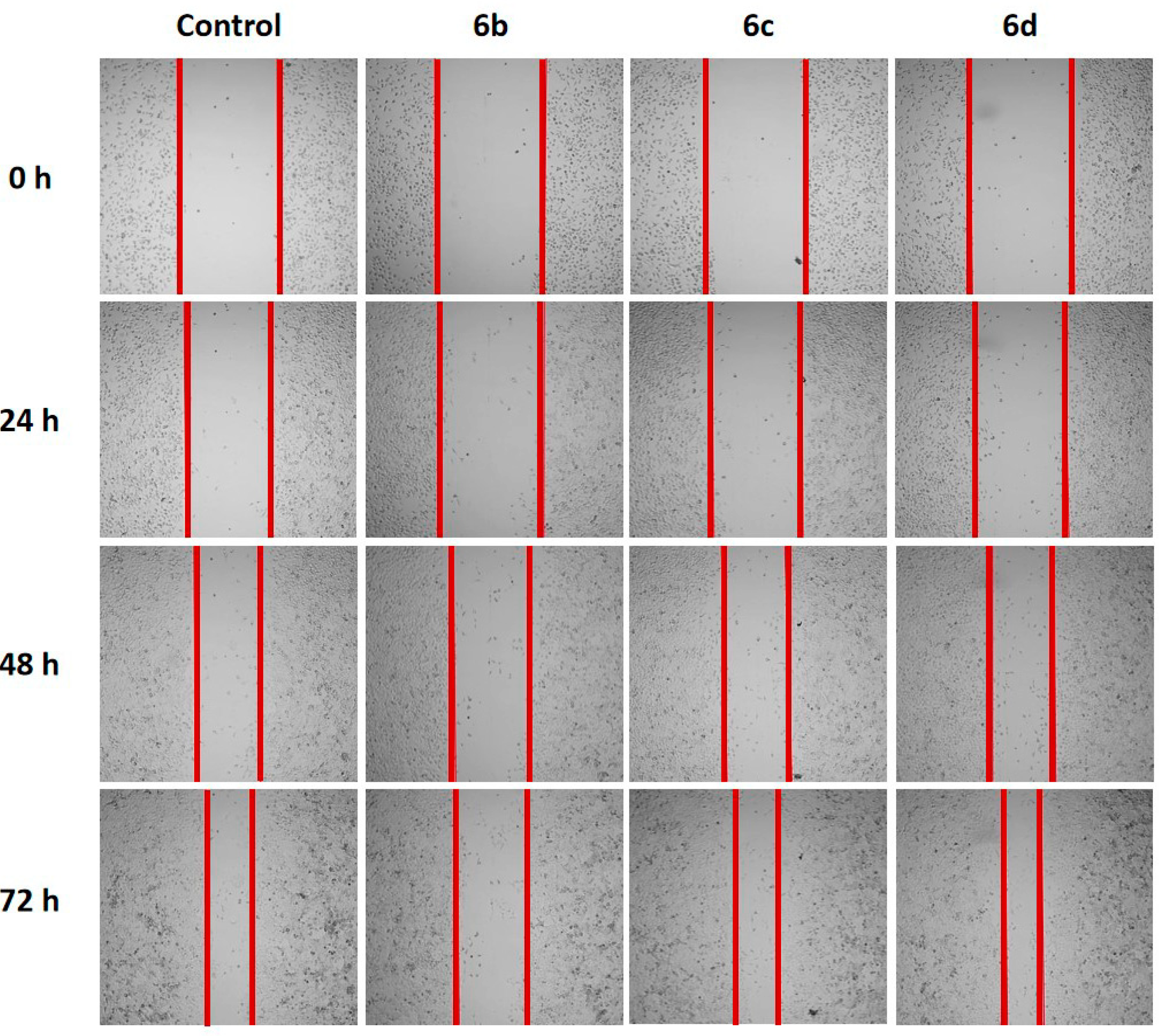
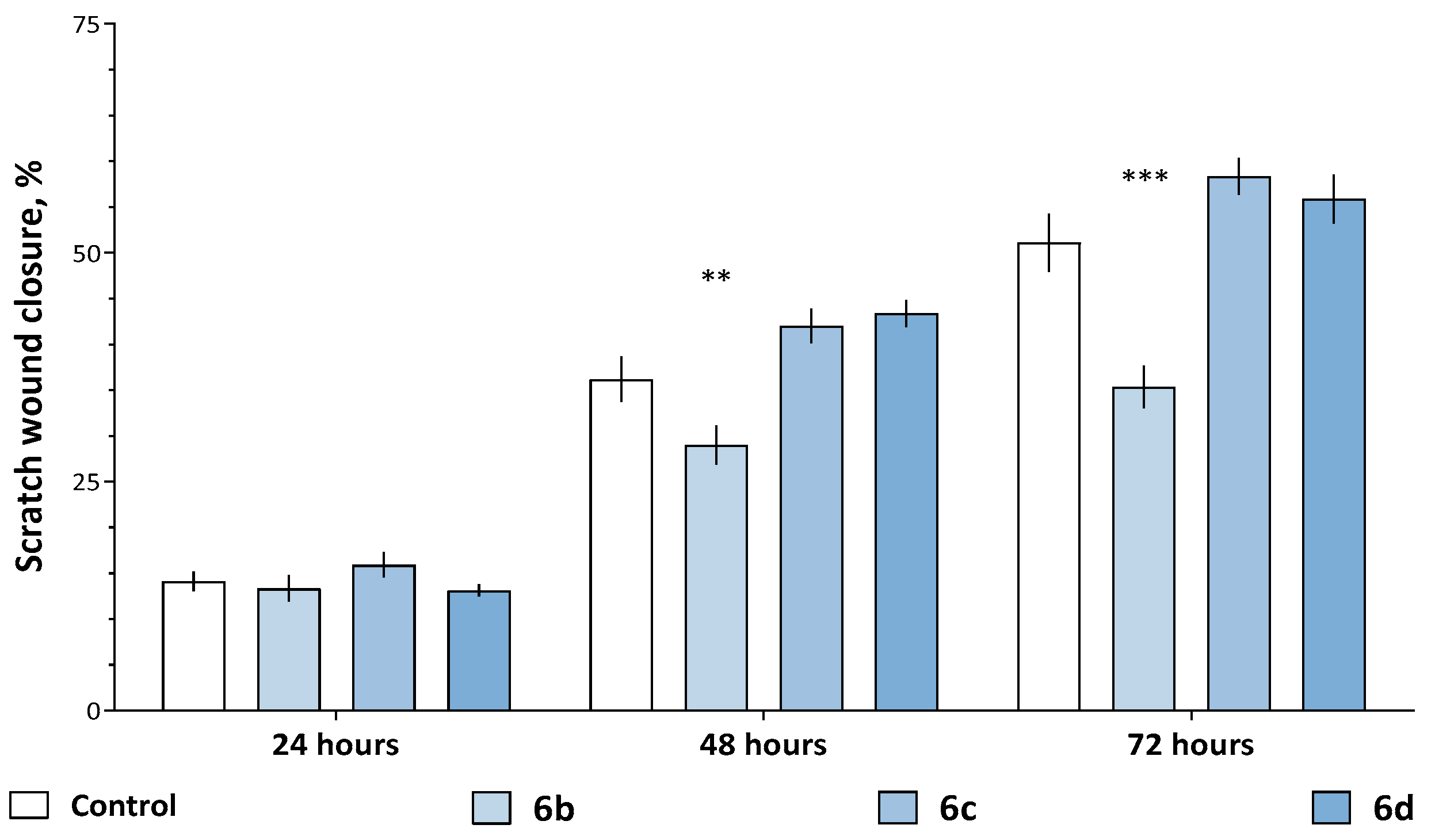
2.2.3. Modulation of the Migration Ability and Antiproliferative Effect
3. Materials and Methods
3.1. Chemistry
3.1.1. Reagents and Materials
3.1.2. Synthesis and General Procedures
- General procedure for the synthesis of 1-(2-nitroaryl)-1H-benzimidazoles (3a,b) and 1-(2-nitroaryl)-1H-benzotriazoles (3c–e).
- K2CO3 (20.70 g, 0.15 mol) and 2a–c (0.1 mol) were added to a solution of 1a, b (0.1 mol) in DMF (100 mL). The reaction mixture was stirred at 110 °C for 2 h in the synthesis of 3a, 3 h in the syntheses of 3c, d or 7 h in the syntheses of 3b, e. The reaction mixture was poured into water. The precipitate was filtered off and crystallized from i-PrOH.
- 1-(2-Nitro-4-(trifluoromethyl)phenyl)-1H-benzimidazole (3a): Yield 98%. m.p. 130−132 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.71 (d, 1H, H3′, J = 1.5 Hz), 8.54 (s, 1H, H2), 8.41 (dd, 1H, H5′, J = 8.3, 1.6 Hz), 8.16 (d, 1H, H6′, J = 8.3 Hz), 7.78–7.82 (m, 1H, H4), 7.29–7.39 (m, 3H, H5, H6, H7). 13C{1H} NMR (126 MHz, DMSO-d6) δ 145.13 (C2′), 143.39 (C2), 143.10 (C4a), 133.71 (C7a), 132.19 (C1′), 131.58 (q, J = 3.4 Hz, C5′), 131.37 (C6′), 130.04 (q, J = 34.2 Hz, C4′), 123.88 (C6), 123.48 (q, J = 3.6 Hz, C3′), 122.90 (C5), 122.78 (q, J = 273.6 Hz, CF3), 120.00 (C4), 109.79 (C7). HRMS (ESI/TOF) m/z calculated for C14H9F3N3O2 [M+H]+: 308.0648. Found: 308.0631.
- 1-(4-Chloro-2-nitrophenyl)-1H-benzimidazole (3b): Yield 94%. m.p. 102–105 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.48 (s, 1H, H2), 8.47 (d, 1H, H3′, J = 2.4 Hz), 8.09 (dd, 1H, H5′, J = 8.5, 2.4 Hz), 7.93 (d, 1H, H6′, J = 8.5 Hz), 7.76–7.81 (m, 1H, H4), 7.27–7.35 (m, 2H, H5, H6), 7.23–7.27 (m, 1H, H7). 13C{1H} NMR (126 MHz, DMSO-d6) δ 145.61 (C2′), 143.63 (C2), 143.01 (C4a), 134.79 (C5′), 134.30 (C4′), 134.05 (C7a), 131.76 (C6′), 127.59 (C1′), 125.88 (C3′), 123.78 (C6), 122.72 (C5), 119.94 (C4), 109.77 (C7). HRMS (ESI/TOF) m/z calculated for C13H9ClN3O2 [M+H]+: 274.0384. Found: 274.0369.
- 1-[2-Nitro-4-(trifluoromethyl)phenyl]-1H-benzotriazole (3c): Yield 93%. m.p. 133–136 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.07 (d, 1H, H3′, J = 2.6 Hz), 8.81 (dd, 1H, H5′, J = 8.8, 2.5Hz), 8.40 (d, 1H, H6′, J = 8.8 Hz), 8.28 (d, 1H, H4, J = 8.4 Hz), 7.85 (d, 1H, H7, J = 8.4 Hz), 7.77 (td, 1H, H6, J = 8.3, 1.0 Hz), 7.61 (td, 1H, H5, J = 8.3, 1.0 Hz). 13C NMR (101 MHz, DMSO-d6) δ 145.93, 145.32, 133.31, 132.30, 132.00, 131.41 (q, J = 35 Hz), 130.32, 129.87, 126.07, 125.99 (q, J = 272 Hz), 124.38, 120.65, 111.03. HRMS (ESI/TOF) m/z calculated for C13H7F3N4O2: 309.2196 [M + H]+. Found: 309.2191.
- Ethyl 4-(1H-benzotriazol-1-yl)-3-nitrobenzoate (3d): Yield 91%. m.p. 115–119 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.70 (d, J = 1.8 Hz, 1H, H2), 8.51 (dd, J = 8.2, 1.9 Hz, 1H, H6), 8.29–8.19 (m, 2H, H4′, H5), 7.80 (dt, J = 8.4, 1.1 Hz, 1H, H7′), 7.72 (td, J = 8.3, 1.0 Hz, 1H, H6′), 7.58 (td, J = 8.3, 1.0 Hz, 1H, H5′), 4.43 (q, J = 7.1 Hz, 2H, CH2), 1.38 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.97, 145.95, 144.97, 135.52, 133.25, 132.58, 132.16, 130.25, 129.15, 127.21, 126.00, 120.66, 110.92, 62.72, 14.71. HRMS (ESI/TOF) m/z calculated for C15H13N4O4: 313.2876 [M + H]+. Found: 313.2873.
- 1-(4-Chloro-2-nitrophenyl)-1H-benzotriazole (3e): Yield 89%. m.p. 134–137 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.51 (d, J = 2.2 Hz, 1H, H3′), 8.23 (d, J = 8.5 Hz, 1H, H4), 8.15 (dd, J = 8.5, 1.5 Hz, 1H, H5′), 8.11 (d, J = 8.5, 1H, H6′), 7.76 (d, J = 8.2 Hz, 1H, H7), 7.69 (td, J = 8.3, 1.0 Hz, 1H, H6), 7.56 (td, J = 8.3, 1.0 Hz, 1H, H5). 13C NMR (101 MHz, DMSO-d6) δ 145.79, 135.97, 135.38, 133.63, 130.49, 130.04, 127.75, 126.75, 125.81, 120.50, 110.88. HRMS (ESI/TOF) m/z calculated for C12H8ClN4O2: 275.6699 [M + H]+. Found: 275.6698.
- General procedure for the synthesis of 1-(2-aminoaryl)-1H-benzimidazoles (4a,b) and 1-(2-aminoaryl)-1H-benzotriazoles (4c–e).
- 2-(1H-benzimidazol-1-yl)-5-(trifluoromethyl)aniline (4a): Yield 97%. m.p. 209−210 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.33 (s, 1H, H2′), 7.75–7.81 (m, 1H, H4′), 7.35 (d, J = 8.1 Hz, 1H, H3), 7.23–7.31 (m, 3H, H5′, H6′, H6), 7.17–7.22 (m, 1H, H7′), 6.98 (dd, J = 8.1, 1.6 Hz, 1H, H4), 5.58 (s, 2H, NH2). 13C{1H} NMR (DMSO-d6, 126 MHz) δ 145.27 (C1), 143.83 (C2′), 143.37 (C4′a), 133.69 (C7′a), 130.12 (q, J = 31.8 Hz, C5), 129.13 (C3), 124.22 (q, J = 272.9 Hz, CF3), 123.07 (C6′), 122.95 (C2), 122.08 (C5′), 119.74 (C4′), 112.29 (q, J = 4.0 Hz, C6), 111.99 (q, J = 4.0 Hz, C4), 110.81 (C7′). HRMS (ESI/TOF) m/z calculated for C14H11F3N3 [M + H]+: 278.0906. Found: 278.0890.
- 2-(1H-benzimidazol-1-yl)-5-chloroaniline (4b): Yield 97%. m.p. 192−195 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.26 (s, 1H, H2′), 7.72–7.79 (m, 1H, H4′), 7.21–7.33 (m, 2H, H5′, H6′), 7.15–7.19 (m, 1H, H7′), 7.13 (d, J = 8.3 Hz, 1H, H3), 6.98 (d, J = 2.3 Hz, 1H, H6), 6.69 (dd, J = 8.4, 2.4 Hz, 1H, H4), 5.39 (s, 2H, NH2). 13C{1H} NMR (126 MHz, DMSO-d6) δ 146.08 (C1), 144.05 (C2′), 143.32 (C4′a), 133.98 (C7′a), 133.93 (C5), 129.69 (C3), 122.92 (C6′), 121.92 (C5′), 119.66 (C4′), 118.81 (C2), 115.55 (C4), 114.94 (C6), 110.69 (C7′). HRMS (ESI/TOF) m/z calculated for C13H11ClN3 [M+H]+: 244.0642. Found: 244.0626.
- 2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)aniline(4c): Yield 98%. m.p. 136–139 °C. 1H NMR (500 MHz, DMSO-d6) δ 8.18 (d, J = 8.3 Hz, 1H, H4′), 7.59 (ddd, J = 8.1, 6.8, 1.0 Hz, 1H, H6′), 7.47–7.53 (m, 2H, H5′, H7′), 7.46 (d, J = 8.2 Hz, 1H, H3), 7.34 (d, J = 2.0 Hz, 1H, H6), 7.01 (dd, J = 8.2, 2.0 Hz, 1H, H4), 5.78 (s, 2H, NH2). 13C NMR (DMSO-d6, 126 MHz) δ 145.91, 145.50, 133.74, 131.76 (q, J = 32 Hz), 129.29, 128.96, 125.02, 124.71 (q, J = 271 Hz, CF3), 123.51 (m), 120.42, 113.44 (q, J = 4.1 Hz), 112.55 (q, J = 4 Hz), 111.46. HRMS (ESI/TOF) m/z calculated for C13H10F3N4: 279.2400 [M + H]+. Found: 279.2398.
- Ethyl 3-amino-4-(1H-benzotriazol-1-yl)benzoate (4d): Yield 95%. m.p. 132–136 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.17 (d, J = 8.2 Hz, 1H, H4′), 7.65 (d, J = 1.9 Hz, 1H, H2), 7.59 (t, J = 8.4 Hz, 1H, H6′), 7.52–7.44 (m, 2H, H5′, H7′), 7.37 (d, J = 8.2 Hz, 1H, H5), 7.29 (dd, J = 8.2, 1.9 Hz, 1H, H6), 5.60 (s, 2H, NH2), 4.35 (q, J = 7.1 Hz, 2H, CH2), 1.34 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (DMSO-d6, 101 MHz) δ 166.15, 145.90, 144.83, 133.66, 132.40, 128.90, 128.25, 125.00, 124.30, 120.22, 117.95, 117.11, 111.53, 61.55, 14.84. HRMS (ESI/TOF) m/z calculated for C15H15N4O: 267.3053 [M + H]+. Found: 267.3051.
- 2-(1H-benzotriazol-1-yl)-5-chloroaniline (4e). Yield 94%. m.p. 154–156 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.16 (d, J = 8.3 Hz, 1H, H4′), 7.58 (dd, J = 8.3, 6.8 Hz, 1H, H6′), 7.49–7.45 (m, 2H, H5′, H7′), 7.24 (d, J = 8.4 Hz, 1H, H3), 7.03 (d, J = 2.3 Hz, 1H, H6), 6.74 (dd, J = 8.4, 2.3 Hz, 1H, H4), 5.55 (s, 2H). 13C NMR (101 MHz, DMSO-d6,) δ 146.38, 145.85, 135.64, 133.97, 129.85, 128.79, 124.88, 120.16, 119.70, 116.20, 116.00, 111.38. HRMS (ESI/TOF) m/z calculated for C12H10ClN4: 245.6871 [M + H]+. Found: 245.6873.
- General procedure for the synthesis of N-(2-(1H-benzimidazol-1-yl)-5-(trifluoromethyl)phenyl)hydroxylamine (8a) and N-(2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)phenyl)hydroxylamine (8b)
- N-(2-(1H-benzimidazol-1-yl)-5-(trifluoromethyl)phenyl)hydroxylamine (8a): Yield 86%. m.p. 153–156 °C. 1H NMR (DMSO-d6, 500 MHz) δ 8.79 (d, J = 1.6 Hz, 1H, OH), 8.61 (s, 1H, NH), 8.31 (s, 1H, H2′), 7.79–7.23 (m, 1H, H4′), 7.59 (d, J = 1.2 Hz, 1H, H6), 7.48 (d, J = 7.9 Hz, 1H, H3), 7.32–7.24 (m, 3H, H5′, H4, H6′), 7.24–7.18 (m, 1H, C7′). 13C{1H} NMR (DMSO-d6, 126 MHz) δ 148.0 (C1), 143.7 (C2′), 143.4 (C4′a), 133.7 (C7′a), 129.9 (q, J = 31.9 Hz, C5), 128.3 (C3), 124.1 (q, J = 272.5 Hz, CF3), 123.5 (C2), 122.9 (C6′), 122.1 (C5′), 119.7 (C4′), 115.6 (q, J = 3.8 Hz, C4), 111.1 (C7′), 110.2 (q, J = 3.8 Hz, C6). HRMS (ESI/TOF) m/z calculated for C14H11F3N3O [M+H]+: 294.0855. Found: 294.0841.
- N-(2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)phenyl)hydroxylamine (8b): Yield 82%. m.p. 151–154 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.87 (s, 1H, OH), 8.68 (s, 1H, NH), 8.18 (dt, J = 8.3, 1.0 Hz, 1H, H4′), 7.64–7.55 (m, 3H, H6, H3, H5′,), 7.55–7.43 (m, 2H, H6′, H7′), 7.30 (dd, J = 8.2, 2.1 Hz, 1H, H4). 13C NMR (101 MHz, DMSO-d6) δ 148.11, 145.96, 133.77, 131.60 (q, J = 31.8 Hz), 128.79, 126.07, 124.98, 123.86, 123.36, 120.22, 115.95, 111.77, 111.05. HRMS (ESI/TOF) m/z calculated for C13H10F3N4O [M+H]+: 295.2394. Found: 295.2389.
- General procedure for the synthesis of N-[2-(1H-benzimidazol-1-yl)-5-R-phenyl]-2-nitro-4-R1-aniline (5a) and N-[2-(1H-benzotriazol-1-yl)-5-R-phenyl]-2-nitro-4-R1-aniline (5b–d).
- N-[2-(1H-benzimidazol-1-yl)-5-(trifluoromethyl)phenyl]-2-nitro-4-(trifluoromethyl)aniline (5a). Yield 93%. m.p. 180–183 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.79 (s, 1H, NH), 8.36 (s, 1H, H2′’), 8.11 (s, 1H, H6′), 8.03 (s, 1H, H3), 7.99–7.92 (m, 2H, H3′, H4′), 7.60–7.53 (m, 1H, H4′’), 7.45–7.38 (m, 2H, H5, H7′’), 7.22–7.14 (m, 2H, H5′’, H6′’), 6.82 (d, J = 8.9 Hz, 1H, H6). 13C NMR (101 MHz, DMSO-d6) δ 143.40, 142.96, 142.73, 135.01, 134.80, 132.43, 132.39, 131.02 (q, J = 3.0 Hz), 129.86 (q, J = 32.6 Hz), 129.25, 126.17 (q, J = 3.6 Hz), 124.58 (q, J = 4.5 Hz), 123.57 (q, J = 272.5 Hz), 123.35, 123.30 (q, J = 271.1 Hz), 123.12 (q, J = 4.4 Hz), 122.50, 119.58, 118.10 (q, J = 33.8 Hz), 118.00, 110.89. HRMS (ESI/TOF) m/z calculated for C21H13F6N4O2: 467.3434 [M + H]+. Found: 467.3435.
- N-[2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)phenyl]-2-nitro-4-(trifluoromethyl)aniline (5b). Yield 92%. m.p. 203–204 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.93 (s, 1H, NH), 8.16 (s, 1H, H6′), 8.08 (d, J = 8.3 Hz, 1H, H3′), 8.04 (s, 1H, H3), 8.02 (d, J = 9.0 Hz, 1H, H4′’), 7.97 (d, J = 8.5 Hz, 1H, H4′), 7.73 (d, J = 8.3 Hz, 1H, H7′’), 7.58–7.47 (m, 2H, H5, H6′’), 7.41 (t, J = 7.8 Hz, 1H, H5′’), 6.88 (d, J = 8.9 Hz, 1H, H6). 13C NMR (101 MHz, DMSO-d6) δ 144.94, 142.46, 134.41, 134.08, 133.06, 131.94, 131.11 (q, J = 2.9 Hz), 130.84 (q, J = 32.4 Hz), 128.71, 128.60, 125.45 (m), 124.69, 124.06 (m), 123.42 (q, J = 272.7 Hz), 123.21 (q, J = 271.1 Hz), 123.12 (q, J = 4.5 Hz), 119.40, 118.59 (q, J = 33.8 Hz), 118.09, 110.87. HRMS (ESI/TOF) m/z calculated for C20H12F6N5O2: 468.3315 [M + H]+. Found: 468.3312.
- Ethyl 4-[2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)anilino]-3-nitrobenzoate (5c). Yield 91%. m.p. 192–196 °C. 1H NMR (400 MHz, DMSO-d6) δ 10.00 (s, 1H, NH), 8.28 (s, 1H, H2), 8.15 (s, 1H, H6′), 8.08 (d, J = 8.3 Hz, 1H, H3′), 8.01 (d, J = 8.3 Hz, 1H, H4′’), 7.97 (d, J = 8.5 Hz, 1H, H4′), 7.74 (d, J = 8.2 Hz, 1H, H7′’), 7.68 (d, J = 9.0 Hz, 1H, H6), 7.53 (t, J = 7.6 Hz, 1H, H6′’), 7.40 (t, J = 7.6 Hz, 1H, H5′’), 6.79 (d, J = 8.9 Hz, 1H, H5), 4.24 (q, J = 7.1 Hz, 2H, CH2), 1.26 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 163.50, 144.77, 142.74, 134.60, 134.23, 133.89, 133.17, 131.84, 130.66 (q, J = 32.8 Hz), 128.47, 128.34, 126.81, 124.95 (q, J = 3.7 Hz), 124.41, 123.76 (q, J = 3.8 Hz), 123.21 (q, J = 273.1 Hz), 119.82, 119.21, 116.75, 110.60, 60.62, 13.76. HRMS (ESI/TOF) m/z calculated for C22H17F3N5O4: 472.3961 [M + H]+. Found: 472.3958.
- N-[2-(1H-benzotriazol-1-yl)-5-chlorophenyl]-2-nitro-4-(trifluoromethyl)aniline (5d). Yield 93%. m.p. 178–181 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.79 (s, 1H, NH), 8.05 (s, 1H, H3), 8.01 (d, J = 8.3 Hz, 1H, H4′’), 7.88 (s, 1H, H6′), 7.87 (d, J = 6.0 Hz, 1H, H3′), 7.71–7.65 (m, 2H, H4′, H7′’), 7.58–7.49 (m, 2H, H5, H6′’), 7.40 (t, J = 7.6 Hz, 1H, H5′’), 6.95 (d, J = 8.9 Hz, 1H, H6). 13C NMR (101 MHz, DMSO-d6) δ 144.86, 142.60, 135.06, 134.79, 132.85, 132.21, 131.21 (q, J = 3.5 Hz), 129.93, 129.15, 128.45, 127.87, 127.41, 124.56, 123.22 (q, J = 271.2 Hz), 123.17 (q, J = 4.3 Hz), 119.35, 118.51 (q, J = 33.8 Hz), 118.28, 110.79. HRMS (ESI/TOF) m/z calculated for C19H12ClF3N5O2: 434.7785 [M + H]+. Found: 434.7781.
- N-[2-(1H-benzimidazol-1-yl)-5-(trifluoromethyl)phenyl]-2-amino-4-(trifluoromethyl)aniline (6a). Yield 96%. m.p. 205–207 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.51 (s, 1H, H2′’), 7.77–7.70 (m, 1H, H4′’), 7.56 (d, J = 8.1 Hz, 1H, H3′), 7.48–7.40 (m, 2H, NH, H7′’), 7.33–7.23 (m, 3H, H4′, H5′’, H6′’), 7.03 (d, J = 8.1 Hz, 1H, H6), 7.01 (d, J = 1.6 Hz, 1H, H3), 6.95 (d, J = 1.6 Hz, 1H, H6′), 6.72 (dd, J = 8.1, 1.6 Hz, 1H, H5), 5.31 (s, 2H, NH2). 13C NMR (101 MHz, DMSO-d6) δ 144.10, 143.44, 143.02, 141.14, 133.62, 129.65 (q, J = 31.7 Hz), 129.07, 128.97, 126.93, 125.52 (q, J = 31.3 Hz), 124.52 (q, J = 271.8 Hz), 124.04, 123.92 (q, J = 272.4 Hz), 123.13, 122.13, 119.61, 115.75 (q, J = 3.9 Hz), 113.10 (m), 112.50 (q, J = 4.2 Hz), 111.08 (q, J = 3.3 Hz), 110.97. HRMS (ESI/TOF) m/z calculated for C21H15F6N4: 437.3605 [M + H]+. Found: 437.3601.
- N-[2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)phenyl]-2-amino-4-(trifluoromethyl)aniline (6b). Yield 94%. m.p. 170–173 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.14 (d, J = 8.4 Hz, 1H, H4′’), 7.77 (d, J = 8.3 Hz, 1H, H7′’), 7.68 (d, J = 8.1 Hz, 1H, H3′), 7.56 (t, J = 7.6 Hz, 1H, H6′’), 7.52 (s, 1H, NH), 7.45 (t, J = 7.6 Hz, 1H, H5′’), 7.33 (d, J = 8.2 Hz, 1H, H4′), 7.07–7.02 (m, 2H, H6′, H6), 6.98 (s, 1H, H3), 6.73 (d, J = 8.1 Hz, 1H, H5), 5.28 (s, 2H, NH2). 13C NMR (101 MHz, DMSO-d6) δ 145.37, 142.92, 140.66, 132.96, 130.82 (q, J = 31.7 Hz), 129.44, 128.53, 128.18, 126.6, 125.63 (q, J = 31.3 Hz), 124.50 (q, J = 271.7 Hz), 124.29, 123.95, 123.81 (q, J = 272.7 Hz), 119.45, 115.53 (q, J = 3.9 Hz), 113.35 (q, J = 3.9 Hz), 112.50 (q, J = 4.3 Hz), 111.39, 111.16 (q, J = 4.1 Hz). HRMS (ESI/TOF) m/z calculated for C20H14F6N5: 438.3485 [M + H]+. Found: 438.3481.
- Ethyl 3-amino-4-[2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)anilino]benzoate (6c). Yield 92%. m.p. 178–181 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.14 (d, J = 8.3 Hz, 1H, H4′’), 7.75 (d, J = 8.3 Hz, 1H, H7′’), 7.69 (d, J = 8.2 Hz, 1H, H3′), 7.56 (t, J = 7.7 Hz, 1H, H6′’), 7.53 (s, 1H, NH), 7.45 (t, J = 7.6 Hz, 1H, H5′’), 7.34 (d, J = 8.0 Hz, 1H, H4′), 7.32 (d, J = 1.7 Hz, 1H, H3), 7.11 (s, 1H, H6′), 7.06 (dd, J = 8.2, 1.7 Hz, 1H, H5), 6.97 (d, J = 8.2 Hz, 1H, H6), 5.09 (s, 2H, NH2), 4.23 (q, J = 7.1 Hz, 2H, CH2), 1.28 (t, J = 7.1 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 165.76, 145.34, 141.86, 140.38, 132.88, 130.73 (q, J = 31.7 Hz), 129.87, 129.34, 128.17, 126.94, 126.14, 124.27, 123.81 (q, J = 272.8 Hz), 122.51, 122.45, 119.44, 117.59, 115.80 (m), 113.92 (m), 111.32, 60.22, 14.22. HRMS (ESI/TOF) m/z calculated for C22H19F3N5O2: 442.4132 [M + H]+. Found: 442.4134.
- N-[2-(1H-benzotriazol-1-yl)-5-chlorophenyl]-2-amino-4-(trifluoromethyl)aniline (6d). Yield 91%. m.p. 191–194 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.13 (d, J = 8.3 Hz, 1H, H4′’), 7.72 (d, J = 8.3 Hz, 1H, H7′’), 7.55 (t, J = 7.6 Hz, 1H, H6′’), 7.49–7.41 (m, 2H, H3′, H5′’), 7.32 (s, 1H, NH), 7.09–7.02 (m, 2H, H6, H4′), 6.98 (s, 1H, H3), 6.78 (d, J = 1.8 Hz, 1H, H6′), 6.75 (d, J = 8.2 Hz, 1H, H5), 5.25 (s, 2H, NH2). 13C NMR (101 MHz, DMSO-d6) δ 145.30, 142.89, 141.52, 135.05, 133.20, 129.86, 128.77, 128.01, 125.54 (q, J = 31.2 Hz), 124.51 (q, J = 271.7 Hz), 124.13, 122.57, 119.37, 118.97, 116.23, 112.58, 112.53, 111.19, 11.14. HRMS (ESI/TOF) m/z calculated for C19H14ClF3N5: 404.7956 [M + H]+. Found: 404.7953.
- General procedure for the synthesis of 1-[2-(1H-benzimidazol-1-yl)phenyl]-1H-benzotriazole (7a–c):
- 1-{4-(trifluoromethyl)-2-[5-(trifluoromethyl)-1H-benzimidazol-1-yl]phenyl}-1H-benzotriazole (7a). Yield 96%. m.p. 124–128 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.61 (s, 1H, H2′’), 8.57 (s, 1H, H3′), 8.35–8.27 (m, 2H, H5′, H6′), 7.97 (d, J = 8.3 Hz, 1H, H4), 7.93 (s, 1H, H4′’), 7.59 (d, J = 8.3 Hz, 1H, H7), 7.47 (t, J = 7.7 Hz, 1H, H6), 7.38–7.31 (m, 2H, H5, H6′’), 7.18 (d, J = 8.5 Hz, 1H, H7′’). 13C NMR (101 MHz, DMSO-d6) δ 146.17, 144.63, 142.24, 135.61, 135.09, 132.35, 131.41 (q, J = 33.6 Hz), 131.16, 129.32, 128.94, 127.92 (q, J = 4.0 Hz), 127.11 (q, J = 3.8 Hz), 124.89, 124.61 (q, J = 272.0 Hz), 123.41 (q, J = 31.8 Hz), 123.22 (q, J = 273.1 Hz), 119.99 (q, J = 3.7 Hz), 119.48, 117.11 (q, J = 4.0 Hz), 110.75, 109.98. HRMS (ESI/TOF) m/z calculated for C21H12F6N5: 448.3434 [M + H]+. Found: 448.3429.
- Ethyl 1-[2-(1H-benzotriazol-1-yl)-5-(trifluoromethyl)phenyl]-1H-benzimidazole-5-carboxylate (7b). Yield 94%. m.p. 100–104 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.61 (s, 1H, H2), 8.57 (s, 1H, H6′), 8.36–8.24 (m, 2H, H3′, H4′), 8.10 (s, 1H, H4), 7.96 (d, J = 8.2 Hz, 1H, H4′’), 7.60–7.51 (m, 2H, H6, H7′’), 7.46 (t, J = 7.4 Hz, 1H, H6′’), 7.34 (t, J = 7.5 Hz, 1H, H5′’), 7.01 (d, J = 8.8 Hz, 1H, H7), 4.25 (q, J = 7.1 Hz, 2H, CH2), 1.28 (t, J = 6.2 Hz, 3H, CH3). 13C NMR (101 MHz, DMSO-d6) δ 165.68, 145.89, 144.61, 142.40, 136.32, 134.97, 132.27, 131.31 (q, J = 33.0 Hz), 131.20, 129.34, 128.91, 127.79 (q, J = 3.5 Hz), 127.02 (q, J = 3.7 Hz), 124.84, 124.34, 124.14, 123.21 (q, J = 273.0 Hz), 121.12, 119.49, 109.92, 109.66, 60.66, 14.14. HRMS (ESI/TOF) m/z calculated for C23H17F3N5O2: 452.4081 [M + H]+. Found: 452.4079.
- 1-{4-Chloro-2-[5-(trifluoromethyl)-1H-benzimidazol-1-yl]phenyl}-1H-benzotriazole (7c). Yield 97%. m.p. 136–141 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.52 (s, 1H, H2′’), 8.28 (d, J = 2.1 Hz, 1H, H3′), 8.09 (d, J = 8.6 Hz, 1H, H6′), 8.02 (dd, J = 8.6, 2.1 Hz, 1H, H5′), 7.95 (d, J = 8.3 Hz, 1H, H4), 7.91 (s, 1H, H4′’), 7.52 (d, J = 8.3 Hz, 1H, H7), 7.44 (t, J = 7.6 Hz, 1H, H6), 7.37–7.30 (m, 2H, H5, H6′’), 7.22 (d, J = 8.5 Hz, 1H, H7′’). 13C NMR (101 MHz, DMSO-d6) δ 146.01, 144.53, 142.14, 135.58, 135.49, 132.57, 131.76, 130.94, 130.75, 129.81, 129.47, 128.74, 124.70, 124.62 (q, J = 271.8 Hz), 123.41 (q, J = 31.7 Hz), 120.03 (q, J = 3.7 Hz), 119.39, 117.07 (q, J = 4.1 Hz), 110.85, 109.83. HRMS (ESI/TOF) m/z calculated for C20H12ClF3N5: 414.7904 [M + H]+. Found: 414.7901.
3.2. Biological Evaluation
3.2.1. Cell Culture
3.2.2. MTT-Assay
- Briefly, cells in the logarithmic growth phase were digested and resuspended to adjust the cell density. The cells were then seeded in 96-well plates at a density of 10,000 cells per well. After 24 h of the incubation of the cells under the standard conditions described above (for the purpose of cell adhesion), the cells were treated with the synthesized compounds at different concentrations (0.1 to 100 µM) or a vehicle alone as a control (1% DMSO) for 24 h. After the incubation time of the cells with synthesized compounds, 5 mg/mL of MTT was added to the cells and incubated for 2 h at 37 °C. Next, the supernatant was discarded; after that, the formazan crystals were dissolved in a DMSO and the absorbance of each well was measured at 570 nm using a multifunctional microplate reader, Cytation3 (Biotech Tools Inc., Winooski, USA).
3.2.3. Animals and Rat Brain Homogenate
3.2.4. TBARS Method
3.2.5. Wound-Healing Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation (WHO). Non-Communicable Diseases. Available online: https://www.who.int/ru/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 25 June 2024).
- World Health Organisation (WHO). Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 25 June 2024).
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, Z.; Prettner, K.; Kuhn, M.; Yang, J.; Jiao, L.; Wang, Z.; Li, W.; Geldsetzer, P.; Barnighausen, T.; et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories from 2020 to 2050. JAMA Oncol 2023, 9, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes. Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Chabner, B.A.; Roberts, T.G., Jr. Timeline: Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Nygren, P.; SBU-Group. Swedish Council on Technology Assessment in Health Care. What is cancer chemotherapy? Acta Oncol. 2001, 40, 166–174. [Google Scholar] [CrossRef]
- Shalini; Kumar, V. Have molecular hybrids delivered effective anti-cancer treatments and what should future drug discovery focus on? Expert. Opin. Drug Discov. 2021, 16, 335–363. [Google Scholar] [CrossRef]
- Gontijo, V.S.; Viegas, F.P.D.; Ortiz, C.J.C.; de Freitas Silva, M.; Damasio, C.M.; Rosa, M.C.; Campos, T.G.; Couto, D.S.; Tranches Dias, K.S.; Viegas, C. Molecular Hybridization as a Tool in the Design of Multi-target Directed Drug Candidates for Neurodegenerative Diseases. Curr. Neuropharmacol. 2020, 18, 348–407. [Google Scholar] [CrossRef]
- Sampath Kumar, H.M.; Herrmann, L.; Tsogoeva, S.B. Structural hybridization as a facile approach to new drug candidates. Bioorganic Med. Chem. Lett. 2020, 30, 127514. [Google Scholar] [CrossRef] [PubMed]
- George, N.; Jawaid Akhtar, M.; Al Balushi, K.A.; Alam Khan, S. Rational drug design strategies for the development of promising multi-target directed indole hybrids as Anti-Alzheimer agents. Bioorganic Chem. 2022, 127, 105941. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.S.; Su, W.Q.; Cheng, J.B.; Xiao, T.; Li, H.Z.; Chen, D.A.; Zhang, Z.L. Benzimidazole hybrids as anticancer drugs: An updated review on anticancer properties, structure-activity relationship, and mechanisms of action (2019–2021). Arch Pharm (Weinheim) 2022, 355, e2200051. [Google Scholar] [CrossRef] [PubMed]
- Malasala, S.; Ahmad, M.N.; Akunuri, R.; Shukla, M.; Kaul, G.; Dasgupta, A.; Madhavi, Y.V.; Chopra, S.; Nanduri, S. Synthesis and evaluation of new quinazoline-benzimidazole hybrids as potent anti-microbial agents against multidrug resistant Staphylococcus aureus and Mycobacterium tuberculosis. Eur. J. Med. Chem. 2021, 212, 112996. [Google Scholar] [CrossRef]
- Karaca Gencer, H.; Acar Cevik, U.; Levent, S.; Saglik, B.N.; Korkut, B.; Ozkay, Y.; Ilgin, S.; Ozturk, Y. New Benzimidazole-1,2,4-Triazole Hybrid Compounds: Synthesis, Anticandidal Activity and Cytotoxicity Evaluation. Molecules 2017, 22, 507. [Google Scholar] [CrossRef]
- Aitha, S.; Thumma, V.; Ambala, S.; Matta, R.; Panga, S.; Pochampally, J. Bis 1,2,3-Triazoles Linked Deoxybenzoin Hybrids as Antimicrobial Agents: Synthesis, In Vitro and In Silico Screening. ChemistrySelect 2023, 8, e202300405. [Google Scholar] [CrossRef]
- Asemanipoor, N.; Mohammadi-Khanaposhtani, M.; Moradi, S.; Vahidi, M.; Asadi, M.; Faramarzi, M.A.; Mahdavi, M.; Biglar, M.; Larijani, B.; Hamedifar, H.; et al. Synthesis and biological evaluation of new benzimidazole-1,2,3-triazole hybrids as potential alpha-glucosidase inhibitors. Bioorganic Chem. 2020, 95, 103482. [Google Scholar] [CrossRef]
- Singh, A.; Sharma, S.; Arora, S.; Attri, S.; Kaur, P.; Kaur Gulati, H.; Bhagat, K.; Kumar, N.; Singh, H.; Vir Singh, J.; et al. New coumarin-benzotriazole based hybrid molecules as inhibitors of acetylcholinesterase and amyloid aggregation. Bioorganic Med. Chem. Lett. 2020, 30, 127477. [Google Scholar] [CrossRef]
- Neganova, M.E.; Aleksandrova, Y.R.; Sharova, E.V.; Smirnova, E.V.; Artyushin, O.I.; Nikolaeva, N.S.; Semakov, A.V.; Schagina, I.A.; Akylbekov, N.; Kurmanbayev, R.; et al. Conjugates of 3,5-Bis(arylidene)-4-piperidone and Sesquiterpene Lactones Have an Antitumor Effect via Resetting the Metabolic Phenotype of Cancer Cells. Molecules 2024, 29, 2765. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Benzimidazole and its derivatives as cancer therapeutics: The potential role from traditional to precision medicine. Acta Pharm. Sin. B 2023, 13, 478–497. [Google Scholar] [CrossRef]
- Ambala, S.; Thumma, V.; Mallikanti, V.; Aitha, S.; Matta, R.; Pochampally, J. Design, Synthesis, and Cytotoxicity of Some New Benzimidazole-Piperazine Conjugate Analogues Against Human Breast Adenocarcinoma. ChemistrySelect 2023, 8, e202302393. [Google Scholar] [CrossRef]
- Harkala, K.J.; Eppakayala, L.; Maringanti, T.C. Synthesis and biological evaluation of benzimidazole-linked 1,2,3-triazole congeners as agents. Org. Med. Chem. Lett. 2014, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Janovec, L.; Janockova, J.; Matejova, M.; Konkolova, E.; Paulikova, H.; Lichancova, D.; Junosova, L.; Hamulakova, S.; Imrich, J.; Kozurkova, M. Proliferation inhibition of novel diphenylamine derivatives. Bioorganic Chem. 2019, 83, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guan, A.; Huang, G.; Liu, C.L.; Li, Z.; Xie, Y.; Lan, J. Design, synthesis and structure-activity relationship of novel diphenylamine derivatives. Bioorganic Med. Chem. 2016, 24, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Riveros, A.; Hernandez-Vazquez, E.; Ramirez-Trinidad, A.; Nieto-Camacho, A.; Miranda, L.D. Multicomponent synthesis and preliminary anti-inflammatory activity of lipophilic diphenylamines. Bioorganic Med. Chem. Lett. 2021, 38, 127860. [Google Scholar] [CrossRef]
- Shah, A.; Desai, K.; Bhanusali, A.; Malek, N.; Naik, N.; Thakar, A.; Shah, A. Molecular modelling, cytotoxicity & biological investigation of novel fluorinated diphenylamine chalcone derivatives. J. Mol. Struct. 2024, 1311, 138379. [Google Scholar]
- Mehton, R.K.; Meshram, V.; Saxena, S.; Chhibber, M. Synthesis and anti-staphylococcal activity of 2, 4-disubstituted diphenylamines. J. Braz. Chem. Soc. 2016, 27, 1236–1244. [Google Scholar] [CrossRef]
- Simon, R.; Wang, S.J. Use of genomic signatures in therapeutics development in oncology and other diseases. Pharmacogenomics J. 2006, 6, 166–173. [Google Scholar] [CrossRef]
- Zubricke, I.; Jonuskiene, I.; Kantminiene, K.; Tumosiene, I.; Petrikaite, V. Synthesis and In Vitro Evaluation as Potential Anticancer and Antioxidant Agents of Diphenylamine-Pyrrolidin-2-one-Hydrazone Derivatives. Int. J. Mol. Sci. 2023, 24, 16804. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Zhang, X.; Sverko, E.; Marvin, C.H.; Jobst, K.J.; Smyth, S.A.; Li, Y.F. Determination of diphenylamine antioxidants in wastewater/biosolids and sediment. Environ. Sci. Technol. 2020, 7, 102–110. [Google Scholar] [CrossRef]
- Thao, P.T.T.; Thong, N.M.; Vo, Q.V.; Van Bay, M.; Quang, D.T.; Nam, P.C. Substituent effects on the antioxidant capacity of monosubstituted diphenylamines: A DFT study. Vietnam. J. Chem. 2020, 58, 742–751. [Google Scholar] [CrossRef]
- Yu, S. A new antioxidant with higher activity at elevated temperature based on multiple intramolecular synergisms. ChemistrySelect 2023, 8, e202300747. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, P.; Du, W.; Xu, R.; Yan, G.; Deng, Y.; Li, X.; Chen, Y. Metabolically stable diphenylamine derivatives suppress androgen receptor and BET protein in prostate cancer. Biochem. Pharmacol. 2020, 177, 113946. [Google Scholar] [CrossRef] [PubMed]
- Tumosienė, I.; Jonuškienė, I.; Kantminienė, K.; Mickevičius, V.; Petrikaite, V. Novel N-Substituted Amino Acid Hydrazone-Isatin Derivatives: Synthesis, Antioxidant Activity, and Anticancer Activity in 2D and 3D Models In Vitro. Int. J. Mol. Sci 2021, 22, 7799. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Fujiwara, Y.; Osawa, T.; Sakai, T.; Kubo, K.; Kubo, K.; Nishitoba, T.; Kimura, K.; Senga, T.; Murooka, H.; et al. Orally active anti-proliferation agents: Novel diphenylamine derivatives as FGF-R2 autophosphorylation inhibitors. Bioorganic Med. Med. Med. Chem. Lett. 2004, 14, 875–879. [Google Scholar] [CrossRef]
- Abou-Seri, S.M. Synthesis and biological evaluation of novel 2, 4′-bis substituted diphenylamines as anticancer agents and potential epidermal growth factor receptor tyrosine kinase inhibitors. Eur. J. Med. Med. Chem. 2010, 45, 4113–4121. [Google Scholar] [CrossRef]
- Abou-Seri, S.M.; Farag, N.A.; Hassan, G.S. Novel diphenylamine 2, 4′-dicarboxamide based azoles as potential epidermal growth factor receptor inhibitors: Synthesis and biological activity. Chem. Pharm. Bull. 2011, 59, 1124–1132. [Google Scholar] [CrossRef][Green Version]
- Rahman, D.E.A. Synthesis, quantitative structure–activity relationship and biological evaluation of 1, 3, 4-oxadiazole derivatives possessing diphenylamine moiety as potential anticancer agents. Chem. Pharm. Bull. 2013, 61, 151–159. [Google Scholar] [CrossRef][Green Version]
- Andrzejewska, M.; Yepez-Mulia, L.; Cedillo-Rivera, R.; Tapia, A.; Vilpo, L.; Vilpo, J.; Kazimierczuk, Z. Synthesis, antiprotozoal and anticancer activity of substituted 2-trifluoromethyl- and 2-pentafluoroethylbenzimidazoles. Eur. J. Med. Chem. 2002, 37, 973–978. [Google Scholar] [CrossRef]
- Apraku, J.; Okoro, C.O. Design, synthesis and anticonvulsant evaluation of fluorinated benzyl amino enaminones. Bioorganic Med. Chem. 2019, 27, 161–166. [Google Scholar] [CrossRef]
- Begunov, R.S.; Egorov, D.O.; Chetvertakova, A.V.; Savina, L.I.; Zubishina, A.A. Antibacterial Activity of the Halogen- and Nitro Derivatives of Benzimidazole Against Bacillus Subtilis. Antibiot. Khimioter Antibiot. Chemother. 2023, 68, 19–24. [Google Scholar] [CrossRef]
- Laudy, A.E.; Moo-Puc, R.; Cedillo-Rivera, R.; Kazimierczuk, Z.; Orzeszko, A. Synthesis and Antimicrobial Activities of New Polyhalogenated Benzimidazoles. J. Heterocycl. Chem. 2012, 49, 1059–1065. [Google Scholar] [CrossRef]
- Ozkay, Y.; Tunali, Y.; Karaca, H.; Isikdag, I. Antimicrobial activity of a new combination system of benzimidazole and various azoles. Arch. Pharm. 2011, 344, 264–271. [Google Scholar] [CrossRef]
- Janeczko, M.; Kazimierczuk, Z.; Orzeszko, A.; Niewiadomy, A.; Krol, E.; Szyszka, R.; Maslyk, M. In Search of the Antimicrobial Potential of Benzimidazole Derivatives. Pol. J. Microbiol. 2016, 65, 359–364. [Google Scholar] [CrossRef]
- Betageri, R.; Zhang, Y.; Zindell, R.M.; Kuzmich, D.; Kirrane, T.M.; Bentzien, J.; Cardozo, M.; Capolino, A.J.; Fadra, T.N.; Nelson, R.M.; et al. Trifluoromethyl group as a pharmacophore: Effect of replacing a CF3 group on binding and agonist activity of a glucocorticoid receptor ligand. Bioorganic Med. Chem. Lett. 2005, 15, 4761–4769. [Google Scholar] [CrossRef]
- Sateesha, K.M.; Pasha, M.; Patil, M.B.; Vetrivelan, V.; Saral, A.; Muthu, S.; Javed, S.; Lokanath, N.K.; Amshumali, M.K. Synthesis, structural and theoretical investigations on 3- diethyl 2-({4-[3-ethoxy-2-(ethoxycarbonyl)-3-oxo-2-phenylpropyl]-2,5-dimethylphenyl}methyl)-2-phenylpropanedioate. J. Indian. Chem. Soc. 2023, 100, 100869. [Google Scholar] [CrossRef]
- Rajput, A.P.; Patil, S.A. Review: Synthesis And Docking Study of Biologically Active Esters. Asian J. Pharm. Technol. Innov. 2016, 4, 140–154. [Google Scholar]
- Kore, K.J.; Bramhakule, P.P.; Rachhadiya, R.M.; Shete, R.V. Evaluation of antiulcer activity of protocatechuic acid ethyl ester in rats. Int. J. Pharm. Life Sci. 2011, 2, 909–915. [Google Scholar]
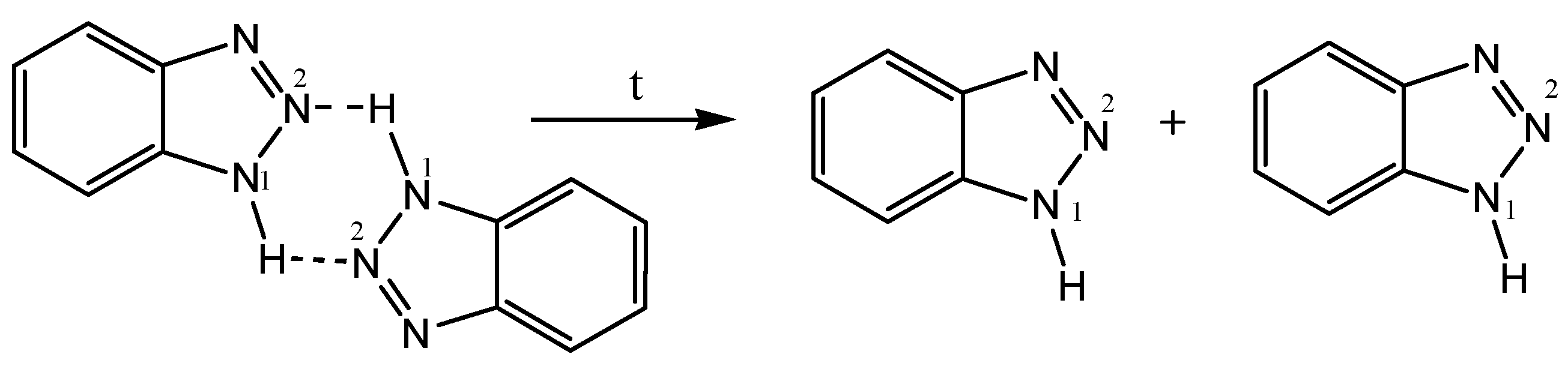
- Begunov, R.S.; Shebunina, T.V.; Yakovleva, Y.S.; Firgang, S.I. An interesting recyclization in the course of reduction of 1-(2-nitro-4-R-phenyl)-1H-benzimidazoles with tin(II) chloride. Mendeleev Commun. 2013, 23, 354–355. [Google Scholar] [CrossRef]
- Begunov, R.S.; Sokolov, A.A.; Fakhrutdinov, A.N. Recyclization-isomerization in the reduction of 1-(2-nitro(het)aryl)benzimidazoles. ChemistrySelect 2020, 5, 3544–3550. [Google Scholar] [CrossRef]
- Begunov, R.S.; Chetvertakova, A.V.; Neganova, M.E. Regioselective synthesis of 2-(1H-benzimidazol-1-yl)-5-nitro- and 2-(5-nitro-1H-benzimidazol-1-yl)anilines. Mendeleev Commun. 2023, 33, 650–652. [Google Scholar] [CrossRef]
- Bhardwaj, V.; He, J. Reactive Oxygen Species, Metabolic Plasticity, and Drug Resistance in Cancer. Int. J. Mol. Sci. 2020, 21, 16804. [Google Scholar] [CrossRef] [PubMed]
- Shuvalova, M.; Dmitrieva, A.; Belousov, V.; Nosov, G. The role of reactive oxygen species in the regulation of the blood-brain barrier. Tissue Barriers 2024, 2361202. [Google Scholar] [CrossRef]
- Giulietti, S.; Bigini, V.; Savatin, D.V. ROS and RNS production, subcellular localization, and signaling triggered by immunogenic danger signals. J. Exp. Bot. 2024, 75, 4512–4534. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, 453–462. [Google Scholar] [CrossRef]
- Kidwell, C.U.; Casalini, J.R.; Pradeep, S.; Scherer, S.D.; Greiner, D.; Bayik, D.; Watson, D.C.; Olson, G.S.; Lathia, J.D.; Johnson, J.S.; et al. Transferred mitochondria accumulate reactive oxygen species, promoting proliferation. Elife 2023, 12, e85494. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Yang, J. The role of reactive oxygen species in angiogenesis and preventing tissue injury after brain ischemia. Microvasc. Res. 2019, 123, 62–67. [Google Scholar] [CrossRef]
- Radomska-Lesniewska, D.M.; Hevelke, A.; Skopinski, P.; Balan, B.; Jozwiak, J.; Rokicki, D.; Skopinska-Rozewska, E.; Bialoszewska, A. Reactive oxygen species and synthetic antioxidants as angiogenesis modulators: Clinical implications. Pharmacol. Rep. 2016, 68, 462–471. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Chua, D.; Tan, N.S. Reactive oxygen species: A volatile driver of field cancerization and metastasis. Mol. Cancer 2019, 18, 65. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, M. Reactive oxygen species in tumor metastasis. Cancer Lett. 2008, 266, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Dharmaraja, A.T. Role of Reactive Oxygen Species (ROS) in Therapeutics and Drug Resistance in Cancer and Bacteria. J. Med. Chem. 2017, 60, 3221–3240. [Google Scholar] [CrossRef]
- Gu, H.; Huang, T.; Shen, Y.; Liu, Y.; Zhou, F.; Jin, Y.; Sattar, H.; Wei, Y. Reactive Oxygen Species-Mediated Tumor Microenvironment Transformation: The Mechanism of Radioresistant Gastric Cancer. Oxid. Med. Cell Longev. 2018, 2018, 5801209. [Google Scholar] [CrossRef]
- Aleksandrova, Y.; Munkuev, A.; Mozhaitsev, E.; Suslov, E.; Tsypyshev, D.; Chaprov, K.; Begunov, R.; Volcho, K.; Salakhutdinov, N.; Neganova, M. Elaboration of the Effective Multi-Target Therapeutic Platform for the Treatment of Alzheimer’s Disease Based on Novel Monoterpene-Derived Hydroxamic Acids. Int. J. Mol. Sci. 2023, 24, 9743. [Google Scholar] [CrossRef]
- Kariri, Y.A.; Aleskandarany, M.A.; Joseph, C.; Kurozumi, S.; Mohammed, O.J.; Toss, M.S.; Green, A.R.; Rakha, E.A. Molecular Complexity of Lymphovascular Invasion: The Role of Cell Migration in Breast Cancer as a Prototype. Pathobiology 2020, 87, 218–231. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Nikolaou, S.; Machesky, L.M. The stressful tumour environment drives plasticity of cell migration programmes, contributing to metastasis. J. Pathol. 2020, 250, 612–623. [Google Scholar] [CrossRef]
- Yan, X.Y.; Leng, J.F.; Chen, T.T.; Zhao, Y.J.; Kong, L.Y.; Yin, Y. Design, synthesis, and biological evaluation of novel diphenylamine derivatives as tubulin polymerization inhibitors targeting the colchicine binding site. Eur. J. Med. Chem. 2022, 237, 114372. [Google Scholar] [CrossRef]
- Nuth, M.; Benakanakere, M.R.; Ricciardi, R.P. Discovery of a potent cytotoxic agent that promotes G(2)/M phase cell cycle arrest and apoptosis in a malignant human pharyngeal squamous carcinoma cell line. Int. J. Oncol. 2022, 60, 41. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, M.; Turnbull, T.; Sebastian, S.; Kempson, I. The MTT Assay: Utility, Limitations, Pitfalls, and Interpretation in Bulk and Single-Cell Analysis. Int. J. Mol. Sci. 2021, 22, 12827. [Google Scholar] [CrossRef] [PubMed]
- Sukumaran, S.K.; Vadakkekuttical, R.J.; Kanakath, H. Comparative evaluation of the effect of curcumin and chlorhexidine on human fibroblast viability and migration: An in vitro study. J. Indian. Soc. Periodontol. 2020, 24, 109–116. [Google Scholar] [PubMed]
- Uysal, K.; Firat, I.S.; Creutz, T.; Aydin, I.C.; Artmann, G.M.; Teusch, N.; Temiz Artmann, A. A Novel In Vitro Wound Healing Assay Using Free-Standing, Ultra-Thin PDMS Membranes. Membranes 2022, 13, 22. [Google Scholar] [CrossRef]
- Schmitt, D.; Andrews, J.; Tan, M. Determination of Breast Cancer Cell Migratory Ability. Methods Mol. Biol. 2016, 1406, 171–180. [Google Scholar] [PubMed]
- Monroe, J.D.; Hodzic, D.; Millay, M.H.; Patty, B.G.; Smith, M.E. Anti-Cancer and Ototoxicity Characteristics of the Curcuminoids, CLEFMA and EF24, in Combination with Cisplatin. Molecules 2019, 24, 3889. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef]
- Suarez-Arnedo, A.; Torres Figueroa, F.; Clavijo, C.; Arbelaez, P.; Cruz, J.C.; Munoz-Camargo, C. An image J plugin for the high throughput image analysis of in vitro scratch wound healing assays. PLoS ONE 2020, 15, e0232565. [Google Scholar] [CrossRef]
- ImageJ. U. S. National Institutes of Health. Available online: https://imagej.nih.gov/ij/h (accessed on 15 August 2024).




| Compound | IC50, μM * | ||||
|---|---|---|---|---|---|
| SH-SY5Y | A549 | MCF-7 | SW-480 | Hek-293 | |
| 3a | 62.20 ± 0.11 | ≥100 | 92.42 ± 1.40 | ≥100 | 77.29 ± 2.12 |
| 3b | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 3c | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 3d | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 3e | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 4a | 66.75 ± 0.41 | 67.17 ± 0.12 | 65.26 ± 0.07 | 84.03 ± 0.38 | 82.89 ± 0.46 |
| 4b | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 4c | 39.59 ± 0.05 | 65.38 ± 0.17 | 75.20 ± 1.43 | ≥100 | ≥100 |
| 4d | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 4e | 99.13 ± 1.47 | 91.87 ± 0.02 | 82.48 ± 0.32 | ≥100 | 87.40 ± 1.42 |
| 5a | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 5b | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 5c | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 5d | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 6a | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 6b | 25.98 ± 0.17 | 26.28 ± 0.02 | 23.29 ± 0.12 | 25.09 ± 0.24 | 22.43 ± 0.41 |
| 6c | 20.94 ± 0.13 | 37.68 ± 0.20 | 77.05 ± 0.67 | ≥100 | ≥100 |
| 6d | 31.56 ± 0.07 | 41.43 ± 0.79 | 33.77 ± 0.64 | 40.38 ± 0.01 | 41.39 ± 0.05 |
| 7a | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 7b | ≥100 | ≥100 | ≥100 | ≥100 | ≥100 |
| 7c | ≥100 | ≥100 | 45.12 ± 0.22 | ≥100 | 36.27 ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aleksandrova, Y.R.; Nikolaeva, N.S.; Shagina, I.A.; Smirnova, K.D.; Zubishina, A.A.; Khlopotinin, A.I.; Fakhrutdinov, A.N.; Khokhlov, A.L.; Begunov, R.S.; Neganova, M.E. N-Aryl Benzimidazole and Benzotriazole Derivatives and Their Hybrids as Cytotoxic Agents: Design, Synthesis and Structure–Activity Relationship Studies. Molecules 2024, 29, 5360. https://doi.org/10.3390/molecules29225360
Aleksandrova YR, Nikolaeva NS, Shagina IA, Smirnova KD, Zubishina AA, Khlopotinin AI, Fakhrutdinov AN, Khokhlov AL, Begunov RS, Neganova ME. N-Aryl Benzimidazole and Benzotriazole Derivatives and Their Hybrids as Cytotoxic Agents: Design, Synthesis and Structure–Activity Relationship Studies. Molecules. 2024; 29(22):5360. https://doi.org/10.3390/molecules29225360
Chicago/Turabian StyleAleksandrova, Yulia R., Natalia S. Nikolaeva, Inna A. Shagina, Karina D. Smirnova, Alla A. Zubishina, Alexander I. Khlopotinin, Artem N. Fakhrutdinov, Alexander L. Khokhlov, Roman S. Begunov, and Margarita E. Neganova. 2024. "N-Aryl Benzimidazole and Benzotriazole Derivatives and Their Hybrids as Cytotoxic Agents: Design, Synthesis and Structure–Activity Relationship Studies" Molecules 29, no. 22: 5360. https://doi.org/10.3390/molecules29225360
APA StyleAleksandrova, Y. R., Nikolaeva, N. S., Shagina, I. A., Smirnova, K. D., Zubishina, A. A., Khlopotinin, A. I., Fakhrutdinov, A. N., Khokhlov, A. L., Begunov, R. S., & Neganova, M. E. (2024). N-Aryl Benzimidazole and Benzotriazole Derivatives and Their Hybrids as Cytotoxic Agents: Design, Synthesis and Structure–Activity Relationship Studies. Molecules, 29(22), 5360. https://doi.org/10.3390/molecules29225360








