Abstract
Pollution remains one of the most significant global challenges. Photocatalysis consists of a new organic pollutant removal technology, with TiO2 widely studied as a photocatalyst in the photocatalytic removal of water pollution. However, intrinsic TiO2 has the disadvantages of weak visible light absorption, low electron separation, and transmission efficiency, as well as few active sites. In this study, anatase-phase Ti3+ self-doped TiO2 (B-TiO2) with a core-shell structure was successfully prepared by forming an amorphous layer rich in oxygen vacancies (OVs) and Ti3+ defects on the TiO2 surface under a nitrogen atmosphere using NaBH4 as a chemical-reducing agent. The visible light absorption performance of the catalyst was notably improved when exposed to light irradiation. The bending of surface energy bands facilitated the separation of photogenerated electron-hole pairs, and the core-shell structure allowed the electron-hole pairs to be transported to the surface of the catalyst and participate in the reaction faster. We observed that 92.86% of Rhodamine B (RhB) was degraded in only 5 min, an increase of 2.73 times that of the degradation rate observed in commercial P25. With extraordinary stability, the photocatalytic efficiency of the catalyst remained at 96.2% after five degradation cycles.
1. Introduction
Pollution is one of the most severe challenges at present. Photocatalytic technology represents a novel approach to the removal of organic pollutants that requires only light to degrade organic pollution into water and carbon dioxide, with the advantages of mild reaction conditions, thorough purification, and universality, making it a truly environmentally friendly green technology [1,2,3,4,5,6,7,8]. The key to photocatalytic technology involves developing efficient photocatalysts. Titanium dioxide (TiO2) is regarded as a highly promising photocatalytic material within the realm of photocatalysts. This is primarily attributed to its wide availability, cost-effectiveness, exceptional chemical stability, non-toxic nature, and overall safety [9,10,11]. Common TiO2 crystal forms include anatase (3.2 eV), rutile (3.0 eV), and slate titanite (2.96 eV). Due to its wide bandgap, it is only sensitive to ultraviolet (UV) light, which makes up only 5 percent of the solar spectrum. Meanwhile, visible light, which accounts for the most significant proportion of solar energy, is not effectively utilized. The low efficiency of the electron-hole pair separation and transport, as well as a small number of reactive sites involved in the catalytic process, jointly limit the photocatalytic performance of TiO2 [12,13].
Haghighat et al. [14] explored the effects of different specific surface areas of TiO2 on photocatalytic performance. The researchers established a positive correlation between the specific surface area (BET) of the catalyst and the amount of active sites. This observation implies that there is a positive correlation between the increase of active sites and the BET, resulting in enhanced photocatalytic performance. Roberto et al. [15] successfully obtained Pt-doped TiO2 nanoporous catalysts using a simple two-step hydrothermal pathway, where the bandgap of Pt-TiO2 decreased to 2.15 eV, and there was a notable increase in the range of visible light absorption. However, the photocatalytic degradation of RhB for 180 min was only 63%. Chen et al. [16] first prepared black TiO2 through the high-pressure hydrogenation of TiO2 nanocrystals at 200 °C for 5 days, with a disordered layer on the surface resulting in a reduced band gap. Wang et al. [17] successfully synthesized Ti3+ self-doped TiO2 microspheres that exhibited exceptional stability. These microspheres possessed a bandgap of 2.89 eV, allowing for enhanced light absorption in the visible spectrum. This desirable property was achieved through the application of high-temperature hydrogen heat treatment. Ti3+ self-doping of TiO2 was also shown to introduce oxygen vacancies (OVs) and Ti3+ defects, affecting the energy band structure, shrinking the band gap, dramatically enhancing the light absorption performance, improving the photocatalytic performance of TiO2, enhancing photogenerated electron and hole separation efficiency, reducing compounding effects, and enhancing photocatalytic performance [17,18,19,20,21,22,23]. Thus, the modulation of TiO2 suffers from high preparation costs, high hazard factors, and low catalytic efficiency.
In this study, Ti3+ self-doped TiO2 (B-TiO2) with a core-shell structure was successfully prepared by using NaBH4 as a chemical reducing agent in a nitrogen environment, which is different from the conventional synthesis method of passing hydrogen at high temperature and high pressure with high safety. This self-doped core-shell structure, without introducing foreign elements, facilitates the separation and movement of electron(e−)-hole(h+) pairs generated by photolysis. Compared to commercial P25, B-TiO2 has a 1.5-fold increase in specific surface area and more active sites. In addition, the light absorption efficiency of B-TiO2 is greatly improved. Our designed and prepared B-TiO2 and its core-shell structure synergistically improved the catalytic performance of conventional TiO2 from various perspectives, such as light trapping, carrier separation, migration, etc. The degradation rate of RhB reached 92.86% after 5 min, which was 2.7 times higher than that of the catalytic performance of commercial P25. This study introduces a synergistic approach and provides a new technology for the synthesis of economical and efficient photocatalysts.
2. Results and Discussion
Figure 1a presents the XRD spectra of TiO2 and B-TiO2, indicating that the precursor of B-TiO2 prepared by the hydrothermal reaction was anatase TiO2, and its corresponding standard PDF card number was PDF#97-017-2916. B-TiO2 was obtained by mixing TiO2 with the reducing agent in ball milling and then calcined in a nitrogen environment, and the results showed that there was no obvious shift in the position of the diffraction peaks as the calcination temperature increased, and the intensity of the diffraction peaks gradually decreased. Table 1 shows the comparison of the diffraction peak parameters of the samples before and after reduction, and the results show that the cellular parameters of the samples increased slightly after the reduction treatment, but they are still tetragonal crystal systems. The full width at half maxima of the diffraction peaks in the highest (101) plane becomes wider with the increase of calcination temperature. Combined with the current study, under high-temperature calcination in a nitrogen atmosphere, NaBH4, which exhibited strong reducing properties, could remove the O atoms in the surface layer of TiO2 to generate OVs and Ti3+ defects, forming an amorphous layer, and the amorphous layer of TiO2 and its encapsulated defect-free core ultimately formed a nuclear shell structure, and the size of the shell becomes thicker with increasing calcination temperature [24,25,26,27]. Since the shell structure itself does not produce XRD peaks, it will shield the X-ray absorption by the core of anatase TiO2, resulting in the phenomenon that the diffraction peaks of the prepared B-TiO2 are weakened with the increase of calcination temperature. As shown in Figure 1b, g = 2.003 of B-TiO2-300 was detected in the EPR experiments. By contrast, the existence of oxygen positions in anatase TiO2 was not detected, and this confirmed the presence of OVs in B-TiO2-300.
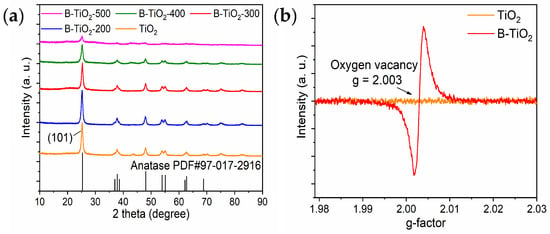
Figure 1.
(a) XRD image and (b) electron paramagnetic resonance (EPR) of anatase TiO2 and B-TiO2.

Table 1.
Cell parameters of the samples and full width at half maxima of the diffraction peak (101) plane.
Figure 2 presents the XPS test results of B-TiO2-300 and TiO2, with Figure 2a showing the O1s fine spectra of elemental oxygen in B-TiO2-300 and TiO2. The peaks corresponding to Ti-O (lattice oxygen), oxygen defects (OVs introduced by calcination), and hydroxyl oxygen (water adsorbed on the surface) appeared in B-TiO2-300 at 529.8, 530.6, and 532.0 eV, respectively. The O1s nuanced spectrum of TiO2 also contained peaks corresponding to Ti-O, OVs, and hydroxyl oxygen. However, the peaks corresponding to its oxygen vacancies were significantly lower than those of B-TiO2-300, indicating that B-TiO2-300 obtained after high-temperature reduction with NaBH4 contained generated oxygen vacancies, which was in accordance with the EPR test outcomes. Figure 2b shows the Ti 2p fine spectra of the Ti elements in B-TiO2-300 and TiO2, indicating that B-TiO2-300 exhibited spikes representing Ti 2p1/2 and Ti 2p3/2 for Ti4+ at 464.1 eV, while the peaks referring to Ti3+ 2p1/2 and 2p3/2 appeared at 463.7 and 457.9 eV. The Ti 2p fine the spectrum of Ti elements in TiO2 only showed peaks that referred to Ti 2p1/2 and Ti 2p3/2 of Ti4+, demonstrating that B-TiO2-300 obtained by NaBH4 high-temperature reduction generated Ti3+, along with OVs generation. The photo-reactivity of the electronic structure was notably influenced by the presence of Ti3+ and OVs, resulting in an augmentation of the light absorption capacities. In addition, Ti3+ was in a defective state, which inhibited the recombining of photogenerated pairs of electron-holes and promoted the splitting of charges to improve conductivity [18,28].
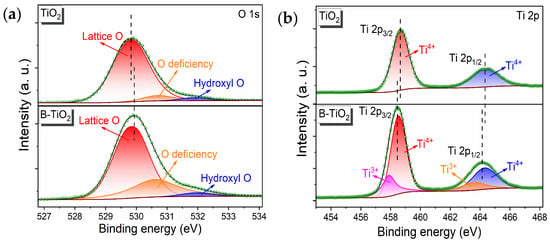
Figure 2.
TiO2 and B-TiO2-300 (a), XPS O1s mapping (b), and XPS Ti2p mapping.
Figure 3a presents the B-TiO2-300 SEM image, indicating that the sample was composed of several tiny aggregated particles. TEM observations of the sample morphology also revealed that the sample’s particles varied in size and shape, as shown in Figure 3b. Ball milling was carried out during the preparation of this sample to uniformly mix NaBH4 and TiO2, and finer TiO2 particles were obtained, which contributed to the excellent catalytic efficacy of the B-TiO2-300 product. Figure 3c shows the HRTEM of B-TiO2-300, indicating that the internal lattice arrangement of the nucleus was ordered. Its lattice spacing value (3.53 Å) was also close to the corresponding value of the standard card of TiO2 (3.51 Å) (Figure 3d), and the outer crystal arrangement is disorderly due to the emergence of OVs and Ti3+ on the TiO2 exterior. Hence, the successful synthesis of B-TiO2, with an inner core and outer shell, was efficiently accomplished [29,30].
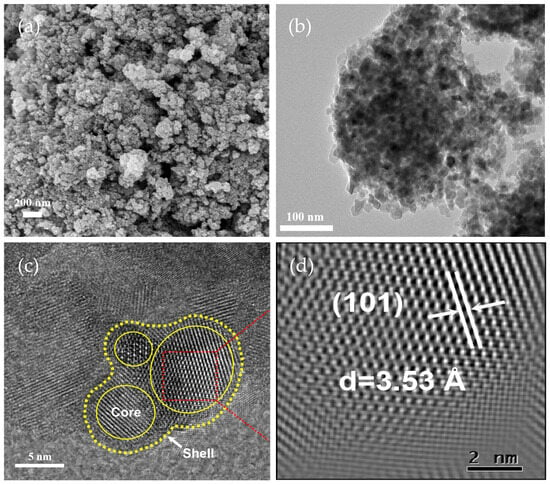
Figure 3.
(a) SEM image, (b) TEM image, and (c,d) HRTEM of B-TiO2-300.
Figure 4a displays the degradation curves of the experimental samples, revealing a trend in the photocatalytic breakdown efficiency of the samples that initially increased and subsequently decreased as the calcination temperature was raised. The performance of the photocatalytic breakdown of B-TiO2-300 was the best, which was significantly improved compared with the unreduced TiO2. After 5 min, the photocatalytic decomposition rate of RhB by B-TiO2-300 was 92.86% (Figure 4c), while the decomposition rates of unreduced TiO2 and commercial P25 were only 50.14% and 34.03%. The findings indicate that the photocatalytic degradation efficiency of B-TiO2-300 exhibited a significantly higher performance compared to the commercially accessible P25. The degradation rates of B-TiO2-300, P25, and TiO2 were compared, as shown in Figure 4b. According to the Langmuir-Hinshelwood model, the breakdown of RhB at low concentrations is seen as a first-order process. Hence, it is possible to simplify the degradation kinetics fitting calculations by employing a linear approach. Moreover, the reaction being examined follows the observed first-order reaction rate equation, Ln(C/C0) = −kt. During the initial phase of the reaction, k represented the apparent rate constant, and Ln(C/C0) was shown to be dependent on the duration of irradiation, denoted as t. The k-values of B-TiO2-300, P25, and TiO2 were 0.136, 0.050, and 0.024 min−1, respectively, which also showed that B-TiO2-300 had the strongest photocatalytic breakdown efficiency, which was substantially greater than untreated TiO2 and also significantly higher than commercial P25. The used B-TiO2-300 photocatalysts were recovered by centrifugal separation, and then repeated 15 min photocatalytic degradation tests were performed. The used B-TiO2-300 photocatalyst was recovered through centrifugal separation and then repeatedly tested for 15 min by photocatalytic degradation. It was observed that B-TiO2-300 had favorable stability, as evidenced by a photocatalytic degradation rate of 96.2% after undergoing five cycles. The exact experimental outcomes are depicted in Figure 4d.
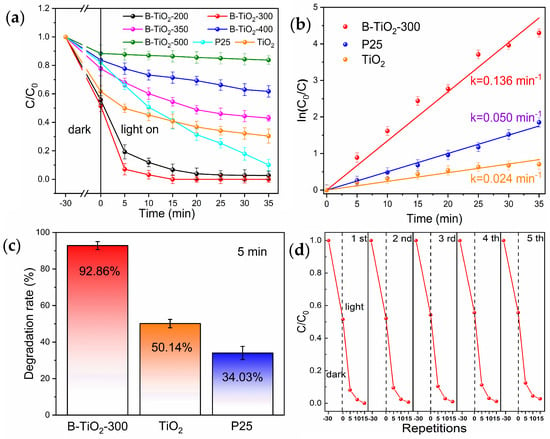
Figure 4.
(a) Photocatalytic degradation, (b) first-order kinetics, (c) 5 min degradation rate, and (d) 15 min cyclic degradation of RhB by P25, TiO2, and B-TiO2-300.
The type IV adsorption isotherms of B-TiO2-300 and P25 on nitrogen, which have H3-type hysteresis loops, are displayed in Figure 5. The BET values of the TiO2 and B-TiO2-300 samples were 75.0 and 69.9 m2/g, respectively, while the BET value of the commercial P25 sample was 46.7 m2/g. This indicated that B-TiO2-300 had a much larger area. Table 2 contrasts the specific surface extent of Ti3+ self-doped TiO2 in some recent research with that of B-TiO2-300. As the data demonstrates, B-TiO2-300 has a comparatively large specific surface area. The larger specific surface area of B-TiO2-300 can provide more active sites for the degradation reaction during the photocatalytic process, thus promoting photocatalytic performance.
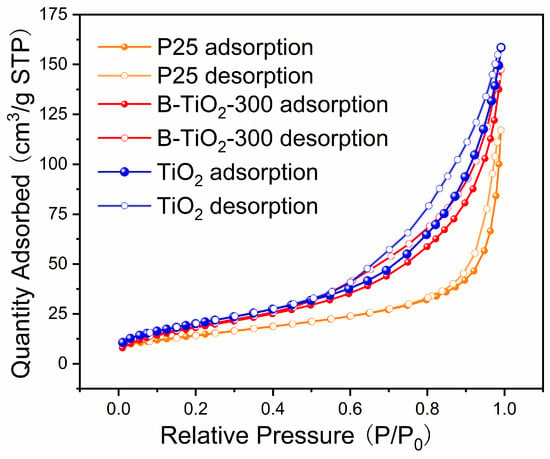
Figure 5.
Nitrogen adsorption/desorption isotherm curves.

Table 2.
Comparison of specific surface area.
As illustrated in Figure 6a, the UV-visible diffuse reflection line of TiO2 and B-TiO2-300 indicated that TiO2 exhibited minimal absorption within the visible light region. By contrast, the light utilization performance of B-TiO2-300 was significantly improved. The primary factor contributing to the improved light absorption properties of B-TiO2-300 is the existence of OVs and Ti3+ defects within the surface layer. The presence of these defects exerts a notable influence on the energy band structure, leading to the formation of defect energy levels, a decline in the valence band boundaries, and a narrowing of the band gap [27,39,40]. In contrast to TiO2, the light absorption edge of B-TiO2-300 did not exhibit a substantial enhancement, indicating that B-TiO2-300 was only partially reduced in the outer shell, and the inner core was still normal TiO2. Figure 6b illustrates the results of the experimental determination of the TiO2 and B-TiO2-300 band gap values, which were measured to be 3.31 eV and 3.28 eV, respectively. This value is acquired using the Kubelka-Munk theory. Additionally, Figure 6c displays the energy band diagram of TiO2, which is derived by DFT calculations. It is evident that TiO2 possesses a band gap of 3.12 eV, a value consistent with the experimental determination of TiO2 (Figure 6c). Figure 6d also shows the energy bands of B-TiO2-300 calculated using the same DFT method, which yielded a theoretical band gap value of 2.20 eV for ideal B-TiO2, and the band gap of B-TiO2-300 was significantly reduced compared with TiO2. The simulation results show that the defect layer of B-TiO2-300 has a lower band gap value, and the photogenerated electron-hole pairs are more easily separated. This accounted for B-TiO2-300′s enhanced visible-range photo-absorption performance, allowing the catalyst to produce more photogenerated electron-hole pairs under the same circumstances and participate in the photocatalytic process, thereby significantly enhancing photocatalytic performance.
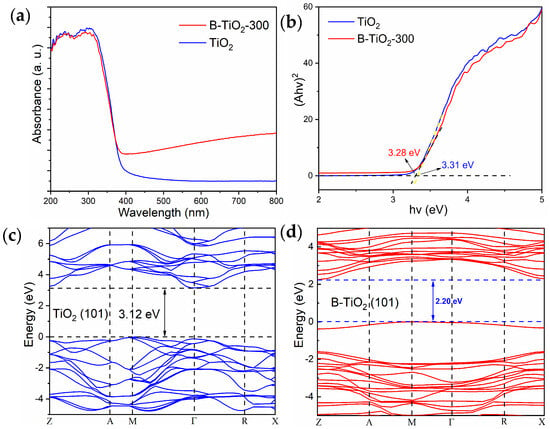
Figure 6.
TiO2 and B-TiO2 (a) UV–visible diffuse reflectance (UDS), (b) TiO2 bandgap, (c) TiO2 DFT energy bands, and (d) B-TiO2 DFT energy bands.
Based on the results of UV photoelectron spectroscopy (UPS) tests, Figure 7 depicts the energy band alterations in TiO2 both before and after reduction. The valence band edge of B-TiO2-300 was 1.810 eV, whereas TiO2′s was 1.913 eV using UPS, as seen in Figure 7a. This difference in valence band edge between the two was the primary cause of B-TiO2-300′s bandgap reduction. The cut-off edge of the secondary electrons of B-TiO2-300 was 18.373 eV, while that of the TiO2 secondary electrons was 18.190 eV, and the ionization potentials IE required for the electron leaps of B-TiO2-300 and TiO2 were calculated as 2.85 and 3.03 eV, respectively, according to Equation (1). Figure 7b presents the energy band changes before and after TiO2 reduction combined with the above data and band gap values. The dashed line indicated the energy band diagram before the formation of homojunctions in TiO2, with the solid line indicating the equilibrium energy band diagram. The Fermi energy level position of B-TiO2 was higher, and the electrons flowed from B-TiO2 to TiO2 to form an accumulating layer for the electrons until the Fermi energy levels were equal. An electron depletion layer is located on the other side. They combine to produce a built-in electric field (qVD) that has the ability to accelerate the pair of e− and h+ separation. After light irradiation, the lower the ionization potential, the easier the electrons could be excited and jump to produce pairs of e− and h+. Furthermore, the effectiveness of the electron-hole pairs’ separation was enhanced under the action of the qVD. The greater the e− and h+ concentration on the catalyst surface, the higher the photocatalytic degradation performance.
where the ionization potential, optical frequency, Planck’s constant, secondary electron cut-off edge, and valence band edge are represented, respectively, by the symbols IE, h, v, E0, and Ef.
IE = hv − (E0 − Ef)
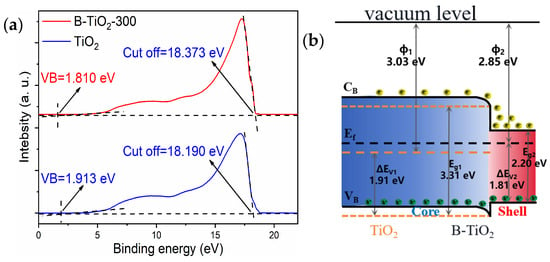
Figure 7.
(a) Ultraviolet photoelectron spectra (UPS) of TiO2 and B-TiO2; (b) energy band diagrams.
Figure 8a shows the instantaneous photocurrent response curves of the experimental samples, and the higher photocurrent indicates the stronger separation of photogenerated electron-hole pairs. From the figure, it can be seen that the photocurrent of B-TiO2-300 is the highest and much larger than that of TiO2, which indicates that the photogenerated electron-hole in B-TiO2-300 is easy to separate, which is also an important reason for the enhancement of photocatalytic degradation performance of B-TiO2-300. Figure 8b shows the electrochemical impedance Nyquist plots of the experimental samples. The migration performance of photogenic electrons and holes is reflected by the curvature radius of the curved arc. A lower curve radius indicates greater migration performance, whereas a larger radius indicates poorer migratory performance. Hence, it can be observed that the migration performance of photogenerated e− and h+ was most optimal in the B-TiO2-300 material. Enhancing the migration efficiency of photogenerated e− and h+ can effectively mitigate the recombination of these charge carriers, hence facilitating the enhancement of photocatalytic performance. On the other hand, photogenerated electrons and holes can migrate faster to the catalyst surface to participate in the reaction, which simultaneously improves the overall photocatalytic performance. Based on recent research findings [41,42,43,44], the enhanced abilities of electron and hole migration and segregation in B-TiO2-300 can be primarily attributed to the development of an amorphous layer containing an abundance of OVs and Ti3+ defects within the topmost layer of TiO2. This amorphous layer is formed by the introduction of NaBH4, which results in a homogeneous core-shell structure with the TiO2 core.
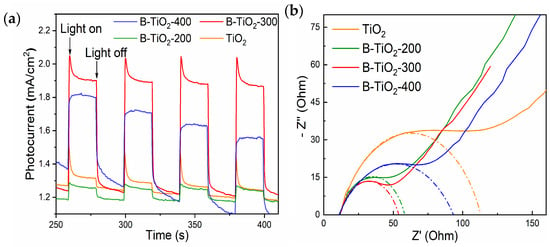
Figure 8.
(a) Transient photocurrent response, and (b) electrochemical impedance Nyquist plots of TiO2 and B-TiO2.
The experiment involved the utilization of DMPO as a radical scavenger to conduct electron paramagnetic resonance (ESR) analysis on B-TiO2-300. The purpose was to investigate the generation of ·O2− and ·OH radicals in the presence of non-light and light illumination conditions. The obtained results are presented in Figure 9a,b. The figure indicates that no ESR signal was detected in the absence of light and that four distinct peaks with an intensity ratio of 1:1:1:1 were seen in the a-figure following exposure to visible light. These peaks were brought on by DMPO’s capture of ·O2−. In the meantime, the b-figure displays four distinct peaks with an intensity ratio of 1:2:2:1, caused by the capture of ·OH by DMPO, suggesting that under light irradiation, the B-TiO2-300 surface produced ·O2− and ·OH radicals. Further capture experiments were conducted in order to examine the active species that were crucial to the photocatalytic degradation process and to learn more about the photocatalytic degradation mechanism of RhB by B-TiO2-300. P-benzoquinone (p-BQ), ammonium oxalate (AO), and isopropanol (IPA) were selected as scavengers of superoxide radicals (·O2−), hydroxyl radicals (·OH), and holes (h+), respectively, and the study illustrated the inhibitory effects of several radical scavengers on the degradation of RhB by B-TiO2-300, as depicted in Figure 9c [45]. Including the p-BQ scavenger greatly lowered the breakdown rate of RhB, adding the AO scavenger slightly reduced the degradation rate of RhB, and the extra of the IPA scavenger barely impacted the breakdown rate of RhB. The findings of this study suggest that the presence of ·O2− had a notable impact on the degradation of RhB, whereas the involvement of ·OH in the degradation process was relatively low. Additionally, it was observed that h+ had minimal influence on the degradation of RhB.
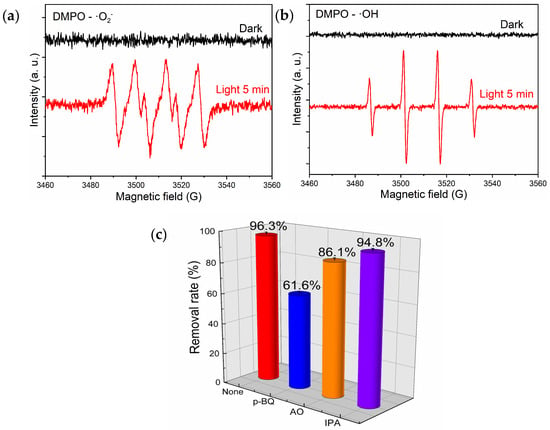
Figure 9.
EPR spectra of the DMPO trapping radicals on B-TiO2-300: (a) DMPO-·O2−, (b) DMPO-·OH, and (c) degradation of RhB by B-TiO2-300 and the addition of different scavengers: p-benzoquinone (p-BQ), ammonium oxalate (AO), and isopropyl alcohol (IPA), after visible light irradiation (λ > 300 nm, 30 s).
3. Materials and Experiment
3.1. Materials
Shanghai Aladdin Biochemical Technology Co., Ltd. provided the sodium hydroxide (NaOH), ethylene glycol ((CH2OH)2), and tetrabutyl titanate (C16H36O4Ti). (Shanghai, China), Tianjin New Technology Industrial Park Chemo Chemical Reagent Co., Ltd. (Tianjin, China) and Xilong Scientific Co., Ltd. (Chengdu, Sichuan, China), respectively. NaBH4 had been purchased from FUCHEN Co., Ltd. (Tianjin, China). Each of the previously stated reagents was used without any prior pre-treatment and had an analytical purity. The water employed in the experimental procedure was prepared in-house and was deionized, originating from the laboratory.
3.2. Synthesis of TiO2
Tetrabutyl titanate was added to 1 M sodium hydroxide liquid and agitated at ambient temperature for 10 min. Tetrabutyl titanate was sonicated for 5 min to homogeneously disseminate in the solution. Following that, the solution underwent the addition of ethylene glycol and was subjected to stirring for a duration of 10 min. The gathered white liquid subsequently went to a 150 mL reaction kettle, which was positioned in a heated oven and held at 150 °C for 12 h. The precipitate was next immersed in 0.1 mol/L hydrochloric acid for 6 h and crashed by filtration with ultrapure water a few times until the pH value reached neutral. The resulting precipitate was subjected to a drying process at a temperature of 60 °C for 10 h. Subsequently, it underwent a calcination process at a temperature of 400 °C for a duration of 4 h, resulting in the production of TiO2 with a significantly elevated specific surface area.
3.3. Synthesis of B-TiO2
To uniformly mix TiO2 with NaBH4, 200 mg of TiO2 and 18.94 mg of NaBH4 were placed into a ball mill (Li-Chen, LC-PTG-6) for 5 min. The obtained powder was then transferred into a square furnace, and the container was put in a tube heater (Kocrystal GSL-1700X-II, Hefei Kejing Material Technology Co., Ltd, Hefei, China) and calcined at 300 °C for 3 h under a nitrogen-filled environment. After that, the tube furnace was subjected to heating at a rate of 10 °C per minute. The resulting powdered product underwent several washes using ethanol and deionized water, followed by drying at a temperature of 60 °C for a duration of 10 h. The final product was denoted B-TiO2-300, with the number 300 indicating the calcination temperature of the tube furnace, and samples B-TiO2-200, B-TiO2-350, B-TiO2-400, and B-TiO2-500 were prepared by changing only the calcination temperature in the same manner.
3.4. Analytical Test Method
The X-ray diffraction (XRD) technique was utilized to ascertain the crystalline composition of the specimens. A Bruker D8 ADVANCE X-ray diffractometer, equipped with a copper (Cu) target, was employed for this purpose. A transmission electron microscope (TEM, Thermo Fisher, model FEI Talos F200X) and the field-emission scanning electron magnifying glass (SEM, Zeiss, model Gemini 300, Oberkochen, Germany) were utilized to observe the microscopic structure of the samples. The UV-Vis absorption spectra were acquired with a UH4150 UV-Vis spectrophotometer produced by Hitachi (Tokyo, Japan). The wavelength range for the measurements was adjusted to 200–800 nm. A McASAP 2460 instrument measured the total area of the surface and pore diameter (BET), and the materials were heated at 200 °C for 6 h, with nitrogen gas used for adsorption and degassing. The samples’ elemental composition and valence states were examined using an Escalab 250XI X-ray photoelectron spectrometer (XPS) manufactured by Thermo Fisher, Waltham, MA, in the United States. EPR (Bruker, Karlsruhe, Germany: A300-10/12) was performed using DMPO and TEMP as the free radicals and oxygen vacancy trapping agents, respectively. An electrochemical workstation (CHI700E series, universal dual constant potential meter) was used to measure the transient photocurrent response and impedance, and a UV–visible spectrophotometer (UV-DRS, L5S) was used to study the optical absorption spectra.
3.5. Photocatalytic Performance Test
The produced photocatalysts were assessed for their ability to degrade organic pollutants using Rhodamine B (RhB) as a simulated substance. A 20 mg/L RhB fluid consisting of 100 mL was poured into a 400 mL beaker, and 20 mg of the test sample was placed in it and sonicated for 30 min at ambient temperature. The entire sonication procedure was carried out in a dark room. Then, the beaker was set under the illumination of a sunlight simulator (Solar-500Q-Xenon lamp, 200–1200 nm, Newbie, Beijing, China), and the brightness of the light source was adjusted so that the light intensity at the liquid surface of the beaker was 600 W/m2. The photocatalytic degradation experiment was conducted for 60 min, with 4 mL of the reaction solution obtained from the centrifuge tube every 5 min. The supernatant was taken after centrifugation, and a UV-visible spectrum analyzer was used to determine the absorbance of the residue, which was recorded as An, while the 20 mg/L RhB solution was recorded as A0. The recorded value for the absorbance of the supernatant following the process of centrifugation was denoted as A1, with the light source’s wavelength set at 554 nm. The RhB concentration in the supernatant was recorded as Cn, C0 indicated the amount of 20 mg/L RhB solution, and C1 was the concentration of centrifugal supernatant after ultrasonication. The relationship between absorbance and concentration was calculated according to:
Cn/C0 = An/A0
3.6. Theoretical Calculations
The researchers employed the Vienna Ab-initio simulation package (VASP) code to perform Density Functional Theory (DFT) computations [45,46,47,48]. The total energy estimates utilized a plane wave cut-off energy of 400 eV. In this study, the representation of ion-electron interactions utilized ultrasoft pseudopotentials, which were incorporated into the projector-augmented wave (PAW) approach [49]. The exchange-correlation function utilized in this study was the Perdew-Burke-Ernzerhof (PBE) functional, which falls under the category of generalized gradient approximation (GGA) [49,50]. The process of integrating over the Brillouin zone was approximated by summing a carefully selected set of k-points. This summation was performed using the Monkhorst-Pack method, with a grid size of 2 × 2 × 1 [51]. The geometries were iteratively adjusted until the energy reached a convergence threshold of 1 × 10−5 eV per atom, and the forces reached a magnitude of 0.02 eV per Ångstrom. A p(1 × 3) supercell was used to model the (101) surface. To mitigate the interactions between the slabs, a vacuum area of 15 Å was established along the z-axis to create separation between them.
4. Conclusions
In summary, we obtained self-doped TiO2 with a high specific surface area by calcining TiO2 mixed with the NaBH4 reductant under a nitrogen atmosphere, which demonstrated outstanding safety compared with traditional H2 and the metal reduction method. An amorphous layer rich in oxygen vacancies (OVs) and Ti3+ defects formed on the TiO2 surface formed a core-shell structure with anatase titanium dioxide nuclei, and a homogeneous junction between the nuclei and shells lowered the bandwidth of B-TiO2-300 and introduced impurity energy levels into the band gap. This greatly improves the absorption efficiency of B-TiO2-300 for visible light and also enhances the separation and mobility properties of photogenerated electron-hole pairs in B-TiO2-300. These changes significantly improve the photocatalytic degradation performance of B-TiO2-300. Notably, the performance of B-TiO2-300 surpassed that of the commercially available P25, with B-TiO2-300 degrading 92.86% of RhB within 5 min, compared to a degradation rate of only 34.03% for commercial P25.
Author Contributions
Conceptualization, S.W. and Y.L. (Yong Li); Data curation, M.Z., M.L. and K.H.; Funding acquisition, S.W. and Y.L. (Yong Li); Investigation, J.W.; Methodology, M.Z., M.L., Y.L. (Yingbin Liang) and Y.L. (Yong Li); Software, M.Z.; Validation, X.Z. and L.H.; Writing–original draft, M.Z. and M.L.; Writing–review and editing, S.W. and Y.L. (Yong Li). All authors have read and agreed to the published version of the manuscript.
Funding
We are grateful for the generous support from the Central Government Funds for Local Scientifific and Technological Development (Grant Nos: XZ202201YD0026C and XZ202101YD0019C), National Natural Science Foundation of China (Grant No: 52062045), the Natural Science Foundation of Tibet Autonomous Region (Grant Nos. XZ202301ZR0020G and XZ202101ZR0121G), and Science and Technology Planning Projects of Lhasa (Grant No. LSKJ202307).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, D.S.; Yi, H.; Lai, C.; Liu, X.G.; Huo, X.Q.; An, Z.W.; Li, L.; Fu, Y.K.; Li, B.S.; Zhang, M.M.; et al. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.H.; Okasha, K.M.; Mahmoud, Y.A.; Elsamahy, T.; Jiao, H.X.; Fu, Y.Y.; Sun, J.Z. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, C.Y.; Ma, Z.B.; Yang, X.M. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Ejraei, A.; Aroon, M.A.; Saravani, A.Z. Wastewater treatment using a hybrid system combining adsorption, photocatalytic degradation and membrane filtration processes. J. Water Process Eng. 2019, 28, 45–53. [Google Scholar] [CrossRef]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Solayman, H.M.; Hossen, M.A.; Aziz, A.A.; Yahya, N.Y.; Leong, K.H.; Sim, L.C.; Monir, M.U.; Zoh, K.D. Performance evaluation of dye wastewater treatment technologies: A review. JECE 2023, 11, 2213–3437. [Google Scholar] [CrossRef]
- Liu, H.X.; Zhang, L.N.; Li, T.D. A Study of Controllable Synthesis and Formation Mechanism on Flower-Like TiO2 with Spherical Structure. Crystals 2018, 8, 466. [Google Scholar] [CrossRef]
- Anand, A.; Aneggi, E.; Boaretti, C.; Lorenzetti, A.; Trovarelli, A.; Modesti, M.; Roso, M. Boosting the photocatalytic performance of PAN-TiO2 nanostructured membranes by mechanosynthesis. JECE 2024, 12, 111595. [Google Scholar] [CrossRef]
- Singh, N.; Song, Y.; Gutiérrez, O.Y.; Camaioni, D.M.; Campbell, C.T.; Lercher, J.A. Electrocatalytic Hydrogenation of Phenol over Platinum and Rhodium: Unexpected Temperature Effects Resolved. ACS Catal. 2016, 6, 7466–7470. [Google Scholar] [CrossRef]
- Saensook, S.; Sirisuk, A. A factorial experimental design approach to obtain defect-rich black TiO2 for photocatalytic dye degradation. JWPE 2022, 45, 102495. [Google Scholar] [CrossRef]
- Yamagishi, T.; Leite, J.; Ueda, S.; Yamaguchi, F.; Suwa, Y. Simultaneous Removal of phenol and Ammonia by an activated sludge process with cross-flow filtration. Water Res. 2001, 35, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.Y.; Yu, S.; Liu, J.P.; Zhang, Y.M.; Wang, Y.C.; Yu, J.Y.; Yuan, M.; Zhang, P.C.; Liu, W.; Zhang, J.X. C, F co-doping Ag/TiO2 with visible light photocatalytic performance toward degrading Rhodamine B. Environ. Res. 2023, 232, 116311. [Google Scholar] [CrossRef] [PubMed]
- Armaković, S.J.; Savanović, M.M.; Armaković, S. Spray-Deposited TiO2 Layers on Aluminum Foil for Sustainable Water Remediation. Crystals 2024, 14, 875. [Google Scholar] [CrossRef]
- Mamaghani, A.H.; Haghighat, F.H.; Lee, C.S. Role of titanium dioxide (TiO2) structural design/morphology in photocatalytic air purification. Appl. Catal. B 2020, 269, 118735. [Google Scholar] [CrossRef]
- Pol, R.; Guerrero, M.; Lecina, E.G.; Altube, A.; Rossinyol, E.; Garroni, S.; Baró, M.D.; Pons, J.; Sort, J.; Pellicer, E. Pt- and (Ni/Pt)-doped TiO2 nanophotocatalysts: A smart approach for sustainable degradation of Rhodamine B dye. Appl. Catal. B 2016, 181, 270–278. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Huang, W.H.; Su, W.N.; Chen, C.L.; Lin, C.J.; Haw, S.C.; Lee, J.F.; Hwang, B.J. Structural evolution and Au nanoparticles enhanced photocatalytic activity of sea-urchin-like TiO2 microspheres: An X-ray absorption spectroscopy study. Appl. Surf. Sci. 2021, 562, 150127. [Google Scholar] [CrossRef]
- Mao, C.Y.; Zuo, F.; Hou, Y.; Bu, X.H.; Feng, P.Y. In Situ Preparation of a Ti3+ Self-Doped TiO2 Film with Enhanced Activity as Photoanode by N2H4 Reduction. Angew. Chem. Int. Ed. 2014, 53, 10485. [Google Scholar] [CrossRef]
- Liu, R.J.; Yang, F.; Xie, Y.L.; Yu, Y. Visible-light responsive boron and nitrogen codoped anatase TiO2 with exposed {0 0 1} facet: Calculation and experiment. Appl. Surf. Sci. 2019, 466, 568–577. [Google Scholar] [CrossRef]
- Shafiee, A.; Aibaghi, B.; Carrier, A.J.; Ehsan, M.F.; Nganou, C.; Zhang, X.; Oakes, K.D. Rapid photodegradation mechanism enabled by broad-spectrum absorbing black anatase and reduced graphene oxide nanocomposites. Appl. Surf. Sci. 2022, 575, 151718. [Google Scholar] [CrossRef]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 1385–8947. [Google Scholar] [CrossRef]
- Huang, H.M.; Zhang, T.T.; Cai, X.N.; Guo, Z.J.; Fan, S.L.; Zhang, Y.J.; Lin, C.W.; Gan, T.; Hu, H.Y.; Huang, Z.Q. In Situ One-Pot Synthesis of C-Decorated and Cl-Doped Sea-Urchin-like Rutile Titanium Dioxide with Highly Efficient Visible-Light Photocatalytic Activity. ACS Appl. Mater. Interfaces 2021, 13, 60337–60350. [Google Scholar] [CrossRef] [PubMed]
- Andronic, L.; Enesca, A. Black TiO2 Synthesis by Chemical Reduction Methods for Photocatalysis Applications. Front. Chem. 2020, 8, 2296–2646. [Google Scholar] [CrossRef] [PubMed]
- Selcuk, S.; Zhao, X.H.; Selloni, A. Structural evolution of titanium dioxide during reduction in high-pressure hydrogen. Nat. Mater. 2018, 17, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, S.W.; Yu, Y.G.; Tian, H.R.; Zhao, Y.L.; Ren, J.C.; Huang, C.; Bian, H.D.; Huang, M.Y.; An, L.; et al. Hydrogen-Location-Sensitive Modulation of the Redox Reactivity for Oxygen-Deficient TiO2. J. Am. Chem. Soc. 2019, 141, 8407–8411. [Google Scholar] [CrossRef]
- Liccardo, L.; Bordin, M.; Sheverdyaeva, P.M.; Belli, M.; Moras, P.; Vomiero, A.; Moretti, E. Surface Defect Engineering in Colored TiO2 Hollow Spheres Toward Efficient Photocatalysis. Adv. Funct. Mater. 2023, 33, 2212486. [Google Scholar] [CrossRef]
- Kim, S.; Cho, Y.; Rhee, R.; Park, J.H. Black TiO2: What are exact functions of disorder layer. Carbon Energy 2020, 2, 44–53. [Google Scholar] [CrossRef]
- Ren, R.; Wen, Z.H.; Cui, S.M.; Hou, Y.; Guo, X.R.; Chen, J.H. Controllable Synthesis and Tunable Photocatalytic Properties of Ti3+-doped TiO2. Sci. Rep. 2015, 5, 10714. [Google Scholar] [CrossRef]
- Xia, T.; Chen, X.B. Revealing the structural properties of hydrogenated black TiO2 nanocrystals. J. Mater. Chem. A 2013, 1, 2983–2989. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Santo, V.D. Effect of Nature and Location of Defects on Bandgap Narrowing in Black TiO2 Nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Yin, H.Y.; Wang, X.L.; Wang, L.; QLin, N.; Zhao, H.T. Self-doped TiO2 hierarchical hollow spheres with enhanced visible-light photocatalytic activity. J. Alloys Compd. 2015, 640, 68–74. [Google Scholar] [CrossRef]
- Wang, G.M.; Wang, H.Y.; Ling, Y.C.; Tang, Y.C.; Yang, X.Y.; Fitzmorris, R.C.; Wang, C.C.; Zhang, J.Z.; Li, Y. Hydrogen-Treated TiO2 Nanowire Arrays for Photoelectrochemical Water Splitting. Nano Lett. 2011, 11, 3026–3033. [Google Scholar] [CrossRef]
- Li, J.; Wu, E.H.; Hou, J.; Huang, P.; Xu, Z.; Jiang, Y.; Liu, Q.S.; Zhong, Y.Q. A facile method for the preparation of black TiO2 by Al reduction of TiO2 and their visible light photocatalytic activity. RSC Adv. 2020, 10, 34775–34780. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.J.; Liu, Q.R.; He, H.; Shi, F.; Huang, G.X.; Xing, B.L.; Jia, J.B.; Zhang, C.X. Coal tar pitch as natural carbon quantum dots decorated on TiO2 for visible light photodegradation of rhodamine B. Carbon 2019, 152, 284–294. [Google Scholar] [CrossRef]
- Wu, Y.C.; Liu, Z.M.; Li, Y.; Chen, J.T.; Zhu, X.X.; Na, P. Construction of 2D-2D TiO2 nanosheet/layered WS2 heterojunctions with enhanced visible-light-responsive photocatalytic activity. Chin. J. Catal. 2019, 40, 60–69. [Google Scholar] [CrossRef]
- Hu, L.M.; Yan, J.T.; Wang, C.L.; Chai, B.; Li, J.F. Direct electrospinning method for the construction of Z-scheme TiO2/g-C3N4/RGO ternary heterojunction photocatalysts with remarkably ameliorated photocatalytic performance. Chin. J. Catal. 2019, 40, 458–469. [Google Scholar] [CrossRef]
- Phongamwong, T.; Barrabés, N.; Donphai, W.; Witoon, T.; Rupprechter, G.; Chareonpanich, M. Chlorophyll-modified Au25(SR)18-functionalized TiO2 for photocatalytic degradation of rhodamine B. Appl. Catal. B Environ. 2023, 325, 122336. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Li, Y.; Yu, H.; Yu, K.; Yu, H.B. Interfacial defective Ti3+ on Ti/TiO2 as visible-light responsive sites with promoted charge transfer and photocatalytic performance. J. Mater. Sci. Technol. 2022, 106, 139–146. [Google Scholar] [CrossRef]
- Liu, Y.; Tian, L.H.; Tan, X.Y.; Li, X.; Chen, X.B. Synthesis, properties, and applications of black titanium dioxide nanomaterials. Sci. Bull. 2017, 62, 431–441. [Google Scholar] [CrossRef]
- Wu, C.Y.; Gao, Z.G.; Gao, S.M.; Wang, Q.Y.; Xu, H.; Wang, Z.Y.; Huang, B.B. Ying Dai, Ti3+ self-doped TiO2 photoelectrodes for photoelectrochemical water splitting and photoelectrocatalytic pollutant degradation. J. Energy Chem. 2016, 25, 726–733. [Google Scholar] [CrossRef]
- Bi, Q.Y.; Song, E.H.; Chen, J.C.; Riaz, M.S.; Zhu, M.H.; Liu, J.J.; Han, Y.F.; Huang, F.Q. Nano gold coupled black titania composites with enhanced surface plasma properties for efficient photocatalytic alkyne reduction. Appl. Catal. B 2022, 309, 121222. [Google Scholar] [CrossRef]
- Yin, G.H.; Huang, X.Y.; Chen, T.Y.; Zhao, W.; Bi, Q.Y.; Xu, J.; Han, Y.F.; Huang, F.Q. Hydrogenated Blue Titania for Efficient Solar to Chemical Conversions: Preparation, Characterization, and Reaction Mechanism of CO2 Reduction. ACS Catal. 2018, 8, 1009–1017. [Google Scholar] [CrossRef]
- Lü, X.J.; Chen, A.P.; Luo, Y.K.; Lu, P.; Dai, Y.M.; Enriquez, E.; Dowden, P.; Xu, H.W.; Kotula, P.G.; Azad, A.K.; et al. Conducting Interface in Oxide Homojunction: Understanding of Superior Properties in Black TiO2. Nano Lett. 2016, 16, 5751–5755. [Google Scholar] [CrossRef] [PubMed]
- Lan, K.; Wang, R.C.; Wei, Q.L.; Wang, Y.X.; Hong, A.; Feng, P.Y.; Zhao, D.Y. Stable Ti3+ Defects in Oriented Mesoporous Titania Frameworks for Efficient Photocatalysis. Angew. Chem. Int. Ed. 2020, 32, 17829–17836. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab-initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, 1133–1138. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).